Abstract
Immune function abnormalities have been implicated in the pathophysiology of schizophrenia (SZ). This is primarily based on the observation that the levels of proinflammatory cytokines are significantly increased in the serum of SZ patients compared with normal control (NC) subjects. However, it is not known if similar cytokines abnormalities are also present in the brain of SZ patients. To further examine the involvement of inflammatory cytokines in the brain of SZ patients, we determined the protein and mRNA levels of TNF-α, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, LTA and IL-1RA in the prefrontal cortex (PFC, Brodmann area 9) of SZ patients. We found that the protein and mRNA expression levels of the cytokines TNF-α and IL-6 are significantly increased and those of IL-10 are significantly decreased in the PFC of SZ patients. No difference in the protein and mRNA levels of IL-1β, IL-13, and IL-1RA was observed between SZ patients and NC subjects. The protein expression levels of IL-8 were significantly decreased and those of LTA were significantly increased in SZ patients, but no significant difference in the mRNA levels of IL-8 and LTA was observed between SZ patients and NC subjects. The levels of IL-2 were undetectable or very low in the postmortem brain of either SZ or NC subjects. These results suggest abnormalities of specific pro- and anti-inflammatory cytokines in the postmortem brain of SZ patients. These observations may have important implications in understanding the role of inflammatory cytokines in the pathophysiology of SZ.
Keywords: schizophrenia, postmortem brain, suicide, cytokines, TNF-α, IL-1β, IL-6
1. Introduction
Immune function abnormalities have been implicated in the etiology and pathophysiology of schizophrenia (SZ). Several studies suggest that prenatal infections act as a risk factor for immune function alterations later in life, such as abnormal cytokine production and marked changes in cognitive and affective behaviors throughout the lifespan (Miller et al., 2013). Several other studies also reported increased microglial activation in SZ patients (Bayer et al., 1999; Schnieder and Dwork, 2011; Steiner et al., 2008).
Cytokines are major mediators of immune function and hence it is not surprising that cytokines have been widely studied in SZ. The suggestion that abnormalities of cytokines are associated with SZ is based on several lines of evidence. For example, the administration of cytokines, such as interferon (IFN)-γ, to rats and humans produces a syndrome known as ‘sickness behavior’ (Dantzer et al., 1999). The development of psychosis with IFN therapy have been generally observed and reported, as reviewed by Cheng et al. (2009) and by Crane et al. (2003). Also, the administration of cytokines to cancer patients is associated with side effects such as anxiety, depression, psychosis, mania and delirium (Capuron et al., 2001). That abnormalities of cytokines are associated with SZ is also supported by the findings that proinflammatory cytokines are abnormally expressed in the serum of SZ patients, as reviewed by Potvin et al. (2008) and by Zakharyan and Boyajyan (2014). The involvement of cytokines in SZ is also based on studies of behavioral effects after peripheral administration of cytokines (Cheng et al., 2009). However, it is not clearly understood if cytokine abnormalities are also present in the brain. Cytokines can either be synthesized in the brain or transported from the periphery (Dantzer et al., 2008). Therefore, it is quite possible that cytokine abnormalities may also be present in the brain and associated with the pathophysiology of SZ. The initial evidence that abnormalities of cytokines may be present in the brain is substantiated by the reports of abnormal levels of cytokines in the cerebrospinal fluid (CSF) of SZ patients (el-Mallakh et al., 1993; Garver et al., 1999; Garver et al., 2003; Soderlund et al., 2009).
Some studies suggest an abnormal expression of cytokines in the postmortem brain of patients with mood disorders and SZ (Fillman et al., 2013; Shelton et al., 2010; Volk et al., 2015). For example, Shelton et al. (2010) found abnormal expression of several proinflammatory cytokines in the postmortem brain (PFC) of depressed subjects. Dean et al. (2010) also reported abnormalities of soluble and membrane-bound tumor necrosis factor (TNF) in the postmortem brain of patients with major depressive disorders (MDD).
In light of this background, it was of interest to examine if alterations in inflammatory cytokines are present in the postmortem brain of SZ patients. Therefore, the aim of the present study was to examine mRNA and protein expression levels of inflammatory cytokines, such as TNF-α, interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, IL-13, and lymphotoxin-A (LTA) in the PFC of SZ patients.
2. Methods
2.1 Subjects
The study was performed in the PFC (Brodmann area 9 [BA9] of 31 SZ patients (16 SZ suicide victims and 15 non-suicide SZ patients) and 24 NC subjects. Brain tissues were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, Maryland. Tissues were collected only after a family member gave informed consent. All tissues from NC and SZ subjects were grossly examined by experienced neuropathologists. Toxicology data were obtained by blood and urine samples analysis. All procedures were approved by the University of Maryland Institutional Review Board (IRB) and by the University of Illinois IRB.
2.2 Diagnostic method
Subject diagnosis was based on the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1992). At least one family member and/or a friend, after giving written informed consent, underwent an interview. Diagnoses were made by a consensus of two psychiatrists from the data obtained in the interview, medical records from the case, and records obtained from the Medical Examiner’s office. Normal control subjects were verified as free from mental illnesses using these consensus diagnostic procedures.
2.3 Determination of mRNA levels
2.3.1 RNA extraction
RNA was isolated from 100 mg of tissue using the TRIZOL (Invitrogen) reagent followed with DNase treatment, as per the manufacturer's instructions. RNA concentration and quality was determined using NanoDrop®ND-1000 (Thermo Scientific, Waltham, MA) and Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA) respectively. RNA integrity number (RIN) showed no significant difference between groups and was >7.0 for all samples.
2.3.2 Quantitative real-time PCR
Gene expression was determined using a two-step real-time RT-PCR (qRT-PCR) method, as previously described (Pandey et al., 2012). Briefly, 1ug of total RNA was reverse transcribed using 200 units MMLV-reverse transcriptase in 50ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, with final reaction volume of 20 μl.
qPCR was performed using Pre-designed Taqman gene expression assays (Applied Biosystems, Foster City, CA) for all target and housekeeping genes, on MX3005p sequence detection system (Agilent, Santa Clara, CA ). The TaqMan assay IDs are listed in Table 1. To determine the stability and optimal number of housekeeping genes we used geNORM version 3.4 (PrimerDesign Ltd, UK) to test twelve commonly used reference genes of different functional classes in 10 samples from each test group (Vandesompele et al., 2002). geNorm analysis was used to determine number and stability of normalizing genes. β-actin and GAPDH ranked as the most stable genes in our samples. PCR efficiency was tested over 5-log dilution series and confirmed that all target genes and housekeeping genes similar amplification efficiencies. For each primer/probe set, the PCR reaction is carried out using 10 μl of cDNA diluted 1:10 fold. Each qPCR plate includes a “no reverse transcriptase” and “no template” control to eliminate non-specific amplification. One sample, from each target gene is run on a gel to confirm specificity and all samples were run in triplicates. Target gene qPCR data is normalized to the geometic mean of β-actin and GAPDH and is expressed relative to the control samples using 2 (ΔΔCt) method, where ΔΔCT = (CT target - CT internal control) Subject - (CT target - CT internal control) Control, and CTinternal control is the geometric mean of ACTB and GAPDH CTs. Outliers were excluded if the normalized (delta Ct) values were greater than 2 standard deviations from the group mean. Relative expression levels are reported as fold change and ΔCt values are used for further statistical analysis (Applied Biosystems User Bulletin No. 2).
Table 1.
TaqMan primers/probes used for qPCR analysis
| TaqMan accession | Probe location (exon boundary) | Assay function | |
|---|---|---|---|
| ACTB | Hs99999903_m1 | 1–1 | House Keeping (HK) |
| GAPDH | Hs99999905_m1 | 3–3 | House Keeping (HK) |
| TNF-α | Hs99999043_m1 | 1–2 | target gene |
| IL-1β | Hs01555410_m1 | 3–4 | target gene |
| IL-1RN (IL-1RA) | Hs00893626_m1 | 4–5 | target gene |
| IL-2 | Hs00174114_m1 | 2–3 | target gene |
| IL-6 | Hs00985639_m1 | 2–3 | target gene |
| IL8 | Hs00174103_m1 | 1–2 | target gene |
| IL10 | Hs00961622_m1 | 4–5 | target gene |
| IL13 | Hs00174379_m1 | 1–2 | target gene |
| LTA | Hs99999086_m1 | 3–4 | target gene |
2.4 Determination of Brain Protein Levels Using ELISA
Levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α) were determined in brain homogenates (100 μL) by enzyme-linked immunosorbent assay (ELISA) using commercially available Quantakine® kits (R & D Systems, Inc., Minneapolis, MN) for human IL-1β, human IL-6, and human TNF-α, according to the manufacturer’s instructions.
2.5 Determination of Brain Protein Levels Using Western Blot Method
Immunolabeling of inflammatory cytokines (IL-8, IL-10, IL-13, IL-1RA, LTA) was determined by the Western blot method in membrane fractions, as described in detail in one of our earlier publication (Dwivedi and Pandey, 2000). Briefly, the brain samples of PFC were homogenized directly in Tris-HCl buffer (50 mM, pH 7.5). Equal volumes of membrane fractions isolated by this procedure (30 μg of protein in 20 μL) were separated from 4–12% Bis-Tris gel (Invitrogen, Grand Island, NY, USA) and transferred to nitrocellulose membranes (Amersham). The membrane was blocked for 1 h at room temperature with 5% non-fat milk in phosphate-buffered saline ( pH 7.4) and the blots were initially developed using the polyclonal primary antibodies (1:1000 dilution) overnight and detected by using anti-rabbit IgG (Amersham) at (1:3000 dilution)for 4 h followed by the application of the ECL chemiluminescence western blotting kit (Amersham).
The membranes were stripped using stripping solution (Chemicon International, Temecula, CA) To normalize the data, β-actin level was measured in the same immunoblot using β-actin as the primary monoclonal antibody (1:5000 for 2 h) and anti-mouse IgG (1:5000 for 2 h) as the secondary antibody. The levels of inflammatory cytokine proteins were calculated as a ratio of the optical density of the primary antibody to the optical density of β-actin antibody. The following polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA): IL-8 (molecular weight 8kDa), IL-10 (molecular weight 37 kDa), IL-13 (molecular weight 13 kDa), and IL-1RA (molecular weight 25 kDa). LTA polyclonal antibody (molecular weight 19 kDa) was purchased from, Abnova (Walnut, CA). Secondary antibodies were purchased from (GE Healthcare Life Sciences, Pittsburgh, PA).
2.6 Determination of IL-2 Protein and mRNA Expression levels
We tried to determine the mRNA levels of IL-2 using the following probe: Hs00174114_m1 (see Table 1). However, we found that either the IL-2 mRNA levels were very low or undetectable. Similarly, we used the Quantikine ELISA kit (purchased from R & D Systems, Inc., Minneapolis, MN) for the immunoassay of human IL-2. Using this method, we found that the IL-2 protein expression levels were either not present or were very low. Because of this we could not quantitate the levels of IL-2 in the postmortem brain samples.
2.7 Statistical Analysis and Effect of Confounding Variables
We analyzed the data using SAS 9.2 statistical software package. First, we used two sample t-test to compare NC subjects with SZ patients. In order to examine the effect of confounding variables, we used generalized linear model (PROC GLM in SAS) for each outcome measure to compare those two groups adjusting for fixed covariates like age, sex and race. To examine the association between group and gender we performed a contingency chi-square test.
We did not observe any significant effect of confounding variables, such as age, race, sex, postmortem interval (PMI) or brain pH on either protein or mRNA expression of the main outcome measures between SZ and NC groups.
3. Results
3.1 mRNA levels of inflammatory cytokines in the PFC of SZ and NC subjects
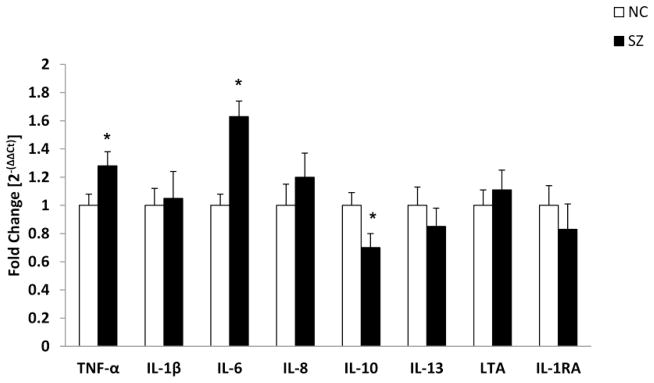
We determined the mRNA levels of TNF-α, LTA, IL-1β, IL-6, IL-8, IL-10, IL-13 and IL-1 receptor antagonist (IL-1RA) in the PFC of SZ patients. The results are shown in Figure 1.
Figure 1.
Mean mRNA expression levels of inflammatory cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-13, lymphotoxin-A (LTA), and IL-1 receptor antagonist (IL-1RA) in the prefrontal cortex (PFC) of schizophrenia (SZ) patients and normal control (NC) subjects. The data are shown as fold change in mRNA levels. Values are fold change ± S.E.M.
*p< .05
3.1.1. TNF-α and LTA in the PFC of SZ and NC subjects
We compared the mRNA levels of TNF-α as well as LTA between SZ and NC subjects. We found that the mRNA levels of TNF-α were significantly increased in SZ compared with NC subjects (Figure 1). The mRNA levels of LTA were not significantly different between the SZ and NC subjects (Figure 1).
3.1.2. IL-1β, IL-2, IL-6 and IL-1RA in the PFC of SZ and NC subjects
We then compared the mRNA levels of IL-1β and IL-6 between the SZ and NC subjects and found that the mRNA levels of IL-6 were significantly increased in the PFC of SZ patients compared with NC subjects (Figure 1). No significant differences in the mRNA levels of IL-1β between SZ and NC subjects were observed (Figure 1).
We could not detect the mRNA levels of IL-2 in the PFC of SZ and NC subjects. There was no significant difference in the mRNA expression levels of IL-1RA between SZ and NC subjects (Figure 1).
3.1.3 IL-8, IL-10 and IL-13 in the PFC of SZ and NC subjects
The mRNA levels of IL-8, IL-10 and IL-13 are shown in Figure 1. When we compared the mRNA levels of IL-10 between SZ and NC subjects we found that these were significantly decreased in SZ patients compared with NC subjects. However, there was no difference in the mRNA levels of IL-8 or IL-13 between the SZ and NC subjects (Figure 1).
These results suggest that the levels of TNF-α and IL-6 are significantly increased in the PFC of SZ patients compared with NC subjects and that the mRNA levels of IL-10 are significantly decreased in the PFC of SZ patients compared with NC subjects. However, there was no significant difference in the mRNA levels of either IL-8 or IL-13 between SZ and NC subjects.
3.2 Protein expression levels of cytokines in the PFC of SZ and NC subjects
Since we observed changes in mRNA levels of several cytokines we studied in the PFC of SZ patient, we also determined the protein expression of these cytokines in SZ patients and NC subjects.
Representative immunoblots of two SZ suicide patients and two NC subjects are shown in Figure 2. As can be seen, protein levels of IL-8, IL-10 and IL-1RA appear to be decreased, while LTA levels appear to be increased in the two SZ suicide subjects compared with the two NC subjects.
Figure 2.
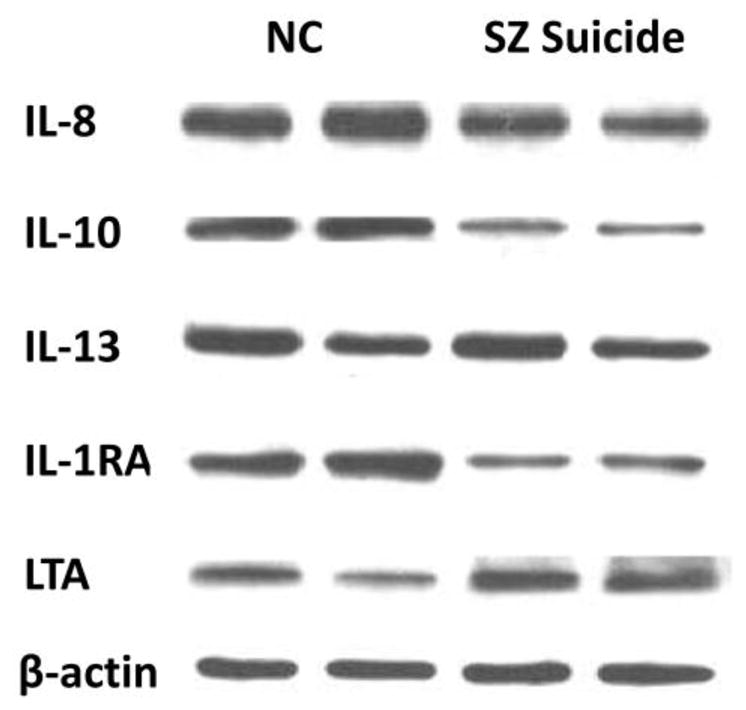
Representative Western blots showing the immunolabeling of IL-8, IL-10, IL-13, IL-1RA, LTA, and β-actin in the PFC of two NC subjects and two SZ suicide patients.
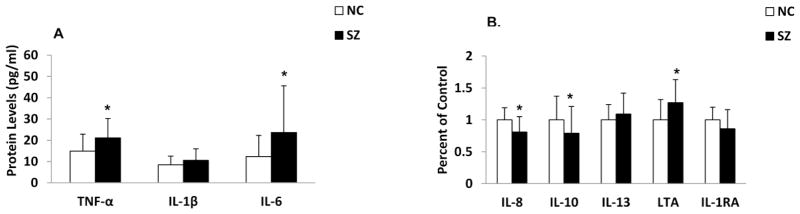
When we compared the protein levels of cytokines between SZ patients and NC subjects, we found that the protein expression levels of TNF-α, IL-6, and LTA were significantly increased and those of IL-8 and IL-10 were significantly decreased in the PFC of SZ patients compared with NC subjects, as shown in Figure 3 (A and B). The protein levels of IL-1β, IL-13 and IL-1RA were not significantly different in the PFC of SZ patients compared with NC subjects, showing that, in general, both protein and mRNA expression levels of the cytokines are altered in the PFC of SZ patients.
Figure 3.
Mean protein expression levels of TNF-α, IL-1β and IL-6 (A) and IL-8, IL-10, IL-13, LTA and IL-1RA (B) in the PFC of SZ patients and NC subjects.
Values are mean ± SD.
*p < .05
3.3 mRNA and protein levels of inflammatory cytokines in SZ suicide, SZ non-suicide and NC subjects
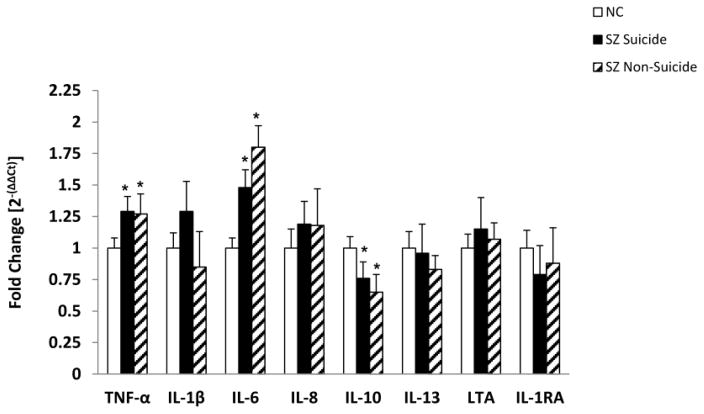
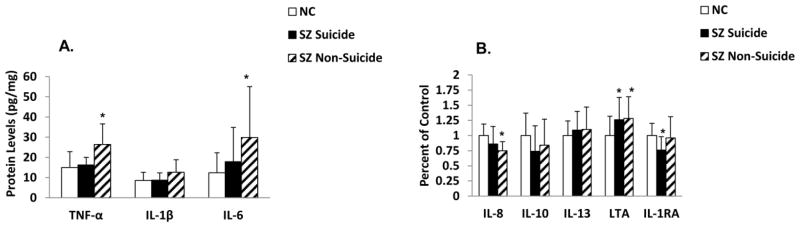
The SZ group consisted of SZ patients who died of natural causes and those who died by suicide. Since some of these inflammatory cytokines have been shown to be altered in the postmortem brain of suicide subjects (Pandey et al., 2012; Tonelli et al., 2008) and in the plasma of suicidal patients [see review by Miller et al.(2013)], it was of interest to examine if the observed changes in the inflammatory cytokines in SZ were specific to suicide or to non-suicide SZ patients. Fifteen SZ patients died of natural causes (i.e., non-suicide) and 16 SZ patients died by suicide. We did not observe any significant differences in the mRNA (Figure 4) and protein (Figure 5) expression of any of the inflammatory cytokines studied between SZ patients who died of natural causes and those who died by suicide. It was interesting to observe that similar to total SZ group, the mRNA expression of TNF-α and IL-6 was significantly increased and that of IL-10 was significantly decreased in both non-suicide SZ and suicide SZ patients compared with NC subjects (Figure 4). In terms of protein expression, both non-suicide and suicide SZ patients showed similar trends and there was no difference in the levels of any of the cytokines between SZ suicide and non-suicide patients (Figure 5). The only exception was that IL-1RA was significantly decreased in SZ suicide but not in SZ non-suicide (Figure 5 B).
Figure 4.
Mean mRNA expression levels of TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-13, LTA and IL-1RA in the PFC of suicidal SZ patients, non-suicidal SZ patients and NC subjects. The data are shown as fold change in mRNA levels. Values are fold change ± S.E.M.
*p< .05
Figure 5.
Mean protein expression levels of TNF-α, IL-1β and IL-6 (A) and IL-8, IL-10, IL-13, LTA and IL-1RA (B) in the PFC of suicidal SZ patients, non-suicidal SZ patients and NC subjects.
Values are mean ± SD.
*p < .05
These results thus show that the significant changes observed in TNF-α, IL-6, IL-8, IL-10, and LTA in SZ patients are not related to suicide, but appear to be related to SZ.
3.4 The effect of psychoactive medication on mRNA and protein levels of inflammatory cytokines in SZ patients
One of the limitations of the study of cytokines in the postmortem brain samples from SZ patients is the presence and history of previous treatment with psychoactive drugs at the time of death. One of the issues is therefore to examine if the observed changes in cytokine levels in the PFC of SZ patients are related to the presence and/or treatment with psychoactive medication. Out of 31 SZ patients, 21 had the presence of psychoactive drugs, primarily neuroleptics, at the time of death and 10 were free of any psychoactive or neuroleptic drugs. We therefore compared the cytokine levels between SZ patients with psychoactive drugs (SZ MEDS) and SZ patients with no psychoactive drugs (SZ No-MEDS) at the time of death. We found that the mRNA levels of IL-1β (p = 0.008) and IL-6 (p = 0.005) were significantly decreased in the PFC of SZ MEDS patients compared with SZ Non-MEDS patients. There was no significant difference in the PFC protein levels of any of the cytokines we studied between SZ MEDS and SZ Non-MEDS group.
A previous meta-analysis of the effect of psychoactive medication in SZ patients has been carried out by Miller et al. (2011). Combining all the studies which examined the effect of neuroleptic treatment on various cytokines, they found that there was a significant increase in sIL-2R and IL-12 levels, and a significant decrease in IL-1β, IL-6 and TGF-β in the blood of SZ patients. Although these studies were carried out in blood samples, it is quite possible that the lack of significant difference for IL-1β observed in the current study between SZ patients and NC subjects could be attributed to the presence of SZ patients who were on neuroleptic treatment. However, the levels of IL- 1β in SZ patients were still higher than in the NC subjects (p = 0.06), but missed the significance.
4. Discussion
In this study, we examined the role of inflammatory cytokines in the pathophysiology of SZ. We determined both the pro and anti-inflammatory cytokines in the PFC obtained from SZ patients and NC subjects. We observed that the protein and gene expression of TNF-α and IL-6 were significantly increased and those of IL-10 were significantly decreased in the PFC of SZ patients compared with NC subjects. In addition, only protein levels of IL-8 were significantly decreased and those of LTA were significantly increased in SZ patients compared with NC subjects. No significant differences were found in the protein and mRNA expression of IL-1β, IL-1RA and IL-13 in the PFC of SZ patients compared with NC subjects.
We found good association between protein and mRNA expression differences in TNF-α, IL-6, and IL-10 levels (i.e., the mRNA and protein expression levels changed in the same direction). However, there was dissociation between protein and mRNA expression levels of LTA and IL-8. Although the reasons for this dissociation are unclear, in some of our previous studies we have observed dissociation between protein and mRNA expression, for example TLR3 (Pandey et al., 2014). Many other investigators find a lack of association or correlation between mRNA and protein expression levels, as reviewed by Maier et al. (2009). A lack of association between mRNA and protein expression has been attributed to many biological factors such as transcription, translation and post-translational changes, such as half-lives of proteins. Abnormalities in the translational and transcriptional efficiency and/or protein half-life or protein turnover may be responsible for the observed dissociation. Sometimes, the methods used for the determination of mRNA or protein levels may also cause this dissociation (Maier et al., 2009). We have used Western blot method for the determination of LTA and IL-8, and Western blot methodology may have contributed towards this dissociation. However, in summary, such dissociations between mRNA and protein expression are not uncommon (Maier et al., 2009).
We also determined the protein and mRNA levels of LTA, also known as TNF-β, which is a member of the TNF superfamily of cytokines. TNF-β is closely related to TNF-α and these share common receptors (Williams-Abbott et al., 1997). It has been suggested that LTA may play a role in the development of SZ (Pae, 2007). LTA causes glutamate secretion, the activation of microglia, (Smith and Maes, 1995) and it plays an important role in the modulation of other cytokines (Goldbach et al., 1997). LTA has been shown to prevent prenatal infections such as toxoplasmosis that may be one of the infections which may increase risk for SZ (Goldbach et al., 1997; Schluter et al., 2003; Smith and Maes, 1995). We therefore also studied LTA in SZ brain and found that only the protein but not the mRNA expression of LTA is increased in SZ brain, suggesting a role of LTA in SZ.
Recently, two studies (Fillman et al., 2013; Volk et al., 2015) investigated inflammatory markers in the postmortem brain of SZ patients. Fillman et al. (2013) used SOLID next generation sequencing to quantify neuroimmune mRNA expression levels in dorsolateral PFC of SZ and matched controls and found several differentially regulated transcripts present in subjects with SZ. They found that among these genes the mRNA levels of IL-6 and IL-8 were significantly increased in dorsolateral PFC of SZ patients but no change in IL-1β. Volk et al. (2015) who studied immune related genes in postmortem brain of SZ subjects found higher mRNA expression levels of IL-6 and IFN-β in the PFC of SZ subjects. Our observation that IL-6 is increased in PFC of SZ patients is consistent with both studies. However, we did not observe changes in IL-8 mRNA levels, as opposed to the observation by Fillman et al. (2013) in SZ.
The suggestion that abnormalities of proinflammatory cytokines may be associated with the pathophysiology of SZ, as indicated before, is primarily based on the observation of increased proinflammatory cytokines in the serum of SZ patients compared with NC subjects, as reviewed by Potvin et al.(2008) and the meta-analysis reported by Miller et al. (2011). We have also previously reported an increase in the mRNA and protein expression levels of IL-1β, IL-6 and TNF-α in the lymphocytes of SZ patients compared with NC subjects (Pandey et al., 2015). Generally, only cytokines protein expression has been determined in the serum of SZ patients and, besides our study, there is only one other study of mRNA expression in the monocytes of SZ patients by Padmos et al. (2008) who, similar to our results, also reported significantly increased mRNA levels of IL-1β, IL-6 and TNF-α in the monocytes of SZ patients. Our present findings indicating increased mRNA and protein levels of TNF-α and IL-6 in the postmortem brain are similar to what we reported in the lymphocytes and plasma of SZ patients, suggesting a similar abnormality in the brain and the periphery. The suggestion that abnormalities of inflammation may be present in the brain of SZ patients is also based on reports of increased microglia activation in the brain of SZ patients using PET techniques by van Berckel et al. (2008) and by Doorduin et al. (2009). Increased microglia density in the postmortem brain of SZ patients has been observed by Steiner et al. (2008) Our studies of inflammatory cytokines in postmortem brain of SZ patients thus support the earlier reports of neuroinflammation.
In this study, we not only observed an increase in the levels of proinflammatory cytokines but also found a decrease in the levels of an important anti-inflammatory cytokine, known as IL-10. This is one of the most important and best characterized anti-inflammatory cytokine. IL-10 antagonizes and balances the activity and/or production of proinflammatory cytokines and thus regulates inflammation (Moore et al., 2001; Murray, 2006). The role of IL-10 in antagonizing the effects of proinflammatory cytokines has been primarily investigated in prenatal exposure to infection (Meyer et al., 2009). The development of normal adult brain function may partly be under the particular influence of a balance between pro- and anti-inflammatory cytokines during the prenatal life (Meyer et al., 2009), and disturbances of this balance would precipitate adult behavior pathology (Meyer et al., 2008). Whereas the induction of both pro- and anti-inflammatory molecules in the fetal brain may diminish each other’s long-term consequences on the brain and behavioral functions, a shift of the balance toward proinflammatory cytokines could be considered more pathologically effective compared to a shift toward the anti-inflammatory side (Meyer et al., 2009). Thus, our observation that there was an increase in the levels of proinflammatory cytokines and a decrease in the levels of the anti-inflammatory cytokine IL-10 suggests that decreased IL-10 levels would exacerbate the behavioral effects of increased proinflammatory cytokines and may promote the development of SZ.
We did not find significant differences in the protein or mRNA expression levels of IL-1β in the PFC of SZ patients. This is consistent with our previous study of IL-1β protein and mRNA expression in the plasma or lymphocytes of SZ patients (Pandey et al., 2015). In that study, we found significant differences in the TNF-α and IL-6 protein and mRNA levels between SZ and NC subjects. The current findings in the PFC of SZ subjects are similar to our earlier findings in the blood of SZ patients. Also, several studies did not find changes in IL-1β protein expression levels in the plasma/serum of SZ patients, but some do (Miller et al., 2011).
We find a good increase or decrease of TNF-α, IL-6 and IL-10 in the PFC of SZ patients. For example, we found about 30% increase in TNF-α and IL-6, and about 30% decrease in IL-10 mRNA and protein expression levels in SZ patients. However, IL-1β was the exception where we did not find pronounced differences between SZ and NC subjects.
Some studies report increased and other report decreased IL-1β levels [see meta-analysis by (Miller et al., 2011) and a review by (Potvin et al., 2008)]. Potvin et al. (2008) found low effect size for IL-1β in 8 studies in SZ. Some studies report decreased IL-1β in the CSF of SZ patients (Miller et al., 2011). Thus, the results of IL-1β studies in SZ are inconsistent (Potvin et al., 2008).
IL-1RA is generally reported to be increased in SZ (Potvin et al., 2008) but we did not find any IL-1RA change in the brain.
The reason for an increase in proinflammatory cytokines and a decrease in anti-inflammatory cytokines in the postmortem brain of SZ patients is unclear. Prenatal maternal infection with several viral and bacterial agents is a major risk factor for SZ (Brown, 2006; Brown and Susser, 2002). This is based on both human and animal studies suggesting that prenatal maternal infection alters cytokine production in placenta and amniotic fluid (Brown, 2006).
The other issue of increased proinflammatory cytokines in the postmortem brain of SZ patients is their functional and behavioral effects. Different cytokines have specific neural developmental effects in the brain as evidenced by in vitro culture studies. For example, IL-1β is most effective in inducing the conversion of mesencephalic progenitor cells into adult genetic phenotype, and IL-6 is effective in decreasing the survival of fetal brain serotonin neurons. Jarskog et al. (1997) have found that, at low concentrations, TNF-α can disrupt cortical neuron dendrite development and has the same effect as the exposure of fetal pre-cortical neurons to higher concentrations of IL-β, IL-6 or TNF-α. These observations show the important role these cytokines play in neural development. Thus, increased levels of proinflammatory cytokines and decreased anti-inflammatory cytokines may affect the neurodevelopment in SZ.
5. Limitations
This study has several strengths. Although brain immune genes have been studied using Affymetrix assay in SZ postmortem brain, our study is probably the first to determine both protein and mRNA of pro- and anti-inflammatory cytokines in postmortem brain of SZ patients individually. The main limitation of this study is that many of the SZ patients were on neuroleptics or on other psychoactive drugs at the time of death. Thus, it is not clear if the observed differences in cytokine levels are due to the illness or result from prior treatment with psychoactive drugs. It appears that the observed changes in inflammatory cytokine levels may not results from previous neuroleptic exposure because many studies show that neuroleptic treatment decreases proinflammatory cytokines (Drzyzga et al., 2006) and increases anti-inflammatory cytokines, such as IL-10, in SZ patients (de Witte et al., 2014). Since we have observed an increase in the levels of proinflammatory cytokines, this effect does not appear to be due to neuroleptic treatment. However, the effect of neuroleptics cannot be ruled out completely.
6. Conclusion
In this study, we show that the protein and mRNA expression of proinflammatory cytokines, such as TNF-α and IL-6, are significantly increased and the levels of anti-inflammatory cytokine IL-10 are significantly decreased. We also report that the protein levels of another member of the TNF superfamily, known as LTA, are also increased in SZ patients. Since both TNF-α and LTA are involved in the prenatal clearance of toxoplasmosis in the brain, this study may suggest that the observed cytokine changes may be a consequence of prenatal infection, which may be one of the risk factors for SZ. Further studies of innate and adaptive immunity in SZ in the brain may clarify this point.
Table 2.
Demographic Characteristics of Subjects
| Group | Age (year s) | Race | Gender | PMI (hours) | Brain pH | Cause of Death | Psychotropic Drugs (at the time of death) | Psychiatric Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|
| Normal Control Subjectsa | |||||||||
| 1. | CONTROL | 19 | Black | Male | 11 | 6.9 | GSW | None | Normal |
| 2. | CONTROL | 22 | Black | Male | 19 | 6.9 | GSW | None | Normal |
| 3. | CONTROL | 42 | White | Female | 23 | 7.2 | Pneumonia | None | Normal |
| 4. | CONTROL | 18 | Black | Male | 11 | 6.2 | GSW | None | Normal |
| 5. | CONTROL | 37 | Black | Male | 5 | 7.1 | ASCVD | None | Normal |
| 6. | CONTROL | 48 | White | Male | 26 | 6.9 | ASCVD | None | Normal |
| 7. | CONTROL | 40 | White | Female | 7 | 7.0 | ASCVD | None | Normal |
| 8. | CONTROL | 23 | Black | Male | 15 | 6.8 | GSW | None | Normal |
| 9. | CONTROL | 38 | Black | Male | 16 | 6.9 | Lung sarcoidosis | None | Normal |
| 10 . | CONTROL | 65 | Black | Female | 23 | 6.9 | ASCVD | None | Normal |
| 11 . | CONTROL | 35 | White | Male | 24 | 6.9 | Crush injury to abdomen and chest | None | Normal |
| 12 . | CONTROL | 63 | White | Female | 30 | 7.1 | Ovarian cancer | None | Normal |
| 13 . | CONTROL | 37 | White | Male | 24 | 7.0 | ASCVD | None | Normal |
| 14 . | CONTROL | 18 | White | Female | 24 | 6.2 | MVA | None | Normal |
| 15 . | CONTROL | 45 | White | Male | 22 | 7.3 | ASCVD | None | Normal |
| 16 . | CONTROL | 18 | Black | Female | 16 | 6.6 | MVA | None | Normal |
| 17 . | CONTROL | 26 | White | Male | 12 | 6.9 | Arrhythmia | None | Normal |
| 18 . | CONTROL | 47 | White | Male | 10 | 7.0 | ASCVD | None | Normal |
| 19 . | CONTROL | 31 | White | Male | 16 | 7.2 | MVA | None | Normal |
| 20 . | CONTROL | 60 | White | Male | 15 | 7.1 | Accidental drowning | None | Normal |
| 21 . | CONTROL | 28 | White | Male | 13 | 6.8 | Electrocution | None | Normal |
| 22 . | CONTROL | 45 | White | Female | 16 | 6.9 | Cardiac arrhythmia | None | Normal |
| 23 . | CONTROL | 62 | White | Male | 19 | 7.0 | Cardiac arrest | None | Normal |
| 24 . | CONTROL | 53 | White | Male | 15 | 6.9 | ASCVD | None | Normal |
| Schizophrenia Subjects b | |||||||||
| 1. | SUICIDE | 45 | Black | Male | 10 | 6.8 | Suicide, stab wound | Haldol | Schizophrenia |
| 2. | SUICIDE | 20 | White | Female | 11 | 6.5 | Jump from height, multiple injuries | Haldol | Schizophrenia |
| 3. | SUICIDE | 54 | White | Male | 12 | 6.6 | Suicide, drowning | Haldol | Schizophrenia |
| 4. | SUICIDE | 20 | White | Male | 23 | 6.4 | OD, Darvocet Intoxication | Fluphenazine | Schizophrenia, Ethanol abuse |
| 5. | SUICIDE | 40 | White | Male | 17 | 6.8 | Jump from height, multiple injuries | Trifluoperazine | Schizophrenia |
| 6. | SUICIDE | 28 | White | Male | 13 | 7.1 | Suicide, hanging | Thioridazine | Schizophrenia, Ethanol abuse |
| 7. | SUICIDE | 35 | White | Female | 7 | 6.9 | Suicide, multiple drugs intoxication | Amitriptyline, amoxapine, loxapine, nortriptyline | Schizophrenia |
| 8. | SUICIDE | 37 | White | Male | 20 | 6.7 | Suicide, drowning | Haldol | Schizophrenia, Ethanol abuse |
| 9. | SUICIDE | 37 | White | Male | 22 | 7.1 | Suicide, GSW to chest | None | Schizophrenia, Ethanol abuse |
| 10. | SUICIDE | 51 | White | Female | 21 | 6.5 | Suicide, overdose | None | Schizophrenia |
| 11. | SUICIDE | 34 | White | Male | 16 | 6.60 | Suicide, jumped from height, multiple injuries | Mesoridazine | Schizophrenia |
| 12. | SUICIDE | 21 | White | Male | 26 | 6.4 | Jumped from height, multiple injuries | Fluphenazine | Schizophrenia |
| 13. | SUICIDE | 23 | White | Male | 20 | 6.6 | Jumped from height, multiple injuries | None | Schizophrenia, Hallucinogen abuse |
| 14. | SUICIDE | 45 | Black | Male | 8 | 6.6 | Suicide, hanging | Olanzapine | Schizophrenia |
| 15. | SUICIDE | 37 | White | Male | 14 | 6.7 | Suicide, electrocution | Risperidone, Fluphenazine | Schizophrenia |
| 16. | SUICIDE | 54 | White | Male | 19 | 6.6 | Suicide, bleeding | None | Schizophrenia |
| 17. | Non- Suicide | 71 | White | Female | 12 | 6.8 | ASCVD | None | Schizophrenia |
| 18. | Non- Suicide | 41 | Black | Female | 16 | 6.6 | Morbidly obese, dilated cardiomyopathy | Perphenazine | Schizophrenia |
| 19. | Non- Suicide | 50 | Black | Female | 11 | 6.9 | Environmental hyperthermia, complication from schizophrenia | None | Schizophrenia |
| 20. | Non- Suicide | 24 | Black | Male | 23 | 6.7 | ASCVD | Olanzapine | Schizophrenia |
| 21. | Non- Suicide | 77 | White | Male | 17 | 6.8 | ASCVD | Risperidone | Schizophrenia |
| 22. | Non- Suicide | 45 | Black | Female | 17 | 6.6 | Diabetic ketoacidosis | Haldol | Schizophrenia |
| 23. | Non- Suicide | 47 | Black | Male | 20 | 7.0 | ASCVD | Fluphenazine | Schizophrenia, Ethanol abuse, Polysubstance abuse |
| 24. | Non- Suicide | 55 | White | Male | 12 | 6.4 | ASCVD | Olanzapine | Schizophrenia, Ethanol abuse |
| 25. | Non- Suicide | 41 | Black | Male | 19 | 6.5 | ASCVD | Haldol | Schizophrenia, Ethanol abuse Schizophrenia, Ethanol abuse, |
| 26. | Non- Suicide | 40 | White | Male | 14 | 6.6 | MVA | None | Cannabis abuse, Cocaine abuse |
| 27. | Non- Suicide | 33 | Black | Male | 12 | 6.2 | Appendicitis/Peritonitis | Olanzapine | Schizophrenia |
| 28. | Non- Suicide | 42 | White | Female | 14 | 6.8 | Liver Cirrhosis | None | Schizophrenia, Cocaine abuse, |
| 29. | Non- Suicide | 53 | White | Male | 14 | 6.1 | ASCVD | None | Polysubstance abuse Schizophrenia |
| 30. | Non- Suicide | 83 | White | Male | 18 | 7.0 | Electrocution, accidental | None | Schizophrenia |
| 31. | Non- Suicide | 57 | White | Male | 11 | 6.2 | Allergic reaction | Haldol | Schizophrenia |
ASCVD = atherosclerotic cardiovascular disease, GSW = gunshot wound, DKA= diabetic ketoacidosis NA = not available
PE/DVT = Pulmonary embolism/deep venous thrombosis.
Mean ± SD age is 38.3 ± 15.2 years; PMI is 17.2 ± 6.3 hours; brain pH is 6.9 ± 0.3; 8 Black, 16 White; 17 Males, 7 Females
Mean ± SD age is 43.2 ± 15.6 years; PMI is 15.8 ± 4.7 hours; brain pH is 6.6 ± 0.3; 9 Black, 22 White; 23 Males, 8 Females
Acknowledgments
Funding
This research was supported by a grant RO1 MH098554 (Dr. Pandey) from the National Institute of Mental Health, Rockville, MD. The funding source had no role in study design, collection, analysis and interpretation of data or the writing of the manuscript.
We thank Runa Bhaumik, Ph.D. for her help with the statistical analyses.
Footnotes
Conflict of interest statement
All authors declare that they have no financial interests or potential conflicts of interest related directly or indirectly to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271(2):126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32(2):200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8(1):51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63(3):376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Chen CC, Ho AS, Chiu NY. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538–542. doi: 10.1176/appi.psy.50.5.538. [DOI] [PubMed] [Google Scholar]
- Crane C, Martin M, Johnston D, Goodwin GM. Does depression influence symptom severity in irritable bowel syndrome? Case study of a patient with irritable bowel syndrome and bipolar disorder. Psychosom Med. 2003;65(5):919–923. doi: 10.1097/01.psy.0000088590.01737.07. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Aubert A, Bluthe R-M, Gheusi G, Cremona S, Laye S, Konsman JP, Parnet P, Kelley KW. Mechanisms of the behavioral effects of cytokines. In: Dantzer R, Wollman EE, Yirmiya R, editors. Cytokines, stress and depression. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 83–105. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, Bahn S. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res. 2014;154(1–3):23–29. doi: 10.1016/j.schres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010;120(1–3):245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20(6):532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Adrenal glucocorticoids modulate [3H]cyclic AMP binding to protein kinase A (PKA), cyclic AMP-dependent PKA activity, and protein levels of selective regulatory and catalytic subunit isoforms of PKA in rat brain. J Pharmacol Exp Ther. 2000;294(1):103–116. [PubMed] [Google Scholar]
- el-Mallakh RS, Suddath RL, Wyatt RJ. Interleukin-1 alpha and interleukin-2 in cerebrospinal fluid of schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17(3):383–391. doi: 10.1016/0278-5846(93)90072-z. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Garver DL, Nair TR, Christensen JD, Holcomb J, Ramberg J, Kingsbury S. Atrophic and static (neurodevelopmental) schizophrenic psychoses: premorbid functioning, symptoms and neuroleptic response. Neuropsychopharmacology. 1999;21(1):82–92. doi: 10.1016/S0893-133X(98)00138-9. [DOI] [PubMed] [Google Scholar]
- Garver DL, Tamas RL, Holcomb JA. Elevated interleukin–6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28(8):1515–1520. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- Goldbach JM, Roth J, Storr B, Zeisberger E. Changes of abdominal temperature and circulating levels of cortisol and interleukin-6 in response to intra-arterial infusions of tumor necrosis factor-alpha or tumor necrosis factor-beta in guinea pigs. Eur J Pharmacol. 1997;334(2–3):249–254. doi: 10.1016/s0014-2999(97)01151-5. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Xiao H, Wilkie MB, Lauder JM, Gilmore JH. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Int J Dev Neurosci. 1997;15(6):711–716. doi: 10.1016/s0736-5748(97)00029-4. [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583(24):3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35(5):959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:92–100. doi: 10.1016/j.pnpbp.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6(4):379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, de Ridder D, Kupka RW, Nolen WA, Drexhage HA. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65(4):395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Pae CU. Potential role of lymphotoxin-alpha (tumor necrosis factor-beta) in the development of schizophrenia. Med Hypotheses. 2007;68(6):1359–1362. doi: 10.1016/j.mehy.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Zhang H. Proinflammatory cytokines and their membrane-bound receptors are altered in the lymphocytes of schizophrenia patients. Schizophr Res. 2015;164(1–3):193–198. doi: 10.1016/j.schres.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Toll-like receptors in the depressed and suicide brain. J Psychiatr Res. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46(1):57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Schluter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, Deckert M. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170(12):6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry. 2011;69(2):134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2010;16(7):751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45(2):135–141. doi: 10.1016/0306-9877(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Soderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, Erhardt S, Engberg G. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry. 2009;14(12):1069–1071. doi: 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117(3):198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry. 2015;172(11):1112–1121. doi: 10.1176/appi.ajp.2015.15010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Abbott L, Walter BN, Cheung TC, Goh CR, Porter AG, Ware CF. The lymphotoxin-alpha (LTalpha) subunit is essential for the assembly, but not for the receptor specificity, of the membrane-anchored LTalpha1beta2 heterotrimeric ligand. J Biol Chem. 1997;272(31):19451–19456. doi: 10.1074/jbc.272.31.19451. [DOI] [PubMed] [Google Scholar]
- Zakharyan R, Boyajyan A. Inflammatory cytokine network in schizophrenia. World J Biol Psychiatry. 2014;15(3):174–187. doi: 10.3109/15622975.2013.830774. [DOI] [PubMed] [Google Scholar]