There is a surprising lack of studies examining the reproducibility of high-resolution CT in asthma. The current study examined reproducibility of airway measurements. In stable well-controlled asthmatic subjects, it is possible to reproducibly image airway luminal areas over time, by region, and by size at total lung capacity throughout the lungs. Therefore, any changes in luminal size on repeat CT imaging are more likely due to changes in disease state and less likely due to normal variability.
Keywords: asthma, computer tomography, precision
Abstract
Brown RH, Henderson RJ, Sugar EA, Holbrook JT, Wise RA, on behalf of the American Lung Association Airways Clinical Research Centers. Reproducibility of airway luminal size in asthma measured by HRCT. J Appl Physiol 123: 876–883, 2017. First published July 13, 2017; doi:10.1152/japplphysiol.00307.2017.—High-resolution CT (HRCT) is a well-established imaging technology used to measure lung and airway morphology in vivo. However, there is a surprising lack of studies examining HRCT reproducibility. The CPAP Trial was a multicenter, randomized, three-parallel-arm, sham-controlled 12-wk clinical trial to assess the use of a nocturnal continuous positive airway pressure (CPAP) device on airway reactivity to methacholine. The lack of a treatment effect of CPAP on clinical or HRCT measures provided an opportunity for the current analysis. We assessed the reproducibility of HRCT imaging over 12 wk. Intraclass correlation coefficients (ICCs) were calculated for individual airway segments, individual lung lobes, both lungs, and air trapping. The ICC [95% confidence interval (CI)] for airway luminal size at total lung capacity ranged from 0.95 (0.91, 0.97) to 0.47 (0.27, 0.69). The ICC (95% CI) for airway luminal size at functional residual capacity ranged from 0.91 (0.85, 0.95) to 0.32 (0.11, 0.65). The ICC measurements for airway distensibility index and wall thickness were lower, ranging from poor (0.08) to moderate (0.63) agreement. The ICC for air trapping at functional residual capacity was 0.89 (0.81, 0.94) and varied only modestly by lobe from 0.76 (0.61, 0.87) to 0.95 (0.92, 0.97). In stable well-controlled asthmatic subjects, it is possible to reproducibly image unstimulated airway luminal areas over time, by region, and by size at total lung capacity throughout the lungs. Therefore, any changes in luminal size on repeat CT imaging are more likely due to changes in disease state and less likely due to normal variability.
NEW & NOTEWORTHY There is a surprising lack of studies examining the reproducibility of high-resolution CT in asthma. The current study examined reproducibility of airway measurements. In stable well-controlled asthmatic subjects, it is possible to reproducibly image airway luminal areas over time, by region, and by size at total lung capacity throughout the lungs. Therefore, any changes in luminal size on repeat CT imaging are more likely due to changes in disease state and less likely due to normal variability.
high-resolution CT (HRCT) is a well-established imaging technology that can noninvasively measure parenchymal and airway morphology in vivo. HRCT imaging can be used to predict clinical outcomes (18, 22), response to treatment (27), and phenotyping (13) in lung disease. With the prominent use of HRCT in obstructive lung disease, there is a surprising lack of studies examining the reproducibility of this imaging method. We are aware of only one study of HRCT repeatability of airway dimensions in humans (17). Determination of the reproducibility of HRCT imaging is essential in the planning and analysis of longitudinal studies and clinical trials using this technology. Therefore, we undertook the current study to determine the precision of repeated HRCT lung and airway measurements.
The CPAP Trial (15) offered an ideal data set to determine HRCT reproducibility. This randomized, double-blind, sham-controlled clinical trial in individuals with asthma examined the effects of nocturnal continuous positive airway pressure (CPAP) or sham treatment on airway reactivity (primary outcome), lung function, and patient reported outcome measures. A subset of participants was enrolled in an ancillary study of HRCT measurements. The lack of a treatment effect of CPAP on clinical or HRCT measures provided an opportunity for the current analysis (15). Specifically, we wanted to assess the reproducibility of the following imaging measurements: lung volumes, air trapping, airway luminal areas, airway wall thicknesses, and airway distensibility, defined as the change in airway luminal size from functional residual capacity (FRC) to total lung capacity (TLC). Because measurements of airway dimensions are an emerging important measure of asthma pathology and little information about these measurements is available, our analysis will help guide future research studies of airway morphometry in asthma.
METHODS
Study design.
The complete study design is described elsewhere (15). Briefly, this was a multicenter, randomized, three-parallel-arm, sham-controlled clinical trial in which asthmatic subjects were instructed to use a CPAP device for 12 consecutive wk to determine the effect of nocturnal CPAP use on airway reactivity to methacholine. The study was conducted at 18 centers of the American Lung Association-Airways Clinical Research Centers Network. All study centers received approval from their respective institutional review boards. The clinical trial was registered at www.clinicaltrials.gov (NCT01629823).
Eligibility.
Major eligibility criteria were as follows: a physician diagnosis of asthma, treatment for asthma for the past 12 mo, stable asthma treatment for ≥8 wk, ≥75% predicted forced expired volume in 1 s (FEV1), methacholine bronchial challenge with provocative concentration that causes 20% fall in FEV1 (PC20) of ≤8 mg/ml, body mass index ≤35, no history of sleep apnea by self-report, absence or low risk of sleep apnea as assessed by multivariable apnea prediction (MAP) index (19), no other known sleep disorders that were under treatment by a sleep specialist, no current pregnancy, and no prior use of CPAP. All participants provided signed consent statements approved by clinical center institutional review boards.
Study treatment.
All participants were randomly allocated to receive a CPAP device (model S9 with heated humidifier, ResMed, Bella Vista, NSW, Australia) that delivered <1 cmH2O (sham), 5 cmH2O, or 10 cmH2O via a nasal mask that was fitted by a trained research coordinator. Patients were instructed to use the device nightly and continue their routine asthma care, including drug therapy and environmental interventions.
Imaging.
Participants in the ancillary study underwent two CT scans at each visit at two lung volumes: TLC and then FRC. Seven of the 18 centers participated in the ancillary imaging study. Prior to subject enrollment, CT scanners at each site were calibrated using a lung phantom (model no. CPT674, The Phantom Laboratory, Salem, NY). While there are some variations between CT scanners, a standardization of the parameters has been developed to allow multiple scanners to output reliable quantitative measures across a given patient population (24, 25) (Table 1). Albuterol was held for ≥6 h before scanning.
Table 1.
Standardized CT scanner parameters
| Scanner Make |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Siemens | Siemens | Siemens | GE | GE | Philips | Philips | Toshiba | ||
| Scanner model | Definition (AS Plus) 128 slice | Definition (DS) 64 slice | Sensation 64 slice | VCT 64 slice/Discovery STE | Discovery CT 750HD 64 slice | Brilliance 64 slice | iCT-256 | Aquilion 64 | |
| Scan type | Spiral | Spiral single source | Spiral | Helical | Helical-standard | Spiral helix | Spiral helix | Helical | |
| Scan FOV | No selection | No selection | No selection | Large | Large | No selection | No selection | Small/medium/large | |
| Rotation time, s | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Detector configuration | (128) 64 × 0.6 | (64) 32 × 0.6 | (64) 32 × 0.6 | 64 × 0.625 | 64 × 0.625 | 64 × 0.625 | 128 × 0.625 | 64 × 0.5 | |
| Pitch | 1.0 | 1.0 | 1.0 | 0.984 | 0.984 | 0.923 | 0.993 | 0.828 | |
| kVp | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | |
| Dose modulation | Care dose Off | Care dose Off | Care dose Off | Auto mA Off | Auto mA Off | Dose right (ACS) Off | Dose right (ACS) Off | SURE Exp (Real EC) Off | |
| Recon 1 algorithm | B35 | B35 | B35 | Standard | Standard | B | B | Standard | |
| Recon 2 algorithm | B30 | B31 | None | Detail | Detail | YB | YB | Detail | |
| Additional image filters | No selection | No selection | No selection | No selection | IQ enhance off | Adaptive filtering off | Adaptive filtering off | No selection | |
| Thickness, mm | 0.75 | 0.75 | 0.75 | 0.625 | 0.625 | 0.67 | 0.67 | 0.5 | |
| Interval, mm | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Iterative recon (noise reduction algorithm) | Do not use IRIS | Do not use IRIS | No selection | Do not use ASIR | Do not use ASIR | Do not use iDOSE | Do not use iDOSE | No selection | |
| Scan time for 30-cm length, s | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | |
| Recon mode | N/A | N/A | N/A | N/A | Full | N/A | N/A | N/A | |
| Smart mA | N/A | N/A | N/A | N/A | Off | N/A | N/A | Off | |
| IQ enhance | N/A | N/A | N/A | N/A | Off | N/A | N/A | N/A | |
| Inspiratory (TLC) | Effective mAs: | Effective mAs: | Effective mAs: | mA: | mA: | mAs: | mAs: | mA: | Effective mAs: |
| Siemens = effective mAs | S: 90 | S: 85 | S: 80 | S: 145 | S: 145 | S: 105 | S: 105 | S: 145 | |
| GE = mA setting | M: 110 | M: 105 | M: 100 | M: 180 | M: 180 | M: 130 | M: 130 | M: 180 | S: 91 |
| Philips = mAs | L: 165 | L: 150 | L: 145 | L: 270 | L: 270 | L: 190 | L: 190 |
L: 270 |
M: 109 L: 164 |
| Expiratory (FRC) | Effective mAs: | Effective mAs: | Effective mAs: | mA: | mA: | mAs: | mAs: | mA: | Effective mAs: |
| Siemens = effective mAs | S: 65 | S: 60 | S: 55 | S: 100 | S: 100 | S: 70 | S: 70 | ||
| GE = mA setting | M: 65 | M: 60 | M: 55 | M: 100 | M: 100 | M: 70 | M: 70 | S: 100 | S: 61 |
| Philips = mAs | L: 90 | L: 85 | L: 80 | L: 145 | L: 145 | L: 105 | L: 105 | M: 100 L: 145 |
M: 61 L: 91 |
FOV, field of view; TLC, total lung capacity; FRC, functional residual capacity.
CT data acquisition.
Scans for each subject at each study site were performed on a single CT machine at baseline and after 12 wk. Settings were based on those previously used in other multicenter studies (9, 12, 14, 24, 26, 28) (Table 1). Scanning started below the lung bases and continued above the apexes of the lungs.
CT analysis.
All images were deidentified, burned onto a DVD disk, and sent to the Imaging Center for analysis. One person, blinded to the treatment arms, performed all image analyses.
Lung volumes.
To ensure consistent lung volume history, all subjects were coached using a set of written instructions for taking a deep breath or passive exhalation; the instructions were read three times (first for practice, then for the CT topogram, and finally for the CT imaging series) by the clinical coordinator at each site. One set of instructions was read for the TLC scans and another set was read for the FRC scans (Table 2).
Table 2.
Breathing instruction script read to all volunteers during CT scan acquisition
| Practice Breathing (TLC) |
| • For the first part of this scan, I am going to ask you to take a couple of deep breaths in and out before we have you hold your breath all the way in |
| • First let’s practice |
| • Take a deep breath in (watch chest to ensure a deep breath as far in as possible) |
| • Let it out (watch chest to ensure air is out) |
| • Take a deep breath in (watch chest to ensure a deep breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Now breathe all the way IN…IN…IN… and hold it (watch chest to ensure a deep breath in as far as possible) |
| • Keep holding your breath—DO NOT BREATHE! (watch chest to ensure spine remains on the table, subject is not shaking—watch for these throughout the study!) |
| • Breathe and relax |
| Scout Views: AP/PA (CT topogram at TLC) |
| • OK let’s get started |
| • Take a deep breath in (watch chest to ensure a deep breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take a deep breath in (watch chest to ensure a deep breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Now breathe all the way IN... IN... IN... and hold it (watch chest to ensure a deep breath as far in as possible) |
| • Keep holding your breath—DO NOT BREATHE! (watch chest to ensure the spine remains on the table, subject is not shaking and then start the scout scan) |
| • Perform scout |
| • At the end of the scout: Breathe and relax |
| Inspiratory CT (TLC) |
| • Now we’re ready again so please |
| • Take a deep breath in (watch chest to ensure a deep breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take a deep breath in (watch chest to ensure a deep breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Now breathe all the way IN...IN...IN as far as possible and hold it in (watch chest to ensure a deep breath in as far as possible) |
| • Keep holding your breath—DO NOT BREATHE! |
| • Perform TLC scan |
| • As the scan ends: Hold your breath for 3…2….1 |
| • At the end of the scan: Breathe and relax |
| Practice Breathing (FRC) |
| • For the second part of this scan, I am going to ask you to breathe normally in and out before we have you hold your breath at the end of breathing out. |
| • First let’s practice |
| • Take a breath in (watch chest to ensure a breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take a breath in (watch chest to ensure a breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take another breath in (watch chest to ensure a breath in) |
| • Let it out, hold your breath |
| • Keep holding your breath—DO NOT BREATHE! (watch chest to ensure spine remains on the table, subject is not shaking—watch for these throughout the study!) |
| • Breathe and relax |
| Expiratory CT (FRC) |
| • Now we’re ready again so please |
| • Take a breath in (watch chest to ensure a breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take a breath in (watch chest to ensure a breath in) |
| • Let it out (watch chest to ensure air is out) |
| • Take another breath in (watch chest to ensure a breath in) |
| • Let it out, hold your breath |
| • Keep holding your breath–DO NOT BREATHE! |
| • Perform FRC scan |
| • As the scan ends: Hold your breath for 3…2….1 |
| • At the end of the scan—Breathe and relax |
Instructions for individual administering the test are shown in italic type. TLC, total lung capacity; FRC, functional residual capacity. AP, anterior-posterior; PA, posterior-anterior.
Airway measurements.
Airway luminal area, airway wall thickness, and airway wall area fraction (wall area as a fraction of the total airway area) at TLC and FRC were calculated using PW software (VIDA Diagnostics, Coralville, IA) based on the lung CT scans. The luminal area was calculated as the average of the measurements along the middle third of the segment. At every centerline voxel position, the wall thickness was measured along rays cast from the center of the airway lumen. Rays were cast every half-degree, for a total of 720 measurements. These 720 wall thickness measurements were averaged into one mean wall thickness. The wall area fraction was calculated as [total airway area (area circumscribed by the outer airway wall) − airway luminal area] ÷ total airway area along the middle third of the airway segment.
Lung volume measurements.
Lung volumes were calculated using PW software (VIDA Diagnostics) based on the lung CT scans. The PW software calculates the total lung volume, as well as the lung air volume and the lung tissue volume. The lung voxels were segmented and smoothed near the mediastinum. The vessels were segmented from the lungs. The lungs were divided into lobes. Air and blood measurements were taken, and parenchymal density was analyzed.
Physiological measurements.
As a measure of parenchymal-airway interdependence, we examined the change in airway luminal size from the expiration (FRC) scan (second) and the inspiration (TLC) scan (first), which we call the distensibility index. Volume history always consisted of the three practice maneuvers (Table 2) before the TLC scan. The distensibility index was defined as the ratio of the airway luminal area at TLC to the airway luminal area at FRC (4–6, 21, 23).
Air trapping measurement.
Air trapping was calculated using PW software (VIDA Diagnostics) based on the lung CT scans. Air trapping was defined as the percentage of voxels with intensity less than −856 HU on the FRC scans (7). Although we refer to the percentage of voxels less than −856 HU as “air-trapping,” there is no clear evidence that this measure represents regions of the lung with small airway closure.
Measures.
The outcome measures were the size of the airway lumen, airway wall thickness, airway wall fraction (wall area as a fraction of the total airway area), distensibility index (defined as the ratio of luminal airway area at inspiration to the luminal airway area at expiration), lung volume at TLC, lung volume at FRC, and percentage of air trapping, from baseline to 12 wk on the FRC and TLC scans.
Data analysis.
The primary statistical analysis was carried out to quantify the reproducibility of airway measurements over time. Intraclass correlation coefficients (ICCs) were calculated for luminal and wall measures of individual airway segments, individual lung lobes, both lungs, and air trapping. To visualize these relationships, Bland-Altman plots and plots of the repeated measurements were created. The overall amount of air trapping in both lungs at baseline and after 12 wk was analyzed using a paired t-test.
RESULTS
Of the 209 participants, 51 enrolled in the imaging substudy at 7 centers and 43 completed the baseline and 12-wk scans (Table 3). Five subjects were lost to follow-up, one dropped out, one declined to participate in the follow-up scan, and one became pregnant during the study. Characteristics of the imaging study participants were similar to those of the parent study participants.
Table 3.
Volunteer demographics
| Characteristic | n (%) |
|---|---|
| Male, n (%) | 15 (35%) |
| Age (yr) at enrollment, median (IQR) | 36 (22, 47) |
| Race or ethnic group, n (%) | |
| White | 26 (60%) |
| Black | 10 (23%) |
| Hispanic | 4 (9%) |
| Other | 3 (7%) |
| Unscheduled health care visits for asthma in previous 12 mo | 3 (7%) |
| Systemic steroid for asthma in past 12 mo | 5 (12%) |
| Daily use of short-acting β-agonist (MDI/nebulizer) | 3 (7%) |
| Daily use of anti-leukotriene | 6 (14%) |
| Age of asthma onset (yr), median (IQR) | 8 (3, 15) |
| Spirometry, median (IQR) | |
| FEV1, %predicted | 91 (83, 98) |
| FVC, %predicted | 101 (90, 109) |
| Tests, median (IQR) | |
| Calculated PC20, mg/ml | 2.06 (0.55, 4.31) |
| Exhaled NO,* ppb | 32.25 (16.75, 63.50) |
| Questionnaires, median (IQR), unless noted | |
| Multivariable apnea prediction score‡ (range 0–18) | 1 (0, 3) |
| Epworth score‡ (range 0–24) | 7 (5, 9) |
| Pittsburgh Sleep Quality Index‡ (range 0–21) | 6 (3, 7) |
| Asthma symptom utility index§ (range 0–1) | 0.89 (0.83, 0.94) |
| Asthma control test§ (range 5–25) | 21 (20, 23) |
| Sinonasal SNQ-6 score‡ (range 0–3) | 1 (1, 2) |
| Berlin score, high risk,† n (%) | 11 (26%) |
A total of 43 subjects were studied. MDI, metered-dose inhaler; IQR, interquartile range; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PC20, provocative concentration that causes 20% fall in FEV1.
9 subjects missing exhaled NO.
1 subject missing Berlin score.
Higher score indicates worse control.
Higher score indicates better control.
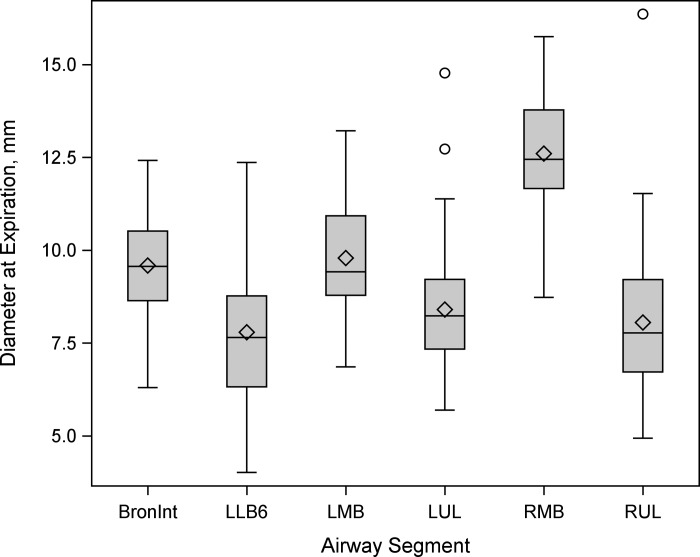
Up to 36 (range 22–36) airways were measured in each participant for a total of 1,593 airways. The software was not able to measure all the airways in every individual at both TLC and FRC. Therefore, it was decided to focus on six airway segments that could be consistently measured by the software across all subjects at FRC and TLC at baseline and after 12 wk: the right upper lobe segment (RUL), the left middle lobe segment (LMB), the bronchus intermedius segment (BronInt), the right middle lobe segment (RMB), the left upper lobe segment (LUL), and the left lower bronchus number 6 segment (LLB6). A total of 258 airways were matched and measured across all four states (baseline, 12 wk, FRC, and TLC); these airways ranged in size from 4.0 to 16.1 mm diameter (Fig. 1).
Fig. 1.
Box-and-whisker plot of luminal airway diameter (mm) at functional residual capacity (FRC) by lung segment at the first visit in the 6 airway segments that were measured across all subjects at both time points. Airway segments included the bronchus intermedius segment (BronInt), left lower bronchus number 6 segment (LLB6), left middle lobe segment (LMB), left upper lobe segment (LUL), right middle lobe segment (RMB), and right upper lobe segment (RUL).
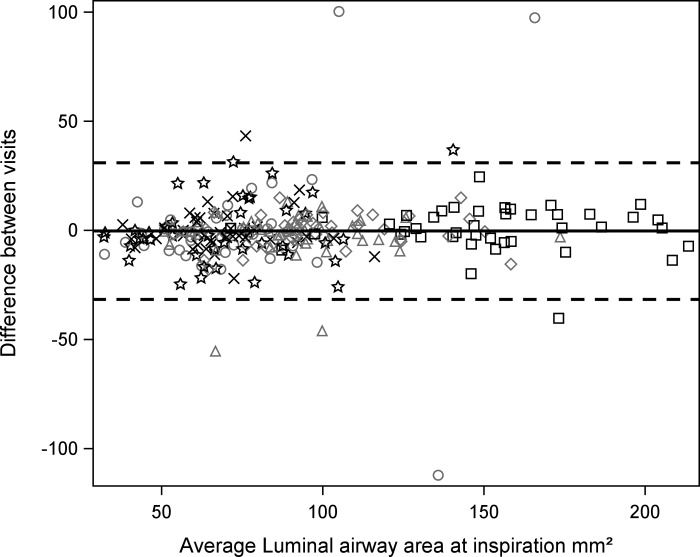
For the six different airway locations, the ICC (95% CI) for the size of the airway lumen from baseline to 12 wk on the TLC scans ranged from 0.95 (0.91, 0.97) to 0.47 (0.27, 0.69) (Table 4, Fig. 2). For the six different airway locations, the ICC (95% CI) for the size of the airway lumen from baseline to 12 wk on the FRC scans ranged from 0.91 (0.85, 0.95) to 0.32 (0.11, 0.65) (Table 4).
Table 4.
ICC for airway measurements
| RUL |
LMB |
BronInt |
RMB |
LUL |
LLB6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ICC | n | ICC | N | ICC | N | ICC | n | ICC | N | ICC | |
| Luminal airway area | ||||||||||||
| Inspiration | 42 | 0.82 (0.70, 0.90) | 43 | 0.95 (0.92, 0.97) | 43 | 0.85 (0.76, 0.91) | 42 | 0.95 (0.91, 0.97) | 42 | 0.93 (0.87, 0.96) | 43 | 0.47 (0.27, 0.69) |
| Expiration | 41 | 0.84 (0.72, 0.91) | 42 | 0.91 (0.85, 0.95) | 42 | 0.88 (0.79, 0.93) | 41 | 0.89 (0.82, 0.94) | 42 | 0.32 (0.11, 0.65) | 40 | 0.53 (0.33, 0.72) |
| Distensibility index | 41 | 0.26 (0.07, 0.61) | 42 | 0.56 (0.36, 0.74) | 42 | 0.47 (0.27, 0.69) | 41 | 0.50 (0.29, 0.70) | 41 | 0.56 (0.35, 0.75) | 40 | 0.34 (0.13, 0.63) |
| Wall thickness | ||||||||||||
| Inspiration | 42 | 0.26 (0.08, 0.59) | 43 | 0.63 (0.44, 0.78) | 43 | 0.56 (0.37, 0.74) | 42 | 0.21 (0.05, 0.58) | 42 | 0.29 (0.10, 0.62) | 43 | 0.08 (0.00, 0.78) |
| Expiration | 41 | 0.13 (0.01, 0.78) | 42 | 0.52 (0.31, 0.72) | 42 | 0.54 (0.35, 0.72) | 41 | 0.23 (0.06, 0.58) | 42 | 0.61 (0.43, 0.77) | 40 | 0.41 (0.20, 0.66) |
| Wall area fraction | ||||||||||||
| Inspiration | 42 | 0.77 (0.62, 0.87) | 43 | 0.65 (0.47, 0.79) | 43 | 0.56 (0.36, 0.73) | 42 | 0.00 (0.00, 1.00) | 42 | 0.49 (0.27, 0.71) | 43 | 0.37 (0.17, 0.64) |
| Expiration | 41 | 0.73 (0.55, 0.85) | 42 | 0.68 (0.50, 0.82) | 42 | 0.71 (0.55, 0.83) | 41 | 0.07 (0.00, 0.84) | 42 | 0.37 (0.16, 0.65) | 40 | 0.39 (0.18, 0.65) |
ICC, intraclass correlation coefficient; RUL, right upper lobe segment; LMB, left middle lobe segment; BronInt, bronchus intermedius segment; RMB, right middle lobe segment; LUL, left upper lobe segment; LLB6, left lower bronchus number 6 segment.
Fig. 2.
Bland-Altman plot showing average of the 2 luminal area measures (mm2) of each of the 258 airways among the 6 airway segments for all the subjects vs. the difference in the 2 size measures (mm2) of each airway at total lung capacity (TLC). △, BronInt; ○, LLB6; ◇, LMB; ×, LUL; □, RMB; ☆, RUL. Note excellent reproducibility at TLC.
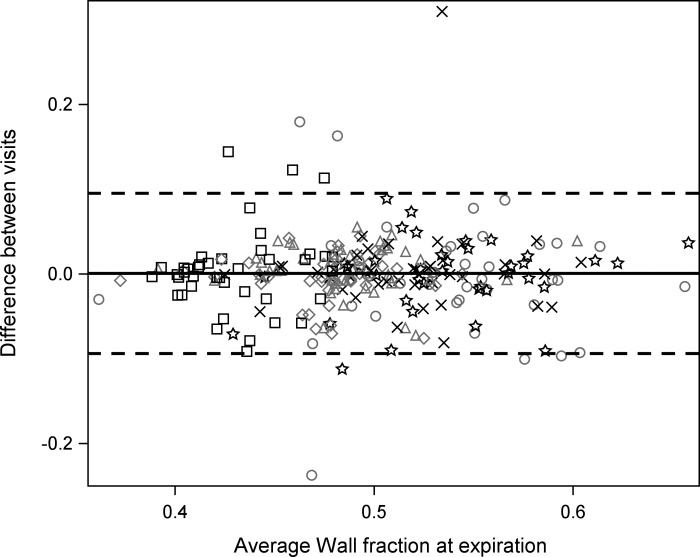
The ICC measurements for all other measurements (distensibility index, wall thickness, and wall area) were lower, ranging from poor (0.08) to moderate (0.63) agreement (Table 4, Fig. 3).
Fig. 3.
Bland-Altman plot showing average of the 2 airway wall areas (as a fraction of total airway area) of each of the 258 airways among the 6 airway segments for all the subjects vs. the difference in the 2 airway wall areas (as a fraction of total airway area) of each airway at TLC. △, BronInt; ○, LLB6; ◇, LMB; ×, LUL; □, RMB; ☆, RUL. Note modest reproducibility.
The ICC for the entire lung volume at TLC and FRC from baseline to 12 wk was 0.82 (0.70, 0.90) and 0.88 (0.80, 0.93), respectively, and varied minimally by lobe (Table 5). Mean (95% CI) air trapping for both lungs was 5.5% (3.6%, 7.4%) at baseline and 4.3% (2.8%, 5.8%) at 12 wk, which was not significantly different (P = 0.11). The ICC for air trapping at FRC from baseline to 12 wk was 0.89 (0.81, 0.94) and varied only modestly by lobe from 0.76 (0.61, 0.87) to 0.95 (0.92, 0.97) (Table 5).
Table 5.
ICC for lung volume measures
| Lung Location | n | Volume at Inspiration, cm3 | Volume at Expiration, cm3 | Air Trapping at Expiration | Inspiratory Capacity, cm3 |
|---|---|---|---|---|---|
| Both lungs | 41 | 0.82 (0.70, 0.90) | 0.88 (0.80, 0.93) | 0.89 (0.81, 0.94) | 0.74 (0.58, 0.86) |
| Left lung | 41 | 0.83 (0.72, 0.91) | 0.85 (0.75, 0.92) | 0.85 (0.74, 0.92) | 0.74 (0.58, 0.86) |
| Left lower lobe | 41 | 0.84 (0.73, 0.91) | 0.83 (0.71, 0.90) | 0.92 (0.85, 0.96) | 0.79 (0.65, 0.88) |
| Left upper lobe | 41 | 0.86 (0.77, 0.92) | 0.86 (0.76, 0.92) | 0.74 (0.56, 0.87) | 0.69 (0.52, 0.83) |
| Right lung | 41 | 0.81 (0.69, 0.89) | 0.90 (0.83, 0.95) | 0.91 (0.85, 0.95) | 0.75 (0.59, 0.86) |
| Right lower lobe | 41 | 0.81 (0.68, 0.89) | 0.86 (0.76, 0.92) | 0.95 (0.92, 0.97) | 0.74 (0.58, 0.86) |
| Right middle lobe | 41 | 0.89 (0.82, 0.94) | 0.89 (0.81, 0.94) | 0.76 (0.61, 0.87) | 0.66 (0.47, 0.81) |
| Right upper lobe | 41 | 0.86 (0.76, 0.92) | 0.87 (0.78, 0.93) | 0.92 (0.85, 0.96) | 0.77 (0.63, 0.87) |
Inspiratory capacity = volume at inspiration − volume at expiration.
Also, we examined the effects of pixel size and reconstruction kernel on reproducibility. We found no consistent significant changes in reproducibility due to either pixel size or kernel. This was likely due to the limited number of subjects studied.
DISCUSSION
Our results demonstrated that luminal area of larger airways at TLC could be reproducibly measured, as demonstrated by excellent ICCs (Table 4). This was also true for lung volume measurements at TLC (Table 5). In contrast, the reproducibility of wall thickness or wall area measurements at TLC or FRC was fair to poor, as was the distensibility index.
One major factor that influenced the size of the airways was the lung volume. It was critical to be able to achieve reproducible lung volumes. For animal studies, we could repeatedly hold the lungs at a constant fixed volume while the animal was anesthetized (2, 3, 6). With awake volunteers, it was necessary to coach the subjects both to consistently stop their ventilation at a predetermined lung volume and to hold their breath for several seconds. For volunteers with moderate-to-severe lung disease, even a short breath hold could be difficult. To ensure lung volume reproducibility, we used a specific script (Table 2) that was read by the clinical coordinator or the radiology technologist every time before and during the scanning procedure. The subjects were coached before the scanning so they could practice the respiratory maneuvers for the TLC and FRC scans. This proved to be a successful process, as shown by our ICC value of 0.78 for the volume of both lungs at TLC. The reproducibility of lung volume at FRC was modestly lower, with an ICC of 0.73. These findings suggest that, for the most consistent lung volume reproduction over time, TLC measurements should be used, but with careful coaching and instruction, reasonable FRC lung volume reproducibility can be achieved. Respiratory gating would be another way to improve lung volume reproducibility during scanning.
As expected, there was an inverse relationship between the ICC and airway luminal size. The ICCs for airway luminal measurements were highest for large airways (RMB and LMB) and lowest for the smaller airways (LLB6 and RUL; Fig. 1, Table 4). As the limit of resolution for airway luminal measurements was reached, accuracy and reproducibility decreased accordingly (10). Although past development of HRCT scanners concentrated on increasing the speed of the scans, allowing complete scans of the entire chest in as short a time as 10 s, more recent development of ultra-high-resolution CT scanners has concentrated on increasing the resolution of the scans (16). The use of these newer-generation scanners should extend the range of airways that can be measured and improve reproducibility of smaller airways.
The lack of significant changes in quantitative measures, such as pulmonary function tests, in the groups randomized to the CPAP treatments compared with the control group (15) supported our observation of no change in airway size among the groups. The consistency in lung function throughout the study allowed us to measure the reproducibility of the luminal size.
Several factors may have led to the high repeatability of the airway luminal area and the low repeatability of airway wall measurements. One possible reason was simply that the larger geometric size of the lumen than the airway wall made for more accurate measurements. The number of pixels counted in the two-dimensional airway luminal area structure was much greater than the number of pixels counted in the one-dimensional airway wall thickness measurement. The wall thickness for some of the airways measured in the current study bordered on the level of resolution of the scanner, approximately one pixel thick, leading to partial volume effect. Another possible reason for greater repeatability of the airway luminal area than repeatability of the airway wall thickness was the method of identification of the wall boundary. For most CT image analyses and the current study, the full-width–half-maximum (FWHM) method is commonly used (29). The change in pixel intensity per unit distance at the boundary of the airway lumen is dramatic: from the very low intensity of air in the lumen to the high intensity of water in the airway wall. For the luminal area measurement, only a single FWHM is calculated. For the wall measurement, a FWHM measurement must be made at the inner and outer boundaries. For the outer wall boundary, the change in pixel intensity is not as large from wall to parenchyma as from lumen to wall, so the FWHM measurement is not as accurate. Furthermore, because of the way the FWHM method calculates the airway wall thickness, the thinner the wall, the less accurate the measurement. Even when we used airway wall area, the reproducibility was, in general, improved over that of airway wall thickness, but was still only modest (Table 4).
A few investigators have analyzed lung volume reproducibility in individuals with chronic obstructive pulmonary disease (COPD). Brown et al. examined the reproducibility of lung volumes over a 9-mo period in patients with emphysema (1). They demonstrated high reproducibility with an ICC of 0.943 for the TLC scans and 0.886 for the residual volume scans. They also showed high correlations for the individual lobes (ICC range 0.873–0.959). The greater reproducibility of lung volumes over time in their COPD group than in our asthma group may be due to the more fixed disease for COPD than for asthma, which is characterized by variability in lung function. Chong et al. also found very high reproducibility in lung volume measurements at TLC in subjects with emphysema over an average of 7 (range 5–17) days, with a concordance correlation coefficient of 0.976 (8). Park et al. found a high correlation of the lung volume repeatability in COPD subjects over an average of 8 mo (12−480 days) with a correlation coefficient of 0.87 (P = 0.74) (20). With proper coaching and imaging techniques, lung volumes can be imaged with a high degree of reproducibility. Under well-controlled conditions, reproducible lung volumes are achievable.
We are aware of only one other study of the reproducibility of airway luminal size in humans. The study was limited to healthy individuals, and no ICC was reported (17). The repeat scans were performed ~5 min after the initial scans. A Bland-Altman plot showed increasing variability with decreasing airway luminal size, presumably due to decreasing resolution of the CT scanner below ~2 mm luminal diameter. On the basis of the software used to analyze the airways in the current study, the smallest airway size we examined was ~4 mm diameter, a size that King et al. showed to have very high reproducibility (17). De Backer et al. showed no change in airway size in the placebo arm of the study as measured by functional respiratory imaging over a 3- to 7-day period (11); they were not specifically examining anatomic reproducibility.
Limitations.
The major limitation of this study was the fact it was not initially designed as a reproducibility study per se. The study was originally designed as a multicenter, randomized, three-parallel-arm, sham-controlled clinical trial in which individuals with asthma were instructed to use a CPAP device for 12 consecutive wk. In addition, we found no significant change in lung function measurements or fractional exhaled nitric oxide within or between groups (15). Since there were no changes in the subjective asthma questionnaires, objective lung function measurements, or HRCT images, we believed that pooling the data to analyze reproducibility of HRCT would be a reasonable undertaking.
In addition, we did not have a healthy control group. The study was originally designed as a sham-controlled trial to examine airway reactivity in asthmatic subjects. On the basis of previous work (5), we would presume that healthy individuals would have less variability in airway size over time and, thus, better airway luminal reproducibility than individuals with asthma. If wall thickness is greater in individuals with asthma than in healthy controls, then reproducibility may actually be better in the asthma population than in the healthy individuals.
The participants were overall well-controlled asthmatic subjects without other comorbidities such as obesity, sleep apnea, or poorly controlled asthma. Whether the airways in individuals with poor control or those with other comorbidities known to affect airway reactivity would demonstrate less reproducibility over time requires further study.
In conclusion, in stable well-controlled asthmatic subjects, it is possible to reproducibly image unstimulated airway luminal areas over time, by region, and by size at TLC throughout the lungs. Therefore, any changes in luminal size on repeat CT imaging are more likely due to changes in disease state and less likely due to normal variability.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant U01 HL-108730 (J. T. Holbrook) and the American Lung Association. ResMed Science Center, ResMed Ltd., provided continuous positive airway pressure devices and technical assistance.
DISCLAIMERS
The National Institutes of Health, American Lung Association, and ResMed Science Center, ResMed Ltd., had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.H.B., J.T.H., and R.A.W. performed experiments; R.H.B., R.J.H., E.A.S., J.T.H., and R.A.W. analyzed data; R.H.B., R.J.H., E.A.S., J.T.H., and R.A.W. interpreted results of experiments; R.H.B. and R.J.H. prepared figures; R.H.B. drafted manuscript; R.H.B., R.J.H., E.A.S., J.T.H., and R.A.W. edited and revised manuscript; R.H.B., R.J.H., E.A.S., J.T.H., and R.A.W. approved final version of manuscript; J.T.H. and R.A.W. conceived and designed research.
ACKNOWLEDGMENTS
We thank the members of the American Lung Association Airways Clinical Research sites for their contribution to the design and conduct of the trial as well as review of the manuscript. The centers include Duke University Medical Center, Durham, NC: Dr. Loretta Que (principal investigator), Dr. Deanna Green (co-principal investigator), Dr. Robert Noveck (coinvestigator), Catherine Foss (principal clinic coordinator), Jessica Ghidorzi (regulatory coordinator), Zongyao Wang, Elise Pangborn, V. Susan Robertson, and Nicholas Eberlein (coordinators), and Dr. Michael Land, Dr. Brian Vickery, Dr. Eveline Wu, Denise Jaggers, Stephanie Allen, and Sabrena Mervin-Blake (former members); National Jewish Health, Denver, CO: Dr. Rohit Katial (principal investigator), Dr. Flavia Hoyte, Maria Rojas (principal clinic coordinator), Holly Currier (coordinator), and Trisha Larson and Nina Phillips (former members); The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus, OH: Dr. John Mastronarde (principal investigator), Dr. Jonathan Parsons (coinvestigator), Janice Drake (principal clinic coordinator), Joseph Santiago and Rachael Compton (coordinators), and Samantha Arrowsmith and Sean Stein (data entry operators); St. Louis Asthma Clinical Research Center, Washington University, St. Louis, MO: Dr. Mario Castro (principal investigator), Dr. Leonard B. Bacharier and Dr. Kaharu Sumino (coinvestigators), Jaime J. Tarsi (principal clinic coordinator), Brenda Patterson (coordinator), Terri Montgomery (data entry operator), and James Kozlowski (CT coordinator); St. Vincent Hospital and Health Care Center, Inc., Indianapolis, IN: Dr. Michael Busk (principal investigator), Debra Weiss (principal clinic coordinator), and Kimberly Sundblad (former member); Vermont Lung Center at the University of Vermont, Colchester, VT: Dr. Charles Irvin (principal investigator), Dr. Anne E. Dixon and Dr. David A. Kaminsky (co-principal investigators), and Stephanie M. Burns (principal clinic coordinator); University of Arizona, Tucson, AZ: Dr. Lynn B. Gerald (principal investigator), Dr. James L. Goodwin and Dr. Mark A. Brown (co-principal investigators), Dr. Tara F. Carr, Dr. Cristine E. Berry, Dr. Kenneth S. Knox, Dr. Wayne J. Morgan, Dr. Cori L. Daines, Dr. Roni Grad, and Dr. Dima Ezmigna (study physicians), Elizabeth A. Ryan (principal clinic coordinator), Monica M. Vasquez, Jesus A. Wences, Silvia S. Lopez, and Janette Priefert (coordinators), Valerie Bloss, Natalie S. Provencio-Dean, Destinee R. Ogas, Clara S. Ehrman, (data system operator), and Monica T. Varela, Rosemary J. Weese, Martha Preciado, Katherine Chee; Andrea Paco (former members); University of California, San Diego, CA: Dr. Stephen I. Wasserman (principal investigator), Dr. Joe W. Ramsdell and Dr. Xavier T. Soler, (co-principal investigators), Katie H. Kinninger (principal clinic coordinator), Paul R. Ferguson and Amber J. Martineau (coordinators), and Tonya Greene and Samang Ung (former members); University of Miami, Miami, FL-University of South Florida, Tampa, FL: Dr. Adam Wanner (principal investigator, Miami), Dr. Richard Lockey (principal investigator, Tampa), Dr. Andreas Schmid and Dr. Michael Campos (co-principal investigators, Miami), Dr. Monroe King (co-principal investigator, Tampa), Dr. Eliana S. Mendes (principal clinic coordinator, Miami), Jeaneen Ahmad (principal clinic coordinator, Tampa), and Patricia D. Rebolledo, Johana Arana, Lilian Cadet, Rebecca McCrery, and Sarah M. Croker (coordinators). University of Missouri-Kansas City School of Medicine, Kansas City, MO: Dr. Gary Salzman (principal investigator), Dr. Asem Abdeljalil, Dr. Abid Bhat, and Dr. Ashraf Gohar (co-principal investigators), and Mary Reed (principal clinic coordinator). Chairman’s Office University of Alabama, Birmingham, AL: Dr. William C. Bailey; Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore, MD: Dr. Robert Wise (center director), Dr. Janet Holbrook (deputy director), Razan Yasin (principal coordinator), Joy Saams, Debra Amend-Libercci, Marie Daniel, Lea Drye, Bethany Grove, Adante Hart, Andrea Lears, Gwen Leatherman, Deborah Nowakowski, Nancy Prusakowski, Alexis Rea, David Shade, Elizabeth Sugar, April Thurman, Anna Adler, Dr. Christian Bime, Ellen Brown, Anne Shanklin Casper, Meng Li, Dr. Sobharani Rayapudi, Suzanna Roettger, Weijiang Shen, Johnson Ukken, and Lucy Wang (members), and Christine Wei (former member); Data and Safety Monitoring Board: Dr. Vernon M. Chinchilli, (chair) and Dr. Paul N. Lanken and Dr. Donald P. Tashkin. National Heart, Lung, and Blood Institute Office: Dr. Gail Weinmann, Michelle Freemer, and Lisa Viviano; and Project Office, American Lung Association, New York, NY: Dr. Norman H. Edelman (scientific consultant), Susan Rappaport and Alexandra Sierra (project officer), and Elizabeth Lancet (former member). The sponsor had a role in the management and review of the study.
REFERENCES
- 1.Brown MS, Kim HJ, Abtin F, Da Costa I, Pais R, Ahmad S, Angel E, Ni C, Kleerup EC, Gjertson DW, McNitt-Gray MF, Goldin JG. Reproducibility of lung and lobar volume measurements using computed tomography. Acad Radiol 17: 316–322, 2010. doi: 10.1016/j.acra.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Brown RH, Kaczka DW, Fallano K, Chen S, Mitzner W. Temporal variability in the responses of individual canine airways to methacholine. J Appl Physiol (1985) 104: 1381–1386, 2008. doi: 10.1152/japplphysiol.01348.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown RH, Kaczka DW, Mitzner W. Effect of parenchymal stiffness on canine airway size with lung inflation. PLoS One 5: e10332, 2010. doi: 10.1371/journal.pone.0010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 163: 994–1001, 2001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- 5.Brown RH, Togias A. Measurement of intraindividual airway tone heterogeneity and its importance in asthma. J Appl Physiol (1985) 121: 223–232, 2016. doi: 10.1152/japplphysiol.00545.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RH, Wizeman W, Danek C, Mitzner W. Effect of bronchial thermoplasty on airway distensibility. Eur Respir J 26: 277–282, 2005. doi: 10.1183/09031936.05.00006605. [DOI] [PubMed] [Google Scholar]
- 7.Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 135: 48–56, 2009. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong D, Brown MS, Kim HJ, van Rikxoort EM, Guzman L, McNitt-Gray MF, Khatonabadi M, Galperin-Aizenberg M, Coy H, Yang K, Jung Y, Goldin JG. Reproducibility of volume and densitometric measures of emphysema on repeat computed tomography with an interval of 1 week. Eur Radiol 22: 287–294, 2012. doi: 10.1007/s00330-011-2277-1. [DOI] [PubMed] [Google Scholar]
- 9.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S, Group SR; SPIROMICS Research Group . Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 69: 491–494, 2014. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dame Carroll JR, Chandra A, Jones AS, Berend N, Magnussen JS, King GG. Airway dimensions measured from micro-computed tomography and high-resolution computed tomography. Eur Respir J 28: 712–720, 2006. doi: 10.1183/09031936.06.00012405. [DOI] [PubMed] [Google Scholar]
- 11.De Backer J, Van Holsbeke C, Vos W, Vinchurkar S, Dorinsky P, Rebello J, Mangale M, Hajian B, De Backer W. Assessment of lung deposition and analysis of the effect of fluticasone/salmeterol hydrofluoroalkane (HFA) pressurized metered dose inhaler (pMDI) in stable persistent asthma patients using functional respiratory imaging. Expert Rev Respir Med 10: 927–933, 2016. doi: 10.1080/17476348.2016.1192464. [DOI] [PubMed] [Google Scholar]
- 12.Diaz AA, Come CE, Ross JC, San José Estépar R, Han MK, Loring SH, Silverman EK, Washko GR; COPDGene Investigators . Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest 141: 736–744, 2012. doi: 10.1378/chest.11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Siddiqui S, Haldar P, Entwisle JJ, Mawby D, Wardlaw AJ, Bradding P, Pavord ID, Green RH, Brightling CE. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax 65: 775–781, 2010. doi: 10.1136/thx.2010.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc 11: 898–907, 2014. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holbrook JT, Sugar EA, Brown RH, Drye LT, Irvin CG, Schwartz AR, Tepper RS, Wise RA, Yasin RZ, Busk MF; American Lung Association Airways Clinical Research Centers . Effect of continuous positive airway pressure on airway reactivity in asthma. A randomized, sham-controlled clinical trial. Ann Am Thorac Soc 13: 1940–1950, 2016. doi: 10.1513/AnnalsATS.201601-043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakinuma R, Moriyama N, Muramatsu Y, Gomi S, Suzuki M, Nagasawa H, Kusumoto M, Aso T, Muramatsu Y, Tsuchida T, Tsuta K, Maeshima AM, Tochigi N, Watanabe S, Sugihara N, Tsukagoshi S, Saito Y, Kazama M, Ashizawa K, Awai K, Honda O, Ishikawa H, Koizumi N, Komoto D, Moriya H, Oda S, Oshiro Y, Yanagawa M, Tomiyama N, Asamura H. Ultra-high-resolution computed tomography of the lung: image quality of a prototype scanner. PLoS One 10: e0137165, 2015. doi: 10.1371/journal.pone.0137165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King GG, Carroll JD, Müller NL, Whittall KP, Gao M, Nakano Y, Paré PD. Heterogeneity of narrowing in normal and asthmatic airways measured by high-resolution CT. Eur Respir J 24: 211–218, 2004. doi: 10.1183/09031936.04.00047503. [DOI] [PubMed] [Google Scholar]
- 18.Lee G, Kim KU, Lee JW, Suh YJ, Jeong YJ. Serial changes and prognostic implications of CT findings in combined pulmonary fibrosis and emphysema: comparison with fibrotic idiopathic interstitial pneumonias alone. Acta Radiol 58: 550–557, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF. A survey screen for prediction of apnea. Sleep 18: 158–166, 1995. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Lee CH, Goo JM, Heo CY, Kim JH. Inter-scan repeatability of CT-based lung densitometry in the surveillance of emphysema in a lung cancer screening setting. Eur J Radiol 81: e554–e560, 2012. doi: 10.1016/j.ejrad.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol (1985) 110: 472–479, 2011. doi: 10.1152/japplphysiol.00603.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salisbury ML, Lynch DA, van Beek EJ, Kazerooni EA, Guo J, Xia M, Murray S, Anstrom KJ, Yow E, Martinez FJ, Hoffman EA, Flaherty KR; IPFnet Investigators . Idiopathic pulmonary fibrosis: the association between the adaptive multiple features method and fibrosis outcomes. Am J Respir Crit Care Med 195: 921–929, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scichilone N, La Sala A, Bellia M, Fallano K, Togias A, Brown RH, Midiri M, Bellia V. The airway response to deep inspirations decreases with COPD severity and is associated with airway distensibility assessed by computed tomography. J Appl Physiol (1985) 105: 832–838, 2008. doi: 10.1152/japplphysiol.01307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieren JP, Hoffman EA, Baumhauer H, Barr RG, Goldin JG, Rennard S. CT Imaging Protocol Standardization for Use in a Multicenter Study: Spiromics. Oak Brook, IL: Radiological Society of North America, 2011. [Google Scholar]
- 25.Sieren JP, Newell JD Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, Couper D, Goldin J, Guo J, Han MK, Hansel NN, Kanner RE, Kazerooni EA, Martinez FJ, Rennard S, Woodruff PG, Hoffman EA, Group SR; SPIROMICS Research Group . SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med 194: 794–806, 2016. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, Donohue KM, Han MK, Hansel NN, Kanner RE, Kleerup E, Rennard S, Barr RG. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 69: 987–996, 2014. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos W, De Backer J, Poli G, De Volder A, Ghys L, Van Holsbeke C, Vinchurkar S, De Backer L, De Backer W. Novel functional imaging of changes in small airways of patients treated with extrafine beclomethasone/formoterol. Respiration 86: 393–401, 2013. doi: 10.1159/000347120. [DOI] [PubMed] [Google Scholar]
- 28.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San José Estépar R, Silverman EK, Rosas IO, Hatabu H. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol 17: 48–53, 2010. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinheimer O, Achenbach T, Bletz C, Duber C, Kauczor HU, Heussel CP. About objective 3-d analysis of airway geometry in computerized tomography. IEEE Trans Med Imaging 27: 64–74, 2008. doi: 10.1109/TMI.2007.902798. [DOI] [PubMed] [Google Scholar]