Abstract
Radial glial cells (RGCs) are the most abundant macroglia in the teleost brain and have established roles in neurogenesis and neurosteroidogenesis; however, their transcriptome remains uncharacterized, which limits functional understanding of this important cell type. Using cultured goldfish RGCs, RNA sequencing and de novo transcriptome assembly were performed, generating the first reference transcriptome for fish RGCs with 17,620 unique genes identified. These data revealed that RGCs express a diverse repertoire of receptors and signaling molecules, suggesting that RGCs may respond to and synthesize an array of hormones, peptides, cytokines, and growth factors. Building upon neuroanatomical data and studies investigating direct neuronal regulation of RGC physiology, differential gene expression analysis was conducted to identify transcriptional networks that are responsive to the conserved secretogranin II-derived neuropeptide secretoneurin A (SNa). Pathway analysis of the transcriptome indicated that cellular processes related to the central nervous system (e.g., neurogenesis, synaptic plasticity, glial cell development) and immune functions (e.g., immune system activation, leukocyte function, macrophage response) were preferentially modulated by SNa. These data reveal an array of new functions that are proposed to be critical to neuronal-glial interactions through the mediator SNa.
Introduction
Radial glial cells (RGCs) are a progenitor subtype in the developing central nervous system (CNS)1 and have a bipolar morphology with radial fibers that serve as scaffolds for neuronal migration2. RGCs are self-renewing through proliferative symmetrical divisions and are multipotent, generating neurons or other glial cells3. In mammals, RGCs are mostly a transient cell type, differentiating into neurons and glia at the end of development. They only persist in two areas of the adult brain, the anterior part of the subventricular zone of the lateral ventricle and subgranular zone of the dentate gyrus, where they serve as progenitor cells. These two areas are the main constitutive neurogenic regions of the adult mammalian brain, which explains the limited capacity for adult neurogenesis in mammals4,5. In contrast, RGCs are abundant throughout teleost development and into adulthood in numerous brain neurogenic zones6, which may explain why teleost fish exhibit the most pronounced and widespread adult neurogenesis of any vertebrate taxon studied thus far7,8. In addition to being a progenitor subpopulation, RGCs are also the only macroglia in teleost fish, as they lack bona fide stellate astrocytes9.
Apart from studies that have established their function in producing neuroestrogens through the expression and function of the steroidogenic enzyme aromatase B10–12, little is known about other functions performed by RGCs and the regulatory factors that control this cell type to maintain brain homeostasis13. While transcriptomics have been used recently to reveal the diversity of human radial glia14,15, fish RGCs remain uncharacterized at the transcriptomic level.
Given that RGCs make direct contact with cerebrospinal fluid and with adjacent neurons, they have the potential to be under regulation by numerous signaling molecules, such as hormones, neurotransmitters, neuropeptides, and cytokines. Previous reports in fish have identified dopaminergic16–18 and serotonergic regulation19 of RGC physiology. Expanding on these neuronal-RGC interactions, our recent study highlighted the neuroanatomical relationship between RGCs and soma of magnocellular and parvocellular neurons immunoreactive for the secretogranin II (SgII)-derived neuropeptide secretoneurin A (SNa) in the goldfish preoptic nucleus20. SN is an evolutionary conserved neuropeptide generated by endoproteolytic processing of its precursor protein SgII and is found in dense-core secretory granules in a wide variety of cell types of the endocrine and nervous systems21–23. SgII belongs to the chromogranin family that have a high proportion of acidic amino acids, the capacity to bind calcium, and the ability to form aggregates at low pH levels24. Teleost fish have two SgII paralogs, SgIIa and SgIIb, likely generated by the whole genome duplication event that occurred in the teleost lineage23.
SN exerts a diverse array of biological functions and controls nervous, endocrine, immune, and vascular systems22,25,26, reflecting the wide distribution of the peptide in the body. In the immune system, SN exerts chemotactic effects on several types of immune cells such as monocytes, eosinophils, natural killer, and endothelial cells26. Moreover, in the CNS, SN acts as a trophic factor stimulating neurite outgrowth27, assists in the growth and repair of neuronal tissue by promoting neuroprotection and plasticity28 and regulates the release of several key neurotransmitters such as glutamate, dynorphin B, dopamine, and γ-aminobutyric acid (GABA)29–31. In addition to its role in these physiological processes, SN is also implicated in many pathophysiological conditions of the CNS including, brain ischemia28,32, Alzheimer disease33,34, and epilepsy35. Although SN has many attributed functions in both the periphery and CNS, nothing is known about how this peptide regulates glia. Therefore, the objective of this study was to generate the first reference RGC transcriptome for fish in order to identify new putative functions that are critical to neuronal-RGC communication through SNa.
Materials and Methods
Experimental animals
All procedures were approved by the University of Ottawa Protocol Review Committee and followed standard Canadian Council on Animal Care guidelines on the use of animals in research. Common adult female goldfish (Carassius auratus) were purchased from a commercial supplier (Mt. Parnell Fisheries Inc.) and allowed to acclimate for at least 3 weeks prior to experimentation. Animals were maintained at 18 °C under a natural stimulated photoperiod and fed standard flaked goldfish food. Sexually mature female goldfish (18–35 g) were anesthetized using 3-aminobenzoic acid ethylester (MS222) for all handling and dissection procedures.
Cell culture and exposure
Cell culture methods have been previously established and validated for RGCs16. Briefly, the hypothalamus and telencephalon were dissected from female goldfish and rinsed twice with Hanks Balanced Salt Solution (HBSS; 400 mg KCl, 600 mg KH2PO4, 350 mg NaHCO2, 8 g NaCl, 48 mg Na2HPO4, and 1 g D-Glucose in 1 L ddH2O) with Antibiotic-Antimycotic solution (Gibco) and minced into small explants. RGCs were dissociated with trypsin (0.25%; Gibco) and cultured in Leibovitz’s L-15 medium (Gibco) with 15% Fetal Bovine Serum (FBS; Gibco) and Antibiotic-Antimycotic (Gibco). Cell culture medium was changed 4–7 days after isolation and then once a week thereafter. RGCs were subcultured by trypsinization (0.125%) for 3 passages, and then were used for experimentation. This results in RGC cultures in which >95% of the cells co-express glial fibrillary acid protein and brain lipid binding protein16. The focus of our study was on goldfish SNa because it is the only fish SN with known biological activity, and we have described the neuroanatomical relationship between RGCs and SNa-positive neurons20. Goldfish SNa was synthesized as reported36. Stock solutions of purified goldfish SNa peptide were made in distilled sterile water and stored at −20 °C until use. Aliquots were thawed on ice then diluted to desired concentrations in serum-free media. Previous studies have shown SN stimulates monocyte migration in a range of 0.01 to 1000 nM37,38 and increases neurite outgrowth at concentrations between 50–300 nM27. Here, we aimed to characterize the response to a maximal dose of SNa, so passage 3 RGCs were exposed to 1000 nM SNa for 24 h.
RNA extraction and Illumina sequencing
Total RNA was extracted using the RNeasy Micro Kit (Qiagen) including an on-column DNase treatment to remove genomic DNA. The concentration of total RNA was determined using the Qubit RNA Assay Kit (Life Technologies). In order to evaluate the quality of the total RNA, RNA integrity number (RIN) was assessed using Agilent RNA 600 Nano Reagents and RNA Nano Chips in Agilent 2100 Bioanalyzer (Agilent Technologies). Sequencing of 10 RGC cultures was performed by MR DNA (www.mrdnalab.com) following the manufacturer’s guidelines (Illumina HiSeq) for paired end sequencing (151 bp). Using oligo(dT) magnetic beads, RNA with poly(A) tails were purified and fragmented into shorter sequences that were used as templates for cDNA synthesis. A total of 10 cDNA libraries were constructed using random-hexamer primers from 1 μg of total RNA from each sample using TruSeq RNA LT Sample Preparation Kits (Illumina). Following the library preparation, the final concentration of the library was measured using the Qubit dsDNA HS Assay Kit (Life Technologies) and the average library size was determined using the Aligent 2100 Bioanalyzer (Aligent Technologies). A total of 5 pM library was clustered using cBot (Illumina) and sequenced paired end for 300 cycles using the HiSeq. 2500 system (Illumina).
De novo transcriptome assembly and annotation
Both de novo assembly and annotation were performed under contract by Genotypic Technologies (http://www.genotypic.co.in). The Illumina paired end raw reads were quality checked using FastQC (http://www.bioinformatics.babraham.ac.uk /projects/fastqc/). Illumina raw reads were processed by GT proprietary tools for adapters and low quality bases trimming towards the 3′-end. Only high-quality reads were considered for further downstream analysis. Biological replicates were pooled together to generate a reference transcriptome using Trinity39 using all default parameters with a k-mer of 25. Trinity partitions the sequence data into individual de Bruijn graphs, each representing the transcriptional complexity at a given gene or locus, and then processes each graph independently to extract full-length splicing isoforms and to identify transcripts derived from paralogous genes. Once assembled, the sequences were clustered based on similarity between sequences with CD-Hit v4.5.440 using the following parameters: 95% coverage and 90% identity, -r 1, and others the default setting. Clustering reduces overall size of the database without removing any sequence information by only removing redundant or highly similar sequences. The longest sequences for each cluster was used as the representative sequence. The clustered transcripts with ≥300 bp in length were considered for the analysis. Transcripts were annotated using the Basic Local Alignment Search Tool (BLAST + 2.2.29) (E-value < 1.0e-4) using Chordata protein sequences from the Uniprot database41. The remaining unannotated transcripts were further annotated using Pfam database. After annotation, gene ontology terms were assigned using protein annotation through evolutionary relationships (PANTHER) in order to classify all transcripts identified in RGC cultures42. Gene ontology categories for biological processes, molecular functions, cellular components, protein classes, and pathways were used to identify the distribution of genes within each gene ontology category in RGC cultures.
Differential gene expression analysis
The differential gene expression (DGE) levels between control (n = 3 cultures) and 1000 nM SNa exposure (n = 3) in RGCs were calculated using the DESeq method43. Briefly, after the variance was calculated for each gene, DGE was calculated assuming a negative binomial distribution for the expression level and a Fisher’s exact test was used to calculate P-value to test for significance between groups for each gene. Once the DGE was calculated, results were separated as up-regulated and down-regulated based on a |log2| > 0.5 cut off. A false discovery rate (FDR) at 5% was used for multiple hypothesis testing. All data have been deposited into the Gene Expression Omnibus (GSE106101), National Center for Biotechnology Information.
qRT-PCR validation of differentially expressed genes
Samples used for qRT-PCR validation were obtained following the same cell culture methods that were used for RNA-Seq (i.e., passage 3 RGCs). The qRT-PCR was conducted using the SYBR green detection system to determine relative gene expression and to validate the DGE analysis. Primers were designed using Primer344 and synthesized by Integrated DNA Technologies (Supplementary File S1). Primer sets were tested for specificity by subjecting the qRT-PCR products to a 1% agarose containing SYBR Safe DNA gel stain (Invitrogen) to ensure a single product was produced from each reaction. Each product was extracted from the gel using NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel) and sequenced to confirm primer specificity by StemCore Laboratories at the Ottawa Hospital Research Institute. The cDNA was prepared from 1 μg of total RNA using the Maxima First Strand cDNA Synthesis Kit for qRT-PCR (Thermo Scientific). The qRT-PCR analyses were conducted using the Maxima SYBR green qPCR Master Mix (Thermo Scientific) and CFX96 Real-Time PCR Detection System (Bio-Rad) to amplify the genes of interest. The thermal cycling parameters were: a single cycle Taq activation step at 95 °C for 3 min, followed by 40 cycles of 95 °C denaturation step for 10 s and one primer annealing temperature (56–63 °C) for 30 s depending on the primer set used. After 40 cycles, a melt curve was performed over a range of 65–95 °C with increments of 0.5 °C to ensure a single amplified product. Data were analyzed using CFX Manager Software package (Bio-Rad). The efficiency of each assay was 100 ± 10% and the R2 of each standard curve was >0.98. Relative mRNA abundance was calculated using the relative standard curve method based on Cq values and normalized using the NORMA-GENE algorithm45. Fold-change was calculated against the average of the control using normalized data. Fold-change for each group were presented as mean + SEM from 4 biological replicates (n = 4) assayed in duplicate. Data were assessed for normality with Shapiro-Wilk’s W test. A Student’s t-test was used to test for differences in mRNA levels between groups.
Gene set enrichment analysis and sub-network enrichment analysis
Gene set and sub-network enrichment analyses were conducted using Pathway Studio 9.0 (Elsevier). Official gene symbols were used to map goldfish genes into Pathway Studio. A total of 28,597 genes were mapped in Pathway Studio and for duplicated gene symbols in the dataset, the gene that showed the “best p-value, highest magnitude fold change” was used for analyses. For gene set enrichment analysis (GSEA), genes were permutated 1000 times using the Kolmogorov-Smirnov classic approach as an enrichment algorithm. The gene set categories examined for enrichment included curated cell processes, cell signaling and receptor signaling pathways. Sub-network enrichment analysis (SNEA) for cell processes was performed to identify gene networks regulated in RGCs following SNa exposure. Utilizing known relationships (i.e., based on expression, binding, and common pathways) between genes, SNEA builds networks focused around gene hubs. For both GSEA and SNEA the enrichment P-value was set at P < 0.05. These analyses have been successfully used to build gene networks in teleost fish46,47.
Results
De novo transcriptome assembly and quality analysis
Before de novo transcriptome assembly, paired-end raw reads were processed to remove adapter fragments and low quality bases generating 161,704,300 clean reads from 163,641,713 raw reads. High-quality reads were subsequently assembled de novo using the Trinity program because there is no goldfish reference genome. Trinity assembler, with a k-mer size of 25 generated 170,967 unigenes with a total sequence length of 150,361,946 bp. Resulting sequences were binned into a non-redundant set of gene-oriented clusters or “unigene” clusters. Each cluster contains sequences that represent a unique gene. Lengths of the clusters ranged from 301 to 20,585 bp with a mean size of 879.5 and an N50 of 1318 bp. The N50 is the contig length such that of equal to or longer that constitutes half of the entire assembly. Among the clusters, 85,984 (47.7%) were 300–499 bp in length, 45,405 (27.4%) were 500–999 bp, 39,523 (24.8%) were 1,000–9,999 bp, and 55 (0.03%) were >10,000 bp in length.
Annotation and gene ontology classification
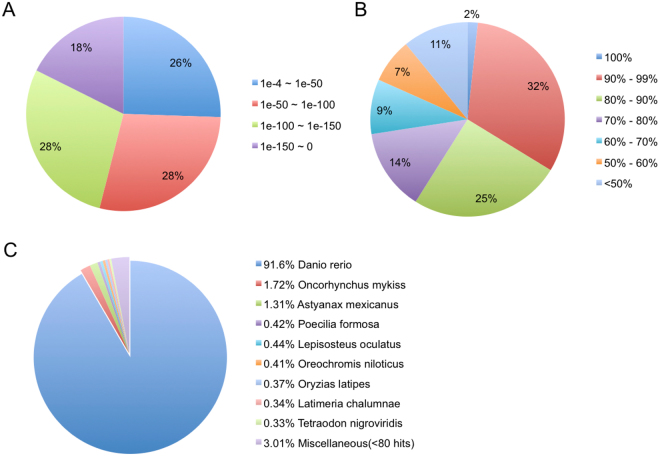
Annotation of the RGC transcriptome was performed by aligning transcripts ≥300 bp in length using BLASTX41 to all available Chordata protein sequences from UniProt database (using a cut off at E-value < 1.0e-4). The remaining unannotated transcripts were then compared to the Pfam database. A total of 67,486 unigenes were annotated (38.05% from UniProt and 1.42% from Pfam) while the remaining 96,145 unigenes (56.24% of all unigenes) could not be annotated, having no significant sequence similarity to any database entries (unknown function). This can be attributed to the lack of genomic information for goldfish, limiting identification of some of the transcripts. Overall, 59% of the transcripts were mapped with >80% identity and 74% of the matched sequence exhibited strong sequence similarity with an E-value < 1e-50 with respect to the E-value distribution pattern (Fig. 1A,B). The BLASTX top-hit species matched in the UniProt database were mostly other fish species, with the top three species being Danio rerio (91.6%), Oncorhynchus mykiss (1.72%), and Astyanax mexicanus (1.31%) (Fig. 1C).
Figure 1.
Annotation summary of assembled Carassius auratus RGC genes against the UniProt database. (A) E-value distribution of BLASTX hits for each transcript with an E-value cut off of 1e-4. (B) Similarity distribution of BLATX hits for each gene. (C) Distribution of the top BLASTX species hits in the UniProt database.
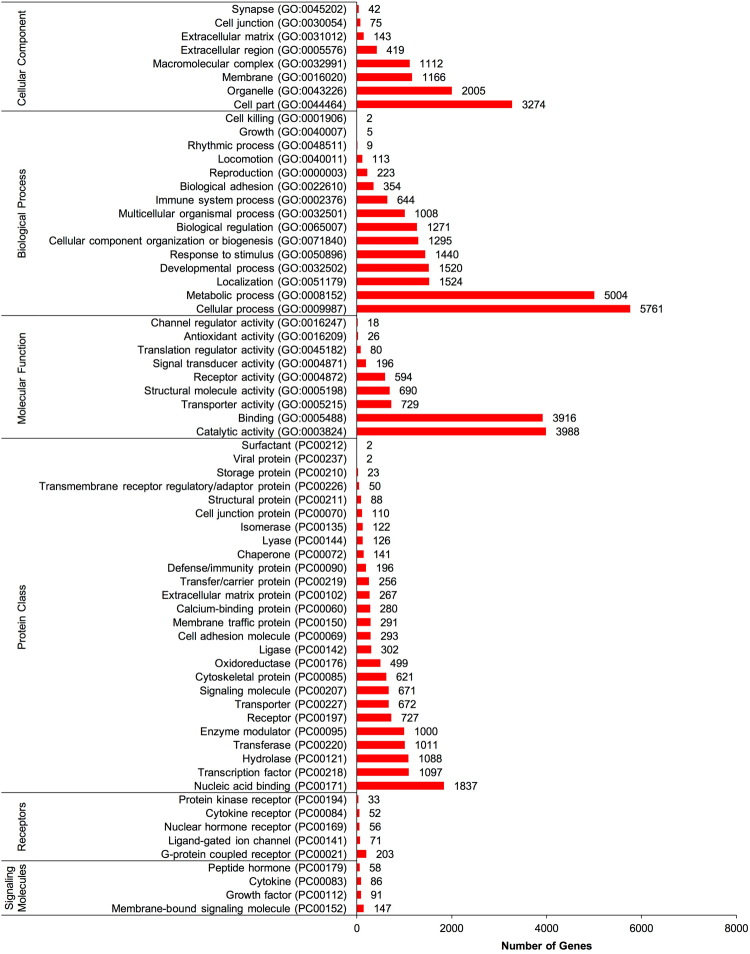
The 17,620 non-redundant genes were assigned gene ontology (GO) terms for functional characterization. These annotated genes were classified in three main ontologies: cellular component (8,236 genes), biological process (20,173), and molecular function (10,237) (Fig. 2). Within the cellular compartment category, the RGC transcriptome was enriched in the cell part (3,274 genes; 39.8%), organelle (2,005; 24.3%), and membrane (1,166; 14.2%). In addition, a few genes were assigned to GO categories such as cell junction (75 genes; 0.9%) and synapse (42; 0.5%). In the biological process category, cellular process (5,761; 28.6%), metabolic process (5,004 genes; 24.8%), and localization (1,524; 7.6%) were predominant, suggesting these genes are involved in metabolism and cell growth. Other notable biological function allocations included transcripts involved in immune system processes (644 genes; 3.2%) and biological adhesion (354; 1.8%). Regarding molecular function, a high proportion of genes were assigned to catalytic activity (3,988, 39.0%), binding (3,916; 38.3%), and nucleic acid binding transporter activity (729; 7.1%).
Figure 2.
Gene ontology (GO) classification of assembled Carassius auratus RGC genes into molecular function, biological function, cellular component, protein class, receptor and signaling molecule categories. The number of genes ascribed to each classification is provided along with GO or protein class (PC) accession number.
The transcriptome was also categorized by protein class ontologies in the online program Panther (Fig. 2). Transcripts were classified as nucleic acid binding proteins (1,837 genes; 15.6%), transcription factors (1,097 genes; 9.3%), and hydrolases (1088 genes; 9.2%). Within protein classes, the receptor and signaling molecule ontologies were investigated (Fig. 2). The RGC transcriptome expresses an array of receptor classes such as G-protein coupled receptors (GPCRs) (203 genes; 48.9%), ligand-gated ion channels (71 genes; 10.1%), nuclear hormone receptors (56 genes; 13.5%), cytokine receptors (52 genes; 12.5%), and protein kinase receptors (3 genes 3; 8.0%). Within the signaling molecule category, genes were cataloged into membrane-bound signaling molecules (140 genes; 38.5%), growth factors (91 genes; 23.8%), cytokines (86 genes; 22.5%), and peptide hormones (58 genes; 15.2%) (Fig. 2). These GO analyses reveal a diverse receptor and signaling molecule profile, suggesting that RGCs can respond to and synthesize an array of hormones, peptides, cytokines, and growth factors.
Overall, a total of 5,344 annotated transcripts were assigned to 162 unique pathway ontologies. Based on the transcripts detected, the Wnt signaling pathway (239 genes; 4.6%) was enriched, as well as gonadotropin-releasing hormone receptor pathway (200 genes; 3.8%), chemokine and cytokine signaling pathways (188 genes; 3.6%), and angiogenesis (170 genes; 3.3%) (Table 1). When considering all pathway ontologies, it was noted that many were neurotransmitter receptor-related such as serotonin (PANTHER pathway accession numbers: P04374, P04373, P04376, P04375), dopamine (P05912), acetylcholine (P00044, P00043, P00042), GABA (P05731), glutamate (P00037, P00039, P00040, P00041), adrenaline (P04378, P04377, P00002, P04379), and histamine (P04385, P04386). A number of genes were assigned to hormone related pathways, including gonadotropin-releasing hormone receptor signaling (P06664), oxytocin receptor signaling (P04391), thyrotropin-releasing hormone receptor signaling (P04394), corticotropin-releasing factor receptor signaling (P04380), opioid proopiomelanocortin pathway (P05917), vasopressin synthesis (P04395), cholesterol biosynthesis (P00014), and androgen/estrogen/progesterone biosynthesis (P02727). In addition, several immune system pathways were identified, for example chemokine and cytokine signaling (P00031), epidermal growth factor signaling (P00018), fibroblast growth factor signaling (P00021), transforming growth factor β (P00052), interleukin signaling (P00036), both T and B cell activation (P00053, P00010), toll receptor signaling (P00054), and interferon γ signaling (P00035). A full list of all pathways, receptors, signaling molecules and other gene ontology classifications can be found in Supplemental File S2.
Table 1.
Top 25 assigned pathway ontologies for assembled Carassius auratus RGC genes based on the number of genes identified in each pathway.
| Pathway Name | Pathway Accession | # of genes identified |
|---|---|---|
| Wnt signaling pathway | P00057 | 239 |
| Gonadotropin releasing hormone receptor pathway | P06664 | 200 |
| Inflammation mediated by chemokine and cytokine signaling pathway | P00031 | 188 |
| Angiogenesis | P00005 | 170 |
| Integrin signaling pathway | P00034 | 166 |
| CCKR signaling map | P06959 | 164 |
| EGF receptor signaling pathway | P00018 | 132 |
| PDGF signaling pathway | P00047 | 128 |
| FGF signaling pathway | P00021 | 118 |
| Huntington disease | P00029 | 110 |
| Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | P00026 | 108 |
| Cadherin signaling pathway | P00012 | 105 |
| Alzheimer disease-presenilin pathway | P00004 | 101 |
| Apoptosis signaling pathway | P00006 | 99 |
| TGF-beta signaling pathway | P00052 | 93 |
| Parkinson disease | P00049 | 93 |
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | P00027 | 85 |
| Ras Pathway | P04393 | 82 |
| Endothelin signaling pathway | P00019 | 74 |
| T cell activation | P00053 | 73 |
| Interleukin signaling pathway | P00036 | 72 |
| p53 pathway | P00059 | 71 |
| B cell activation | P00010 | 70 |
| Cytoskeletal regulation by Rho GTPase | P00016 | 68 |
| VEGF signaling pathway | P00056 | 66 |
Differential gene expression analysis following SNa treatment
With a fold-change cut off |log2| > 0.5, the DGE analysis revealed 1,776 differentially expressed genes (DEGs) after SNa treatment at P < 0.05 and 123 DEGs at P < 0.001. A total of 42 DEGs, 31 up-regulated and 11 down-regulated, passed the false discovery rate (FDR; < 0.05) correction for multiple hypothesis testing. All of the 31 up-regulated DEGs could not be annotated at this time, thus likely representing novel transcripts with no detected similarities to other species. Of the 11 down-regulated DEGs in goldfish RGCs, 7 were annotated (Table 2). Transcripts that were differentially down-regulated (FDR < 0.05) genes included: mothers against decapentaplegic homolog 6 (smad6b), fibroblastic growth factor 4 (fgf4), NGFI-A binding protein 1a (nab1a), BAI1-associated protein 2b (baiap2b), GRB2-related adaptor protein a (grapa), phosphodiesterase 8 A (pde8a), tRNA nucleotidyl transferase (trnt1), and heat shock protein beta 11 (hspb11).
Table 2.
List of differentially expressed genes compared to control in Carassius auratus RGC culture after 24 h 1000 nM SNa treatment (FDR < 0.05).
| Gene symbol | Gene Name | Uniprot Accession | Fold Change | P-Value | FDR Adjusted P-Value |
|---|---|---|---|---|---|
| smad6b | Mothers against decapentaplegic homolog 6 | tr|Q1L8Y3|Q1L8Y3_DANRE | 0.40 | 7.34E-11 | 2.80E-07 |
| fgf4 | Fibroblast growth factor 4 | tr|Q9DFC9|Q9DFC9_DANRE | 0.29 | 5.03E-10 | 1.78E-06 |
| nab1a | NGFI-A binding protein 1a | tr|Q1LWI4|Q1LWI4_DANRE | 0.28 | 9.87E-07 | 2.33E-03 |
| baiap2b | BAI1-associated protein 2b | tr|U3JAA2|U3JAA2_DANRE | 0.46 | 1.65E-06 | 3.63E-03 |
| grapa | GRB2-related adaptor protein a | tr|Q503S8|Q503S8_DANRE | 0.35 | 3.21E-06 | 6.43E-03 |
| pde8a | Phosphodiesterase 8 A | tr|H9GZ87|H9GZ87_DANRE | 0.31 | 1.25E-05 | 2.08E-02 |
| trnt1 | tRNA nucleotidyl transferase | tr|A7MCH7|A7MCH7_DANRE | 0.08 | 1.30E-05 | 2.14E-02 |
| hspb11 | Heat shock protein beta 11 | sp|A5JV83|HSPBB_DANRE | 0.33 | 2.60E-05 | 3.88E-02 |
qRT-PCR validation of DGE analysis
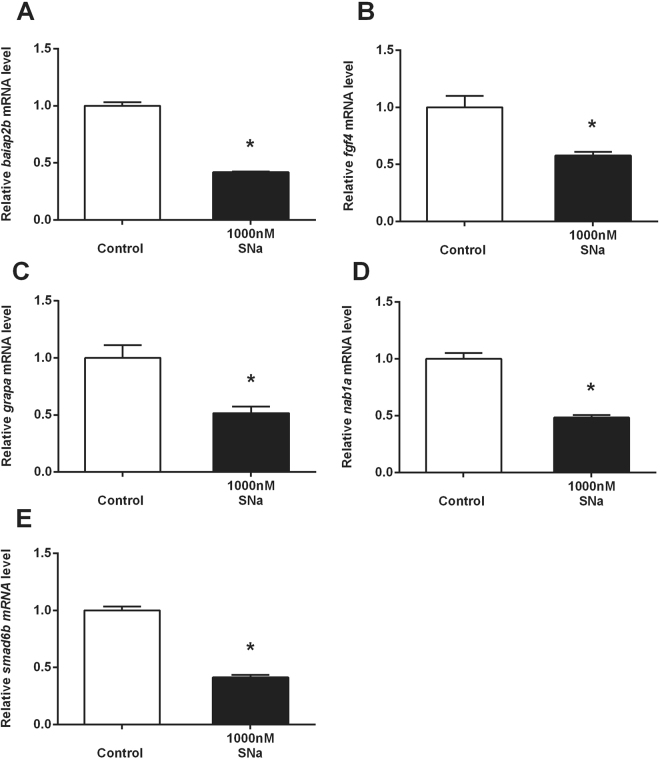
In order to validate the expression of annotated DEGs identified by RNA-Seq, 5 primer pairs were designed based on the corresponding assembled sequence. The fold-changes quantified by RNA-Seq and qPCR, respectively for these 5 DEGs were: 0.46 and 0.42 (P < 0.05) for baiap2b, 0.29 and 0.58 (P < 0.05) for fgf4, 0.35 and 0.52 (P < 0.05) for grapa, 0.28 and 0.48 (P < 0.05) for nab1a, and 0.40 and 0.41(P < 0.05) for smad6b (Fig. 3). Overall, the expression profiles obtained by RNA-Seq analysis and qPCR were highly comparable in the direction of change.
Figure 3.
Quantitative real-time PCR analysis for (A) baiap2b, (B) fgf4, (C) grapa, (D) nab1a and (E) smad6b mRNA in Carassius auratus RGC culture exposed to 1000 nM SNa. Data were normalized and defined as fold-change relative to control. Bars represent the mean + SEM (n = 4). Treatment groups marked by asterisks have significantly different mRNA levels compared to control (P < 0.05).
Gene set enrichment analysis
GSEA revealed a total of 17 pathways that were statistically significant (P < 0.05) and had a median fold change >5% (Table 3). Pathways were grouped by three categories: cell processes (4 pathways), cell signaling (2 pathways), and receptor signaling (11 pathways). Cell processes involved in adherens and tight junction assembly such as nectins, claudins, cadherins, and junctional adhesion molecules (JAMs) were all significantly increased (10–17% or 1.10- to 1.17-fold) indicating that SNa up-regulates gene networks involved in cell-cell junctions. GSEA for cell signaling pathways revealed that actin cytoskeleton regulation and T cell activation pathways were significantly increased in SNa-treated RGCs. SNa significantly increased genes involved in four different T-cell receptor signaling pathways. Besides the T-cell receptor, other receptors such as glucagon receptor, dopamine D1 receptor, tumor necrosis factor receptor (TNFR), tumor necrosis factor receptor superfamily member 1 A (TNFSF1A), and neural cell adhesion molecule I (NCAMI) all shared similar CREB/ELK-SRF signaling. In addition, interleukin 6 receptor (ILR) and ephrin receptor (ephrinR) signaling pathways were also responsive to SNa treatment. A complete list of all enriched pathways is provided in Supplementary File S3.
Table 3.
Gene set enrichment analysis (GSEA) for transcripts in Carassius auratus RGC cultures treated with 1000 nM SN (P < 0.05).
| Gene Set Category | Total # of entities | # of measured entities | Median change | P-value | |
|---|---|---|---|---|---|
| Ariadne Cell Process Pathways | Adherens Junction Assembly (Nectin) | 104 | 57 | 1.10 | 0.045 |
| Tight Junction Assembly (Claudins) | 137 | 62 | 1.13 | 0.022 | |
| Adherens Junction Assembly (Cadherins) | 133 | 53 | 1.14 | 0.016 | |
| Tight Junction Assembly (JAMs) | 119 | 55 | 1.17 | 0.024 | |
| Ariadne Cell Signaling Pathways | Actin Cytoskeleton Regulation | 551 | 357 | 1.10 | 0.009 |
| T Cell Activation | 957 | 490 | 1.11 | 0.003 | |
| Ariadne Receptor Signaling Pathways | IL6R - > STAT signaling | 8 | 8 | 1.08 | 0.047 |
| EphrinR - > actin signaling | 216 | 143 | 1.12 | 0.020 | |
| T-cell receptor - > NF-kB signaling | 176 | 33 | 1.15 | 0.000 | |
| T-cell receptor - > AP-1 signaling | 180 | 33 | 1.17 | 0.025 | |
| T-cell receptor - > CREBBP signaling | 176 | 25 | 1.19 | 0.027 | |
| GlucagonR - > CREB/ELK-SRF/SP1 signaling | 42 | 27 | 1.20 | 0.040 | |
| DopamineR1 - > CREB/ELK-SRF signaling | 40 | 25 | 1.20 | 0.016 | |
| T-cell receptor - > ATF/CREB signaling | 195 | 45 | 1.21 | 0.021 | |
| TNFR - > CREB/ELK-SRF signaling | 45 | 30 | 1.23 | 0.050 | |
| TNFRSF1A - > CREB/ELK-SRF signaling | 41 | 32 | 1.23 | 0.014 | |
| NCAM1 - > CREB/ELK-SRF/MYC signaling | 27 | 21 | 1.26 | 0.046 |
Only those pathways with a median fold-change greater than 5% are presented. The number of measured entities in the pathway is reported along with the number of measured entities and median fold-change for the pathway.
Sub-network enrichment analysis
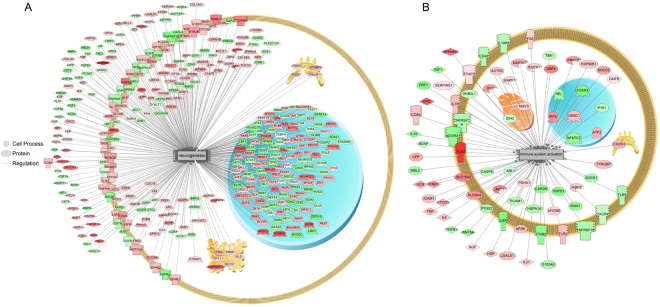
Using SNEA, a total of 192 cellular processes were significantly responsive to SNa exposure. Sub-networks that included processes related to CNS and immune system were major biological themes regulated by SNa (Table 4). All 19 sub-networks involved in processes related to the CNS were increased (7–70% or 1.07- to 1.70-fold). Examples of these sub-networks included neurogenesis, glial cell development, neuronal activity, synaptic plasticity, axon guidance and extension. A total of 19 sub-networks were associated with cellular processes of the immune system indicating that this is another major theme in the transcriptomic response to SNa. There were 18 sub-networks that were increased (5–53% or 1.05- to 1.53-fold) and 1 sub-network that was decreased (−13% or −1.13 fold), indicating that SNa has a largely stimulatory effect on diverse processes in RGCs. These processes included immune system activation, immunoreactivity, lymphocyte activation, macrophage response leukocyte function and differentiation. Neurogenesis (Fig. 4A) and immune system activation (Fig. 4B) are prominent examples of sub-networks that were significantly regulated by SNa. All identified sub-networks are presented in Supplementary File S3.
Table 4.
Cell processes identified by sub-network enrichment analysis (SNEA) that were regulated by 1000 nM SNa in primary Carassius auratus RGC cultures.
| Gene Set Seed | Total # in pathway | # of Measured Neighbors | Median change | P-value | |
|---|---|---|---|---|---|
| Processes related to CNS | Axon guidance | 210 | 160 | 1.17 | 0 |
| Transmission of nerve impulse | 598 | 321 | 1.1 | 0 | |
| Cognition | 212 | 126 | 1.11 | 0.004 | |
| Innervation | 172 | 113 | 1.08 | 0.005 | |
| Brain function | 150 | 89 | 1.14 | 0.006 | |
| Cerebellum development | 14 | 8 | 1.44 | 0.007 | |
| Neuronal activity | 263 | 133 | 1.06 | 0.008 | |
| Neuroproliferation | 6 | 5 | 1.7 | 0.01 | |
| Hindbrain development | 15 | 11 | 1.44 | 0.013 | |
| Synaptic transmission | 509 | 288 | 1.13 | 0.013 | |
| Synaptic plasticity | 454 | 284 | 1.1 | 0.013 | |
| Schwann cell formation | 15 | 12 | 1.26 | 0.016 | |
| Memory | 721 | 385 | 1.08 | 0.016 | |
| Neuron development | 103 | 69 | 1.1 | 0.029 | |
| Dentate gyrus development | 6 | 6 | 1.34 | 0.03 | |
| Neurogenesis | 561 | 383 | 1.11 | 0.031 | |
| Central nervous system function | 25 | 15 | 1.26 | 0.041 | |
| Axon extension | 74 | 59 | 1.16 | 0.042 | |
| Glial cell development | 17 | 11 | 1.25 | 0.044 | |
| Processes related to immunity | B-cell activation | 233 | 105 | 1.1 | 0.001 |
| Leukocyte function | 158 | 74 | 1.09 | 0.003 | |
| Granulocyte adhesion | 52 | 27 | 1.11 | 0.009 | |
| Peritoneal macrophage function | 10 | 5 | 1.34 | 0.014 | |
| Immunoreactivity | 463 | 260 | 1.13 | 0.014 | |
| Eosinophil degranulation | 43 | 15 | 1.31 | 0.015 | |
| T-cell homeostases | 100 | 50 | 1.05 | 0.016 | |
| Respiratory burst | 185 | 92 | 1.1 | 0.017 | |
| NK cell mediated cytotoxicity | 367 | 133 | 1.09 | 0.018 | |
| Leukocyte accumulation | 84 | 43 | 1.1 | 0.019 | |
| Leukocyte differentiation | 9 | 5 | 1.53 | 0.022 | |
| B lymphocyte proliferation | 277 | 148 | 1.07 | 0.022 | |
| Lymphocyte activation | 277 | 131 | 1.11 | 0.023 | |
| Lymphocyte aggregation | 10 | 6 | 1.32 | 0.027 | |
| Macrophage response | 48 | 19 | 1.1 | 0.028 | |
| Immune system activation | 163 | 76 | 1.07 | 0.033 | |
| Leukocyte tethering / rolling | 106 | 58 | 1.11 | 0.041 | |
| Phagocyte activity | 167 | 80 | 1.1 | 0.046 | |
| Granulosa cell differentiation | 46 | 34 | −1.13 | 0.047 |
Only those sub-networks with a median fold-change greater than 5% in the cell process are presented (P < 0.05). Reported here are only some major cell processes affected by SNa in RGCs.
Figure 4.
Subnetwork enrichment analysis (SNEA) indicated that genes involved in (A) neurogenesis and (B) immune system activation were significantly enriched in Carassius auratus RGC cultures following 24 h exposure to 1000 nM SNa treatment (P < 0.05). Red indicates that gene expression was increased and green indicates that gene expression was decreased. All abbreviations are provided in Supplemental File S4.
Discussion
Given what has been reported previously about teleost RGCs13,48, we predicted that transcriptomic profiling would identify genes related to steroidogenesis and neurogenesis, in addition to several receptors for classical neurotransmitters. Goldfish RGCs do indeed express genes related to these key processes, but also express a much wider range of cell surface and nuclear receptors, including numerous neurogenesis- and immune-related genes. A total of 17,620 non-redundant unigene clusters were identified by RNA sequencing and GO analysis revealed a diverse receptor and signaling molecule profile. This suggests that teleost RGCs can respond to and synthesize an array of hormones, peptides, cytokines, and growth factors.
A reference transcriptome for cultured teleost RGCs
We extend our observations on the expression of glial fibrillary acidic protein (gfap), brain fatty acid binding protein (blbp), aromatase B (cyp191b), and related proteins16 to transcriptomic characterization of goldfish RGCs. Further validating this culture system, several critical markers previously reported to be expressed in zebrafish RGCs in the adult telencephalon and diencephalon13 were identified by RNA-Seq in goldfish RGCs. These include the well-documented cyp191b, blbp, gfap, and also calcium binding protein β (s100b), connexin 43 (cx43), glial high-affinity glutamate transporter (slc1a3a), glutamine synthase (glula), nestin (nes), sex determining region Y box 2 (sox2), and vimentin (vim). These glial markers have been used to characterize RGCs in zebrafish and provide the cell with the abilities of neurotransmitter uptake (slc1a3a, glula), estrogen production (cyp19a1b), and generation of calcium waves (cx32)13. Identification of several steroidogenic enzyme transcripts such as steroidogenic acute regulatory protein (star), 17α-hydroxylase (cyp17a1), 17β-hydroxysteroid dehydrogenase (hsd17b10), aromatase B (cyp19a1b), and 5α-reductase (srd5a1), suggest that these cells are capable of de novo estrogen production from cholesterol and are able to produce pregnenolone and progesterone intermediates, confirming previous observations that these are steroidogenic cells48. Here we also show that cultured goldfish RGCs express progesterone receptor (pgr), androgen receptor (ar), G-protein coupled estrogen receptor (gper1), estrogen receptor α (esr1), estrogen receptor β1 (esr2b), and estrogen receptor β2 (esr2a). These data support other reports in zebrafish that in vivo RGCs express Pgr protein49 as well as observations for the distribution of ar, esr1, esr2a, and esr2b mRNAs with aromatase B expression in RGCs50. The expression of nuclear estrogen receptor mRNAs has been documented before in goldfish RGC cultures17; however, to the best of our knowledge, this is the first report demonstrating that gper1 is expressed by RGCs. The combination of numerous steroidogenic enzymes and steroid receptors expressed by RGCs strongly support the hypothesis that RGCs are both a source and target of neurosteroids50.
RGCs express multiple receptors
We report that RGCs express receptors for various neurotransmitters including 5-HT (1 A, 2B, 2 C, 6), DA (D2, D4), GABA (GABAA, GABAB), acetylcholine (M4, M5), adrenaline (α2a, α2c, β1, β2), adenosine (A1, A2B), and histamine (H1, H3). Our previous studies showed that DA D1 receptor activation in RGCs can increase aromatase B and regulate protein networks associated with progenitor cell functions16,18. Furthermore, RGCs may also be under the direct control of 5-HT, as 5-HT neurons share a close distribution with RGCs in the zebrafish paraventricular organ and in vivo inhibition of 5-HT synthesis decreases RGC proliferation in zebrafish19. In support of our RNA-Seq data, goldfish RGCs have previously been documented to express adenosine A1 receptors51 and activation of this receptor in rabbit RGCs causes ATP-evoked increases in calcium that regulate proliferation52. In mice, RGC fate is under the control of GABA excitation where high levels of activation result in quiescence, while low levels of excitation cause either symmetrical or asymmetrical cell divisions53. Histamine H1, H2, and H3 receptors are expressed by mammalian astrocytes and regulate astrocytic functions such as calcium influx, energy metabolism, neurotransmitter clearance, neurotrophic activity, and immune response54. Together, this panel of expressed neurotransmitter receptors suggest that goldfish RGCs are in communication with numerous neuronal subtypes. Goldfish RGCs also express receptors for growth hormone (ghra), growth hormone-releasing hormone (ghrh), thyroid hormone (thraa, thrab, thrb), thyrotropin releasing-hormone (trhr2), prolactin-releasing peptide (prlhr2a), and prolactin (prlrb), amongst others. Few studies have investigated hormonal control of glial cells. Best described are the stimulatory effects of thyroid hormones on neural progenitor proliferation in mammals55,56 and estrogenic stimulation of aromatase B expression in zebrafish RGCs57. We have found that both thyroid hormone and estrogen receptors are expressed in female goldfish RGCs in our cultures. The response of these cells to thyroid hormones has not been investigated, but we have shown previously that goldfish RGCs respond directly to estradiol17. These new transcriptomic data reported here are required to fully investigate hormonal regulation of this progenitor cell type in the CNS.
RGCs express multiple immune system genes
The GO analysis also identified the presence of many immune system pathways, cytokines, and cytokine receptors in RGCs, indicating that fish RGCs may have important functions in brain immune reactivity. In fish brain, RGCs are the main macroglia due to the lack of typical stellate astrocytes9, thus RGCs likely share some functions with mammalian astrocytes in order to maintain brain homeostasis and to regulate neuroinflammation58–60. The transcriptome of goldfish RGCs reveal key proinflammatory molecules and receptors, including interleukin/interleukin receptors, toll-like receptors, tumor necrosis factor/tumor necrosis factor receptors, and transforming-growth factor β (TGFβ). Although their roles in neuroinflammation are less established than those in astrocytes, RGCs in fish brain respond to inflammatory cues to initiate the regenerative response. Unlike mammals, in which brain injury and neuroinflammation can result in glial scarring that obstructs neurogenesis61, neuroinflammation in fish directly activates RGC proliferation and subsequent neurogenesis through leukotriene C4 signaling62. In addition, the expression of chemokine/chemokine receptors implicates RGCs in the control of processes such as neural migration, axonal guidance, and neural regeneration that involve chemotaxis63–65. Zebrafish RGCs express the chemokine Cxcl2 and its receptor Cxcr465 and also the chemokine receptor Cxcr5, which regulates RGC proliferation and differentiation66. Thus, the immune system appears to be a prominent functional theme based this transcriptomic study.
The secretogranin-derived peptide SNa regulates multiple functions in cultured goldfish RGCs
The close neuroanatomical relationship between abundant RGCs and soma of SNa-immunoreactive neurons in the preoptic nucleus of female goldfish20 led us to postulate that SNa is a regulator of RGC physiology. It is known that these preoptic SNa-positive cells are the magnocellular and parvocellular neurons expressing the nonapeptides isotocin and vasotocin67,68, the fish homologs of mammalian oxytocin and vasopressin, respectively. Our data have identified the expression of both oxytocin (oxtr), and vasopressin (avpr2l) in RGCs providing further support for potential interactions between these neuropetidergic neurons and RGCs.
We report that SNa decreased the expression of smad6b, nab1a, and fgf4 in cultured goldfish. The mammalian homologue Smad6 is a negative regulator of bone morphogenic proteins69 and this gene has been linked to the regulation of stem/progenitor cell behaviours70. Our study showed that SNa decreased nab1a mRNA, the orthologue to mammalian nab1. Nerve growth factor-induced gene A binding protein 1 (NAB1; also called EGR1 binding protein 1) is an active corepressor that negatively regulates the transcriptional activity of nerve growth factor-induced gene A (NGFI-A)71. Furthermore, NGFI-A is an immediate-early gene and is responsive to various extracellular stimuli such as growth factors, hormones, and neurotransmitters in order to regulate the growth, proliferation, and differentiation of a variety of cell types72, including astrocyte growth and proliferation73,74. As a corepressor, NAB1 completely blocks transcription facilitated by NGFI-A71. Therefore, SNa-induced decreases in NAB1A may modulate NGFI-A-dependent processes in RGCs. It was also shown here that SNa reduced the relative expression levels of fgf4. The Fgfs are polypeptides that regulate cell proliferation, differentiation, and migration in the developing and mature CNS75, including mammalian RGC self-renewal and differentiation76,77. These observations and our pathway analyses all support the proposal that SNa regulates networks implicated in glial cell development, neurogenesis and neuronal proliferation.
Many SNa-regulated transcripts in RGCs are related to cell-cell junctions and cell-cell communication. Transcripts involved in both tight (claudins, JAMs) and adherens (nectin, cadherins) junction assembly were significantly affected by SNa. Cell-cell adhesion molecules are important in neuronal cell migration, axon guidance, synapse formation, and in forming glial networks78. As SNa regulates genes involved in the assembly of adherens and tight junctions, this neuropeptide is thus implicated in regulating processes that involve glial-glial and/or glial-neuron interactions.
A major finding in this study is that multiple immune response pathways in RGCs were affected by SNa exposure. Gene set enrichment revealed that IL-6R signaling, TNFR signaling, T-cell activation, and several T-cell receptor signaling pathways were preferentially affected by SNa. It has been established that other neuropeptides such as vasoactive intestinal peptide79, substance P80, and somatostatin81 can affect IL-6 expression in astrocytes, and SNa may fall into this category. SNa also increased many immune system processes based upon the transcriptome profiles, some of which included immune system activation, phagocyte activity, leukocyte function, macrophage response, lymphocyte aggregation, and activation. Regulation of immune system responses has been previously reported for SN in other cell types. For example, after experimental autoimmune encephalomyelitis is induced in the rat brain, there is a close correlation between SN-immunoreactivity and macrophage infiltration, indicating that SN may play a role in leukocyte recruitment82. Both in vivo and in vitro, SN can stimulate the migration of human monocytes, also acting synergistically with other sensory neuropeptides like substance P or somatostatin37. SN has also been shown to be a chemoattractant for human eosinophils, comparable in activity to interleukin-883 and SN can stimulate natural killer cell migration and cytokine release84. We propose that SNa may be acting as a proinflammatory regulator of goldfish RGCs, although further studies are needed to directly test this hypothesis.
A number of pathways related to CNS processes were regulated by SNa in cultured RGCs, demonstrating that SNa may have diverse functional roles in the brain. These included memory, neurogenesis, transmission of nerve impulses, synaptic transmission and plasticity, axon extension and guidance, and neuron and glial cell development among others. Overall, pathways in this general biological theme of “CNS-related” showed increased expression compared to untreated RGCs, suggesting that SNa has a largely stimulatory effect on these biological processes. RGCs are neuronal precursors in the adult fish CNS and are involved in the guidance of migrating young neurons85. Mammalian SN has been shown to act as a trophic peptide stimulating neurite outgrowth27 and can also activate the Jak2/Stat3 pathway to promote neuroprotection and enhance neurogenesis in murine models of stroke28. Perturbations in the gene networks identified here are consistent with documented neurogenic and neuroprotective effects of SN. It is tempting to speculate on the importance of SNa-regulated transcripts in RGCs that may be involved in some aspects of neurogenesis and synaptic plasticity. To the best of our knowledge, there are no comparable data available showing effects of SN on a glial cell type. Therefore, it will be necessary to specifically address this question in future research. Nevertheless, key regulators such as neurogenic differentiation factor 286 and myelin transcription factor-like 187 among others were expressed in goldfish RGCs and may be regulated by SNa.
Conclusion
RGCs express an array of hormone, neurotransmitter, and neuropeptide receptors, suggesting a multiplicity of new functions critical to neuronal-glial communication. The identification of immune system pathways, proinflammatory signals and receptors indicate that RGCs may also be involved in regulating neuroinflammation processes in the fish brain. It will be important to determine the neuroanatomical localization of many of these genes to link them to in vivo functions. We show that exposure to the neuropeptide SNa modulated the expression of multiple genes in pathways associated with CNS and immune function. Several independent lines of evidence suggest that the yet unidentified SN receptor is G-protein coupled88. How key components of GPCR signaling pathways are linked to a putative membrane SN receptor in RGCs will be the focus of our future research. We hope that our annotated RGC transcriptome will provide a resource for future studies.
Electronic supplementary material
Acknowledgements
Funding from the NSERC Discovery program (VLT), NSERC, CGSM, and Michael Smith Foreign Study Award (DDF), University of Ottawa International Research Acceleration Program (to VLT and WH), University of Ottawa Research Chair in Neuroendocrinology (VLT) and the Ontario Trillium Schoralship (LX) are acknowledged with appreciation. The help of Timea Martin with preliminary transcriptome assembly is acknowledged with appreciation.
Author Contributions
D.D.F., X.L., W.H. and V.L.T. designed and performed the experiments, D.D.F., C.M., A.P. and N.C. analysed the data. D.D.F. and V.L.T. wrote the manuscript. All authors contributed to the writing and reviewing of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14930-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Götz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 2.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 3.Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141:2182–94. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 4.Brunne B, et al. Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus. Glia. 2010;58:1553–69. doi: 10.1002/glia.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ming G, Song H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandel H, Brand M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 2013;223:131–47. doi: 10.1007/s00427-012-0425-5. [DOI] [PubMed] [Google Scholar]
- 7.Zupanc GKH. Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol. 2006;192:649–670. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 8.Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. Trans. R. Soc. B Biol. Sci. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp L, Wolburg H, Mack AF. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010;518:4277–4287. doi: 10.1002/cne.22481. [DOI] [PubMed] [Google Scholar]
- 10.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–55. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrini E, et al. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen. Comp. Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Tong S-K, et al. A cyp19a1b-gfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis. 2009;47:67–73. doi: 10.1002/dvg.20459. [DOI] [PubMed] [Google Scholar]
- 13.Than-Trong E, Bally-Cuif L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia. 2015;63:1406–1428. doi: 10.1002/glia.22856. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MB, et al. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat. Neurosci. 2015;18:637–646. doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen ER, et al. Fixed single-cell transcriptomic characterization of human radial glial diversity. Nat. Methods. 2015;13:87–93. doi: 10.1038/nmeth.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing L, McDonald H, Da Fonte DF, Gutierrez-Villagomez JM, Trudeau VL. Dopamine D1 receptor activation regulates the expression of the estrogen synthesis gene aromatase B in radial glial cells. Front. Neurosci. 2015;9:1–12. doi: 10.3389/fnins.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing L, Esau C, Trudeau VL. Direct Regulation of Aromatase B Expression by 17β-Estradiol and Dopamine D1 Receptor Agonist in Adult Radial Glial Cells. Front. Neurosci. 2015;9:504. doi: 10.3389/fnins.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing L, Martyniuk CJ, Esau C, DaFonte DF, Trudeau VL. Proteomic profiling reveals dopaminergic regulation of progenitor cell functions of goldfish radial glial cells in vitro. J. Proteomics. 2016;144:123–132. doi: 10.1016/j.jprot.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Pérez MR, et al. Relationships between radial glial progenitors and 5-HT neurons in the paraventricular organ of adult zebrafish - potential effects of serotonin on adult neurogenesis. Eur. J. Neurosci. 2013;38:3292–3301. doi: 10.1111/ejn.12348. [DOI] [PubMed] [Google Scholar]
- 20.Da Fonte, D. F., Xing, L., Mikwar, M. & Trudeau, V. L. Secretoneurin-A inhibits aromatase B (cyp19a1b) expression in female goldfish (Carassius auratus) radial glial cells. Gen. Comp. Endocrinol. 10.1016/j.ygcen.2017.04.014 (2017). [DOI] [PubMed]
- 21.Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H. Secretoneurin—a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C) Neuroscience. 1993;53:359–365. doi: 10.1016/0306-4522(93)90200-Y. [DOI] [PubMed] [Google Scholar]
- 22.Fisher-Colbrie R, Laslop A, Kirchmair R. Secretogranin II: Molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog. Neurobiol. 1995;46:49–70. doi: 10.1016/0301-0082(94)00060-U. [DOI] [PubMed] [Google Scholar]
- 23.Zhao E, Hu H, Trudeau VL. Secretoneurin as a hormone regulator in the pituitary. Regul. Pept. 2010;165:117–122. doi: 10.1016/j.regpep.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa H, Takata K. The granin family–its role in sorting and secretory granule formation. Cell Struct. Funct. 1995;20:415–20. doi: 10.1247/csf.20.415. [DOI] [PubMed] [Google Scholar]
- 25.Zhao E, Zhang D, Basak A, Trudeau VL. New insights into granin-derived peptides: evolution and endocrine roles. Gen. Comp. Endocrinol. 2009;164:161–74. doi: 10.1016/j.ygcen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Fischer-Colbrie R, Kirchmair R, Kähler CM, Wiedermann CJ, Saria A. Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr. Protein Pept. Sci. 2005;6:373–85. doi: 10.2174/1389203054546334. [DOI] [PubMed] [Google Scholar]
- 27.Gasser MC, Berti I, Hauser KF, Fischer-Colbrie R, Saria A. Secretoneurin promotes pertussis toxin-sensitive neurite outgrowth in cerebellar granule cells. J. Neurochem. 2003;85:662–9. doi: 10.1046/j.1471-4159.2003.01677.x. [DOI] [PubMed] [Google Scholar]
- 28.Shyu W-C, et al. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J. Clin. Invest. 2008;118:133–148. doi: 10.1172/JCI32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saria A, et al. Secretoneurin releases dopamine from rat striatal slices: a biological effect of a peptide derived from secretogranin II (chromogranin C) Neuroscience. 1993;54:1–4. doi: 10.1016/0306-4522(93)90377-R. [DOI] [PubMed] [Google Scholar]
- 30.Agneter E, et al. Sustained dopamine release induced by secretoneurin in the striatum of the rat: a microdialysis study. J. Neurochem. 1995;65:622–5. doi: 10.1046/j.1471-4159.1995.65020622.x. [DOI] [PubMed] [Google Scholar]
- 31.You ZB, et al. Effects of secretogranin II-derived peptides on the release of neurotransmitters monitored in the basal ganglia of the rat with in vivo microdialysis. Naunyn. Schmiedebergs. Arch. Pharmacol. 1996;354:717–24. doi: 10.1007/BF00166897. [DOI] [PubMed] [Google Scholar]
- 32.Hasslacher J, et al. Secretoneurin as a marker for hypoxic brain injury after cardiopulmonary resuscitation. Intensive Care Med. 2014;40:1518–1527. doi: 10.1007/s00134-014-3423-4. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann WA, et al. Synaptic loss reflected by secretoneurin-like immunoreactivity in the human hippocampus in Alzheimer’s disease. Eur. J. Neurosci. 1998;10:1084–1094. doi: 10.1046/j.1460-9568.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 34.Lechner T, et al. Chromogranin peptides in Alzheimer’s disease. Exp. Gerontol. 2004;39:101–113. doi: 10.1016/j.exger.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Marti E, Blasi J, Ferrer I. Early induction of secretoneurin expression following kainic acid administration at convulsant doses in the rat and gerbil hippocampus. Hippocampus. 2002;12:174–185. doi: 10.1002/hipo.1103. [DOI] [PubMed] [Google Scholar]
- 36.Zhao E, Basak A, Trudeau VL. Secretoneurin stimulates goldfish pituitary luteinizing hormone production. Neuropeptides. 2006;40:275–282. doi: 10.1016/j.npep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Reinisch N, et al. Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett. 1993;334:41–44. doi: 10.1016/0014-5793(93)81676-Q. [DOI] [PubMed] [Google Scholar]
- 38.Kong C, et al. Secretoneurin and chemoattractant receptor interactions. J. Neuroimmunol. 1998;88:91–98. doi: 10.1016/S0165-5728(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 39.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu B, Fu L, Sun S, Li W. Artificial and natural duplicates in pyrosequencing reads of metagenomic data. BMC Bioinformatics. 2010;11:187. doi: 10.1186/1471-2105-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44:D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anders S, et al. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Untergasser A, et al. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115–e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heckmann L-H, Sørensen PB, Krogh PH, Sørensen JG. NORMA-Gene: a simple and robust method for qPCR normalization based on target gene data. BMC Bioinformatics. 2011;12:250. doi: 10.1186/1471-2105-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martyniuk CJ, Denslow ND. Exploring Androgen-Regulated Pathways in Teleost Fish Using Transcriptomics and Proteomics. Integr. Comp. Biol. 2012;52:695–704. doi: 10.1093/icb/ics072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martyniuk CJ, Alvarez S, Lo BP, Elphick JR, Marlatt VL. Hepatic Protein Expression Networks Associated with Masculinization in the Female Fathead Minnow (Pimephales promelas) J. Proteome Res. 2012;11:4147–4161. doi: 10.1021/pr3002468. [DOI] [PubMed] [Google Scholar]
- 48.Xing L, Goswami M, Trudeau VL. Radial glial cell: critical functions and new perspective as a steroid synthetic cell. Gen. Comp. Endocrinol. 2014;203:181–5. doi: 10.1016/j.ygcen.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Diotel N, et al. Nuclear Progesterone Receptors Are Up-Regulated by Estrogens in Neurons and Radial Glial Progenitors in the Brain of Zebrafish. PLoS One. 2011;6:e28375. doi: 10.1371/journal.pone.0028375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diotel N, et al. The brain of teleost fish, a source, and a target of sexual steroids. Front. Neurosci. 2011;5:137. doi: 10.3389/fnins.2011.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beraudi A, et al. Distribution and expression of A1 adenosine receptors, adenosine deaminase and adenosine deaminase-binding protein (CD26) in goldfish brain. Neurochem. Int. 2003;42:455–64. doi: 10.1016/S0197-0186(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 52.Uckermann O, Grosche J, Reichenbach A, Bringmann A. ATP-evoked calcium responses of radial glial (Müller) cells in the postnatal rabbit retina. J. Neurosci. Res. 2002;70:209–218. doi: 10.1002/jnr.10406. [DOI] [PubMed] [Google Scholar]
- 53.Song J, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jurič DM, Kržan M, Lipnik-Stangelj M. Histamine and astrocyte function. Pharmacol. Res. 2016;111:774–83. doi: 10.1016/j.phrs.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 55.Mohan V, et al. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp. Neurol. 2012;237:477–488. doi: 10.1016/j.expneurol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Stenzel D, Wilsch-Brauninger M, Wong FK, Heuer H, Huttner WB. Integrin αvβ3 and thyroid hormones promote expansion of progenitors in embryonic neocortex. Development. 2014;141:795–806. doi: 10.1242/dev.101907. [DOI] [PubMed] [Google Scholar]
- 57.Coumailleau P, et al. Aromatase, estrogen receptors and brain development in fish and amphibians. Biochim. Biophys. Acta. 2015;1849:152–62. doi: 10.1016/j.bbagrm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyritsis N, et al. Acute Inflammation Initiates the Regenerative Response in the Adult Zebrafish Brain. Science (80-.). 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 63.Stumm RK, et al. A Dual Role for the SDF-1/CXCR4 Chemokine Receptor System in Adult Brain: Isoform-Selective Regulation of SDF-1 Expression Modulates CXCR4-Dependent Neuronal Plasticity and Cerebral Leukocyte Recruitment after Focal Ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stumm R, Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J. Mol. Endocrinol. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- 65.Diotel N, et al. Cxcr4 and Cxcl12 expression in radial glial cells of the brain of adult zebrafish. J. Comp. Neurol. 2010;518:4855–76. doi: 10.1002/cne.22492. [DOI] [PubMed] [Google Scholar]
- 66.Kizil C, et al. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 2012;7:27. doi: 10.1186/1749-8104-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canosa LF, et al. Forebrain mapping of secretoneurin-like immunoreactivity and its colocalization with isotocin in the preoptic nucleus and pituitary gland of goldfish. J. Comp. Neurol. 2011;519:3748–65. doi: 10.1002/cne.22688. [DOI] [PubMed] [Google Scholar]
- 68.Pouso P, et al. The secretogranin-II derived peptide secretoneurin modulates electric behavior in the weakly pulse type electric fish, Brachyhypopomus gauderio. Gen. Comp. Endocrinol. 2015;222:158–166. doi: 10.1016/j.ygcen.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 69.Wrana JL. Regulation of Smad Activity. Cell. 2000;100:189–192. doi: 10.1016/S0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 70.Ding Z-Y, et al. Smad6 suppresses the growth and self-renewal of hepatic progenitor cells. J. Cell. Physiol. 2014;229:651–60. doi: 10.1002/jcp.24488. [DOI] [PubMed] [Google Scholar]
- 71.Thiel G, et al. The human transcriptional repressor protein NAB1: Expression and biological activity. Biochim. Biophys. Acta - Gene Struct. Expr. 2000;1493:289–301. doi: 10.1016/S0167-4781(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 72.O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/S0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- 73.Hu RM, Levin ER. Astrocyte Growth Is Regulated By Neuropeptides Through Tis-8 and Basic Fibroblast Growth-Factor. J. Clin. Invest. 1994;93:1820–1827. doi: 10.1172/JCI117167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayer SI, Rossler OG, Endo T, Charnay P, Thiel G. Epidermal-growth-factor-induced proliferation of astrocytes requires Egr transcription factors. J. Cell Sci. 2009;122:3340–3350. doi: 10.1242/jcs.048272. [DOI] [PubMed] [Google Scholar]
- 75.Reuss B. & von Bohlen und Halbach, O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- 76.Kang W, Wong LC, Shi S-H, Hébert JM. The transition from radial glial to intermediate progenitor cell is inhibited by FGF signaling during corticogenesis. J. Neurosci. 2009;29:14571–80. doi: 10.1523/JNEUROSCI.3844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahara S, O’Leary DDM. Fgf10 Regulates Transition Period of Cortical Stem Cell Differentiation to Radial Glia Controlling Generation of Neurons and Basal Progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Togashi H, Sakisaka T, Takai Y. Cell adhesion molecules in the central nervous system. Cell Adh. Migr. 2009;3:29–35. doi: 10.4161/cam.3.1.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maimone D, et al. Norepinephrine and vasoactive intestinal peptide induce IL-6 secretion by astrocytes: Synergism with IL-1β and TNFα. J. Neuroimmunol. 1993;47:73–81. doi: 10.1016/0165-5728(93)90286-8. [DOI] [PubMed] [Google Scholar]
- 80.Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J. Neuroimmunol. 1994;51:101–8. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 81.Grimaldi M, Florio T, Schettini G. Somatostatin inhibits interleukin 6 release from rat cortical type I astrocytes via the inhibition of adenylyl cyclase. Biochem. Biophys. Res. Commun. 1997;235:242–8. doi: 10.1006/bbrc.1997.6513. [DOI] [PubMed] [Google Scholar]
- 82.Storch MK, et al. Co-localization of secretoneurin immunoreactivity and macrophage infiltration in the lesions of experimental autoimmune encephalomyelitis. Neuroscience. 1996;71:885–893. doi: 10.1016/0306-4522(95)00476-9. [DOI] [PubMed] [Google Scholar]
- 83.Dunzendorfer S, Schratzberger P, Reinisch N, Kähler CM, Wiedermann CJ. Secretoneurin, a novel neuropeptide, is a potent chemoattractant for human eosinophils. Blood. 1998;91:1527–32. [PubMed] [Google Scholar]
- 84.Feistritzer C, Mosheimer BA, Colleselli D, Wiedermann CJ, Kähler CM. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul. Pept. 2005;126:195–201. doi: 10.1016/j.regpep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Zupanc GKH, Clint SC. Potential role of radial glia in adult neurogenesis of teleost fish. Glia. 2003;43:77–86. doi: 10.1002/glia.10236. [DOI] [PubMed] [Google Scholar]
- 86.Lee J, et al. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science (80-.). 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 87.Mall M, et al. Myt1l safeguards neuronal identity by actively repressing many non-neuronal fates. Nature. 2017;544:245–249. doi: 10.1038/nature21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trudeau VL, et al. Is secretoneurin a new hormone? Gen. Comp. Endocrinol. 2012;175:10–8. doi: 10.1016/j.ygcen.2011.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.