Abstract
An individual’s socioeconomic status (SES) is often viewed as a proxy for a host of environmental influences. SES disparities have been linked to variance in brain structures particularly the hippocampus, a neural substrate of learning and memory. However, it is unclear whether the association between SES and hippocampal volume is similar in children and adults. We investigated the relationship between hippocampal volume and SES in a group of children (n = 31, age 8–12 years) and a group of young adults (n = 32, age 18–25 years). SES was assessed with four indicators that loaded on a single factor, therefore a composite SES scores was used in the main analyses. Hippocampal volume was measured using manual demarcation on high resolution structural images. SES was associated with hippocampal volume in the children, but not in adults, suggesting that in childhood, but not adulthood, SES-related environmental factors influence hippocampal volume. Additionally, hippocampal volume, but not SES, was associated with scores on a memory task, suggesting that net effects of postnatal environmental factors, captured by SES, are more distal determinants of memory performance than hippocampal volume. Longitudinal investigation of the association between SES, hippocampal volume and cognitive functioning may further our understanding of the putative neural mechanisms underlying SES-related environmental effects on cognitive development.
Keywords: SES, childhood, hippocampus, structural MRI
Introduction
Childhood is a period of robust gains in knowledge and cognitive abilities (Bjorklund, 2012). This period is also characterized by complex changes in the brain (Gogtay et al., 2004; Giedd et al., 1999) that differ across individuals in pace and magnitude. The sources of individual differences in cognitive development are, at least partially, linked to environmental factors that may shape typical brain growth. For example, growing up in poverty (Evans, 2004; Evans and Schamberg, 2009) and sustaining emotional or physical trauma and deprivation (Perry, 2002; Schore et al., 2001) can adversely influence brain development. Socioeconomic status (SES) has been extensively studied as a proxy measure of the myriad environmental factors that shape development. SES was found to be associated with children’s health outcomes, both physical and mental, as well as with cognitive and brain development (Cohen, Janicki-deverts, Chen & Matthews, 2010; Conroy, Sandel & Zuckerman, 2010; Hackman, Farah & Meaney, 2010; Jednoróg et al., 2012; Mackey et al., 2015).
Disparity in childhood SES is associated with individual differences in cognitive abilities. In children and adolescents, full scale IQ correlates with family income and parental education (Lange, Froimowitz, Bigler, Lainhart & Brain Developmental Cooperative Group, 2010). Children from lower SES also obtain lower scores on tests of language, math, executive function, and memory (Farah et al., 2006; Noble, Norman & Farah, 2005; Noble, McCandliss & Farrah, 2007; Herrmann and Guadagno, 1997; Hackman and Farah, 2009; Jednoróg et al., 2012). Moreover, individuals raised in lower SES households exhibit lower performance in the classroom than their higher SES counterparts (Brooks-Gunn and Duncan, 1997; Feinstein, 2003), are more likely to fail academically in later childhood (Feinstein, 2003), and obtain lower scores on standardized academic achievement tests during adolescence (Mackey et al., 2015).
The biological mechanisms underlying the relation between early life SES and cognitive abilities in both childhood and adulthood are not yet fully understood. However, early-life SES may account for variability in brain development and, potentially, lasting cognitive effects in adulthood. Indeed, low SES is associated with thinner frontal and cingulate cortices (Lawson, Duda, Avants, Wu & Martha, 2013; Noble, Korgaonkar, Grieve & Brickman, 2013; Noble et al., 2015), as well as smaller cerebellar (Cavanagh et al., 2013) and cortical gray matter volume (Jednoróg et al., 2012; Luby et al., 2015; Mackey et al., 2015). Recently, smaller cortical thickness in adolescents from lower income background has been linked to poorer standardized test performance in multiple cognitive domains (Mackey et al., 2015).
The hippocampus, is sensitive to both adverse (e.g., stress: McEwen, 1999; Carrion, Weems & Reiss, 2007; Hanson et al., 2015; Alfarez, Joe & Krugers, 2003; Mirescu and Gould, 2006) and protective (e.g., enriched environment: Brown et al., 2003; Kempermann, Kuhn & Gage, 1997; Miller, Colella, Mikulis, Maller & Green, 2013) effects of childhood, SES-related, factors. Due to its known role in memory functioning (Scoville and Milner, 1957; Tulving and Markowitsch, 1998, Chaddock et al., 2010; see Van Petten, 2004 for findings of a meta-analysis across the lifespan), this structure may partially confer SES-related effects on learning and memory functions during early life. Total hippocampal volume is thought to be stable after the age of four, yet pronounced individual differences that are independent of age have been documented in both children (Gogtay et al., 2006; Daugherty, Bender, Raz & Ofen, 2016) and adults (Raz et al., 2005). These findings may suggest that brain anatomy is modified by early factors throughout the lifespan. Indeed, children from households of lower SES have smaller hippocampal volume as compared to counterparts in higher SES households (Hanson, Chandra, Wolfe & Pollak, 2011; Jednoróg et al., 2012; Noble, Houston, Kan & Sowell, 2012b; Hanson et al., 2015). Among those in the lowest range of estimated SES measured, relatively higher parental education is correlated with larger hippocampal volume (Noble et al., 2015).
SES may impact the developing hippocampus and, in turn, cognitive performance, via a confluence of adverse and favorable environmental influences whose final biological effect may be assumed to fall along a continuum. At one end of this continuum, one may consider the result of deleterious environmental factors such as the elevated stress level. Indeed, animal studies have shown that the hippocampus is vulnerable to stress (see McEwen, 1999 for review), and that stress-related elevated cortisol levels can interfere with plasticity and neurogenesis (McEwen, 1999; Mirescu and Gould, 2006), the posited cellular mechanics of learning and memory functions. Research in human has shown effects of stress on hippocampal structure (Duman, 2002; Hackman et al., 2010; Carrion et al., 2007; Hanson et al., 2015) and function (Sheridan et al., 2013). Moreover, low SES has been linked to higher levels of salivary cortisol in elementary school children (Lupien, King, Meaney & McEwen, 2001; Sheridan, How, Araujo, Schamberg & Nelson, 2013). Finally, studies of the long-term effects of childhood maltreatment, another deleterious factor that is potentially associated with lower levels of SES (Cancian, Slack, Yang, 2010), have also documented reduced hippocampal volume in children exposed to maltreatment (McLaughlin et al., 2016). On the opposite end of the continuum, higher SES may furnish positive environmental factors such as increased quality of parental care, enriched home, pre-academic and academic environments, and facilitated access to health services and an overall healthier lifestyle, factors that may contribute to healthy brain and cognitive development. Indeed, in animal studies, researchers have shown that an enriched living environment may promote neural plasticity and higher rates of neurogenesis in the hippocampus (Brown et al., 2003; Kempermann et al., 1997). It was also shown that the maternal care of offspring may buffer adverse effects of stress on the hippocampus (Weaver et al., 2004; Francis, Diorio, Liu & Meaney, 1999; Liu et al., 1997). Protective effects of higher level of life enrichment and of maternal care has also been documents in children and adults (Gunnar, 1998; Miller et al., 2013). Thus, the impact of early life SES on hippocampal functional development may have a lasting impact into adulthood. Yet, little is known of this presumed association, in part due to the shortcomings of methods that limit valid comparisons of hippocampal volume across ages.
Although studies have been published on the relationship between childhood SES and hippocampal volume, the findings in the extant literature rely chiefly on hippocampal volume measures obtained from semi-automatic segmentation methods using FreeSurfer (Noble et al., 2015; Noble et al., 2012a; Noble et al., 2012b; Jednoróg et al., 2012), voxel-based morphometry (Hanson et al., 2011; Jednoróg et al., 2012), and SPM (Rao et al., 2010). When compared to gold-standard manual tracing, however, the convergent validity of these semi-automatic segmentation methods is questionable (Shen et al., 2010; Oscar-Berman & Song, 2011; Mechelli, Price, Friston & Ashburner, 2005). For example, Morey et al. (2009) found poor to moderate percent volume overlap (0.77–0.82) between FreeSurfer, FSL-FIRST, and manual demarcation in adults. Dewey et al. (2010) showed poor percent volume overlap (0.37–0.75) when comparing FreeSurfer and IBASPM with an auto-assisted manual tracing in HIV-infected adults. Pipitone et al. (2014) found moderate Dice’s Similarity Coefficient in older adults and patients with first episode psychosis (0.87–0.89), and low correlation (r<=0.70) between Multiple Automatically Generated Templates and manual demarcation. Importantly, none of the above studies provided evidence of the validity of FreeSurfer hippocampal segmentation in children. In a sample of 6–11-year old children, poor agreement between two automated methods (FreeSurfer and FSL-FIRST) and manual demarcation was found by Schoemaker et al. (2016). Importantly, Wenger et al., (2014) found that an overestimation bias by FreeSurfer systematically varies with age when comparing groups of younger and older adults. Therefore, age-related effects obtained using such methods may be spurious and should be interpreted with caution. We aim to address these limitations by employing a manual demarcation procedure, performed by raters with confirmed high inter-rater reliability for the demarcation of the hippocampus (ICC(2) > 0.9). We note that another study that used reliable manual segmentation showed an association between hippocampal volume and SES in a sample of children (see Hanson et al., 2015). However, a similar comparison was not conducted in adults. Hence, the extent to which SES may account for differences in hippocampal volume and mnemonic correlates across age groups remains unknown.
In the present study, we investigated the relation of SES to hippocampal volume in typically developing children (ages 8–12 years) and in young adults (ages 18–25 years). We then examined age group as a possible moderator of the magnitude of this relationship. We predicted that because children may be more vulnerable to adverse environmental influences, hippocampal volume would be differentially related to SES in children compared to adults, with a stronger association observed in children. In addition, in this sample we also examined the association of hippocampal volume to performance on a memory task.
Method
Participants
Thirty-one healthy, typically developing children (ages 8–12 years, M = 10.49, SD = 1.36; 42% female; 19% African American, 74% Caucasian, 7% more than one race) and 32 young adults (ages 18–25 years, M = 21.71, SD = 1.94; 50% female; 19% Asian, 19% African American, 56% Caucasian, 3% more than one race, 3% Other/Unknown) were recruited. The two groups did not differ in IQ (children: M = 111.71, SD = 11.77; adults: M = 109.81, SD = 11.14; t(61) = 0.66, p = 0.51). Participants were recruited from the Metro Detroit area as part of a larger study of cognitive and brain development. Participants were self-reported right-hand dominant, spoke English as a native language, had no reported developmental or neurological disorders, and no history of head trauma. For MRI compatibility and safety, participants had no metallic implants, braces, or permanent retainers. Participants were consented in accord with procedures approved by Wayne State University Institutional Review Board, which included parental consent for minors.
Socioeconomic Status
Four variables were measured to reflect the participants’ SES. Subjective SES rating was assessed through self-report on MacArthur Scale of Subjective Social Status (MAS; http://www.macses.ucsf.edu/). The MAS measures perception of one’s own social status in relation to the population of the United States. This measure consists of a 10-point Likert scale displayed as a vertical ladder. Participants were told that the ladder represented the social standing of people in the United States, with individuals having the most money and education and most respected jobs occupying the top rungs, and those with the least money and education and least respected or no job at the bottom. Participants indicated the rung that best matched their subjective rating of their relative social status. Placement on the rungs was coded as corresponding to a number between 1 and 10, with a score of 1 given to the rung at the very bottom of the ladder. This self-report measure was completed by the parents of minor participants. Adult participants were administered the same questions and instructed to respond regarding their parents’ household. MAS data were collected from 56 participants (29 children and 27 adults). We note that MAS data ranged between 3 to 9 out of a possible range of 1 to 10, suggesting that in this sample there was no representation of the lowest end of the subjective SES scale. In addition, to the MAS, we obtained information about participants total yearly family income, as well as the father’s and the mother’s levels of education. Both income and education data were collected in bins, and education data were recoded into ordinal variables (Table 1). Family income data were collected from 53 participants (29 children and 24 adults), and father’s and mother’s education from 58 participants (30 children and 28 adults). To account for the number of members per household, income-to-needs ratio for each participant was calculated as the median of the selected income bin, divided by the federal poverty level, based on the family size for the year of data collection (see Table 2 for descriptive statistics of the four SES measures). The complete set of four SES measures were collected from a total of 52 participants (29 children and 23 adults). To reduce the number of SES measures, with the data from these 52 participants we conducted a Principle Component Analysis with orthogonal rotation. The four measures loaded on a single factor (loadings ranged between 0.70 – 0.81). Therefore, we calculated the standardized weighted factor composite score which was used in subsequent analyses. In addition, we verified that the scores on each of the four SES measures were normally distributed (z value of skewness and kurtosis < |1.81|).
Table 1.
Recorded income and education levels.
| Income Level | Education Level |
|---|---|
| Less than 5,000 | None of below (1) |
| 5,000 – 11,999 | Less than high school (2) |
| 12,000 – 15,999 | High school (3) |
| 16,000 – 24,999 | Associate degree (4) |
| 25,000 – 34,999 | Bachelor’s degree (5) |
| 35,000 – 49,999 | Master’s degree (6) |
| 50,000 – 74,999 | Ph.D/MD (7) |
| 75,000 – 99,999 | – |
| 100,000 and greater | – |
Participants indicated income and education data in discrete levels listed here. The median value of each income level was used in calculation of income-to-needs ratio. Education was entered as the number in parenthesis next to each level.
Table 2.
Descriptive statistics of the four measures used to capture socioeconomic status in the total sample, and by age group.
| Total Sample | Children | Adults | |||||
|---|---|---|---|---|---|---|---|
| SES measure | mean (SD) |
range | mean (SD) |
range | mean (SD) |
range |
ˆGroup Diff.: t, p |
| MacArthur Scale | 6.3 (1.3) | [3, 9] | 6.2 (1.6) | [3, 9] | 6.3 (1.1) | [4, 8] | −0.4, 0.69 |
| Income-to-needs ratio | 2.9 (1.4) | [0.3, 5.4] | 2.8 (1.2) | [0.3, 5.1] | 3.0 (1.5) | [0.3, 5.4] | −0.3, 0.73 |
| Father’s Education | 4.2 (1.3) | [1, 7] | 4.2 (1.2) | [1, 6] | 4.3 (1.5) | [1, 7] | −0.5, 0.66 |
| Mother’s Education | 4.9 (1.4) | [2, 7] | 5.3 (1.3) | [3, 7] | 4.4 (1.4) | [2, 7] | 2.6, 0.02* |
| Composite SES score | 0.0 (1.0) | [−2.2, 1.6] | 0.1 (1.0) | [−2.2, 1.6] | −0.1 (1.0) | [−1.7, 1.4] | N.A. |
Group differences (right column) were evaluated with a 2-tailed t-test.
MRI Acquisition and Post-Acquisition Processing
Hippocampal volume measures were taken from a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence that was collected using a 32-channel head coil in a 3 Tesla Siemens Verio scanner (Siemens Medical AG, Erlangen, Germany). The 3D sequence was acquired in the coronal plane, perpendicular to the anterior-posterior commissural axis with the following parameters: echo time = 4.26 ms; repetition time = 2200 ms; inversion time = 1200 ms; flip angle = 9.0°; pixel bandwidth = 130 Hz/pixel; GRAPPA acceleration factor PE = 2; interpolated voxel size 0.5 mm × 0.5 mm × 1.0 mm.
Prior to hippocampal manual demarcation, the T1 MPRAGE image set was corrected for inhomogeneity, resampled to a 0.5 mm3 isotropic voxel and manually realigned to be perpendicular to the longitudinal axis of the hippocampus, aligning the interhemispheric fissure. Individual differences in tilt and roll were also corrected manually. All preprocessing and manual demarcation were completed with Analyze v11.0 (Biomedical Imaging Resource, Mayo Clinic College of Medicine, Rochester, MN, USA).
Hippocampal Volumetry
Manual demarcation procedures were modified from Raz et al. (2004). Images were displayed (magnified × 2) on a 21-in. digitizing tablet (Wacom Cintiq) and manually demarcated with a stylus by three independent raters (Q.Y., M.N., and W.L.). The reliability between independent raters was tested using an intra-class correlation coefficient with the assumption of random raters (ICC(2); Shrout and Fleiss, 1979) of at least 0.90 for all raters. See Figure 1 top for an example of manual demarcation.
Figure 1. Example of manual demarcation of the hippocampus.
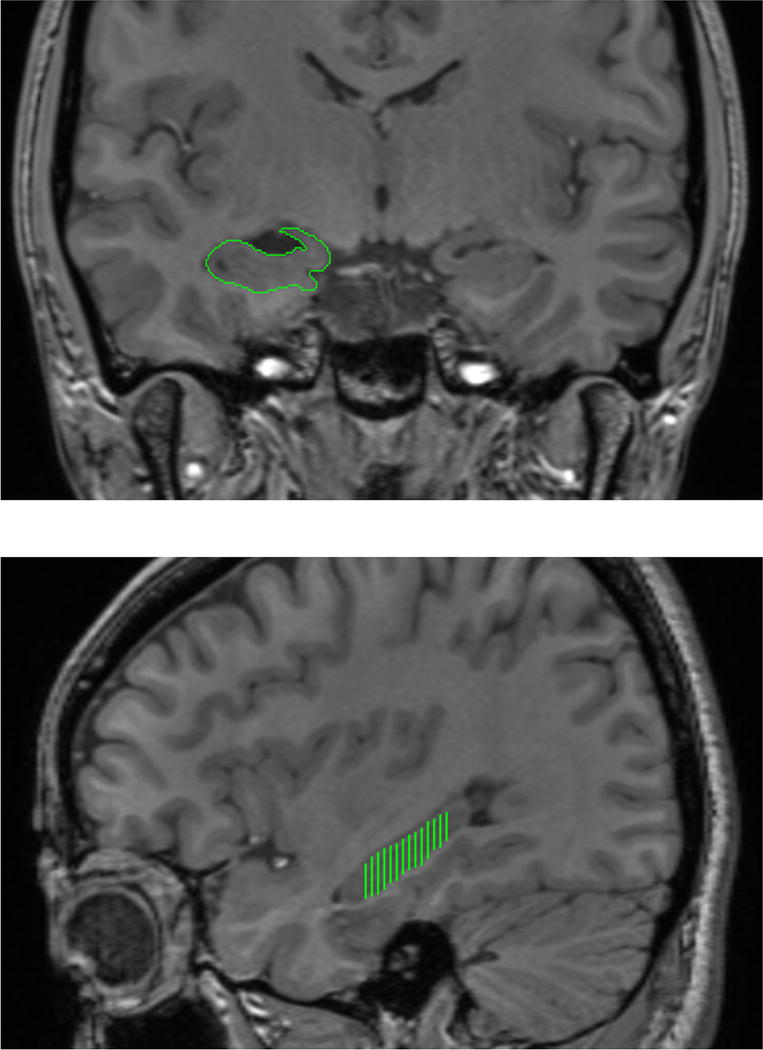
The hippocampus was traced bilaterally in the coronal plane and an example of the tracing is shown on the right hippocampus (top). The range, anterior-to-posterior used in tracing the hippocampus is shown on a saggital (bottom). Images are presented in standard radiological orientation.
The hippocampus was measured in the coronal plane on every third slice extending from the mammilary bodies to the most posterior slice on which the pulvinar nucleus was still visualized, for a total of 15–22 slices (see Figure 1 bottom for an example of the anterior-posterior range). Reducing the number of slices sampled within the range has little impact on the accuracy and reliability as compared to measuring from every 0.5 mm slice (Eritaia et al., 2000). Volume was calculated as the sum area across traced slices, multiplied by the thickness between two consecutive traced slices. Per the specific protocol instructions, we did not trace on each slice, rather on every third slice, and volume is calculated based on the distance between traced slices. Multiplying by the thickness between two consecutive traced slices assures that the computed volume includes the extent of the traced range.
Intracranial Volume Measurement and Volumetry Correction
Intracranial volume (ICV) was measured from the T1 MPRAGE that was aligned to the anterior-posterior commissures with resampled voxel size to 0.5 mm3 during post-processing. Independent raters manually demarcated ICV following procedures adapted from Raz et al. (2004) with high reliability (ICC(2) > 0.90). ICV was measured on every 20th slice, beginning with the most dorsal slice on which brain tissue was visualized and extending 10 slices ventrally. The hippocampal volume was corrected for differences in ICV via analysis of covariance (Jack et al., 1989) and the equation: volumeadj = volumei – b (ICVi– ICVmean), where i denotes certain individual, b is the unstandardized coefficient of whole sample volume regressed on ICV, and ICVmean is the sample mean. The slope of regional volumes regressed on ICV was similar between children and adults (F(1, 56) = 0.19, p = 0.66); thus, the assumption of homogeneous slopes across age groups was met and the same correction was applied to the whole sample.
Memory Performance
To assess, within this sample, the possible relationship between hippocampal volume and memory performance, all participants were given the Visual-Auditory Learning task, a subtest of the Woodcock-Johnson III cognitive tasks (Woodcock and McGrew, 2001). This task was selected because it likely tests participants’ ability of associative learning and efficient integration of multi-modal associations, aspects of cognitive function supported by the hippocampus (Achim, Bertrand, Montoya, Malla, & Lepage, 2007; Duff & Brown-Schmidt, 2012). Indeed, performance on this task has been shown to be influenced by hippocampal integrity (Lancelot et al., 2005).
In short, during a learning phase, participants were presented with several visual stimuli (pictures) and simultaneously provided the associated individual auditory stimulus (words) that are paired with each picture. Participants were then asked to recall the words that were associated with each picture. The picture-word pairs were arranged in short, meaningful sentences, and participants were instructed to ‘read’ the pictures during each recall phase. Recall phase was completed immediately following the learning phase, and again after a delay (M = 57.22 minutes, SD = 16.81). The number of errors was recorded for each recall phase and converted, using the provide norms for the subtest, to a Standard Score used in all analyses.
Statistical Analyses
Prior to evaluating associations between hippocampal volume and other variables, we evaluated possible differences in hippocampal volume between the hemispheres with a general linear model (GLM). Hemispheric hippocampal volumes were included as a 2-level repeated dependent variable (left, right), with age group, sex, and the age group by sex interaction included as independent variables. When neither hemispheric effect nor hemisphere by age group effect were significant, the total hippocampal volume was used in subsequent analyses.
To examine the association between SES, age and hippocampal volume, GLMs were conducted with hippocampal volume as the dependent variable, and age group, SES composite score, and their interaction (SES × age group) as independent variables. Sex was entered in the models as a control variable, because we were not expecting sex differences in memory performance or hippocampal volume. In addition, we were not specifically interested in the interaction between sex and SES in predicting hippocampal volume. Evidence of a significant SES × age group interaction was further investigated with a post-hoc Fischer’s Z test to evaluate the difference in the correlations of SES with hippocampal volume between children and young adults.
Finally, to evaluate the functional relevance of SES-related differences in hippocampal volume we conducted a secondary GLM analysis. In this analysis, we examined the association between hippocampal volume and cognitive ability, assessed by the Woodcock Johnson III Visual-Auditory Learning task. Age group, hippocampal volume and SES composite score were included as predictors in the model, and sex was included as a covariate.
All GLMs were bootstrapped with bias correction (5000 draws of the original sample) to produce 95% confidence intervals (CI) so to avoid spurious effects related to smaller sample size. Examination of the 95% CI that do not include zero can be interpreted as evidence in support of a significant effect at p < 0.05.
Results
Preliminary analyses
No Hemispheric Difference in Hippocampal Volume Estimation
Using GLM with right and left hippocampal volumes as dependent variables, there was no significant difference between hemispheres (F(1, 60) = 0.16, p = 0.69), nor did the difference between hemispheres vary by age group (F(1, 60) = 0.11, p = 0.74). Therefore, we used total hippocampal volume as the dependent variable in all subsequent analyses. Importantly, total hippocampal volume was not different between age groups (F(1, 59) = 1.03, p = 0.32) or between sexes (F(1, 59) = 0.001, p = 0.97).
No Age Group Difference in Composite Socioeconomic Status Score
Before examining whether the association between SES and hippocampal volume was dependent on age group, we evaluated the relation between age and SES. In a GLM controlling for sex, the SES composite score did not differ between age groups (F(1, 49) = 0.06, p = 0.80). Within the individual age group, the SES composite score did not correlate with age, controlling for sex (children: r(26) = −0.17, p = 0.39; adults: r(20) = −0.26, p = 0.24).
Major Findings
SES Interacted with Age to Predict Hippocampal Volume
When controlling for sex and the unique effects of SES and age group, age group interacted with the composite SES score (F(1, 47) = 9.11, p = 0.004; 95% CI −484.74/−130.15) to explain a significant portion of the variance in hippocampal volume (see Figure 2). Post-hoc analyses within age groups (controlled for sex) yielded a significant association between the SES score and hippocampal volume in children (r(26) = 0.54, p = 0.003), but not in adults (r(20) = - 0.25, p = 0.26) (Figure 2). The association in children was significantly greater than that in adults (Fischer’s Z = 2.71, one-tailed p = 0.007). Thus, a higher SES composite score was associated with increased hippocampal volume in children, yet this pattern was absent in adults.
Figure 2. The relation between socioeconomic status (SES) and hippocampal volume differed in children and adults.

Composite SES score was positively correlated with hippocampal volume in children (filled circles, solid line) but not in adults (open circles, dashed line). Hippocampal volume represents participant’s total hippocampal volume corrected for ICV via the analysis of covariance (see Methods for the full description).
The interaction between age group and SES in predicting hippocampal volume was also tested using the four individual SES measures. Similar interaction patterns were found for the individual measures, although the interaction between age group and mother’s education did not reach statistical significance (see supporting materials).
Hippocampal Volume, but not SES was linked to Memory Performance
We examined the relevance of SES score and hippocampal volume to immediate and delayed visual-auditory learning performance. In GLM analysis, age group, SES score, hippocampal volume, all three 2-way interactions, and the 3-way interaction between these variables were entered as predictors for immediate visual-auditory learning, controlled for sex. To simplify the model, interaction terms with a p value greater than 0.10 were excluded from the model. Thus, the final model included age group, SES score, hippocampal volume, and a 2-way interaction between age group and SES score (p = 0.08). Hippocampal volume was significantly related to task performance (F(1, 46) = 4.60, p = 0.04; 95% CI 0.0005/0.011) (Figure 3). In contrast, SES score was not related to task performance (F(1, 46) = 1.78, p = 0.19; 95% CI −12.87/2.61).
Figure 3. Hippocampal volume was related to memory performance.
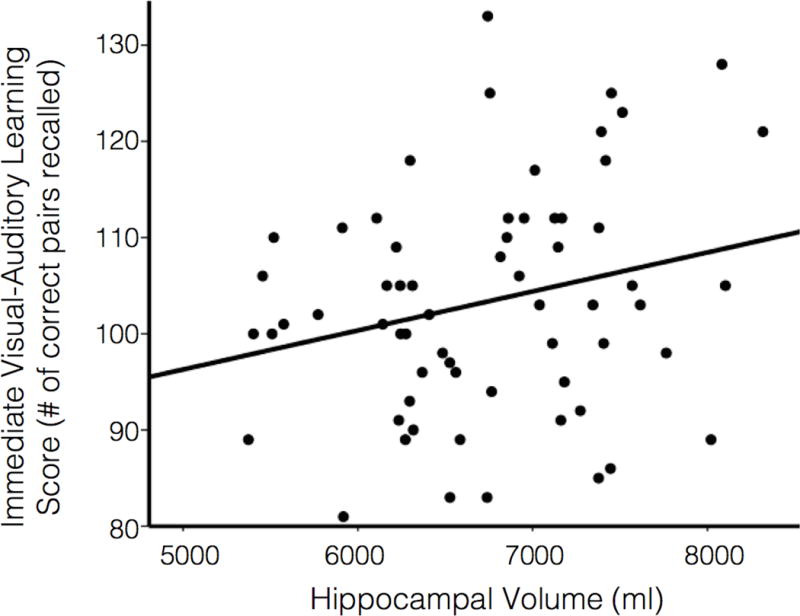
Hippocampal volume represents participant’s total hippocampal volume corrected for ICV via the analysis of covariance (see Methods for the full description). Immediate visual-auditory learning scores were calculated as the number of correct visual-auditory pairs recalled. When controlled for sex and age, hippocampal volume significantly predicted immediate visual-auditory learning performance.
To assess the relevance of SES score and hippocampal volume to delayed visual-auditory learning performance, another GLM analysis was conducted with delayed visual-auditory learning performance as a dependent variable. Similarly, all possible interaction terms were included, but interaction terms with a p value greater than 0.10 were excluded from the model. We found that neither hippocampal volume (F(1, 45) = 0.15, p = 0.70; 95% CI −0.005/0.008), nor SES score (F(1, 45) = 0.54, p = 0.47; 95% CI −3.127/6.733) were related to delayed learning performance.
In sum, the interaction between age group and SES explained a unique portion of the variance in hippocampal volume. The latter variable, in turn, accounted for a unique portion of the variance in immediate visual-auditory learning performance, while SES did not.
Discussion
Our chief objective was to evaluate whether the relationship between socioeconomic status and hippocampal volume is age-dependent. For this purpose, we measured hippocampal volumes in typical children and young adults. We found that a significant portion of the individual variance in hippocampal volume was explained by an interaction between age group and the composite SES score—indexed based on measures of subjective SES rating, income-to-needs ratio and parents’ education. An association between SES and hippocampal volume was observed in the children’s group only. Specifically, higher SES composite score in children, but not adults, was associated with greater hippocampal volume. This finding may indicate that the effects of SES disparity on hippocampal volume that exist in childhood are mitigated in adulthood. Moreover, larger hippocampal volume, but not higher SES, was significantly associated with improved performance on a visual-auditory learning task. The latter findings are consistent with the notion that a variable indexing brain anatomy is a more proximal predictor of memory performance than a sociodemographic factor.
The relation between SES and hippocampal volume during childhood likely reflects the cumulative effects of both adverse and protective environmental factors associated with family SES. Adverse factors, such as acute and chronic stress, can impair hippocampus-related learning and memory processes, as shown in rodents (Alfarez et al., 2003). Human neuroimaging studies also show that increased stress experienced during pregnancy and early postnatal life is related to smaller hippocampal volume (Carrion et al., 2007; Hanson et al., 2015). In contrast, protective environmental factors may buffer the ill-effects of stress on the developing hippocampus in animal models (Weaver et al., 2004; Francis, Diorio, Liu & Meaney, 1999; Liu et al., 1997), and a similar effect has been shown in humans (Gunnar, 1998; Luby et al., 2016). Higher SES has been linked to an increased level of parental support that, in turn, accounts for larger hippocampal volumes (Luby et al., 2015). In addition, an enriched environment can benefit the development of the rodent hippocampus (Brown et al., 2003; Kempermann et al., 1997), and in humans, can mitigate hippocampal atrophy following traumatic brain injury (Miller et al., 2013). In sum, these findings both underscore the sensitivity of the hippocampus to environmental influences and suggest that SES, as a proxy measure of the environment, warrants further investigation in relation to hippocampal structure and function.
The relation between SES and hippocampal volume was absent in young adults in the present study. Coupled with the pronounced effect we identified in children, this finding may indicate that the effects of SES-related environmental factors on developmental trajectories in typical individuals are most relevant in early life, and that the effects are minimized, perhaps negated, in young adulthood. However, based on studies of clinical populations, SES may partially determine sensitivity to pathology and traumatic injury during adulthood (Miller et al., 2013). In this regard, the role of environmental factors, for which SES serves as a proxy, may have differential relevance across the lifespan. In children, environmental influences may have a more direct impact on neural and cognitive development whereas in adulthood, such influences serve as modifiers of factors relevant to meeting challenges associated with quality of life, such as access to quality medical care (Conroy et al., 2010). Our findings are consistent with other reports of adverse exposure during an early developmental period that may delay, but not stunt, typical development (Weaver et al., 2004; Francis et al., 1999; Liu et al., 1997). The differential association between SES and hippocampal volume by age also echoes a proposal that the negative effects of low SES may be reversible (Hackman et al., 2010), which has largely been supported by animal models (Lemaire, Lamarque, LeMoal, Piazza & Abrous, 2006). The differential association between SES and hippocampal volume at different ages demonstrated here calls for future studies investigating this issue over the lifespan. More critically, longitudinal studies are needed to establish these developmental effects with more sensitive instruments to measure SES constituents or correlates.
Economic circumstances, parental education and social tier perceptions are all crude proxies for the environmental influences on cognitive and brain development. SES is commonly measured by a composite of multiple indicators including parental education, household income-to-need ratio, and individual’s subjective perception of belonging to a certain social tier (Oakes and Rossi, 2003). Although these indicators are highly correlated, they may differentially relate to the multitude of factors that shape development (Duncan and Magnuson, 2012; Brito and Noble, 2014). Indeed, income may relate more directly to the material resources available to the family, whereas parental education may more directly relate to the nature of parent-child interaction (Duncan and Magnuson, 2012; Noble et al., 2015). In this study, we used a composite SES score that was determined by factor analysis showing that all four SES measures loaded highly on a single factor. When examining the individual measures, we found similar pattern of relationship with hippocampal volume as the relationship observed for the composite score. However, the nature of the self-report in this study should be considered. For the children’s group, parents completed the survey and ranked their social tier, whereas in the young adult group the participants themselves completed the selection. The social tier ranking is highly subjective and the reporting from parents versus young adults indicating familial SES may be differentially accurate. Nonetheless, we believe this had little, or negligible impact on the effects observed between age groups. First, the rating of a social tier was one of four measures that strongly loaded onto a single SES factor used in all our analyses. Second, in supplemental analyses we assessed the interaction between age and SES for each of the four SES indicators separately (see supporting materials). The pattern of age group by SES interaction in predicting hippocampal volume, by which a link between SES and hippocampal volume was found only in children, was similar across individual SES measures, except the effect of mother’s education which failed to reach significance. Taken together, the findings presented here and in our supplemental analyses suggest that the SES indices used in our study measure a unitary construct, that may be robust to identifying a link between SES and hippocampal volume across age.
We found that hippocampal volume was related to immediate visual-auditory learning performance but not delayed performance, which may reflect the relevance of the hippocampus in the process of forming new associations. Ostby et al. (2012), to the contrary, found that hippocampal volume was related to delayed, but not immediate, memory recall. There are several possible reasons for the contradictory findings. First, the discrepancy may be the result of the different stimuli used in the current study. While we required participants to memorize verbal-visual associations, the task used by Ostby et al. (2012) required recall of pictures with geometrical figures. Second, the definitions of “immediate” were different. The immediate recall in the current study was administered right after encoding, in contrast to about 30-minutes delay in the study by Ostby et al. (2012). Participants’ completed our delayed task about an hour after the immediate recall, roughly matching the timing of the “immediate” task in the study by Ostby et al. (2012). Thus our findings are consistent with Ostby et al. (2012) in that participants’ performance in our delayed task was not related to hippocampal volume. As for the one-week delayed retain in the study by Ostby et al. (2012), we do not have a similar measure for comparison. Finally, Ostby et al. (2012) used FreeSurfer to estimate hippocampal volume, which may be another factor that contributed to divergent results, considering the uncertain validity of anatomical indices obtained with this technique in different populations (Wenger et al., 2014).
Although in the current study we showed that larger hippocampal volume predicted better memory performance, and that higher SES explained larger hippocampal volumes in children, SES did not explain unique variability in memory function. Taken together, the net effects of postnatal environmental factors that are captured by SES may be more distal determinants of memory performance when compared to hippocampal volume. This is in contrast with other reports (Noble et al., 2007) and may reflect reduced sensitivity of the subtest used here to gauge SES-related differences in cognitive abilities. The null finding may be the result of our sample size and the limited representation of participants from the lowest SES ranks, where effects of SES on cognition are strongest (Noble et al., 2015). Future studies need to employ reliable and valid measures of hippocampal volume in larger samples. Additional behavioral outcome measures are also needed to investigate a mediating effect of hippocampal volume on the relation between SES and cognitive development. Future studies may also evaluate the mediating effects of other brain regions on the relation between SES and cognitive development (Lawson et al., 2013; Noble et al., 2013; Noble et al., 2015; Jednoróg et al., 2012) with the goal of gaining further insight into the putative mechanisms underlying SES effects.
The reported association between SES and hippocampal volume in children is largely consistent with prior findings (Hanson et al., 2011; Jednoróg et al., 2012; Noble et al., 2012a; Noble et al., 2015) and those suggesting that the effects are age-dependent (Staff et al., 2012; Noble et al., 2012a). However, a few limitations should be noted. First, our findings are based on cross-sectional data, and thus the age group differences in the association between SES and hippocampal volume cannot be interpreted as evidence of a developmental change. Cross-sectional studies are incapable of providing reliable estimates of change (Lindenberger, von Oertzen, Ghisletta & Hertzog, 2011) or its mediators (Maxwell and Cole, 2007). Additional longitudinal studies are necessary to determine whether the age-moderated effect reported here is a true developmental effect. The intriguing possibility that adverse effects of low SES during early development may be mitigated as one enters adulthood can only be assessed with longitudinal data. Moreover, only longitudinal data can provide additional support for the specific neural substrates underlying the age-dependent associations between SES and hippocampal volume, and offer insights regarding sensitive periods when low SES may exert particular deleterious effects on development. Longitudinal studies with specific interventions targeted at relevant SES-related factors such as parental care or income (Hackman et al., 2010) may be best situated to elucidate the neural mechanisms mediating environmental effects on development. In addition, given the unavailability of childhood SES data for the current adult sample, it is also possible that the differential SES-hippocampal volume associations in the adults, versus children’s, sample was a result of discrepant childhood SES experienced by the adults’ sample from their own experience as children. Investigating the effect of childhood SES on the SES-hippocampal volume association in early adulthood would be an interesting direction for future research. Finally, one must acknowledge the inherent difficulty in interpreting the null effect in adults. The tests within the relatively small sample size may be underpowered to detect an association between SES and hippocampal volume in this group. Though care was taken to recruit a representative and matched sample (confirmed by similar overall SES and IQ in the two groups), the relatively small sample size across groups calls for caution in interpreting the interaction between age and SES in predicting hippocampal volume. It is possible that with larger samples, and a broader representation of lower SES scores, we could have found an association between SES and hippocampal volume in adults.
We used a gold-standard approach to derive hippocampal volume measures, it is possible, however, that the effect of SES on the hippocampus may differ across the hippocampal subfields (Daugherty et al., 2016) or its anterior-posterior subregions (Daugherty, Yu, Flinn & Ofen, 2015). For example, dentate gyrus, is characterized by persistent postnatal neurogenesis (Eriksson et al., 1998) and appears to demonstrate protracted development (Daugherty et al., 2016). The dentate, may be more vulnerable to the adverse effects of low SES compared to other subfields, an effect otherwise obscured when testing association with the total volume of the hippocampus. Furthermore, volumes of hippocampal subfields (Bender, Daugherty & Raz, 2013) and subregions (Riggins, Blankenship, Mulligan, Rice & Redcay, 2015) show differential relation to memory across age. The relation between SES and human hippocampal subfields or subregions has yet to be investigated. With the lack of a-priori hypothesis about the effect of SES on the sub structures of the hippocampus, any results this relatively small data set may provide would be difficult to interpret. However, exploration of this topic has become feasible with the advent of high-resolution imaging and for future studies with larger sample size.
In conclusion, we found evidence for an association between SES and hippocampal volume in childhood, but not in young adulthood. This finding seems to be consistent with the notion that low SES effects on the brain may be transient. We also found that hippocampal volume accounted for individual difference in memory performance, although, SES was unrelated to performance on a memory task. The latter findings are consistent with the notion that the net effects of SES are more distal determinants of memory performance in comparison to the effects of hippocampal volume. Future investigation using longitudinal designs, additional brain measures and diverse cognitive tasks may provide further insight into the age-dependent effects of low SES on brain development and the intriguing possibility that adverse effects of low SES early in life may be subsequently mitigated.
Supplementary Material
Research Highlights.
Socioeconomic status (SES), hippocampal volume assessed in children and adults.
Hippocampal volume measured using manual demarcation with confirmed high reliability.
Positive relation between SES and hippocampal volume in children but not in adults.
Consistent with prior work, hippocampal volume was positively related to memory performance.
Acknowledgments
The authors would like to thank Raphael Serota, Carson Miller Rigoli, Moria Aviv-Karavany, Sadia Ghazi, Priya Sam and Robert Flinn for help with data collection, to the Wayne State University MR research center for technical support, and for the Institute of Gerontology and Wayne State University for funding provided for this project. We would also like to thank two anonymous reviewers for helpful comment on an earlier version of this manuscript, and the children and the families that participated in the study.
References
- Achim AM, Betrand M, Montoya A, Malla AK, Lepage M. Medial temporal lobe activations during associative memory encoding for arbitrary and semantically related object pairs. Brain Research. 2007;1161:46–55. doi: 10.1016/j.brainres.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joe M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. European Journal of Neuroscience. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Bender AR, Daugherty AM, Raz N. Vascular risk moderates associations between hippocampal subfield volumes and memory. Journal of Cognitive Neuroscience. 2013;25(11):1851–1862. doi: 10.1162/jocn_a_00435. [DOI] [PubMed] [Google Scholar]
- Bjorklund D. Children’s thinking: Cognitive development and individual differences. 5th. Thompson Wadsworth; Belmont, CA: 2012. [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The future of children. 1997;7(2):55–71. [PubMed] [Google Scholar]
- Brito NH, Noble KG. Socioeconomic status and structural brain development. Frontiers in Neuroscience. 2014;8(276):1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn C, Kempermann G, Praag H, Winkler J, Gage F, Kuhn H. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cancian M, Slack KS, Yang MY. The effect of family income on risk of child maltreatment. Institute for Research on Poverty. 2010;1385-10:1–18. [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children : a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Krishnadas R, Batty GD, Burns H, Deans KA, Ford I, Sattar N. Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterized population sample. The Cerebellum. 2013;12:882–91. doi: 10.1007/s12311-013-0497-4. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Conroy K, Sandel M, Zuckerman B. Poverty grown up : How childhood socioeconomic status impacts adult health. Journal of Developmental & Behavioral Pediatrics. 2010;31(2):154–160. doi: 10.1097/DBP.0b013e3181c21a1b. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Bender A, Raz N, Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26:220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A, Yu Q, Flinn R, Ofen N. A reliable and valid method for manual demarcation of hippocampal head, body, and tail. International Journal of Developmental Neuroscience. 2015;41:115–122. doi: 10.1016/j.ijdevneu.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, Mccaffrey D, Harezlak J, the HIV Neuroimaging Consortium Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. NeuroImage. 2010;51(4):1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Frontiers in human neuroscience. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: The concept of synaptic plasticity. European Psychiatry. 2002;17(Suppl 3):306–10. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Duncan G, Magnuson K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage GH. Neurogenesis in the adult human hippocampus. Nature. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, Pantelis C. An optimized method for estimating intracranial volume from magnetic resonance images. Magnetic Resonance in Medicine. 2000;44:973–977. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Hurt H. Childhood poverty: Specific associations with neurocognitive development. Brain Research. 2006;1:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Feinstein L. Inequality in the early cognitive development of British children in the 1970 cohort. Economica. 2003;70(277):73–97. [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J, Lusk L, Hayashi K, Greenstein D, Vaituzis A, Thompson P. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, III, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions : potential effects on the developing human brain. Preventive Medicine. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Davidson RJ. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann D, Guadagno M. Memory Performance and Socio-Economic Status. Applied Cognitive Psychology. 1997;11:113–120. [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(3):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Lancelot C, Ahad P, Noulhiane M, Hasboun D, Baulac M, Samson S. Loss of memory for auditory-spatial associations following unilateral medial temporal-lobe damage. Neuropsychologia. 2005;(43):1975–1982. doi: 10.1016/j.neuropsychologia.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE, Brain Developmental Cooperative Group Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Developmental Neuropsychology. 2010;35(3):296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Martha FJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16(5):641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal Stimulation of the Pups Counteracts Prenatal Stress-Induced Deficits in Hippocampal Neurogenesis. Biological Psychiatry. 2006;59(9):786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What’s change got to do with it? Psychology and Aging. 2011;26:34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal response to stress. Stress. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Barch D. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2015;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Proceedings of the National Academy of Sciences. 2016. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development; p. 201601443. doi.org/10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ. Can poverty get under your skin ? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Finn AS, Leonard JA, Jacoby-senghor DS, West MR, Gabrieli CFO, Gabrieli JDE. Neuroanatomical correlates of the income-achievement gap. Psychological Science. 2015;26(6):925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, Pine DS. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2015.365. doi.org/10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain : Methods and applications. Current Medical Imaging Reviews. 2005;1(2):105–113. [Google Scholar]
- Miller LS, Colella B, Mikulis D, Maller J, Green REA. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Frontiers in Human Neuroscience. 2013;7(506):1–8. doi: 10.3389/fnhum.2013.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and neurogenesis. Hippocampus. 2006;16(3):233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, Mccarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012a;6(307):1–10. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012b;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science. 2013;16(5):653–664. doi: 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farrah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Oakes JM, Rossi PH. The measurement of SES in health research: Current practice and steps toward a new approach. Social Science & Medicine. 2003;56:769–784. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Song J. Brain volumetric measures in alcoholics : a comparison of two segmentation methods. Neuropsychiatric Disease and Treatment. 2011;7:65–75. doi: 10.2147/NDT.S13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Walhovd KB. Dissociating memory processes in the developing brain: The role of hippocampal volume and cortical thickness in recall after minutes versus days. Cerebral Cortex. 2012;22(2):381–390. doi: 10.1093/cercor/bhr116. [DOI] [PubMed] [Google Scholar]
- Perry BD. Childhood experience and the expression of genetic potential : what childhood neglect tells us about nature and nurture. Brain and Mind. 2002;3:79–100. [Google Scholar]
- Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Chakravarty MM. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. NeuroImage. 2014;101:494–512. doi: 10.1016/j.neuroimage.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Farah MJ. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Riggins T, Blankenship SL, Mulligan E, Rice K, Redcay E. Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Development. 2015;86(6):1710–1718. doi: 10.1111/cdev.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, Pruessner JC. Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage. 2016;129:1–14. doi: 10.1016/j.neuroimage.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal. 2001;22(1–2):201–269. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Saykin AJ, Kim S, Firpi HA, West JD, Risacher SL, Flashman LA. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging and Behavior. 2010;4(1):86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Developmental Science. 2013;16(5):665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology. 2012;71(5):653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wenger E, Martensson J, Noack H, Bodammer NC, Kuhn S, Schaefer S, Lovden M. Comparing manual and automatic segmentation of hippocampal volumes: Reliability and validity issues in younger and older brains. Human Brain Mapping. 2014;35(8):4236–4248. doi: 10.1002/hbm.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R, McGrew K. Woodcock-Johnson III tests of achievement. Riverside Pub.; Itasca, IL: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


