We show that heat shock protein 90 functionally contributes to the heat loss response of cutaneous vasodilation during exercise in the heat, and this response is mediated through the activation of nitric oxide synthase. Therefore, interventions that may activate heat shock protein 90 may facilitate an increase in heat dissipation through an augmentation of cutaneous perfusion. In turn, this may attenuate or reduce the increase in core temperature and therefore the level of heat strain.
Keywords: chaperone, nitric oxide, thermoregulation, heat loss, microcirculation
Abstract
While the mechanisms underlying the control of cutaneous vasodilation have been extensively studied, there remains a lack of understanding of the different factors that may modulate cutaneous perfusion during an exercise-induced heat stress. We evaluated the hypothesis that heat shock protein 90 (HSP90) contributes to the heat loss response of cutaneous vasodilation via the activation of nitric oxide synthase (NOS) during exercise in the heat. In 11 young males (25 ± 5 yr), cutaneous vascular conductance (CVC) was measured at four forearm skin sites that were continuously treated with 1) lactated Ringer solution (control), 2) NOS inhibition with 10 mM NG-nitro-l-arginine methyl ester (l-NAME), 3) HSP90 inhibition with 178 μM geldanamycin, or 4) a combination of 10 mM l-NAME and 178 μM geldanamycin. Participants rested in a moderate heat stress (35°C) condition for 70 min. Thereafter, they performed a 50-min bout of moderate-intensity cycling (~52% V̇o2peak) followed by a 30-min recovery period. We showed that NOS inhibition attenuated CVC (~40–50%) relative to the control site during pre- and postexercise rest in the heat (P ≤ 0.05); however, no effect of HSP90 inhibition was observed (P > 0.05). During exercise, we observed an attenuation of CVC with the separate inhibition of NOS (~40–50%) and HSP90 (~15–20%) compared with control (both P ≤ 0.05). However, the effect of HSP90 inhibition was absent in the presence of the coinhibition of NOS (P > 0.05). We show that HSP90 contributes to cutaneous vasodilation in young men exposed to the heat albeit during exercise only. We also show that the HSP90 contribution is due to NOS-dependent mechanisms.
NEW & NOTEWORTHY We show that heat shock protein 90 functionally contributes to the heat loss response of cutaneous vasodilation during exercise in the heat, and this response is mediated through the activation of nitric oxide synthase. Therefore, interventions that may activate heat shock protein 90 may facilitate an increase in heat dissipation through an augmentation of cutaneous perfusion. In turn, this may attenuate or reduce the increase in core temperature and therefore the level of heat strain.
the regulation of core temperature during exercise in the heat requires the transfer of heat from the body to the environment, which is mediated through an increase in cutaneous vasodilation. The mechanisms underpinning the regulation of cutaneous perfusion during an exercise-induced stress have been extensively studied. Several key factors, such as nitric oxide synthase (NOS) (12, 21, 35, 36), cyclooxygenase (32), K+ channels (2, 30, 31), and neurotransmitters released from sympathetic cholinergic nerves (26), have been shown to play an important role in the regulation of cutaneous vasodilation. Despite this knowledge, there remain important gaps in our understanding of the mechanism(s) governing the regulation of cutaneous vasodilation during exercise.
Heat shock protein 90 (HSP90) is a member of a family of molecular chaperone proteins that can be upregulated by various stressors, including local skin heating (44). Shastry and Joyner (39) showed that changes in intracellular HSP90 content may contribute to cutaneous vasodilation as evidenced by an increase in cutaneous perfusion during local heating of skin to 42°C. Although their results demonstrated a potential role of HSP90 in the regulation of the cutaneous vascular response in humans in vivo, it has been shown that the mechanisms underpinning the regulation of cutaneous vasodilation differ between local vs. whole body heating (20). For example, although endothelial NOS contributes to cutaneous vasodilation during local heating, neuronal, but not endothelial, NOS is involved in the regulation of cutaneous vasodilation during whole body heating at rest (25). Furthermore, adenosine receptors contribute to cutaneous vasodilation during local heating (6) but not whole body passive heating at rest (7). As such, it remains unclear if HSP90 can modulate the regulation of cutaneous vasodilation during a whole body heat stress such as that associated with exercise in the heat.
It is well established that HSP90 activates NOS, thus increasing NO production (16, 29, 38). Therefore, if HSP90 is involved in the regulation of cutaneous vasodilation during heat stress, it is plausible that this response may be mediated via the activation of NOS. As noted above, Shastry and Joyner (39) demonstrated that HSP90 contributes to cutaneous vasodilation during local skin heating to 42°C. Given that local heating-induced cutaneous vasodilation has been shown to be largely mediated by NOS (37), their findings may indirectly support the possibility that HSP90 mediates cutaneous vasodilation during heat stress through NOS-dependent mechanisms. Thus, the purpose of this study was to elucidate the contribution of HSP90 in the regulation of cutaneous vasodilation during exercise in the heat and to determine whether this contribution acts through the NOS pathway.
MATERIALS AND METHODS
Ethical approval.
This study was approved by the University of Ottawa Health Sciences and Science Research Ethics Board and complied with the guidelines set forth by the Declaration of Helsinki. All participants gave verbal and written informed consent before participation in the study.
Participants.
Eleven young habitually active men (25 ± 5 yr) participated in the study. All participants were screened for cardiovascular, respiratory, and metabolic diseases before participating. Moreover, the participants were nonsmoking and not currently taking any prescription medicines. Participants’ body mass, height, and surface area were (mean ± SD) 79.8 ± 10.5 kg, 1.77 ± 0.06 m, and 1.97 ± 0.1 m2, respectively.
Experimental design.
All participants completed one screening and one experimental session. Participants were required to refrain from engaging in heavy exercise and consuming over-the-counter medications (e.g., nonsteroidal anti-inflammatory agents, vitamins, minerals) 48 h before the sessions. Furthermore, they were restricted from consuming all caffeinated beverages and alcohol at least 12 h before the commencement of the sessions. On the day of the experimental session, participants were allowed to consume food up to 2 h before the start of the experiment.
During the screening session, body mass was measured using a digital weight scale platform (model CBU150X; Mettler Toledo, Schwerzenbach, Switzerland) with a weighing terminal (model IND560; Mettler Toledo, Mississauga, ON, Canada) while height was assessed using an eye-level physician stadiometer (model 2391; Detecto, Webb City, MO). These two measurements were then used to calculate body surface area according to the DuBois and DuBois equation (5). Body fat percentage was estimated through body density via the hydrostatic weighing technique. Peak oxygen uptake was determined through an incremental cycling exercise protocol. Participants seated on a semirecumbent cycling ergometer maintained a pedaling rate of 60–100 revolutions/min at a starting resistance of 80 W, which was increased by 20 W/min until volitional fatigue. Ventilatory and metabolic data were collected using an automated indirect calorimetry system (Medgraphic Ultima; Medical Graphic, St. Paul, MN).
The experimental session was performed on a day separated from the screening session by a minimum of 48 h. Upon arrival at the laboratory, participants voided their bladder after which pretrial body mass was indexed on a weighing terminal (Mettler Toledo). Following the measurement of body mass, participants were seated on a reclining surgical bed in a non-heat-stress environment (~25°C). Four microdialysis fibers (30 kDa cutoff, 10 mm membrane) (MD2000; Bioanalytical Systems, West Lafayette, IN) were inserted in the dermal layer of the skin on the left dorsal forearm. Under aseptic conditions, a 25-gauge needle was inserted subcutaneously in the skin (~2.5 cm in length). Subsequently, the microdialysis fiber was threaded through the lumen of the needle, after which the needle was removed from the skin, leaving behind the fiber embedded in the forearm. Each fiber was secured with surgical tape and separated by at least 4 cm. After the insertion of the fibers, the participant was transferred to an adjacent thermal chamber (Can-Trol Environmental Systems, Markham, ON, Canada) regulated at 25°C and 20% relative humidity where they remained resting while seated on a semirecumbent cycle ergometer. At this time, the perfusion of the pharmacological agents was started at each of the four microdialysis sites at a rate of 4 μl/min with a microinfusion pump (model 400; CMA Microdialysis, Solna, Sweden). The sites were perfused with either 1) lactated Ringer solution (Baxter, Deerfield, IL) (control), 2) 10 mM NG-nitro-l-arginine methyl ester (l-NAME, a specific inhibitor of NOS), 3) 178 μM geldanamycin (an inhibitor of HSP90), or 4) a combination of 10 mM l-NAME and 178 μM geldanamycin (NOS and HSP90 inhibition). l-NAME and geldanamycin were acquired from Sigma-Aldrich (St. Louis, MO) and Cayman Chemical (Ann Arbor, MI), respectively. Dimethyl sulfoxide (DMSO, Sigma-Aldrich) was necessary as an organic solvent for dissolving geldanamycin (39). To offset any potential influence of DMSO on responses, all of the above agents were dissolved in 5% DMSO solution. In our pilot work, we demonstrated that 5% DMSO solution had no effect on cutaneous vascular response compared with the control site during exercise in the heat. Concentrations of l-NAME (4, 11, 19, 27, 47, 48) and geldanamycin (39) were determined on the basis of previous studies employing the intradermal microdialysis technique. The drug perfusion continued for a minimum of 60 min to ensure maximal inhibition of NOS and/or HSP90 within the current experimental conditions. Furthermore, this time period has been shown to be sufficient to ensure that any redness associated with fiber insertion had subsided (1). Drug perfusion continued throughout the entire experimental protocol to ensure the separate and combined continuous blockade of NOS and HSP90.
Following the ≥60-min habituation period in a non-heat-stress ambient temperature condition of 25°C, participants were monitored for an additional 10 min to acquire baseline measurements. Thereafter, room temperature was increased to 35°C during which time the participant remained resting on the semirecumbent cycle ergometer for at least 70 min. Baseline resting measurements were recorded during the final 10 min. Participants then cycled for 50 min at a moderate intensity equivalent to 52 ± 3% of their predetermined peak oxygen uptake, which was followed by a 30-min recovery bout. Thereafter, 50 mM sodium nitroprusside (Sigma-Aldrich) was infused for 20–25 min at all four sites at a rate of 6 μl/min until maximum values for cutaneous perfusion were achieved for a minimum of 2 min. Upon completion of the experimental session, the participant’s body mass was measured.
Measurements.
Cutaneous blood flow (expressed in perfusion units) was measured at four local sites on the forearm using laser-Doppler flowmetry (PeriFlux System 5000; Perimed, Stockholm, Sweden) at a sampling rate of 32 Hz. Integrated seven-laser array laser-Doppler probes (model 413; Perimed) were situated directly above the center of the membrane of the microdialysis fibers. Cutaneous vascular conductance (CVC) was evaluated as laser-Doppler flux, an index of cutaneous blood flow divided by mean arterial pressure, and expressed as a percent maximum using values from the maximal absolute CVC protocol. Blood pressure was determined every 5 min using a manual mercury column sphygmomanometer (Baumonometer Standby Model; WA Baum, Copiague, NY), and mean arterial pressure was calculated as diastolic arterial pressure plus one-third the difference between systolic and diastolic pressures.
Core temperature was estimated by aural canal temperature measured at 5-min intervals using an ear thermometer (Braun Thermoscan Pro 6000; Welch Allyn, Skaneateles Falls, NY) inserted in the aural canal. Aural canal temperature has been shown to closely track changes in esophageal temperature, especially in the heat (42). Skin temperature was measured continuously at four sites (calf, quadriceps, chest, and biceps) using thermocouple disks (Concept Engineering, Old Saybrook, CT) attached to the skin with adhesive rings and surgical tape. Mean skin temperature was estimated as a weighted mean using the local skin temperatures of the calf (20%), quadriceps (20%), biceps (30%), and chest (30%) (15). All temperature data were collected using a data acquisition model (model 34970A; Agilent Technologies Canada, Mississauga, ON, Canada) and displayed and recorded using LabVIEW software (National Instruments, Austin, TX). With the use of these values, mean body temperature was calculated as 0.9 × core temperature + 0.1 × mean skin temperature (13). Heart rate was measured continuously using a Polar coded WearLink and transmitter, Polar RS400 interface, and Polar Trainer 5 software (Polar Electro, Kempele, Finland).
Metabolic energy expenditure was measured via indirect calorimetry using electrochemical gas analyzers (AMETEK model S-3A/1 and CD3A; Applied Electrochemistry, Pittsburgh, PA) to determine O2 and CO2 concentrations in expired air. Participants wore a fitted facemask (model 7600 V2; Hans-Rudolph, Kansas City, MO) that was attached to a two-way T-shape nonbreathing valve (model 2700; Hans-Rudolph). Oxygen uptake and respiratory exchange ratio were calculated from O2 and CO2 concentrations in expired air and sampled every 30 s to estimate metabolic rate.
Data analysis.
Based on CVC data obtained in our previous work (9), with an 80% power and a significance level of 0.05, a minimal sample size of n = 9 for CVC was determined. For all variables, a 5-min average was taken for each of the two baseline resting periods [under non-heat-stress (25°C) and heat stress (35°C) conditions] and at 10-min intervals during exercise and the subsequent recovery. For the measurement of maximal cutaneous blood flow during the sodium nitroprusside treatment, responses were measured over a 2-min interval. Blood pressure and aural canal temperature were manually recorded every 5 min, and an average of two values measured during a 10-min interval were used for data analysis. The percent contribution of NOS and HSP90 to cutaneous vasodilation was evaluated as [(CVC at the control site – CVC at treatment site)/CVC at the control site] × 100 in accordance with a previous report (45). This evaluation was performed at rest, during, and following exercise in the heat only using a last 5-min average data at each period.
Statistical analyses.
CVC data were analyzed with a two-way repeated-measures analysis of variance with the factors of treatment site (control, NOS inhibition only, HSP90 inhibition only, combined NOS and HSP90 inhibition) and stage (baseline at 25 and 35°C and every 10 min during and following exercise). Maximum absolute CVC (perfusion units, mmHg−1) and percent contribution of NOS and HSP90 were analyzed with a one-way repeated-measures analysis of variance with a factor of treatment site. Secondary variables (body temperatures, heart rate, and mean arterial pressure) were analyzed with a one-way repeated-measures analysis of variance with a factor of stage. When detecting a significant interaction, or a main effect, post hoc multiple comparisons were carried out using a modified version of Bonferroni correction (Holm-Bonferroni’s method). In addition, Student’s t-tests were also employed where applicable. The level of significance for all analyses was set at P ≤ 0.05. All values are reported with a mean ± 95% confidence interval (1.96 × SE). Statistical analyses were conducted using SPSS 24 (IBM, Armonk, NY).
RESULTS
Cutaneous vascular response.
There were no between-site differences in CVC during rest in a non-heat-stress condition (i.e., room maintained at 25°C) (P > 0.05, Fig. 1). Resting CVC increased during the subsequent exposure to a heat stress (i.e., room maintained at 35°C) at all skin sites (P ≤ 0.05, Fig. 1). Relative to the control site, CVC during heat stress at rest was attenuated with NOS inhibition (P ≤ 0.05), whereas it tended to be reduced by the combined inhibition of NOS and HSP90 (P = 0.07) (Fig. 1). However, no effect of HSP90 inhibition was observed relative to the control site (P > 0.05, Fig. 1).
Fig. 1.
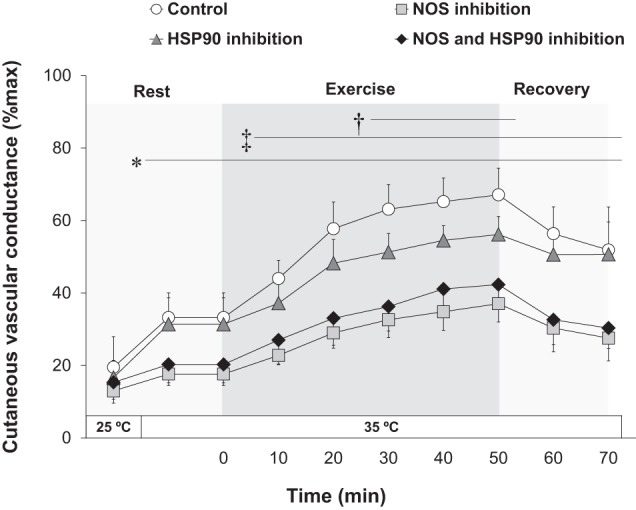
Cutaneous vascular conductance at rest (25 and 35°C) and during and following exercise in the heat (35°C). Four intradermal forearm skin sites were continuously treated with either: 1) lactated Ringer solution (control), 2) nitric oxide synthase (NOS) inhibition, 3) heat shock protein 90 (HSP90) inhibition, or 4) NOS and HSP90 inhibition. P ≤ 0.05, control vs. NOS inhibition (*), control vs. HSP90 inhibition (†), and control vs. NOS and HSP90 inhibition site (‡). Cutaneous vascular conductance did not differ between NOS inhibition and NOS and HSP90 inhibition site (P > 0.05). All values are expressed as means ± 95% confidence interval (n = 11). All values were obtained by averaging over the final 5 min of each time interval.
During exercise in the heat, CVC increased above preexercise baseline resting levels at all treatment sites (P ≤ 0.05, Fig. 1). Upon cessation of exercise, CVC at all skin sites declined but remained elevated above preexercise levels for the duration of the 30-min recovery (P ≤ 0.05, Fig. 1). During both the exercise and recovery periods, NOS inhibition attenuated CVC compared with the control site (P ≤ 0.05, Fig. 1); this response was observed regardless of coinhibition of HSP90. A reduction in CVC was measured during the mid-to-late stages of exercise (≥30 min into exercise) with the inhibition of HSP90 alone compared with the control site (P ≤ 0.05, Fig. 1). Conversely, no difference in CVC was measured between the NOS inhibition site compared with the combined NOS- and HSP90-inhibited site (P > 0.05, Fig. 1). Taken together, these results demonstrate that HSP90 contributes to cutaneous vasodilation during exercise via the activation of NOS.
Percent contribution of NOS alone to cutaneous vasodilation during 35°C rest was greater than that of HSP90 alone (Fig. 2). At the end of exercise and subsequent recovery, the percent contribution of NOS only and NOS and HSP90 in combination was greater than that of HSP90 only (Fig. 2). Maximum absolute CVC obtained with sodium nitroprusside did not differ between sites (P > 0.05, Table 1).
Fig. 2.
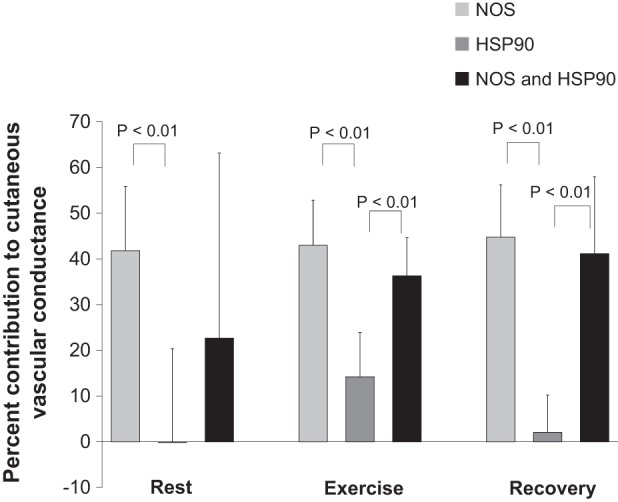
Percent contribution of NOS and HSP90 to cutaneous vasodilation at rest as well as during and following exercise in the heat (35°C). All values were obtained by averaging over the final 5 min of each time interval. All values are expressed as means ± 95% confidence interval (n = 11).
Table 1.
Maximum absolute cutaneous vascular conductance at the four skin sites
| Perfusion Units, mmHg−1 | |
|---|---|
| Control | 2.44 ± 0.69 |
| NOS inhibition | 2.19 ± 0.56 |
| HSP90 inhibition | 2.51 ± 0.68 |
| NOS and HSP90 inhibition | 2.21 ± 0.76 |
Values are expressed as means ± 95% confidence interval; n = 11 experiments. NOS, nitric oxide synthase; HSP90, heat shock protein 90. There were no between-site differences (P = 0.30 for a main effect of treatment site).
Body temperature and cardiovascular responses.
Resting core and skin temperatures and heart rate increased during the transition from the non-heat-stress to the heat stress ambient condition, with a further increase measured during exercise (P ≤ 0.05, Table 2). Responses remained elevated above baseline resting values during the postexercise recovery period (P ≤ 0.05, Table 2). No differences in resting mean arterial pressure were measured between the non-heat-stress and heat stress conditions, whereas responses were significantly elevated above preexercise resting levels during exercise (P ≤ 0.05, Table 2). However, postexercise mean arterial pressure was reduced below preexercise resting conditions in the heat (P ≤ 0.05, Table 2).
Table 2.
Thermal and cardiovascular responses during baseline resting, exercise, and postexercise recovery
| Baseline Resting |
||||
|---|---|---|---|---|
| 25°C | 35°C | Exercise | Recovery | |
| Core temperature, °C | 36.57 ± 0.17 | 36.99 ± 0.12* | 38.00 ± 0.33*† | 37.64 ± 0.33*† |
| Mean skin temperature, °C | 31.64 ± 0.20 | 34.43 ± 0.25* | 35.87 ± 0.22*† | 34.99 ± 0.18*† |
| Mean body temperature, °C | 36.08 ± 0.16 | 36.73 ± 0.11* | 37.79 ± 0.30*† | 37.37 ± 0.30*† |
| Heart rate, beats/min | 60 ± 2 | 64 ± 3* | 147 ± 10*† | 89 ± 7*† |
| Mean arterial pressure, mmHg | 91 ± 4 | 95 ± 4 | 101 ± 4*† | 90 ± 4† |
All values are presented as means ± 95% confidence interval; n = 11. All values were obtained by averaging over the final 5 min of each time interval.
P ≤ 0.05 vs. Baseline resting at 25°C.
P ≤ 0.05 vs. Baseline resting at 35°C.
Body weight.
Following the experiment, body weight was reduced by 1.6 ± 0.2% (P ≤ 0.05).
DISCUSSION
We evaluated for the first time the role of HSP90 in the regulation of cutaneous vasodilation during exercise in the heat. We showed that, while HSP90 inhibition alone did not modulate cutaneous vasodilation under resting in either the non-heat-stress or heat stress conditions, it did attenuate cutaneous vasodilation during exercise in the heat. This was evidenced as a reduction in CVC relative to the control site. However, the attenuation of CVC with the combined NOS and HSP90 inhibition was not different from that achieved by NOS inhibition alone. Furthermore, as in the preexercise resting periods, no effect of HSP90 inhibition was observed during the postexercise recovery period under a heat-stressed condition. Taken together, we show that HSP90 contributes to cutaneous vasodilation via NOS-dependent mechanisms in young habitually active men during exercise in the heat.
Cutaneous vascular response.
Consistent with a previous study by Shastry and Joyner (39), we showed that HSP90 inhibition had no effect on CVC during exposure to a non-heat-stress ambient temperature of 25°C (Fig. 1). Similarly, no effect of NOS inhibition was measured during this period (Fig. 1). Although this is in keeping with some previous studies (10, 26, 46), it does contrast others who have reported a role for NOS in the regulation of cutaneous blood flow in young adults during resting in normothermic conditions (3, 17, 22). Although the reasons underpinning the disparate findings are unclear, this may be because of the high variability of resting cutaneous blood flow as previously suggested (43).
During exposure to a whole body heat stress associated with resting in a room regulated at an ambient temperature of 35°C, we observed an increase in CVC above normothermic resting conditions at all skin sites (Fig. 1), a response that paralleled the elevations in core and skin temperatures (Table 2). The increase in CVC was largely mediated by NOS (i.e., 40–50%), since NOS inhibition with l-NAME greatly diminished the increase in CVC (Figs. 1 and 2). In contrast, HSP90 did not contribute to cutaneous vasodilation during this period, since CVC did not differ between the control and HSP90 inhibition sites (Figs. 1 and 2). This is in contrast to the previous study by Shastry and Joyner (39) who demonstrated that HSP90 in part mediates cutaneous vasodilation elicited by local heating of skin to 42°C. The discrepancy could be the result of differences in local forearm skin temperature. In the current study, although we did not directly measure skin temperature at the forearm-treated sites (because of the instrumentation covering the area), we showed that mean skin temperature remained at ~34.5°C (Table 2), which was markedly lower than the forearm skin temperature of 42°C employed by Shastry and Joyner (39). It is possible therefore that a threshold for inducing the activation of HSP90 exists (i.e., between 34.5 and 42°C), and this threshold was not exceeded in the present study.
At the initiation of exercise, CVC at the control site increased from preexercise resting levels in the heat, reaching ~70% of maximal levels attained at the end of exercise (Fig. 1), which paralleled the exercise-induced increases in core and skin temperatures (Table 2). Importantly, during the mid-to-late stages of exercise (≥30 min into exercise), we showed that HSP90 inhibition reduced CVC compared with the control site (Fig. 1). Our observation provides new evidence demonstrating an important functional role of HSP90 in the regulation of cutaneous vasodilation during exercise in the heat in young men. Noteworthy, we show that the contribution of HSP90 was not observed in the early stages of exercise (≤20 min into exercise) wherein nonthermal factors associated with performing exercise such as central command, mechanoreflex, baroreceptors, and others have been shown to play an important role in modulating the heat loss response of cutaneous vasodilation (28).
While speculative, it may be that the influence of HSP90 during the mid-to-late stages of exercise in the heat is affected in part or in whole by the elevation of body temperature and therefore the level of hyperthermia of the individual. By the end of exercise, mean skin temperature increased to ~36°C (Table 2), which may have been sufficient to induce an effect of HSP90 on cutaneous perfusion. As mentioned above, increases in local skin temperature can induce HSP90 (39). Alternatively, there may be a core temperature threshold above which a contribution of HSP90 to the regulation of cutaneous vasodilation is initially observed. In the present study, we observed a contribution of HSP90 when core temperature increased to 37.7 ± 0.3°C at 30 min into exercise, and was subsequently preserved for the remaining duration of exercise. Furthermore, the activation of HSP90 may be related to acetylcholine released from sympathetic nerves. Heat stress activates sympathetic cholinergic nerves that in turn release acetylcholine, which is known to contribute to cutaneous vasodilation during a whole body heat stress (23, 41). Indeed, Shastry and Joyner (39) demonstrated that acetylcholine-induced cutaneous vasodilation is in part due to the activation of HSP90. Further study is required to directly delineate whether acetylcholine released from sympathetic nerves directly activates HSP90 during exercise, a response that could be examined using a simultaneous acetylcholine receptor blockade.
In accordance with several previous studies (2, 12, 18, 21, 35, 36, 40), we showed that NOS is involved in the regulation of cutaneous vasodilation. However, our study findings extend our understanding of the role of NOS in the regulation of CVC by demonstrating that HSP90 modulates cutaneous vasodilation mainly via activating NOS. This was evidenced by our observation that the contribution of HSP90 to the cutaneous vasodilation during exercise was abolished when NOS was simultaneously inhibited (i.e., CVC did not differ between the NOS inhibition vs. the combined NOS and HSP90 inhibition sites) (Fig. 1). Although not directly assessed in the current study, HSP90 may stimulate endothelial NOS, thereby eliciting cutaneous vasodilation during exercise in the heat. Indeed, Shastry and Joyner (39) suggested that HSP90 is an important modulator in inducing a sustained cutaneous vasodilation during local heating, a response that heavily depends on endothelial NOS (24). In addition, recent studies have shown that endothelial NOS mediates NOS-dependent cutaneous vasodilation during exercise (12, 35). Moreover, a similar link between HSP90 and endothelial NOS was reported in animal and in vitro studies (16, 29, 38).
Upon cessation of exercise, CVC at the control site declined rapidly (Fig. 1). In parallel, the contribution of HSP90 to the cutaneous vasodilation disappeared in the early minutes of recovery (i.e., within the first 10 min) (Fig. 1). The diminished HSP90 contribution observed during the postexercise period may be a consequence of a rapid reduction in core temperature and/or withdrawal of cutaneous vasodilation. More work is required to elucidate the mechanisms underpinning the diminished HSP90 contribution postexercise.
Study limitations.
We employed aural canal temperature to estimate core temperature. Although it is generally thought that aural canal temperature provides a poor estimate of core temperature, this temperature tracks changes in esophageal temperature well, especially in the heat (42). Hence we believe our estimation of core temperature based on the aural canal temperature measurement is valid in the present study.
Although we found HSP90 to be an important modulator of the heat loss response of cutaneous vasodilation, it remains unclear whether increases in body temperature per se and or performing exercise alone is/are required for the upregulation of HSP90. To the best of our knowledge, there are no studies demonstrating that mechanisms underpinning the heat loss response of cutaneous vasodilation during exercise in the heat are different compared with those observed during a passive heat stress. Further studies employing passive heat stress at rest are required to elucidate this response.
As a first step, we tested cutaneous vascular responses in young males only. Increasing evidence has shown that the cutaneous vascular response differs between males and females (8, 14). As such, future studies in young females would be an important next step to delineate potential sex-related differences in the contribution of HSP90 to the regulation of cutaneous vasodilation during heat stress.
Perspective and Significance
Cutaneous vasodilation is an integral response in the dissipation of heat and therefore the regulation of core temperature during exercise in the heat. It is well established that NOS plays a critical role in the regulation of the heat loss response of cutaneous vasodilation. We show that the contribution of NOS represents ~40–50% of total cutaneous vasodilation during exercise. Thus, factors that may modulate the activation of NOS can potentially play an important role in affecting heat dissipation. In the present study, we suggest that HSP90 activates NOS, contributing significantly to the regulation of cutaneous blood flow during exercise in the heat in healthy and habitually active young men. Hence further research is warranted to examine how the activation of HSP90 may enhance cutaneous perfusion and thereby heat loss.
Conclusion.
We showed that HSP90 inhibition attenuated cutaneous vasodilation during exercise in the heat, but this response was not measured with the simultaneous inhibition of NOS. We therefore conclude that HSP90 contributes to cutaneous vasodilation via NOS-dependent mechanisms in young men exercising in the heat.
GRANTS
This study was supported by the Natural Sciences and Engineering Research Council of Canada (Discover Grant RGPIN-06313–2014 and Discovery Grants Program—Accelerator Supplement RGPAS-462252-2014; funds held by Dr. Glen P. Kenny). G. P. Kenny is supported by a University of Ottawa Research Chair Award. N. Fujii was supported by the Human and Environmental Physiology Research Unit. B. D. McNeely was supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.F. and G.P.K. conceived and designed research; N.F., S.Y.Z., and B.D.M. performed experiments; N.F. and S.Y.Z. analyzed data; N.F., S.Y.Z., B.D.M., T.N., and G.P.K. interpreted results of experiments; N.F. and S.Y.Z. prepared figures; N.F. and S.Y.Z. drafted manuscript; N.F., S.Y.Z., B.D.M., T.N., and G.P.K. edited and revised manuscript; N.F., S.Y.Z., B.D.M., T.N., and G.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate all of the volunteers for taking time to participate in this study. We thank Mr. Michael Sabino of Can-Trol Environmental Systems Limited (Markham, ON, Canada) for support. We thank Lyra Halili and Mercy O. Danquah for help with data collection.
Present address for N. Fujii: University of Tsukuba, Institute of Health and Sport Sciences, Tsukuba City, Japan.
REFERENCES
- 1.Anderson C, Andersson T, Wårdell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol 102: 807–811, 1994. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- 2.Brunt VE, Fujii N, Minson CT. No independent, but an interactive, role of calcium-activated potassium channels in human cutaneous active vasodilation. J Appl Physiol (1985) 115: 1290–1296, 2013. doi: 10.1152/japplphysiol.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res 110: 43–47, 2017. doi: 10.1016/j.mvr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) 17: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 6.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 7.Fieger SM, Wong BJ. No direct role for A1/A2 adenosine receptor activation to reflex cutaneous vasodilatation during whole-body heat stress in humans. Acta Physiol (Oxf) 205: 403–410, 2012. doi: 10.1111/j.1748-1716.2012.02426.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N, Halili L, Singh MS, Meade RD, Kenny GP. Intradermal administration of ATP augments methacholine-induced cutaneous vasodilation but not sweating in young males and females. Am J Physiol Regul Integr Comp Physiol 309: R912–R919, 2015. doi: 10.1152/ajpregu.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii N, McGinn R, Paull G, Stapleton JM, Meade RD, Kenny GP. Cyclooxygenase inhibition does not alter methacholine-induced sweating. J Appl Physiol (1985) 117: 1055–1062, 2014. doi: 10.1152/japplphysiol.00644.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii N, McNeely BD, Zhang SY, Abdellaoui YC, Danquah MO, Kenny GP. Activation of protease-activated receptor 2 mediates cutaneous vasodilatation but not sweating: roles of nitric oxide synthase and cyclo-oxygenase. Exp Physiol 102: 265–272, 2017. doi: 10.1113/EP086092. [DOI] [PubMed] [Google Scholar]
- 11.Fujii N, Meade RD, Akbari P, Louie JC, Alexander LM, Boulay P, Sigal RJ, Kenny GP. No effect of ascorbate on cutaneous vasodilation and sweating in older men and those with type 2 diabetes exercising in the heat. Physiol Rep 5: 5, 2017. doi: 10.14814/phy2.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii N, Meade RD, Alexander LM, Akbari P, Foudil-Bey I, Louie JC, Boulay P, Kenny GP. iNOS-dependent sweating and eNOS-dependent cutaneous vasodilation are evident in younger adults, but are diminished in older adults exercising in the heat. J Appl Physiol (1985) 120: 318–327, 2016. doi: 10.1152/japplphysiol.00714.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagge AP, Gonzalez RR. Mechanisms of heat exchange: biophysics and physiology. In: Handbook of Physiology Environmental Physiology. Bethesda, MD: Am Physiol Soc, 1996, p. 45–84. [Google Scholar]
- 14.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Lack of limb or sex differences in the cutaneous vascular responses to exogenous norepinephrine. J Appl Physiol (1985) 117: 1417–1423, 2014. doi: 10.1152/japplphysiol.00575.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy JD, Dubois EF. The technic of measuring radiation and convection. J Nutr 15: 461–475, 1938. [Google Scholar]
- 16.Harris MB, Ju H, Venema VJ, Blackstone M, Venema RC. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen Pharmacol 35: 165–170, 2000. doi: 10.1016/S0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 17.Hodges GJ, Sparks PA. Noradrenaline and neuropeptide Y contribute to initial, but not sustained, vasodilatation in response to local skin warming in humans. Exp Physiol 99: 381–392, 2014. doi: 10.1113/expphysiol.2013.075549. [DOI] [PubMed] [Google Scholar]
- 18.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 19.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985) 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg DL Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995. doi: 10.1161/01.RES.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 24.Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellogg DL Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol (1985) 107: 1438–1444, 2009. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985) 113: 1512–1518, 2012. doi: 10.1152/japplphysiol.00859.2012. [DOI] [PubMed] [Google Scholar]
- 27.Kellogg DL Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol (1985) 98: 629–632, 2005. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol 3: 1689–1719, 2013. doi: 10.1002/cphy.c130011. [DOI] [PubMed] [Google Scholar]
- 29.Lin LY, Lin CY, Ho FM, Liau CS. Up-regulation of the association between heat shock protein 90 and endothelial nitric oxide synthase prevents high glucose-induced apoptosis in human endothelial cells. J Cell Biochem 94: 194–201, 2005. doi: 10.1002/jcb.20195. [DOI] [PubMed] [Google Scholar]
- 30.Louie JC, Fujii N, Meade RD, Kenny GP. The roles of the Na+/K+-ATPase, NKCC, and K+ channels in regulating local sweating and cutaneous blood flow during exercise in humans in vivo. Physiol Rep 4: 4, 2016. doi: 10.14814/phy2.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louie JC, Fujii N, Meade RD, McNeely BD, Kenny GP. The roles of KCa, KATP, and KV channels in regulating cutaneous vasodilation and sweating during exercise in the heat. Am J Physiol Regul Integr Comp Physiol 312: R821–R827, 2017. doi: 10.1152/ajpregu.00507.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- 35.McNamara TC, Keen JT, Simmons GH, Alexander LM, Wong BJ. Endothelial nitric oxide synthase mediates the nitric oxide component of reflex cutaneous vasodilatation during dynamic exercise in humans. J Physiol 592: 5317–5326, 2014. doi: 10.1113/jphysiol.2014.272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meade RD, Fujii N, Alexander LM, Paull G, Louie JC, Flouris AD, Kenny GP. Local infusion of ascorbate augments NO-dependent cutaneous vasodilatation during intense exercise in the heat. J Physiol 593: 4055–4065, 2015. doi: 10.1113/JP270787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Ou J, Fontana JT, Ou Z, Jones DW, Ackerman AW, Oldham KT, Yu J, Sessa WC, Pritchard KA Jr. Heat shock protein 90 and tyrosine kinase regulate eNOS NO· generation but not NO· bioactivity. Am J Physiol Heart Circ Physiol 286: H561–H569, 2004. doi: 10.1152/ajpheart.00736.2003. [DOI] [PubMed] [Google Scholar]
- 39.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol 282: H232–H236, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol (1985) 88: 467–472, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol (1985) 93: 1947–1951, 2002. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- 42.Taylor NA, Tipton MJ, Kenny GP. Considerations for the measurement of core, skin and mean body temperatures. J Therm Biol 46: 72–101, 2014. doi: 10.1016/j.jtherbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Tew GA, Klonizakis M, Moss J, Ruddock AD, Saxton JM, Hodges GJ. Reproducibility of cutaneous thermal hyperaemia assessed by laser Doppler flowmetry in young and older adults. Microvasc Res 81: 177–182, 2011. doi: 10.1016/j.mvr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Wilson N, McArdle A, Guerin D, Tasker H, Wareing P, Foster CS, Jackson MJ, Rhodes LE. Hyperthermia to normal human skin in vivo upregulates heat shock proteins 27, 60, 72i and 90. J Cutan Pathol 27: 176–182, 2000. doi: 10.1034/j.1600-0560.2000.027004176.x. [DOI] [PubMed] [Google Scholar]
- 45.Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 304: R651–R656, 2013. doi: 10.1152/ajpregu.00464.2012. [DOI] [PubMed] [Google Scholar]
- 46.Wong BJ, Fieger SM. Transient receptor potential vanilloid type 1 channels contribute to reflex cutaneous vasodilation in humans. J Appl Physiol (1985) 112: 2037–2042, 2012. doi: 10.1152/japplphysiol.00209.2012. [DOI] [PubMed] [Google Scholar]
- 47.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol (1985) 95: 504–510, 2003. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- 48.Yamazaki F, Takahara K, Sone R, Johnson JM. Influence of hyperoxia on skin vasomotor control in normothermic and heat-stressed humans. J Appl Physiol (1985) 103: 2026–2033, 2007. doi: 10.1152/japplphysiol.00386.2007. [DOI] [PubMed] [Google Scholar]


