Abstract
Erythrocytes are vital to human adaptation under hypoxic conditions because of their abundance in number and irreplaceable function of delivering oxygen (O2). However, although multiple large-scale altitude studies investigating the overall coordination of the human body for hypoxia adaptation have been conducted, detailed research with a focus on erythrocytes was missing due to lack of proper techniques. The recently maturing metabolomics profiling technology appears to be the answer to this limitation. Metabolomics profiling provides unbiased high-throughput screening data that reveal the overall metabolic status of erythrocytes. Recent studies have exploited this new technology and provided novel insight into erythrocyte physiology and pathology. In particular, a series of studies focusing on erythrocyte purinergic signaling have reported that adenosine signaling, coupled with 5′ AMP-activated protein kinase (AMPK) and the production of erythrocyte-enriched bioactive signaling lipid sphingosine 1-phosphate, regulate erythrocyte glucose metabolism for more O2 delivery. Moreover, an adenosine-dependent “erythrocyte hypoxic memory” was discovered that provides an explanation for fast acclimation upon re-ascent. These findings not only shed new light on our understanding of erythrocyte function and hypoxia adaptation, but also offer a myriad of novel therapeutic possibilities to counteract various hypoxic conditions.
Keywords: erythrocyte, hypoxia adaptation, adenosine signaling, sphingosine 1-phosphate, hypoxic memory
hypoxia, defined as having inadequate oxygen (O2) supply to the whole body or a region of the body, is a well-accepted central feature of a myriad of human diseases, including anemia, myocardial ischemia, stroke, chronic obstructive pulmonary disease, and pulmonary hypertension (3–5). Hypoxic conditions associated with these diseases are dangerous because decreased O2 availability promotes multiple end-organ damage and failure. Hypoxia also frequently occurs in healthy individuals when they go to high altitude, or when exposed to a low-O2 content environment. After ascent to altitudes higher than 2,500 m, acute mountain sickness can happen anytime from day 1 to day 5 and can result in high-altitude cerebral edema and high-altitude pulmonary edema. If not treated promptly, these conditions that began with symptoms including headache, lassitude, dizziness, and nausea can become fatal, if the end organs are severely damaged (25). The ability and speed to adapt to high-altitude hypoxia differ among people. Over the last century, a large body of clinical, genetic, and demographic evidence collected in humans colonized at multiple high-altitude locales, including the Tibetan Plateau, the Andean Altiplano, and the Semien Plateau of Ethiopia (32), have shed light on the chronic adaptation to high-altitude hypoxia. However, in recent years, new technologies represented by unbiased high-throughput metabolomics screening (14) have provided novel insights into this intriguing field by identifying key metabolic changes in erythrocytes on exposure to low-O2 conditions.
The erythrocyte is the most abundant cell in the human body and the only cell type that carries O2 from the lungs to peripheral tissues. Because of their role in O2 delivery, erythrocytes rapidly sense and respond to O2 insufficiency by increasing their capability of releasing O2. Erythrocyte O2 release is regulated by pH (the Bohr effect), temperature, and the erythrocyte-specific metabolite 2,3-bisphosphoglycerate (2,3-BPG). 2,3-BPG is an allosteric modulator that binds to hemoglobin and causes conformational changes that lead to O2 unloading. It is synthesized by the Rapoport-Luebering Shunt from 1,3-bisphosphoglycerate of the glycolytic pathway, the only pathway erythrocytes rely on for energy production. However, the generation of every 2,3-BPG molecule costs the erythrocyte the opportunity to generate one adenosine triphosphate (ATP), which makes 2,3-BPG production very “energy costing” to erythrocytes. Nonetheless, ~20~25% of glucose is used for 2,3-BPG production in erythrocytes, and that number goes even higher in response to hypoxia (28). Therefore, one of the biggest puzzles regarding erythrocyte metabolism is what factors dictate the programming of erythrocyte glucose metabolism and 2,3-BPG production.
Recently, through accurately measuring functional phenotypes that are the net result of genomic, transcriptomic, and proteomic changes, metabolomics profiling has become particularly useful to study mature erythrocytes. Metabolomic profiling bypasses genomic study limitations in erythrocytes due to their lack of a nucleus and de novo protein synthesis machinery. It has led to the discovery of substantial metabolic alterations in erythrocytes from sickle cell disease (SCD) patients (15, 29) and animal models (42). SCD is a hemolytic anemia with severe hypoxia (36). Of note, a group of metabolites that showed the most significant elevation in SCD screening results, including adenosine (24), 2,3-BPG (24, 38), and sphingosine 1-phosphate (S1P) (38), also increased significantly in the erythrocytes and plasma of 21 young and healthy low-land individuals exposed to high-altitude hypoxia for 16 days (14). Here, we review the role and mechanisms of these metabolites in the acute adaptation of humans to high-altitude hypoxia.
Adenosine Metabolism and Signaling
Adenosine metabolism.
As a critical precursor for the synthesis of ATP and nucleic acids, adenosine is produced and metabolized both in the cytosol in all cell types and in the extracellular space. Extracellularly, adenosine can be generated from ATP through the action of two enzymes, ecto-nucleoside triphosphate diphosphohydrolase (CD39), which converts ATP to ADP and AMP, and ecto-5′-nucleotidase (CD73), which hydrolyzes AMP to adenosine (13). Inside cells, adenosine is derived from S-adenosylhomocysteine, a by-product of the methionine cycle. Because adenosine is the ligand of four G protein-coupled receptors that regulate various important cellular functions, extracellular adenosine levels are finely regulated by the generating enzymes, degrading enzymes and transporters (20). Adenosine is deaminated to inosine by the critical enzyme adenosine deaminase (ADA), the genetic deficiency of which causes a lethal immunodeficiency (8). Extracellular adenosine can also be transported into the cells through a family of equilibrative nucleoside transporters (ENTs). Together, these molecules maintain a critical balance of adenosine metabolism for a variety of physiological and pathological functions.
Adenosine signaling.
Adenosine has been well characterized as a potent hypoxia indicator in tissue and cell damage. It regulates a myriad of cellular, physiological, and pathological activities, including apoptosis, vasodilation, cardiac rhythm, and inflammation, among many others (20, 22). Adenosine orchestrates the physiological response to hypoxia predominantly through the activation of its four G protein coupled receptors that are referred to as A1, A2A, A2B, and A3. These four receptors are coupled to two different G proteins and have distinctive expression and distribution profiles among different cells and tissues (11). A1 and A3 are coupled to Gi proteins, whereas A2A and A2B couple with Gs proteins. Moreover, the four receptors also vary in their affinity to adenosine. In particular, A2B has the lowest affinity and is usually activated in conditions in which adenosine levels are very high, such as inflammation and severe organ and tissue damage (18). Once activated by adenosine, the receptors can induce a broad spectrum of cellular signaling components, including some key kinases such as protein kinase A (PKA), protein kinase C, extracellular signal-regulated kinase (ERK), and mitogen-activated protein kinases (21, 23, 31). Many of these signaling molecules are involved in controlling the metabolism of cells.
Elevated Adenosine A2B-AMPK Signaling Contributes to Human Adaptation to High-Altitude Hypoxia
Elevated plasma adenosine is beneficial for hypoxia adaptation.
Realizing that current strategies to counteract hypoxia are limited by a lack of fundamental understanding of molecular mechanisms underlying adaptation to hypoxia, Liu et al. (24) recruited 21 young, healthy human volunteers and conducted high-altitude studies in which the volunteers stayed for 16 days at 5,260 m. Following 16 days at 5,260 m, the volunteers displayed 1) an increase in arterial oxygenation and hemoglobin; 2) no acute mountain sickness; 3) improved cognitive function; and 4) improved exercise performances (35). To identify metabolic alterations in erythrocytes in response to hypoxia that accompany acclimatization to high altitude, metabolomic profiling was conducted. The results showed that erythrocyte 2,3-BPG levels rapidly increased in the volunteers following ascent to high-altitude and continued to increase to day 16 (24). These data confirmed a study conducted ~50 yr ago in which a similar increase in 2,3-BPG was observed (17). Along with elevated 2,3-BPG, increased O2 release was also revealed by calculating P50, a conventional measure for hemoglobin O2 affinity where the O2 partial pressure is measured at 50% of hemoglobin saturation. Moreover, Liu et al. also found that plasma adenosine levels, as well as soluble CD73 activity, were also increased in the circulation of the volunteers in this high-altitude hypoxic environment. In the accompanying mouse studies, mice with genetic deficiency of CD73 were tested under hypoxic conditions (10% O2, similar to that at 5,260 m) intended to mimic the level of hypoxia associated with the human high-altitude study (24). The results indicate that elevated plasma CD73 activity and adenosine levels play protective roles in counteracting tissue hypoxia, inflammation, and lung injury in mice by increasing erythrocyte 2,3-BPG production and O2 release.
A2B underlies the protective role of adenosine for hypoxia adaptation.
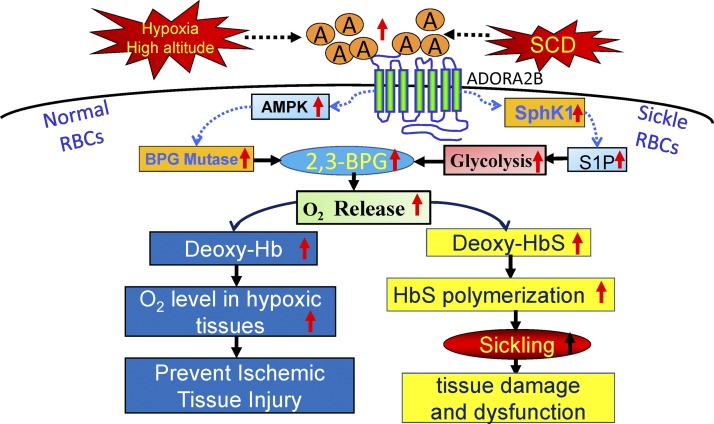
A previous study revealed a detrimental role of elevated adenosine in SCD through increased 2,3-BPG production that contributes to sickling in the unique disease setting (42). According to Liu et al. (24), the adenosine A2B signaling-induced 2,3-BPG production also occurs in normal mouse exposed to hypoxia. In both in vivo and in vitro studies, Liu et al. were able to observe the critical role of A2B signaling in mediating hypoxia-induced 2,3-BPG elevation. In particular, in mice specifically lacking the A2B receptor in erythrocytes, hypoxia caused more severe tissue damage due to the inability of erythrocytes to release more O2 (24). This piece of evidence is the first to show that erythrocyte adenosine A2B signaling plays an important part in counteracting hypoxia-induced tissue injury, including inflammation and lung damage.
AMPK-mediated 2,3-BPG mutase activation accounts for the protective role of adenosine A2B signaling.
Like adenosine, AMPK plays an essential role in multiple cellular functions, especially under conditions of energy depletion or limited O2 availability (19). Noticing that adenosine A2B signaling induces 2,3-BPG production and that 2,3-BPG mutase, the 2,3-BPG generating enzyme, is a substrate of AMPK (39), Liu et al. (24) exploited various pharmacological tools and showed that AMPK functions downstream of erythrocyte A2B and underlies hypoxia-induced 2,3-BPG production by phosphorylation and activation of 2,3-BPG mutase. Moreover, they also revealed that erythrocyte p-AMPK and 2,3-BPG mutase activity are induced in humans at high altitude, and that AMPK activation induces erythrocyte 2,3-BPG mutase activity, 2,3-BPG production, and O2 release in cultured human erythrocytes. This work is the first to connect adenosine signaling with AMPK activation in regulating erythrocyte metabolism and shed light on potentially novel therapeutic possibilities, since both are easy targets for drug development.
S1P Regulates Hypoxia-Induced Glycolysis Enhancement in Erythrocytes
Erythrocyte S1P levels and Sphk1 activity increase in humans at high altitude.
S1P is a versatile signaling lipid (34) with high abundance in erythrocytes. It regulates a wide spectrum of physiological processes, including immune response (12), bone marrow cells trafficking (6), vascular integrity (9), and cell survival and proliferation (2). However, its function in erythrocytes is largely unknown. Interestingly, in the same high-altitude human studies discussed above, erythrocyte S1P levels increased rapidly within 12 h at high altitude and increased further as the volunteers remained at the high altitude. Similarly, the enzyme that generates S1P in mature erythrocytes, Sphk1, was also activated by hypoxia (38), indicating that S1P may play a role in the hypoxic response of erythrocytes.
Sphk1 increases O2 release from mouse erythrocytes to counteract hypoxia.
Hypothesizing that S1P may play a role in regulating hypoxia adaptation by increasing erythrocyte 2,3-BPG production and O2 release, Sun et al. (38) exposed wild-type and Sphk1-deficient (Sphk1−/−) (1) mice to hypoxia (10% O2, similar to that encountered by the human volunteers at 5,260 m) and found a similar increase in erythrocyte S1P levels and Sphk1 activity in wild-type but not Sphk1−/− mice. Sphk1−/− mice also showed lower 2,3-BPG levels and O2 release ability and had more severe tissue hypoxia in heart and kidney. These results were validated in a set of reciprocal bone marrow transplanted mice with specific deficiency of Sphk1 in erythrocytes and other cells coming from the bone marrow (38), suggesting that erythrocyte Sphk1 is the major player.
Erythrocyte Sphk1 directs glucose flux to glycolysis in hypoxia.
Since 2,3-BPG is generated through the glycolytic arm of glucose metabolism in erythrocytes (28) and glycolysis was enhanced in erythrocytes of humans in high-altitude hypoxia, Sun et al. (38) tested if erythrocyte Sphk1 promotes glycolysis and thereby boosts 2,3-BPG production. In vivo genetic and in vitro chemical tracing experiments collectively demonstrated that activation of Sphk1 and increased S1P production direct erythrocyte glucose metabolism to glycolysis rather than the pentose phosphate pathway; another arm of glucose metabolism in erythrocytes. Evidence was also provided that indicates the role of Sphk1 in regulating erythrocyte glucose metabolism is dependent on intracellular S1P rather than extracellular S1P signaling (38).
S1P regulates the subcellular translocation of GAPDH.
It has been well documented that glycolytic enzymes, including the rate-limiting GAPDH, translocate from membrane to cytosol in erythrocytes on exposure to hypoxia (10, 30). However, factors other than O2 availability that can regulate such a process remain unknown. Since S1P is a well-known signaling lipid that can be found both in the aqueous cytosol and lipid bilayers, Sun et al. tested if S1P is such a factor. Mouse genetic studies coupled with biochemical experiments revealed that S1P can directly bind to deoxygenated Hb and facilitate the replacement of glycolytic enzymes on the membrane (38). In this way, the authors have shown for the first time that the erythrocyte enriched lipid molecule S1P plays a role in erythrocyte function by regulating glucose metabolism for hypoxia adaptation.
Adenosine A2B Signaling Activates Erythrocyte Sphk1
Of note, the regulation of erythrocyte 2,3-BPG production by both S1P and adenosine A2B signaling is not merely a coincidence. In fact, a study published in 2015 by Sun et al. has revealed that, in both normal and SCD erythrocytes, adenosine A2B signaling activates Sphk1 through the PKA-ERK1/2 signaling cascade (37). In this study, multiple genetic models were used, including a mouse strain that lacks both ADA and A2B (43), which allows accumulation of high levels of plasma adenosine, while missing the A2B signaling cascade. Erythrocytes from these mice lack activated Sphk1, despite the high plasma adenosine levels. Therefore, the adenosine A2B signaling regulates erythrocyte O2 delivery ability in multiple ways.
ENT1-Mediated Hypoxic Memory in Erythrocytes
Since the erythrocyte adenosine signaling pathway plays such an important role in hypoxia adaptation, one might wonder if we can find a fast way to increase plasma adenosine levels for faster acclimatization to hypoxia. In fact, a most recent study (33) has provided a very promising answer to that question by proposing the novel concept of “erythrocyte hypoxic memory” endowed by the erythrocyte equilibrative nucleoside transporter 1 (eENT1).
Plasma adenosine associates with altitude acclimatization.
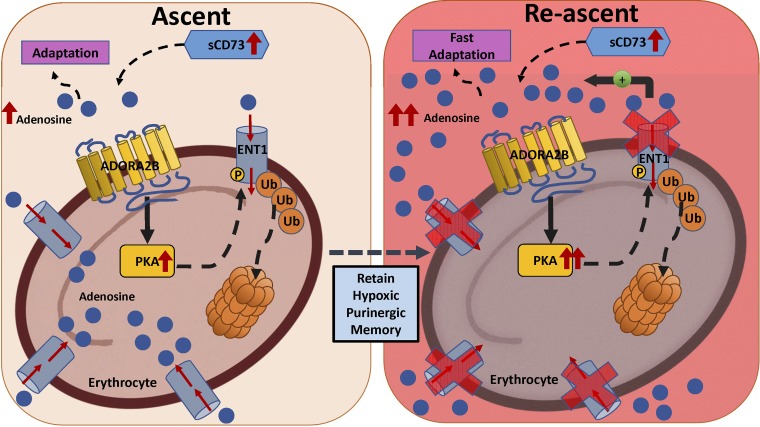
In this study, the same aforementioned volunteers from the AltitudeOmics study spent 7 or 21 days at 1,525 m before re-ascent to 5,260 m (14). Interestingly, their plasma adenosine levels were much higher upon re-ascent than on day 1 of the first ascent. Thus Song and colleagues (14) showed that increased plasma adenosine levels accompanied the initial acclimatization to high altitude and that even higher levels of plasma adenosine were associated with the rapid acclimatization on re-ascent.
eENT1 regulates extracellular adenosine under acute hypoxia.
After ruling out the possibility of ADA and CD73 in regulating the rapid accumulation of plasma adenosine levels upon re-ascent, Song et al. (33) focused on the adenosine transporters and demonstrated that eENT1 accounts for 90% of adenosine uptake in circulation. Using genetically modified mice with erythrocyte-specific knockout of ENT1, the authors showed that that the loss of eENT1 leads to quicker and higher accumulation of extracellular adenosine under acute hypoxia, resulting in reduced hypoxia-induced tissue inflammation and damage. Intriguingly, hypoxia-mediated eENT1 ubiquitination and degradation were observed in mouse hypoxia experiments mimicking the human high-altitude study. These results explain to a large extent the fast increase in plasma adenosine levels on re-exposure to hypoxia (33).
Adenosine A2B-PKA signaling pathway mediates eENT1 degradation in hypoxia.
In an effort to explain the eENT1 degradation in hypoxia, Song et al. (33) tested the possibility of a feed-forward loop that could be accountable for the fast increase of plasma adenosine levels. First, they found genetic evidence that sCD73-dependent elevation of plasma adenosine underlies hypoxia-mediated downregulation of eENT1 levels, thus reducing eENT1 activity. Second, using erythrocytes from four adenosine receptor-deficient mice, Song et al. revealed that A2B underlies hypoxia-mediated reduction of ENT activity resulting from decreased ENT1 levels in erythrocytes. Moreover, they were able to show pharmacologically that PKA signaling accounted for such effects. Finally, the results from the mouse study were also seen in erythrocytes from the human high-altitude volunteers (33). Altogether, Song et al. defined that adenosine A2B-PKA-induced proteasome-mediated degradation of ENT1 on erythrocytes, the most abundant circulating cell, is a major cellular purinergic signaling regulatory component underlying initial hypoxia-induced adenosine response, and that erythrocytes with reduced eENT1 retain a “hypoxic puringeric memory” for quicker adaptation to subsequent hypoxia.
Conclusion
There are ~25 trillion erythrocytes circulating in our body, with over 2 million new erythrocytes produced every second (7). Indeed, as the most abundant cell in our body and the cell capable of delivering O2 to tissues, understanding mechanisms regulating O2 delivery by erythrocytes is pivotal to understanding human physiology and pathology, especially pertaining to hypoxia. In this review, we have summarized recent important findings based on human high-altitude studies coupled with cutting-edge, high-throughput, nonbiased metabolomics screening, which has provided tremendous new insights into how our body adapts to hypoxia via regulating erythrocyte metabolism and O2 release (Fig. 1). The adenosine-activated signaling pathways in erythrocytes regulate hemoglobin O2 affinity and thereby affect O2 delivery to every organ, tissue, and cell in our body (22). The studies summarized above were the first to focus on the cellular metabolism of erythrocytes and show that the activation of these pathways in mice protects against tissue hypoxia in lungs, kidneys, and hearts and reduces inflammation in response to hypoxia challenge. Of note, the novel concept of “erythrocyte hypoxic memory” provides a completely new angle to understand the response of erythrocytes to hypoxia. Because erythrocytes are cells without a nucleus or translational machinery, they were long considered to be a bag of hemoglobin molecules. Recent studies reviewed above revealed that erythrocytes have sophisticated signaling and metabolism regulatory mechanisms that are critical to the erythrocyte response to hypoxia adaptation (Fig. 2).
Fig. 1.
Beneficial and detrimental role of adenosine A2B signaling in normal individuals and SCD patients. Hypoxia-induced adenosine A2B signaling activates AMPK and Sphk1. The former activates 2,3-BPG, generating enzyme BPG mutase, whereas the latter promotes glycolysis. Together, both in high-altitude and in SCD, adenosine A2B activation increases erythrocyte 2,3-BPG levels and in turn increases O2 release. In normal erythrocytes, this pathway helps release more O2 to counteract tissue hypoxia and injury, whereas, in SCD erythrocytes, this pathway leads to more sickling and disease progression. RBCs, red blood cells.
Fig. 2.
Erythrocyte ENT1 mediated rapid adenosine accumulation on re-ascent. eENT1 is degraded in erythrocytes previously exposed to high altitude (ascent) or hypoxia. On re-ascent or second hypoxic exposure, the absence of eENT1 allows for fast adenosine accumulation, which increases 2,3-BPG levels and O2 release to facilitate fast hypoxia adaptation. [Modified from Song et al. (33).]
Interestingly, the same adenosine-activated erythrocyte signaling pathways resulting in increased 2,3-BPG that are beneficial for adaptation of normal humans to high-altitude hypoxia (Fig. 1) are detrimental for individuals with SCD (42). The detrimental effect of increased 2,3-BPG in SCD is due to the underlying mutation in SCD that results in the substitution of valine for glutamic acid at the sixth position of β-hemoglobin. Because of this mutational alteration, sickle hemoglobin (HbS) forms insoluble polymers when it releases O2 and becomes deoxy-HbS. The deoxy-HbS polymers grow in length, resulting in the deformation of erythrocytes to form the characteristic sickle shape. Normal human hemoglobin tetramers, HbA, do not form insoluble polymers following release of O2. Thus the same adenosine-activated signaling pathways promote O2 release from hemoglobin in normal individuals and those with SCD. However, it is the physical properties of deoxy-HbS that account for the detrimental outcome in the case of SCD.
Moreover, the physiological importance of these novel findings regarding adenosine-activated signaling pathways in erythrocytes goes beyond normal human adaptation to high-altitude hypoxia. The newly identified adenosine A2B-AMPK/Sphk1/ENT1 signaling pathways also offer tremendous therapeutic possibilities. Therapeutic enhancement of this pathway will lead to new and exciting possibilities in ameliorating hypoxia-related diseases in various organs and tissues, such as myocardial (27) and cerebral ischemia (40) and tumorigenesis (16). These new findings also present possibilities for safer and more efficient ways to store and distribute blood for trauma patients requiring large blood transfusions (41). Finally, the discovery of signaling pathways that regulate O2 release from erythrocytes may provide strategies for enhancement of athletic performance (26). Possible therapeutic approaches to enhance 2,3-BPG production and O2 release include the use of selective A2B receptor agonists and AMPK-activating drugs, such as metformin. Some of these drugs have already received Food and Drug Administration approval for use in other medical conditions. In conclusion, investigation of erythrocyte metabolism and function can provide novel insights into our understanding of human physiological and pathological response to hypoxia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-114457 (project 3 to Y. Xia), HL-136969 (to Y. Xia), and HL-113574 (to Y. Xia). Funding for the overall AltitudeOmics study was provided, in part, by grants from the U.S. Department of Defense [W81XWH-11-2-0040 Telemedicine & Advanced Technology Research Center (TATRC)] to RCR, the Cardiopulmonary & Respiratory Physiology Laboratory, University of Oregon; and the Charles S. Houston Endowed Professorship at the Altitude Research Center, School of Medicine, University of Colorado Anschutz Medical Campus.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S. and J.M.M. prepared figures; K.S. drafted manuscript; K.S., H.L., A.S., J.M.M., A.D., K.C.H., R.E.K., H.K.E., M.R.B., R.C.R., and Y.X. edited and revised manuscript; Y.X. approved final version of manuscript.
REFERENCES
- 1.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 2.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem 275: 288–296, 2000. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JD, Honigman B. The effect of altitude-induced hypoxia on heart disease: do acute, intermittent, and chronic exposures provide cardioprotection? High Alt Med Biol 12: 45–55, 2011. doi: 10.1089/ham.2010.1021. [DOI] [PubMed] [Google Scholar]
- 4.Bärtsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation 116: 2191–2202, 2007. doi: 10.1161/CIRCULATIONAHA.106.650796. [DOI] [PubMed] [Google Scholar]
- 5.Bärtsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med 369: 1666–1667, 2013. doi: 10.1056/NEJMc1309747. [DOI] [PubMed] [Google Scholar]
- 6.Bendall LJ, Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr Opin Hematol 20: 281–288, 2013. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- 7.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S. An estimation of the number of cells in the human body. Ann Hum Biol 40: 463–471, 2013. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn MR, Datta SK, Kellems RE. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem 273: 5093–5100, 1998. doi: 10.1074/jbc.273.9.5093. [DOI] [PubMed] [Google Scholar]
- 9.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119: 1871–1879, 2009. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus 8, Suppl 3: s53–s58, 2010. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov 12: 265–286, 2013. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci 32: 16–24, 2011. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Nemkov T, Sun K, Liu H, Song A, Monte AA, Subudhi AW, Lovering AT, Dvorkin D, Julian CG, Kevil CG, Kolluru GK, Shiva S, Gladwin MT, Xia Y, Hansen KC, Roach RC. AltitudeOmics: red blood cell metabolic adaptation to high altitude hypoxia. J Proteome Res 15: 3883–3895, 2016. doi: 10.1021/acs.jproteome.6b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darghouth D, Koehl B, Madalinski G, Heilier JF, Bovee P, Xu Y, Olivier MF, Bartolucci P, Benkerrou M, Pissard S, Colin Y, Galacteros F, Bosman G, Junot C, Roméo PH. Pathophysiology of sickle cell disease is mirrored by the red blood cell metabolome. Blood 117: e57–e66, 2011. doi: 10.1182/blood-2010-07-299636. [DOI] [PubMed] [Google Scholar]
- 16.Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5: e190, 2016. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton JW, Brewer GJ. The relationship between red cell 2,3-diphosphoglycerate and levels of hemoglobin in the human. Proc Natl Acad Sci USA 61: 756–760, 1968. doi: 10.1073/pnas.61.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61: 443–448, 2001. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509: 310–317, 2014. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal 8: 419–436, 2012. doi: 10.1007/s11302-012-9294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) 91: 173–181, 2013. doi: 10.1007/s00109-013-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Xia Y. Beneficial and detrimental role of adenosine signaling in diseases and therapy. J Appl Physiol (1985) 119: 1173–1182, 2015. doi: 10.1152/japplphysiol.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, Sun K, Li J, Cheng NY, Huang A, Edward Wen Y, Weng TT, Luo F, Nemkov T, Sun H, Kellems RE, Karmouty-Quintana H, Hansen KC, Zhao B, Subudhi AW, Jameson-Van Houten S, Julian CG, Lovering AT, Eltzschig HK, Blackburn MR, Roach RC, Xia Y. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 134: 405–421, 2016. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 26: 160096, 2017. doi: 10.1183/16000617.0096-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol 4: 332, 2013. doi: 10.3389/fphys.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirtschink P, Krek W. Hypoxia-driven glycolytic and fructolytic metabolic programs: Pivotal to hypertrophic heart disease. Biochim Biophys Acta 1863: 1822–1828, 2016. doi: 10.1016/j.bbamcr.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Mulquiney PJ, Bubb WA, Kuchel PW. Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: in vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13C and 31P NMR. Biochem J 342: 567–580, 1999. doi: 10.1042/bj3420567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers SC, Ross JG, d’Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood 121: 1651–1662, 2013. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers SC, Said A, Corcuera D, McLaughlin D, Kell P, Doctor A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J 23: 3159–3170, 2009. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachdeva S, Gupta M. Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J 21: 245–253, 2013. doi: 10.1016/j.jsps.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonson TS. Altitude adaptation: a glimpse through various lenses. High Alt Med Biol 16: 125–137, 2015. doi: 10.1089/ham.2015.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song A, Zhang Y, Han L, Yegutkin GG, Liu H, Sun K, D’Alessandro A, Li J, Karmouty-Quintana H, Iriyama T, Weng T, Zhao S, Wang W, Wu H, Nemkov T, Subudhi AW, Jameson-Van Houten S, Julian CG, Lovering AT, Hansen KC, Zhang H, Bogdanov M, Dowhan W, Jin J, Kellems RE, Eltzschig HK, Blackburn M, Roach RC, Xia Y. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat Commun 8: 14108, 2017. doi: 10.1038/ncomms14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 35.Subudhi AW, Bourdillon N, Bucher J, Davis C, Elliott JE, Eutermoster M, Evero O, Fan JL, Jameson-Van Houten S, Julian CG, Kark J, Kark S, Kayser B, Kern JP, Kim SE, Lathan C, Laurie SS, Lovering AT, Paterson R, Polaner DM, Ryan BJ, Spira JL, Tsao JW, Wachsmuth NB, Roach RC. AltitudeOmics: the integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS One 9: e92191, 2014. doi: 10.1371/journal.pone.0092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Curr Opin Hematol 20: 215–221, 2013. doi: 10.1097/MOH.0b013e32835f55f9. [DOI] [PubMed] [Google Scholar]
- 37.Sun K, Zhang Y, Bogdanov MV, Wu H, Song A, Li J, Dowhan W, Idowu M, Juneja HS, Molina JG, Blackburn MR, Kellems RE, Xia Y. Elevated adenosine signaling via adenosine A2B receptor induces normal and sickle erythrocyte sphingosine kinase 1 activity. Blood 125: 1643–1652, 2015. doi: 10.1182/blood-2014-08-595751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun K, Zhang Y, D’Alessandro A, Nemkov T, Song A, Wu H, Liu H, Adebiyi M, Huang A, Wen YE, Bogdanov MV, Vila A, O’Brien J, Kellems RE, Dowhan W, Subudhi AW, Jameson-Van Houten S, Julian CG, Lovering AT, Safo M, Hansen KC, Roach RC, Xia Y. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun 7: 12086, 2016. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thali RF, Tuerk RD, Scholz R, Yoho-Auchli Y, Brunisholz RA, Neumann D. Novel candidate substrates of AMP-activated protein kinase identified in red blood cell lysates. Biochem Biophys Res Commun 398: 296–301, 2010. doi: 10.1016/j.bbrc.2010.06.084. [DOI] [PubMed] [Google Scholar]
- 40.Vespa PM. Brain hypoxia and ischemia after traumatic brain injury: is oxygen the right metabolic target? JAMA Neurol 73: 504–505, 2016. doi: 10.1001/jamaneurol.2016.0251. [DOI] [PubMed] [Google Scholar]
- 41.Yan EB, Hellewell SC, Bellander BM, Agyapomaa DA, Morganti-Kossmann MC. Post-traumatic hypoxia exacerbates neurological deficit, neuroinflammation and cerebral metabolism in rats with diffuse traumatic brain injury. J Neuroinflammation 8: 147, 2011. doi: 10.1186/1742-2094-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Zhang W, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 17: 79–86, 2011. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Mohsenin A, Morschl E, Young HW, Molina JG, Ma W, Sun CX, Martinez-Valdez H, Blackburn MR. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J Immunol 182: 8037–8046, 2009. doi: 10.4049/jimmunol.0900515. [DOI] [PMC free article] [PubMed] [Google Scholar]