Abstract
The hippocampus and the medial prefrontal cortex (mPFC) are traditionally associated with regulating memory and executive function, respectively. The contribution of these brain regions to food intake control, however, is poorly understood. The present study identifies a novel neural pathway through which monosynaptic glutamatergic ventral hippocampal field CA1 (vCA1) to mPFC connectivity inhibits food-motivated behaviors through vCA1 glucagon-like peptide-1 receptor (GLP-1R). Results demonstrate that vCA1-targeted RNA interference-mediated GLP-1R knockdown increases motivated operant responding for palatable food. Chemogenetic disconnection of monosynaptic glutamatergic vCA1 to mPFC projections using designer receptors exclusively activated by designer drugs (DREADDs)-mediated synaptic silencing ablates the food intake and body weight reduction following vCA1 GLP-1R activation. Neuropharmacological experiments further reveal that vCA1 GLP-1R activation reduces food intake and inhibits impulsive operant responding for palatable food via downstream communication to mPFC NMDA receptors. Overall these findings identify a novel neural pathway regulating higher-order cognitive aspects of feeding behavior.
Keywords: GLP-1, ventral hippocampus, reward, feeding, obesity, impulsivity
Introduction
The hippocampus and the medial prefrontal cortex (mPFC) are brain regions associated with the “higher-order” control of memory and executive function. Recent findings reveal that these brain regions are structurally and functionally interconnected 1–4. For example, ventral hippocampus (vHP) inputs to the prelimbic (PL) and infralimbic (ILA) regions of the mPFC are involved in the control of contextual fear conditioning and pharmacological antidepressant responses 3, 5, 6. However, the contributions of the vHP to mPFC neural pathways in controlling other fundamental motivated behaviors are less understood. Emerging research reveals that hippocampal and mPFC neural processes independently have a critical impact on energy balance and food-motivated behaviors 7–14. For example, ventral hippocampus neural activity has potent effects on several aspects of feeding behavior through integration of endocrine feeding signals (e.g., ghrelin, leptin) with external environmental and interoceptive stimuli 14. Similarly, the PL and ILA regions of the mPFC control feeding through dopaminergic and opioid systems 15–17 and regulate environmental cue-driven feeding 12, 13, 18 and impulsive responding for food 19–21. Whether a vHP to mPFC neural pathway orchestrates higher-order and cognitive aspects of feeding behavior is unknown.
Feeding behavior is regulated, in part, via various peripherally-derived endocrine and centrally-derived neuropeptidergic signals 22, 23 that communicate to neurons in the hypothalamus and caudal brainstem 24. Glucagon-like peptide-1 (GLP-1) is a hormone that is primarily derived from the distal small intestine and from neurons in nucleus tractus solitarius (NTS) of the hindbrain 25. Peripheral and central activation of GLP-1 receptors (GLP-1R) produces glucose-dependent insulin secretion and reduces food intake and body weight 26–30. Importantly, GLP-1 analogs were recently FDA approved for weight loss and this system is of high interest for the development of future anti-obesity pharmacotherapies 29, 31, 32. Current research into the neural substrates mediating GLP-1R induced hypophagia has expanded from traditional lower-order hypothalamic and hindbrain “feeding centers” 33–36 to include structures within the mesolimbic reward system and other forebrain sites 37–42. For example, GLP-1Rs are expressed in the vHP 43, 44 and recent work from our laboratory demonstrates that pharmacological activation of vHP GLP-1Rs potently reduces caloric intake and body weight45. The extent to which endogenous vHP GLP-1R signaling controls food-motivated behaviors, however, is unknown. Moreover, the neural circuits through which the GLP-1R system suppresses motivated responding for palatable food have not been investigated.
The present study examines whether vHP GLP-1R signaling engages downstream mPFC signaling to control higher-order aspects of feeding behavior. Specifically, we hypothesize the presence of a novel functional neural pathway whereby vHP field CA1 (vCA1) glutamatergic pyramidal neurons are activated by GLP-1R agonists, which in turn engages downstream activation of mPFC glutamate receptors to inhibit motivated and impulsive responding for palatable food. This hypothesis is investigated using vHP targeted RNA-interference (RNAi) mediated GLP-1R knockdown, classic and virally-mediated neural pathway tracing, fluoroscent in situ hybridization, and pathway-specific synaptic silencing via inhibitory DREADDs (Designer Receptor Exclusively Activated by Designer Drugs) in combination with behavioral neuropharmacology.
Materials and Methods
Animals
Male Sprague-Dawley rats (Envigo, Indianapolis, IN; PND 60; 320–450g) were individually housed in a temperature controlled vivarium with ad libitum access (except where noted) to water and food (LabDiet 5001, LabDiet, St. Louis, MO) on either 12h:12h light/dark cycle or 12h:12h reverse light/dark cycle. For all surgical procedures, rats were anesthetized via IM ketamine 90mg/kg and xylazine, 2.8mg/kg and acepromazine, 0.72 mg/kg injections, followed by SC injections for analgesia (Ketoprofen, 5mg/kg). All procedures were approved by the Institute of Animal Care and Use Committee at the University of Southern California.
Viral preparations and central drug injections
Central drug injections
Experiments involving central drug injections included unilateral vHP injections of exendin-4 (American Peptide) and mPFC injections of clozapine-n oxide (CNO, National Institutes of Health), AP-5 (Sigma), or CNQX (R&D Systems). Drugs were dissolved in artificial cerebral spinal fluid (aCSF) except for CNO, which was dissolved in 33% DMSO and 66% aCSF. Drugs were administered using a microinfusion pump (Harvard Apparatus, Holliston, MA) connected to a 33-gauge microsyringe injector through the indwelling guide cannulae. Flow rate was calibrated and set to 5μl/min and 100nl injection volume. Injectors were left in place for 30-sec to allow for complete infusion of the drug. See Supplementary Materials for further details regarding stereotaxic placement of vHP and mPFC guide cannulae and central drug administration.
AAV-mediated RNA interference for GLP-1R
For in vivo knockdown of GLP-1R expression, short hairpin RNA targeting GLP-1R mRNA was cloned and packaged into an adeno-associated virus (AAV1) under the control of a U6 promoter and co-expressing green fluorescent protein (GFP) downstream of a Cb7 promoter (titer = 5.22e12), described in more detail in 46, 47. A GFP-expressing AAV1 downstream of a Cb7 promoter (titer = 5.22e12) was used as a control. AAVs or aCSF were then delivered bilaterally to the vHP (AP: −4.9 ML: +/− 4.8 DV: −7.8) at an injection volume of 200nl via pressure injection with the microinfusion pump setup described above.
Following behavioral procedures (~60 days), animals were euthanized and brains were removed and flash frozen in dry-ice cooled hexane until processing as described in 47, 48. Tissues processing involved simultaneous histological verification and region of interest tissue micropunch collection (see Supplementary Materials); collected vHP tissue was then used for qPCR analyses for GLP-1R mRNA, described in more detail in Supplementary Materials.
AAV2-CaMKIIa hM4di DREADDs
CaMKIIa-promoted hM4di DREADDs (AAV2-CaMKIIa-HA-hM4Di-IRES-mCitrine) were obtained from the UNC Gene Therapy Vector Core. Animals received chronic indwelling cannulae targeting the vHP and mPFC. Following recovery, animals received 200nl pressure injections of the AAV-CaMKIIa-hM4di DREADDs or a control virus (AAV2-CaMKIIa-GFP) to the vHP through the indwelling guide cannulae.
Immunohistochemistry (IHC)
Animals were anesthetized, then transcardially perfused with ice-cold 0.9% saline, followed by 4% paraformaldehyde in 0.1M borate buffer (pH 9.5). Brains were removed and immersed in 12% sucrose in PFA fixative for 20–24 hr at 4°C. The brains were then flash-frozen in dry-ice cooled isopentane before sectioning on a sliding microtome at 30 μm. Sections are stored in antifreeze solution at −20°C until IHC processing. IHC processing details are described in Supplementary Materials. Primary and secondary antibodies utilized are described in each experiment below. Photomicrographs were acquired using a Nikon 80i (Nikon DS-QI1, 1280×1024 resolution, 1.45 megapixel) under epifluorescent illumination.
Fluorescent in situ hybridization (FISH) for GLP-1R
Animals were fixation perfused and tissue was harvested and processed as described above in IHC methods. Flash-frozen brains were sectioned as above at 20 μm and stored at −20°C until FISH processing. Detailed FISH procedures are described in Supplementary Materials.
Anterograde neural pathway tracing
Neural pathway tracing experiments utilized two tracing strategies: anterograde tracing from the vHP field CA1 following unilateral iontophoretic injections of PHAL (Vector Labs, Burlingame, CA; 2.5% in 0.1 M sodium phosphate-buffered saline, pH 7.4) or AAV1-GFP (AAV1-hSyn-eGFP-WPRE-bGH) 49–51 (Penn Vector Core). Iontophoresis was performed using a precision current source (digital midgard precision current source, Stoelting) as described previously 52.
Experiment 1: the effects of vHP GLP-1R knockdown on energy balance and food-motivated behaviors
Animals (n=32) received one of the following 200nl, bilateral injections to the vHP as described above: AAV-GLP-1R shRNA (referred to as vHP GLP-1R KD group; n=11), control AAV (n=11), or aCSF (n=10). After surgical procedures, food intake (spillage accounted for) and body weight were tracked daily for 40 days. Next, a representative subset of animals (based on representing median body weight group averages from the viral groups) (vHP GLP-1R KD n=6, control AAV n=6) underwent meal pattern analyses in chambers equipped with automated food intake monitors (Med Associates). aCSF controls were not included in this analyses because of equipment limitations at the time of the study, however this group of animals did not differ in GLP-1R mRNA expression or other behavioral measures from the control AAV control group (Fig. 1). The automated food intake monitors records the weight of the food hopper every 10 sec, allowing for ongoing analysis of meal patterns. Animals were first habituated to the chambers for 24 hr with ad libitum access to food and water. Analyzed data are from a second 24 hr period immediately following the habituation session. Meals were defined as an episode of feeding where at least 0.25g was ingested, with meal termination defined as the beginning of a pause in ingestion of at least 10min 45. Data were objectively calculated with a custom Microsoft Excel macro 45.
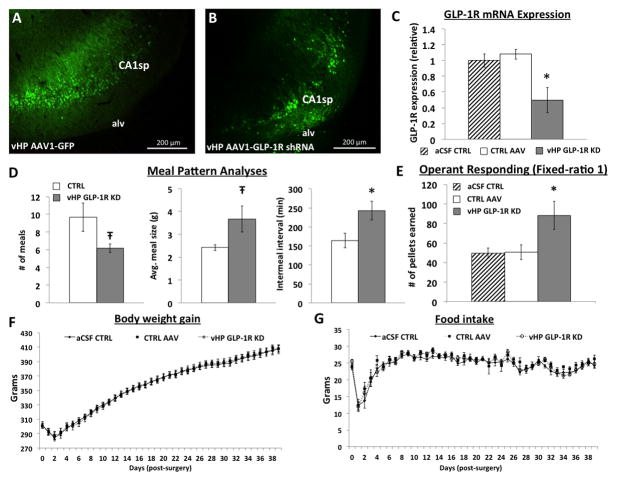
Figure 1.
vHP targeted A) AAV-GFP and B) AAV-GLP-1R shRNA delivery transfects vHP neurons. C) vHP AAV-GLP-1R shRNA significantly reduces GLP-1R mRNA expression. D) Meal pattern analyses in vHP GLP-1R KD vs. CTRL AAV rats. E) Operant responding on a FR1 reinforcement schedule in vHP GLP-1R KD vs. CTRL animals. F) Body weight gain and G) food intake in vHP GLP-1R KD vs. CTRL groups. CA1sp: CA1 pyramidal cells; alv = alveus. *p<0.05 vs CTRL AAV or aCSF controls; Ŧp<0.07. Data are mean ± SEM.
To measure food motivated behaviors, animals (vHP GLP-1R KD n=10, control AAV n=11, aCSF control n=9) were trained to lever press in operant conditioning boxes (Med Associates) for a palatable 45mg food pellet (35% kcal fat enriched with sucrose, F05989, Bio-Serv) on a FR1 reinforcement schedule. Training occurred in 1 hr sessions over a 5-day period. This procedure included one “active” lever (right) that produced the food reinforcement, and a left “inactive” lever that served as a dummy lever for the measurement of general lever pressing activity.
Experiment 2: Investigation of vHP projections to the mPFC
Rats received either a unilateral iontophoretic injection of PHAL (n=4) or AAV1-GFP (n=2) targeting the vHP. Following a survival period (10 days for PHAL and 14 days for AAV1-GFP), animals were fixation-perfused and tissue was harvested and processed as described above. IHC detection of PHAL proceeded according to the described procedures using goat anti-PHAL primary antibody (1:5000, Vector Labs), followed by a donkey anti-goat secondary antibody conjugated to Cy3 (1:500, Jackson Immunoresearch). AAV1-GFP was detected based on a combination of native fluorescence of GFP and amplification of signal using a chicken anti-GFP primary antibody (1:500, Abcam) followed by a donkey anti-chicken secondary antibody conjugated to AF488 (1:500, Jackson Immunoresearch). Representative images were obtained from animals with PHAL or AAV1-GFP injection sites that were most confined to the vCA1.
Experiment 3: Investigation of mPFC inputs from vHP GLP-1R expressing neurons
Rats (n=4) received a unilateral 100nl pressure injection of fluorogold (FG, Fluorochrome LLC; 2% in 0.9 % NaCl) targeting the mPFC. Following a survival period of 10 days, animals were fixation-perfused and tissue was harvested and processed as described in the Methods section. A representative animal with fluorogold back-labeled neurons in the vHP (field CA1) was selected for GLP-1R FISH analyses (See Methods and Supplementary materials). In order to observe separate GLP-1R mRNA in vHP cell bodies (as fluorogold and DAPI autofluoresce in the same channel), the protocol above was repeated in a separate untreated animal where DAPI (ACD, 320851) was placed on the slide prior to coverslipping.
Experiment 4: The effects of vHP to mPFC disconnection with inhibitory DREADDs on vHP GLP-1R mediated feeding
Rats were implanted bilaterally with vHP and mPFC-targeted cannulae (n=10) and received injections of AAV2-CaMKIIa-hM4Di DREADDs to the vHP as described above. After an incubation period of 30 days, animals were given unilateral injections of CNO (3mM) or vehicle to the mPFC, which was followed by unilateral vHP administration of exendin-4 (0.03μg) 1 hr into the light cycle. Food intake (spillage accounted for) and body weight were measured 24 hrs later. This study utilized a counter-balanced within-subjects design with 2–3 intervening days between treatments. Doses for CNO and exendin-4 were based on 53, 54 and 45 respectively. As a control experiment, a separate cohort of animals (n=7), received vHP AAV2-CaMKIIa-GFP and the design above was replicated.
Experiment 5: Investigating the effects of mPFC glutamate receptor blockade on vHP GLP-1R mediated food intake
Rats (n=11) were implanted bilaterally with vHP and mPFC-targeted cannulae. 1 hr into the light cycle, a NMDA receptor antagonist, AP-5 (0.5μg), or vehicle was delivered unilaterally into the mPFC, which was then followed by vHP exendin-4 (0.03μg) or vehicle delivery. Food intake (spillage accounted for) and body weight were measured 24 hrs later. This study utilized a counter-balanced within-subjects design with 2–3 intervening days between treatments. This design was then replicated in a separate cohort of rats (n=9) using an AMPA/Kainate receptor antagonist, CNQX (0.3μg), delivered to the mPFC. Dose selection for AP-5 and CNQX are based on 40.
Experiment 6: Investigating the molecular mechanisms underlying vHP GLP-1R to mPFC NMDA-R signaling
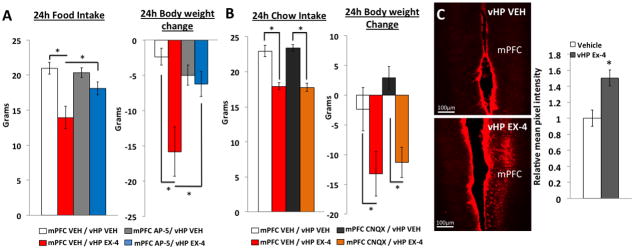
Animals received unilateral vHP delivery of either exendin-4 (0.03μg, n=6) or vehicle (n=6). 90min later, animals were perfused and tissue was harvested as described above. IHC detection of phosphorylated NR2B (pNR2B) was performed according to the procedures above using rabbit anti-pNR2B (1:1000, Abcam) followed by a donkey anti-rabbit secondary antibody conjugated to biotin. The tissue was incubated in ABC reagent (1:1000, Vector) for 5 hours, washed in KPBS, and then incubated in biotinylated tyramine (1:1000) for 10 minutes. The tissue is then incubated overnight in a Cy3-conjugated streptavidin (1:500) complex. Quantification of pNR2B signal intensity was performed using ImageJ v1.48, where the mPFC region of interest was selected and mean pixel intensity was determined for each animal. Data are represented as the mean pixel intensity relative to the vehicle group.
Experiment 7: The effects of mPFC NMDA receptor blockade on the vHP GLP-1R regulated food impulsivity behavior
To measure food impulsivity behaviors, we adapted protocols for a task known as “differential reinforcement of low rates of responding” (DRL) 20, 21, 55. Briefly, rats (n=10) were trained to lever press in operant conditioning boxes (Med Associates) for a palatable 45mg food pellet (35% kcal fat enriched with sucrose, F05989, Bio-Serv) on a DRL 0 reinforcement schedule, where animals are not required to wait an allotted time between lever presses and are reinforced immediately upon a lever press. After 5 days of DRL 0, animals are then placed on DRL 5. Here, animals are required to withhold responding for 5 sec before the subsequent lever press response results in food reinforcement. A lever press that occurs before the 5 sec period resets the timer and no reinforcement is provided. After 5 days of DRL 5, animals underwent 5 days of DRL 10 (10 sec between reinforced lever presses), and finally 10 days of DRL 20 (20 sec between reinforced lever presses). During all 45min training sessions, a stimulus light turned on for reinforced lever presses. Efficiency in each DRL training schedule was calculated by the number of pellets earned/total number of lever presses.
Two days after the last day of DRL 20 training, animals were given 4 tests (within-subjects design, 2–3 intervening days). Prior to each test, animals were 24 hr food deprived to induce high-impulsive drive 21. Unilateral mPFC injections of AP-5 (0.5μg) or vehicle and subsequent unilateral vHP injections of exendin-4 (0.03μg) were given 3 hrs before being tested in the DRL 20 task.
Data analysis
All experiments, except for GLP-1R qPCR analyses, met assumptions of normality and equality of variance and were analyzed using parametric repeated measures or one-way analysis of variance (ANOVA) followed by Fisher least significant difference post hoc comparisons when significant main effects were obtained to compare individual treatments with control groups. When assumptions were not met, non-parametric Mann-Whitney tests were used. Data analyses were conducted with computer software (Statistica V7; Statsoft). The α level for significance was 0.05. All quantifiable data are displayed as mean +/− SEM. To ensure the minimum sample size required to be reasonably likely to detect the hypothesized effect, power analyses were conducted for each proposed experiment based on our pilot and recently published experiments using Statsoft software (Statistica 64, V7). Alpha level was set at 0.05 for power analyses; the Root Mean Square Standardized Effect (RMSSE) was estimated separately for each experiment.
Results
Endogenous vHP GLP-1R signaling regulates feeding and food motivated behaviors
To examine the relevance of endogenous vHP GLP-1R-mediated feeding and palatable food-motivated responding, animals received bilateral vCA1 administration of AAV1-GLP-1R shRNA (vHP GLP-1R KD; n=11), AAV1-GFP (AAV control; n=10), or artificial cerebral spinal fluid (aCSF control; n=10). The latter control group is necessary in light of recent evidence that under some conditions AAVs can be neurotoxic 56, and thus the control/scrambled AAV treatment can potentially have unintended neural or behavioral outcomes. However, in this case the aCSF control and AAV control groups did not differ significantly for any of the dependent variables (all p values > 0.4). Following experimental procedures (~60 days post-surgery), histological analyses confirmed successful transfection of both AAV-GFP and AAV-GLP-1R shRNA in vCA1 neurons (visualized with GFP, Figure 1A–B). One animal was omitted from analyses because the injection site did not include vCA1 and one KD outlier animal was removed based on excessive GLP-1R mRNA expression. Consistent with recently published work utilizing this AAV-GLP-1R shRNA knockdown approach 46, 47, qPCR analyses of vHP AAV-GLP-1R shRNA transfected tissue showed a significant (~50%) reduction of GLP-1R mRNA compared to either CTRL AAV or aCSF controls (Figure 1C, F(1,16)=8, p<0.01). vHP GLP-1R KD had no significant effects on long term food intake and body weight during the 5-week post-surgery period (Figure 1F–G). However, microstructural feeding analyses revealed that vHP GLP-1R KD alters meal patterning, evidenced by a trend towards greater meal size ( F(1,10)=4.5, p<0.07) with a compensatory decrease in meal frequency (F(1,10)=4.7, p<0.07) in the vHP GLP-1R KD group (n=6), as well as a significant increase in inter-meal interval (F(1,10)=6.3, p<0.05) compared to AAV-controls (n=6) (Figure 1D). No group differences were detected for satiety ratio, calculated as inter-meal interval/meal frequency (data not shown).
To examine the endogenous relevance of vHP GLP-1R signaling on food motivated behaviors, rats were trained to lever press for a palatable 45mg food pellet (35% kcal fat enriched with sucrose) on a fixed ratio-1 reinforcement (FR1) schedule. Results show a significant main effect of group (F(2,26)=4.39, p<0.05) where vHP GLP-1R KD animals (n=10) earned a significantly greater number of pellets across a 5-day training period compared to either CTRL AAV (n=11) or aCSF controls (n=9) (p<0.05) (Figure 1E). Collectively, these data indicate that vHP GLP-1R signaling endogenously regulates meal patterning and effort-based responding for palatable food.
The vCA1 sends a monosynpatic input to the ipsilateral ILA and PL regions of the mPFC
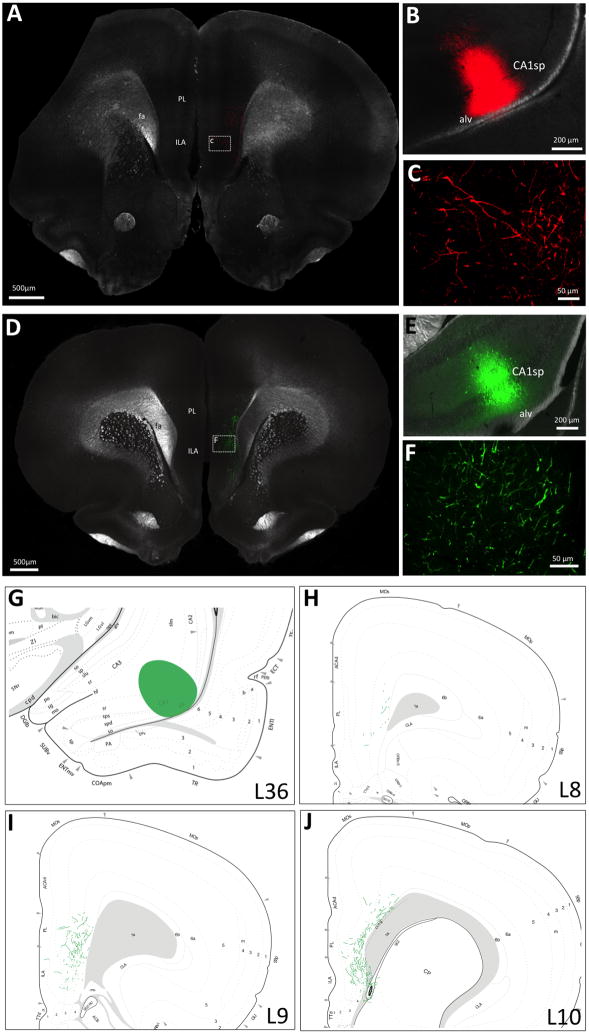
Extending the findings of previous work1, 2, our results reveal an exclusively ipsilateral monosynaptic connection from the vHP to the mPFC (ILA and PL regions) using both traditional and virus-based anterograde pathway tracing methods. Animals received a unilateral vHP field CA1 (vCA1) iontophoretic injection of the anterograde pathway tracer Phaseolus vulgaris leocoagglutinin (PHAL) or an AAV that labels axons in an anterograde fashion (AAV1-hSyn-eGFP-WPRE-bGH; henceforth referred to as “AAV1-GFP”) (representative injection sites depicted in Figure 2B and E). This AAV is used by the Allen Institute for Brain Science 51 and in the literature 50 for anterograde tracing purposes. Results demonstrate extensive PHAL immunoreactive and AAV1-GFP axon terminal fields in the PL and ILA that were exclusively ipsilateral to the vCA1 injection (Figure 2A and C; D and F). Figure 2G–J displays a schematic representation of the distribution AAV1-GFP axon terminal fields in the mPFC. We note that while previous reports have investigated vHP to mPFC projections 1, here we examine a novel vCA1 subregion that is caudal and lateral to previously investigated vHP anterograde projection pathways. Moreover, this is the first report to our knowledge to directly compare PHAL with AAV1-GFP anterograde neural pathway tracing from the same injection area.
Figure 2.
vCA1 neurons provide monosynaptic and unilateral input to the mPFC. Unilateral iontophoretic vCA1 delivery of either PHAL (red) or AAV1-GFP (green) (representative injection sites in B and E respectively) reveals A, C) PHAL and D, F) AAV1-GFP fluorescent axon terminal fields in the mPFC ipsilateral to the vCA1 injection. A schematic representation of mPFC AAV1-GFP axon field terminals from the vHP is shown in G-J, where G) displays a representative vCA1 AAV1-GFP injection site and H-J) shows mPFC AAV1-GFP axon terminal field distribution (Swanson atlas levels 8–10). CA1sp: CA1 pyramidal layer; alv = alveus; PL = prelimbic area, ILA = infralimbic area; fa = corpus callosum, anterior forceps.
vHP GLP-1R expressing neurons project to the mPFC
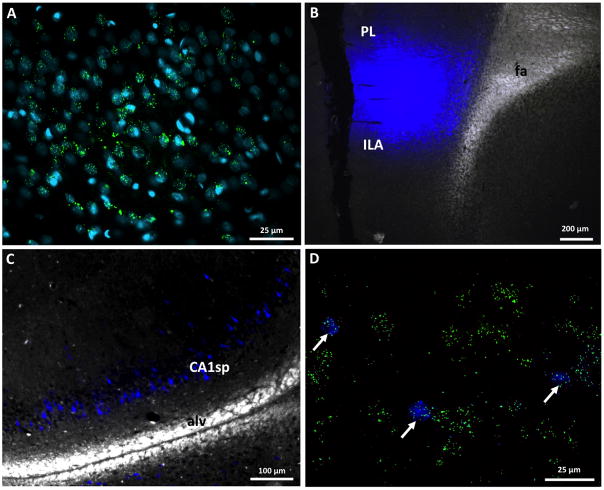
We detected GLP-1R mRNA expression in vHP (field CA1) pyramidal layer neurons (Figure 3A) via fluorescent in situ hybridization (FISH), an outcome consistent with previous work utilizing a mouse model with transgenic GFP expression in GLP-1R-expressing neurons 44 and with a publication demonstrating radio-labeled in situ hybridization signal in this region43. In all rats that received mPFC fluorogold (FG) injections (n=4; representative injection site in Figure 3B), extensive FG expression in back-labeled vHP neurons was observed (representative FG-labeled cell bodies in Figure 3C). These data support the presence of a monosynaptic pathway between the vCA1 and mPFC, as also suggested in Figure 2. Moreover, vCA1 FISH analyses combined with native FG immunoreactivity from a representative mPFC FG-injected animal revealed that all FG back-labeled vCA1 cell bodies co-expressed GLP-1R mRNA (Figure 3D). Collectively, these data provide proof-of-principle evidence that vCA1 neurons express GLP-1Rs, and that mPFC-projecting vCA1 neurons are predominantly GLP-1R positive.
Figure 3.
A) GLP-1R mRNA expression (green) in vCA1 cell bodies (DAPI, teal). B) Representative mPFC fluorogold injection site C) Distribution of backlabeled fluorogold vCA1 neurons that project to the mPFC. D) Representative vCA1 neurons that project to the mPFC (fluorogold, blue) co-express GLP-1R mRNA (green).
Reversible chemogenetic disconnection of vHP to mPFC neural circuitry abolishes vHP GLP-1R mediated food intake reduction
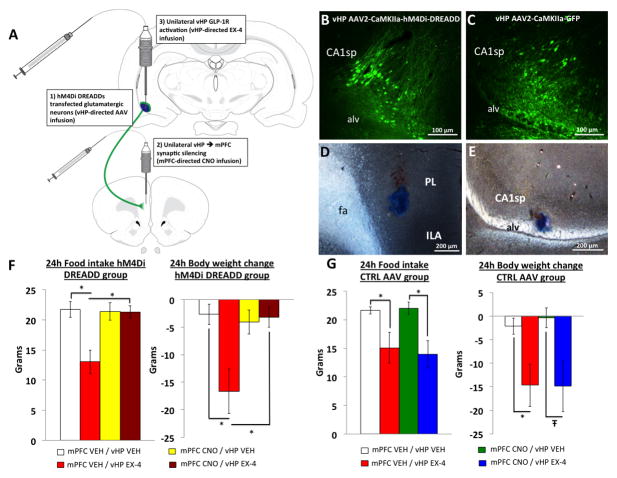
Taking advantage of the ipsilateral monosynaptic connection between the vHP GLP-1R expressing neurons and the mPFC, we utilized an hM4Di inhibitory DREADDs technique to silence synaptic communication to the mPFC from the vHP to investigate the functional relevance of this circuit to food intake. Recent findings show that AAV-hM4Di DREADDs are trafficked to axon terminals, and administration of the DREADDs ligand, clozapine-n oxide (CNO) targeted to axon terminals silences synaptic communication to post-synaptic cells without affecting the firing rate of the presynaptic neurons53, 54. Utilizing the design outlined schematically in Figure 4A, animals (n=10) first received AAV2-CaMKIIA-hM4Di DREADDs in the vCA1, where the CamKIIa promoter allows for the selective expression of hM4Di in glutamatergic pyramidal neurons in the vCA1 (Figure 4B). Using a counterbalanced, within-subjects design, vHP hM4Di DREADDs-expressing animals received unilateral CNO (3mM) or vehicle (VEH) targeted to the ILA and PL (representative injection site in Figure 3D following 100nl Chicago Skyblue ink administration). Rats then received unilateral administration of the selective GLP-1R agonist, exendin-4 (EX4), or vehicle to the ipsilateral vCA1 (representative injection site in Figure 3E) and 24hr food intake and body weight were recorded. The dose of EX4 (0.03μg) was selected with reference to previous work 45 showing it to be without effects on feeding and body weight when delivered to the lateral ventricle. Results reveal a significant main effect of drug (F(3,27)>4.4, p<0.01), where the food intake and body weight reduction that is observed with vHP GLP-1R activation (Figure 4F, mPFC VEH/vHP VEH vs. mPFC VEH/vHP EX4, p<0.05), is eliminated when synaptic communication from the vHP to the mPFC is disconnected via mPFC CNO delivery (Figure 4F, mPFC VEH/vHP EX4 vs. mPFC CNO / vHP EX4, p<0.05). In contrast, animals receiving a control virus (AAV2-CaMKIIa-GFP; representative transfection in Figure 3C) in the vHP retain the food intake and body weight reduction resulting from vHP GLP-1R activation when pre-treated with CNO (Figure 4G, mPFC CNO/vHP VEH vs. mPFC CNO/vHP EX4, F(3,18)=5.5, p<0.05; body weight mPFC CNO/vHP VEH vs. mPFC CNO/vHP EX4, F(3,18)=4.2, p<0.07). Taken together, these data suggest that a monosynaptic neural pathway from the vHP to the mPFC is critical in regulating GLP-1R mediated food intake.
Figure 4.
Reversible vHP to mPFC disconnection with inhibitory DREADDs. A) Shows the experimental design involving DREADDs mediated vHP-mPFC synaptic disconnection and pharmacological activation of vHP GLP-1Rs with exendin-4. B) AAV2-CaMKIIa hM4Di DREADDs and C) AAV2-CaMKIIa-GFP transfects vHP pyramidal neurons. D) Representative mPFC and E) vHP injection sites. F) 24 hr food intake and body weight following mPFC administration of CNO or vehicle (VEH) and vHP administration of exendin-4 (EX4) or VEH in vHP hM4Di DREADDs transfected animals. G) 24hr food intake and body weight following mPFC administration of CNO or VEH and vHP administration of EX4 or VEH in vHP CTRL AAV animals. *p<0.05; Ŧp<0.07. Data are mean ± SEM.
vHP GLP-1R-mediated hypophagia requires mPFC NMDA receptor signaling
We next examined the role of different glutamate receptor subtypes in the mPFC in vHP-GLP-1R-mediated feeding. To determine the functional relevance of mPFC NMDA receptors (NMDAR) in the vHP GLP-1R-mediated hypophagic response, rats (n=11) first received a unilateral mPFC injection of a NMDA receptor antagonist, AP-5 (0.5μg) or vehicle. Immediately afterwards, animals received unilateral and ipsilateral vHP injections of EX4 (0.03μg). Treatments were counterbalanced using a within-subjects design. Results showed a main effect of drug (F(3,30)=9.8, p<0.01), where vHP EX4 administration significantly reduced food intake and body weight when animals were pretreated with mPFC vehicle (Figure 5A, p<0.01). In contrast, pretreatment with mPFC AP-5 resulted in the attenuation of vHP EX4 induced food intake and body weight reduction (Figure 5A, mPFC VEH/vHP EX4 vs. mPFC AP-5/vHP EX4, p<0.01). Utilizing a similar experimental design (n=9), mPFC pretreatment with an AMPA/Kainate receptor antagonist, CNQX (0.3μg) had no effect on the food intake and body weight reduction induced by vHP exendin-4 administration (main effect of drug, F(3,18)=6.3, Figure 5B, mPFC CNQX/vHP VEH vs. mPFC CNQX/vHP EX4, p<0.05). These data indicate that the full expression of vHP GLP-1R mediated feeding requires mPFC NMDA-R signaling, but not AMPA/Kainate receptor signaling. Consistent with these findings, unilateral vHP EX4 (n=6; 0.03μg) administration significantly increased phosphorylation of NR2B, an NMDA-R subunit, in the mPFC 90 minutes after injection compared to vehicle treatment (n=6; Figure 5C, F(1,10)=12.6, p<0.01). Overall these data provide behavioral, neuroanatomical, and molecular evidence consistent with the hypothesis that mPFC NMDA receptors (NMDA-R) are a downstream target for vHP GLP-1R-mediated anorectic effects.
Figure 5.
A) 24 hr food intake and body weight following mPFC administration of AP-5 or vehicle (VEH) and vHP administration of exendin-4 (EX-4) or VEH. B) 24 hr food intake and body weight following mPFC administration of CNQx or VEH and vHP administration of EX-4 or VEH. C) Phosphorylated NR2B expression in the mPFC following unilateral vHP delivery of exendin-4 or vehicle. *p<0.05. Data are mean ± SEM.
vHP GLP-1R to mPFC NMDA-R neural signaling inhibits impulsive responding for palatable food
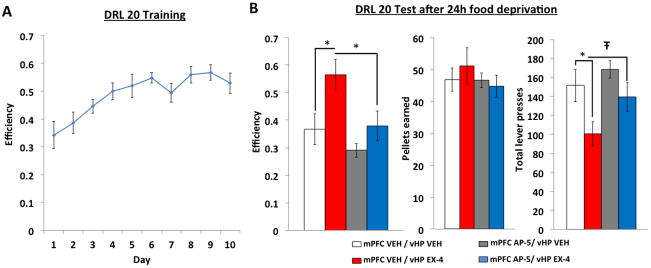
To investigate the role of a vHP GLP-1R to mPFC NMDA-R neural pathway in food impulsivity, we utilized a behavioral procedure known as “differential reinforcement of low rates of responding” (DRL) as a measure of inhibitory control 20, 21, 55. Briefly, animals (n=10) first learn to lever press for a palatable food reward (35% kcal fat enriched with sucrose). Following a series of training phases with escalating DRL delay criteria, rats acquired stable responding after 10 days of DRL 20 schedule, where lever presses are only reinforced if an animal waits 20 sec between lever presses. Responses made before the 20 sec period ends result in no food reinforcement and the timer is reset. Our data show that animals increase their efficiency (measured by pellets earned/total number of lever presses) in this task across a 10 day DRL 20 training period (Figure 6A, significant main effect of day, F(9,108)=11.9, p<0.01).
Figure 6.
A) DRL 20 efficiency across a 10 day training period. B) DRL 20 test session after 24 hr food deprivation in animals receiving mPFC AP-5 or vehicle and vHP exendin-4 or vehicle. *p<0.05; Ŧp<0.07. Data are mean ± SEM.
During test sessions in which impulsive food drive is upregulated via 24 hr food restriction 21, results show that vHP EX4 administration (0.03μg) alone significantly increases efficiency in the DRL 20 task (main effect of drug, F(3,30)=6.3, p<0.01) (Figure 6B, mPFC VEH/vHP VEH vs. mPFC AP-5/vHP EX4, p<0.01), suggesting that vHP GLP-1R activation reduces impulsive responding for food. Importantly, this effect is generated by a significant decrease in total lever presses (Figure 6B, mPFC VEH/vHP VEH vs. mPFC VEH/vHP EX4, p<0.05) with no differences in pellets earned, suggesting that vHP GLP-1R signaling did not entirely suppress appetitive drive, but rather increased the efficiency in earning food reinforcement. When combined with mPFC NMDA-R blockade via AP-5 pre-treatment, the increased efficiency elicited by vHP GLP-1R activation was significantly attenuated (Figure 6B, mPFC VEH/vHP EX4 vs. mPFC AP-5/vHP EX4, p<0.01), as demonstrated by a trend towards an increase in total lever presses for the mPFC AP-5/vHP EX4 treatment compared to mPFC VEH/vHP EX4 (p<0.07) with no differences found in the number of pellets earned. Together these data suggest that a vHP GLP-1R to mPFC NMDA-R neural pathway regulates impulsive responding for palatable food.
Discussion
The hippocampus is known for its role in regulating mnemonic processes, including those relating to declarative and visuospatial memory. More recently this brain region has emerged as an important center for controlling higher-order aspects of feeding behavior 8, 14. Hippocampal neurons respond to several types of gastrointestinal satiation signals 57, 58 and utilize endocrine signals to bi-directionally control energy balance and conditioned food-motivated behaviors 14. Using vHP-targeted RNA interference to chronically knockdown vHP gene expression of GLP-1R (a G protein-coupled receptor of clinical relevance for obesity), the present findings extend previous work by providing evidence that vHP GLP-1R signaling is endogenously relevant for both normal feeding behavior and for effort-based responding for palatable food. Relative to controls, rats with vHP GLP-1R KD demonstrated increased lever-press responding (FR1 operant schedule) for a palatable high-fat, sugar-enriched food under ad libitum conditions. vHP GLP-1R KD also altered meal patterning by significantly increasing inter-meal interval (driven by trends towards increased meal size and compensatory decreases in meal frequency) relative to controls. This is consistent with our recent work 45 showing that pharmacological vHP GLP-1R activation decreases meal size, and with other work demonstrating that peripheral and central GLP-1R activation reduces food intake primarily through a reduction in meal size 27, 59–62. Despite these alterations in feeding behavior, GLP-1R KD did not influence long-term body weight or total caloric intake when animals were maintained on chow. It is unknown whether total caloric intake and/or body weight would be impacted with a greater GLP-1R KD magnitude (~40% KD in the present study), and/or with an “obesogenic” dietary challenge (e.g., cafeteria diet).
GLP-1 is produced in L cells in the ileum, as well as in NTS neurons that have extensive ascending projections throughout the brain. The endogenous source of GLP-1 signaling to the vHP is unknown, but given that GLP-1-expressing NTS neurons do not project to the hippocampus 45, 63, GLP-1 acting on vHP neurons is likely to originate from either peripheral transport of GLP-1 across the blood-brain barrier 64, or via “volume transmission” from GLP-1 released in the cerebral spinal fluid (CSF) from hindbrain GLP-1 neurons 45. Consistent with the latter possibility, our recent work detected active levels of GLP-1 in both the vHP parenchyma and in the CSF, and GLP-1-immunoreactive terminals are abundant in the ependymal layer lining the cerebral ventricles at multiple rostral-caudal levels of the neuraxis 45. Moreover, ~30% of GLP-1-producing neurons are co-labeled following injection of a retrograde pathway tracer in the lateral cerebral ventricle, suggesting a subset of these neurons have axon terminals innervating the ventricular space.
To investigate the neural pathways downstream of vHP GLP-1R mediated-feeding, we utilized complementary anterograde neural pathway-tracing methods to investigate a vHP to mPFC neural connection: PHAL and AAV1-GFP pathway-tracing. Building on previous work 1, both methods revealed a robust monosynaptic connection from vCA1 to the PL and ILA that are exclusively ipsilateral (Figure 2A–F). Furthermore, combination of FISH and retrograde neuronal tracing revealed evidence that vHP neurons that project to the mPFC also express GLP-1R (Figure 3D). To examine the functional relevance of this monosynaptic vHP GLP-1R to mPFC neural connection in the control of feeding behaviors, we combined neuropharmacology with reversible chemogenetic CaMKIIa hM4di DREADDs-mediated synaptic disconnection. Results showed that vHP to mPFC disconnection eliminates the food intake reduction that accompanies vHP GLP-1R activation, suggesting that the mPFC is a critical downstream target for vHP GLP-1R mediated feeding. Importantly these effects were not secondary to confounds arising from parenchymal CNO 65 and AAV delivery 56 because animals with vHP AAV-CaMKIIa-GFP controls were unaffected by mPFC CNO delivery.
While the current study, along with previous neuroanatomical work 1, 2, presents strong support for a direct relationship between vHP GLP-1R signaling and the mPFC, we recognize the possibility that multisynaptic and other downstream pathways are also involved. The vCA1 sends inputs to other feeding-related regions, including the lateral hypothalamic area (LHA) and amygdala 1, 66. Furthermore, amygdala-LHA and mPFC-LHA circuitry has been shown to regulate external cue-driven feeding 12, 67. Interestingly, recent work from our lab has demonstrated interactions between vHP ghrelin-mediated hyperphagia and the LHA 68 and other data from 69 has shown that activation of a vHP to lateral septum pathway reduces feeding. Additional studies will be required to determine the involvement of multisynaptic pathways and other downstream neural targets modulating vHP GLP-1R-mediated feeding effects.
vHP pyramidal neurons are primarily glutamatergic and provide an excitatory input to the mPFC 1, 2, and hippocampal glutamatergic signaling has been shown to interact with mPFC ionotropic glutamate receptors such as NMDA and AMPA/Kainate receptors 2, 70, 71. Here we demonstrate that a glutamatergic pathway from the vHP to the mPFC is functionally relevant to feeding behaviors. First, mPFC NMDA receptor, but not AMPA/Kainate receptor blockade attenuated the food intake and body weight reduction following vHP GLP-1R activation, suggesting that downstream mPFC NMDA receptor signaling is required for the full expression of vHP GLP-1R mediated feeding behaviors. At the molecular level, our data further suggests that vHP GLP-1R activation increases phosphorylation of the NMDA receptor subunit, NR2B, in the mPFC. mPFC NMDA receptor blockade attenuated but did not completely reverse the vHP GLP-1R induced food intake reduction, whereas DREADDs mediated vHP to mPFC synaptic silencing completely reversed the effects. These findings suggest that other downstream mPFC neurochemical systems are likely involved. For example, research has shown that mPFC neurons utilize metabotropic glutamate receptors (e.g. mGluR 2/3, mGluR 5) to control executive functions 72–74 and mGluR 2/3 activation has been shown to interact with mPFC serotonin receptor signaling to control impulsive behaviors 75. Furthermore, research has implicated dopaminergic 11, 16, 17, 20, 55, 76 and opioid systems acting in the mPFC 15, 21 in appetite control. Thus, the extent to which other neurochemical signals in the mPFC interact with vHP GLP-1R signaling requires further investigation.
The “differential reinforcement of low rates of responding” (DRL) task is a well-established measure of food impulsive response control in rats 20, 55. mPFC pyramidal neuron activation has been shown to reduce impulsive feeding behaviors 19 and mPFC opioid systems play a role in regulating impulsive feeding behaviors, particularly when animals are in a “high-drive” impulsive state such as during food restriction 21. Our results demonstrate that vHP GLP-1R signaling is involved in controlling impulsive responding for palatable food, as vHP-targeted EX4 administration reduced impulsivity in the DRL task when animals were in a high-impulsive state induced by 24 hr food deprivation. mPFC NMDA-R blockade attenuated the vHP GLP-1R induced impulsivity reduction, indicating these downstream receptors regulate both the food intake reduction and the reduced impulsive responding for palatable food triggered by vHP GLP-1R activation. Importantly we found that this effect was primarily driven by changes in lever press responses and no differences were found in pellets earned between all treatments, suggesting that vHP GLP-1R to mPFC NMDA-R signaling does not eliminate appetitive drive, suggesting a more selective effect on food impulsivity. We note that parallel analyses of impulsive responding for palatable food were not possible in the vHP GLP-1R KD animals, as these rats had increased FR1 lever-press responding at baseline (DRL 0), therefore precluding interpretation of potential group differences with longer DRL delays. These results taken together suggest that vHP GLP-1R to mPFC NMDA-R pathways are modulating higher-order aspects of feeding behavior, particularly in the realm of inhibitory control.
Both vHP and mPFC neural processing, as well as CNS GLP-1R signaling are capable of regulating both food and non-food motivated behaviors, particularly anxiety and stress 3–7, 77–79. However, our results indicate that vHP GLP-1R activation does not impact anxiety-like behaviors in the Zero maze task (Supplemental Figure S1), an established rodent assay for anxiety-like behaviors. Moreover, our recent work 45 demonstrated that vHP GLP-1R activation does not induce nausea-associated behaviors (assessed via conditioned flavor avoidance). These findings suggest that vHP GLP-1R-mediated reductions in food-motivated behaviors are not secondary to alterations in visceral state or anxiety. However, comprehensive additional studies are required to carefully assess other non-food-motivated behaviors that might be regulated by vHP GLP-1R to mPFC NMDAR pathways. Moreover, provided that Experiment 1 demonstrated that vHP GLP-1R KD increases operant FR1 responding for palatable food, it is likely that activation of the novel identified vHP GLP-1R to mPFC NMDAR pathway reduces various food motivated behaviors rather than specifically reducing impulsive responding for palatable food. Other aspects of motivational feeding behaviors (e.g. cue-driven feeding), as well as other measures of food impulsivity (e.g., impulsive choice tested via delay discounting 80) that are potentially inhibited by this pathway will require further research.
The GLP-1 system is clinically relevant for obesity treatment based on its potent food intake and body weight reducing effects following CNS GLP-1R activation 29. Results from the present study identify a neural pathway where vHP glutamatergic neurons are activated by GLP-1R signaling which in turn engages NMDA-R signaling in the mPFC to regulate food-motivated behaviors. Collectively these data illuminate novel neurobiological targets for the control higher-order feeding behaviors, and reveal a novel functional output of vHP-mPFC neural connectivity.
Supplementary Material
Time spent in open arms in the Zero maze anxiety task following vHP exendin-4 or vehicle administration.
Acknowledgments
We thank the following individuals for notable contributions to this work: Dr Alan G. Watts, Dr Larry W. Swanson, Brian Zingg, Sandhya Prathap, Emily Nakamoto, Lilly Taing, Hrant Gevorgian, Joanna Liang, Ryan Usui, Mehul Trivedi, Agustina Kim, Allison Apfel, Kaitlin Sontag, Anish Reddy, and Natalie Demirjian. This study was supported by the National Institute of Health grants: DK104897 (SEK), DK096139 (MRH), DK107333 (TMH) and pilot grant funding from the USC Diabetes and Obesity Research Institute (SEK). We report no biomedical financial interests or potential conflicts of interest.
References
- 1.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain research reviews. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little JP, Carter AG. Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(37):12808–12819. doi: 10.1523/JNEUROSCI.1616-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Jin J, Maren S. Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiology of learning and memory. 2016;134(Pt A):38–43. doi: 10.1016/j.nlm.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot K, Kimura S, Bai J, Kemp A, Manahan-Vaughan D, Giros B, et al. Modulation of hippocampus-prefrontal cortex synaptic transmission and disruption of executive cognitive functions by MK-801. Cerebral cortex. 2015;25(5):1348–1361. doi: 10.1093/cercor/bht329. [DOI] [PubMed] [Google Scholar]
- 5.Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, et al. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Molecular psychiatry. 2016;21(9):1298–1308. doi: 10.1038/mp.2015.176. [DOI] [PubMed] [Google Scholar]
- 6.Beeman CL, Bauer PS, Pierson JL, Quinn JJ. Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learning & memory. 2013;20(6):336–343. doi: 10.1101/lm.031161.113. [DOI] [PubMed] [Google Scholar]
- 7.Valdes JL, Maldonado P, Recabarren M, Fuentes R, Torrealba F. The infralimbic cortical area commands the behavioral and vegetative arousal during appetitive behavior in the rat. The European journal of neuroscience. 2006;23(5):1352–1364. doi: 10.1111/j.1460-9568.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 8.Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(10):R701–713. doi: 10.1152/ajpregu.00496.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horst NK, Laubach M. Reward-related activity in the medial prefrontal cortex is driven by consumption. Frontiers in neuroscience. 2013;7:56. doi: 10.3389/fnins.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldo BA, Spencer RC, Sadeghian K, Mena JD. GABA-Mediated Inactivation of Medial Prefrontal and Agranular Insular Cortex in the Rat: Contrasting Effects on Hunger- and Palatability-Driven Feeding. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(4):960–970. doi: 10.1038/npp.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(19):RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(36):8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(9):3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, et al. Medial prefrontal D1 dopamine neurons control food intake. Nature neuroscience. 2014;17(2):248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(2):497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(24):6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warthen DM, Lambeth PS, Ottolini M, Shi Y, Barker BS, Gaykema RP, et al. Activation of Pyramidal Neurons in Mouse Medial Prefrontal Cortex Enhances Food-Seeking Behavior While Reducing Impulsivity in the Absence of an Effect on Food Intake. Frontiers in behavioral neuroscience. 2016;10:63. doi: 10.3389/fnbeh.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain research. 1994;642(1–2):20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- 21.Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. Endogenous Opioid Signaling in the Medial Prefrontal Cortex is Required for the Expression of Hunger-Induced Impulsive Action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(10):2464–2474. doi: 10.1038/npp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterson MJ, Horvath TL. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell metabolism. 2015;22(6):962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Moran TH. Gut peptides in the control of food intake. International journal of obesity. 2009;33(Suppl 1):S7–10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 24.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell metabolism. 2012;16(3):296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(10):R885–895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, et al. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(12):3531–3540. doi: 10.1523/JNEUROSCI.3579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayanaswami V, Dwoskin LP. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacology & therapeutics. 2016 doi: 10.1016/j.pharmthera.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuffer WA, Trujillo JM. Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy. 2015;35(10):926–934. doi: 10.1002/phar.1639. [DOI] [PubMed] [Google Scholar]
- 33.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1427–1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 34.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain research. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(4):R465–470. doi: 10.1152/ajpregu.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreisler AD, Rinaman L. Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(10):R906–916. doi: 10.1152/ajpregu.00243.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology. 2012;153(2):647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(20):6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, et al. Role of lateral septum glucagon-like peptide 1 receptors in food intake. American journal of physiology Regulatory, integrative and comparative physiology. 2016;311(1):R124–132. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS one. 2015;10(3):e0119034. doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403(2):261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular metabolism. 2015;4(10):718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(2):327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(7):1917–1928. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, et al. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016. Endogenous Glucagon-Like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. American journal of physiology Endocrinology and metabolism. 2012;303(4):E496–503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain research. 1998;793(1–2):169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris JA, Oh SW, Zeng H. Adeno-associated viral vectors for anterograde axonal tracing with fluorescent proteins in nontransgenic and cre driver mice. Current protocols in neuroscience. 2012;Chapter 1(Unit 1):20–21. 18. doi: 10.1002/0471142301.ns0120s59. [DOI] [PubMed] [Google Scholar]
- 51.Allen Mouse Brain Connectivity Atlas. alleninstitute.org. Allen Institute for Brain Science; 2016. [Google Scholar]
- 52.Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain research reviews. 2010;64(1):14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature neuroscience. 2014;17(4):577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus-->midbrain pathway for feeding behavior. Neuron. 2014;82(4):797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. The European journal of neuroscience. 2013;37(11):1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Gestel MA, van Erp S, Sanders LE, Brans MA, Luijendijk MC, Merkestein M, et al. shRNA-induced saturation of the microRNA pathway in the rat brain. Gene therapy. 2014;21(2):205–211. doi: 10.1038/gt.2013.76. [DOI] [PubMed] [Google Scholar]
- 57.Min DK, Tuor UI, Chelikani PK. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 2011;179:151–158. doi: 10.1016/j.neuroscience.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 58.Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One. 2012;7(5):e34844. doi: 10.1371/journal.pone.0034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R983–987. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 60.Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299(3):R945–952. doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. American journal of physiology Endocrinology and metabolism. 2013;304(12):E1314–1320. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013;305(11):E1367–1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. The Journal of comparative neurology. 2013;521(10):2235–2261. doi: 10.1002/cne.23282. [DOI] [PubMed] [Google Scholar]
- 64.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. Journal of molecular neuroscience : MN. 2002;18(1–2):7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 65.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, et al. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96(4):2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- 67.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(19):8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife. 2015:4. doi: 10.7554/eLife.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nature communications. 2015;6:10188. doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory Amino Acid Pathway from the Hippocampus to the Prefrontal Cortex. Contribution of AMPA Receptors in Hippocampo-prefrontal Cortex Transmission. The European journal of neuroscience. 1992;4(12):1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 71.Jay TM, Burette F, Laroche S. NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. The European journal of neuroscience. 1995;7(2):247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 72.Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, et al. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(4):1196–1201. doi: 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. Journal of neurophysiology. 2011;106(2):960–973. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cerebral cortex. 2011;21(3):727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behavioural pharmacology. 2011;22(8):805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- 76.Macedo CE, Angst MJ, Gobaille S, Schleef C, Guignard B, Guiberteau T, et al. Prefrontal dopamine release and sensory-specific satiety altered in rats with neonatal ventral hippocampal lesions. Behavioural brain research. 2012;231(1):97–104. doi: 10.1016/j.bbr.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 77.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(30):10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Maniscalco JW, Rinaman L. Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiology & behavior. 2017 doi: 10.1016/j.physbeh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebral cortex. 2006;16(1):106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time spent in open arms in the Zero maze anxiety task following vHP exendin-4 or vehicle administration.