Abstract
Bone morphogenetic protein (BMP) 10, a cardiac-restricted BMP family member, is essential in cardiomyogenesis, especially during trabeculation. Crossveinless-2 [CV2, also known as BMP endothelial cell precursor derived regulator (BMPER) ] is a BMP-binding protein that modulates the activity of several BMPs. The objective of this study was to examine the combined effects of BMP10 and CV2 on cardiomyocyte differentiation using mouse dedifferentiated fat (mDFAT) cells, which spontaneously differentiate into cardiomyocyte-like cells, as a model.
Our results revealed that CV2 binds directly to BMP10, as determined by co-immunoprecipitation, and inhibits BMP10 from initiating SMAD signaling, as determined by luciferase reporter gene assays. BMP10 treatment induced mDFAT cell proliferation, whereas CV2 modulated the BMP10-induced proliferation. Differentiation of cardiomyocyte-like cells proceeded in a reproducible fashion in mDFAT cells, starting with small round Nkx2.5-positive progenitor cells that progressively formed myotubes of increasing length that assembled into beating colonies and stained strongly for Troponin I and sarcomeric alpha-actinin. BMP10 enhanced proliferation of the small progenitor cells, thereby securing sufficient numbers to support formation of myotubes. CV2, on the other hand, enhanced formation and maturation of large myotubes and myotube-colonies and was expressed by endothelial-like cells in the mDFAT cultures. Thus BMP10 and CV2 have important roles in coordinating cardiomyogenesis in progenitor cells.
Keywords: Adipocyte-derived stem cells, bone morphogenetic protein 10, crossveinless 2, cardiomyocyte differentiation
1. Introduction
The bone morphogenetic proteins (BMPs), a sub-group of the transforming growth factor-beta superfamily, play critical roles during cardiogenesis (Morrell et al., 2016; Wang et al., 2011). BMP10, a cardiac-restricted BMP, is essential for cardiomyocyte proliferation and trabecular formation during midgestation (Chen et al., 2004; Neuhaus et al., 1999). It has also been shown to induce cardiomyocyte proliferation after myocardial infarction (Sun et al., 2014) and contribute to hypertension-induced cardiac hypertrophy and cardiac remodeling due to intensity-controlled exercise training (Nakano et al., 2007; Waring et al., 2014). BMP10 is closely related to BMP9, which is central in angiogenesis and maintenance of vascular quiescence (David et al., 2008; Tillet and Bailly, 2014). We previously showed that BMP9 is regulated by crossveinless-2 [CV2, also known as BMP endothelial cell precursor derived regulator (BMPER) ], a BMP-binding protein that antagonizes several BMPs and plays an important role in angiogenesis (Moser et al., 2003; Walsh et al., 2010; Yao et al., 2012). Interestingly, CV2 is also expressed in early cardiogenesis and may help specify cardiac mesodermal lineage (Harada et al., 2008; Willis et al., 2013). Deletion of the CV2 gene in mice causes a reduction in cardiomyocyte size and cardiac wall thickness, and an increase in vascular density (Willis et al., 2013). Thus, BMP10 and CV2 have separately been shown to influence cardiomyocyte differentiation.
Here, we hypothesize that BMP10 and CV2 interact and help coordinate cardiomyocyte differentiation in a model of adipocyte-derived stem cells referred to as dedifferentiated fat (DFAT) cells. Our results reveal that CV2 binds BMP10 and inhibits its activity, similar to interactions between CV2 and BMP9. Furthermore, treatment with BMP10 enhances proliferation of early cardiac progenitors derived from mDFAT cells, whereas CV2 supports subsequent formation of myotubes. The two effects are additive when the treatments are applied sequentially. Taken together, the data support that mDFAT cells can be used to model where cardiomyogenesis is regulated in at least two steps, and that treatment with BMP10 and CV2 might be helpful in optimizing cardiomyocyte differentiation in stem cells.
2. Methods
2.1. Collection of Adipose Tissue
For collection of mouse adipose tissue used to isolate cells, wild type C57BL/6J mice were euthanized at 8–10 weeks of age by inhalation of isoflurane followed by cervical dislocation, and adipose tissue were collected postmortem. The studies were reviewed and approved by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
2.2. Isolation of Adipocytes and Culture of mDFAT Cells
Lipid-filled white adipocytes were isolated from 2 g of mouse subcutaneous adipose tissue as previously described (Jumabay et al., 2014; Matsumoto et al., 2008). After the adipocytes had been isolated, they were washed three times in culture medium [Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FBS, HyClone, Logan, UT) and 0.5% of antibiotic-antimycotic solution (Mediatech, Manassas, VA) ] before they were used for further analysis or culture. In order to generate DFAT cells, isolated adipocytes were pre-incubated (floated) on top of medium in culture dishes or 50-ml plastic tubes with loosened caps for 24 hours to allow for potential remaining non-adipocyte cells to detach and sink to the bottom. Adipocytes (30–50 ml of the floating creamy layer) were then added to culture medium in 6- or 12-well plates fitted with filter inserts (pore size 70 μm) and incubated for 5 days. DFAT cells (emerging from the adipocytes) passed through the inserts and attached to the bottom of the culture dishes. After 5 days, the inserts and remains of the adipocytes were removed. This method of preparing DFAT cells did not include attachment of the adipocytes to plastic surfaces or ceiling culture as previously described (Matsumoto et al., 2008; Sugihara et al., 1989). The method allowed the separation of the DFAT cells from the adipocytes as soon as they passed through the filter and attached to the bottom of the dish.
2.3. Cardiomyocyte Differentiation from mDFAT Cells
Freshly generated, un-passaged DFAT cells in 6- or 12-well dishes were used for experiments on day 5 after the adipocytes had first been placed in culture. The mDFAT cells were washed with DMEM once, and treated for 3 days (corresponding to day 5-7) with culture medium containing (1) control vehicle, (2) recombinant mouse BMP10 (25 ng/ml), (3) recombinant mouse CV2 (50 ng/ml), or (4) BMP10 (25 ng/ml) followed by CV2 (50 ng/ml) for 3 additional days (corresponding to day 8-10) and referred to as BMP10/CV2 (BMP10 and CV2 were from R&D Systems, Minneapolis, MN). Where indicated, the experiments also included treatment with CV2 (50 ng/ml) on day 8-10, without the preceding BMP10, or neutralizing anti-BMP10 antibodies on day 5-7 (100 ng/ml, R&D Systems, MAB2926) to confirm that BMP10 from FBS or cells did not affect the experiments. After completion of the treatments, the culture medium was renewed every 3 days.
The BMP10 concentration (25 ng/ml) was selected as the lowest concentration that achieved optimal cardiomyocyte differentiation from a range of 0-50 ng/ml. The CV2 concentration (50 ng/ml) was selected as the lowest concentration that provided BMP10 inhibition. There was no detectable BMP10 in the culture medium (20% FBS) when tested by BMP10 DuoSet ELISA (R&D Systems, mature bovine BMP10 is 100% identical to human BMP10).
It has previously been reported that BMP10 can replace BMP9 in endothelial differentiation, but is unable to replace BMP10 in cardiac development (Chen et al., 2013; Jiang et al., 2016; Ricard et al., 2012). BMP9 levels of approximately 6 ng/ml have been reported in FBS (Herrera and Inman, 2009), but BMP9 was barely detectable in our culture medium (20% FBS) by BMP9 DuoSet ELISA (R&D Systems, mature bovine BMP9 is >92% identical to human BMP9). Separate experiments were performed to rule out potential effects of BMP9 on cardiomyocyte differentiation, using BMP9 (10 ng/ml) and neutralizing anti-BMP9 antibodies (100 ng/ml, R&D Systems, MAB3209). BMP9 did not significantly affect the cardiomyocyte differentiation in the mDFAT cells (data not shown).
2.4. RNA Analysis
RNA was isolated using RNeasy® minikits (Qiagen, Valencia, CA) following the manufacturer's instructions. Two μg of total RNA was subsequently reverse-transcribed using High Capacity cDNA Reverse Transcription kits (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Quantitative PCR (qPCR) was performed as previously described (Bostrom et al., 2004) using an Applied Biosystems ViiA(TM) 7 Real-Time PCR System (Applied Biosystems). The probes used for qPCR for mouse Nkx2.5, Troponin I, and CV2 were pre-designed and purchased from Applied Biosystems as part of Taqman® Gene Expression assays.
2.5. Proliferation Assay
Mouse DFAT cells (passage 1-3) were seeded at a density of 5,000 cells per well in triplicates in 24 well dishes. After 24 hours, the cells were treated as described for the cardiomyocyte differentiation experiments. On day 3, 5, 7, 10 and 14, cells were trypsinized and collected from 3 wells of each treatment and counted using a Countess® II Automated Cell Counter (Thermo Fisher Scientific).
2.6. Luciferase Assay
Luciferase assays were performed as previously described (Yao et al., 2012; Yao et al., 2006). Briefly, transient transfections of bovine aortic endothelial cells (BAECs) were performed in triplicates in 24-well plates. The cells were plated in DMEM (10% FBS) 24 hours prior to the transfection at 10,000-20,000 cells per well in order to yield target confluency of 40-80%. The cells were transiently transfected using 1.5 μl of FuGene 6 reagent (Promega, Madison, WI) and 500 ng of DNA per well. The cells were transfected with a BMP-responsive luciferase reporter gene (BRE-Luc) and Renilla luciferase (Promega) for normalization, treated as indicated in the results section in DMEM (10% FBS), and analyzed 20-24 hours after start of the treatment. The cells were lysed in 100 μl of Passive Lysis Buffer (Promega) per well, freeze-thawed twice and agitated for 15 min. Luciferase activity was determined using an AutoLumat LB 953 luminometer (Thermo Fisher Scientific, Canoga Park, CA) and expressed as mean from quadruplicate transfections after normalization to Renilla.
2.7. Co-Immunoprecipitation
Co-immunoprecipitations of recombinant proteins were performed as previously described (Yao et al., 2012; Yao et al., 2009). We used recombinant human CV2 and BMP10 (0.2 or 1 μg/ml; R&D Systems). We used antibodies to CV2 and BMP10 (0.4 μg/ml or 2 μg/ml, respectively; R&D Systems, MAB2926) and non-specific IgG (0.4 μg/ml; Santa Cruz Biotechnology). Immunoprecipitated complexes were analyzed by immunoblotting as described below.
2.8. Immunoblotting
Immunoblotting was performed as previously described (Yao et al., 2010; Yao et al., 2012). Equal amounts of cellular protein or culture medium were used. Blots were incubated with specific antibodies to Troponin I (0.2 μg/ml; Santa Cruz Biotechnology, H-170) or sarcomeric alpha-actinin (0.2 μg/mL; Sigma Aldrich, A7811), or CV2 (1:1000; Sigma, AV52569).
2.9. Immunofluorescence
Immunostaining was performed as previously described in detail (Jumabay et al., 2012). Briefly, cells grown in chamber slides were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 10% goat serum and 1% bovine serum albumin (BSA) in PBS, and incubated overnight at 4°C with the appropriate primary antibodies or non-specific immunoglobulin G (IgG) control antibodies, diluted 1:200 in 1% BSA in PBS. The following day, the cells were incubated with secondary Alexa Fluor® 488-conjugated (green fluorescence) or Alexa Fluor® 594-conjugated (red fluorescence) goat anti-mouse or anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR). The cells were washed with PBS, and the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma Aldrich, St. Louis, MO) and visualized by fluorescence microscopy. Control staining using non-specific IgG antibodies showed no staining and are not included in the figures due to space considerations. We used the following antibodies for immunostaining: anti-sarcomeric alpha-actinin (1:500; Sigma-Aldrich, A7811), anti-Nkx2.5 [1:200, Biorbyt (Berkeley, CA), orb100801], anti-Troponin I (1:200, Santa Cruz Biotechnology, H-170), and anti-CV2 (1:200; Sigma AV52569). The next day, the cells were washed three times with PBS, incubated for 1 h at room temperature with secondary antibodies, washed three times with PBS, and visualized by fluorescence microscopy.
2.10. Statistical Analysis
Data were analyzed for statistical significance by the non-parametric Kruskal-Wallis test with Dunn's test for post hoc analysis using the GraphPad Prism® software (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered significant. All experiments were repeated a minimum of three times.
3. Results
3.1. MDFAT Cells as A Model of Cardiomyocyte Differentiation
We previously reported that mDFAT cells, derived from subcutaneous white adipose tissue, spontaneously generate beating cardiomyocytes (Jumabay et al., 2010). In this cell model, the differentiation of cardiomyocytes follows a reproducible sequence. After 5-6 days, groups of rounded cells are detected (Figure 1A, top panels). After 8-10 days, short myotubes have formed with expression and striated staining for sarcomeric alpha-actinin and Troponin I, and beating of individual cells (Figure 1A, middle panels, and 1B, top panels). After 15 days or more, the cardiomyocyte-like cells have lengthen and formed distinct colonies, with striated staining for sarcomeric alpha-actinin and Troponin I, now with coordinated beating of many of the cells in each colony (Figure 1A, bottom panels, and 1B, middle and bottom panels) (see Supplemental Data for video of spontaneously beating cardiomyocytes). Several characteristics confirmed the cardiomyocyte differentiation, including the central position of the nuclei, branching of the cardiomyocytes (Figure 1C), the formation of colonies, and the spontaneous beating (Supplemental Video).
Figure 1. Mouse DFAT cells as a model of cardiomyogenesis.
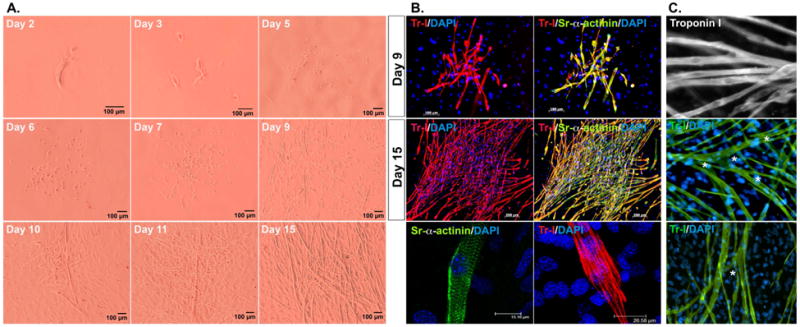
Freshly prepared, unpassaged mDFAT cells were grown in culture medium only, and examined by (A) bright field microscopy on day 2-15, or (B) immunostaining of the cardiac markers Troponin I (Tr, red) and sarcomeric alpha-actinin (alpha-Sr, green) at day 9 and 15. (C) Central location of the nuclei (top panel), and branching (marked by stars, middle and bottom panels) in mDFAT-derived cardiomyocytes stained for Troponin I (Tr, green). DAPI (blue) was used to visualize nuclei.
3.2. CV2 interferes with BMP10 activity
We examined expression of BMP10 and CV2 in white adipocytes and mDFAT cells on day 3-15. We were unable to detect BMP10 expression by qPCR, immunostaining, or ELISA of the medium (data not shown). However, CV2 expression was detected in the adipocytes and increased in mDFAT cells as determined by qPCR, and visualized by immunostaining (Figure 2A) where at least part of the CV2 appeared to be cell-associated. It supports that CV2 would be able to modulate BMP signaling at an early stage.
Figure 2. CV2 interferes with BMP10 activity.
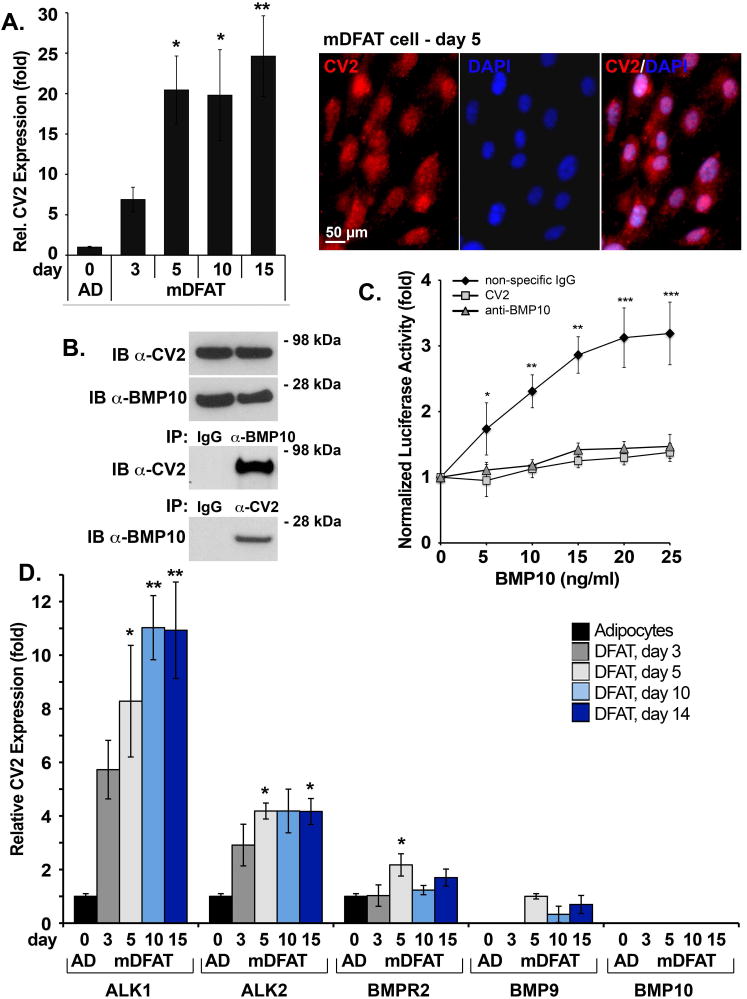
(A) (left) CV2 expression in mDFAT cells by qPCR (representative of 3 experiments), and (right) by immunostaining for CV2 (red) and DAPI (blue, for visualization of nuclei) (day 5).
(B) Interactions between BMP10 and CV2 (50 ng of each protein) were examined using recombinant proteins by immunoprecipitation (IP) followed by immunoblotting (IB) with non-specific IgG, and anti-BMP10 and anti-CV2 antibodies as indicated. IB of the starting material for the IP is shown in the top panels.
(C) Response of BMP-responsive luciferase reporter gene (BRE-luc) in BAEC treated with BMP10 (0-25 ng/ml), alone or together with CV2 (100 ng/ml), anti-BMP10 antibodies (200 ng/ml), or non-specific control IgG (200 ng/ml) (representative of 3 experiments, n=4).
(D) Expression of ALK1, ALK2, BMPR2, BMP9 and BMP10 in mDFAT cells by qPCR (representative of 3 experiments, n=3).
*<0.05, **<0.01, ***<0.001.
In previous studies, we showed that CV2 interacts with BMP9 (Yao et al., 2012), which is closely related to BMP10 (Tillet and Bailly, 2014). To investigate if CV2 and BMP10 interacts on a protein level, we combined recombinant human CV2 (50 ng) and BMP10 (50 ng). We performed immunoprecipitations of BMP10 (∼17 kDa) and CV2 (∼80 kDa) using antibodies against BMP10 or CV2. The results showed co-immunoprecipitation of BMP10 and CV2 regardless of which antibodies were used for immunoprecipitation (Figure 2B), suggesting direct interaction between BMP10 and CV2.
To test if CV2 affects BMP10 activity, we performed luciferase assays by transfecting endothelial cells with a BMP-responsive luciferase reporter gene (BRE-luc), as previously described (Yao et al., 2012), and treated the cells with BMP10 (0-25 ng/ml) together with CV2 (100 ng/ml), neutralizing anti-BMP10 antibodies (200 ng/ml), or non-specific control IgG (200 ng/ml). The result showed that CV2 blocked BMP10-induced luciferase activity similarly to the anti-BMP10 antibodies (Figure 2C), suggesting that CV2 antagonizes BMP10 activity, and that CV2 may modulate BMP10-stimulated proliferation.
We also screened for expression of the activin receptor-like kinase 1 (ALK1), ALK2, the BMP type II receptor (BMPR2), BMP9 and BMP10 in mDFAT cells on day 3-14. The expression of all proteins that were expressed reached a plateau around day 5 (Figure 2D), which was the time point selected for initiating treatments of mDFAT cells in subsequent experiments.
3.3. Effect of BMP10 and CV2 on Proliferation and Expression of Cardiomyocyte Markers in mDFAT Cells
To examine how BMP10 and CV2 might stimulate the transition of mDFAT cells from multipotent cells to cardiomyocyte-like cells, we treated the mDFAT cells with 4 different treatments for 3 days, starting on day 5 when filter inserts were removed from the mDFAT cells: (1) vehicle control, (2) BMP10 (25 ng/ml), (3) CV2 (50 ng/ml, referred to as CV2/5-7), and (4) BMP10 (25 ng/ml), which was followed by CV2 (50 ng/ml) for 3 additional days. The cells were monitored for up to 3 weeks, and bright field photographs were obtained after 5, 7, 10 and 14 days (Figure 3). We found that cells treated with BMP10 generated a large number of small rounded cells (Figure 3, row 2), whereas CV2 limited the number of such cells, but supported the emergence of myotubes (Figure 3, row 3). When BMP10 and CV2 were added sequentially (BMP10/CV2), both the number of rounded cells and mature myotubes increased (Figure 3, row 4), suggesting a synergistic effect of BMP10 and CV2.
Figure 3. Bright field morphological observation of mDFAT cells after 5-14 days of treatment.
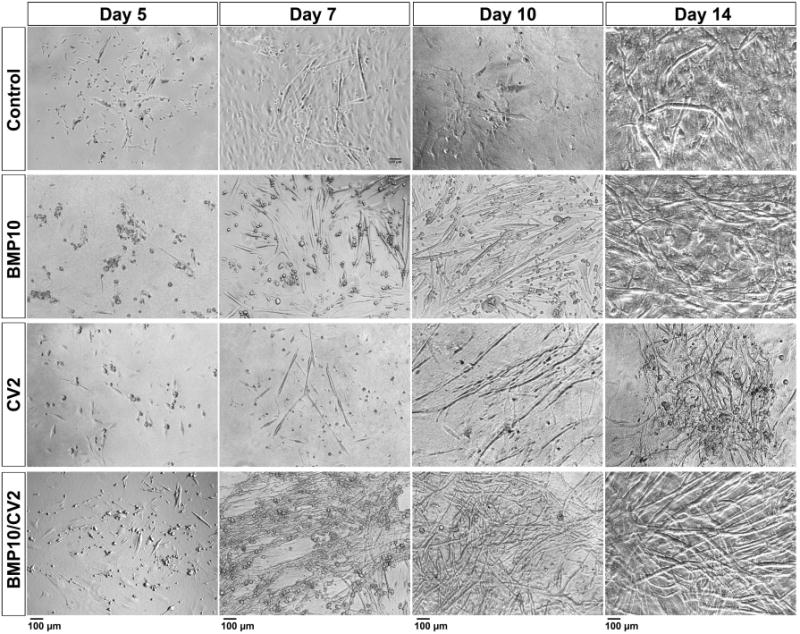
Mouse DFAT cells were treated with growth medium supplemented with control vehicle, BMP10 (25 ng/ml), CV2 (50 ng/ml), or BMP10 for 3 days followed by CV2 for 3 days (BMP10/CV2). Photos were obtained after 5, 7, 10 and 14 days.
Expression of the cardiomyocyte markers Nkx2.5 and Troponin I was monitored by qPCR for 3 weeks after treatment start, and cell proliferation was monitored by direct cell counting for 2 weeks after treatment start. In these experiments, control cells were also treated with “late” CV2 treatment (referred to as CV2/8-10), which corresponded to the CV2 phase of the combined BMP10/CV2 treatment. Other control cells were treated with neutralizing anti-BMP10 antibodies to ensure that potential BMP10 derived from the FBS would not affect the differentiation.
The results revealed that BMP10 or CV2/5-7 separately enhanced expression of both markers, whereas the combination of BMP10/CV2 had an additive or synergistic effect on the marker expression (Figure 4A). Control cells with the late CV2/8-10 treatment showed a mild increase in marker expression only observed after 3 weeks, and control cells treated with anti-BMP10 antibodies were indistinguishable from vehicle-treated controls at all time points (Figure 4A). This is consistent with the absence of BMP10 in FBS by ELISA. The late CV2/8-10 and anti-BMP10 antibody treatments were not included in the subsequent experiments.
Figure 4. Cardiac marker expression after 7 days of treatment.
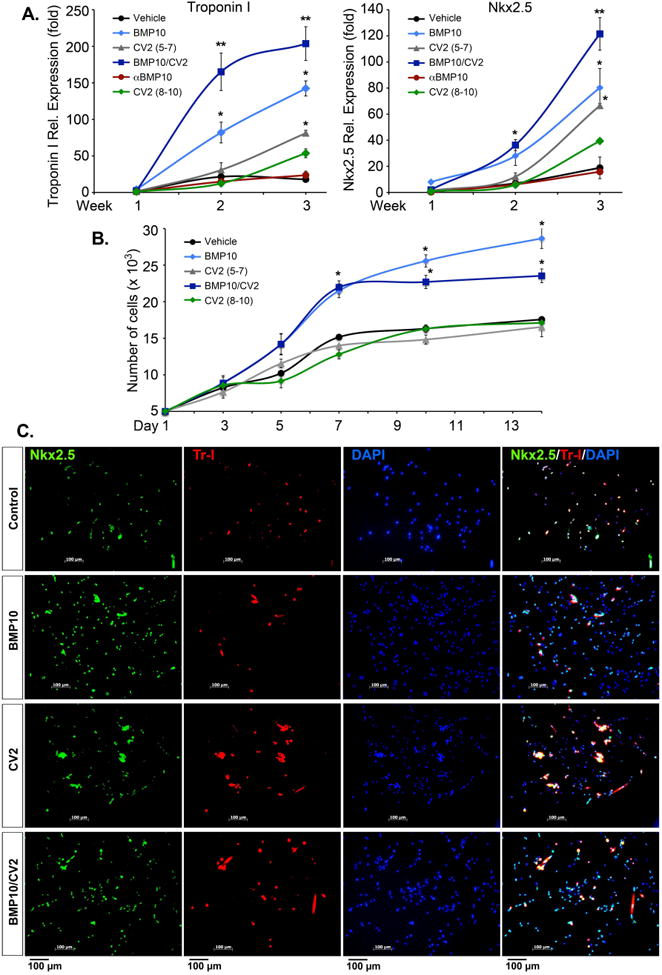
Mouse DFAT cells were treated with growth medium supplemented with control vehicle (day 5-7), BMP10 (25 ng/ml, day 5-7), CV2/5-7 (50 ng/ml, day 5-7), BMP10 (day 5-7) followed by CV2 (day 8-10) (BMP10/CV2), anti-BMP10 antibodies (100 ng/ml, day 5-7), or CV2/8-10 (50 ng/ml, day 8-10).
(A) Expression of Nkx2.5 and Troponin I was determined by qPCR.
(B) Proliferation was determined by direct cell counting.
(C) Localization of Nkx2.5 (green) and Troponin I (Tr, red) was determined by immunofluorescence. DAPI (blue) was used to visualize nuclei.
*<0.1, **<0.01 (n=3).
In regards to proliferation, BMP10 alone stimulated the proliferation for up to 14 days compared to control and CV2 treatments (Figure 4B). However, BMP10/CV2 caused a decrease in proliferation after 7 days as compared to BMP10 alone (Figure 4B), suggesting that the enhancement of cardiomyocyte markers seen with the BMP10/CV2 treatment was associated with a reduction in proliferation.
The endothelial lineage markers CD31 and VE-cadherin were also examined at the end of the differentiation experiments, i.e. after 3 weeks. There was a 1.5-2.5-fold increase in the endothelial markers as shown in Supplemental Figure 1, consistent with the known capacity of mDFAT cells to undergo endothelial differentiation (Jumabay et al., 2012). There was also a small increase in BMP9 expression in the cells treated with BMP10/CV2 as shown in the same figure. Finally, we repeated the experiments in media with 5% or 10% FBS in attempt to minimize FBS-derived BMPs that might interfere. However, the experiments in 5% FBS could not be completed due to poor cell survival. Similarly, the cells did not perform well in 10% FBS, but the expression of Nkx2.5 and Troponin I showed a trend towards the same pattern as in cells grown in 20% FBS (Supplemental Figure 2).
3.4. Effect of BMP10 and CV2 on Staining of Cardiomyocyte Markers in mDFAT Cells
Immunofluorescence localized the expression of Nkx2.5 to the rounded precursor cells (Figure 4B). As the cells grew larger, Nkx2.5 co-localized with Troponin I (Figure 4B). DAPI staining was used to visualize nuclei. On day 10-14, long myotubes that co-expressed Troponin I and sarcomeric alpha-actinin were detected in all treatments (Figure 5), although large numbers of rounded cells were still present in cells treated with BMP10 alone or BMP10/CV2 (Figure 5, row 2 and 4). More mature myotubes dominated in cells treated with CV2 alone or preceeded by BMP10 (Figure 5, row 3 and 4). After 3 weeks, few rounded cells remained and the cardiomyocyte colonies were well developed (Figure 6). The largest colonies were seen in cells treated with BMP10 alone or BMP10/CV2 (Figure 6, row 2 and 4), whereas the longest myotubes were found in cells treated with CV2 alone or preceded by BMP10 (Figure 6, row 3 and 4).
Figure 5. Cardiac marker expression after 14 days of treatment.
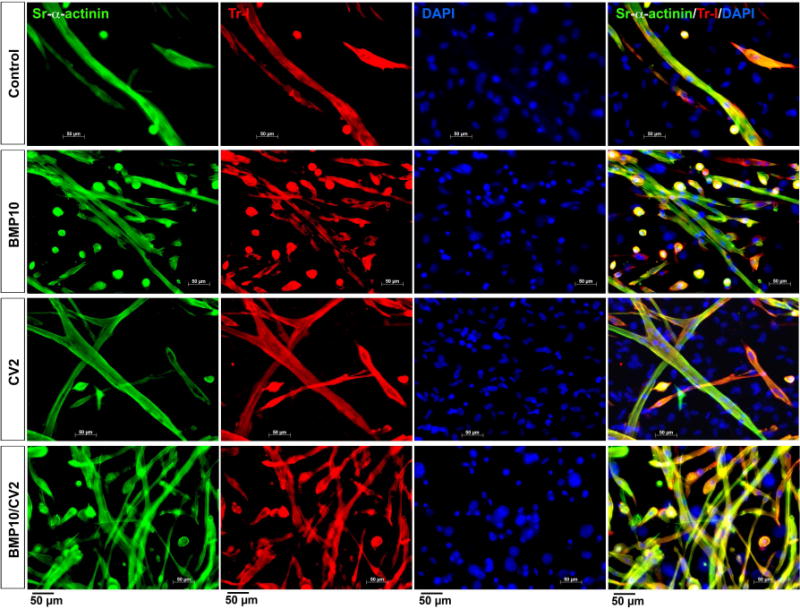
Mouse DFAT cells were treated with growth medium supplemented with control vehicle (day 5-7), BMP10 (25 ng/ml, day 5-7), CV2/5-7 (50 ng/ml, day 5-7), BMP10 (day 5-7) followed by CV2 (day 8-10) (BMP10/CV2). Cell morphology and expression of sarcomeric alpha-actinin (Sr-alpha-actinin, green) and Troponin I (Tr-I, red) were examined after 14 days by immunofluorescence. DAPI (blue) was used to visualize nuclei.
Figure 6. Cardiac marker expression after 3 weeks of treatment.
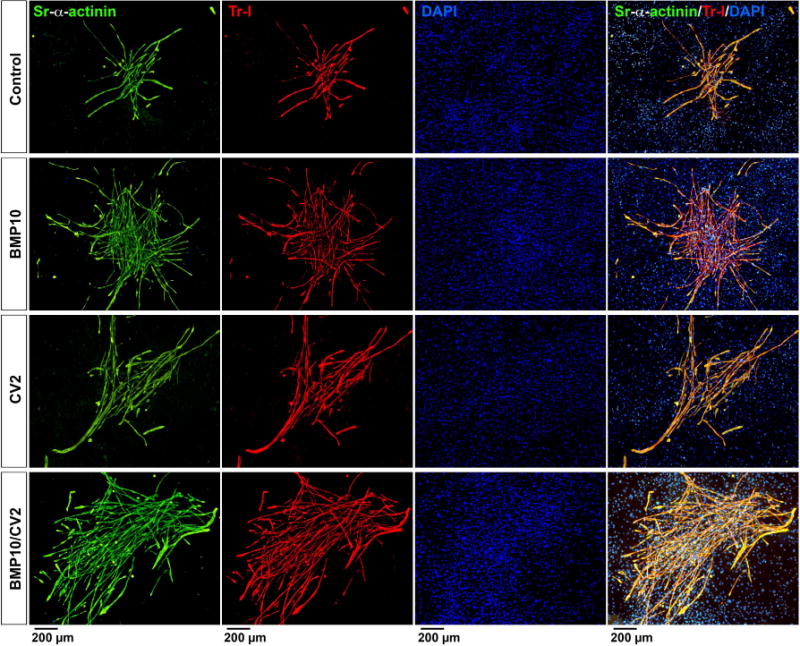
Mouse DFAT cells were treated with growth medium supplemented with control vehicle (day 5-7), BMP10 (25 ng/ml, day 5-7), CV2/5-7 (50 ng/ml, day 5-7), BMP10 (day 5-7) followed by CV2 (day 8-10) (BMP10/CV2). Cell morphology and expression of sarcomeric alpha-actinin (Sr-alpha-actinin, green) and Troponin I (Tr-I, red) were examined after 3 weeks by immunofluorescence. DAPI (blue) was used to visualize nuclei.
We then quantified the number of cardiomyocyte-like cells and colonies after each treatment. The cells were plated and treated for 3 weeks in 6-well culture dishes, and then immunostained for Troponin I. For each treatment, all stained cells and colonies in an entire 6-well were counted by fluorescence microscopy. The cells were classified as small round cells (<50 μm), or short (50-200 μm), medium (200-500 μm), or long myotubes (>500 μm). The colonies were classified as small (1-25 myotubes), medium (25-50 myotubes) or large (>50 myotubes) colonies. The results revealed that cells treated with BMP10 or BMP10/CV2 had the largest number of single rounded cells and small myotubes, whereas inclusion of CV2 subsequent to BMP10 promoted the formation of longer myotubes (Figure 7A). We also determined the cell diameters after each treatment, but no significant differences were seen between treatments (Supplemental Figure 3). In addition, BMP10 promoted the formation of small to medium colonies, whereas inclusion of CV2 subsequent to BMP10 increased the colony size (Figure 7B).
Figure 7. Cell numbers and cardiac marker expression after 3 weeks of treatment.
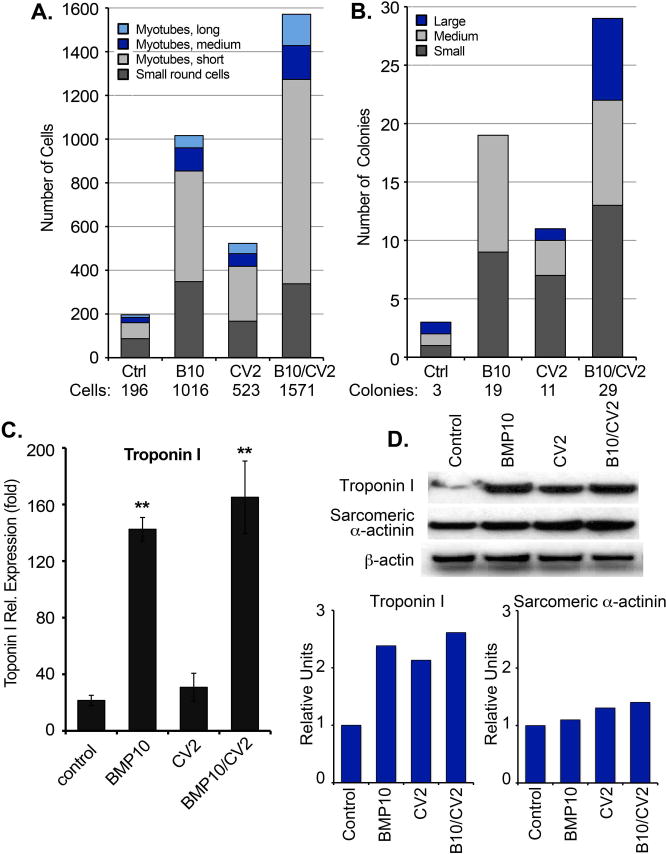
(A, B) Mouse DFAT cells were plated in 6-well culture dishes and treated with growth medium supplemented with control vehicle, BMP10 (25 ng/ml), CV2 (50 ng/ml), or BMP10 for 3 days followed by CV2 for 3 days (BMP10/CV2). After 3 weeks, the cells were immunostained for Troponin I. For each treatment, all stained cells and colonies in one entire 6-well were counted by fluorescence microscopy. (A) The cells were classified as small round cells (<50 μm), or short (50-200 μm), medium (200-500 μm), or long myotubes (>500 μm), and (B) colonies were classified as small (1-25 myotubes), medium (25-50 myotubes) or large (>50 myotubes) colonies (representative of 3 experiments).
(C) Expression of Troponin I expression by qPCR. **<0.01 (n=3).
(D) Expression of Troponin I and sarcomeric alpha-actinin by immunoblotting with densitometry normalized to betaactin.
Furthermore, we compared the expression of Troponin I by qPCR and immunoblotting after 3 weeks. BMP10 or BMP10/CV2 enhanced expression of Troponin I (Figure 7C). Immunoblotting confirmed high levels of Troponin I protein in all samples but with less differences between the samples than qPCR (Figure 7D), possibly due to variation in the turnover of Troponin I mRNA and protein, respectively (Martin, 1981). In contrast, expression of the late cardiomyocyte marker sarcomeric alpha-actinin was highest in the cells treated with CV2 alone or preceded by BMP10 (Figure 7D), supporting that CV2 promotes cardiomyocyte maturation.
Together, our results supports a model where BMP10 stimulates proliferation of early cardiac progenitors and CV2 promotes cardiomyocyte maturation potentially by regulating BMP10 and other BMPs. A combined treatment with BMP10 and CV2 might be useful in optimizing cardiomyocyte differentiation in cardiac regeneration.
4. Discussion
In our study, we show for the first time evidence of interaction and coordination between BMP10 and CV2 using the mDFAT cells as a model of cardiomyocyte differentiation. The white mature adipocytes lose their fat and transition into multipotent DFAT with the ability to differentiate into a variety of lineages including cardiomyogenesis (Jumabay et al., 2010), and the differentiation of cardiomyocyte-like cells proceeds in a reproducible fashion, starting with small round progenitor cells that progressively form myotubes of increasing length and assemble in contracting colonies. We show that BMP10 and CV2 are able to directly interact on a protein-level and support different stages of the differentiation. When sequentially applied, BMP10 and CV2 have a synergistic effect in that BMP10 first secures the proliferation of sufficient progenitors to support the formation of long myotubes in colonies.
Previous reports have shown that BMP9 and BMP10 are functionally equivalent ligands of ALK1 in cardiovascular development (David et al., 2007; Levet et al., 2015), and we show that CV2 interacts with BMP10 in addition to BMP9 (Yao et al., 2012) and BMP10. Although BMP10 can substitute for BMP9 in vascular development via ALK1-dependent signaling in endothelial cells, it has a unique function in cardiac development, which cannot be substituted by BMP9 (Chen et al., 2013; Ricard et al., 2012). Therefore, we did not include BMP9 in this study, although ourprevious studies showed that BMP9 promotes endothelial cell differentiation in mDFAT cells (Jumabay et al., 2012).
The BMP10/Myocardin pathway is crucial for cardiac development and a block in BMP10 signaling produced a block in cardiomyocyte proliferation (Huang et al., 2012). Our data support the previous findings that BMP10 enhances proliferation and increases the number of Nkx2.5-positive progenitor cells in vitro and in vivo (Lichtner et al., 2013; Sun et al., 2014). Nkx2.5 is a transcriptional activator, which is essential in cardiomyogenesis and has previously been shown to be stimulated by BMP4 signaling and the SMAD1 pathway (Jamali et al., 2001; Takei et al., 2009). Our finding that the mDFAT cells are BMP10-responsive cardiac progenitors could be used for further elucidation of BMP-related pathways in cardiomyogenic differentiation.
In the present study, mDFAT cells express significant levels of CV2 at baseline, which likely assist in the spontaneous differentiation of cardiomyocyte-like cells observed in this cell population (Jumabay et al., 2010). Addition of exogenous CV2 further enhanced the cardiomyogenesis, especially subsequent to BMP10, and resulted in larger colonies of cardiomyocyte-like cells. CV2 is known to regulate BMP2, BMP4, and BMP7 (Moser et al., 2003; Serpe et al., 2008), all of which are involved in cardiac development (Harada et al., 2008; Wang et al., 2011). The CV2 effect is also context dependent, and it is possible that if one BMP is antagonized, the activity of another BMP promoted. CV2 also appears to play a role in balancing myocardial vessel density and cardiomyocyte size (Willis et al., 2013). Consistent with the latter findings, we found that addition of CV2 increased the size of the myotubes generated from mDFAT cells. It is possible that CV2 produced by cells other than cardiomyocytes, such as endothelial cells, can regulate the size of neighboring cardiomyocytes.
Overall, this study suggests that BMP10 and CV2 together can promote cardiomyogenesis in two steps, in that BMP10 enhances proliferation and induction of early cardiomyocyte progenitors, whereas CV2 plays a role in the size of myotubes and coordination of cells into larger aggregates. Our findings improve our understanding of cardiomyogenic mechanisms, and may enhance our ability to generate cardiomyocytes from progenitor cells for the purpose of regeneration.
Supplementary Material
Acknowledgments
Funding for this work was provided in part by NIH/NHLBI: grant number HL30568 (A.M.F., K.I.B., L.I.-A.), NIH/NHLBI: grant number HL81397 (K.I.B.), and NIH/NHLBI: grant number HL112839 (K.I.B.), and the American Heart Association: grant number 13SDG17190013 (M.J.).
Footnotes
Disclosures: None declared.
References
- Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279(51):52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H, Roose-Girma M, Zhang G, Shou W, Yan M. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci U S A. 2013;110(29):11887–11892. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- Harada K, Ogai A, Takahashi T, Kitakaze M, Matsubara H, Oh H. Crossveinless-2 controls bone morphogenetic protein signaling during early cardiomyocyte differentiation in P19 cells. J Biol Chem. 2008;283(39):26705–26713. doi: 10.1074/jbc.M801485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Elicker J, Bowens N, Liu X, Cheng L, Cappola TP, Zhu X, Parmacek MS. Myocardin regulates BMP10 expression and is required for heart development. J Clin Invest. 2012;122(10):3678–3691. doi: 10.1172/JCI63635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Karamboulas C, Rogerson PJ, Skerjanc IS. BMP signaling regulates Nkx2-5 activity during cardiomyogenesis. FEBS Lett. 2001;509(1):126–130. doi: 10.1016/s0014-5793(01)03151-9. [DOI] [PubMed] [Google Scholar]
- Jiang H, Salmon RM, Upton PD, Wei Z, Lawera A, Davenport AP, Morrell NW, Li W. The Prodomain-bound Form of Bone Morphogenetic Protein 10 Is Biologically Active on Endothelial Cells. J Biol Chem. 2016;291(6):2954–2966. doi: 10.1074/jbc.M115.683292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumabay M, Abdmaulen R, Ly A, Cubberly MR, Shahmirian LJ, Heydarkhan-Hagvall S, Dumesic DA, Yao Y, Bostrom KI. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem cells translational medicine. 2014;3(2):161–171. doi: 10.5966/sctm.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, Roos KP, Yao Y, Bostrom KI. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J Mol Cell Cardiol. 2012;53(6):790–800. doi: 10.1016/j.yjmcc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumabay M, Zhang R, Yao Y, Goldhaber JI, Bostrom KI. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc Res. 2010;85(1):17–27. doi: 10.1093/cvr/cvp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levet S, Ouarne M, Ciais D, Coutton C, Subileau M, Mallet C, Ricard N, Bidart M, Debillon T, Faravelli F, Rooryck C, Feige JJ, Tillet E, Bailly S. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc Natl Acad Sci U S A. 2015;112(25):E3207–3215. doi: 10.1073/pnas.1508386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner B, Knaus P, Lehrach H, Adjaye J. BMP10 as a potent inducer of trophoblast differentiation in human embryonic and induced pluripotent stem cells. Biomaterials. 2013;34(38):9789–9802. doi: 10.1016/j.biomaterials.2013.08.084. [DOI] [PubMed] [Google Scholar]
- Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem. 1981;256(2):964–968. [PubMed] [Google Scholar]
- Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215(1):210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Bloch DB, Ten Dijke P, Goumans MJ, Hata A, Smith J, Yu PB, Bloch KD. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13(2):106–120. doi: 10.1038/nrcardio.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23(16):5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Hori H, Abe M, Shibata H, Arimura T, Sasaoka T, Sawabe M, Chida K, Arai T, Nakahara K, Kubo T, Sugimoto K, Katsuya T, Ogihara T, Doi Y, Izumi T, Kimura A. Interaction of BMP10 with Tcap may modulate the course of hypertensive cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293(6):H3396–3403. doi: 10.1152/ajpheart.00311.2007. [DOI] [PubMed] [Google Scholar]
- Neuhaus H, Rosen V, Thies RS. Heart specific expression of mouse BMP-10 a novel member of the TGF-beta superfamily. Mech Dev. 1999;80(2):181–184. doi: 10.1016/s0925-4773(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119(25):6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O'Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14(6):940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y. A simple culture method of fat cells from mature fat tissue fragments. J Lipid Res. 1989;30(12):1987–1995. [PubMed] [Google Scholar]
- Sun L, Yu J, Qi S, Hao Y, Liu Y, Li Z. Bone morphogenetic protein-10 induces cardiomyocyte proliferation and improves cardiac function after myocardial infarction. J Cell Biochem. 2014;115(11):1868–1876. doi: 10.1002/jcb.24856. [DOI] [PubMed] [Google Scholar]
- Takei S, Ichikawa H, Johkura K, Mogi A, No H, Yoshie S, Tomotsune D, Sasaki K. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am J Physiol Heart Circ Physiol. 2009;296(6):H1793–1803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- Tillet E, Bailly S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front Genet. 2014;5:456. doi: 10.3389/fgene.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20(5):244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Wang J, Greene SB, Martin JF. BMP signaling in congenital heart disease: new developments and future directions. Birth Defects Res A Clin Mol Teratol. 2011;91(6):441–448. doi: 10.1002/bdra.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2014;35(39):2722–2731. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MS, Dyer LA, Ren R, Lockyer P, Moreno-Miralles I, Schisler JC, Patterson C. BMPER regulates cardiomyocyte size and vessel density in vivo. Cardiovasc Pathol. 2013;22(3):228–240. doi: 10.1016/j.carpath.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107(4):485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119(21):5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Watson AD, Ji S, Bostrom KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res. 2009;105:575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281(45):33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


