Obese, insulin-resistant men responded to 16 wk of progressive resistance training with muscle hypertrophy and increased strength and a shift in muscle fiber composition toward fast-twitch, type IIx fibers. Activation of muscle mTOR was increased by 8 wk but did not increase further at 16 wk despite continued augmentation of peak power and rate of force generation.
Keywords: metabolic syndrome, fiber hypertrophy, fiber shift, insulin resistance, euglycemic clamp
Abstract
Resistance training of healthy young men typically results in muscle hypertrophy and a shift in vastus lateralis composition away from type IIx fibers to an increase in IIa fiber content. Our previous studies of 8 wk of resistance training found that many metabolic syndrome men and women paradoxically increased IIx fibers with a decrease in IIa fibers. To confirm the hypothesis that obese subjects might have muscle remodeling after resistance training very different from healthy lean subjects, we subjected a group of nine obese male volunteers to progressive resistance training for a total of 16 wk. In these studies, weight loss was discouraged so that muscle changes would be attributed to the training alone. Detailed assessments included comparisons of histological examinations of needle biopsies of vastus lateralis muscle pretraining and at 8 and 16 wk. Prolonging the training from 8 to 16 wk resulted in increased strength, improved body composition, and more muscle fiber hypertrophy, but euglycemic clamp-quantified insulin responsiveness did not improve. Similar to prior studies, muscle fiber composition shifted toward more fast-twitch type IIx fibers (23 to 42%). Eight weeks of resistance training increased the muscle expression of phosphorylated Akt2 and mTOR. Muscle GLUT4 expression increased, although insulin receptor and IRS-1 expression did not change. We conclude that resistance training of prediabetic obese subjects is effective at changing muscle, resulting in fiber hypertrophy and increased type IIx fiber content, and these changes continue up to 16 wk of training.
NEW & NOTEWORTHY Obese, insulin-resistant men responded to 16 wk of progressive resistance training with muscle hypertrophy and increased strength and a shift in muscle fiber composition toward fast-twitch, type IIx fibers. Activation of muscle mTOR was increased by 8 wk but did not increase further at 16 wk despite continued augmentation of peak power and rate of force generation.
human skeletal muscle fiber type plasticity is a well-established phenomenon (22). Resistance exercise training of healthy young men and women has consistently resulted in a shift within fast-twitch fibers from type IIx to type IIa (17, 24). Training and detraining reports show shifts back and forth between types IIa and IIx, with increased IIa after resistance training being found consistently (10, 23). Endurance training increases type I slow-twitch fibers, with proportional decreases in type II fibers (11). Twelve weeks of bike training in older, nonobese men has been reported to cause a shift away from IIx to IIa fibers (9). In contrast, there have been shifts away from type I to type IIa and IIx with intense training of sprint runners (12). These studies and many others have found that both endurance and resistance training or detraining have resulted in shifts in muscle fiber composition along a continuum of type I to type IIa to type IIx and back again (17, 18).
The resistance training protocol that was used for 16 wk was a modification of block periodization (21). Block periodization is a program of sequential stages of exercise training that is designed to provide optimal training efficiency among athletes (6, 7, 16). The three stages of block periodization typically move a participant through more general fitness to more specific targeted characteristics. The stages, called “accumulation,” “transmutation,” and “realization”, constitute sequential programming of exercises (loads, reps, sets, etc.). Accumulation emphasizes higher volume but less specific training that results in improved overall work capacity and basic strength. Transmutation entails more specific exercises with lower volumes and higher intensities of training that can increase the maximum strength achieved for a target exercise. Realization typically deals with very specific exercises with increasing intensity and further decreases in reps and sets, resulting in a taper of volume (sets × reps × load) toward the end of this stage.
In our previous studies of exercise training, 8-wk duration using an accumulation phase was ineffective at changing the insulin resistance of metabolic syndrome men and women (15, 27, 28). Longer and more extensive training might be needed in subjects who were profoundly sedentary before beginning an exercise intervention that was intended to decrease insulin resistance as a way of decreasing the risk of developing type 2 diabetes. The studies described in this report repeated our previous 8-wk resistance training and then added another 8 wk of progressively increasing intensity and volume training for nine obese, prediabetic men.
In contrast to studies of healthy, lean young people, our prior reports of 8 wk of either resistance or endurance training of previously sedentary metabolic syndrome men and women showed a shift from type IIa to IIx fibers (15, 27, 28). The design of the study we report was intended to test the hypothesis that longer and more intense training would bring obese, prediabetic men closer to a status similar to healthy, lean men, and this would move the composition of their further hypertrophied muscle back away from type IIx fibers toward more type IIa fibers.
METHODS
Subject Selection
Eleven men who were obese (BMI >3.0 kg/m2) and had a close family member with type 2 diabetes were recruited. A detailed pretraining physical activity questionnaire was completed by each subject. None had performed regular exercise for ≥6 mo. No subject was taking any prescribed medications. Specifically, there were no antihypertensive or antidiabetic medications. Each subject had a pretraining assessment, with the assessments repeated after 8 and 16 wk of resistance training. These measurements included body composition, V̇o2max, strength quantifications, insulin responsiveness by euglycemic clamp, and percutaneous needle biopsy of the vastus lateralis muscle. At each of the assessment time points, fasting glucose, insulin, and lipids were measured. Two subjects did not complete all of the protocol, and their data are not included in the analysis. The average age was 40, with a range of 30 to 54. Pretraining aerobic fitness of these subjects (26.9 ± 1.0 ml·kg−1·min−1) was similar to previous groups of men and women that we have studied (15, 28), where sedentary lean control subjects in these previous studies averaged 30.6 ± 1.8. This study and the written, informed consent documents were approved by the East Tennessee State University Institutional Review Board.
Block Periodization (Accumulation Phase) Training Protocol
All subjects spent 1 wk being oriented to the testing and training equipment and then having measurements of body composition, maximum isometric strength and rate of force development, unloaded and loaded jump heights, and V̇o2max. In the second week, euglycemic insulin infusion studies and percutaneous muscle biopsies were performed. The third week of the protocol was the first week of resistance training. Training consisted of supervised specific exercises each of the 5 weekdays (21). Saturdays were considered a very light training day, and subjects were instructed to perform specific stretching exercise on their own. Table 1 lists the training exercises distributed through each week. Each day the exercises were performed in the sequence indicated. During weeks 1–4, the target set and repetition scheme was 3 × 10; during weeks 5–8, the target was 4 × 5; during weeks 9–12, the target was 4 × 10; and during weeks 13–16, the target was 4 × 5. Thus training volume was maintained at relatively high levels throughout the 16 wk. Training was similar to that carried out by strength power athletes (15) and consisted primarily of large muscle mass multijoint exercises such as squats and pulling movements (e.g., clean pulls). Training occurred 5 days/wk, with light days devoted to midsection work on Tuesdays and Thursdays during weeks 1–4 and 9–12 and on Wednesdays during weeks 5–8 and 9–13. Subjects were closely supervised by trained National Strength and Conditioning Association- or USA Weighlifting-certified strength and conditioning coaches. Weights were adjusted by the subjects and coaches such that the intensity (load lifted) was steadily progressed, resulting in a slow but steady increase in work volume. Fatigue was managed by using heavy and light days (15) and by observation of the coaches.
Table 1.
Resistance training protocol: 4 blocks of 4 wk each
| Exercise Sequence No. | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| Block I (weeks 1–4), 10 repetitions × 3 sets | ||||||
| 1 | Squats | Bent-legged situps | Shoulder shrugs | Bent-legged situps | Squats | Stretch |
| 2 | Bench press | Supine windshield wipers | Mid-thigh pulls | Supine windshield wipers | Bench press | |
| 3 | Seated dumbbell press | Stretch | Stiff-legged Deadlifts | Stretch | Seated dumbbell Press | |
| 4 | Front Raises | Bentover rows | Front raises | |||
| 5 | Bicep curls | |||||
| Block II (weeks 5–8), 5 repetitions × 4 sets | ||||||
| 1 | Squats* | Shoulder shrugs | Basket hangs 3 × 10 | Shoulder shrugs | Squats* | Stretch |
| 2 | Squat press | Mid-thigh Pulls* | Supine windshield wipers 3 × 10 | Mid-thigh pulls* | Squat press | |
| 3 | Dumbbell incline press* | Stiff-legged deadlifts | Stretch | Stiff-legged deadlifts | Dumbbell incline press* | |
| 4 | Lateral raises | Upright row | Upright row | Lateral raises | ||
| 5 | Assisted pullups | Assisted pullups | ||||
| Block III (weeks 9–12), 10 repetitions × 4 sets | ||||||
| 1 | Squats | Bent-legged situps | Shoulder shrugs | Bent-legged situps | Squats | Stretch |
| 2 | Incline press, 45° | Supine windshield wipers | Mid-thigh pulls | Supine windshield wipers | Incline press, 45° | |
| 3 | Seated dumbbell press | Leg lifts | Stiff-legged deadlifts | Leg lifts | Seated dumbbell press | |
| 4 | Front raises | Stretch | Bentover rows | Stretch | Front raises | |
| 5 | Bicep curls | |||||
| Block IV (weeks 13–16), 5 repetitions × 4 sets | ||||||
| 1 | Squats* | Shoulder shrugs | Basket hangs 3 × 10 | Shoulder shrugs | Squats* | Stretch |
| 2 | Squat press | Mid-thigh pulls* | Supine windshield wipers 3 × 10 | Mid-thigh pulls* | Squat press | |
| 3 | Dumbbell incline press* | Stiff-legged deadlifts | Stretch | Stiff-legged deadlifts | Dumbbell incline press* | |
| 4 | Lateral raises | Upright row | Upright row | Lateral raises | ||
| 5 | Assisted pullups | Assisted pullups | ||||
Followed by a down set 1 × 5.
Methods
Euglycemic hyperinsulinemic clamp.
After a 2-h baseline period, an insulin infusion at 40 mU·m2·min−1 was performed for 2 h to achieve a physiological insulin concentration of ∼50 μU/ml and a steady-state glucose infusion rate (ssGIR) to quantify insulin sensitivity (26).
Fasting serum glucose and insulin.
After a 12-h overnight fast, blood was obtained in triplicate for measuring glucose and insulin, as previously described (19).
Body composition.
Body fat mass and lean body mass was measured by air displacement plethysmography (BodPod, Concord, CA).
Strength testing.
Maximum leg and hip strength was assessed isometrically using a custom-built lifting rack and standard methods (15). Data were collected and analyzed for peak force and rate of force development using custom Labview 8.6 software (National Instruments, Upper Saddle River, NJ). Strength testing was done before and after the 8- and 16-wk programs.
Quantification of jump height.
Maximal jump height was measured unloaded and with a 20-kg load, as described previously (14). The studies were performed on a 91.4 × 91.4-cm force plate (Rice Lake Weighing Systems, Rice Lake, WI) sampling at 1,000 Hz. Subjects were instructed to squat to a knee angle of 90°. A 3-s countdown was given followed by the “jump” command. Two unloaded practice jumps were performed, followed by two full effort trials. Subjects performed the jumps using two different external loads (0-kg PVC pipe and 20-kg Olympic barbell) held on their backs across their shoulders. One minute of rest was given after the first trial and after each subsequent trial. Jump height and power output were recorded and averaged for the two trials under each respective loading condition.
Aerobic capacity testing.
V̇o2max was measured using a Monark Ergomedic 874E cycle ergometer (Monark Exercise, Vansbro, Sweden), using a 10-min modified Astrand protocol of increasing resistance settings on the ergometer. Expired air was analyzed using a TrueOne 2400 Metabolic Measurement System (ParvoMedics, Sandy, UT).
Muscle biopsies.
Percutaneous needle biopsies of vastus lateralis were performed after an overnight fast and 2 h of quiet recumbency using a Bergstrom-Stille 5-mm muscle biopsy needle with suction, as described previously (29). To evaluate the chronic effects of exercise training rather than an acute effect from an exercise bout, muscle biopsies were delayed until 24–48 h after the last training session. The sample was divided in two, with one piece frozen immediately in liquid nitrogen for later analysis. The second piece for microscopy was mounted on cork under a dissecting microscope to orient the specimen for transverse sectioning and quickly frozen in a slurry of isopentane cooled by liquid nitrogen. These specimens were stored at −80°C until the day of sectioning with a Leica cryostat.
Quantification of muscle fiber type composition and fiber size.
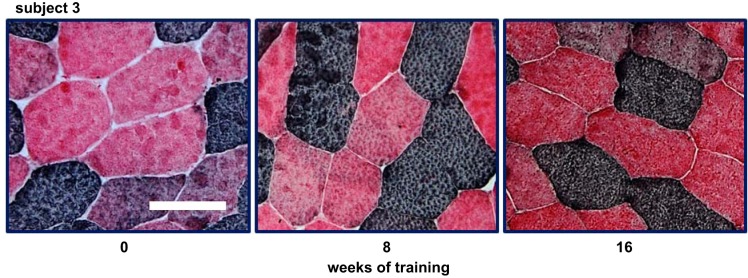
Fiber composition was determined using methods described by Behan et al. (3). This was a bright-field staining method using labeled fast and slow myosin antibodies from Sigma-Aldrich (A4335) and Fisher (50-174-899, NC9996165, and NC9799788). All sections were coded and then quantified independently by two observers who were unaware of which subject the image represented. Figure 1 displays sample bright-field images from biopsies performed on one subject at 0, 8, and 16 wk of training. All fiber size data for the current study were calculated using the minimum diameter measured for each fiber (8). Fiber content is based on counting the number of fibers of each type in images from stained slides. At least 200 fibers were counted for determining percent fiber composition. Fiber diameter was measured in at least 30 fibers of each fiber type for each subject.
Fig. 1.
Examples of fiber type proportions in a prediabetic man undergoing 16 wk of resistance training. Images displayed show examples of a single subject’s fiber type proportions in 3 separate biopsies. The proportions were determined by counting ≥200 transverse-cut fibers for each biopsy, usually 3 or more sections (see methods). This individual was calculated to have 29, 40, and 47% type IIx fibers at 0, 8, and 16 wk of training, respectively. In these images, type I fibers appear dark, type IIx fibers are red, and type IIa fibers appear intermediate or purple. The white bar shown in pretraining image (left) represents 100 µm.
Immunoblot quantification of factors affecting insulin action and fiber hypertrophy in skeletal muscle.
Western blots were performed as described previously (15, 25). Each subject had three separate muscle biopsies pretraining and after 8 and 16 wk of training. For these measurements, muscle was homogenized and subjected to PAGE, transferred to nitrocellulose membranes, and incubated overnight at 4°C with specific antibodies directed at the factor of interest. Images were prepared using a Bio-Rad Image Box and analyzed using Bio-Rad’s Quantity One software. Each quantification was the average of at least two separate blots. For each gel electrophoresis, 10 µg of protein from muscle homogenates was applied to each lane, except for AMPK and GLUT4, where 5 µg of protein was used. Usually, 10% polyacrylamide gels were used, but for mTOR, 3–8% gradient gels were employed. Loading equivalence was verified using α-actin antibodies to identify this housekeeping marker. Antibodies against AMPK, phosphorylated (p)-AMPK, mTOR, p-mTOR, IRS-1, IRS-1 p-Tyr896, and IRS-1 p-Ser636 were purchased from Cell Signaling Technology (Danvers, MA). The antibodies against p-Akt2, 4E-BP1, p-4E-BP1, and the insulin receptor β-subunit antibodies came from Millipore (Bellerica, MA). The antibodies for GLUT4 were obtained from Alpha Diagnostics (San Antonio, TX).
Statistics.
All comparisons for these studies were done using ANOVA with repeated measures (Holm-Sidak method) using SigmaPlot 13 software (Systat Software, San Jose, CA). Significant differences were associated with a P value of <0.05.
RESULTS
Prediabetic Volunteers for this Study Exhibited Profound Obesity and Insulin Resistance
Table 2 lists the baseline and posttraining characteristics of the nine men who completed the 16 wk of progressive resistance training. At baseline, all nine subjects had BMIs >30.0 kg/m2, seven had waist circumferences >102 cm, six had impaired fasting glucose (>100 mg/dl), two had elevated systolic blood pressure (>130 mmHg), and all nine had dyslipidemia Three of nine subjects had both high LDL (>130 mg/dl) and low HDL (<40 mg/dl), four had only low HDL, and two subjects had only high LDL. After 8 and 16 wk of training, weight was not statistically different, and there was no change in fasting glucose or insulin. Body composition did change with an increase in lean body mass and a decrease in fat mass.
Table 2.
Resistance training-related changes in the characteristics of participants
| Training Duration |
||||||
|---|---|---|---|---|---|---|
| 0 wk | P Value (Compare 0 With 8 Wk) | 8 wk | P Value (Compare 8 With 16 Wk) | 16 Wk | P Value (Compare 0 With 16 wk) | |
| BMI | 34.3 ± 1.1 | NS | 34.5 ± 1.3 | NS | 34.6 ± 1.3 | NS |
| LBM, kg | 70.0 ± 2.7 | 0.007 | 71.3 ± 2.9 | 0.042 | 72.2 ± 2.9 | 0.002 |
| Fat mass, kg | 39.1 ± 3.2 | 0.010 | 37.6 ± 3.2 | 0.169 | 36.9 ± 3.2 | <0.001 |
| Fat, % | 35.1 ± 1.7 | NS | 34.1 ± 1.6 | NS | 33.5 ± 1.6 | 0.002 |
| FBS, mg/dl | 97.4 ± 3.4 | NS | 93.7 ± 1.7 | NS | 96.8 ± 3.6 | NS |
| Insulin, μU/ml | 12.5 ± 2.5 | NS | 11.3 ± 2.0 | NS | 15.0 ± 3.0 | NS |
Data shown are the means ± SE for 9 subjects. BMI, body mass index, LBM, lean body mass, FBS, fasting serum glucose concentration; NS, not significant. Comparisons were made using ANOVA with repeated measurements.
Resistance Training Increased Muscle Fiber Size and the Proportion of Type IIx Fibers
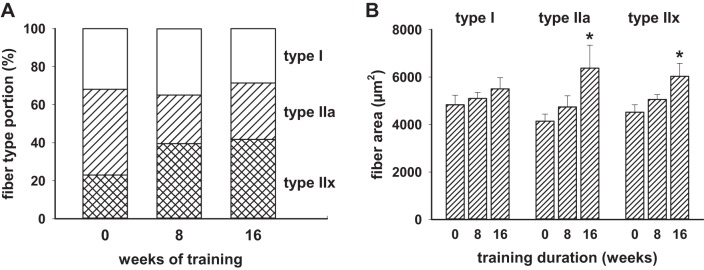
Biopsies of vastus lateralis were performed at baseline, 8 wk of training, and 16 wk of training. Fiber composition at baseline was 35 ± 5% type I, 43 ± 5% type IIa, and 22 ± 5% type IIx. As shown in Fig. 2A, the proportion of type IIa fibers decreased and the type IIx fibers increased by 8 wk (P = 0.007 and P = 0.002, respectively), and this predominance of type IIx fibers was maintained in the 16-wk biopsies (P = 0.021 and P < 0.001, respectively, relative to time 0,). Figure 2B displays the fiber cross-sectional areas for each fiber type. Both type IIa and IIx increased significantly by 16 wk.
Fig. 2.
Skeletal muscle type II fiber proportion shifted away from type IIa to type IIx, and fiber size was increased by resistance training. Nine men with the metabolic syndrome had muscle fiber type proportions and fiber cross-sectional areas assessed after 8 and 16 wk of progressive resistance training. A: as shown graphically, the proportion of type IIa fibers was lower than baseline at 8 (P = 0.007) and 16 wk (P = 0.021), and the %type IIx fibers was significantly higher at 8 (P = 0.002) and 16 wk (P < 0.001). The type I fiber proportion did not change at the 8- or 16-wk times. B: the 9 men showed progressive increases in type II fiber areas that were statistically significant at 16 wk of training.
Sustained Resistance Exercise Training of Prediabetic Obese Men Results in Continued Increase in Strength
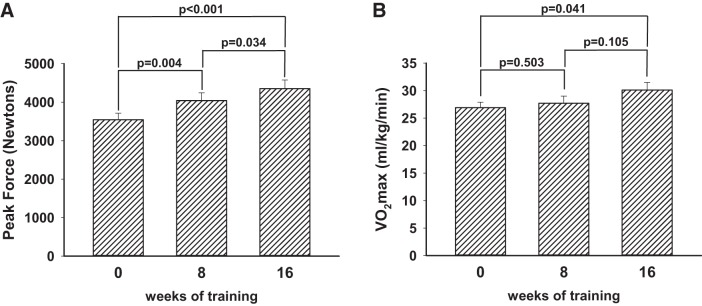
Nine men completed 16 wk of progressive resistance training. Peak force significantly increased at 8 wk and again at 16 wk, as shown in Fig. 3. The rate of force development data showed the same trend, increasing from 4,449 ± 582 Newtons/s to 4,768 ± 483 at 8 wk and 5,611 ± 508 at 16 wk. For the rate of force development, only the difference from baseline to 16 wk was statistically significant (P = 0.048). Aerobic fitness quantified by V̇o2max was increased by 16 wk (Fig. 3B).
Fig. 3.
The impact on strength and endurance of extending resistance training beyond 8 wk to a total of 16 wk. Nine men with the metabolic syndrome completed a total of 16 wk of progressive resistance training. A: change in strength as quantified by peak force generation using a static pull apparatus. By 8 wk, peak force was increased over the baseline data, and at 16 wk there was further statistically significant enhancement. Increases in the rate of force development were similar to the static pull data, with a 7% apparent increase (P = 0.113) at 8 wk and a 26% increase (P = 0.038) at 16 wk. B: the resistance training also resulted in a significant increase in V̇o2max by 16 wk, suggesting increased aerobic capacity as well.
Other dynamic strength testing included quantifying jump height. Each subject performed jump height without loading and with a 20-kg bar across their shoulders. Figure 4 displays these data, indicating improved performance with training at 8 and 16 wk.
Fig. 4.
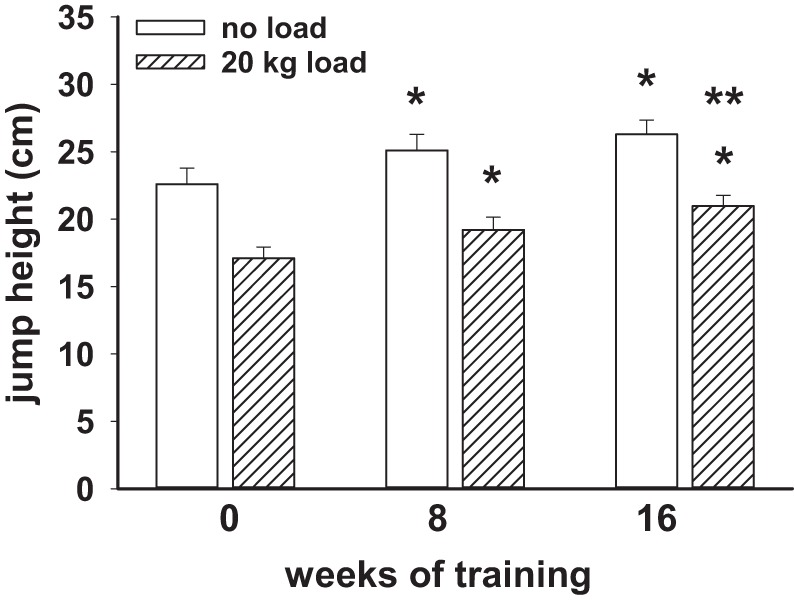
The impact of 8 and 16 wk of resistance training on jump height in 9 obese men. The achieved jump height with no loading was significantly increased at 8 and 16 wk compared with the pretraining data. Jump height with loading by a 20-kg bar across the shoulders was also increased at 8 and 16 wk of training. The loaded jump height at 16 wk was significantly increased over the 8-wk data as well. *Significant difference (P < 0.05) from time 0; **significant increase from the 8-wk data.
The Impact of Progressive Strength Training on Insulin Responsiveness in Prediabetic Obese Men
Euglycemic clamp data showed profound insulin resistance that changed very little at 8 and 16 wk of progressive resistance training (Fig. 5).
Fig. 5.
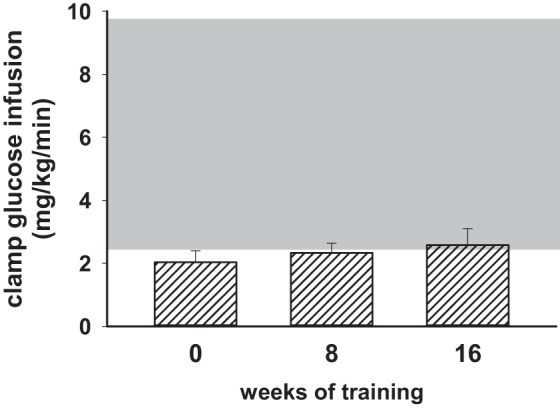
Insulin responsiveness quantified by euglycemic clamps did not change significantly after 8 or 16 wk of resistance training. Shaded area represents the mean ± 2 SD of the steady-state glucose infusion rate determined in 16 lean but sedentary control subjects from previously published studies. The group of 9 men with the metabolic syndrome did not increase their insulin responsiveness at 8 or 16 wk of training despite increases in strength and aerobic capacity that demonstrated the effectiveness of the training program on changing these 2 parameters.
Expression of Muscle FiberHypertrophy-Related Factors and Insulin Action Components After 8 and 16 Wk of Resistance Training
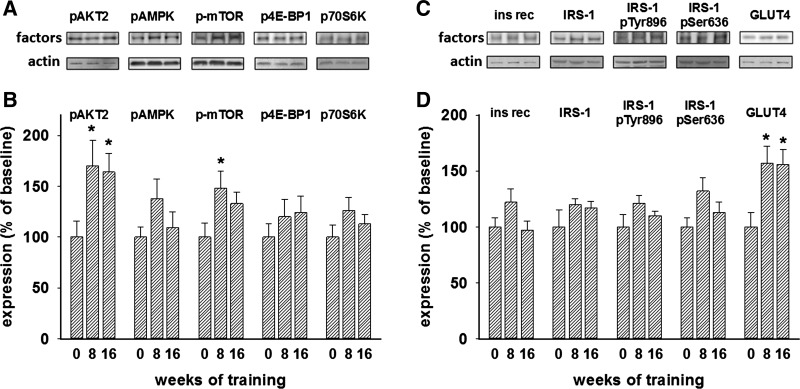
Figure 6, A and B, displays the relative expression of four activated kinases and a target of mTOR in the muscle of the nine obese men who underwent a total of 16 wk of progressive resistance training. Phosphorylated Akt2 and mTOR were increased at 8 wk but did not further increase despite continued increases in strength and fiber cross-sectional areas determined at 16 wk of training (Figs. 2B and 3A). The potential increases in phosphorylation of targets downstream of mTOR, 4E-BP1 and S6K, did not achieve statistical significance in these studies. Figure 6, C and D, shows that expression of insulin receptors and insulin receptor substrate-1 (IRS-1) did not significantly change at 8 or 16 wk of training. Levels of phosphorylation of IRS-1 at Tyr896 and Ser636 after an overnight fast were not changed by the training intervention. GLUT4 in muscle increased 54% at 8 wk compared with pretraining expression, and this increase was maintained at 16 wk. The representative immunoblots in Fig. 6, A and C, are each from the three consecutive samples from single subjects at 0, 8, and 16 wk of training.
Fig. 6.
Expression of fiber hypertrophy-related factors and insulin action pathway components in muscle of prediabetic obese men who participated in progressive resistance training for a total of 16 wk. A and C: sample blot images for each of the components represented by the bars at the bottom. Each of these representative images contains 3 lanes from a single subject at times 0, 8, and 16 wk of training. Below the blot samples for “factors” are lanes from blots probed for actin to verify consistent protein loading. B: summary data related to the muscle hypertrophy pathways. These data are the phosphorylated proteins indicated. Each bar shown represents the relative expression and SE for 9 subjects before training, after 8 wk of training, and after 16 wk of training. The image analysis software assigned intensity on an arbitrary scale. The pretraining biopsy was termed baseline. The mean of the pretraining band intensity was assigned 100% for each of the proteins quantified in this way. The activated Akt2 was expressed higher than baseline at 8 and 16 wk, and phosphorylated mTOR was significantly greater than baseline after 8 wk of training. D: summary data for insulin action pathway factors assessed in Western blots of muscle homogenates. Bars and SE shown here represent the data from at least 2 separate immunoblots for each subject for each of the factors shown. Only the 8- and 16-wk expression of muscle GLUT4 was significantly different from the pretraining sample determinations. The pretraining quantification was arbitrarily assigned 100% scale. *Significant increase (P > 0.05) from pretraining baseline using ANOVA with repeated measurements.
DISCUSSION
Sixteen weeks of progressive resistance training in men with the metabolic syndrome using a modified block periodization protocol resulted in increased strength, increased lean body mass, muscle fiber hypertrophy, and an increased proportion of type IIx fibers in vastus lateralis muscle. Body weights were maintained at the preintervention level, and insulin resistance of these men was unchanged by the exercise training intervention.
Eight weeks of resistance training in these subjects resulted in significantly increased activation (phosphorylation) of muscle mTOR, but we did not detect an increase in the phosphorylation of 4E-BP1 or S6K, both of which are downstream targets of activated mTOR. Akt2 phosphorylation was increased at 8 wk, but phosphorylation of AMPK did not achieve a significant increase. Increased activation of AMPK is seen primarily in aerobic training (2). Aerobic fitness increased by 16 wk, although the proportion of type I muscle fibers did not change. This increase in V̇o2max was seen in a prior resistance training protocol (15) and may also be an indicator of muscle remodeling after a general increase in physical activity.
Akt2 is a downstream kinase target of IRS-1-activated phosphatidylinositol-3 kinases (PI-3Ks) that is involved in the translocation of GLUT4 in response to insulin (4). This substantial change in activation was unexpected in view of the lack of a corresponding improvement in the whole body insulin responsiveness.
The change in muscle fiber composition that these obese men manifested was different from the expected result based on prior studies of healthy young men and women. Hather et al. (10) and Adams et al. (1) performed long-term resistance training in 13 young men showing a 30% increase in type IIa and a near-disappearance of type IIx fibers after 19 wk of training. Staron et al. (23) performed a series of heavy resistance training and detraining protocols in six lean young women that showed fiber shifting back and forth. After 20 wk of training, all three fiber types increased cross-sectional areas, and the percent of type IIx fibers declined. Five months of detraining resulted in decreased type IIa and increased type IIx fiber proportions, but 6 wk of retraining again shifted the fiber composition away from type IIx back to type IIa fibers. When four women continued training for another 7 wk, the cross-sectional areas further increased and type IIx fibers decreased to the point that they were no longer detectable in the final biopsies (23).
Although resistance training generally has not changed the proportion of type I fibers (10, 23), Jansson et al. (12) found a shift away from type I fibers to increased type IIa and IIx fibers after stationary bike sprint training in young men. On the other hand, endurance training has caused an increase in type I fibers, with decreases in type IIa and IIx fibers (11). In cross-sectional assessments of trained athletes, Costill et al. (5) showed that men and women distance runners had more type I slow-twitch fibers than middle-distance runners, who had more than untrained subjects, who had more than sprint runners. Jansson et al. (13) found that runners decreased their portion of type I fibers by detraining and increased their type I fibers by retraining, suggesting that the different muscle fiber composition of various elite athletes (5) may have been achieved at least in part by their long-term training.
The investigators at the Human Performance Laboratory (HPL) at Ball State University have published the results of several studies that involved resistance or endurance training in young and older men and women. Some of these reports included data on muscle fiber shifts induced by the training programs. Williamson et al. (30) subjected six young women (average age 21) and six young men (average age 25) to 12 wk of progressive resistance training and found a drop in type IIx fibers and a proportionate increase in type IIa fibers. In a separate report, Williamson et al. (31) trained older men (average age 74) with resistance protocols for 12 wk. In this study, there was no significant change in type IIa or IIx fiber proportions. In both young (average age 20) and older (average age 74) men who underwent 12 wk of primarily aerobic stationary bike training, Harber et al. (9) also found increased type IIa fibers and decreased type IIx fibers. In contrast, our subjects in this report were sedentary, insulin resistant, obese men whose ages were in between those of the HPL groups. The methods used to determine muscle fiber composition were different from those we employed. We used immunohistochemistry with antibodies that recognize fast and slow fibers, whereas the investigators at the HPL used either ATPase histology (31) or SDS-PAGE and silver staining of MHCs from muscle homogenates (9, 30). It is unlikely that the method of fiber typing can account for our contrasting result in fiber shifts, but it is likely that the differences in the characteristics of our subjects (obese, sedentary, and middle-aged) are the cause.
Previous studies of exercise training in obese subjects when weight loss was not allowed have failed to decrease insulin resistance (15, 27, 28). These reports described the effects of 8 wk of either resistance (15) or predominantly endurance training (28). The exercise training programs were effective at increasing strength (15) or increasing aerobic fitness (28), demonstrating that the failure of improving insulin responsiveness was not because the training was inadequate in producing the desired physical outcome. The current study was designed to determine whether longer-duration resistance training with additional increases in training intensity could improve insulin responsiveness, still maintaining the subjects’ weights unchanged. It was found that 16 wk of resistance training did not significantly increase insulin responsiveness in the nine obese, prediabetic men. Extending the training for a second block of 8 wk did further increase measures of strength and aerobic fitness (V̇o2max). Body composition data showed an increase in lean body mass. Muscle fibers increased in size, and there was a shift in muscle fiber composition to an increase in purely fast-twitch type IIx fibers. This study involved only obese men, in contrast to our previous reports that included obese men and women and lean control subjects.
Our earliest study of 8 wk of resistance training did not show statistically significant changes in muscle fiber composition (15), but more recent protocols did find shifts of fiber types (27, 28). Eight weeks of bike training showed that metabolic syndrome subjects undergoing the same training had very different fiber shifts than those seen in lean control subjects. Controls showed a decrease in type IIx fibers and an increase in type IIa fibers, whereas the obese metabolic syndrome subjects significantly increased the proportion of type IIx fibers, with a net decrease in type I fibers (28). We speculate that these opposite fiber shifts were related to the different pretraining fitness levels of the two groups, and the pretraining type IIa content was much higher in the obese subjects. Our recent report found that those subjects with the metabolic syndrome who underwent 8 wk of either resistance or stationary bike training increased their type IIx fiber content (27). Of the 30 subjects included in this analysis, the half that showed some improvement in insulin responsiveness significantly decreased type IIa and increased type IIx fibers (27). The more modest apparent increase in type IIx fibers among the other half of subjects did not reach statistical significance. The data in this current report show a significant shift away from type IIa fibers to type IIx fibers at 8 wk, and that proportion was maintained at 16 wk. The stronger statistical significance in this current study is likely due to the more homogeneous group that included only obese men.
The fiber shift data taken from participants with 8 wk of resistance or aerobic training with weight loss prohibited differ from previous reports from other groups. Stationary bike training of sedentary lean control subjects caused a shift from type IIx to type IIa fibers (28), but the metabolic syndrome subjects trained side by side with the controls demonstrated a shift in fiber composition away from type I and type IIa to type IIx fibers (28). The current study also found a shift from type IIa to IIx fibers by 8 wk of resistance training. It may be that training of either resistance or aerobic protocols initially causes muscle remodeling that is needed to transition from very sedentary muscle to muscle that is preparing for further changes to foster increased, physically challenging activity. The enlarged cross-sectional areas result is consistent with the concept of the accumulation stage of block periodization; however, the shift toward faster fibers was unexpected.
Another potential contributing factor for our more recent data being different from older studies is that the method for identifying and quantifying fiber composition is quite different from most studies before 2002. The two-antibody, bright-field microscopy method described by Behan et al. (3) had very consistent results, and their methods were not as technically challenging as some of the prior methods for identifying fiber types that require multiple staining steps at varying pHs for modifying ATPase activity (8) or relative mobility of silver-stained bands of PAGE blots (20).
In the current study, obese prediabetic men volunteered to undergo resistance training with assessments before training and again at 8 and 16 wk. Peak force and rate of power generation increased. Body fat decreased, and lean body mass increased. There was a shift in muscle fiber composition from type IIa to type IIx fibers and an increase in the size of the type IIa and IIx fibers. Extending progressive resistance training without weight loss from 8 to 16 wk did not significantly improve insulin responsiveness. We conclude that resistance training in prediabetic obese men was effective at decreasing body fat, increasing their muscle size, and shifting the skeletal muscle fiber type toward more fast-twitch type IIx fibers, but in the absence of weight loss, whole body insulin responsiveness did not change.
GRANTS
These studies were funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases DK-080488 (C. A. Stuart) and a grant from the East Tennessee State University Research Development Committee (C. A. Stuart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.S., M.L.L., and M.H.S. conceived and designed research; C.A.S., M.L.L., M.A.S., M.E.H., and M.H.S. performed experiments; C.A.S., M.L.L., M.A.S., M.E.H., and M.H.S. analyzed data; C.A.S., M.L.L., M.A.S., M.E.H., and M.H.S. interpreted results of experiments; C.A.S. prepared figures; C.A.S. drafted manuscript; C.A.S., M.L.L., M.A.S., M.E.H., and M.H.S. edited and revised manuscript; C.A.S., M.L.L., M.A.S., M.E.H., and M.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank several Department of Exercise Science graduate students who worked closely with our study participants through their training and assessments. Furthermore, we express appreciation for the work of our research nurse Susie Whitaker, who was instrumental in recruitment, scheduling, and assisting with procedures.
REFERENCES
- 1.Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol (1985) 74: 911–915, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc 38: 1939–1944, 2006. doi: 10.1249/01.mss.0000233799.62153.19. [DOI] [PubMed] [Google Scholar]
- 3.Behan WM, Cossar DW, Madden HA, McKay IC. Validation of a simple, rapid, and economical technique for distinguishing type 1 and 2 fibres in fixed and frozen skeletal muscle. J Clin Pathol 55: 375–380, 2002. doi: 10.1136/jcp.55.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridges D, Saltiel AR. Phosphoinositides: Key modulators of energy metabolism. Biochim Biophys Acta 1851: 857–866, 2015. doi: 10.1016/j.bbalip.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976. [DOI] [PubMed] [Google Scholar]
- 6.DeWeese BH, Hornsby G, Stone ME, Stone MH. The training process: Planning for strength—power training in track and field. Part I: Theoretical aspects. J Sport Health Sci 4: 308–317, 2015. doi: 10.1016/j.jshs.2015.07.003. [DOI] [Google Scholar]
- 7.DeWeese BH, Hornsby G, Stone ME, Stone MH. The training process: Planning for strength—power training in track and field. Part II: Practical and applied aspects. J Sport Health Sci 4: 318–324, 2015. doi: 10.1016/j.jshs.2015.07.002. [DOI] [Google Scholar]
- 8.Dubowitz V, Sewry CA. Normal muscle. In: Muscle Biopsy: A Practical Approach (Houston MJ and Cook L, editors). Philadelphia, PA: Saunders Elsevier, 2007. doi: 10.1016/B978-1-4160-2593-1.50008-5. [DOI] [Google Scholar]
- 9.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985) 113: 1495–1504, 2012. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 143: 177–185, 1991. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 11.Hudlicka O. The response of muscle to enhanced and reduced activity. Baillieres Clin Endocrinol Metab 4: 417–439, 1990. doi: 10.1016/S0950-351X(05)80063-1. [DOI] [PubMed] [Google Scholar]
- 12.Jansson E, Esbjörnsson M, Holm I, Jacobs I. Increase in the proportion of fast-twitch muscle fibres by sprint training in males. Acta Physiol Scand 140: 359–363, 1990. doi: 10.1111/j.1748-1716.1990.tb09010.x. [DOI] [PubMed] [Google Scholar]
- 13.Jansson E, Sjödin B, Tesch P. Changes in muscle fibre type distribution in man after physical training. A sign of fibre type transformation? Acta Physiol Scand 104: 235–237, 1978. doi: 10.1111/j.1748-1716.1978.tb06272.x. [DOI] [PubMed] [Google Scholar]
- 14.Kraska JM, Ramsey MW, Gregory GH, Nate F, Sands WA, Stone ME, Stone MH. Relationship between strength characteristics and unweighted and weighted vertical jump height. Int J Sports Physiol Perform 4: 461–473, 2009. doi: 10.1123/ijspp.4.4.461. [DOI] [PubMed] [Google Scholar]
- 15.Layne AS, Nasrallah S, South MA, Howell ME, McCurry MP, Ramsey MW, Stone MH, Stuart CA. Impaired muscle AMPK activation in the metabolic syndrome may attenuate improved insulin action after exercise training. J Clin Endocrinol Metab 96: 1815–1826, 2011. doi: 10.1210/jc.2010-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Painter KB, Haff GG, Ramsey MW, McBride J, Triplett T, Sands WA, Lamont HS, Stone ME, Stone MH. Strength gains: block versus daily undulating periodization weight training among track and field athletes. Int J Sports Physiol Perform 7: 161–169, 2012. doi: 10.1123/ijspp.7.2.161. [DOI] [PubMed] [Google Scholar]
- 17.Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol 170: 143–223, 1997. doi: 10.1016/S0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- 18.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115: 359–372, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Reeds DN, Stuart CA, Perez O, Klein S. Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metabolism 55: 1658–1663, 2006. doi: 10.1016/j.metabol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol (1985) 77: 493–501, 1994. [DOI] [PubMed] [Google Scholar]
- 21.South MA, Layne AS, Stuart CA, Triplett NT, Ramsey M, Howell ME, Sands WA, Mizuguchi S, Hornsby WG III, Kavanaugh AA, Stone MH. Effects of short-term free-weight and semiblock periodization resistance training on metabolic syndrome. J Strength Cond Res 30: 2682–2696, 2016. doi: 10.1519/JSC.0000000000001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol 22: 307–327, 1997. doi: 10.1139/h97-020. [DOI] [PubMed] [Google Scholar]
- 23.Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol (1985) 70: 631–640, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA. Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol 60: 71–79, 1990. doi: 10.1007/BF00572189. [DOI] [PubMed] [Google Scholar]
- 25.Stuart CA, Howell ME, Cartwright BM, McCurry MP, Lee ML, Ramsey MW, Stone MH. Insulin resistance and muscle insulin receptor substrate-1 serine hyperphosphorylation. Physiol Rep 2: 1–8, 2014. doi: 10.14814/phy2.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart CA, Howell ME, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab 94: 3535–3542, 2009. doi: 10.1210/jc.2009-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart CA, Lee ML, South MA, Howell ME, Cartwright BM, Ramsey MW, Stone MH. Pre-training muscle characteristics of subjects who are obese determine how well exercise training will improve their insulin responsiveness: exercise training and muscle of obese subjects. J Strength Cond Res 31: 798–808, 2017. doi: 10.1519/JSC.0000000000001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart CA, South MA, Lee ML, McCurry MP, Howell ME, Ramsey MW, Stone MH. Insulin responsiveness in metabolic syndrome after eight weeks of cycle training. Med Sci Sports Exerc 45: 2021–2029, 2013. doi: 10.1249/MSS.0b013e31829a6ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart CA, Yin D, Howell MEA, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab 291: E1067–E1073, 2006. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- 30.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol (1985) 91: 1955–1961, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol (1985) 88: 627–633, 2000. [DOI] [PubMed] [Google Scholar]