Abstract
Objective
We investigated whether rheumatoid arthritis (RA) diagnosis influences smoking behavior changes and whether these changes were associated with mortality.
Methods
We identified an incident RA cohort in the Nurses’ Health Study (NHS, 1976–2012). Behavioral data were collected through biennial questionnaires. We created a comparison cohort, matching RA cases to women without RA by age and calendar year at the index date of RA diagnosis. To investigate smoking behavior changes in the early RA period, sustained cessation was defined as permanently quitting within four years of RA/index date. We used Cox regression to obtain HRs for mortality, comparing sustained smoking cessation to continued smoking.
Results
Among 121,701 women in the NHS, we identified 938 with incident RA matched to 8,951 non-RA comparators. Among current smokers, 40.0% with RA permanently quit smoking in the early RA period compared to 36.1% of comparators (OR for sustained cessation 1.18, 95%CI 0.88–1.58). There were 313 (33.4%) deaths in the RA cohort and 2,042 (22.8%) among comparators. Compared to continued smoking, sustained cessation was associated with similarly decreased mortality in both the RA (HR 0.58, 95%CI 0.33–1.01) and comparison (HR 0.47, 95%CI 0.39–0.58) cohorts. Women with RA had higher mortality for >5 post-RA pack-years (HR 3.67, 95%CI 2.80–4.81) than comparators with >5 post-index pack-years (HR 1.88, 95%CI 1.62–2.17; pinteraction<0.001, reference: ever smoker non-RA women with 0 post-index pack-years).
Conclusion
Sustained smoking cessation within four years of RA diagnosis reduced mortality risk, with a similar effect observed among non-RA comparators. Smoking >5 pack-years after RA diagnosis significantly increased mortality beyond the risk of non-RA comparators.
Keywords: cigarette smoking, rheumatoid arthritis, mortality, epidemiology
Smoking remains a major public health problem, responsible for 480,000 premature deaths and $289 billion in losses from health care expenditures and decreased productivity per year in the U.S.(1). While smoking rates have declined in the past few decades, 16.8% of Americans currently smoked in 2014(1). Diagnosis of a smoking-related chronic disease, such as cancer, chronic obstructive pulmonary disease, diabetes mellitus, and coronary heart disease, may lead to sustained smoking cessation and reduce subsequent mortality risk by 20–50%(2–4). However, the effect of a new diagnosis of rheumatoid arthritis (RA) on smoking behavior changes, and whether these changes affect subsequent mortality, have not been investigated.
Cigarette smoking is recognized as an important risk factor for RA development and is associated with increased risk of comorbidities that are leading causes of death in RA, including cardiovascular and respiratory diseases(5–10). Smoking cessation at any time after RA diagnosis was associated with decreased mortality risk in a recent large study in the U.K.(11). However, comparators without RA were unavailable and the timing of smoking cessation in relation to RA diagnosis was not investigated. Therefore, it remains unclear whether smoking contributes to mortality differently for patients with RA than those without RA or whether being diagnosed with RA affects smoking behavior changes.
To address this, we aimed to quantify the influence of the early period around RA diagnosis upon sustained smoking cessation, compared to those without RA, while controlling for factors such as secular trends and age at diagnosis. We then aimed to determine whether smoking behavior changes around the time of diagnosis and smoking intensity after diagnosis were associated with subsequent mortality risk. We compared women who developed incident RA and those who did not in the Nurses’ Health Study (NHS), where repeated measures of smoking have been collected throughout 36 years of prospective follow-up, allowing for detailed analyses on smoking behaviors before and after RA diagnosis.
SUBJECTS AND METHODS
Study population
The NHS enrolled 121,701 female registered nurses in the U.S., aged 30–55 years at baseline in 1976. Women in the NHS completed questionnaires at baseline and every two years, providing data on behaviors, sociodemographics, diet quality, medications, and diseases. Follow-up in the NHS has been high, with >90% returning questionnaires each cycle. All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
Incident RA cohort
We excluded participants who reported prevalent RA or other connective tissue diseases (CTD) prior to the NHS baseline in 1976. Women who self-reported a physician diagnosis of RA or other CTD after the initial questionnaire were mailed a screening questionnaire(12). For those who screened positive, medical records were obtained and reviewed independently by two rheumatologists to confirm RA meeting the 1987 American College of Rheumatology classification criteria(13). Details on RA characteristics at diagnosis, including date of diagnosis, serologic status, radiographic changes/erosions, and nodules, were obtained from medical record review. Seropositivity was defined as positive rheumatoid factor (RF) or cyclic citrullinated peptide (CCP) antibodies ordered through routine medical care with the upper limit of normal varying by each clinical site and assay used.
Matched non-RA comparison cohort
We matched each RA case with up to ten non-RA comparators based on age and calendar year in order to investigate smoking and mortality, controlling for secular trends. We matched on age and calendar year due to the emerging data occurring during follow-up on the hazardous health effects of smoking and the known effects of age on mortality. We did not match on other lifestyle or clinical variables to maximize sample size and maintain otherwise expected differences between the RA and comparison cohorts that could be adjusted for using multivariable regression. We defined the index date for matching as the date of RA diagnosis. Participants in the NHS were eligible to be a comparator if they had never reported RA or other CTD prior to or on the index date. Since some women developed RA late in the NHS follow-up, exactly ten comparators with complete exposure data were not available for every RA case.
Ascertainment of smoking status and cumulative smoking intensity
Information on smoking behaviors was self-reported on the mailed questionnaires administered every two years since 1976 and previously validated as accurate (14, 15). On the initial NHS questionnaire, participants reported whether they were current smokers or had ever smoked in the past and the age at which they began to smoke. Current smokers reported the number of cigarettes smoked per day, and past smokers reported the age at which they stopped smoking and number of cigarettes smoked per day before quitting. On each subsequent questionnaire, participants reported whether they were never, current, or past smokers as well as the number of cigarettes smoked per day. We calculated pack-years (years of smoking multiplied by packs of cigarettes per day) for past and current smokers. Since women were surveyed repeatedly during the NHS follow-up, we were able to prospectively update time-varying smoking status as well as the timing and accrual of pack-years. We also classified smoking pack-years that occurred before/after the index date as pre-RA, post-RA, pre-index, and post-index pack-years, for women with RA and non-RA comparators.
Definitions of smoking behavior changes around RA/index date
To identify smoking behavior changes occurring around the index date of RA diagnosis, we defined a peri-RA period for smoking cessation that included two NHS questionnaire cycles (2–4 years) prior to the date of RA diagnosis to capture early symptoms of RA prior to clinical diagnosis and two questionnaire cycles (2–4 years) after the date of RA diagnosis to capture the early RA period (Figure 1). We defined an analogous peri-index period for comparators.
Figure 1.
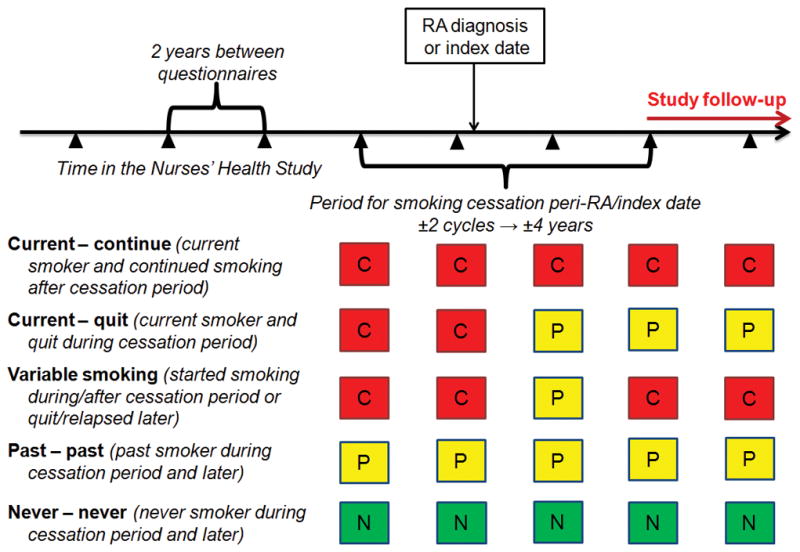
Schematic illustrating definitions of smoking behaviors according to the period for smoking cessation around rheumatoid arthritis diagnosis or matched index date. Wedges indicate questionnaire cycles during follow-up in the Nurses’ Health Study. C, current smoker at questionnaire; N, never smoker at questionnaire; P, past smoker at questionnaire; RA, rheumatoid arthritis.
We defined sustained smoking cessation (“current – quit”) as a current smoker that permanently quit smoking during the peri-RA/index period. Those who continued smoking (“current – continue”) were current smokers throughout the peri-RA/index period and afterward. “Past – past” smokers were those who had already quit smoking before the peri-RA/index period and never smoked again during in follow-up. “Never – never” smokers maintained their never smoking status before, during, and after the peri-RA/index period. “Variable smoking” was defined as all other smoking behavior patterns: current smokers at RA diagnosis/index date then quit >4 years after RA diagnosis/index date, quit at RA diagnosis/index date then later started smoking again, or quit/relapsed smoking; past smokers at RA diagnosis who later started smoking again; and never smokers at RA diagnosis/index date who later started smoking. RA cases and comparators were required to have smoking behavior status assessed at the beginning of the peri-RA/index period to be included in the analysis.
Identification of deaths
Deaths were identified by systematic searches of the National Death Index and state vital records, as previously described(16). The NHS searches for participants who do not respond to biennial questionnaires or other contact attempts. This search is supplemented by postal authority and family reports. These methods ascertain >98% of deaths in the NHS(17). Women who died during the smoking cessation period were not included in the analysis since they may have had pre-existing serious comorbidities and did not have the opportunity to quit smoking. Complete death data were available until 2012, so this served as the end of the study period.
Covariates
We considered covariates as confounders based on their association with both RA risk and mortality based on prior literature. Covariates were included at the start earliest year of the peri-RA/index period. Physical activity was measured starting in 1980 with a validated survey and converted into continuous weekly hours of moderate/vigorous activity (18). Body mass index (BMI) was categorized according to the World Health Organization as underweight, normal, overweight, or obese(19). Dietary factors were assessed using semi-quantitative food frequency questionnaires in 1980, 1984, 1986, and every four years until 2010(20). Participants were classified into tertiles of the Alternative Healthy Eating Index by methods previously described(21). For comorbidities at baseline, we used the previously validated Multimorbidity Weighted Index (MWI) that was derived using the NHS and related cohorts and is composed of 74 distinct prevalent and serious conditions (such as cancer, diabetes, and cardiovascular disease) by self-report, with each condition weighted by the effect on physical health-related quality of life(22). We modified the MWI for this analysis to include a total of 64 conditions, excluding conditions affecting only men, premenopausal conditions that were never assessed in the NHS, and RA or other CTD since RA was an exposure of interest. RA severity factors were seropositivity (defined as positive RF or CCP through review of medical records from routine care) as well as radiographic changes/erosions and nodules collected from medical records around RA diagnosis. Data on medications were not available, but participants were not yet diagnosed with RA at the baseline period two years prior to index date.
Statistical analysis
We reported descriptive statistics according to the five smoking behaviors during the peri-RA/index period (current – continue, current – quit, variable smoking, past – past, never – never) separately for the RA and comparison cohorts. Among all current smokers at the index date, we performed multivariable logistic regression to obtain odds ratios (OR) and 95% confidence intervals (CI) for sustained smoking cessation for those with RA compared to women without RA. The multivariable analysis was adjusted for age, calendar year, BMI, physical activity, diet quality, income, and MWI.
We investigated the effect of smoking behavior changes on mortality. We initially performed these analyses separately in the RA and comparison cohorts. Our primary interest was to compare mortality risk in the extreme smoking behaviors in current smokers prior to RA diagnosis: sustained smoking cessation occurring in the early RA period vs. continued smoking throughout follow-up. We hypothesized that sustained smoking cessation during the early RA period would have an inverse association (hazard ratio <1) with mortality compared to continued smoking. Person-years accrued from the end of the period for smoking cessation (Figure 1) to the end of follow-up, date of death, or date of censor from loss to follow-up, whichever came first. We used Cox regression models to obtain hazard ratios (HR) and 95%CIs for the association between smoking behavior changes and mortality, adjusting for age and questionnaire period. Since current smokers tend to have lower BMI, a separate model was performed additionally adjusting for BMI category and age. The final multivariable models adjusted for age, questionnaire period, BMI category, physical activity (hours/week), Alternative Healthy Eating Index tertiles, U.S. census-tract income, and MWI at baseline. We adjusted for RA severity factors at diagnosis (serostatus, radiographic changes/erosions, and nodules each as binary variables), only for analyses restricted to the RA cohort since these variables were not present in the comparison cohort. We did not adjust for time-varying covariates after baseline since these factors are likely mediate, rather than confound, the association of smoking behavior changes with mortality so it would be inappropriate to include them in multivariable analyses. We did not include smoking intensity (pre-RA/index pack-years) in this model since this focused on smoking status changes rather than smoking intensity.
To examine whether smoking had a differential effect among RA patients compared to comparators on mortality risk, we combined both cohorts into a single analysis using an indicator variable and investigated whether there was a multiplicative interaction between smoking behaviors and RA/comparator status for mortality using an interaction term between these variables.
We investigated whether post-RA/index pack-years were associated with mortality. We calculated pack-years after index date. The exposure in this analysis was time-varying such that women were categorized as having 0 (reference), >0 to 5, or >5 post-RA/index pack-years throughout follow-up. We chose 5 pack-years as a cutpoint after examining cubic spline curves for continuous post-RA pack-years and mortality rates in the RA cohort, which was our primary cohort of interest. Since the sample size was limited and many women that were diagnosed with RA were advanced in age, we were unable to examine higher post-RA pack-years cutpoints. We hypothesized that post-RA pack-years would have a positive association (HR >1) with mortality compared to no smoking after RA diagnosis. We performed similar multivariable models as already detailed, adjusting for pre-RA/index pack-years (categorized as never, >0–10, >10–20, >20 pack-years).
We investigated whether there was effect modification for post-RA/index pack-years by RA/comparator status for mortality risk. We combined both cohorts into a single cohort using an indicator variable for RA/comparator status at the index date. We performed this analysis only among ever smokers since never smokers were unlikely to start smoking after index date. We tested for an interaction by adding a multiplicative interaction term for post-RA/index pack-years and RA/comparator status. We also tested for additive interactions, reporting the attributable proportions (AP) due to interaction and 95%CIs. Since testing for additive interaction requires two binary comparisons, we investigated two separate additive interactions: 1) RA/comparator status and 0 vs. >0 to 5 post-RA/index pack-years and 2) RA/comparator status and 0 vs. > 5 post-RA/index pack-years.
Lastly, since smoking may be causally related to mortality, we investigated the population attributable risk (PAR) for mortality of smoking after RA/index date. PARs and 95%CIs were calculated according to methods previously described(23). We considered any smoking after RA or index date and analyzed RA and comparators separately in these analyses.
We tested for the proportional hazards assumption by comparing nested models with and without interaction terms with follow-up time using likelihood ratio tests. The proportional hazards assumption was met in all analyses. For all analyses, two-sided p<0.05 was considered statistically significant. Analyses were performed using SASv9.3.
RESULTS
Among 121,701 women in the NHS, we identified 938 incident RA cases and 8,951 matched non-RA comparators. The characteristics of both the RA and comparator cohorts at the beginning of the peri-RA/index period are presented in Table 1, stratified by smoking behaviors as defined in Figure 1. There were fewer never smokers in the RA cohort (33.9%) than in the comparison cohort (43.4%). Current smokers who continued smoking throughout follow-up were younger than past or never smokers in both cohorts. Those who continued smoking had lower BMI compared to current smokers who quit permanently in the peri-RA or peri-index period. Current smokers who continued smoking had the least healthy diets in both cohorts as measured by the Alternative Healthy Eating Index.
Table 1.
Characteristics two years prior to index date according to smoking behaviors during the smoking cessation period in the Nurses’ Health Study.
| RA cohort (n=938) | Current – continue (n=41) | Current – quit (n=90) | Variable smoking* (n=119) | Past – past (n=370) | Never – never (n=318) |
|---|---|---|---|---|---|
| Mean age, years (SD) | 52.8 (9.0) | 54.1 (8.0) | 50.4 (8.9) | 57.7 (9.1) | 55.4 (10.3) |
| Mean household income, $1000 USD (SD) | 65.8 (23.1) | 60.5 (23.7) | 62.9 (20.4) | 66.3 (25.1) | 63.2 (24.1) |
| Moderate to vigorous physical activity, hours per week (SD)** | 1.6 (2.5) | 2.3 (2.4) | 2.6 (3.1) | 2.5 (3.1) | 2.2 (2.7) |
| Mean body mass index, kg/m2 (SD) | 23.1 (4.1) | 25.9 (5.1) | 26.0 (4.7) | 26.3 (5.3) | 25.6 (4.5) |
| Body mass index category, % | |||||
| Underweight (<18.5 kg/m2) | 8.5 | 1.8 | 1.2 | 1.1 | 2.0 |
| Normal (18.5 to 24.9 kg/m2) | 69.7 | 46.4 | 45.4 | 49.0 | 49.4 |
| Overweight (25.0 to 29.9 kg/m2) | 12.9 | 30.9 | 41.2 | 29.7 | 34.1 |
| Obese (≥30.0 kg/m2) | 8.9 | 18.5 | 12.3 | 20.2 | 14.5 |
| Alternative Healthy Eating Index tertiles, %*** | |||||
| Tertile 1 – least healthy | 35.0 | 27.3 | 26.4 | 23.4 | 31.3 |
| Tertile 2 | 26.0 | 27.1 | 27.0 | 35.3 | 30.4 |
| Tertile 3 – most healthy | 12.3 | 30.9 | 31.2 | 33.5 | 25.6 |
| Pack-years of cigarette smoking, % | |||||
| Never | 0.0 | 1.3 | 7.2 | 0.0 | 100.0 |
| >0 to ≤10 | 1.5 | 4.9 | 7.6 | 37.1 | 0.0 |
| >10–20 | 6.5 | 14.1 | 15.9 | 20.6 | 0.0 |
| >20 | 92.0 | 79.0 | 66.3 | 38.2 | 0.0 |
| Cancer, % | 10.9 | 7.1 | 8.3 | 11.4 | 7.7 |
| Diabetes, % | 4.3 | 5.4 | 4.0 | 3.5 | 3.7 |
| Cardiovascular disease, % | 0.0 | 3.3 | 2.3 | 3.9 | 2.1 |
| Chronic obstructive pulmonary disease, % | 7.1 | 10.9 | 5.5 | 7.8 | 3.3 |
| Asthma, % | 2.1 | 7.9 | 6.1 | 8.1 | 7.8 |
| Mean Multimorbidity Weighted Index (SD)**** | 2.1 (2.8) | 3.1 (3.4) | 3.1 (4.1) | 3.7 (4.0) | 3.1 (3.4) |
| Seropositive, % | 64.2 | 68.6 | 57.2 | 58.0 | 59.5 |
| Rheumatoid nodules, % | 18.6 | 21.7 | 15.1 | 10.2 | 10.3 |
| Radiographic changes/erosions, % | 19.9 | 30.0 | 42.1 | 29.4 | 30.0 |
| Comparison cohort (n=8,951) | Current – continue (n=460) | Current – quit (n=717) | Variable smoking* (n=988) | Past – past (n=2,899) | Never – never (n=3,887) |
|---|---|---|---|---|---|
| Mean age, years (SD) | 53.2 (9.2) | 53.6 (9.0) | 49.7 (8.4) | 57.1 (9.4) | 55.6 (9.7) |
| Mean household income, $1000 USD (SD) | 59.8 (21.6) | 63.4 (25.0) | 63.2 (26.3) | 65.8 (25.1) | 62.6 (24.8) |
| Moderate to vigorous physical activity, hours per week (SD)** | 1.9 (2.7) | 2.0 (2.5) | 2.4 (2.8) | 2.6 (3.2) | 2.5 (3.2) |
| Mean body mass index, kg/m2 (SD) | 24.0 (4.4) | 24.4 (4.3) | 24.6 (4.6) | 25.9 (5.2) | 25.7 (5.0) |
| Body mass index category, % | |||||
| Underweight (<18.5 kg/m2) | 4.5 | 2.8 | 3.4 | 1.0 | 1.6 |
| Normal (18.5 to 24.9 kg/m2) | 62.9 | 60.1 | 59.3 | 51.5 | 50.6 |
| Overweight (25.0 to 29.9 kg/m2) | 22.0 | 27.1 | 26.0 | 29.9 | 29.9 |
| Obese (≥30.0 kg/m2) | 10.6 | 10.0 | 10.8 | 17.3 | 17.3 |
| Alternative Healthy Eating Index tertiles, %*** | |||||
| Tertile 1 – least healthy | 33.5 | 28.8 | 29.0 | 22.0 | 26.7 |
| Tertile 2 | 24.2 | 24.7 | 26.8 | 27.3 | 25.4 |
| Tertile 3 – most healthy | 16.3 | 24.3 | 23.4 | 33.0 | 25.0 |
| Pack-years of cigarette smoking, % | |||||
| Never | 0.0 | 1.6 | 7.5 | 0.0 | 100.0 |
| >0 to ≤10 | 2.9 | 14.9 | 11.0 | 47.3 | 0.0 |
| >10–20 | 11.2 | 14.1 | 12.6 | 21.2 | 0.0 |
| >20 | 83.4 | 66.4 | 67.4 | 28.2 | 0.0 |
| Cancer, % | 8.7 | 6.8 | 9.3 | 9.1 | 7.8 |
| Diabetes, % | 2.2 | 4.9 | 6.6 | 5.2 | 4.8 |
| Cardiovascular disease, % | 3.2 | 4.5 | 1.9 | 3.3 | 2.6 |
| Chronic obstructive pulmonary disease, % | 8.7 | 6.7 | 4.8 | 4.4 | 2.6 |
| Asthma, % | 3.8 | 5.8 | 5.4 | 7.2 | 5.0 |
| Mean Multimorbidity Weighted Index (SD)**** | 2.7 (3.2) | 2.6 (3.8) | 2.3 (3.3) | 2.8 (3.6) | 2.4 (3.1) |
Each RA case was matched with up to 10 women without RA on age and calendar year at index date to form the comparison cohort.
Missing values are not reported.
“Variable smoking” includes current smokers at RA diagnosis then quit >4 years after RA diagnosis/index date (n=66 in RA cohort; n=514 in comparison cohort), quit at RA diagnosis/index date then later started smoking again (n=1 in RA cohort; n=18 in comparison cohort), or quit/relapsed smoking (n=27 in RA cohort; n=278 in comparison cohort); past smokers at RA diagnosis who later started smoking again (n=19 in RA cohort; n=135 in comparison cohort); and never smokers at RA diagnosis/index date who later started smoking (n=6 in RA cohort; n=43 in comparison cohort).
Physical activity was first assessed in 1980.
Alternative Healthy Eating Index was first assessed in 1984.
The previously validated Multimorbidity Weighted Index (MWI) is composed of prevalent/serious conditions, each condition weighted by the effect on physical health-related quality of life. We did not include rheumatoid arthritis and other connective tissue diseases, male conditions, and premenopausal female conditions that were not assessed so the MWI included a total of 64 conditions for these analyses.
RA, rheumatoid arthritis; SD, standard deviation.
RA diagnosis and smoking cessation
Among 225 current smokers at the beginning of the peri-RA/index period, 90 (40.0%) quit smoking permanently in the peri-RA window (Table 2). In the comparison cohort, 36.1% quit smoking in the analogous peri-index window. Among current smokers, those with RA had an OR for sustained smoking cessation of 1.21 (95%CI 0.91–1.61) compared to comparators, adjusted for age and calendar year. There was no statistical association of RA with sustained smoking cessation compared to comparator status when additionally adjusting for BMI, physical activity, diet quality, income, and MWI (OR 1.18, 95%CI 0.88–1.58).
Table 2.
Among current smokers at the beginning of the peri-RA/index period, frequencies for smoking behavior changes and odds ratio for sustained smoking cessation.
| Current – continue | Current – quit | Current then variable smoking* | Age- and year-adjusted OR (95% CI) for sustained cessation among current smokers | Multivariable adjusted OR (95% CI) for sustained cessation among current smokers** | |
|---|---|---|---|---|---|
| Comparison cohort (n=1,987) | 460 (23.2%) | 717 (36.1%) | 810 (40.8%) | 1.00 (Ref) | 1.00 (Ref) |
| RA cohort (n=225) | 41 (18.2%) | 90 (40.0%) | 94 (41.8%) | 1.21 (0.91–1.61) | 1.18 (0.88–1.58) |
Each RA case was matched with up to 10 women without RA on age and calendar year at index date to form the comparison cohort.
“Current then variable smoking” includes current smokers at RA diagnosis/index date then quit >4 years after RA diagnosis/index date, quit at RA diagnosis/index date then later started smoking again, or quit/relapsed smoking.
Adjusted for age (years), questionnaire period, BMI category (underweight, normal, overweight, obese), physical activity (hours of moderate or vigorous exercise per week, continuous), census-tract household family income (<$40K, $40K+ per year), Alternative Healthy Eating Index score (tertiles), and Multimorbidity Weighted index (continuous).
CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis.
Smoking behavior changes around RA/index date and mortality risk
There were 313 deaths (33.4%) during 16,318 person-years after the peri-RA window in the RA cohort and 2,042 (22.8%) during 161,881 person-years after the peri-index window in the comparison cohort. The absolute mortality rate of the RA cohort (1,286 to 3,670 deaths/100,000 person-years) was consistently higher than in the comparison cohort (1,024 to 2,599 deaths/100,000 person-years). Compared to those who continued smoking, sustained cessation in the peri-RA period was inversely associated with mortality in the age- and period-adjusted model (HR 0.52, 95%CI 0.30–0.89, Table 3). This association remained statistically significant after adjusting for BMI categories (HR 0.53, 95%CI 0.31–0.91) and had a trend toward statistical significance when additionally adding income, physical activity, diet quality, and MWI(HR 0.58, 95%CI 0.33–1.01). Results were similar when additionally adjusting for RA severity factors at diagnosis (HR 0.60, 95%CI 0.34–1.05). In the comparison cohort, there was a similar protective effect of sustained smoking cessation around the index date for mortality that was statistically significant (multivariable HR 0.47, 95%CI 0.39–0.58) compared to continued smoking. Never smokers had decreased mortality risk in both the RA (multivariable HR 0.21, 95%CI 0.13–0.36) and comparison (multivariable HR 0.24, 95%CI 0.21–0.29) cohorts compared to those who continued smoking. When combining both cohorts into a single analysis, there was no interaction between smoking behaviors and RA/comparator status for mortality risk (p=0.20).
Table 3.
Hazard ratios for total mortality according to smoking behaviors during the smoking cessation period in the Nurses’ Health Study.
| Deaths/Person-years | Mortality rate* | Age-adjusted HR (95% CI) | Age and BMI-adjusted HR (95% CI)1 | Multivariable HR (95% CI)2 | |
|---|---|---|---|---|---|
| RA cohort (n=938) | |||||
| Current – continue | 23/627 | 3,670 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Current – quit | 50/1,586 | 3,152 | 0.52 (0.30–0.89) | 0.53 (0.31–0.91) | 0.58 (0.33–1.01) |
| Variable smoking | 57/2,552 | 2,234 | 0.49 (0.29–0.84) | 0.50 (0.29–0.86) | 0.55 (0.32–0.95) |
| Past – past | 109/5,798 | 1,880 | 0.28 (0.17–0.46) | 0.28 (0.17–0.47) | 0.31 (0.19–0.52) |
| Never – never | 74/5,755 | 1,286 | 0.20 (1.12–0.33) | 0.20 (0.12–0.34) | 0.21 (0.13–0.36) |
|
| |||||
| Comparison cohort (n=8,951) | |||||
| Current – continue | 182/7,002 | 2,599 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Current – quit | 207/13,147 | 1,575 | 0.44 (0.36–0.54) | 0.45 (0.37–0.55) | 0.47 (0.39–0.58) |
| Variable smoking | 304/22,954 | 1,324 | 0.44 (0.36–0.52) | 0.44 (0.37–0.53) | 0.47 (0.39–0.56) |
| Past – past | 621/47,679 | 1,302 | 0.31 (0.26–0.37) | 0.31 (0.26–0.36) | 0.30 (0.26–0.36) |
| Never – never | 728/71,099 | 1,024 | 0.25 (0.21–0.30) | 0.24 (0.20–0.29) | 0.24 (0.21–0.29) |
Each RA case was matched with up to 10 women without RA on age and calendar year at index date to form the comparison cohort.
Per 100,000 person-years.
Adjusted for age (years), questionnaire period, and body mass index category (underweight, normal, overweight, obese).
Adjusted for age (years), questionnaire period, BMI category (underweight, normal, overweight, obese), physical activity (hours of moderate or vigorous exercise per week, continuous), census-tract household family income (<$40K, $40K+ per year), Alternative Healthy Eating Index score (tertiles), and Multimorbidity Weighted Index (continuous).
BMI, body mass index; CI, confidence interval; HR, hazard ratio; RA, rheumatoid arthritis; SD, standard deviation.
Cumulative smoking after RA/index date and mortality risk
Among the subset of those who ever smoked (n=597 in the RA cohort; n=4,914 in the comparison cohort), we examined whether cumulative smoking after index date was associated with mortality. Compared to those without post-RA pack-years, women with >5 pack-years after RA had a three-fold increased mortality in age- and period-adjusted models (HR 2.97, 95%CI 2.12–4.15, Table 4). This association was slightly attenuated when adjusting for pre-RA pack-years (HR 2.60, 95%CI 1.81–3.74), BMI (HR 2.63, 95%CI 1.81–3.80), and income, physical activity, diet quality, and MWI (HR 2.43, 95%CI 1.64–3.58). Results were similar when additionally adjusting for RA severity factors at diagnosis (HR 2.50, 95%CI 1.69–3.70). Smoking >0 to 5 pack-years after RA diagnosis had a trend towards a modest association with mortality that was not statistically significant after multivariable adjustment (HR 1.33, 95% 1.13–1.57), compared to ever smokers who did not smoke after RA diagnosis.
Table 4.
Among ever smokers, hazard ratios for total mortality according to cumulative smoking pack-years after RA diagnosis/index date in the Nurses’ Health Study.
| Pack-years after RA or index date | Deaths/Person-years | Mortality rate* | Age-adjusted HR (95% CI) | Age and pre-RA/index smoking adjusted HR (95% CI)1 | Age, pre-RA/index smoking, and BMI adjusted HR (95% CI)2 | Multivariable HR (95% CI)3 |
|---|---|---|---|---|---|---|
| RA cohort (n=597) | ||||||
| 0 | 122/6,267 | 1,947 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| >0 to 5 | 47/2,095 | 2,243 | 1.59 (1.12–2.25) | 1.45 (1.01–2.08) | 1.38 (0.96–2.00) | 1.33 (0.91–1.95) |
| >5 | 67/1,692 | 3,724 | 2.97 (2.12–4.15) | 2.60 (1.81–3.74) | 2.63 (1.81–3.80) | 2.43 (1.64–3.58) |
|
| ||||||
| Comparison cohort (n=4,914) | ||||||
| 0 | 688/53,678 | 1,282 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| >0 to 5 | 215/16,548 | 1,299 | 1.44 (1.23–1.68) | 1.21 (1.03–1.42) | 1.25 (1.06–1.47) | 1.33 (1.13–1.57) |
| >5 | 361/16,514 | 2,186 | 2.09 (1.84–2.38) | 1.62 (1.40–1.86) | 1.69 (1.46–1.95) | 1.93 (1.66–2.24) |
Each RA case was matched with up to 10 women without RA on age and calendar year at index date to form the comparison cohort.
Per 100,000 person-years.
Adjusted for age, questionnaire period, and cumulative smoking prior to RA diagnosis/index date (never, >0–10, >10–20, >20 pack-years).
Adjusted for age, questionnaire period, cumulative smoking prior to RA diagnosis/index date (never, >0–10, >10–20, >20 pack-years), and BMI category (underweight, normal, overweight, obese).
Adjusted for age, questionnaire period, cumulative smoking prior to RA diagnosis/index date (never, >0–10, >10–20, >20 pack-years), BMI category (underweight, normal, overweight, obese), physical activity (hours of moderate or vigorous exercise per week, continuous), census-tract household family income (<$40K, $40K+ per year), Alternative Healthy Eating Index score (tertiles), and Multimorbidity Weighted Index (continuous).
BMI, body mass index; CI, confidence interval; HR, hazard ratio; RA, rheumatoid arthritis; SD, standard deviation.
In the comparison cohort, there was also a statistically significantly increased risk for mortality for those that smoked >5 pack-years compared to no smoking after index date. However, this effect was less pronounced than in the RA cohort (HR 1.93, 95%CI 1.66–2.24). The cohorts also differed in absolute mortality risk among ever smokers. In the RA cohort, those with >5 post-RA pack-years had a mortality rate of 3,724 compared to 1,947 per 100,000 person-years in ever smokers without post-RA pack-years. The comparison cohort had lower mortality rates among ever smokers: 2,186 with >5 post-index pack-years and 1,282 with no post-index pack-years (both per 100,000 person-years). Comparators with light smoking after index date had a modest but statistically significantly increased mortality risk compared to ever smokers with no smoking after index date (multivariable HR 1.27, 95%CI 1.08–1.50).
We found a significant additive interaction for >5 post-RA and >5 post-index pack-years with RA/comparator status, with AP for mortality of 0.37 (95%CI 0.19–0.56, p=0.0001; reference: 0 post-index pack-years, Figure 2). Therefore, smoking >5 pack-years after RA diagnosis conferred 37% additional mortality risk beyond the independent effects of having RA and smoking >5 pack-years after index date. There was no multiplicative interaction between post-RA/index pack-years and RA/comparator status on mortality risk (p=0.24).
Figure 2.
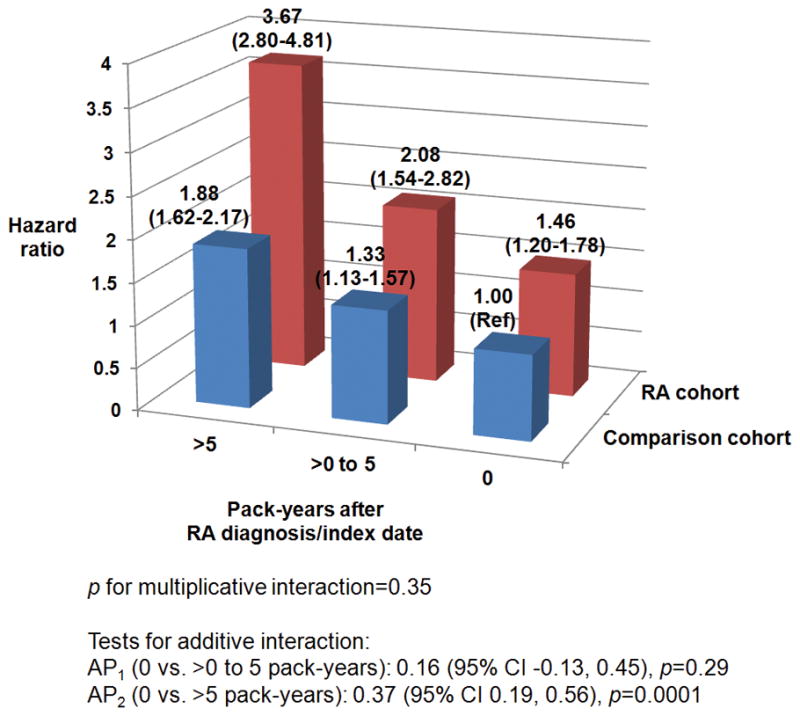
Among ever smokers, multivariable adjusted hazard ratios for total mortality according to cumulative smoking pack-years after RA diagnosis/index date for RA cases and comparators combined into a single analysis (n=5,511).
Population attributable risk for mortality of post-RA/index smoking
In the RA cohort, the PAR for mortality from smoking after RA diagnosis among ever smokers was 18% (95%CI 7–28%). Therefore, assuming smoking was causally related to death, 18% of deaths in RA could be prevented by sustained smoking cessation. In the comparison cohort, the PAR for mortality from smoking after index date was lower at 14% (95%CI 10–19%) among ever smokers, also suggesting that smoking after RA diagnosis contributes more to mortality for RA patients than those without RA.
DISCUSSION
We found that sustained smoking cessation within 4 years of RA diagnosis decreased the risk of subsequent mortality by 42% compared to continued smoking. There was a similar protective effect of smoking cessation on mortality risk among a similar population of non-RA comparators while controlling for secular changes in smoking according to calendar time. Smoking more than 5 pack-years after diagnosis of RA increased the risk of mortality by nearly three-fold and that this effect was higher than the comparator cohort. While quitting smoking similarly reduced mortality risk in RA and matched non-RA comparators, the risk of death appeared higher for patients with RA once a threshold of 5 pack-years after diagnosis was reached. These findings underscore the importance of emphasizing smoking cessation to decrease mortality risk among patients with RA.
We also performed these investigations in a similar cohort without RA and many potential confounders were available for adjustment. Therefore, we were able study the specific effect of RA diagnosis on smoking behaviors and subsequent mortality. While the multivariable results for mortality risk comparing sustained cessation to continued smoking were borderline statistically significant in the RA cohort (HR 0.58, 95%CI 0.33–1.01), the analogous analyses were highly significant in the comparator cohort, with a similar effect size estimate (HR 0.47, 95%CI 0.39–0.58). We therefore suspect with sufficient power we would be able to detect this effect in the RA cohort as well. Women with RA had higher absolute mortality rates than matched non-RA comparators, as previously shown(8, 9, 24–26). A large study performed in the U.K. among only RA patients showed that current smokers had two-fold increased mortality risk compare to never smokers(11). This study also demonstrated that smoking cessation was associated with decreased mortality risk; for each year of smoking cessation, the hazard ratio for death decreased by 10–15%(11). Our study differs by defining a peri-RA diagnosis period for smoking cessation and by using non-RA comparators to evaluate whether smoking behaviors and mortality risk are different among patients with RA compared to a similar population without RA.
We report that cumulative smoking after RA diagnosis increased mortality risk beyond the effect observed in matched comparators. Women with RA who smoked >5 pack-years after diagnosis had nearly three-fold increased risk of mortality, compared to ever smokers without post-RA smoking. Comparators had a statistically significant increase as well, but less pronounced than the RA cohort. These findings were robust to adjustment for potential confounders including pre-RA/index pack-years, BMI, physical activity, diet quality, multimorbidities, and RA severity factors. When combining both cohorts into a single analysis, we found a significant additive interaction, such that there was a 37% excess risk for mortality for having RA and smoking >5 pack-years. We found that smoking cessation may prevent a large proportion of deaths, particularly among those with RA.
Smoking influences transitions between preclinical phases in the development of RA(27–29). Smoking may contribute to RA pathogenesis by interacting with the HLA-DRB1 shared epitope to increase RA risk, perhaps through the formation of citrullinated neo-antigens resulting in loss of tolerance, autoimmunity, and systemic inflammation eventually resulting in polyarthritis(30–33). Smoking cessation may decrease RA risk, though it may take up to 20 years for the risk of a former smoker to return to the level of a never smoker(14, 34).
Smoking is associated with worse RA severity and outcomes, including the development of erosions and nodules, higher disease activity, and extra-articular manifestations such as interstitial lung disease(35–37). These factors are associated with an increased risk of mortality among patients with RA, perhaps explaining the differential effect for RA on mortality compared to the general population(38, 39). Since we did not observe this increased risk until after five post-RA pack-years accumulated, there may be a cumulative effect of smoking on mortality after this threshold, perhaps mediated through these RA-specific manifestations, increased burden of comorbidities, and decreased physical activity. While there was no statistical association of >0 to 5 post-RA smoking with mortality (HR 1.33, 95%CI 0.91–1.95), the analogous analyses in the comparison cohort did show a modest association of >0 to 5 post-index smoking with mortality (HR 1.33, 95%CI 1.13–1.57). Thus, we suspect that the lack of statistical association in the RA cohort was due to being underpowered and that there is likely an increased risk for mortality even among smokers with >0 to 5 pack-years after RA diagnosis. Recent data associated increased levels of circulating cytokines with mortality risk in a group of mostly male patients with RA and who were heavy smokers; thus, smoking likely contributes to mortality risk in RA regardless of sex(40). Since we adjusted for pre-RA/index pack-years, smoking prior to RA diagnosis is less likely to explain these findings though residual confounding from pre-RA/index smoking may remain.
We defined behavior changes occurring around RA diagnosis while also analyzing these changes in non-RA comparators to control for secular trends. This method has the advantage of attributing smoking behavior change to the early RA period in order to better understand the effects of RA and smoking on mortality. Previous studies were unable to determine the effect of RA diagnosis on sustained smoking cessation without a non-RA comparison group and repeated measures of smoking. While women diagnosed with RA had slightly increased rates of sustained smoking cessation than comparators, this difference was not statistically significant. Strengths of the study include the inclusion of a comparator cohort, the focus on the peri-RA period for smoking behavior change, and the prospective assessment of smoking with higher accuracy than indirect methods such as billing codes, pharmacologic smoking cessation aids, or using data obtained from medical records. Since we assessed behaviors regularly throughout follow-up, our study was able to detect women with sustained smoking cessation around RA diagnosis. Further, we were able to adjust for important confounders important for RA and mortality risk, including pre-RA smoking intensity and multimorbidities such as cardiovascular disease and cancer.
The NHS includes mostly white U.S. women aged 30–55 years who were healthy, educated, and working at the inception of the study in 1976. While smoking rates were higher during many of the years of this study, we were able to control for these secular trends using matched comparators. These women may have been more willing to quit smoking since they were health professionals. It is possible that the decreasing prevalence of smoking may make these results less applicable to current smoking trends(1). However, the large sample size and high smoking prevalence in the early years of the NHS allowed for well-powered analyses of smoking behavior changes occurring during follow-up. While we were able to adjust for pre-RA/index pack-years, it is possible that those with longer smoke-free intervals prior to index date may have lower mortality even with similar pre-RA/index pack-years. While detailed data were available for many covariates, RA-specific data on disease activity and treatment were unavailable. RA severity factors at diagnosis were available and our findings were similar after adjustment for serostatus, radiographic changes/erosions, and nodules, although residual confounding is possible. We chose the smoking cessation period to capture the entire early RA period, composed of early symptoms before diagnosis and the first few years after diagnosis, but another definition may have yielded different estimates. While our aim was to investigate the extremes of sustained smoking vs. sustained cessation, it is unclear whether patients that quit smoking in the early RA period will have sustained cessation. RA-specific smoking cessation programs may be needed to further encourage those diagnosed with RA to quit smoking beyond the motivating effect of the RA diagnosis alone(41).
In conclusion, sustained smoking cessation was associated with lower mortality risk compared to continued smoking. We found that smoking >5 pack-years after RA diagnosis increased mortality risk beyond the effect observed in non-RA comparators. These findings suggest that smoking may be an important contributor to the excess mortality observed for RA. Interventions for smoking cessation at RA diagnosis may substantially decrease the mortality burden of RA.
SIGNIFICANCE & INNOVATIONS.
We used data from the Nurses’ Health Study consisting of detailed assessment of smoking during lengthy follow-up to determine smoking behavior changes in the early rheumatoid arthritis (RA) period while also forming a comparator cohort without RA.
Among current smokers, only 40.0% of women diagnosed with RA permanently quit smoking in the early RA period, compared to 36.1% of women without RA that permanently quit smoking in a similar matched period.
Compared to sustained smoking, women who permanently quit smoking in the early RA period had 42–53% reduced mortality. Smoking >5 pack-years after RA diagnosis significantly increased mortality beyond the risk of non-RA comparators. For patients with RA, smoking after diagnosis attributed to 18% of total mortality.
These results demonstrate the mortality burden from smoking for RA patients, beyond the effect observed in the general population.
Acknowledgments
Funding/Support: This work was supported by the Rheumatology Research Foundation Disease-Targeted Innovative Award (Dr. Choi) and Scientist Development Awards (Dr. Sparks and Dr. Barbhaiya), the National Institutes of Health (grant numbers K24 AR052403, P60 AR047782, L30 AR066953, L30 AR070514, R01 AR049880, UM1 CA186107, K23 AR069688, K01 AR064351, and T32 AR007530). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
We thank the participants of the NHS for their dedicated participation in this longitudinal study as well as the NHS staff members at the Channing Division of Network Medicine (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School). We also thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Chang, Nguyen, Zhang, Choi, Karlson
Acquisition of data. Sparks, Barbhaiya, Tedeschi, Costenbader, Karlson
Analysis and interpretation of data. Sparks, Chang, Nguyen, Barbhaiya, Tedeschi, Lu, Costenbader, Zhang, Choi, Karlson
References
- 1.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–40. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Jiang B, Li LS, Li LS, Sun DL, Wu L, et al. Changes in smoking behavior and subsequent mortality risk during a 35-year follow-up of a cohort in Xi’an, China. Am J Epidemiol. 2014;179(9):1060–70. doi: 10.1093/aje/kwu011. [DOI] [PubMed] [Google Scholar]
- 3.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Manschot A, van Oostrom SH, Smit HA, Verschuren WM, Picavet HS. Diagnosis of diabetes mellitus or cardiovascular disease and lifestyle changes - the Doetinchem cohort study. Prev Med. 2014;59:42–6. doi: 10.1016/j.ypmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 6.Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: Results from the Nurses’ Health Study. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. doi: 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine. 2013;80(1):29–33. doi: 10.1016/j.jbspin.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 9.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(1):36–45. doi: 10.1002/acr.22642. [DOI] [PubMed] [Google Scholar]
- 10.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16(2):R61. doi: 10.1186/ar4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph RM, Movahedi M, Dixon WG, Symmons DP. Smoking-related mortality in patients with early rheumatoid arthritis - a retrospective cohort study using the Clinical Practice Research Datalink. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001–11. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 22.Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse Cumulative Impact of Chronic Diseases on Physical Health-Related Quality of Life: Implications for a Measure of Multimorbidity. Am J Epidemiol. 2016;184(5):357–65. doi: 10.1093/aje/kwv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 25.Mikuls TR, Saag KG, Criswell LA, Merlino LA, Kaslow RA, Shelton BJ, et al. Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women’s Health Study. Ann Rheum Dis. 2002;61(11):994–9. doi: 10.1136/ard.61.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuller LH, Mackey RH, Walitt BT, Deane KD, Holers VM, Robinson WH, et al. Determinants of mortality among postmenopausal women in the women’s health initiative who report rheumatoid arthritis. Arthritis Rheumatol. 2014;66(3):497–507. doi: 10.1002/art.38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks JA, Chang SC, Deane KD, Gan RW, Demoruelle MK, Feser ML, et al. Associations of smoking and age with inflammatory joint signs among first-degree relatives without rheumatoid arthritis: Results from the Studies of the Etiology of RA. Arthritis Rheumatol. 2016 doi: 10.1002/art.39630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokkonen H, Brink M, Hansson M, Lassen E, Mathsson-Alm L, Holmdahl R, et al. Associations of antibodies against citrullinated peptides with human leukocyte antigen-shared epitope and smoking prior to the development of rheumatoid arthritis. Arthritis Res Ther. 2015;17:125. doi: 10.1186/s13075-015-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1654–8. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Jiang X, Cui J, Lu B, Costenbader KH, Sparks JA, et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol. 2015;67(10):2611–23. doi: 10.1002/art.39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41(12):1319–24. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 32.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 34.Di Giuseppe D, Orsini N, Alfredsson L, Askling J, Wolk A. Cigarette smoking and smoking cessation in relation to risk of rheumatoid arthritis in women. Arthritis Res Ther. 2013;15(2):R56. doi: 10.1186/ar4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saevarsdottir S, Rezaei H, Geborek P, Petersson I, Ernestam S, Albertsson K, et al. Current smoking status is a strong predictor of radiographic progression in early rheumatoid arthritis: results from the SWEFOT trial. Ann Rheum Dis. 2015;74(8):1509–14. doi: 10.1136/annrheumdis-2013-204601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restrepo JF, del Rincon I, Battafarano DF, Haas RW, Doria M, Escalante A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol. 2015;34(9):1529–36. doi: 10.1007/s10067-015-3025-8. [DOI] [PubMed] [Google Scholar]
- 37.Inoue Y, Nakajima A, Tanaka E, Inoue E, Kobayashi A, Hoshi D, et al. Effect of Smoking on Remission Proportions Differs Between Male and Female Patients with Rheumatoid Arthritis: A Study Based on the IORRA Survey. J Rheumatol. 2015;42(7):1083–9. doi: 10.3899/jrheum.140376. [DOI] [PubMed] [Google Scholar]
- 38.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chehata JC, Hassell AB, Clarke SA, Mattey DL, Jones MA, Jones PW, et al. Mortality in rheumatoid arthritis: relationship to single and composite measures of disease activity. Rheumatology (Oxford) 2001;40(4):447–52. doi: 10.1093/rheumatology/40.4.447. [DOI] [PubMed] [Google Scholar]
- 40.England BR, Sokolove J, Robinson WH, Thiele GM, Ganti AK, Sayles H, et al. Associations of circulating cytokines and chemokines with cancer mortality in men with rheumatoid arthritis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39735. [DOI] [PubMed] [Google Scholar]
- 41.Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a Rheumatoid Arthritis-Specific Smoking Cessation Programme; a Pilot Randomized Controlled Trial. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22960. [DOI] [PubMed] [Google Scholar]


