Abstract
Multiple sclerosis (MS) is a chronic, autoimmune, inflammatory, demyelinating disorder of the central nervous system (CNS), which ultimately leads to axonal loss and permanent neurological disability. Current treatments for MS are largely comprised of medications that are either immunomodulatory or immunosuppressive and are aimed at reducing the frequency and intensity of relapses. Neural stem cells (NSCs) in the adult brain can differentiate into oligodendrocytes in a context-specific manner, and shown to be involved in the remyelination in these patients. NSCs may exert their beneficial effects not only through oligodendrocyte replacement but also by providing trophic support and immunomodulation, a phenomenon now known as “therapeutic plasticity”. In this review, we first provided an update on the current knowledge regarding MS pathogenesis and the role of immune cells, microglia, and oligodendrocytes in MS disease progression. Next, we reviewed the current progress on research aimed towards stimulating endogenous NSC proliferation and differentiation to oligodendrocytes in vivo and in animal models of demyelination. In addition, we explored the neuroprotective and immunomodulatory effects of transplanted exogenous NSCs on T cell activation, microglial activation, and endogenous remyelination, and their effects on the pathological process and prognosis in animal models of MS. Finally, we examined various protocols to generate genetically engineered NSCs as a potential therapy for MS. Overall, this review highlights the studies involving the immunomodulatory, neurotrophic, and regenerative effects of NSCs, and novel methods aiming at stimulating the potential of NSCs for the treatment of MS.
Keywords: neural stem cell, neural progenitor cell, microglia, oligodendrocyte, multiple sclerosis
Introduction
Multiple sclerosis (MS) is one of the most common neurological disorders of the central nervous system (CNS) in young adults. The pathological hallmarks of the disease are the appearance of multifocal inflammatory lesions in the CNS separated in time and space, demyelination, and axonal transaction [1, 2]. Relapsing-remitting multiple sclerosis (RRMS) is the most common form of MS, and has a biphasic disease course marked by alternating episodes of acute neurological deficits and/or worsening of a given neurological function (i.e. relapse), followed by a complete or partial recovery (i.e. remission). Generally after 15–25 years, ~70% of the RRMS patients develop secondary progressive MS (SPMS) which is characterized by progressive neurological decline independent of relapses (inflammation) [3]. Around 10–15% of the MS patients present primary progressive disease (PPMS) characterized by the steady progressive deterioration in neurological function from the onset of symptoms, without preceding or concomitant relapses [4].
Etiology and pathology of MS
MS is an immune-mediated disease in which the body’s immune system mistakenly attacks myelin in the CNS. Apart from the major histocompatibility complex (MHC) loci, many other non-MHC genetic variants involved in MS pathogenesis have been recently identified [5]. Notably, broad complex-tramtrack-bric-a-brac (BTB) and Cap’n’collar (CNC) Homology 1 basic leucine zipper transcription factor 2 (BACH2), which is required for efficient formation of regulatory T (Treg) cells, is found to be downregulated in blood cells of MS patients compared to healthy subjects, which may be responsible for the impaired Treg functions in MS patients [6]. Treg cells have been recognized as the critical immunomodulators of the adaptive immune system in MS. Deletion of Treg cells causes spontaneous autoimmune disease in mice, whereas augmentation of Treg-cell function can prevent the development of or attenuate the signs in the experimental autoimmune encephalomyelitis (EAE), the animal model of MS [7]. MS is also associated with impaired maturation of Treg cells [8]. Remission in RRMS has been shown to correspond with increased proportions of FoxP3+ Treg cells in the blood [9]. Thus, Treg cells are being considered as potential therapeutic targets in MS [10, 11].
Several environmental candidates such as nicotine smoking, low serum vitamin D levels [12, 13] and viral infection were found to increase the risk of developing MS, by inhibition of mitochondrial respiratory chain in the CNS and contributing to demyelination [14], activation of potentially encephalitogenic T cells and their trafficking to the CNS [15], and increased production of proinflammatory cytokine interleukin-6 (IL-6) [16]. Loss of self-tolerance may be triggered by an environmental antigen, virus, or other factors discussed above [17]. Epstein-Barr virus (EBV )[18] and human herpes virus (HHV)-6 [19] has been consistently linked with MS pathogenesis, and 99% of MS patients are EBV seropositive [20]. The adoptive transfer of in vitro-expanded autologous EBV-specific CD8+ T cells into a patients with severe SPMS could reduce disease activity and decrease intrathecal immunoglobulin production of EBV-infected autoreactive B cells [21].
Immunopathology of MS
Two model theories of lesion development in MS have proposed: the Outside-In model and the Inside-Out model [22]. In the Outside-In model, MS lesions develop from the outside (myelin) to the inside (axons); in the Inside-Out model, the lesions develop from the inside (axons) to the outside (myelin). The Outside-In model refers to a primary CNS demyelination, usually induced by anti-myelin autoimmune cells generated in the periphery, while the Inside-Out model refers to a primary CNS axonal degeneration and subsequent recruitment of systemic/adaptive immune cells [23, 24].
Denuded axons are vulnerable and start degenerating as the disease progresses [25, 26]. Despite the extensive axonal loss in acute MS lesions, relapses are reversible by the potent compensatory mechanisms in the brain [27, 28]. The conversion of RRMS to SPMS is thought to occur when the brain exhausts its capacity to compensate for further axonal loss [29, 30]. Chronically demyelinated axons have an increased energy requirement to maintain conduction velocity in the absence of myelin [31, 32]. Mitochondrial density and activity were increased within demyelinated axons in MS lesions which coincided with increased oxidative stress [33, 34].
Remyelination failure in MS
Remyelination is the regenerative process by which demyelinated axons are reinvested with new myelin sheaths. Spontaneous and robust remyelination occurs at the early stages of MS [35], occurring within a month or two after active demyelination [36]. Experimental animal models of CNS demyelination indicate remyelination is not performed by pre-existing mature oligodendrocytes [37], but involves new remyelinating oligodendrocytes derived from the maturation of quiescent oligodendrocyte progenitor cells (OPCs) distributed throughout the adult CNS [38, 39]. In the corpus callosum, remyelinating oligodendrocytes can also be derived from neural stem and precursor cells of the adult subventricular zone as shown in animal models [40, 41]. Moreover, it has also been observed that both the numbers and the differentiation stages of OPCs and mature oligodendrocytes are highly variable within lesions of different patients and in different lesion stages [42].
The eventual failure of remyelination that occurs as MS progresses, results from multiple factors such as the generation of a non-permissive environment which prevents OPCs differentiation, and also from a slowly progressive loss of the OPCs pool from established lesions [43, 44].
Parenchymal OPCs are mostly responsible for oligodendrogenesis and remyelination in MS [45]. These OPCs are present in robust densities inside the lesions during early phases of MS pathology [46], although in chronic MS lesions their number become significantly lower [47, 48].
Within a demyelinating lesion, activated CD4+ and CD8+ T cells, as well as macrophages, are thought to act in concert with reactive microglia to release a milieu of proinflammatory factors that lead to oligodendrocyte dysregulation and apoptosis [49]. Oligodendrocytes are particularly vulnerable to antigen recognition and cytotoxicity by CD8+ cytotoxic T-lymphocytes since they express MHC class I antigens under certain inflammatory conditions [50, 51].Postmortem study of the brain tissue from some RRMS patients revealed that very early MS lesions exhibit extensive oligodendrocytes apoptosis in myelinated tissue containing few or no lymphocytes [52], which raises the possibility of a non-immune-related toxic effects directly against the oligodendrocytes. Oxidative damage is another common contributor to oligodendrocyte loss under many pathological conditions like MS [53, 54].
Current limitations of the disease-modifying treatments for MS
Currently available treatments for MS primarily target the underlying immunologic etiology of the disease [55]. While significantly effective in preventing the frequency of relapses, these treatment options have little benefit for SPMS patients since they do not prevent the continuous axon loss, and progression and irreversible disability. Secondly, a shift from adaptive to innate immunity characterized by abnormalities of dendritic cells (DCs) activation or maturation may underlie the transition to the progressive phase of the disease [56]. Current immunomodulatory drugs are directed primarily against the cells and mediators of the adaptive immune system [57]. Thus, preventing this transition, perhaps by acting at the level of the innate immune system, is an important therapeutic strategy.
Development of therapies to benefit progressive MS patients will require a more comprehensive understanding of the pathogenesis of progressive MS. It is suggested that during the late stages of the disease, the inflammation is relatively less, but the susceptibility of the target tissue to neurodegeneration and axonal degeneration increases [1]. Therefore, we argue that an essential strategy for MS therapy is to target the axonal pathology aiming for neuroprotective as well as neuroregenerative outcomes.
Models to study MS pathology
Various animal models such as T cell mediated (EAE), toxin or virus induced demyelination, and genetic models of demyelination are now used to understand the pathological and etiological aspects of MS.
EAE offers a practical strategy for reproducing certain distinct adaptive immune-mediated pathologic features of demyelination. EAE shares many pathological features with MS including chronic neuroinflammation, multi-focal autoimmune demyelination, and axonal loss, and is triggered by an autoimmune attack on the CNS [58].
Theiler’s Murine Encephalomyelitis Virus (TMEV) induced demyelinating disease (TMEV-IDD) is the most widely studied virally induced demyelinating disease (in mice) which can be explained by the Inside-Out model [59]. Following TMEV infection, axonal degeneration precedes demyelination [60]. In this model, mice develop chronic progressive demyelinating disease without remission, similar to the disease course of PPMS. Epidemiological studies suggest that viral models are useful in understanding the possible viral etiology [61], the process of the axonal injury/repair in MS [62], and the interplay between genetic predisposition and environmental insults [26]. It is also important to evaluate the therapeutic potential of engrafted neural stem cells (NSCs) in the presence of a persistent viral infection that is associated with chronic neuroinflammation and demyelination [63].
Cuprizone-induced demyelination model is a useful model of non-inflammatory demyelination which acts as a pre-clinical tool for screening candidate drugs for remyelination-promoting effects. Also, focal injection of lysolecithin into the spinal cord white matter of mice produces a discrete demyelinating lesion followed by spontaneous and complete remyelination [64].
Animal models that enable the study of remyelination in the presence of ongoing inflammation are needed to examine whether current or new therapies can promote remyelination in face of the inhibitory cues present in the MS plaque microenvironment. An innovative animal model combines cuprizone-induced demyelination with the transfer of myelin-reactive T helper 17 (Th17) cells which delays the endogenous repair process. The IFN-/IL-17-secreting T cells in the corpus callosum extend the period of demyelination and open the window to test beneficial effects of available putative remyelinating therapies [65]. Recently it has been showed that cerebrospinal fluid from SPMS patients injected in mice could induce inflammatory demyelination, axonal loss, and astrogliosis [66].
All the models mentioned above mimic only a part of MS pathology, and they act in a complementary way. Treatments should be assessed in multiple models to reflect their various aspects on adaptive and innate immune systems, demyelination and remyelination, short-term effect and long-term prognosis. For example, interferon-β (IFN-β) could alleviate inflammation and reduce demyelination in EAE models. However, in cuprizone-treated mice, IFN-β exerts side-effects regarding remyelination in the absence of an immune-mediated demyelination, which questions their long-term use as a possible MS treatment [32].
Participation of endogenous NSCs in remyelination: studies in animal models of MS
In the last decade, growing interest has focused on utilizing neural stem cells (NSCs) to promote remyelination. In the adult CNS, tissue-specific germinal niches, such as the subventricular zone(SVZ) of the lateral ventricles and the subgranular zone of the dentate gyrus (DG) of the hippocampus, contain multipotent NSCs with the capacity to self-renew and differentiate into functional neurons and glia [57, 67]. Multipotent NSCs have also been isolated from a subcortical white matter of the adult human brain [68]. A recent study revealed the existence of dormant ependymal CD133+ NSCs lining the surface of the fourth ventricle in mice which could be mitotically activated and differentiated into neurons and glia upon stimulation [69, 70].
NSCs in the adult mammalian brain have been shown to give rise to rapidly dividing neural progenitor cells (NPCs) to produce neurons, astrocytes, and oligodendrocytes, and functionally contribute to (although modest) cognition and repair processes after injury [69, 71]. For example, neuroblasts in the adult mice SVZ can be primarily directed to an oligodendrocyte fate upon lysolecithin induced demyelination of the corpus callosum [72, 73]. In EAE, NSCs can become activated, migrate to the lesions and differentiate into oligodendrocytes, providing another source of myelinating oligodendrocytes [68, 74]. Retroviral-mediated Mash1/Ascl1 misexpression redirects neurogenic intermediate progenitors to an exclusive oligodendrocyte lineage in the adult subgranular zone (SGZ) [71, 75]. It is now believed that radial glia cells not only serve as progenitors for many neurons and glial cells soon after birth, but also give rise to adult SVZ stem cells that continue to produce astrocytes, neurons [71, 76], and, to a lesser extent, oligodendrocytes [77]. Neurogenic capacity is disrupted during aging, while the ability to produce new oligodendrocytes is not compromised in the human brain [78]. In the aged SVZ, proliferation is reduced due to loss of stem cell numbers, inability to self-renew or increases in cell cycle length [79]. The remaining actively proliferating NSCs in SVZ and DG decrease over time in the aged brain, transforming into astrocytes [80, 81].
The participation of SVZ derived progenitors in remyelination has been demonstrated in several experimental mouse models of demyelination [82, 83]. Acute EAE results in enhanced migration of SVZ-derived NPCs to the olfactory bulb and triggers their mobilization in the periventricular white matter. The mobilized cells give rise to oligodendrocytes in the inflammatory demyelinating lesioned white matter to replace the dysfunctional or dying oligodendrocytes [74]. In contrast, during the chronic/nonremitting phase of EAE (analogous to the progressive form of MS), NSC and NPC proliferation is attenuated in the SVZ and hippocampus [84].
In the TMEV-IDD model in mice, progenitors in the SVZ is mobilized to undergo oligodendrogenesis and migrate towards demyelinated areas close to the lateral ventricles in the corpus callosum to participate in remyelination [40].
In the cuprizone-induced demyelination model, large numbers of NPCs were shown to migrate into the corpus callosum where the majority of these cells differentiated into oligodendrocytes and exhibited robust capacity to remyelinate, especially in the rostral regions adjacent to the SVZ. These NPC-derived oligodendrocytes reestablished the nodes of Ranvier and g-ratios, and newly formed myelin was equivalent to those of healthy control mice [41]. However, in a chronic model when demyelination is sustained over a period of time (after long-term cuprizone administration), SVZ derived NPCs minimally contribute to myelin repair [84]. This is associated with an exhaustion of the pool of SVZ progenitors which have a limited self-renewal potential [85], a drastic drop of their proliferation and mitochondrial dysfunction in NPCs [86].
The NPCs and OPCs play a key role in augmenting the endogenous myelin/neuronal repair capacity in MS-like disease, likely via CXCL12/CXCR4 autocrine signaling post inflammation [87]. Generally, CNS inflammation in MS patients is associated with upregulation of the chemokine ligand CXCL12 expression. In EAE mice, CXCL12 expression in the DG and corpus callosum was persistently increased following spontaneous recovery even though CNS inflammation had subsided, and the numbers of NPCs in both regions increased correspondingly. A significant portion of the NPCs and OPCs express the CXCL12 and CXCL12 receptor CXCR4.Thus the increased levels of CXCL12 expression in the DG and corpus callosum of EAE-recovering mice may be associated with the promotion of neuro/oligodendrogenesis generating CXCR4+ CXCL12+ NPCs and OPCs endowed with intrinsic neuro/oligondendroglial differentiation potential.
Therapeutic strategies utilizing endogenous NSCs has a great potential since it avoids the intricate procedure of generating exogenous generation of NSCs which involves lengthy differentiation protocols [88]. Currently, available drugs and recombinant cytokines or soluble factors need an intensive study to exploit their potential in booting endogenous remyelination.
Vitamin D3 may directly enhance proliferation of NSCs, and their differentiation into neurons and oligodendrocytes in EAE mice. NSCs constitutively expressing the vitamin D receptor (VDR) exhibited increased expression of neurotrophic factors NT-3 and BDNF after exposure to vitamin D3 [89]. Increased remyelination in hippocampus by endogenous progenitor cells was observed in rats received vitamin D3 following ethidium bromide (EB)-induced demyelination [90]. 1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) has an immunomodulatory effect and has been implicated in the pathogenesis of MS. There are several additional benefits to administering vitamin D. Vitamin D3 induces human DCs to adopt a tolerogenic phenotype, characterized by decreased expression of CD40, CD80, and CD86, low interleukin-12 (IL-12) release, and enhanced anti-inflammatory interleukin-10 (IL-10) secretion [91]. It also reduces the serum levels of pathogenic IL- 17 in RRMS patients [92].
Limitation of endogenous NSC towards remyelination
In general, the microenvironment at and around the lesion site during demyelination appears to favor astrogliogenesis rather than oligodendrogenesis from SVZ derived cells. This has been evidenced in several studies. For example, epidermal growth factor (EGF) plays a dual role in MS and EAE. In the lysolecithin-induced demyelination model, intravenous (i.v.) infusion of EGF dramatically promoted the proliferation and migration of SVZ NSCs as well as their differentiation into oligodendrocytes in the corpus callosum [93]. However, in chronic MS lesions, EGF signaling is associated with astrogliosis and glial scar formation. In fact, EGF was shown to play a pivotal role in astrogliogenesis at the expense of oligodendrogenesis [94]. Interestingly, EAE mice injected (i.v.) with anti-EGF neutralizing antibody at day 9 after the initial proliferation phase of SVZ-derived NSCs had significantly ameliorated EAE symptoms via induction of neurogenesis and oligodendrogenesis in the SVZ [95]. Similarly, an up-regulation of bone morphogenetic protein 4 (BMP4) protein levels is usually detected during active demyelination, and NSCs treated with BMP4 produced more astrocytes in vitro. Intraventricular infusion of Noggin, an endogenous antagonist of BMP4, increased the number of Olig2- positive oligodendrocytes and decreased astrocyte numbers in the SVZ after cuprizone-induced demyelination in mice [96].
Fingolimod (FTY720) is a sphingosine-1-phosphate (S1P) receptor modulator, and the first oral treatment option available for RRMS [97]. However, FTY720 did not promote remyelination in lysolecithin-induced demyelination animal models [98]. Administration of FTY720 to JHM strain of mouse hepatitis virus (JHMV)-infected mice resulted in enhanced migration and increased proliferation of transplanted NPCs after spinal cord engraftment, yet failed to improve disease or increase remyelination [99].
Treatment with IL-4 and IL-10 upregulated the surface adhesion molecule lymphocyte function-associated antigen 1 (LFA-1), and chemokine receptors CXCR4 on NSCs, thus facilitating migration of NSCs towards the CNS inflammatory foci [100]. Overall, it is apparent that stimulation of endogenous NSCs with beneficial factors is a promising approach for the treatment of MS and requires further research to reveal its therapeutic potential and the timing, does and safety of each candidate. However, NSC derived oligodendrogenesis is limited compared to astrogliogenesis.
NSCs-microglia cross talk: effect on NSC survival and differentiation, and immunomodulation
Microglia, the resident macrophages in the CNS parenchyma, are a heterogeneous group of monocyte-derived cells serving multiple roles within the brain [101]. They have actively involved in MS pathogenesis both, in early as well as in late stages of MS lesions formation [102]. Intrinsic triggers such as subtle pathological changes in the CNS induce the formation of clusters of activated microglia [103], which adopt a cytotoxic phenotype when exposed to proinflammatory molecules by releasing reactive oxygen species (ROS) and nitric oxide (NO) [104]. This further aggravates the imbalance between increased energy demand and decreased energy supply in chronically demyelinated axons [105].
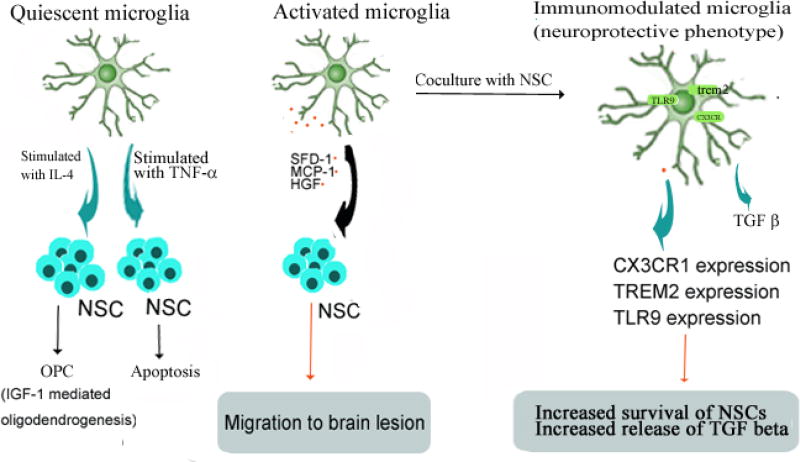
Phagocytosis and removal of damaged myelin seem to be one of the major roles of microglia in MS and removal of myelin debris is a prerequisite of successful remyelination [106]. In response to inflammation and infection in the CNS, oligodendrocytes release cytokines that recruit microglia to phagocytosis inhibitory molecules present in the lesion microenvironment [107], thereby aiding repair and regeneration [108]. Inactive lesions in SPMS comprised an external border of activated microglia. Impaired phagocytosis of myelin fragments on the surface of microglia was in part responsible for the failure of remyelination [109] (Figure 1).
Figure 1. NSC survival, differentiation, and immunomodulation are shaped by NSC-microglia cross talk.
Microglial derived signals determine NSC survival and differentiation in EAE. Conversely, NSC derived signals cause immunomodulation in microglia via paracrine factors and signaling pathways. Resting microglia stimulated by IL-4 in vitro, promotes Insulin-like growth factor-1 (IGF-1) mediated oligodendrogenesis from adult NPCs in mice [120]. On the other hand, microglia-derived tumor necrosis factor-alpha (TNF-α) induced the expression of the BH3 (Bcl-2 homology domain-3) in NPCs by an NF-k B (nuclear factor- kB)-dependent mechanism and, increases NPC apoptosis by a mitochondrial pathway [121]. Soluble factors released from mouse microglial cells direct the migration of NPCs in vitro and in vivo [117]. In the EAE brain, microglia produce stromal cell-derived factor-1 (SDF-1), monocyte chemo-attractant protein-1 (MCP-1) and hepatocyte growth factor (HGF), responsible for the inflammation-induced attraction of transplanted NPCs into white matter lesions [118]. In an allogeneic co-culture model, both human NPCs and microglia showed increased survival and proliferation, and the release of transforming growth factor-β (TGF-β) was also upregulated. NSCs can induce a significant up-regulation of the surface molecules CX3CR1 on microglia which is associated with a neuroprotective phenotype, and triggering receptor expressed on myeloid cells-2 (TREM2) [119, 122, 123, 124].
Microglia are also important modulators of the inflammatory milieu in the CNS in MS [110]. During the active phase of the MS, activated microglia produce proinflammatory mediators [111], chemokines and oxidizing radicals which are potentially detrimental to oligodendrocytes, suggesting a correlation between microglial activity and oligodendrocyte damage in MS [105]. Resident microglia can establish a cross-talk with infiltrated immune cells, including IL-17+ γδ T cells, regulating their recruitment, activation, and function within the CNS [112, 113]. 18β-glycyrrhetinic acid (GRA) effectively reduced CNS inflammation and myelin damage in EAE in C57BL/6 mice through inhibition of microglia activation via the suppression of mitogen-activated protein kinase (MAPK) signal pathway which plays an important role in the IFN-γ-induced expression of proinflammatory genes in activated microglia. GRA-modulated microglia downregulated production of proinflammatory cytokines and chemokines, which reduced the recruitment of encephalitogenic T cells into the CNS [114], and promoted remyelination [115].
NSC survival and differentiation
Microglia is thought to play a role in the migration of NSCs, as well as in effecting their survival and differentiation. In both acute and chronic EAE, microglia number was significantly higher in CNS regions containing transplanted NPCs [116]. Soluble factors released from mouse microglial cells direct the migration of NPCs in vitro and in vivo [117]. In the EAE brain, microglia produces stromal cell-derived factor-1 (SDF-1), monocyte chemo-attractant protein-1(MCP-1) and hepatocyte growth factor (HGF), accounting for the inflammation-induced attraction of transplanted NPCs (which constitutively expressed cognate receptors for these chemokines) into white matter tracts [118]. In an allogeneic co-culture model, both human NPCs and microglia showed increased survival and proliferation, and the release of transforming growth factor-β (TGF-β) was also upregulated. However, differentiations of NPCs were hindered by microglia [119]. Depleting microglia from hippocampal cultures reduces NSCs survival and proliferation. Microglia stimulated by IL-4 in vitro, encouraged Insulin-like growth factor-1 (IGF-1) mediated oligodendrogenesis from adult NPCs in mice [120]. On the other hand, microglia-derived tumor necrosis factor-alpha (TNF-α) induced the expression of the BH3 (Bcl-2 homology domain-3) only family member Puma in NPCs by an NF-kB (nuclear factor-kB)-dependent mechanism and increases NPC apoptosis by a mitochondrial pathway [121].
NSC-induced modulation of microglial function
Novel treatment strategies should utilize NSCs to modulate host microglial phenotypes and functions to benefit neuroprotection and repair. NSCs or NPCs may not only be shaped by microglia but they, in turn, are capable of manipulating microglia functions and activity. NSCs can transform microglia from a harmful to a neuroprotective phenotype by significantly increasing the expression of molecules associated with a neuroprotective phenotype in adult mouse brain [122]. For example, NSCs can induce a significant up-regulation of the surface molecules CX3CR1 on microglia which is associated with a neuroprotective phenotype [123], and triggering receptor expressed on myeloid cells-2 (TREM2) [124]. Injection of primary mouse NPCs into the striatum of C57BL/6 mice cause a significant increase in an absolute number of Iba-1+ microglia with activated morphology, those effects were mainly exerted through vascular endothelial growth factor (VEGF), which is secreted by grafted NPCs in significant amounts [125].
NSCs have been shown to improve host neuronal viability in mouse organotypic brain slice cultures by switching microglia from a detrimental to a neuroprotective phenotype, through the microglial Toll-like receptor 9 (TLR9)-extracellular-regulated protein kinases 1/2 (ERK1/2) pathway. These beneficial modulatory effects of NSCs were abrogated by the microglial inhibitor minocycline [122]. NSCs that were preconditioned with minocycline in vitro before transplantation had upregulated expression of Nrf2-regulated antioxidant genes, and enhanced the survival of grafted cells and released of paracrine mediators, such as brain derived neurotrophic factor (BDNF) and VEGF [126]. Conversely, microglial activation improved regenerative potential in the SVZ in the chronic phase of EAE. In vivo treatment with minocycline increased NSCs proliferation and their differentiation into mature oligodendrocytes in the SVZ by inhibiting the activation of microglia [127].
Tissue and cellular sources for NSCs: utility and limitations
Various cell types may serve as a source of NSCs or NPCs, for example, human embryonic stem (ES) cells (hESCs) [128], fetal and adult brain SVZ cells, and postmortem human CNS tissue [129]. Autologous mesenchymal stem cells (MSCs) are another source of neural stem cells for MS because they are readily obtained from adult bone marrow (BM) [130]. Experiments showed that the therapeutic effects of bone marrow-derived NSCs (BM-NSCs) and SVZ-NSCs were almost identical in EAE models, BM-NSCs also exhibited comparable morphological properties and possess a similar ability to differentiate into neurons, astrocytes, and oligodendrocytes both in vitro and in vivo [131].
The generation of induced pluripotent stem cells (iPSCs) from adult skin fibroblasts has heralded the possibility of autologous transplants that would circumvent histocompatibility barriers and ethical problems [132]. iPSCs can differentiate efficiently into NSCs and, subsequently, into specific neural lineages [133]. The gene expression profiles of iPSCs derived NSCs is comparable to that of human fetal-derived NSCs and these iPSCs-NSCs could be differentiated into neurons, astrocytes, and oligodendrocytes [134]. A research group used Sendai virus constructs encoding four iPSC transcriptional factors (Sox2, Oct4, Klf4 and c-Myc) to derive neural stem cells from CD34+ cells from both cord blood cells and adult peripheral blood [135]. Experiments demonstrated that mouse iPSCs-derived NPCs (miPSCs-NPCs) differentiated into mature oligodendrocytes in demyelinated Shiverer mice and generated compact myelin around host axons and restored nodes of Ranvier and conduction velocity as efficiently as CNS-derived NPCs [136].
However, several aspects of human iPSCs may be impacted by epigenetic mechanisms. A recent study demonstrated that human iPSCs derived NPCs from patients with schizophrenia (SZ) had perturbations in canonical WNT signaling, which may be caused in part by increased oxidative stress within the nervous systems commonly observed in MS patients [137]. NPCs differentiated from iPSCs that collected from blood samples of PPMS patients provided no neuroprotection against active CNS demyelination compared to NPCs from control iPSC lines [138].
Several recent reports indicate that NSCs and NPCs can be directly generated from skin fibroblasts by direct reprogramming [139]. Plasmid vectors containing the EBV-derived oriP/EBNA1 defined expression factors and a small hairpin directed against p53 could reprogram adult human fibroblasts to induced NSCs (iNSCs) without the addition of small molecules [140]. Direct conversion of somatic cells into stably expandable iNSCs and induced NPCs (iNPCs) may prove to be highly efficient, safe and labor-saving, compared with the circuitous two-step strategy used during the conversion of somatic cells to iPSCs and subsequent differentiation into neural stem cells [141]. iNPCs could be induced directly from human fibroblasts by overexpression of SRY-box 2 (SOX2) protein in combination with a chemical cocktail under 3D sphere culture conditions [142]. Highly expandable human NSCs with multipotent neural differentiation potential can also be directly generated from human fibroblasts by lentiviral transduction with four to five reprogramming genes [143].
Mouse fibroblasts derived tripotent iNSCs could be differentiated not only into neurons and astrocytes but also into oligodendrocytes capable of integration into dysmyelinated Shiverer brain [144]. Future experiments will be necessary to help define the potential of these cells in the context of inflammation and their tissue tropism in MS. The therapeutic potential of human NPCs may differ greatly depending on the method of derivation and expansion [145]. The expression of neurotrophic factors in NPCs usually decreases with time in culture [146], and long-term cultured NPCs lose their capacity to restrain the proliferation of pathogenic immune cells in vitro [147]. Therefore, it is imperative to obtain enough quantity of stem or progenitor cells within a short time before the quality of individual cell decreases. This presents a significant challenge for the technologies concerning iPSCs derived NSCs, and directly induced NSCs.
Route of administration
Mostly preferred routes for the delivery of MSCs or NSCs are the intravenous (i.v.) and intrathecal delivery routes since they can cross the blood-brain barrier (BBB) [148]. However, syngeneic naïve NPCs injected subcutaneously and intravenously in EAE mice were low invasive in the CNS. Most of the injected NPCs were found in the liver, gut, spleen, lung and kidney, which inevitably reduced the number of NPCs in secondary lymphoid organs and CNS [149, 150]. Focal injection of NSCs in the CNS is not practical in MS, where a multifocal, chronic, and spatially disseminated CNS damage accumulates over time. This would require multiple local injections to reach the multifocal lesions [151]. Intrathecal administration to lesions might be hindered by the limited capacity of grafted NSCs to migrate over long distances within the CNS parenchyma [152].
NSCs delivery directly into the cerebrospinal fluid (CSF) circulation by intracerebroventricular (i.c.v.) injection to specifically target the CNS in mice and rats has been tested [153]. Newborn rat NPCs, which were transplanted i.c.v at the peak of disease in EAE migrated exclusively into the inflamed white matter (but not into adjacent gray matter regions), and subsequently differentiated into oligodendrocytes [154].
Intranasal (i.n.) delivery of NSCs is another noninvasive method of delivery. NSCs have shown to migrate into the CNS directly via the nasal route and result in functional recovery, and confer immunomodulation and remyelination in EAE in mice [155]. In mice, NSCs injected in the carotid artery promoted cell homing to the area of stroke lesion, and improved behavioral recovery [156]. Intra-carotid delivery of NSC has not been reported in EAE. It has been shown that exogenous NSCs interact more closely with the infiltrating pathogenic immune cells rather than with those in the periphery. Therefore suppression of inflammation in CNS by NSCs is likely to be more effective by targeted local delivery rather than their interaction at the periphery [155].
Therapeutic mechanisms of action of transplanted NSCs: studies in animal models of demyelination
NSCs and NPCs have been shown to exert their beneficial effects through a) immunomodulation, b) by cell replacement, c) by providing trophic support, and d) by stimulation of endogenous remyelination (Figure 2) [157]. For the NSC therapy to be successful in MS, the cells need to be plastic enough to accommodate and survive in the non-permissive inflammatory environment, highly migratory to reach multiple lesion sites in the CNS and can differentiation into myelinating oligodendrocytes, through multiple mechanisms of action (Table 1).
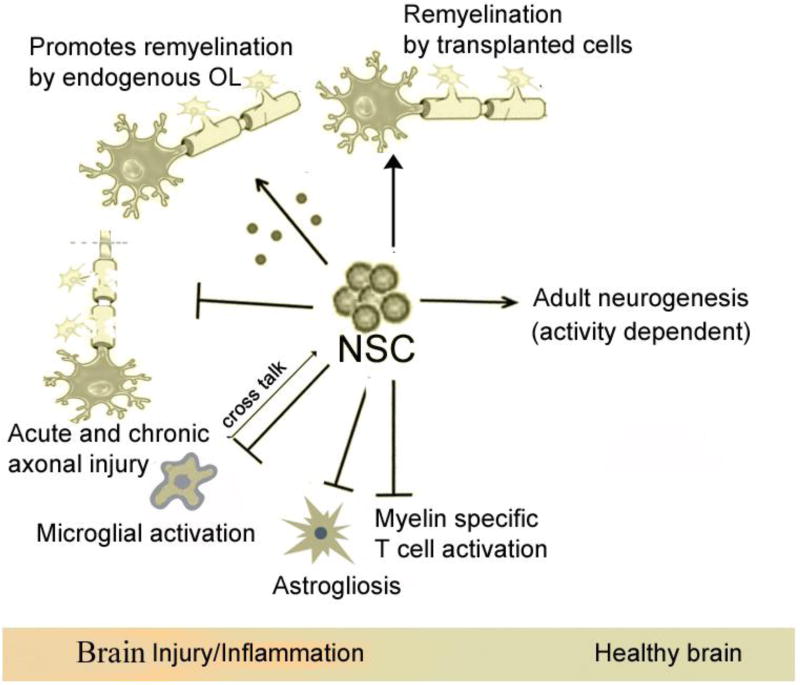
Figure 2. Functions of adult NSCs in healthy and EAE (MS) brain.
NSCs in the adult mammalian brain have been shown to give rise to rapidly dividing neural progenitor cells (NPCs) to produce neurons, astrocytes, and oligodendrocytes, and functionally contribute to (although modest) cognition and repair processes after injury. In EAE, NSCs have been shown to exert their beneficial effects through a) immunomodulation b) by cell replacement [153], c) by providing trophic support, d) by stimulation of endogenous remyelination [166, 167, 168]. Transplanted NPCs can stimulate endogenous remyelination by inducing the proliferation and terminal differentiation of host OPCs, likely via CXCL12/CXCR4 autocrine signaling post inflammation [87]. NSCs inhibit MOG and MBP specific CD4+T cell activation, proliferation, increased number of FOXP3+ Tregs cells [145, 150, 158, 160]. Intraspinally transplanted NPCs in postnatal mice can differentiate into mature oligodendrocytes and functionally incorporate throughout the demyelinated white matter tracts in JHMV-infected demyelination model [97]. NSCs can transform microglia from a harmful to a neuroprotective phenotype by significantly increasing the expression of molecules associated with a neuroprotective phenotype in adult mouse brain [119, 122, 123, 124]. Transplanted NSCs can indirectly suppress astrocyte gliosis in EAE.
Table 1.
Therapeutic mechanisms of action of transplanted NSCs in animal models of demyelination
| Species/Cell | Route | Type of EAE |
Mechanism of action | Clinical outcomes |
Ref. | |
|---|---|---|---|---|---|---|
| NSC to OPC differentiation |
Immune modulation |
|||||
| Mouse/Adult SVZ NSCs | iv | Chronic EAE/Mouse | Did not observe glial or neuronal differentiation | Inhibition of encephalitogenic TH17 cell differentiation | Improvement in locomotor activity | [192] |
| Adult SVZ NSPCs (IL-10 producing) | icv. and iv. | Chronic EAE / Mouse | Oligodendroglial and neuronal differentiation | Inhibition of peripheral and CNS-confined inflammation; induction of apoptosis of CNS-infiltrating T cells | Improvement of locomotor activity (EAE score) | [197] |
| Mouse/Adult SVZ NSCs | icv. and iv. | Chronic EAE / Mouse | Oligodendrocyte differentiation | Rescue of endogenous OPCs and modulation of neurotrophic and/or growth factors | Attenuation of EAE disease score, and improvement of locomotor activity | [153] |
| Mouse/Adult SVZ NSCs | iv. | Relapsing EAE / Mouse | Not reported/tested | Modulation of apoptosis of CNS-infiltrating T lymphocytes | Improvement of locomotor activity | [158, 163] |
| Rat/Neonatal striatal derived NSC | icv. | Acute EAE Rat | Not reported | Inhibition of MOG-specific lymphocyte proliferation | Improvement in locomotor activity, and Attenuation of clinical score | [154] |
| Mouse/EB derived | icv | Acute EAE Viral-Mouse | Remyelination was correlated with regulatory T Cell induction | [145] | ||
| hESCs derived were selected based itive transcriptomic | Intra spinal | Chronic EAE JHMV strain of mouse | NPC survival beyond 8 days post transplantation not seen | Decreased accumulation of CD4+ T cells, reduced demyelination, and increase in CD4+FOXP3+ regulatory T cells (Tregs) in lymphatics | Significant neurological recovery | [160] |
| Mouse/MSC-NSC | Sc. | Acute EAE/Mouse | 1) Suppressed the activation of myeloid DCs to APCs, 2) reduced the proliferation and activation of MOG specific encephalitogenic T cells. | [150] | ||
| Mouse/MSC-Sox2βGeo NPC | inthrathecal | Acute EAE Mouse | 1)exerted a neuroprotective effect in via secretion of leukemia inhibitory factor (LIF), 2)was able to support the in vivo survival and differentiation of resident and oligodendrocytes, 3) Attenuation of CNS inflammation reduced tissue injury | Amelioration clinical and pathological features of the disease. | [167] | |
| Rat /fetal NSCs DO (rfNSCs-IDO) | iv | Acute EAE MOG-Mouse | No glial cell differentiation | 1) Inhibition of T cell proliferation in peripheral lymph nodes, 2) increase in regulatory T cell numbers | Attenuated clinical scores (signs ) and faster remission | [214] |
| Mice/Bone marrow NSCs overexpression T-3, and LINGO-1-Fc | iv. | Acute EAE MOG-Mouse | Glial cell differentiation seen. Blockade of further demyelination and the promotion of remyelination. | Attenuation of CNS inflammation and promote an M2 phenotype in macrophages/microglia. | Faster attenuation of clinical signs compared to control NSC. | [213] |
| Rat/GDNF gene-modified NSCs (GDNF/NSCs) | icv | Acute EAE MOG-Mouse | More glial and neuronal survival and differentiation compared to control NSC | Suppression of inflammation in the CNS | Attenuated the clinical signs, and promotion of functional recovery. | [212] |
Effect on T cell function
The immunomodulatory effects are mainly exerted by undifferentiated stem cells by releasing a milieu of neuroprotective molecules at the site of tissue lesion [158]. MSCs-NPCs have been shown to suppress T-cell proliferation and to promote the expansion of FoxP3+ Treg cells in vitro [159]. NPCs induced from a human iPSCs line were intraspinal transplantation into demyelinated mice due to viral infection, decreased the accumulation of CD4+ T cells in the CNS along with reduced demyelination at the site of injection were correlated with a transient increase in Treg cells in the peripheral lymphatics [145].
A recent study described long lasting clinical recovery along with dampened neuroinflammation and remyelination after transplantation of NPCs derived from human ESCs, in a viral model of MS [160]. The human NPCs (hNPCs) used in that study were derived by a novel direct differentiation method (direct differentiation, DD-NPCs) and cells were selected for intraspinal transplantation based on a definitive transcriptomic signature. The same group then wanted to determine whether NPCs differentiated using conventional methods would be similarly effective in improving clinical outcome under neuroinflammatory demyelinating conditions. hNPCs were differentiated from a human iPSC line via the conventional embryoid body intermediate stage (EB-NPCs). Intraspinal transplantation of EB-NPCs into mice infected with the neurotropic JHMV resulted in decreased accumulation of CD4+ T cells in the central nervous system that was concomitant with reduced demyelination at the site of injection. Dampened neuroinflammation and remyelination was correlated with a transient increase in Treg cells concentrated within the peripheral lymphatics. However, compared to their earlier study, pathological improvements were modest and did not result in significant clinical recovery. It was concluded that the genetic signature of NPCs is critical to their effectiveness in this model. More importantly, there is a need for rigorous characterization and selection of therapeutically valuable NSC types derived from human iPSC for the treatment of MS [161].
Trophic support
MSC-NPCs are known to secrete trophic factors such as IGF-1, VEGF, HGF and SDF1 in vitro [159]. In EAE mice that were injected subcutaneously with NPCs prior to disease onset, the NPCs accumulated in the draining lymph nodes which hindered the activation of myeloid DCs to antigen presenting cells (APCs) by a BMP-4-dependent mechanism, that reduced the proliferation and activation of encephalitogenic T cells [150]. Mac-3-, CD3-, and CD4-positive cells in the inflamed CNS were also diminished [162]. In chronic EAE, SVZ derived syngenic NSCs promoted neuroprotection through secretion of immunomodulatory molecules and neurotrophic factors [163].
Intraventricular injections of newborn rat derived NPCs into adult rats with acute EAE were shown to ameliorate the clinical severity and signs of EAE. Grafted NPCs migrated into the inflamed white matter and attenuated brain inflammation by inducing a reduction in perivascular infiltrates [164]. Syngeneic adult NSCs injected in the lateral ventricular were capable of long-distance migration into demyelinating areas inside an inflamed CNS in the EAE mice. Within these areas, OPCs of donor origin increased significantly and remyelinated axons actively [153].
Cell replacement
Intraspinally transplanted NPCs in postnatal mice can differentiate into mature oligodendrocytes and functionally incorporate throughout the demyelinated white matter tracts in JHMV-infected demyelination model [97]. NPCs transplantation did not alter the accumulation of T cells or macrophages within the CNS nor cytokine/chemokine gene expression in the CNS. Presumably, the enhanced remyelination was not depended on bystander effects of grafted cells [98]. Transplantation of oligodendrocyte transcription factor 1(Olig1) gene knockout NPCs (Olig1−/−) into JHMV-infected mice resulted in similar NSCs survival, proliferation, and selective migration to areas of demyelination but exhibited poor remyelination. The majority of transplanted Olig1−/− NPCs differentiated into astrocyte lineage. These suggested that improved clinical symptoms might be associated with remyelination by the donor NSCs via formation of myelinating oligodendrocytes [165].
Stimulation of endogenous remyelination
Transplanted NPCs can stimulate endogenous remyelination by inducing the proliferation and terminal differentiation of host OPCs. NPCs that were transplanted into the lateral ventricles of cuprizone-fed mice, were shown to exert a trophic effect on endogenous OPCs, remyelination in the corpus callosum was performed exclusively by resident OPCs which failed to remyelinate in chronic MS [166]. Intrathecal injection of MSCs-NPCs at the onset of the chronic phase of disease increased the number of endogenous OPCs in EAE mice and accelerated remyelination [167]. These effects were manly exerted through the secretion of leukemia inhibitory factor (LIF) that promotes survival, differentiation and the remyelination capacity of endogenous OPCs and mature oligodendrocytes [168].
There are many differences in the inherent mechanisms between human NSCs and other mammal species derived counterparts which should be worth of serious consideration in the translation of experimental research to the clinical setting. Intraspinal transplantation of human ES-NPCs in a viral model of MS resulted in dramatic reduction in neuroinflammation and sustained clinical recovery, although human NPCs were rejected within a relatively short period. Unlike the mouse NPCs, hNPCs had powerful immunomodulatory effects and induced an increased number of FOXP3+ Treg cells within the spinal cords [160]. There are more challenges to be tackled before NSCs therapy in animal models can be safely and successfully translated to human therapy for MS [169].
The absence of CD95L in human NPCs during inflammation is unlikely to result in the massive T-cell apoptosis reported in the mouse counterparts, whereas human NPCs have a higher capacity of generating oligodendrocytes cells in inflammatory conditions which are compatible with a therapeutic transplantation of NPCs for the treatment of MS [170].
Current issues with NSC transplantation: effect of the inflammatory environment on NSC survival and differentiation
In MS and EAE, remyelination takes place within an inflammatory environment containing signals and chemicals that are intrinsically hostile to the survival and differentiation of oligodendrocyte [171]. In the adult brain, endogenous NSCs that within the specialized germinal niches in the CNS are thought to provide support and maintenance to the endogenous OPCs. Direct physical contact and diffusible signals are the two major mechanisms that are thought to regulate the proliferation and differentiation of endogenous NSCs [172]. The in vivo differentiation of NSCs is highly dependent on the environmental cues within the CNS [173]. Identifying the mechanisms and signals responsible for blocking NSCs differentiation in CNS in MS warrants further investigation since manipulating these signals could promote oligodendrocyte production and remyelination, ultimately resulting in more effective CNS repair. Inflammation is permissive for the recruitment and migration of NSCs [74] while at the same time inhibitory to their proliferation and differentiation. The Taiep rat is a myelin mutant that shows many features of chronic demyelination in MS. The induction of acute inflammation in the non-remyelinating situation owing to a lack of the stimuli required to activate OPCs to generate remyelinating oligodendrocytes results in remyelination [174]. An anti-inflammatory environment seems to be a prerequisite for the differentiation of NSCs into myelinating oligodendrocytes [175]. For example, the pro-inflammatory cytokine TNF- reduces the proliferative ability of NSCs and NPCs but induces their migration [173], whereas the anti-inflammatory cytokine IL-10 maintained NSCs in the adult brain of mice in undifferentiated yet highly proliferative state [176]. Interferon gamma (IFN-γ), an important cytokine for the clearance of CNS infections, inhibits proliferation of NSPCs in inflammatory conditions through dephosphorylation of the tumor suppressor Retinoblastoma protein (pRb), which is dependent on activation of signal transducers and activators of transcription-1 (STAT1) signaling pathways [177]. From the foregoing discussion, it is apparent that inflammation is a double-edged sword as it could exert both detrimental and beneficial effects. Therefore, it is of great importance to determine the correct time of intervention, and design more refined therapies that aim at micro-manipulating the inflammatory milieu in the CNS, and to offset the negative effects, and maximize the beneficial outcomes [178].
Differentiation arrest of transplanted and endogenous NPCs is the result of the persistent inflammatory environment prevailing in EAE and MS. Natural killer (NK) cells were in close proximity to NSCs in SVZ during the chronic phase of MS. NSCs produced interleukin-15 (IL-15) and sustain functionally competent NK cells which limited the neuro-repair capacity of NSCs following brain inflammation [179]. At the acute phase of EAE, only a small fraction of NPCs injected in lateral ventricle succeed to differentiate, whereas at chronic phase most of them followed a differentiation process [180].
NPCs display CNS pathotropism upon transplantation [181]. The clinical value of cell transplantation in a chronic, multifocal disease like MS will depend on the ability of transplanted cells to migrate to the multiple disease foci in the brain. The inflammatory process may attract targeted migration of transplanted cells into the inflammatory lesions. NSCs express CXCR4, the cognate receptor for SDF-1, and this inflammatory chemoattractant SDF1/CXCR4 signaling involves in the mobilization of NSCs towards the injury sites [182] and their differentiation into OPCs and mature oligodendrocytes upon focal transplantation into JHMV-infected mice with established demyelination[183].
The cellular densities and proliferative signals are significantly higher in MS SVZ as seen in postmortem MS brains [184]. Therefore, prolonged exposure of SVZ cells to repetitive inflammatory insults may not exhaust their proliferative potential. However, their migratory capability and oligodendrogenesis remain limited, implying that strategies aiming at promoting these phenomena need to be developed.
The progressive decline in the rate of proliferation of NSCs with aging raises the questions of whether the precursor cells eventually become unresponsive to cellular niche cues, or whether the cellular niche provides less positive stimuli for evoking proliferation or provides more negative cues [185]. Persistent CNS inflammation significantly impairs proliferation of stem/ precursor cells in the SVZ of EAE mice by hindering their entry into the cell cycle by upregulation of cell cycle inhibitors, while these SVZ resident cells return to normal kinetics once the inflammation subsides [186].
The continual and dual role of the neuroinflammatory response leaves it difficult to decipher upon a single modulatory strategy. To maximize the therapeutic effect of cell-based therapies, treatments must be specific to the injury and also be personalized for each patient [187].Therefore, developing a microenvironment conducive to the survival and proper differentiation of NSCs and in vitro induction prior to transplantation are of great importance for the application of NSCs to treat MS.
Genetically modified NSCs
Genetic manipulation of NSCs holds great promise for improving the survivability of NSCs in vivo. Using various tools such as in vitro gene transfer, NSCs can be been manipulated for cell immortalization as well as control of proliferation. Genetically modified NSCs that overexpress pro-survival signaling molecules or paracrine factors, or critical glial cell lineage determining transcription factors may enhance the therapeutic effects of NSC transplantation therapy. Trophic factors that are responsible for enhancing the survival, proliferation and migration of transplanted NSCs provide neuroprotection, reduce astrogliosis, promote remyelination, and modulate inflammation. Specifically, neurotrophin-3(NT-3), glial cell line-derived neurotrophic factor (GDNF), BDNF, IL-10, LIF, and olig2 have been studied as a potential candidate for genetic transduction to strengthen the efficacy and differentiation potential of NSCs into oligodendrocytes [188].
OPCs can be efficiently generated from human fetal NSCs by concurrent or sequential in vitro exposure to combinations of NT3 and growth factors[189]. BM-NSCs transduced with NT-3 attenuated CNS inflammation and neurological deficits in active EAE significantly more than naive NSCs [190]. BM-NSCs exhibited efficient proliferation and differentiation into oligodendrocytes and neurons, and nominal differentiation into astrocytes, thus promoting remyelination and neuronal repopulation, and reducing the degree of astrogliosis [188]. NT-3 induced BM-NSCs also secrete the anti-inflammatory cytokine IL-10, thus modulating a hostile host environment into a microenvironment supportive of remyelination [190].
GDNF gene-modified NSCs transplanted in the lateral ventricle of EAE rats significantly promoted functional recovery, profoundly suppressed brain inflammation, differentiated into more neurons and oligodendrocytes, improved density of myelin, and reduced the clinical signs[191].
BDNF has been shown to plays a key role in axon protection and disease attenuation during chronic EAE in mice [192]. BDNF was found to be elevated in the CSF of MS patients compared to control individuals, and CSF derived from both SPMS and PPMS patients significantly stimulated human embryonic-derived NPCs differentiate into more oligodendrocytes in vitro [193]. Transplantation of human BDNF-NSCs significantly improved neurological motor function following traumatic brain injury (TBI) [194], and in middle cerebral artery occlusion model (MCAo) [195]. Human BM-NSCs and nanoparticle carriers encapsulated with BDNF and integrated into the biodegradable injectable 3D scaffolds, increased secretion of LIF and chemokines by NSCs in the CNS, and showed a sustained release of bioactive BDNF and enhanced their tissue repair [196].
Recent research demonstrates that adult mice CNS derived NSCs engineered to secrete the anti-inflammatory cytokine IL-10 (IL-10-NSCs) exhibited enhanced peripheral immunosuppressive effects in EAE mice compared to naïve NSCs [197]. IL-10–NSCs also promoted apoptosis of infiltrating T cells in the CNS through a Fas/FasL pathway, and converted a hostile environment to a relatively more supportive of remyelination. Additionally, transplanted IL-10-NSCs differentiated primarily into oligodendrocytes at the expense of astrocyte generation. This was associated with significant attenuation of clinical signs and pathology in acute EAE compared to mice treated with control NSCs [198].
IGF-1 is critical for oligodendrocyte differentiation, survival, and myelination in neonatal and adult mice brain. IGF-1 produced by microglia and reactive astrocytes display protective effects on oligodendrocytes following cuprizone induced toxic demyelination [199]. Transgenic mice that overexpressed IGF-1 demonstrated significantly less apoptosis of mature oligodendrocytes and exhibited rapid remyelination after cuprizone induced demyelination [200, 201]. The IGF-1-overexpressing neonatal rats spinal cord-derived NSCs exhibited higher viability, and efficiently differentiated into oligodendrocytes in a mouse spinal cord injury model [202]. The effects were shown to be mediated by extracellular signal regulated kinases1 and 2 (ERK1/2) pathway.
NSCs normally express low levels of indoleamine 2,3-dioxygenase (IDO), a tryptophan-metabolizing enzyme which has potent immune suppressive activities. In an EAE animal model, systemic injections of NSCs expressing IDO resulted in significant local immune suppression in the cervical lymph nodes and CNS by recruiting regulatory T lymphocytes and reducing the number of activated T lymphocytes during the inflammation in the CNS which induced significantly fewer clinical symptoms and faster recovery [203].
Genetically altered NSCs that expressed the critical oligodendrocyte lineage transcription factor Olig2 promoted the functional recovery by contributing to remyelination, and completely abrogating relapses when administered early after onset of EAE [204]. Most intraventricularly injected mice Olig2-NSCs differentiated into OPCs, in contrast to the control NSCs which largely remained undifferentiated [199]. Similarly, overexpression of Olig2 in mice SVZ progenitor cells increased the generation of OPCs which migrated and differentiated into mature oligodendrocytes after transplantation [205]. NSCs within the DG do not spontaneously differentiate into oligodendrocytes and endogenous remyelination is limited after injury [206, 207]. However retroviral mediated expression of the transcription factor Ascl1- into the DG of adult mice converted them into mature oligodendrocytes and enhanced there myelination in the DG in diphtheria-toxin (DT)-inducible, a genetic model for demyelination [207].
The chemokine (C-C motif) receptor 5 (CCR5) is a receptor for chemokines CCL3, CCL4 and CCL5, that are abundantly produced in CNS-inflamed foci of MS/EAE. CCR5 over expressing mouse BM derived NSCs (CCR5-NSCs)were rapidly attracted towards inflamed foci in active EAE (in mice) in larger numbers, and more effectively suppressed CNS inflammatory infiltration, thus reducing the extent of early myelin/neuron damage by creating a less hostile environment for host remyelinating cells [205].
NSCs could also be engineered to produce a “cocktail” of potential therapeutic molecules effectively targeting the major mechanisms underlying the chronicity of EAE and MS, such as persistent inflammation, deficiency of trophic support for differentiation, and accumulation of neuroregeneration inhibitors. Soluble LINGO-1 protein (LINGO-1-Fc), an antagonist of LINGO-1, is a key part of the common receptor complex which blocks the harmful effect of neuroregeneration inhibitors on OPCs/oligodendrocytes and attenuates myelin inhibition [208]. At the chronic stage of EAE, NSCs engineered to produce IL-10 (for immunosuppression), NT-3 (for neurotrophy), and LINGO-1-Fc (for inhibition of negative effects) migrated into the inflamed foci and induced M2 macrophages/microglia in CNS, thus reducing astrogliosis and promoting endogenous oligodendrocyte/neuron differentiation which represents a novel and potentially effective therapy for the chronic stage of MS [209].
Immortalized human NSC cell lines can be generated by a retroviral vector encoded with a v-myc oncogene. These immortalized NSCs exhibited potent migration capability and differentiation potential into neurons and glial cells in animal models of human neurological disorders. Multipotent neural cell lines can engraft and participate in the development of mouse cerebellum [210]. The continuously multiplying cell may exist as a limitless supply of neurons and oligodendrocytes for treatment for MS [211]. Although Fas-deficient NPCs had significantly higher survival and increased differentiation capabilities compared to wild type NPCs in vitro, this did not translate to better terminal differentiation and post-transplantation survival in vivo. The environmental factors in the CNS prevented the differentiation of grafted NPCs, regardless of their inherent differentiation capacities ex vivo [212].
Genetically engineered NSC can boost and influence multiple gene networks and interacts with endogenous neural and immune cells to improve cognitive and motor behavior. Expression of specific, transcription factors, or ligands or receptors in NSC can induce relatively more significant changes in synaptic plasticity, mitochondrial and lysosomal function, and affect both innate and adaptive immunity resulting in better functional recovery. Alternatively, they can be generated as more fate restrictive, to directed then to generate more glial cells for remyelination.
Clinical research on NSC-based cell therapies
Safety is the primary concern of stem cells therapies; clinical researches on NSCs in MS have not been reported to date. In an early study, 15 patients with amyotrophic lateral sclerosis (ALS) receiving an intraspinal transplantation of escalating doses of NSCs safely tolerated the cells at high doses [213]. A recent pilot study investigated the safety and tolerability of autologous MSC-NPCs treatment for MS. Six patients with progressive MS who were refractory to conventional treatments were treated with intrathecal injections of MSC-NPCs and there were no serious adverse events in the following 7 years and some patients showed a measurable clinical improvement [214]. Same authors reported a Phase 1 safety trial involving 20 MS patients with established disability, in which MSC-NPs administered intrathecally in three doses of up to 10 million cells per injection, spaced three months apart, resulted in improved Expanded Disability Status Scale (EDSS), improved 9-Hole Peg Test (9-HPT), and better bladder function clinically (reported as abstract and oral presentation at the 68th Annual Meeting of the American Academy of Neurology). A Phase I, open-label, single-site, safety study of human spinal cord-derived neural stem cell (supplied by Stem Cell Incorporation) transplantation for the treatment of chronic spinal cord injury has been initiated in 4 spinal cord injury (SCI) patients in 2016, is well tolerated. Data is still being collected. A Phase I safety study was conducted by Dr. David Rowitch’ s group for testing human fetal CNS derived neural stem cells transplantation in 4 Pelizaeus-Merzbacher disease (PMD) subjects. The cells were fairly tolerated with no serious or fatal outcomes. A fraction of the patients had a modest but clear gain in motor functions, which are not seen for such a progressive and severe neurodegenerative disease [215]. Based on increasing evidence demonstrating the robust regenerative potential of human NSCs, this mode of cell therapy could provide a feasible clinical intervention in stopping neurodegeneration. In theory, a combination therapy with existing immunomodulatory therapies may be beneficial, i.e., simultaneously replacing cells, regulating autoimmunity, and promoting regeneration in MS patients.
Conclusions
The present review delineates several aspects of the MS pathology, endogenous remyelination, and results of NSC transplantation in animal models that must be taken into consideration in the development of an NSC-based cell therapy for MS. We briefly summarized the current understanding of MS pathogenesis, namely the different types pathological lesions in the CNS, immune cell mediated inflammatory demyelination, apoptosis of oligodendrocytes, axonal degeneration, and oxidative stress. The current consensus regarding an effective therapeutic regimen that effective treatment should contain a combination of anti-inflammatory, regenerative, and neuroprotective strategies. The success of NSC transplantation primarily depends on the cell fate pre-commitment of transplanted NSCs into OPCs, while at the same time the endogenous differentiation of OPCs needs to be boosted in chronic stages of the disease. Preclinical data suggests that NSCs and NPCs may be competent in simultaneously exerting an immunomodulatory action, as well as activation of the endogenous NSC pool. Modulation of microglial function in CNS is an important target for NSCs. However, the activity of microglia in a different stage of MS is different, therefore optimum timing of interventions need to be carefully explored. The extent of cell replacement is currently not clear and needs further exploration. However, several complex issues need to be addressed. First, large scale generation of NSCs or NPCs from human iPSCs or by direct conversion of somatic cells into iNSCs must be developed. There is also a need for rigorous characterization and selection of therapeutically valuable NSC types derived from human iPSCs. Lastly, the ideal route and time of NSC injection are of great importance since the fate of transplanted cells, the therapeutic mechanisms and efficacy in vivo are critically dependent on these factors. Genetically modified NSCs expressing trophic or survival factors could improve the microenvironments, enhancing the survival and appropriate differentiation of NSCs. The behavior and efficacy of exogenous NSCs in different types of the animal model need comprehensive analysis to deduce the real features of NSCs before translation into clinical trials. Assisting the endogenous stem cells to overcome the obstacles of proliferation, migration, and differentiation in the lesions is another interesting approach, and humanized mice models are needed to simulate the scenarios.
Acknowledgments
We thank the funding support from the National Natural Science Foundation of China (81601373), the Hubei Provincial Natural Science Foundation of China (2016CFB407), the Bureau of Xiangyang City Science and Technology projects (No. [2014] 6–7), the Project for Discipline Groups Construction of Food New–type Industrialization of Hubei University of Arts and Science, National Institutes of Health (R01HD087566), National Multiple Sclerosis Society, and Shriners Hospitals for Children.
Footnotes
Author contributions
All authors researched the data for the article, provided substantial contributions to discussions of the content, and wrote the article.
Competing financial interests
The authors declare no competing financial interests related to this work.
References
- 1.Lassmann H. Pathology and disease mechanisms in different stages of multiple sclerosis. Journal of the neurological sciences. 2013;333(1–2):1–4. doi: 10.1016/j.jns.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kishore A, Kanaujia A, Nag S, Rostami AM, Kenyon LC, Shindler KS, Das Sarma J. Different mechanisms of inflammation induced in virus and autoimmune-mediated models of multiple sclerosis in C57BL6 mice. BioMed research international. 2013;2013:589048. doi: 10.1155/2013/589048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. The New England journal of medicine. 2000;343(20):1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 4.Miller DH, Leary SM. Primary-progressive multiple sclerosis. The Lancet Neurology. 2007;6(10):903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 5.Fugger L, Friese MA, Bell JI. From genes to function: the next challenge to understanding multiple sclerosis. Nature reviews Immunology. 2009;9(6):408–417. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- 6.Perga S, Montarolo F, Martire S, Berchialla P, Malucchi S, Bertolotto A. Anti-inflammatory genes associated with multiple sclerosis: a gene expression study. Journal of neuroimmunology. 2015;279:75–78. doi: 10.1016/j.jneuroim.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. Journal of immunology. 2002;169(9):4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 8.Eliseeva DD, Zavalishin IA, Karaulov AV, Bykovskaia SN. [The role of regulatory T cells in the development of autoimmune process in multiple sclerosis] Vestnik Rossiiskoi akademii meditsinskikh nauk / Rossiiskaia akademiia meditsinskikh nauk. 2012;(3):68–74. [PubMed] [Google Scholar]
- 9.Peelen E, Damoiseaux J, Smolders J, Knippenberg S, Menheere P, Tervaert JW, Hupperts R, Thewissen M. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. Journal of neuroimmunology. 2011;240–241:97–103. doi: 10.1016/j.jneuroim.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Lifshitz GV, Zhdanov DD, Lokhonina AV, Eliseeva DD, Lyssuck EY, Zavalishin IA, Bykovskaia SN. Ex vivo expanded regulatory T cells CD4+CD25+FoxP3+CD127Low develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity. 2016;49(6):388–396. doi: 10.1080/08916934.2016.1199020. [DOI] [PubMed] [Google Scholar]
- 11.Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nature clinical practice Neurology. 2008;4(7):384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 12.Koch MW, Metz LM, Agrawal SM, Yong VW. Environmental factors and their regulation of immunity in multiple sclerosis. Journal of the neurological sciences. 2013;324(1–2):10–16. doi: 10.1016/j.jns.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 14.Haider L. Inflammation, Iron, Energy Failure, and Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Oxidative medicine and cellular longevity. 2015;2015:725370. doi: 10.1155/2015/725370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flugel-Koch C, Flugel A. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488(7413):675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 16.Correale J, Farez MF. Smoking worsens multiple sclerosis prognosis: two different pathways are involved. Journal of neuroimmunology. 2015;281:23–34. doi: 10.1016/j.jneuroim.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 17.De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A. Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology. 2008;70(13 Pt 2):1113–1118. doi: 10.1212/01.wnl.0000294325.63006.f8. [DOI] [PubMed] [Google Scholar]
- 18.Cepok S, Zhou D, Srivastava R, Nessler S, Stei S, Bussow K, Sommer N, Hemmer B. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. The Journal of clinical investigation. 2005;115(5):1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virtanen JO, Farkkila M, Multanen J, Uotila L, Jaaskelainen AJ, Vaheri A, Koskiniemi M. Evidence for human herpesvirus 6 variant A antibodies in multiple sclerosis: diagnostic and therapeutic implications. Journal of neurovirology. 2007;13(4):347–352. doi: 10.1080/13550280701381332. [DOI] [PubMed] [Google Scholar]
- 20.Pohl D. Epstein-Barr virus and multiple sclerosis. Journal of the neurological sciences. 2009;286(1–2):62–64. doi: 10.1016/j.jns.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Pender MP, Csurhes PA, Smith C, Beagley L, Hooper KD, Raj M, Coulthard A, Burrows SR, Khanna R. Epstein-Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Multiple sclerosis. 2014;20(11):1541–1544. doi: 10.1177/1352458514521888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsunoda I, Fujinami RS. Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination. Springer seminars in immunopathology. 2002;24(2):105–125. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato F, Martinez NE, Stewart EC, Omura S, Alexander JS, Tsunoda I. “Microglial nodules” and “newly forming lesions” may be a Janus face of early MS lesions; implications from virus-induced demyelination, the Inside-Out model. BMC neurology. 2015;15:219. doi: 10.1186/s12883-015-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagemeier K, Bruck W, Kuhlmann T. Multiple sclerosis - remyelination failure as a cause of disease progression. Histology and histopathology. 2012;27(3):277–287. doi: 10.14670/HH-27.277. [DOI] [PubMed] [Google Scholar]
- 26.Stohlman SA, Hinton DR. Viral induced demyelination. Brain pathology. 2001;11(1):92–106. doi: 10.1111/j.1750-3639.2001.tb00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. The New England journal of medicine. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 28.Joshi DC, Zhang CL, Lin TM, Gusain A, Harris MG, Tree E, Yin Y, Wu C, Sheng ZH, Dempsey RJ, Fabry Z, Chiu SY. Deletion of mitochondrial anchoring protects dysmyelinating shiverer: implications for progressive MS. 2015;35(13):5293–5306. doi: 10.1523/JNEUROSCI.3859-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annual review of neuroscience. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 30.Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. Journal of neuroscience research. 2004;78(2):157–166. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- 31.Andrews H, White K, Thomson C, Edgar J, Bates D, Griffiths I, Turnbull D, Nichols P. Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. Journal of neuroscience research. 2006;83(8):1533–1539. doi: 10.1002/jnr.20842. [DOI] [PubMed] [Google Scholar]
- 32.Trebst C, Heine S, Lienenklaus S, Lindner M, Baumgartner W, Weiss S, Stangel M. Lack of interferon-beta leads to accelerated remyelination in a toxic model of central nervous system demyelination. Acta neuropathologica. 2007;114(6):587–596. doi: 10.1007/s00401-007-0300-z. [DOI] [PubMed] [Google Scholar]
- 33.Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJ, De Vries HE, van der Valk P, van Horssen J. Enhanced number and activity of mitochondria in multiple sclerosis lesions. The Journal of pathology. 2009;219(2):193–204. doi: 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- 34.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annual review of pathology. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 35.Grade S, Bernardino L, Malva JO. Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2013;31(7):692–700. doi: 10.1016/j.ijdevneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Annals of neurology. 1993;33(2):137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- 37.Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Molecular and cellular neurosciences. 2004;27(3):247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. Journal of neuroimmunology. 2006;176(1–2):162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45(1):41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Mecha M, Feliu A, Carrillo-Salinas FJ, Mestre L, Guaza C. Mobilization of progenitors in the subventricular zone to undergo oligodendrogenesis in the Theiler’s virus model of multiple sclerosis: implications for remyelination at lesions sites. Experimental neurology. 2013;250:348–352. doi: 10.1016/j.expneurol.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Xing YL, Roth PT, Stratton JA, Chuang BH, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(42):14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staugaitis SM, Chang A, Trapp BD. Cortical pathology in multiple sclerosis: experimental approaches to studies on the mechanisms of demyelination and remyelination. Acta neurologica Scandinavica Supplementum. 2012;(195):97–102. doi: 10.1111/ane.12041. [DOI] [PubMed] [Google Scholar]
- 43.Dulamea AO. Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis. Advances in experimental medicine and biology. 2017;958:91–127. doi: 10.1007/978-3-319-47861-6_7. [DOI] [PubMed] [Google Scholar]
- 44.Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. The American journal of pathology. 2004;164(5):1673–1682. doi: 10.1016/S0002-9440(10)63726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell stem cell. 2010;6(6):578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(17):6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. Journal of neuropathology and experimental neurology. 2006;65(3):245–256. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. Journal of neurocytology. 2002;31(6–7):523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- 49.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain pathology. 2007;17(2):210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain pathology. 2004;14(1):43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, Waisman A, Lassmann H, Liblau RS, Mars LT. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. Journal of immunology. 2008;181(3):1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- 52.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Annals of neurology. 2004;55(4):458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 53.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of neurochemistry. 2008;107(1):1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 54.Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. Journal of neurochemistry. 1996;67(3):1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- 55.Tavazzi E, Rovaris M, La Mantia L. Drug therapy for multiple sclerosis. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2014;186(11):833–840. doi: 10.1503/cmaj.130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Annals of neurology. 2009;65(3):239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 57.Fitzner D, Simons M. Chronic progressive multiple sclerosis - pathogenesis of neurodegeneration and therapeutic strategies. Current neuropharmacology. 2010;8(3):305–315. doi: 10.2174/157015910792246218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Annals of neurology. 2006;60(1):12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 59.Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nature reviews Neuroscience. 2012;13(7):507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- 60.Sato F, Tanaka H, Hasanovic F, Tsunoda I. Theiler’s virus infection: Pathophysiology of demyelination and neurodegeneration. Pathophysiology : the official journal of the International Society for Pathophysiology / ISP. 2011;18(1):31–41. doi: 10.1016/j.pathophys.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oikonen M, Laaksonen M, Aalto V, Ilonen J, Salonen R, Eralinna JP, Panelius M, Salmi A. Temporal relationship between environmental influenza A and Epstein-Barr viral infections and high multiple sclerosis relapse occurrence. Multiple sclerosis. 2011;17(6):672–680. doi: 10.1177/1352458510394397. [DOI] [PubMed] [Google Scholar]
- 62.Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G. Animal models of Multiple Sclerosis. European journal of pharmacology. 2015;759:182–191. doi: 10.1016/j.ejphar.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nature reviews Microbiology. 2003;1(2):151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- 64.Keough MB, Jensen SK, Yong VW. Experimental demyelination and remyelination of murine spinal cord by focal injection of lysolecithin. Journal of visualized experiments : JoVE. 2015;(97) doi: 10.3791/52679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baxi EG, DeBruin J. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. 2015;35(22):8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cristofanilli M, Rosenthal H, Cymring B, Gratch D, Pagano B, Xie B, Sadiq SA. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Experimental neurology. 2014;261:620–632. doi: 10.1016/j.expneurol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 68.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine. 2003;9(4):439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 69.Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, Shi Z, Zhang K, Li C, Cui Y, Lin H, Luo D, Wang J, Lin C, Dai Z, Zhu H, Zhang J, Liu J, Liu H, deVellis J, Horvath S, Sun YE, Li S. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161(5):1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andressen C. Neural stem cells: from neurobiology to clinical applications. Current pharmaceutical biotechnology. 2013;14(1):20–28. [PubMed] [Google Scholar]
- 71.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Baron-Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. The European journal of neuroscience. 1999;11(12):4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 73.Jablonska A, Kozlowska H, Markiewicz I, Domanska-Janik K, Lukomska B. Transplantation of neural stem cells derived from human cord blood to the brain of adult and neonatal rats. Acta neurobiologiae experimentalis. 2010;70(4):337–350. doi: 10.55782/ane-2010-1806. [DOI] [PubMed] [Google Scholar]
- 74.Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nature neuroscience. 2008;11(8):888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cerebral cortex. 2003;13(6):580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 77.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capilla-Gonzalez V, Herranz-Perez V, Garcia-Verdugo JM. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Frontiers in cellular neuroscience. 2015;9:365. doi: 10.3389/fncel.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging and disease. 2011;2(1):49–63. [PMC free article] [PubMed] [Google Scholar]
- 80.Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quinones-Hinojosa A. Age-related changes in astrocytic and ependymal cells of the subventricular zone. Glia. 2014;62(5):790–803. doi: 10.1002/glia.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell stem cell. 2011;8(5):566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526(7573):448–452. doi: 10.1038/nature14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klingener M, Chavali M, Singh J, McMillan N, Coomes A, Dempsey PJ, Chen EI, Aguirre A. N-cadherin promotes recruitment and migration of neural progenitor cells from the SVZ neural stem cell niche into demyelinated lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(29):9590–9606. doi: 10.1523/JNEUROSCI.3699-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Brousse B, Magalon K, Durbec P, Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biology open. 2015;4(8):980–992. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calzolari F, Michel J, Baumgart EV, Theis F, Gotz M, Ninkovic J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nature neuroscience. 2015;18(4):490–492. doi: 10.1038/nn.3963. [DOI] [PubMed] [Google Scholar]
- 86.Lee Y, Oh SB, Park HR, Kim HS, Kim MS, Lee J. Selective impairment on the proliferation of neural progenitor cells by oxidative phosphorylation disruption. Neuroscience letters. 2013;535:134–139. doi: 10.1016/j.neulet.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 87.Zilkha-Falb R, Kaushansky N, Kawakami N, Ben-Nun A. Post-CNS-inflammation expression of CXCL12 promotes the endogenous myelin/neuronal repair capacity following spontaneous recovery from multiple sclerosis-like disease. Journal of neuroinflammation. 2016;13:7. doi: 10.1186/s12974-015-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaneko N, Kako E, Sawamoto K. Prospects and limitations of using endogenous neural stem cells for brain regeneration. Genes. 2011;2(1):107–130. doi: 10.3390/genes2010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang GX. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Experimental and molecular pathology. 2015;98(2):240–245. doi: 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cellular and molecular neurobiology. 2010;30(2):289–299. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 92.Toghianifar N, Ashtari F, Zarkesh-Esfahani SH, Mansourian M. Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Journal of neuroimmunology. 2015;285:125–128. doi: 10.1016/j.jneuroim.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 93.Cantarella C, Cayre M, Magalon K, Durbec P. Intranasal HB-EGF administration favors adult SVZ cell mobilization to demyelinated lesions in mouse corpus callosum. Developmental neurobiology. 2008;68(2):223–236. doi: 10.1002/dneu.20588. [DOI] [PubMed] [Google Scholar]
- 94.Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathology and applied neurobiology. 2003;29(5):434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 95.Amir-Levy Y, Mausner-Fainberg K, Karni A. Treatment with Anti-EGF Ab Ameliorates Experimental Autoimmune Encephalomyelitis via Induction of Neurogenesis and Oligodendrogenesis. Multiple sclerosis international. 2014;2014:926134. doi: 10.1155/2014/926134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cate HS, Sabo JK, Merlo D, Kemper D, Aumann TD, Robinson J, Merson TD, Emery B, Perreau VM, Kilpatrick TJ. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. Journal of neurochemistry. 2010;115(1):11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- 97.Totoiu MO, Nistor GI, Lane TE, Keirstead HS. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Experimental neurology. 2004;187(2):254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hardison JL, Nistor G, Gonzalez R, Keirstead HS, Lane TE. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Experimental neurology. 2006;197(2):420–429. doi: 10.1016/j.expneurol.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanc CA, Grist JJ, Rosen H, Sears-Kraxberger I, Steward O, Lane TE. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. The American journal of pathology. 2015;185(10):2819–2832. doi: 10.1016/j.ajpath.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guan Y, Jiang Z, Ciric B, Rostami AM, Zhang GX. Upregulation of chemokine receptor expression by IL-10/IL-4 in adult neural stem cells. Experimental and molecular pathology. 2008;85(3):232–236. doi: 10.1016/j.yexmp.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 101.Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology. 2012;33(2):191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzumura A. [Microglia in pathophysiology of neuroimmunological disorders] Nihon rinsho Japanese journal of clinical medicine. 2013;71(5):801–806. [PubMed] [Google Scholar]
- 103.van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L, Gerritsen W, Kooi EJ, Witte ME, Geurts JJ, de Vries HE, Peferoen-Baert R, van den Elsen PJ, van der Valk P, Amor S. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. Journal of neuroinflammation. 2012;9:156. doi: 10.1186/1742-2094-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature reviews Neurology. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 105.Al-Shamsi M, Shahin A, Ibrahim MF, Tareq S, Souid AK, Mensah-Brown EP. Bioenergetics of the spinal cord in experimental autoimmune encephalitis of rats. BMC neuroscience. 2015;16:37. doi: 10.1186/s12868-015-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lampron A, Larochelle A, Laflamme N, Prefontaine P, Plante MM, Sanchez MG, Yong VW, Stys PK, Tremblay ME, Rivest S. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. The Journal of experimental medicine. 2015;212(4):481–495. doi: 10.1084/jem.20141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. Journal of neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141(3):302–313. doi: 10.1111/imm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Annals of neurology. 2001;50(5):646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 110.Marin-Teva JL, Cuadros MA, Martin-Oliva D, Navascues J. Microglia and neuronal cell death. Neuron glia biology. 2011;7(1):25–40. doi: 10.1017/S1740925X12000014. [DOI] [PubMed] [Google Scholar]
- 111.Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain research Brain research reviews. 2004;46(3):261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Derkow K, Kruger C, Dembny P, Lehnardt S. Microglia Induce Neurotoxic IL-17+ gammadelta T Cells Dependent on TLR2, TLR4, and TLR9 Activation. PloS one. 2015;10(8):e0135898. doi: 10.1371/journal.pone.0135898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Almolda B, Gonzalez B, Castellano B. Are Microglial Cells the Regulators of Lymphocyte Responses in the CNS? Frontiers in cellular neuroscience. 2015;9:440. doi: 10.3389/fncel.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nature medicine. 2005;11(2):146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 115.Zhou J, Cai W, Jin M, Xu J, Wang Y, Xiao Y, Hao L, Wang B, Zhang Y, Han J, Huang R. 18beta-glycyrrhetinic acid suppresses experimental autoimmune encephalomyelitis through inhibition of microglia activation and promotion of remyelination. Scientific reports. 2015;5:13713. doi: 10.1038/srep13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muja N, Cohen ME, Zhang J, Kim H, Gilad AA, Walczak P, Ben-Hur T, Bulte JW. Neural precursors exhibit distinctly different patterns of cell migration upon transplantation during either the acute or chronic phase of EAE: a serial MR imaging study. Magnetic resonance in medicine. 2011;65(6):1738–1749. doi: 10.1002/mrm.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cohen ME, Fainstein N, Lavon I, Ben-Hur T. Signaling through three chemokine receptors triggers the migration of transplanted neural precursor cells in a model of multiple sclerosis. Stem cell research. 2014;13(2):227–239. doi: 10.1016/j.scr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 119.Liu J, Hjorth E, Zhu M, Calzarossa C, Samuelsson EB, Schultzberg M, Akesson E. Interplay between human microglia and neural stem/progenitor cells in an allogeneic co-culture model. Journal of cellular and molecular medicine. 2013;17(11):1434–1443. doi: 10.1111/jcmm.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Molecular and cellular neurosciences. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 121.Guadagno J, Xu X, Karajgikar M, Brown A, Cregan SP. Microglia-derived TNFalpha induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell death & disease. 2013;4:e538. doi: 10.1038/cddis.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu HM, Zhang LF, Ding PS, Liu YJ, Wu X, Zhou JN. Microglial activation mediates host neuronal survival induced by neural stem cells. Journal of cellular and molecular medicine. 2014;18(7):1300–1312. doi: 10.1111/jcmm.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. The Journal of experimental medicine. 2005;201(4):647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nature neuroscience. 2012;15(11):1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(10):3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasmussen S, Imitola J, Ayuso-Sacido A, Wang Y, Starossom SC, Kivisakk P, Zhu B, Meyer M, Bronson RT, Garcia-Verdugo JM, Khoury SJ. Reversible neural stem cell niche dysfunction in a model of multiple sclerosis. Annals of neurology. 2011;69(5):878–891. doi: 10.1002/ana.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nature biotechnology. 2001;19(12):1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 129.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. Journal of the neurological sciences. 2008;265(1–2):26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 130.Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. Journal of cell science. 117;(19):4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 131.Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, Rostami A, Zhang GX. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. The American journal of pathology. 2010;177(4):1989–2001. doi: 10.2353/ajpath.2010.091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hew M, O’Connor K, Edel MJ, Lucas M. The Possible Future Roles for iPSC-Derived Therapy for Autoimmune Diseases. Journal of clinical medicine. 2015;4(6):1193–1206. doi: 10.3390/jcm4061193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem cell research & therapy. 2013;4(5):115. doi: 10.1186/scrt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, Zhan M, Davis J, Bharti K, Zeng X, Rao M, Malik N, Vemuri MC. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem cells translational medicine. 2013;2(11):862–870. doi: 10.5966/sctm.2013-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang T, Choi E, Monaco MC, Campanac E, Medynets M, Do T, Rao P, Johnson KR, Elkahloun AG, Von Geldern G, Johnson T, Subramaniam S, Hoffman D, Major E, Nath A. Derivation of neural stem cells from human adult peripheral CD34+ cells for an autologous model of neuroinflammation. PloS one. 2013;8(11):e81720. doi: 10.1371/journal.pone.0081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mozafari S, Laterza C, Roussel D, Bachelin C, Marteyn A, Deboux C, Martino G, Baron-Van Evercooren A. Skin-derived neural precursors competitively generate functional myelin in adult demyelinated mice. The Journal of clinical investigation. 2015;125(9):3642–3656. doi: 10.1172/JCI80437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG. Wnt/beta-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. The Journal of biological chemistry. 2013;288(24):17214–17224. doi: 10.1074/jbc.M112.445965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicaise AM, Banda E, Guzzo RM, Russomanno K, Castro-Borrero W, Willis CM, Johnson KM, Lo AC, Crocker SJ. iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Experimental neurology. 2016;288:114–121. doi: 10.1016/j.expneurol.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 139.Lee ST, Chu K, Jung KH, Song YM, Jeon D, Kim SU, Kim M, Lee SK, Roh JK. Direct generation of neurosphere-like cells from human dermal fibroblasts. PloS one. 2011;6(7):e21801. doi: 10.1371/journal.pone.0021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Capetian P, Azmitia L, Pauly MG, Krajka V, Stengel F, Bernhardi EM, Klett M, Meier B, Seibler P, Stanslowsky N, Moser A, Knopp A, Gillessen-Kaesbach G, Nikkhah G, Wegner F, Dobrossy M, Klein C. Plasmid-Based Generation of Induced Neural Stem Cells from Adult Human Fibroblasts. Frontiers in cellular neuroscience. 2016;10:245. doi: 10.3389/fncel.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, Brustle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell stem cell. 2012;10(4):473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 142.Mirakhori F, Zeynali B, Rassouli H, Shahbazi E, Hashemizadeh S, Kiani S, Salekdeh GH, Baharvand H. Induction of Neural Progenitor-Like Cells from Human Fibroblasts via a Genetic Material-Free Approach. PloS one. 2015;10(8):e0135479. doi: 10.1371/journal.pone.0135479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miura T, Sugawara T, Fukuda A, Tamoto R, Kawasaki T, Umezawa A, Akutsu H. Generation of primitive neural stem cells from human fibroblasts using a defined set of factors. Biology open. 2015;4(11):1595–1607. doi: 10.1242/bio.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(7):2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Plaisted WC, Zavala A, Hingco E, Tran H, Coleman R, Lane TE, Loring JF, Walsh CM. Remyelination Is Correlated with Regulatory T Cell Induction Following Human Embryoid Body-Derived Neural Precursor Cell Transplantation in a Viral Model of Multiple Sclerosis. PloS one. 2016;11(6):e0157620. doi: 10.1371/journal.pone.0157620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hawryluk GW, Mothe AJ, Chamankhah M, Wang J, Tator C, Fehlings MG. In vitro characterization of trophic factor expression in neural precursor cells. Stem cells and development. 2012;21(3):432–447. doi: 10.1089/scd.2011.0242. [DOI] [PubMed] [Google Scholar]
- 147.Fainstein N, Einstein O, Cohen ME, Brill L, Lavon I, Ben-Hur T. Time limited immunomodulatory functions of transplanted neural precursor cells. Glia. 2013;61(2):140–149. doi: 10.1002/glia.22420. [DOI] [PubMed] [Google Scholar]
- 148.Martino G, Franklin RJ, Baron Van Evercooren A, BKerr DA. Stem Cells in Multiple Sclerosis Consensus G. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nature reviews Neurology. 2010;6(5):247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 149.Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Annals of neurology. 2007;61(3):209–218. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 150.Pluchino S, Zanotti L, Brambilla E, Rovere-Querini P, Capobianco A, Alfaro-Cervello C, Salani G, Cossetti C, Borsellino G, Battistini L, Ponzoni M, Doglioni C, Garcia-Verdugo JM, Comi G, Manfredi AA, Martino G. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PloS one. 2009;4(6):e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) British journal of pharmacology. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Donega M, Giusto E, Cossetti C, Schaeffer J, Pluchino S. Systemic injection of neural stem/progenitor cells in mice with chronic EAE. Journal of visualized experiments : JoVE. 2014;(86) doi: 10.3791/51154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 154.Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41(1):73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- 155.Wu S, Li K, Yan Y, Gran B, Han Y, Zhou F, Guan YT, Rostami A, Zhang GX. Intranasal Delivery of Neural Stem Cells: A CNS-specific, Non-invasive Cell-based Therapy for Experimental Autoimmune Encephalomyelitis. Journal of clinical & cellular immunology. 2013;4(3) doi: 10.4172/2155-9899.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, Schaar B, Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d–positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke; a journal of cerebral circulation. 2008;39(4):1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 157.Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nature neuroscience. 2012;15(8):1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- 158.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nature reviews Neuroscience. 2006;7(5):395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 159.Harris VK, Faroqui R, Vyshkina T, Sadiq SA. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem cells translational medicine. 2012;1(7):536–547. doi: 10.5966/sctm.2012-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen L, Coleman R, Leang R, Tran H, Kopf A, Walsh CM, Sears-Kraxberger I, Steward O, Macklin WB, Loring JF, Lane TE. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem cell reports. 2014;2(6):825–837. doi: 10.1016/j.stemcr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Ruscio A, Patti F, Welner RS, Tenen DG, Amabile G. Multiple sclerosis: getting personal with induced pluripotent stem cells. Cell death & disease. 2015;6:e1806. doi: 10.1038/cddis.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ravanidis S, Poulatsidou KN, Lagoudaki R, Touloumi O, Polyzoidou E, Lourbopoulos A, Nousiopoulou E, Theotokis P, Kesidou E, Tsalikakis D, Karacostas D, Grigoriou M, Chlichlia K, Grigoriadis N. Subcutaneous Transplantation of Neural Precursor Cells in Experimental Autoimmune Encephalomyelitis Reduces Chemotactic Signals in the Central Nervous System. Stem cells translational medicine. 2015;4(12):1450–1462. doi: 10.5966/sctm.2015-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436(7048):266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 164.Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Molecular and cellular neurosciences. 2003;24(4):1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 165.Whitman LM, Blanc CA, Schaumburg CS, Rowitch DH, Lane TE. Olig1 function is required for remyelination potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Experimental neurology. 2012;235(1):380–387. doi: 10.1016/j.expneurol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Einstein O, Friedman-Levi Y, Grigoriadis N, Ben-Hur T. Transplanted neural precursors enhance host brain-derived myelin regeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(50):15694–15702. doi: 10.1523/JNEUROSCI.3364-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harris VK, Yan QJ, Vyshkina T, Sahabi S, Liu X, Sadiq SA. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. Journal of the neurological sciences. 2012;313(1–2):167–177. doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 168.Laterza C, Merlini A, De Feo D, Ruffini F, Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, Quattrini A, Taveggia C, Farina C, Cattaneo E, Martino G. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nature communications. 2013;4:2597. doi: 10.1038/ncomms3597. [DOI] [PubMed] [Google Scholar]
- 169.Reekmans K, Praet J, De Vocht N, Daans J, Van der Linden A, Berneman Z, Ponsaerts P. Stem cell therapy for multiple sclerosis: preclinical evidence beyond all doubt? Regenerative medicine. 2012;7(2):245–259. doi: 10.2217/rme.12.5. [DOI] [PubMed] [Google Scholar]
- 170.Ricci-Vitiani L, Lombardi DG, Signore M, Biffoni M, Pallini R, Parati E, Peschle C, De Maria R. Human neural progenitor cells display limited cytotoxicity and increased oligodendrogenesis during inflammation. Cell death and differentiation. 2007;14(4):876–878. doi: 10.1038/sj.cdd.4402078. [DOI] [PubMed] [Google Scholar]
- 171.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews Neuroscience. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 172.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. The Journal of pathology. 2009;217(2):169–180. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 173.Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Molecular and cellular neurosciences. 2003;24(3):623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 174.Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain : a journal of neurology. 2005;128(3):528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- 175.Molina-Holgado E, Vela JM, Arevalo-Martin A, Guaza C. LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. The European journal of neuroscience. 2001;13(3):493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- 176.Perez-Asensio FJ, Perpina U, Planas AM, Pozas E. Interleukin-10 regulates progenitor differentiation and modulates neurogenesis in adult brain. Journal of cell science. 2013;126(18):4208–4219. doi: 10.1242/jcs.127803. [DOI] [PubMed] [Google Scholar]
- 177.Kulkarni A, Scully TJ, O’Donnell LA. The antiviral cytokine interferon-gamma restricts neural stem/progenitor cell proliferation through activation of STAT1 and modulation of retinoblastoma protein phosphorylation. Journal of neuroscience research. 2016 doi: 10.1002/jnr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kizil C, Kyritsis N, Brand M. Effects of inflammation on stem cells: together they strive? EMBO reports. 2015;16(4):416–426. doi: 10.15252/embr.201439702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nature neuroscience. 2016;19(2):243–252. doi: 10.1038/nn.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Giannakopoulou A, Grigoriadis N, Polyzoidou E, Lourbopoulos A, Michaloudi E, Papadopoulos GC. Time-dependent fate of transplanted neural precursor cells in experimental autoimmune encephalomyelitis mice. Experimental neurology. 2011;230(1):16–26. doi: 10.1016/j.expneurol.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 181.Mueller FJ, Serobyan N, Schraufstatter IU, DiScipio R, Wakeman D, Loring JF, Snyder EY, Khaldoyanidi SK. Adhesive interactions between human neural stem cells and inflamed human vascular endothelium are mediated by integrins. Stem cells. 2006;24(11):2367–2372. doi: 10.1634/stemcells.2005-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abdanipour A, Sagha M, Noori-Zadeh A, Pakzad I, Tiraihi T. In vitro study of the long-term cortisol treatment effects on the growth rate and proliferation of the neural stem/precursor cells. Neurological research. 2015;37(2):117–124. doi: 10.1179/1743132814Y.0000000431. [DOI] [PubMed] [Google Scholar]
- 186.Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, Garcia-Verdugo JM, Comi G, Khoury SJ, Martino G. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain : a journal of neurology. 2008;131(10):2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair. Pharmacology & therapeutics. 2014;141(1):21–31. doi: 10.1016/j.pharmthera.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 188.Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(35):7924–7933. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neri M, Maderna C, Ferrari D, Cavazzin C, Vescovi AL, Gritti A. Robust generation of oligodendrocyte progenitors from human neural stem cells and engraftment in experimental demyelination models in mice. PloS one. 2010;5(4):e10145. doi: 10.1371/journal.pone.0010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang J, Yan Y, Xia Y, Kang T, Li X, Ciric B, Xu H, Rostami A, Zhang GX. Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22(2):440–450. doi: 10.1038/mt.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Gao X, Deng L, Wang Y, Yin L, Yang C, Du J, Yuan Q. GDNF Enhances Therapeutic Efficiency of Neural Stem Cells-Based Therapy in Chronic Experimental Allergic Encephalomyelitis in Rat. Stem cells international. 2016;2016:1431349. doi: 10.1155/2016/1431349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, Gerhardt E, Neumann H, Sendtner M, Luhder F, Gold R. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain : a journal of neurology. 2010;133(8):2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 193.Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F, Hong J, Shi Y, Leung S, Dong C, Zhang JZ. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35(2):273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 194.Ma H, Yu B, Kong L, Zhang Y, Shi Y. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochemical research. 2012;37(1):69–83. doi: 10.1007/s11064-011-0584-1. [DOI] [PubMed] [Google Scholar]
- 195.Chang DJ, Lee N, Choi C, Jeon I, Oh SH, Shin DA, Hwang TS, Lee HJ, Kim SU, Moon H, Hong KS, Kang KS, Song J. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) in a rodent model of middle cerebral artery occlusion. Cell transplantation. 2013;22(8):1441–1452. doi: 10.3727/096368912X657323. [DOI] [PubMed] [Google Scholar]
- 196.Kandalam S, Sindji L, Delcroix GJ, Violet F, Garric X, Andre EM, Schiller PC, Venier-Julienne MC, des Rieux A, Guicheux J, Montero-Menei CN. Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta biomaterialia. 2016 doi: 10.1016/j.actbio.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 197.Klose J, Schmidt NO, Melms A, Dohi M, Miyazaki J, Bischof F, Greve B. Suppression of experimental autoimmune encephalomyelitis by interleukin-10 transduced neural stem/progenitor cells. Journal of neuroinflammation. 2013;10:117. doi: 10.1186/1742-2094-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, Rostami A, Zhang GX. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. The Journal of clinical investigation. 2009;119(12):3678–3691. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gudi V, Skuljec J, Yildiz O, Frichert K, Skripuletz T, Moharregh-Khiabani D, Voss E, Wissel K, Wolter S, Stangel M. Spatial and temporal profiles of growth factor expression during CNS demyelination reveal the dynamics of repair priming. PloS one. 2011;6(7):e22623. doi: 10.1371/journal.pone.0022623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mason JL, Ye P, Suzuki K, D’Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(15):5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sabo JK, Aumann TD, Kilpatrick TJ, Cate HS. Investigation of sequential growth factor delivery during cuprizone challenge in mice aimed to enhance oligodendrogliogenesis and myelin repair. PloS one. 2013;8(5):e63415. doi: 10.1371/journal.pone.0063415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shi B, Ding J, Liu Y, Zhuang X, Zhuang X, Chen X, Fu C. ERK1/2 pathway-mediated differentiation of IGF-1-transfected spinal cord-derived neural stem cells into oligodendrocytes. PloS one. 2014;9(8):e106038. doi: 10.1371/journal.pone.0106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee YE, An J, Lee KH, Kim SS, Song HJ, Pyeon H, Nam H, Kang K, Joo KM. Correction: The Synergistic Local Immunosuppressive Effects of Neural Stem Cells Expressing Indoleamine 2,3-Dioxygenase (IDO) in an Experimental Autoimmune Encephalomyelitis (EAE) Animal Model. PloS one. 2016;11(2):e0148720. doi: 10.1371/journal.pone.0148720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sher F, Amor S, Gerritsen W, Baker D, Jackson SL, Boddeke E, Copray S. Intraventricularly injected Olig2-NSCs attenuate established relapsing-remitting EAE in mice. Cell transplantation. 2012;21(9):1883–1897. doi: 10.3727/096368911X637443. [DOI] [PubMed] [Google Scholar]
- 205.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 206.Geurts JJ, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van der Valk P. Extensive hippocampal demyelination in multiple sclerosis. Journal of neuropathology and experimental neurology. 2007;66(9):819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 207.Braun SM, Pilz GA, Machado RA, Moss J, Becher B, Toni N, Jessberger S. Programming Hippocampal Neural Stem/Progenitor Cells into Oligodendrocytes Enhances Remyelination in the Adult Brain after Injury. Cell reports. 2015;11(11):1679–1685. doi: 10.1016/j.celrep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 208.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature neuroscience. 2004;7(3):221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 209.Li X, Zhang Y, Yan Y, Ciric B, Ma CG, Gran B, Curtis M, Rostami A, Zhang GX. Neural Stem Cells Engineered to Express Three Therapeutic Factors Mediate Recovery from Chronic Stage CNS Autoimmunity. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24(8):1456–1469. doi: 10.1038/mt.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 211.Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain & development. 2007;29(4):193–201. doi: 10.1016/j.braindev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 212.Hackett C, Knight J, Mao-Draayer Y. Transplantation of Fas-deficient or wild-type neural stem/progenitor cells (NPCs) is equally efficient in treating experimental autoimmune encephalomyelitis (EAE) American journal of translational research. 2014;6(2):119–128. [PMC free article] [PubMed] [Google Scholar]
- 213.Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, Bordeau J, Fournier C, Johe K, Hazel T, Cudkowicz M, Atassi N, Borges LF, Rutkove SB, Duell J, Patil PG, Goutman SA, Feldman EL. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology. 2016;87(4):392–400. doi: 10.1212/WNL.0000000000002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18(12):1476–1482. doi: 10.1016/j.jcyt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 215.Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. 2012;4(155):155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]