Sudden infant death syndrome (SIDS) occurs during sleep and is associated with central serotonin (5-HT) deficiency. We report that rat pups deficient in central 5-HT (TPH2−/−) are profoundly more apneic in active sleep (AS) but not quiet sleep (QS). Unlike control pups, the arousal of TPH2−/− pups in air, CO2, and hypoxia was delayed in AS compared with QS. Thus for infants deficient in central 5-HT, the risk of SIDS may be higher in AS than in QS.
Keywords: apnea, arousal, breathing, serotonin, SIDS
Abstract
Sudden infant death syndrome (SIDS), occurring during sleep periods, is highly associated with abnormalities within serotonin (5-HT) neurons, including reduced 5-HT. There is evidence that future SIDS cases experience more apnea and have abnormal arousal from sleep. In rodents, a loss of 5-HT neurons is associated with apnea in early life and, in adulthood, delayed arousal. As the activity of 5-HT neurons changes with vigilance state, we hypothesized that the degree of apnea and delayed arousal displayed by rat pups specifically lacking central 5-HT varies with state. Two-week-old tryptophan hydroxylase 2-deficient (TPH2−/−) and wild-type (WT) rat pups were placed in plethysmographic chambers supplied with room air. At the onset of active (AS) or quiet (QS) sleep, separate groups of rats were exposed to hypercapnia (5% CO2) or mild hypoxia (~17% O2) or maintained in room air. Upon arousal, rats received room air. Apnea indexes and latencies to spontaneous arousal from AS and QS were determined for pups exposed only to room air. Arousal latencies were also calculated for TPH2−/− and WT pups exposed to hypoxia or hypercapnia. Compared with WT, TPH2−/− pups hypoventilated in all states but were profoundly more apneic solely in AS. TPH2−/− pups had delayed arousal in response to increasing CO2, and AS selectively delayed the arousal of TPH2−/− pups, irrespective of the gas they breathed. Thus infants who are deficient in CNS 5-HT may be at increased risk for SIDS in AS because of increased apnea and delayed arousal compared with QS.
NEW & NOTEWORTHY Sudden infant death syndrome (SIDS) occurs during sleep and is associated with central serotonin (5-HT) deficiency. We report that rat pups deficient in central 5-HT (TPH2−/−) are profoundly more apneic in active sleep (AS) but not quiet sleep (QS). Unlike control pups, the arousal of TPH2−/− pups in air, CO2, and hypoxia was delayed in AS compared with QS. Thus for infants deficient in central 5-HT, the risk of SIDS may be higher in AS than in QS.
sudden infant death syndrome (SIDS), the leading cause of death from 1 mo to 1 yr of age, occurs in vulnerable infants during periods of sleep (26). Prospective studies have shown that future SIDS cases have increased obstructive and mixed apneas (21, 24) and impaired arousal from sleep (23). Together, apnea and impaired arousal potentially underlie the markers of tissue hypoxia evident in SIDS cases at autopsy (25, 39, 42). Defects in serotonin (5-hydroxytryptamine; 5-HT) neurons including reduced 5-HT and its biosynthetic enzyme tryptophan hydroxylase 2 (TPH2) are consistently identified in SIDS cases (14, 38). 5-HT-deficient neonatal rodents, like future SIDS cases, display more apnea than controls (10, 22), suggesting a potential pathophysiological link between serotonergic defects and apnea in SIDS cases.
The impact of 5-HT deficiency on the control of breathing and arousal may depend on the state of vigilance. 5-HT neurons are most active in wakefulness, have reduced firing in non-rapid eye movement (NREM) sleep [or quiet sleep (QS) in infants], and are practically silent in REM sleep [or active sleep (AS) in infants] (32). It may be that the respiratory phenotypes associated with 5-HT deficiency only manifest in QS, when these neurons still fire and, in normal animals, release synaptic 5-HT. On the other hand, respiratory neurons also lose drive from major groups of noradrenergic neurons (e.g., those in the locus coeruleus) in AS (28, 46), making infants especially susceptible to obstructive and central apneas in this state (16). As 5-HT can signal through extrasynaptic, metabotropic G protein-coupled receptors, it may contribute to respiratory stability over long time frames, including in AS, despite the fact that the serotonergic neurons are silent.
In addition to the medulla, 5-HT neurons also reside in the dorsal raphe within the pons and midbrain, promoting wakefulness via ascending projections to the thalamus and forebrain. These neurons increase their firing in response to increasing CO2 (40), allowing them to promote the arousal of adult animals (6, 40). 5-HT released from these neurons also helps terminate REM sleep by inhibiting cholinergic neurons in the pontine tegmentum (18, 33). To date, there have been no studies that have examined the specific role of central 5-HT in arousal or sleep architecture in animals at an age relevant to SIDS.
Here we hypothesized that the degree to which TPH2-deficient (TPH2−/−) rat pups display apnea and compromised arousal depends on the state of vigilance. Our findings suggest that for infants deficient in central 5-HT the risk of SIDS may be higher in AS than QS because of higher apnea incidence and delayed arousal.
MATERIALS AND METHODS
Ethical Approval
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Missouri at Columbia, in accordance with national guidelines.
Animals and Treatments
The generation of the TPH2−/− mutant rat lines on a Dark Agouti background has been described previously (22). Briefly, the rat TPH2 locus was targeted with zinc finger nucleases to generate a short (10 bp) deletion in exon 7. We set up TPH2+/− male and female rats to generate the wild-type (WT) and TPH2−/− pups used in this study. Genotyping was performed with the TPH2 forward primer (5′-GGCCTTTAGGTCCTGAGGTT-3′) and the TPH2 reverse primer (5′-CCCTTCTCCACAGAAGTGCT-3′). PCR generated a 306-bp WT band and a 295-bp mutant band, which were analyzed on a 15% polyacrylamide gel. TPH2+/− dams were fed ad libitum on standard rat chow and kept on a 12:12-h light-dark cycle. Rat pups between postnatal days (P)14 and 16 were used for all experiments. Three separate groups of animals were used: group 1 (normoxia): 10 WT, 8 TPH2−/−; group 2 (hypercapnia): 8 WT, 7 TPH2−/−; and group 3 (hypoxia): 8 WT, 8 TPH2−/−. Average body weights (all animals combined) were 24.4 ± 0.6 g and 14.9 ± 0.4 g for WT and TPH2−/−, respectively (P < 0.001). On any given day, at least one TPH2−/− and one littermate WT were tested. Groups 1–3 contained both male and female pups, and because no sex-specific differences were identified data from both sexes were combined for analyses.
Experimental Setups
To minimize stress and allow for normal sleeping, we monitored breathing with whole body plethysmography, with constant flow through 20-gauge needles, acting as critical resistors, inserted into rubber stoppers at each end of the chamber. In this way, the chamber was semisealed to maximize the signal related to breathing while providing the animal with a constant supply of gas. To avoid variability in the respiratory signal arising from changes in environmental humidity, all gases were bubbled through a flask of water before being delivered to the chamber.
A programmable heated pump delivered warmed water to the jacketed glass animal chamber (volume = 200 ml) to maintain ambient temperature at 31°C—within the thermoneutral range for pups in the second postnatal week (36). A thermometer attached to a thermocouple placed within the chamber continually monitored chamber temperature. Air or hypercapnic gas was directed into the chamber via premixed cylinders (Airgas, Holts Summit, MO). Mildly hypoxic gas (~16.5–17.5% O2, balance N2) was produced by mixing gas from separate N2 and O2 tanks (Airgas); flow from each tank was adjusted, with flowmeters used to produce the desired level of hypoxia. Mild hypoxia was used because we observed in preliminary experiments that both WT and TPH2−/− pups woke up immediately upon introduction of moderate hypoxia (10% O2); thus a milder stimulus was used in an effort to resolve any potential effect of genotype on the response. Gas passed through a common flowmeter before entering the chamber at a flow of ~250 ml/min. Chamber pressure was kept just above atmospheric by pulling gas via a wall vacuum at a similar flow rate from the opposite end of the chamber. Before experiments, we ensured that switching gases (e.g., from normoxia to hypercapnia or hypoxia) elicited no significant pressure change within the chamber that might have induced arousal. Breathing was detected with a differential pressure transducer (Validyne) connected to the animal chamber on one side and an empty reference chamber on the other. To account for thermal drift, we connected the two chambers via an ~10-cm length of small-diameter tubing. All analog signals were fed into a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO) and analyzed in LabChart 7 and 8 (ADInstruments).
Determining Sleep State and Arousal
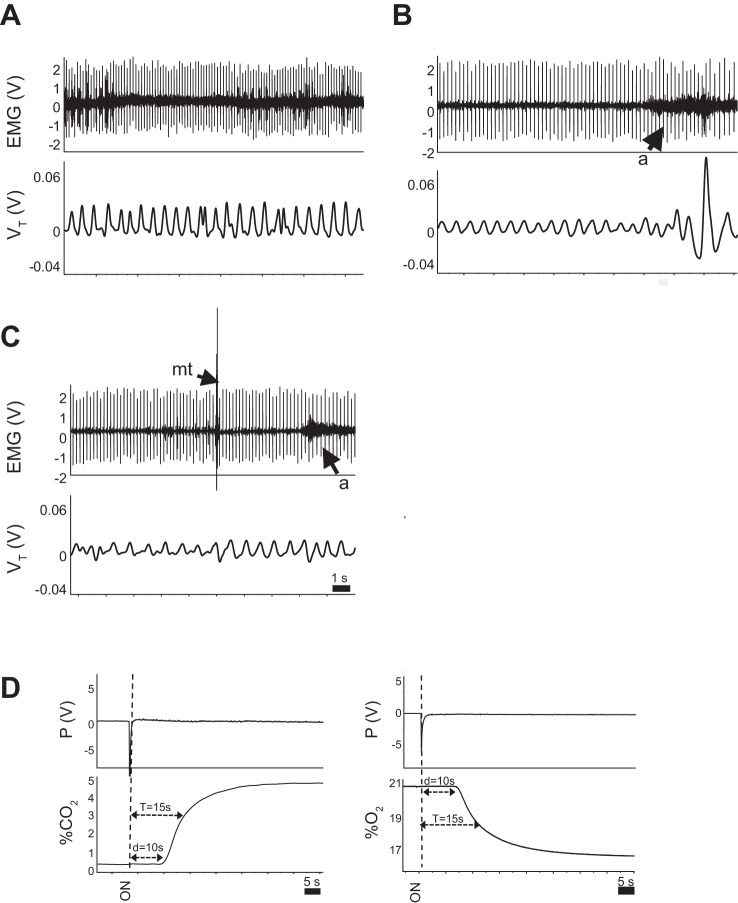
Electrodes were implanted under the nuchal muscles to determine sleep state. Pups were removed from the dam, anesthetized initially with ~2% isoflurane, and maintained on ~1% isoflurane. Noxious paw pinch was used to assess depth of anesthesia. Each pup was implanted with two insulated stainless steel wire electrodes (part no. E363/76, PlasticsOne, Roanoke, VA); one was sutured in place under the nuchal muscle, while another was placed in the flank as a ground electrode. Sleep states and arousal were determined using the changes in nuchal EMG activity along with the standard behavioral criteria associated with vigilance state in pups, as described previously (11). Quiet wakefulness (QW) was associated with relatively high EMG amplitude with few or no gross body movements (Fig. 1A). QS was associated with lower EMG amplitude, immobility, and the head resting on the forelimbs or the floor of the chamber (Fig. 1B). EMG amplitude decreased further in AS, when bursts of motor activity associated with myoclonic twitching also appeared (Fig. 1C). Arousals from sleep were identified by sustained increases in EMG activity (Fig. 1, B and C) that were accompanied by stereotypical behaviors including forelimb withdrawal and lifting of the head (11).
Fig. 1.
Methodology for assessing sleep state and arousal from sleep. A–C: nuchal EMG activity in a wild-type pup while awake (A), during quiet sleep (QS, B), and during active sleep (AS, C). Note the decrease in amplitude of the EMG in QS, compared with wakefulness, with a further decrease in AS. The appearance of bursts of EMG activity in AS is due to myoclonic twitching (mt), further distinguishing AS from QS. Arousals from sleep (a) are easily discernible on the records by a sustained increase in EMG amplitude. Spikes on the EMG record are the result of cardiac electrical activity, which we used to determine resting heart rate in each sleep state. D: dynamics of gas washin to the chamber are shown for hypercapnia (left) and hypoxia (right). d, delay; T, time constant.
Experimental Protocol
After surgery, animals were immediately transferred to the prewarmed glass chamber and allowed to recover over a 20- to 30-min period in room air. Recovery was deemed complete when the frequency of breathing had stabilized and pups displayed stereotypical sleep behavior (described above). For group 1 (normoxia), pups were allowed to sleep and arouse without intervention over a 2-h period. Groups 2 and 3 were used to test arousal responses to hypercapnia and hypoxia, respectively. We used separate groups because in preliminary experiments we observed that prior exposure to hypercapnia altered spontaneous arousal in the intervening room air periods. Pups were exposed to intermittent episodes of hypercapnia or mild hypoxia, also over a 2-h period. Hypercapnic and hypoxic episodes were interspersed with a minimum of 2 min of room air until the commencement of the next sleep episode, before a subsequent gas exposure. Hypercapnic or hypoxic gas was delivered as soon as the experimenter determined that the animal had fallen asleep, using EMG and behavioral criteria as described above (determined within ~10 s).
Data and Statistical Analysis
Cardiorespiratory and metabolic variables.
Respiratory and metabolic variables were recorded and analyzed with LabChart 7 (ADInstruments). Analysis of breathing was done with peak detection on the raw respiratory record. We measured respiratory frequency (fB), tidal volume (Vt), and ventilation (V̇i) in periods of QW, QS, and AS. Vt was calculated with the Fenn equation, using the amplitude of the raw respiratory signal, the amplitude produced by calibration injections of 100 μl of air into the chamber at the normal respiratory frequency, as well as the difference between body and ambient temperatures (13). As the air was bubbled through water before entering the chamber (i.e., 100% humidified), the respiratory signal was generated solely by the degree of heating of the air as it entered the airways. The degree of heating depends on the difference between body and ambient temperatures. All experiments were performed with the chamber held at thermoneutral ambient temperature (~31°C) (36). Body temperature in TPH2−/− and WT littermates was determined in a separate set of animals (n = 4 WT and 6 TPH2−/−) kept within the 31°C chamber for 45 min, after which rectal temperature was immediately measured with a fine thermocouple and thermometer (Omega Engineering, Norwalk, CT). Body temperature was 35.5 ± 0.3°C and 34.5 ± 0.5°C in WT and TPH2−/− pups, respectively. These temperatures were used to calculate Vt and V̇i. Fractional expired () and inspired () CO2 were used to determine metabolic CO2 production (V̇co2) with the equation V̇co2 = ( − ) × flow rate (ml/min)/mass (kg). Heart rate (HR) was determined by using the background electrocardiographic activity contained in the nuchal EMG tracings (Fig. 1, A–C).
We identified apneas in QS and AS by identifying any breath with a respiratory period lasting ≥150% of the average respiratory period for that animal. Apnea indexes for each animal were defined as the number of apneas per hour. The coefficient of variation of the respiratory period [CV-P (%)] was calculated to obtain another index of respiratory stability with the formula CV-P (%) = standard deviation of Period/average Period × 100. Two-factor repeated-measures ANOVAs were performed in SigmaPlot to determine significant main effects of, and interactions between, genotype and sleep state on respiratory and metabolic variables. Data for apnea indexes were not normally distributed, so data were rank transformed before statistical analyses with two-factor repeated-measures ANOVA. When significant effects were identified, pairwise multiple comparisons were performed with Tukey’s post hoc analysis. Effects were deemed statistically significant when P values were <0.05.
Sleep architecture, arousal, and respiratory responses to hypoxia and hypercapnia.
Duration of each sleep episode was measured from the initial fall in EMG activity associated with sleep to the stereotypical behavior and sustained increase in EMG activity associated with arousal (described above). The dynamics of gas washin (for groups 2 and 3) are shown in Fig. 1D. After the manual switch of gas from air to either hypercapnia or hypoxia, there was a delay of 10 s before the gas arrived at the chamber and was detected by gas analyzers. The time constant (τ) for gas washin was 15 s. For all animals, both the delay and τ were included in the arousal latencies. Given the 10-s delay, sleep episodes in which arousals occurred <10 s after the gas switch were not included in the analysis.
To determine whether genotype-specific differences in arousal responses might be associated with, and potentially caused by, differences in respiratory responses to each stimulus, we measured the change in V̇i of both WT and TPH2−/− pups in response to hypoxia and hypercapnia. For hypercapnic responses, we measured fB, Vt, and V̇i across ~10 s of breathing (~15 breaths) immediately before the introduction of the gas as well as after 1 min of exposure (from 55 to 65 s after the introduction of the gas). For hypoxia, we measured these respiratory variables immediately before gas exposure and from 25 to 35 s after the introduction of the gas (most pups woke up within 1 min of hypoxic exposure).
Effects of genotype, sleep state, and gas exposure (normoxia, hypoxia, hypercapnia) on sleep episode duration were assessed with a three-factor repeated-measures ANOVA (state as the repeated measure) (SPSS, IBM, Armonk, NY), followed by Tukey’s post hoc analyses when significant effects were found. Effects were deemed statistically significant when P values were <0.05.
RESULTS
A Loss of Central 5-HT Results in Hypoventilation as Well as Apnea Solely in AS
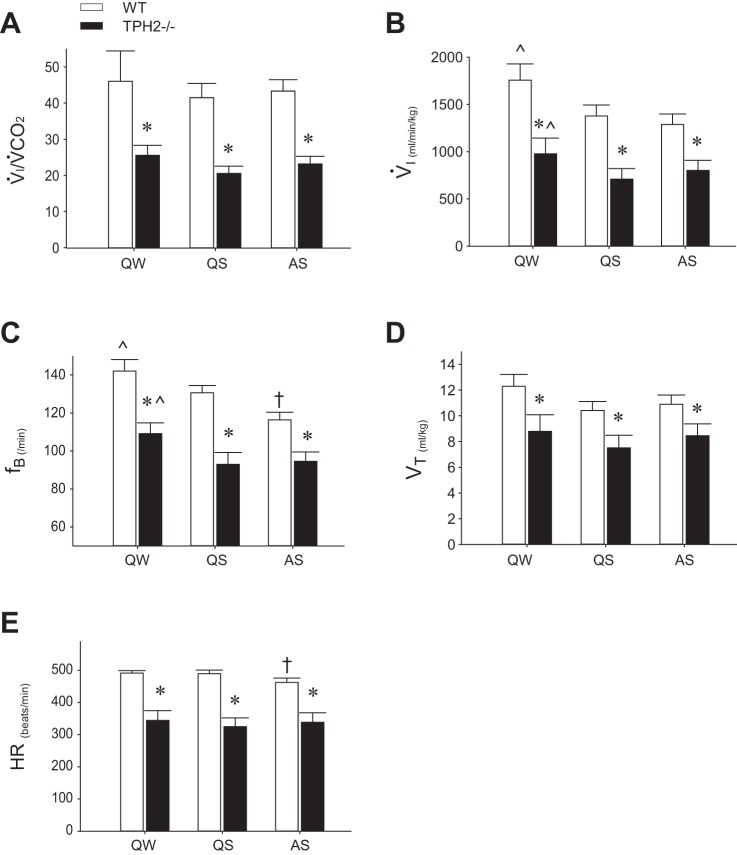
We used whole body plethysmography coupled with nuchal electromyography and continuous behavioral observation to monitor and record the respiratory pattern of TPH2−/− pups and WT littermates in QW, QS, and AS. Compared with their WT littermates, TPH2−/− pups hypoventilated in all vigilance states: the V̇i/V̇co2 of TPH2−/− pups was ~50% lower than WT (P < 0.001; Fig. 2A) because of reduced V̇i (P < 0.001; Fig. 2B) with no difference in V̇co2 (WT: 41, 39, and 37 ml·kg−1·min−1 in QW, QS, and AS, respectively; TPH2−/−: 41, 37, and 37 ml·kg−1·min−1, respectively). The reduced V̇i of TPH2−/− pups was due to significantly reduced fB (P < 0.001; Fig. 2C) and Vt (P = 0.002; Fig. 2D). Note that compared with QW the fB and overall V̇i of TPH2−/− and WT pups was reduced in both sleep states (P < 0.001 for both variables). The fB of WT pups was further reduced in AS compared with QS (genotype × state: P = 0.026; Fig. 2C). TPH2−/− pups had a lower HR than WT across all states (P < 0.001; Fig. 2E). As was the case for fB, the HR of WT, but not TPH2−/−, pups was significantly reduced in AS compared with QS (genotype × state: P < 0.001; Fig. 2E).
Fig. 2.
Resting cardiorespiratory variables in sleeping TPH2−/− and TPH2+/+ pups. Ventilatory equivalent (V̇i/V̇co2, A), ventilation (V̇i, B), respiratory frequency (fB, C), tidal volume (Vt, D), and heart rate (HR, E) for TPH2+/+ (WT, n = 10) and TPH2−/− (n = 8) littermates in quiet wakefulness (QW), quiet sleep (QS), and active sleep (AS). Note that all variables are lower in TPH2−/− compared with TPH2+/+, irrespective of sleep state. *Significant difference between TPH2+/+ and TPH2−/− (P < 0.001). ^V̇i and fB significantly higher in QW compared with QS and AS. †fB and HR of TPH2+/+ lower in AS compared with QS (effect of state within TPH2+/+: P < 0.001).
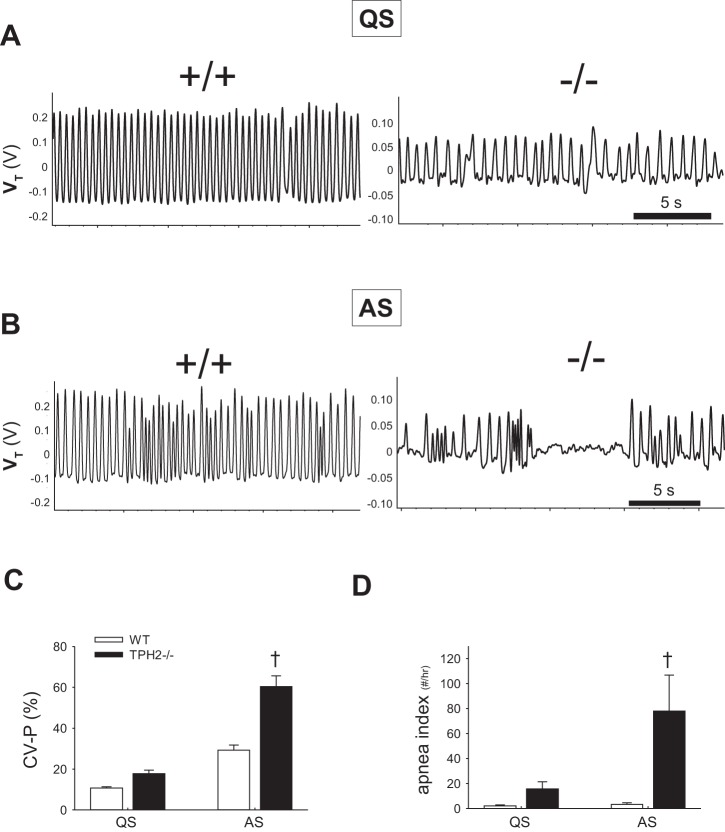
Both sleep state and genotype influence the regularity of the breathing pattern, as measured by the CV-P% and the apnea indexes. The breathing pattern of both TPH2−/− and WT littermates became more irregular after the transition from QS to AS (effect of state within WT and KO on CV-P%: P < 0.001; compare Fig. 3A with Fig. 3B and Fig. 3C). However, the effect of central 5-HT deficiency on stability was only apparent in AS (genotype × sleep state on CV-P%: P < 0.001; Fig. 3C). Similarly, the apnea index of TPH2−/− pups in AS was significantly greater than WT, an effect not observed in QS. On average, 5-HT-deficient pups experienced ~75 more apneas per hour in AS than WT littermates (genotype × state: P = 0.009; Fig. 3D). Notably, the apneas displayed by TPH2−/− pups were on occasion >5 s long (Fig. 3B).
Fig. 3.
TPH2−/− pups are prone to severe apnea in active sleep. A: respiratory pattern of a TPH2+/+ (left) and a TPH2−/− (right) pup in quiet sleep (QS). B: respiratory pattern of a +/+ and a −/− pup in active sleep (AS). C and D: coefficient of variation of the respiratory period (CV-P%, C) and apnea index (no. of apneas/h, D) of TPH2+/+ (WT, n = 10) and TPH2−/− (n = 8) pups in quiet sleep (QS) and active sleep (AS). †CV-P% and apnea index of TPH2−/− higher than TPH2+/+ in AS only (effect of genotype within AS: P < 0.001).
Central 5-HT Deficiency Leads to Prolonged Episodes of Sleep in Room Air (Group 1)
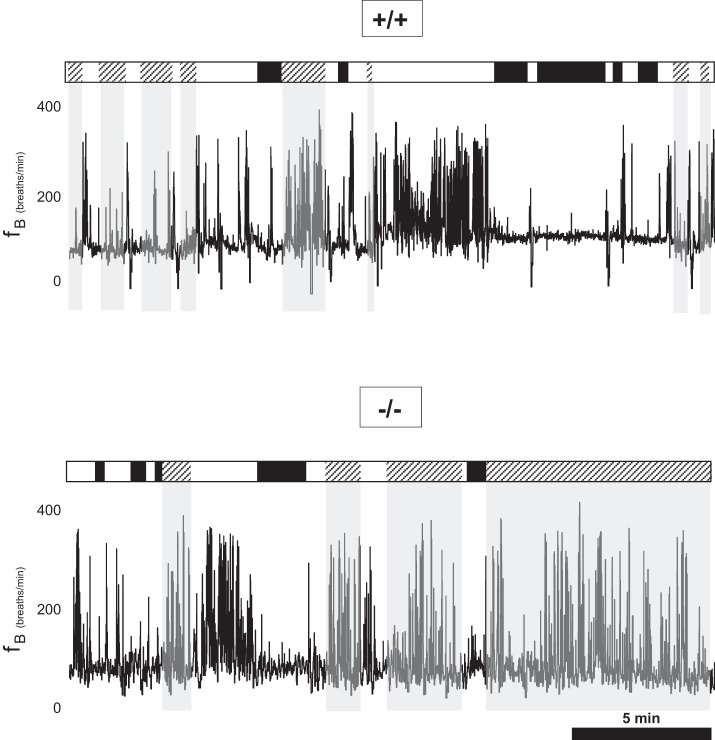
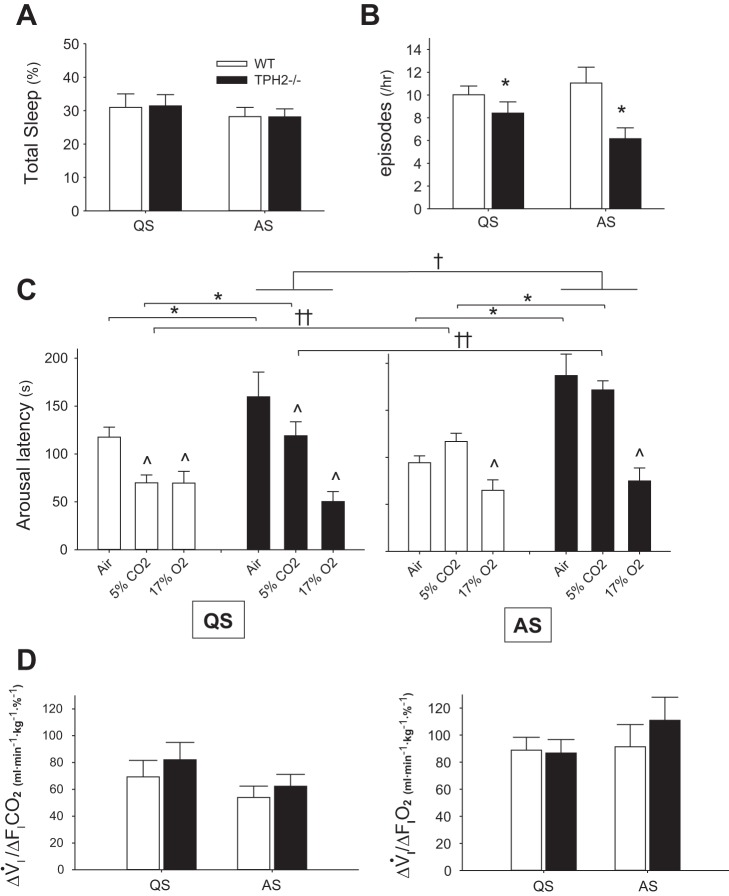
We recorded the nuchal EMG activity of pups over a 2-h period in room air (group 1), and, coupled with behavioral observation, we measured the number and duration of QS and AS episodes. The overall sleep architectures of a WT and a TPH2−/− littermate are shown in Fig. 4. In addition, fB across vigilance states is shown for each pup, demonstrating unstable respiratory rhythm of TPH2−/− pups compared with WT littermates, particularly in AS. Overall, a loss of central 5-HT had no influence over the amount of time spent in QS and AS (Fig. 5A). However, sleep architecture in room air was markedly altered by central 5-HT deficiency. Compared with WT, TPH2−/− pups had fewer episodes of QS and AS while in room air (P = 0.007; Fig. 5B). However, these episodes of sleep were significantly longer in the absence of central 5-HT (P < 0.001; Fig. 5C). This was especially evident in AS, when TPH2−/− pups had episodes in room air that were approximately twice as long as those experienced by WT littermates (Fig. 5C, right).
Fig. 4.
Altered sleep architecture in TPH2−/− pups. Schematic demonstrating state transitions over 20 min of sleep in a TPH2+/+ (top) and a TPH2−/− (bottom) littermate. White bars, wakefulness; black bars, quiet sleep; hatched bars, active sleep. Note that TPH2−/− pups display fewer, but longer, episodes of active sleep. Also shown is the continuous record of respiratory frequency (fB). Note the relatively destabilized respiratory pattern in the TPH2−/− pup in AS compared with TPH2+/+.
Fig. 5.
Arousal is delayed by 5-HT deficiency and active sleep. A: total time spent in quiet (QS) and active (AS) sleep while in room air as % of total time in the chamber for TPH2+/+ (WT) and TPH2−/−. B: no. of QS and AS episodes per hour for each genotype. *Significant difference between TPH2+/+ and TPH2−/− (P = 0.007). C: latencies to arousal from quiet sleep (QS, left) and active sleep (AS, right) of TPH2+/+ and TPH2−/− littermates in room air (n = 10 +/+, 8 −/−) and in response to CO2 (n = 8 +/+, 7 −/−) or hypoxia (n = 8 +/+, 8 −/−). *Significant effect of genotype on arousal latencies in air and in response to increasing CO2 (genotype × gas: P = 0.02). ^Significant effect of CO2 or hypoxia on arousal latencies, compared with spontaneous arousal latencies in air (gas: P < 0.001). †Significant effect of AS on arousal latencies of TPH2−/− pups only (genotype × sleep state: P = 0.01). ††Significant effect of AS on arousal latencies of both genotypes in response to increasing CO2 (state × gas: P = 0.001). D: ventilatory response [ΔV̇i/Δfractional concentration of inspired CO2 (, left) or O2 (, right)] of TPH2+/+ and TPH2−/− littermates.
Central 5-HT Deficiency and AS Compromise Arousal in Response to Hypercapnia but Not Hypoxia (Groups 2 and 3)
In addition to spontaneous arousal in room air, we assessed how central 5-HT deficiency influenced arousal from QS and AS in response to hypoxia and hypercapnia, as would be experienced during sleep apnea. At the beginning of episodes of AS and QS, we switched the gas entering the chamber from air to either hypoxia or hypercapnia and measured the delay (or latency) to arousal (Fig. 5C). Compared with spontaneous arousals in room air, hypoxia hastened arousal from QS and AS (P < 0.001), an effect not influenced by central 5-HT deficiency. Unlike hypoxia, CO2 only hastened arousal from QS; for both genotypes, hypercapnia was ineffective at hastening arousal from AS compared with its effect in QS (state × gas: P = 0.001). In both QS and AS, central 5-HT deficiency delayed arousal in response to hypercapnia but not to hypoxia (genotype × gas: P = 0.023). Furthermore, AS selectively delayed the arousal of TPH2−/− pups, irrespective of the gas breathed (state × genotype: P = 0.01).
We assessed whether the deleterious effects of AS and 5-HT deficiency on arousal might be explained by reduced afferent feedback from the airways or chest wall associated with reduced V̇i responses. Both WT and TPH2−/− pups increased V̇i in response to hypercapnia and hypoxia, and there were no significant effects of genotype or sleep state on the magnitude of the responses (Fig. 5D).
DISCUSSION
Neurochemical abnormalities suggesting brain stem 5-HT system dysfunction are consistently identified in SIDS cases, and prospective studies have shown that SIDS cases experience an unusually high number of apneic events before death. Given that SIDS occurs predominantly during periods of sleep (27), and because 5-HT neuron firing varies across vigilance states (32), here we addressed the hypothesis that in infancy the extent to which central 5-HT deficiency compromises breathing and arousal depends on the specific state of vigilance. Our major findings suggest that there is a critical interaction between sleep state and 5-HT deficiency on the control of breathing and arousal; infant rats deficient in central 5-HT display apnea only in AS, and their arousal responses are selectively delayed in AS compared with QS, phenotypes not observed in WT littermates.
Apnea of TPH2−/− Pups Emerges in Active Sleep
Our data indicate that a loss of central 5-HT leads to profound hypoventilation in infant rats across all states. Our findings extend those of Kaplan and colleagues, who first reported the reduced ventilation of TPH2−/− rat pups (22). Importantly, the present data indicate that the reduced breathing of TPH2−/− pups is not secondary to reduced metabolic drive; rather, they are hypoventilating, indicated by a considerably reduced V̇i-to-V̇co2 ratio. Notably, TPH2−/− pups hypoventilate across vigilance states, including AS when 5-HT neuronal activity is lowest, suggesting that 5-HT exerts an excitatory influence within respiratory circuits in a manner that is independent of the firing pattern of the serotonergic neurons. This could occur because 5-HT signals largely through metabotropic G protein-coupled receptors, as well as by volume transmission through en passant varicosities. Thus 5-HT has the potential to signal over relatively long time frames that may not necessarily mirror the firing pattern of the serotonergic neurons.
Our study confirms the findings of Kaplan and colleagues, who first described apnea in TPH2−/− pups (22). Our novel finding is that 5-HT deficiency leads to apnea in AS but not in QS. It may be that the apnea of TPH2−/− pups is due in part to a loss of serotonergic inputs to respiratory patterning neurons in the dorsolateral pons. There are inhibitory 5-HT1A receptors expressed by neurons within the Kölliker-Fuse region (KF) (4), and indeed in adult mice the blockade of these receptors within the KF increases the occurrence of spontaneous apnea (12). However, those observations cannot fully explain why TPH2−/− pups are apneic only in AS. It may be that in QS there is compensation provided for a loss of 5-HT by neuromodulators that are coreleased with 5-HT (TRH, substance P, or glutamate) or that there is redundancy provided by other groups of neurons that help stabilize the breathing pattern in QS. Catecholaminergic neurons within the A5 and A6 (locus coeruleus), for example, contribute to respiratory patterning (46) and could conceivably compensate for reduced 5-HT signaling in QS. However, as the activity of these neurons also ceases during AS (3), so too could their compensatory potential. Interestingly, decreased respiratory stability solely in REM sleep also emerges after a broad lesioning of brain stem catecholaminergic neurons (28). Alternatively, it may be that 5-HT facilitates or inhibits specific types of neurons that change their activity during the transition from QS to AS. Keeping in mind 1) the prolonged episodes of AS displayed by TPH2−/− pups (see below), 2) the role of acetylcholine as a driver of AS/REM sleep, and 3) the excitatory effect of acetylcholine on respiratory neurons (37), another possibility is that a loss of 5-HT leads to abnormally high cholinergic drive to respiratory patterning neurons (2, 29). Again, the KF and other regions of the dorsolateral pons are worth exploring in this regard, as they send projections to the entire extent of the ventral respiratory group and are key contributors to respiratory patterning (35).
5-HT-Deficient Pups Display Prolonged Episodes of Active Sleep
We discovered that the spontaneous arousal of TPH2−/− pups from both QS and AS was delayed compared with WT. Central 5-HT appears to be particularly crucial for arousal from AS; TPH2−/− pups had episodes of AS that were twice as long as WT pups, while their QS episodes were only slightly prolonged. Solarewicz and colleagues showed that adult TPH2−/− mice have longer episodes of NREM sleep (i.e., the equivalent of QS in infants) compared with WT, but the effect of central 5-HT depletion on the duration of REM sleep (the equivalent of AS in infants) was not reported (41). As we recorded breathing and sleep patterns in unanesthetized WT and TPH2−/− pups ~30 min after surgery for EMG implantation with isoflurane anesthesia, it is possible that the altered sleep architecture demonstrated by TPH2−/− pups is related to the residual effects of isoflurane. However, we note that the respiratory variables and HR we report for TPH2−/− pups are close to those reported previously for pups tested without prior anesthesia (9, 22).
With regard to the mechanism(s), cholinergic neurons of the laterodorsal (LDT) and pedunculopontine (PPT) tegmentii send projections to, and activate, glutamatergic neurons with the pontine reticular formation to initiate and maintain AS (5). Serotonergic neurons within the dorsal raphe nuclei project to the LDT and PPT (17). 5-HT reduces REM sleep when it is applied to the LDT, likely acting through inhibitory 5-HT1A receptors (18, 43). Taken together, these findings strongly suggest that the prolonged episodes of AS demonstrated by TPH2−/− pups is due to prolonged LDT and/or PPT activation resulting from a loss of inhibitory drive provided by serotonergic dorsal raphe neurons, neurons that increase their activity toward the end of REM sleep episodes (19, 32).
Despite having considerably longer episodes of AS, overall TPH2−/− pups spend the same amount of time in AS and QS, as they have fewer episodes of sleep compared with WT pups. With respect to AS, the LDT and PPT express not only inhibitory 5-HT1A receptors but also excitatory 5-HT2 receptors (34). And there is evidence from adult animals that the activation of 5-HT2A receptors increases the number of REM sleep episodes (1). Thus it may be that a loss of pontine 5-HT reduces not only the ability of these cholinergic neurons to turn off once activated (due to a loss of 5-HT1A signaling) but also their ability to turn on (due to a loss of 5-HT2A receptor signaling). The consequences for TPH2−/− rat pups are fewer, but longer, AS episodes.
We showed that the arousal of TPH2−/− pups was delayed in AS, irrespective of the gas they breathed, an effect not observed in WT pups. This suggests that central 5-HT is necessary for the appropriate timing of arousal from AS, in normal conditions and in response to hypoxia and hypercapnia. It appears that other components of the ascending arousal system (e.g., orexigenic, noradrenergic, histaminergic neurons), while potentially contributing to arousal in AS, are incapable of fully compensating for a loss of central 5-HT. Thus AS may represent a particularly dangerous state for an infant with central serotonergic dysfunction because of the simultaneous emergence of apnea and delayed arousal.
Arousal in Response to Hypercapnia Is Delayed by Central 5-HT Deficiency
Arousal from sleep and the associated increase in sympathetic and respiratory motor output is vital for maintaining homeostasis in response to the hypoxia and hypercapnia associated with apnea and other life-threatening cardiorespiratory events. A failure to arouse in response to such events is likely involved in the pathogenesis of SIDS. Here we show that in both QS and AS central 5-HT deficiency delays arousal in response to hypercapnia but not hypoxia. This finding supports those of Buchanan and Richerson, who showed a similar effect in adult Lmx1b−/− mice lacking central 5-HT neurons (6). The effect we describe in the TPH2−/− pups was not due to reduced feedback from lung or airway receptors secondary to a reduced hypercapnic ventilatory response. Instead, their delayed arousal to hypercapnia is likely due to a lack of 5-HT2A receptor signaling in the pons, thalamus, or other targets of the ascending arousal system (7). These regions are normally innervated by CO2-sensitive serotonergic neurons originating in the dorsal raphe nuclei (7, 40, 45).
For both genotypes, the latencies to arousal in response to increasing CO2 were longer in AS compared with QS and were not different from the latencies to spontaneous arousal in room air. These results are somewhat different from those of Buchanan and Richerson, who showed that WT mice exhibit robust arousal from REM sleep in response hypercapnia while Lmx1b−/− mice essentially fail to arouse in response to the challenge (6). Others have found that the arousal of young animals in response to hypercapnia is delayed in AS compared with QS (15, 20). It appears that in early postnatal life groups of neurons that normally terminate AS are, unlike respiratory neurons that promote the hypercapnic ventilatory response, insensitive to inputs originating from central CO2 chemoreceptive sites.
Despite the delayed arousal in response to hypercapnia displayed by pups deficient in central 5-HT, our data also show that for both genotypes arousal occurred more quickly in response to hypoxia than it did in response to hypercapnia. Thus, by itself, delayed arousal in response to hypercapnia (at least for these rat pups) would be unlikely to trigger sudden death during sleep, as the subsequent drop in Po2 would promptly initiate arousal. That said, human infants, unlike rodents, usually arouse more quickly in response to hypercapnia than hypoxia (31, 44), and infants nearly dying of SIDS have blunted arousal responses to hypercapnia as well as hypoxia (31). The relevance of the delayed arousal of TPH2−/− pups in response to hypercapnia for SIDS is therefore unclear.
Significance: Infants with Serotonergic Dysfunction May Have Increased Risk for SIDS During AS
Defects within the 5-HT system of the brain stem, including reduced 5-HT and TPH2, are pathological features of SIDS cases (30, 38). SIDS occurs during sleep periods, and there is evidence that future SIDS cases experience apnea, incomplete arousal from sleep, and increased amounts of AS (8, 21, 23, 24). However, there have been few, if any, studies in young animals examining how sleep state could modify the influence of serotonergic dysfunction on the control of breathing or arousal. Our data suggest that central 5-HT deficiency in early life delays spontaneous arousal and, in both QS and AS, arousal in response to hypercapnia. A key additional finding was that the deleterious effects of central 5-HT deficiency on breathing and arousal from sleep only fully emerge in AS. Unlike WT pups, TPH2−/− pups became apneic in AS and, compared with their arousal from QS, their arousal from AS was delayed, irrespective of the nature of the chemical stimulus. Thus, unlike normal infants, infants deficient in central 5-HT, or who have other serotonergic system defects, may be at higher risk for SIDS while in AS because of apnea and arousal responses that are delayed relative to QS.
GRANTS
Funding for this work was provided by American Heart Association Scientist Development Grant 14SDG18560022 (principal investigator: K. J. Cummings), National Heart, Lung, and Blood Institute Grant HL-122358 (principal investigator: M. R. Hodges), and University of Missouri College of Veterinary Medicine Faculty Research Grants (K. J. Cummings).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.O.Y., A.M.G., M.R.H., and K.J.C. conceived and designed research; J.O.Y. and K.J.C. performed experiments; J.O.Y. and K.J.C. analyzed data; J.O.Y., M.R.H., and K.J.C. interpreted results of experiments; J.O.Y., M.R.H., and K.J.C. drafted manuscript; J.O.Y., A.M.G., M.R.H., and K.J.C. approved final version of manuscript; A.M.G., M.R.H., and K.J.C. edited and revised manuscript; K.J.C. prepared figures.
ACKNOWLEDGMENTS
We thank Jane Chen for technical assistance and animal husbandry and Dr. Hannah C. Kinney (Harvard Medical School) for critical feedback on the manuscript.
REFERENCES
- 1.Amici R, Sanford LD, Kearney K, McInerney B, Ross RJ, Horner RL, Morrison AR. A serotonergic (5-HT2) receptor mechanism in the laterodorsal tegmental nucleus participates in regulating the pattern of rapid-eye-movement sleep occurrence in the rat. Brain Res 996: 9–18, 2004. doi: 10.1016/j.brainres.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 216: 53–68, 1983. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1: 876–886, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnard S, Khemiri H, Masse F, Denise P, Verdaguer M, Gestreau C. Differential respiratory control of the upper airway and diaphragm muscles induced by 5-HT1A receptor ligands. Sleep Breath 16: 135–147, 2012. doi: 10.1007/s11325-010-0466-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev 92: 1087–1187, 2012. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107: 16354–16359, 2010. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan GF, Smith HR, MacAskill A, Richerson GB. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol 114: 233–243, 2015. doi: 10.1152/jn.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornwell AC, Feigenbaum P, Kim A. SIDS, abnormal nighttime REM sleep and CNS immaturity. Neuropediatrics 29: 72–79, 1998. doi: 10.1055/s-2007-973539. [DOI] [PubMed] [Google Scholar]
- 9.Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol 296: R1783–R1796, 2009. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1−/− mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol 298: R1333–R1342, 2010. doi: 10.1152/ajpregu.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnall RA, Schneider RW, Tobia CM, Zemel BM. Arousal from sleep in response to intermittent hypoxia in rat pups is modulated by medullary raphe GABAergic mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R551–R560, 2012. doi: 10.1152/ajpregu.00506.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhingra RR, Dutschmann M, Dick TE. Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Respir Physiol Neurobiol 226: 110–114, 2016. doi: 10.1016/j.resp.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 14.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303: 430–437, 2010. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fewell JE, Baker SB. Arousal and cardiopulmonary responses to hyperoxic hypercapnia in lambs. J Dev Physiol 12: 21–26, 1989. [PubMed] [Google Scholar]
- 16.Gaultier C. Cardiorespiratory adaptation during sleep in infants and children. Pediatr Pulmonol 19: 105–117, 1995. doi: 10.1002/ppul.1950190206. [DOI] [PubMed] [Google Scholar]
- 17.Honda T, Semba K. Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res 647: 299–306, 1994. doi: 10.1016/0006-8993(94)91329-3. [DOI] [PubMed] [Google Scholar]
- 18.Horner RL, Sanford LD, Annis D, Pack AI, Morrison AR. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. J Neurosci 17: 7541–7552, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev 43: 563–578, 1991. [PubMed] [Google Scholar]
- 20.Johnston RV, Grant DA, Wilkinson MH, Walker AM. The effects of repeated exposure to hypercapnia on arousal and cardiorespiratory responses during sleep in lambs. J Physiol 582: 369–378, 2007. doi: 10.1113/jphysiol.2007.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Polain DL, Wayenberg JL. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep 15: 287–292, 1992. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan K, Echert AE, Massat B, Puissant MM, Palygin O, Geurts AM, Hodges MR. Chronic central serotonin depletion attenuates ventilation and body temperature in young but not adult Tph2 knockout rats. J Appl Physiol (1985) 120: 1070–1081, 2016. doi: 10.1152/japplphysiol.01015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Franco P, Groswasser J, Scaillet S, Kelmanson I, Togari H, Kahn A. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med 168: 1298–1303, 2003. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 24.Kato I, Groswasser J, Franco P, Scaillet S, Kelmanson I, Togari H, Kahn A. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am J Respir Crit Care Med 164: 1464–1469, 2001. doi: 10.1164/ajrccm.164.8.2009001. [DOI] [PubMed] [Google Scholar]
- 25.Kinney HC, Burger PC, Harrell FE Jr, Hudson RP Jr. “Reactive gliosis” in the medulla oblongata of victims of the sudden infant death syndrome. Pediatrics 72: 181–187, 1983. [PubMed] [Google Scholar]
- 26.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 114: 234–238, 2004. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 28.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol Regul Integr Comp Physiol 264: R544–R554, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol 117: 257–265, 2009. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 31.McCulloch K, Brouillette RT, Guzzetta AJ, Hunt CE. Arousal responses in near-miss sudden infant death syndrome and in normal infants. J Pediatr 101: 911–917, 1982. doi: 10.1016/S0022-3476(82)80009-7. [DOI] [PubMed] [Google Scholar]
- 32.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101: 569–575, 1976. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 33.Monti JM, Monti D. Role of dorsal raphe nucleus serotonin 5-HT1A receptor in the regulation of REM sleep. Life Sci 66: 1999–2012, 2000. doi: 10.1016/S0024-3205(99)00649-9. [DOI] [PubMed] [Google Scholar]
- 34.Morilak DA, Ciaranello RD. 5-HT2 receptor immunoreactivity on cholinergic neurons of the pontomesencephalic tegmentum shown by double immunofluorescence. Brain Res 627: 49–54, 1993. doi: 10.1016/0006-8993(93)90747-B. [DOI] [PubMed] [Google Scholar]
- 35.Mörschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol (1985) 85: 84–90, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep 28: 801–807, 2005. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- 38.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 39.Rognum TO, Saugstad OD. Hypoxanthine levels in vitreous humor: evidence of hypoxia in most infants who died of sudden infant death syndrome. Pediatrics 87: 306–310, 1991. [PubMed] [Google Scholar]
- 40.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 41.Solarewicz JZ, Angoa-Perez M, Kuhn DM, Mateika JH. The sleep-wake cycle and motor activity, but not temperature, are disrupted over the light-dark cycle in mice genetically depleted of serotonin. Am J Physiol Regul Integr Comp Physiol 308: R10–R17, 2015. doi: 10.1152/ajpregu.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashima S, Becker LE. Developmental abnormalities of medullary “respiratory centers” in sudden infant death syndrome. Exp Neurol 90: 580–587, 1985. doi: 10.1016/0014-4886(85)90155-4. [DOI] [PubMed] [Google Scholar]
- 43.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci 18: 5490–5497, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Hal AL, Rodriguez AM, Sargent CW, Platzker AC, Keens TG. Hypoxic and hypercapneic arousal responses and prediction of subsequent apnea in apnea of infancy. Pediatrics 75: 848–854, 1985. [PubMed] [Google Scholar]
- 45.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79: 161–169, 1997. doi: 10.1016/S0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 46.Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol 164: 123–130, 2008. doi: 10.1016/j.resp.2008.06.016. [DOI] [PubMed] [Google Scholar]