Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in patients with refractory epilepsy. SUDEP frequently occurs during the night, which has been attributed to an effect of sleep. We have shown that sleep state does indeed influence survival following a seizure. That SUDEP occurs during the night could also implicate a circadian influence. In this study we found that time of day independently affects the physiological consequences of seizures.
Keywords: seizure, SUDEP, circadian, epilepsy, mouse, death
Abstract
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in refractory epilepsy patients. Although specific mechanisms underlying SUDEP are not well understood, evidence suggests most SUDEP occurs due to seizure-induced respiratory arrest. SUDEP also tends to happen at night. Although this may be due to circumstances in which humans find themselves at night, such as being alone without supervision or sleeping prone, or to independent influences of sleep state, there are a number of reasons why the night (i.e., circadian influences) could be an independent risk factor for SUDEP. We explored this possibility. Adult male WT mice were instrumented for EEG, EMG, and EKG recording and subjected to maximal electroshock (MES) seizures during wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep during the nighttime/dark phase. These data were compared with data collected following seizures induced during the daytime/light phase. Seizures induced during the nighttime were similar in severity and duration to those induced during the daytime; however, seizures induced during the nighttime were associated with a lesser degree of respiratory dysregulation and postictal EEG suppression. Seizures induced during REM sleep during the nighttime were universally fatal, as is seen when seizures are induced during REM during the daytime. Taken together, these data implicate a role for time of day in influencing the physiological consequences of seizures that may contribute to seizure-induced death.
NEW & NOTEWORTHY Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in patients with refractory epilepsy. SUDEP frequently occurs during the night, which has been attributed to an effect of sleep. We have shown that sleep state does indeed influence survival following a seizure. That SUDEP occurs during the night could also implicate a circadian influence. In this study we found that time of day independently affects the physiological consequences of seizures.
epilepsy is a heterogeneous group of disorders in which afflicted individuals have spontaneous seizures. Seizures can often be prevented with antiepileptic medications; however, ~35% of patients with epilepsy are refractory to medications (Kwan and Brodie 2000). These patients are at highest risk for sudden unexpected death in epilepsy (SUDEP), the leading cause of death in patients with epilepsy (Devinsky et al. 2016). Although the exact mechanisms for how SUDEP occurs are not well understood, a preponderance of evidence suggests SUDEP occurs due to seizure-induced respiratory and/or cardiac dysfunction (Ryvlin et al. 2013); however, there is a recent report of a small series of SUDEP cases that occurred without a precedent seizure (Lhatoo et al. 2016). Another consistent feature of SUDEP is that it occurs frequently during the night (Lamberts et al. 2012; Nobili et al. 2011; Ryvlin et al. 2013).
A number of theories exist for why SUDEP happens during the night. One is that there is reduced supervision during the night, and therefore the patient cannot be resuscitated if needed (Nashef et al. 1998). Earlier intervention has been shown to reduce seizure length and the degree of seizure-associated oxygen desaturation (Seyal et al. 2013). Having others in the room is also associated with reduced SUDEP risk (Nashef et al. 1998). Another theory is that following a nighttime seizure, patients end up in the prone position, which increases the likelihood of suffocation (Liebenthal et al. 2015; Tao et al. 2010, 2015). Finally, there is evidence to suggest that there is a sleep-state association (Hajek and Buchanan 2016; Ryvlin et al. 2013). Seizures and epileptiform discharges occur more commonly in NREM sleep in humans and experimental animal models (Bazil and Walczak 1997; Malow et al. 1998). Breathing and cardiac activity are regulated in a sleep state-dependent fashion (Buchanan 2013). In the largest published collection of SUDEP cases from epilepsy monitoring units, the majority of cases occurred during the night, and the majority of these for which there was adequate data occurred during sleep (Ryvlin et al. 2013). We recently demonstrated that seizures are more likely to be fatal when induced during sleep, especially in REM sleep in a mouse maximal electroshock seizure model (Hajek and Buchanan 2016). Seizures induced during sleep in this model are also associated with greater respiratory dysfunction compared with seizures induced during wakefulness (Hajek and Buchanan 2016). Interestingly, seizures are also regulated in a time-of-day-, or circadian-, dependent fashion (Durazzo et al. 2008; Hofstra et al. 2009; Loddenkemper et al. 2011a, 2011b; Quigg et al. 1998; Zarowski et al. 2011), as are breathing (Buchanan 2013) and cardiac activity (Hastings et al. 2003).
In this study we set out to determine in the same MES model in mice whether there is a circadian influence on electrocerebral, cardiac, and respiratory function following a seizure, and the likelihood of survival from the seizure. Seizures were induced via MES in adult male mice during different vigilance states during the nighttime/dark phase with concomitant measurement of EEG, EMG, EKG, and breathing via whole body plethysmography and were compared with data collected similarly during the daytime/light phase (Hajek and Buchanan 2016). We found that, in this model, the time of day, or circadian phase, during which the seizure was induced differentially affected respiratory and electrocerebral function and that baseline respiratory rhythm irregularity predicted risk of seizure-induced death.
METHODS
Ethical approval.
All procedures and protocols were approved by the Institutional Animal Care and Use Committee at Yale University School of Medicine and the University of Iowa Carver College of Medicine.
Experimental animals.
Adult male (22–35 g) mice from our colony were housed in a 12:12-h light-dark (LD) regimen (lights on 6:00 PM to 6:00 AM for dark studies; lights on 6:00 AM to 6:00 PM for light studies) in standard cages with food and water available ad libitum. The mice used in this study were Lmx1bf/f mice from our serotonin neuron-deficient (Lmx1bf/f/p × Lmx1bf/f) breeding line (Buchanan and Richerson 2010; Zhao et al. 2006), backcrossed to C57BL/6J for six generations, making them ~98% congenic for C57BL/6J. Lmx1bf/f mice carry two copies of the floxed Lmx1b gene but generally have been found to be phenotypically normal (Buchanan and Richerson 2010; Buchanan et al. 2014; Hodges et al. 2008; Zhao et al. 2006). To verify that these mice were phenotypically comparable to our previous cohorts and to C57BL/6 mice, we determined 24-h (6:00 AM to 6:00 AM; 12:12-h LD cycle) sleep architecture and free-running circadian period for these mice, because these phenotypes are relevant to this study. We found that these mice have similar sleep architecture (total: wake, 51.79 ± 2.75%; NREM, 41.62 ± 2.63%; REM, 6.67 ± 1.74%; light: wake, 45.25 ± 2.18%; NREM, 47.14 ± 2.02%; REM, 7.82 ± 1.65%; dark: wake, 57.89 ± 1.95%; NREM, 35.27 ± 2.45%; REM, 7.11 ± 1.40%; n = 8) to that which we previously reported for earlier generations of Lmx1bf/f mice (Buchanan and Richerson 2010) and to that published for C57BL/6J mice (Franken et al. 1998). We also found these mice to have a similar free-running circadian period in wheel-running behavior in constant darkness (23.82 ± 0.02 h; n = 6) compared with that published for C57BL/6 mice (Schwartz and Zimmerman 1990).
All seizure induction studies were conducted between 8:00 AM and 12:00 PM. This corresponds to Zeitgeber time (ZT) 14 to 18 for dark studies and ZT 2 to 6 for light studies, with ZT0 being the time of lights on. Light studies were previously published (Hajek and Buchanan 2016). Breeding and genotyping was performed as previously described (Buchanan et al. 2014; Hajek and Buchanan 2016; Zhao et al. 2006). All experiments during the dark phase of the LD cycle were conducted under dim red light.
EEG/EMG headmount and EKG electrode implantation.
EEG/EMG headmounts (no. 8201; Pinnacle Technology, Lawrence, KS) were implanted as previously described (Buchanan and Richerson 2010). Briefly, under isoflurane (0.5–2% inh) anesthesia, the skull was exposed, four small holes were bored into the skull with a 23-gauge hypodermic needle 2 mm anterior to bregma and 2 mm anterior to lambda ± 2 mm from the midline, and the headmount was attached to the skull with stainless steel machine screws (no. 000-120; 0.1 in. anterior, 0.125 in. posterior; Pinnacle Technology). EMG wires protruding from the posterior of the headmount were sutured into the bilateral nuchal muscles ±1–2 mm from the midline. EKG leads (MS303-76; Plastics One, Roanoke, VA) were implanted during the same surgery in the left chest wall and right axilla in a modified lead II configuration, and a temperature probe (IPTT-300; Bio Medic Data Systems, Seaford, DE) was implanted subcutaneously over the scapulae. The headmount base, screw heads, and EMG wires were secured with dental cement (Jet Acrylic; Lang Dental, Wheeling, IL), and the skin was sutured closed, leaving only the socket of the headmount exposed on the top of the head. Animals received pre- and postoperative analgesia with meloxicam (1.0 mg/kg sc preoperatively; 1.0 mg·kg−1·day−1 postoperatively either subcutaneously for 2 days or in the drinking water for 5–7 days) and were allowed to recover for 7–10 days before being studied.
Seizure induction with MES.
Before being studied, animals were acclimated to the recording apparatus for at least 1 h per day on at least 3 consecutive days. On the experimental trial day, baseline data were recorded for at least 30 min before the animal received a single electroshock stimulation (50 mA, 0.2 s, 60-Hz sine wave pulses) via ear-clip electrodes (modified, toothless, stainless steel alligator clips coated with saline-moistened gauze) attached to a Rodent Shocker (Harvard Apparatus) during the vigilance state of interest. Many mice succumbed to the seizure when these stimulus parameters were used, as shown previously (Buchanan et al. 2014; Hajek and Buchanan 2016). The extension-to-flexion ratio (E/F ratio), which is the length of time the hindlimbs were extended beyond 90° divided by the length of time the hindlimbs were flexed (≤90°), was used to assess seizure severity. Measurements of E/F ratio were made off-line by post hoc video review. Higher E/F ratios correlate with widespread propagation of epileptiform activity (Anderson et al. 1986). MES thresholds were determined for this mouse strain previously via incremental stimulus presentations (Buchanan et al. 2014).
EEG/EMG/EKG data acquisition.
EEG, EMG, and EKG data were acquired as described previously (Buchanan and Richerson 2010; Buchanan et al. 2014; Hajek and Buchanan 2016). Briefly, a preamplifier (no. 8202-SL; Pinnacle Technology) was attached to the implanted headmount, and the animals were introduced to the recording chamber and allowed to acclimate as described. Preamplifier leads were then passed through a commutator (no. 8204; Pinnacle Technology) and into a conditioning amplifier (model 440 instrumentation amplifier; Brownlee Precision, San Jose, CA). EEG and EMG signals were amplified (50,000 times), bandpass filtered (0.3 to 200 Hz for EEG; 10 to 300 Hz for EMG), and digitized (1,000 samples per second) with an analog-to-digital (A-D) converter (PCI-6221; National Instruments, Austin, TX) on a desktop computer (Dell) and acquired using software custom written in MATLAB (The MathWorks, Natick, MA). EKG signals were passed through a separate conditioning amplifier (Grass model LP511 AC; Astro-Med, West Warwick, RI), where they were amplified (20,000 times), bandpass filtered (0.3 to 300 Hz), and then digitized with the A-D converter as described above. Body temperature signals from the implanted telemeter were sampled periodically with a telemetry reader wand (DAS-7007S; Bio Medic Data Systems).
Sleep-wake determination.
Sleep state was assessed online in real-time before delivery of the stimulation. A standard approach based on the EEG/EMG frequency characteristics was used to assign vigilance state (Buchanan and Richerson 2010; Franken et al. 1998) as follows: wake: low-amplitude, high-frequency (7–13 Hz) EEG with high EMG power; NREM: high-amplitude, low-frequency (0.5–4 Hz) EEG with moderate to low EMG power and lack of voluntary motor activity; and REM: moderate-amplitude, moderate-frequency (4.5–8 Hz) EEG with minimal EMG power except for brief bursts and minimal activity correlating with EMG bursts. Electroshocks were delivered when the animals were determined to be in the vigilance state of interest for at least 60 s. Vigilance states were verified off-line post hoc using custom software written in MATLAB. Fast-Fourier transform (FFT) power spectra were created with MATLAB for each 10-s epoch of data and used along with EEG and EMG characteristics to verify scoring.
Breathing plethysmography.
To aid in quantification of respiratory parameters, the recording chamber was fit with a high-sensitivity/ultra-low-pressure transducer (DC002NDR5; Honeywell International, Minneapolis, MN). The analog output from the pressure transducer was digitized by the A-D converter (PCI-6221; National Instruments) and displayed on a computer monitor in real time using the acquisition program custom written in MATLAB. A mechanical ventilator (Mini-Vent; Harvard Apparatus) was used to deliver metered breaths (300 μl; 150 breaths/min) to the recording chamber to calibrate the breathing signal. Individual breaths were identified and measured using custom software written in MATLAB to aid in assessment of breathing parameters including respiratory rate (RR), tidal volume (VT), and minute ventilation (VE) as previously described (Buchanan et al. 2014; Hajek and Buchanan 2016; Hodges et al. 2008). Relative humidity, ambient temperature, body temperature and atmospheric pressure (obtained from Weather Underground (https://www.wunderground.com) were used to calculate VT using standard methods (Buchanan et al. 2014; Drorbaugh and Fenn 1955).
EKG analysis.
Heart rate (HR) and measurements of heart rate variability (HRV) were determined using custom software written in MATLAB, Kubios HRV 2.2 (https://www.kubios.com) (Tarvainen et al. 2014), and Microsoft Excel (Redmond, WA) as previously described (Hajek and Buchanan 2016). HRV indexes included the standard deviation of all R-R intervals (SDNN) and the root mean square of the standard deviation of the differences between R-R intervals (RMSSD). These are standard measurements to assess autonomic function (Stein et al. 1994) and may be important in prediction of SUDEP risk (DeGiorgio et al. 2010; Kalume et al. 2013).
Wheel-running behavior.
Mice were housed in modified home cages equipped with running wheels (11.5-cm diameter). Wheel cages were housed in light-tight, black, cast acrylic chambers. Lighting within chambers could be independently regulated. Inflow/outflow fans on chambers allowed adequate airflow. A rare earth magnet (5-mm diameter × 0.75 mm; neodymium; K&J Magnetics, Plumsteadville, PA) attached to the wheel activated a hermetically sealed reed switch (MITI-3v1-6-12.5; Littelfuse, Schipfol, The Netherlands) with each wheel revolution. Wheel revolution data were transmitted to a multiplexer (ACTI-556B; Actimetrics, Wilmette, IL) and ultimately to a computer running ClockLab acquisition software (Actimetrics). Mice were initially entrained to a 12:12-h (6:00 AM to 6:00 PM lights on) LD cycle and then released into constant darkness (DD). The slope of the best-fit line through the daily onsets of wheel running activity was used to determine the free-running period (tau) with the aid of ClockLab analysis software (Actimetrics). Data represent the mean during 3 weeks in DD for 6 animals.
Statistics.
Interactions between time of day (i.e., light vs. dark), vigilance state, respiratory parameters, and cardiac measures were analyzed for all physiological variables using two-way analysis of variance (ANOVA), paired t-test, or two-tailed t-test, assuming unequal variance as appropriate. Logistic regression was performed for survival analyses. The significance threshold was set at P < 0.05 for all conditions. Analyses were accomplished using Microsoft Excel, OriginPro 9.0 (OriginLab, Northampton, MA), and Systat 11.0. Data expressed as x ± y represent means ± SE. Error bars represent SE.
RESULTS
Seizures induced across vigilance states during the nighttime dark phase were similar in severity and duration to those induced during daytime light phase.
To determine whether the time of day during which a seizure occurred had any effect on survival, seizures were induced during the nighttime dark phase during wakefulness, NREM sleep, and REM sleep (n = 12 per state) in separate groups of mice. Data were compared with those collected during the daytime and previously reported (Hajek and Buchanan 2016). As was seen for seizures induced during the daytime, there was a significant effect of sleep state on survival when seizures were induced during the nighttime (Fig. 1; P < 0.001; logistic regression analysis). All (100%) seizures induced during the nighttime during REM sleep were fatal (Fig. 1), as was seen when seizures were induced during the daytime during REM sleep (Hajek and Buchanan 2016). Fifty percent of seizures induced during NREM sleep during the nighttime and 25% of seizures induced during wakefulness during the nighttime were fatal (Fig. 1). Although a smaller number of mice died when seizures were induced during wakefulness (25% vs. 50%) or NREM sleep (50% vs. 67%) during the nighttime compared with the daytime, this difference was not statistically significant in a logistic regression analysis (P = 0.143; daytime values from Hajek and Buchanan 2016).
Fig. 1.
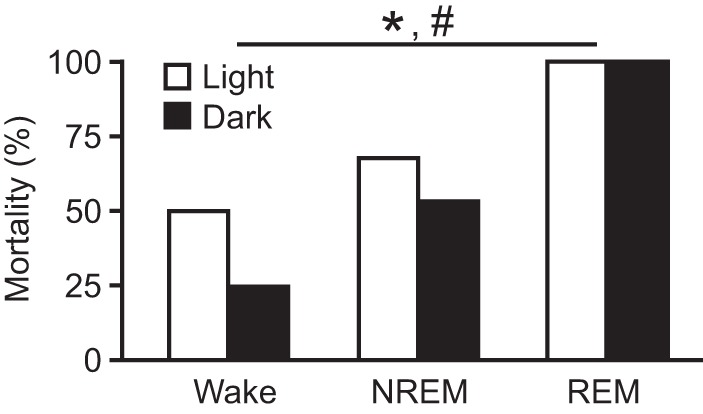
Seizures induced via MES during REM sleep during the dark phase and light phase are universally fatal. Mortality rates for seizures induced during Wake, NREM, and REM; n = 12 per sleep state. *P < 0.001 among vigilance states during the light. #P < 0.001 among vigilance states during the dark. P = 0.143 for light vs. dark. Data from seizures induced during the light phase are replotted from Hajek and Buchanan (2016) for comparison.
That we found significant mortality from MES-induced seizures in the Lmx1bf/f mice studied presently that have been backcrossed onto C57BL/6J for a number of generations is consistent with our previous mortality rates in this genotype (Buchanan et al. 2014). However, we previously found that one C57BL/6 substrain, the C57BL/6N strain from Harlan, displayed no seizure-induced mortality (Buchanan et al. 2014). There can be wide strain variation in how mice perform in different seizure models. Although strain variation between C57BL/6J and C57BL/6N has not to our knowledge been reported for epilepsy, these substrains display quite a number of phenotypic variations (Sturm et al. 2015; Wolf et al. 2016). To investigate the possibility of substrain effect, we subjected eight male C57BL/6J mice to MES-induced seizures during wakefulness during the daytime and found 37.5% survival. This is comparable to what we have seen previously Lmx1bf/f mice and starkly different from the 100% survival seen in C57BL/6N mice (Buchanan et al. 2014).
Seizures induced during the nighttime were associated with higher E/F ratios, indicating increased severity, when induced during NREM (20.67 ± 2.54) or REM sleep (21.54 ± 2.34) compared with wakefulness (11.53 ± 1.91; P < 0.05; Fig. 2A). This trend was similar to that observed when seizures were induced during the daytime (Hajek and Buchanan 2016). Seizures during the nighttime were also longer in duration when induced during NREM (29.90 ± 3.73 s) or REM sleep (30.98 ± 5.26 s) compared with wakefulness (15.19 ± 4.12 s; P < 0.05; Fig. 2B). This trend was also similar to that observed when seizures were induced during the daytime (Hajek and Buchanan 2016). There was no significant difference in severity (P = 0.846) or duration (P = 0.741) between seizures induced during NREM or REM sleep. Also similarly to seizures induced during the daytime, there was no significant difference in seizure severity [E/F ratios: wake (W), 8.10 ± 1.85 survive vs. 6.85 ± 1.72 die, P = 0.118; NREM (N), 20.04 ± 2.04 survive vs. 21.30 ± 2.31 die, P = 0.495; Fig. 2A] or duration (W, 14.36 ± 2.85 s survive vs. 16.02 ± 4.69 s die, P = 0.679; N, 28.98 ± 4.02 s survive vs. 30.82 ± 2.33 s die, P = 0.794; Fig. 2B) among animals that survived and those that died when seizures were induced during a given vigilance state during the nighttime. There was no statistically significant difference in the severity (W, P = 0.156; N, P = 0.094; R, P = 0.073; Fig. 2A) or duration (W, P = 0.188; N, P = 0.127; R, P = 0.859; Fig. 2B) of seizures induced during a given vigilance state during the daytime or the nighttime (Fig. 2).
Fig. 2.
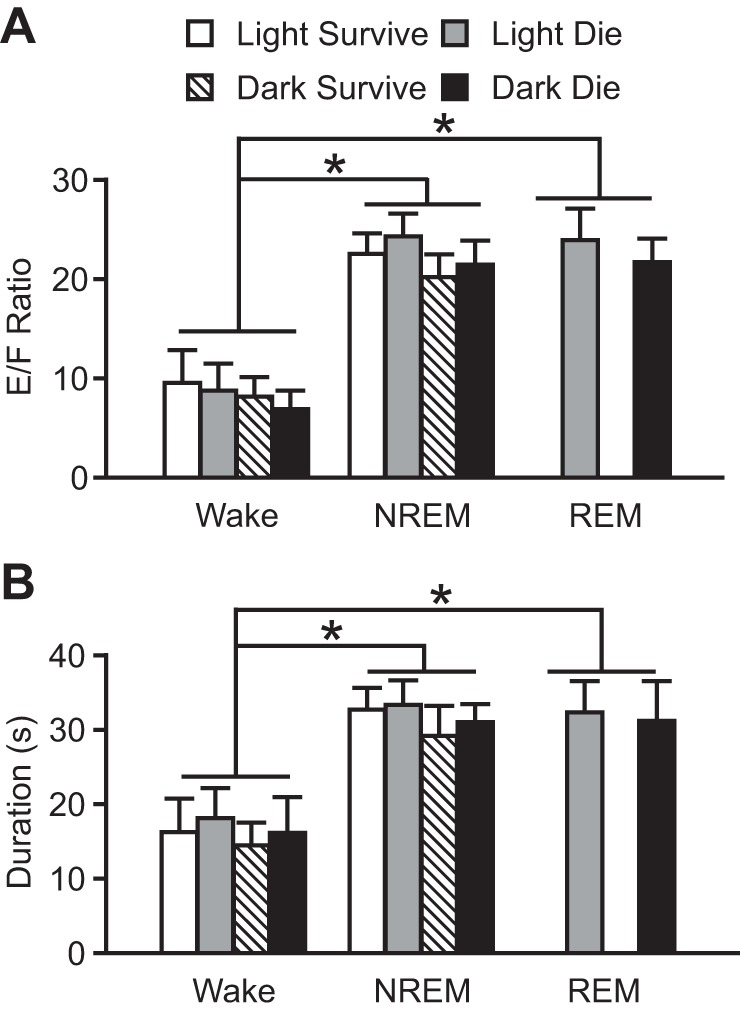
Increased severity and duration of seizures induced via MES during sleep during both dark and light phases. Severity (A) and duration (B) of seizures induced during Wake, NREM, and REM in mice that survived during the light (open bars; n = 6, 4, and 0, respectively) and dark (hatched bars; n = 9, 6, and 0, respectively) and those that died during the light (shaded bars; n = 6, 8, and 12, respectively) and dark (solid bars; n = 3, 6, and 12, respectively). Data are means ± SE. *P < 0.05. E/F ratio, extension-flexion ratio. Data from seizures induced during the light phase are replotted from Hajek and Buchanan (2016) for comparison.
Seizures induced during the nighttime were associated with less respiratory dysfunction compared with those induced during the daytime.
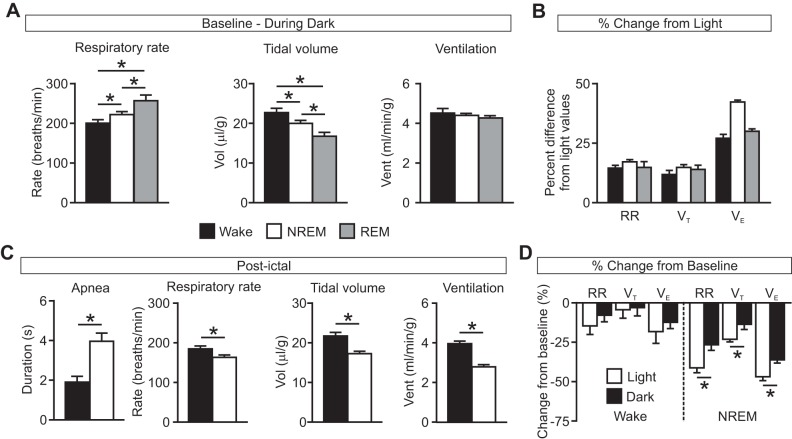
Similarly to data collected during the daytime light phase (Hajek and Buchanan 2016), there was again normal vigilance-state dependent variability in breathing parameters during the nighttime dark phase with increased respiratory rate (RR; W, 198.91 ± 5.14 breaths/min; N, 220.24 ± 4.86 breaths/min; R, 255.08 ± 11.48 breaths/min; n = 12 per group; P < 0.05 for all comparisons) and reduced tidal volume (VT; W, 22.56 ± 0.70 μl/g; N, 19.81 ± 0.46 μl/g; R, 16.61 ± 0.86 μl/g; n = 12 per group; P < 0.05 for all comparisons) during NREM and REM sleep compared with wakefulness (Fig. 3A). There was a trend toward a small reduction in minute ventilation during NREM and REM sleep compared with wakefulness (VE; W, 4.48 ± 0.17 ml·min−1·g−1; N, 4.37 ± 0.16 ml·min−1·g−1; R, 4.19 ± 0.23 ml·min−1·g−1; n = 12 per group), but this was not statistically significant (P = 0.321 for W vs. N, P = 0.156 for W vs. R, P = 0.261 for N vs. R; Fig. 3A). Across vigilance states, there was an increase in RR (W: P < 0.001; N: P < 0.001; R: P < 0.05), VT (W: P < 0.05; N: P < 0.001; R: P < 0.05) and VE (W: P < 0.001; N: P < 0.001; R: P < 0.001) at baseline during the nighttime compared with the daytime [Fig. 3B; P values for comparisons between dark data (Fig. 3A) and previously published light data (Hajek and Buchanan 2016)]. Consistent with our previous reports (Buchanan et al. 2014; Hajek and Buchanan 2016), we again noted that all seizures were associated with respiratory arrest during the seizure. Similarly to seizures induced during the daytime, seizures induced during the nighttime during NREM sleep were associated with a greater duration of postictal apnea (W, 1.87 ± 0.28 s, n = 9; N, 3.93 ± 0.39 s, n = 6; P < 0.05) and a reduced RR (W, 182.35 ± 4.88 breaths/min, n = 9; N, 161.85 ± 3.68 breaths/min, n = 6; P < 0.05), reduced VT (W, 21.66 ± 0.78 μl/g, n = 9; N, 17.11 ± 0.32 μl/g, n = 6; P < 0.05), and reduced VE (W, 3.95 ± 0.089 ml·min−1·g−1, n = 9; N, 2.77 ± 0.058 ml·min−1·g−1, n = 6; P < 0.05) compared with seizures induced during wakefulness (Fig. 3C). When these data were compared with daytime data (Hajek and Buchanan 2016), there was no significant difference in the duration of postictal apnea when seizures were induced during wake during the nighttime vs. the daytime (P = 0.281); however, but there was a significant effect of time of day on duration of postictal apnea when seizures were induced during NREM sleep (P < 0.05). Seizures induced during the nighttime during NREM sleep were also associated with a greater percent reduction in RR (W, −7.77 ± 3.58%, n = 9; N, −26.26 ± 2.74%, n = 6; P < 0.05) and VE (W, −12.20 ± 3.66%, n = 9; N, −35.67 ± 1.69%, n = 6; P < 0.001) from baseline compared with seizures induced during wakefulness. There was a trend toward greater percent reduction from baseline in VT (W, −2.99 ± 4.70%, n = 9; N, −13.48 ± 2.82%, n = 6; P = 0.0607) following seizures induced during NREM sleep compared with those induced during wakefulness, but this was not statistically significant. Percent reduction in RR [light (L), −40.73 ± 2.28%, n = 4; dark (D), −26.26 ± 2.74%, n = 6; P < 0.05], VT (L, −22.76 ± 0.94%, n = 4; D, −13.48 ± 2.82%, n = 6; P < 0.05), and VE (L, −46.26 ± 1.72%, n = 4; D, −35.67 ± 1.69%, n = 6; P < 0.05) was smaller when seizures were induced during NREM sleep in the nighttime compared with those induced during the daytime (Fig. 3D; Hajek and Buchanan 2016). There was no significant difference in percent reduction in RR (L, −14.47 ± 5.25%, n = 6; D, −7.77 ± 3.58%, n = 9; P = 0.147), VT (L, −4.32 ± 5.56%, n = 6; D, −2.99 ± 4.70%, n = 9; P = 0.429), and VE (L, −17.98 ± 7.55%, n = 6; D, −12.20 ± 3.66%, n = 9; P = 0.229) when seizures were induced during wakefulness in the nighttime compared with those induced during the daytime (Fig. 3D; Hajek and Buchanan 2016). As was seen for seizures induced during the daytime (Hajek and Buchanan 2016), fatal seizures induced during the nighttime also occurred in mice that had greater baseline respiratory rate variability [coefficient of variance of inter-breath interval (CV IBI): W, fatal 19.33 ± 2.28%, n = 3, nonfatal 13.11 ± 1.14%, n = 9, P < 0.05; N, fatal 22.71 ± 2.33%, n = 6, nonfatal 12.05 ± 2.02%, n = 6, P < 0.05; R, fatal 21.44 ± 1.93%, n = 12; Fig. 4].
Fig. 3.
Greater degree of respiratory suppression following seizures induced during NREM sleep during the light phase compared with the dark phase. A: baseline respiratory rate (left), tidal volume (Vol; middle), and minute ventilation (Vent; right) during Wake (solid bars), NREM (open bars), and REM (shaded bars) recorded during the dark phase. *P < 0.05; n = 12 per state. B: respiratory parameters during the dark phase expressed as percent difference from light phase values. RR, respiratory rate; VT, tidal volume; VE, ventilation. All values significantly different compared with corresponding light phase values (RR: W, P < 0.001; N, P < 0.001; R, P < 0.05; VT: W, P < 0.05; N, P < 0.001; R, P < 0.05; VE: W, P < 0.001; N, P < 0.001; R, P < 0.001). C: postictal apnea (left), respiratory rate (left middle), tidal volume (right middle), and minute ventilation (right) following seizures induced during the dark phase during Wake (black) and NREM (white). *P < 0.05; n = 9 for Wake; n = 6 for NREM. D: effects of seizures on breathing when seizures were induced during Wake and NREM during the light (open bars; n = 6 and 4, respectively) or dark phase (solid bars; n = 9 and 6, respectively) expressed as percent change from baseline. *P < 0.05. All data are means ± SE.
Fig. 4.
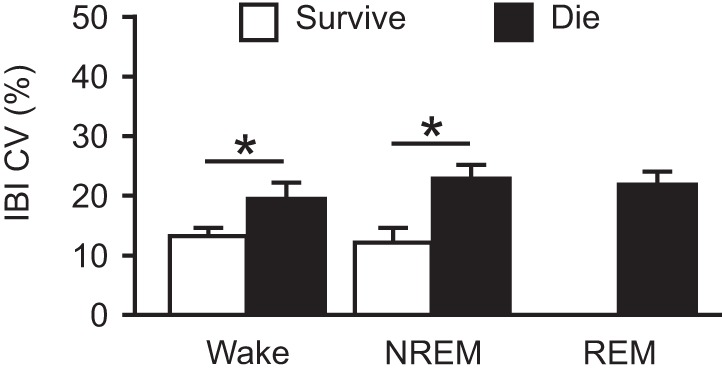
Increased baseline respiratory rhythm dysregulation in mice that ultimately died from a seizure induced by MES during the dark phase. Coefficient of variance of the inter-breath interval (IBI CV) is shown for mice that survived (open bars; n = 9, 6, and 0, respectively) and mice that died (solid bars; n = 3, 6 and 12, respectively) from seizures induced by MES during Wake, NREM, or REM during the dark phase. Data are means ± SE. *P < 0.05.
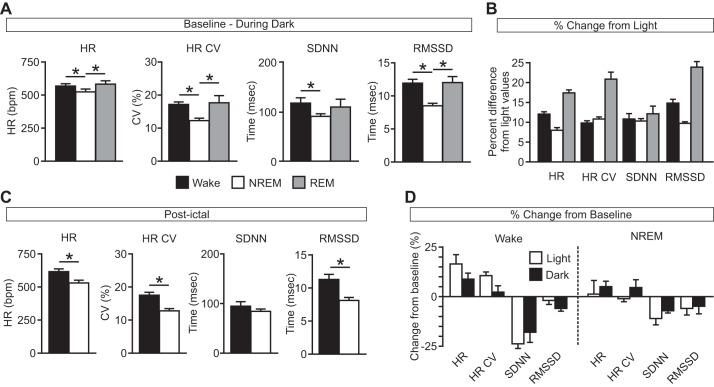
Similarly to the daytime (Hajek and Buchanan 2016), the expected vigilance-state dependent variation in baseline heart rate was observed during the nighttime (HR: W, 564.07 ± 10.86 beats/min; N, 519.69 ± 11.43 beats/min; R, 577.99 ± 18.79 beats/min; P < 0.05 for W vs. N and N vs. R; P = 0.273 for W vs. R; HR CV: W, 17.01 ± 0.47%; N, 12.18 ± 0.44%; R, 17.63 ± 1.88%; P < 0.05 for W vs. N and N vs. R; P = 0.381 for W vs. R; SDNN: W, 116.22 ± 10.13 ms; N, 89.76 ± 3.18 ms; R, 108.40 ± 14.25 ms; P < 0.05 for W vs. ; P = 0.336 for W vs. R; P = 0.117 for N vs. R; RMSSD: W, 11.85 ± 0.49 ms; N, 8.43 ± 0.23 ms; R, 12.00 ± 0.73 ms; P < 0.05 for W vs. N and N vs. R; P = 0.434 for W vs. R; Fig. 5A). Across vigilance states, HR (W: P < 0.001; N: P < 0.05; R: P < 0.05) was increased at baseline during the nighttime dark phase compared with the daytime light phase (Fig. 5B). Also similarly to findings for seizures induced during the daytime, although there were differences in HR (W, 611.79 ± 12.70 beats/min, n = 9; N, 544.50 ± 13.80 beats/min, n = 6; P < 0.05), HR CV (W, 17.38 ± 0.68%, n = 9; N, 12.68 ± 0.50%, n = 6; P < 0.001), and RMSSD (W, 11.20 ± 0.61 ms, n = 9; N, 8.06 ± 0.29 ms, n = 6; P < 0.05) depending on when the seizure was induced (Fig. 5C), there were no significant differences in the percent change of cardiac measures for seizures induced during NREM sleep compared with those induced during wakefulness during the nighttime (HR: W, 8.71 ± 3.02%, n = 9; N, 4.98 ± 2.89%, n = 6; P = 0.206; HR CV: W, 4.51 ± 3.92%, n = 9; N, 2.26 ± 2.80%, n = 6, P = 0.340; SDNN: W, −17.68 ± 4.98%, n = 9; N, −7.01 ± 0.74%, n = 6; P = 0.069; RMSSD: W, −4.49 ± 0.89%, n = 9; N, −4.79 ± 3.60%, n = 6; P = 0.467). There were no significant differences in the effects on cardiac function of seizures induced during wakefulness (HR: L, 16.22 ± 4.44%, n = 6; D, 8.71 ± 3.02%, n = 9; P = 0.085; HR CV: L, 10.42 ± 3.92%, n = 6; D, 2.26 ± 2.80%, n = 9, P = 0.137; SDNN: L, −23.43 ± 2.37%, n = 6; D, −17.68 ± 4.98%, n = 9; P = 0.173; RMSSD: L, −1.89 ± 2.21%, n = 6; D, −4.49 ± 0.89%, n = 9; P = 0.118) or NREM sleep (HR: L, 1.28 ± 6.62%, n = 4; D, 4.98 ± 2.89%, n = 6; P = 0.288; HR CV: L, −1.01 ± 1.12%, n = 4; D, 4.51 ± 3.92%, n = 6, P = 0.196; SDNN: L, −10.89 ± 3.10%, n = 4; D, −7.01 ± 0.74%, n = 6; P = 0.094; RMSSD: L, −5.86 ± 3.65%, n = 4; D, −4.79 ± 3.60%, n = 6; P = 0.434) during the daytime vs. the nighttime (Fig. 5D).
Fig. 5.
Minimal effect of seizures induced during different vigilance states during the dark phase on cardiac function. A: baseline heart rate (HR), coefficient of variance of the inter-heartbeat interval (HR CV), and heart rate variability (SDNN, RMSSD) during Wake (solid bars), NREM (open bars), and REM (shaded bars) during the dark phase. *P < 0.05; n = 12 for each state. B: cardiac measures (as in A) during the dark phase expressed as percent change from light phase values. C: postictal cardiac measures (as in A) following seizures induced during Wake (solid bars) and NREM (open bars) during the dark phase. D: effects of seizures on cardiac measures when seizures were induced during Wake and NREM during the light (open bars; n = 6 and 4, respectively) or dark phase (solid bars; n = 9 and 6, respectively) expressed as percent change from baseline. Data are means ± SE. bpm, beats/min; SDNN, standard deviation of R-R intervals; RMSSD, root mean square of the differences between consecutive R-R intervals.
Seizures induced during the nighttime were associated with a shorter duration of PGES compared with those induced during the daytime.
As was observed for seizures induced during the daytime, fatal seizures induced during the nighttime were associated with complete suppression of EEG power following the seizure consistent with death (data not shown). Similarly to nonfatal seizures induced during the daytime, nonfatal seizures induced during the nighttime during wakefulness or NREM sleep were associated with a transient reduction in EEG power that recovered over time and that was longer in duration when seizures were induced during NREM sleep (W, 2.27 ± 0.23 min, n = 9; N, 4.02 ± 0.24 min, n = 6; P < 0.001; Fig. 6). Compared with seizures induced during the daytime, seizures induced during wakefulness (L, 3.04 ± 0.27 min, n = 6; D, 2.27 ± 0.23 min, n = 9; P < 0.05) or NREM sleep (L, 5.19 ± 0.36 min, n = 6; D, 4.02 ± 0.24 min, n = 9; P < 0.05) during the nighttime were associated with a shorter duration of postictal EEG suppression (Fig. 6). The degree of suppression of EEG power following seizures induced during the dark was similar for seizures induced during wakefulness and NREM sleep, reducing normalized total EEG power to 0.454 ± 0.0391 and 0.476 ± 0.0715, respectively (P < 0.362). These values were comparable to the reduction in EEG power seen when seizure were induced during the light during wakefulness or NREM sleep (Hajek and Buchanan 2016).
Fig. 6.
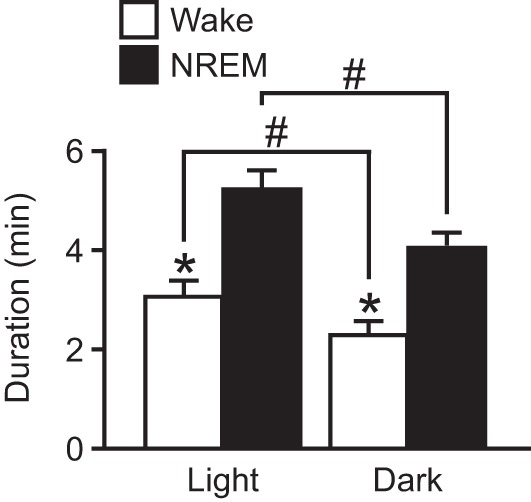
Longer duration suppression of EEG activity following nonfatal seizures induced during NREM sleep during the light phase compared with the dark phase. Duration of suppression of EEG power is shown when a seizure was induced during Wake (open bars; n = 6 light and 9 dark) or NREM (solid bars; n = 4 light and 6, dark) during the light (left) or the dark phase (right). Data are means ± SE. *P < 0.001, Wake vs. NREM; #P < 0.05, light vs. dark. Data from seizures induced during the light phase are represented from Hajek and Buchanan (2016) for comparison.
DISCUSSION
SUDEP occurs more frequently during the night. This is often attributed to a combination of sleep state, prone sleeping position, and a lack of supervision and/or monitoring during the night; however, night as an independent risk factor for SUDEP is often not considered. In this study we provide evidence in a mouse model that time of day can be an independent influence on the physiological changes associated with a seizure.
Time of day differentially influences the respiratory consequences of seizures.
It is significant to note that effects of seizures on breathing are more profound during the light phase of the light-dark cycle, which corresponds to the less active period for the nocturnal mice used in this study and others. Most studies in rodents are conducted during the light phase out of convenience for investigators (i.e., normal working hours, normal lighting conditions, etc.). On the basis of the results of this study, this indeed may actually be the more relevant time to study these phenomena in rodents.
We found that circadian differences in baseline breathing and cardiac activity in our mouse strain were similar to those that have been reported for a variety of nocturnal rodents, namely, faster rates and larger volumes during the nighttime, active phase (Friedman et al. 2004). In humans, breathing indexes and heart rate also increase during the active phase, but in the case of humans this is the daytime. Thus it stands to reason that the worsening of seizure-related effects on breathing, cortical activity, and survival for mice during the daytime (mouse inactive period) will translate to worsened effects on these parameters in humans during the nighttime (human inactive period).
There is a well-described circadian variation in seizure propensity in animals and epilepsy patients that depends on the model employed and/or the epilepsy semiology (Durazzo et al. 2008; Loddenkemper et al. 2011a; Quigg et al. 1998). Specifically, for MES it has been shown that there is a higher MES threshold when seizures are induced during the dark phase (Gerstner et al. 2014). We did not formally examine the threshold to development of maximal tonic extension seizures in our study, but rather employed a single “maximal” stimulus that was sufficient to consistently induce a maximal seizure in every animal. We did not observe statistically significant differences in seizure severity or duration when seizures were induced during the dark phase vs. the light phase. Although our data indicate that sleep state has a significant effect on survival from an MES-induced seizure regardless of whether the seizure is induced during the daytime or the nighttime, we did not find a statistically significant difference in overall survival when seizures were induced during the nighttime vs. the daytime. There are a number of possible explanations for this. First, we used a relatively small sample size and could have been underpowered to detect the difference. Second, we sampled narrow time windows during the light and dark phases. Although we were consistent between experiments, we might have found an effect by using a larger sampling window or a greater number of sampling windows. Finally, this could simply be true, and there is not a time of day difference in survival from MES.
Although circadian rhythms are known to be regulated by an intricate ensemble of “clock” genes, these genes have a variety of functions beyond simply regulating circadian timing. In some instances, loss of these genes or other circadian transcription factors can lead to an increase in excitability and seizure susceptibility (Gachon et al. 2004). Thus the relationship between circadian rhythms and epilepsy could be quite complicated, with clock genes being involved in both temporal regulation of seizures and tempering of excitability. Whether there are clock gene variations in SUDEP cases compared with controls is not known. It also is not known whether patients with epilepsy, with or without increased SUDEP risk, display normal circadian respiratory rhythms.
A large body of data implicates serotonin (5-HT) in SUDEP (Richerson and Buchanan 2011). In addition to 5-HT’s role in breathing, seizures, and sleep-wake regulation, it is an important modulator of the central circadian timing system (Medanic and Gillette 1992; Mintz et al. 1997; Morin et al. 1990; Yannielli and Harrington 2004). The hypothalamic suprachiasmatic nucleus (SCN), which houses the mammalian central circadian oscillator (Moore and Eichler 1972; Stephan and Zucker 1972), receives serotonergic inputs from the raphe nuclei that modulate light-induced phase shifts and also have a role in nonphotic entrainment (Yannielli and Harrington 2004). 5-HT function is also regulated in a circadian fashion (Quay 1968). 5-HT and the rate-limiting enzyme in its production, tryptophan hydroxylase, are differentially expressed in the raphe nuclei at different times of day (Malek et al. 2004). Additionally, the serotonergic content of the structures to which the raphe projects, including the SCN, oscillates with time of day (Cagampang and Inouye 1994). Lesions to the raphe nuclei in rats eliminate the free-running rhythm of motor activity (Levine et al. 1986). Thus the raphe nuclei are a crucial component in the central timing system and have diurnal variations in activity.
In addition to 5-HT, neurotransmitters such as norepinephrine and others may play a role in day-night differences in seizure-induced respiratory arrest. Norepinephrine levels vary with circadian time and reach a nadir in the early hours of the morning in humans (Linsell et al. 1985), and they also vary in rodents (Cagampang et al. 1994). Circadian variations in norepinephrine levels might contribute to the day-night differences in seizure-induced respiratory arrest seen in this study and the circadian variation in human SUDEP (Lamberts et al. 2012).
Circadian variations in vulnerability to seizure-induced respiratory arrest may also be explicable by vagally mediated parasympathetic innervation of respiratory tissues. Unilateral vagotomy abolishes rhythms in clock protein levels and mucin secretion on the ipsilateral side (Bando et al. 2007). This finding suggests that the peripheral oscillators found in respiratory tissues are regulated by the central timing system via the vagus nerve. These likely would not play a major role in respiratory rhythmogenesis but may be responsible for modulating breathing in a circadian manner.
As we found previously for seizures induced by MES during the daytime (Hajek and Buchanan 2016), mice that died when seizures were induced during the nighttime had a greater CV IBI. This is a somewhat surprising finding, because respiratory variability is thought to be desirable and represent a healthy ability to adapt to change (Gutierrez et al. 2013; Wysocki et al. 2006). One possible explanation is that the variability seen in our mice that died from seizures is more profound than that which would be considered healthy variability. Another possibility is that CV IBI alone may not be the best measure to assess respiratory variability. In the future it will be interesting to see if similar findings will be observed in other mouse seizure models.
We also noted in this study that there was a shorter duration of postictal generalized EEG suppression (PGES) following seizures induced during the nighttime compared with seizures induced during the daytime. Ictal increases in brain stem adenosine are thought to be important for seizure discontinuation; however, these increases may also cause severe PGES and respiratory dysregulation (Shen et al. 2010; Ryvlin et al. 2013). Adenosine levels in the brain have a bimodal distribution in rodents over the course of the 24-h day with the highest and longest sustained peak coming during the day (Chagoya de Sánchez 1995). Increases in the baseline levels of adenosine during the day may result in a more severe and longer lasting PGES following a seizure.
Limitations.
From a circadian perspective, one limitation to this work is that we only sampled two time points, one during the light phase and one during the dark phase, although we were quite consistent in the time window during which each animal experienced seizures. To truly understand the contribution of the circadian timing system, it would be better to sample more time points. For instance, perhaps there would be a difference between early and late night as there is for sensitivity of the circadian clock to phase shifting to certain stimuli such as light (Ding et al. 1994), and then perhaps we would uncover statistically significant differences in seizures severity and/or duration, and in survival from a seizure. Similarly, we maintained all animals in a light-dark cycle. Therefore, their circadian clocks were maintained daily by the light/dark clues. To truly assess the animals’ intrinsic clock, it would be more prudent to maintain them in constant darkness and use an output measure such as wheel-running behavior to assess timing with respect to the animals’ individual free-running rhythm (Buchanan and Gillette 2005). We used a nonstandard mouse strain in this study. We have compared some phenotypes in this strain against a more standard inbred strain, C57BL/6J, and believe these mice to perform comparably in our assays. Nevertheless, this should be taken as a caveat. All this being said, our data provide first-pass evidence that time of day can be an independent factor that modulates the impact of a seizure on physiology.
Conclusions.
SUDEP is a devastating outcome that befalls a significant proportion of refractory epilepsy patients. Whereas there is a strong relationship between sleep and epilepsy, and the fact that SUDEP happens more commonly at night could reflect the influence of sleep on epilepsy, there is substantial evidence to support that circadian phase should be considered as an independent risk factor for SUDEP. We provide evidence in one mouse model to support this and demonstrate that this is independent of the effects of vigilance state. More work is needed to truly understand how circadian phase might impact SUDEP in patients.
GRANTS
This work was supported by The Christopher Donalty and Kyle Coggins Memorial SUDEP Research Fund from Citizens United for Research on Epilepsy (to G. F. Buchanan) and National Institutes of Health Grants K08NS069667 (to G. F. Buchanan), R01NS095842 (to G. F. Buchanan), TL1TR000151 (to M. A. Hajek), and T32NS007421 (to B. S. Purnell). G. F. Buchanan is also supported by the Beth and Nate Tross Epilepsy Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.F.B. conceived and designed research; B.S.P., M.A.H., and G.F.B. performed experiments; B.S.P., M.A.H., and G.F.B. analyzed data; B.S.P. and G.F.B. interpreted results of experiments; B.S.P. and G.F.B. prepared figures; B.S.P. and G.F.B. drafted manuscript; B.S.P., M.A.H., and G.F.B. edited and revised manuscript; B.S.P., M.A.H., and G.F.B. approved final version of manuscript.
REFERENCES
- Anderson RE, Howard RA, Woodbury DM. Correlation between effects of acute acetazolamide administration to mice on electroshock seizure threshold and maximal electroshock seizure pattern, and on carbonic anhydrase activity in subcellular fractions of brain. Epilepsia 27: 504–509, 1986. doi: 10.1111/j.1528-1157.1986.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil CW, Walczak TS. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia 38: 56–62, 1997. doi: 10.1111/j.1528-1157.1997.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Buchanan GF. Timing, sleep, and respiration in health and disease. Prog Mol Biol Transl Sci 119: 191–219, 2013. doi: 10.1016/B978-0-12-396971-2.00008-7. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Gillette MU. New light on an old paradox: site-dependent effects of carbachol on circadian rhythms. Exp Neurol 193: 489–496, 2005. doi: 10.1016/j.expneurol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 592: 4395–4410, 2014. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107: 16354–16359, 2010. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagampang FR, Inouye ST. Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res 639: 175–179, 1994. doi: 10.1016/0006-8993(94)91780-9. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Okamura H, Inouye S. Circadian rhythms of norepinephrine in the rat suprachiasmatic nucleus. Neurosci Lett 173: 185–188, 1994. doi: 10.1016/0304-3940(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V. Circadian variations of adenosine and of its metabolism. Could adenosine be a molecular oscillator for circadian rhythms? Can J Physiol Pharmacol 73: 339–355, 1995. doi: 10.1139/y95-044. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, Gornbein J, Harper RM. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav 19: 78–81, 2010. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 15: 1075–1088, 2016. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266: 1713–1717, 1994. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD, Zaveri HP. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 70: 1265–1271, 2008. doi: 10.1212/01.wnl.0000308938.84918.3f. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol Regul Integr Comp Physiol 275: R1127–R1137, 1998. [DOI] [PubMed] [Google Scholar]
- Friedman L, Haines A, Klann K, Gallaugher L, Salibra L, Han F, Strohl KP. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol (1985) 97: 1787–1795, 2004. doi: 10.1152/japplphysiol.01394.2003. [DOI] [PubMed] [Google Scholar]
- Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev 18: 1397–1412, 2004. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner JR, Smith GG, Lenz O, Perron IJ, Buono RJ, Ferraro TN. BMAL1 controls the diurnal rhythm and set point for electrical seizure threshold in mice. Front Syst Neurosci 8: 121, 2014. doi: 10.3389/fnsys.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G, Das A, Ballarino G, Beyzaei-Arani A, Türkan H, Wulf-Gutierrez M, Rider K, Kaya H, Amdur R. Decreased respiratory rate variability during mechanical ventilation is associated with increased mortality. Intensive Care Med 39: 1359–1367, 2013. doi: 10.1007/s00134-013-2937-5. [DOI] [PubMed] [Google Scholar]
- Hajek MA, Buchanan GF. Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol 115: 2286–2293, 2016. doi: 10.1152/jn.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4: 649–661, 2003. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra WA, Grootemarsink BE, Dieker R, van der Palen J, de Weerd AW. Temporal distribution of clinical seizures over the 24-h day: a retrospective observational study in a tertiary epilepsy clinic. Epilepsia 50: 2019–2026, 2009. doi: 10.1111/j.1528-1167.2009.02044.x. [DOI] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest 123: 1798–1808, 2013. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 342: 314–319, 2000. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53: 253–257, 2012. doi: 10.1111/j.1528-1167.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- Levine JD, Rosenwasser AM, Yanovski JA, Adler NT. Circadian activity rhythms in rats with midbrain raphe lesions. Brain Res 384: 240–249, 1986. doi: 10.1016/0006-8993(86)91160-1. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Nei M, Raghavan M, Sperling M, Zonjy B, Lacuey N, Devinsky O. Nonseizure SUDEP: sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 57: 1161–1168, 2016. doi: 10.1111/epi.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal JA, Wu S, Rose S, Ebersole JS, Tao JX. Association of prone position with sudden unexpected death in epilepsy. Neurology 84: 703–709, 2015. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab 60: 1210–1215, 1985. doi: 10.1210/jcem-60-6-1210. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Lockley SW, Kaleyias J, Kothare SV. Chronobiology of epilepsy: diagnostic and therapeutic implications of chrono-epileptology. J Clin Neurophysiol 28: 146–153, 2011a. doi: 10.1097/WNP.0b013e31821213d4. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Vendrame M, Zarowski M, Gregas M, Alexopoulos AV, Wyllie E, Kothare SV. Circadian patterns of pediatric seizures. Neurology 76: 145–153, 2011b. doi: 10.1212/WNL.0b013e318206ca46. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Pévet P, Raison S. Circadian change in tryptophan hydroxylase protein levels within the rat intergeniculate leaflets and raphe nuclei. Neuroscience 125: 749–758, 2004. doi: 10.1016/j.neuroscience.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Malow BA, Lin X, Kushwaha R, Aldrich MS. Interictal spiking increases with sleep depth in temporal lobe epilepsy. Epilepsia 39: 1309–1316, 1998. doi: 10.1111/j.1528-1157.1998.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol 450: 629–642, 1992. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Gillespie CF, Marvel CL, Huhman KL, Albers HE. Serotonergic regulation of circadian rhythms in Syrian hamsters. Neuroscience 79: 563–569, 1997. doi: 10.1016/S0306-4522(96)00696-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin LP, Michels KM, Smale L, Moore RY. Serotonin regulation of circadian rhythmicity. Ann N Y Acad Sci 600: 418–426, 1990. doi: 10.1111/j.1749-6632.1990.tb16898.x. [DOI] [PubMed] [Google Scholar]
- Nashef L, Garner S, Sander JW, Fish DR, Shorvon SD. Circumstances of death in sudden death in epilepsy: interviews of bereaved relatives. J Neurol Neurosurg Psychiatry 64: 349–352, 1998. doi: 10.1136/jnnp.64.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev 15: 237–246, 2011. doi: 10.1016/j.smrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Quay WB. Differences in circadian rhythms in 5-hydroxytryptamine according to brain region. Am J Physiol 215: 1448–1453, 1968. [DOI] [PubMed] [Google Scholar]
- Quigg M, Straume M, Menaker M, Bertram EH 3rd. Temporal distribution of partial seizures: comparison of an animal model with human partial epilepsy. Ann Neurol 43: 748–755, 1998. doi: 10.1002/ana.410430609. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52, Suppl 1: 28–38, 2011. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 12: 966–977, 2013. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci 10: 3685–3694, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia 54: 377–382, 2013. doi: 10.1111/j.1528-1167.2012.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia 51: 465–468, 2010. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J 127: 1376–1381, 1994. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm M, Becker A, Schroeder A, Bilkei-Gorzo A, Zimmer A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav 14: 292–300, 2015. doi: 10.1111/gbb.12208. [DOI] [PubMed] [Google Scholar]
- Tao JX, Qian S, Baldwin M, Chen XJ, Rose S, Ebersole SH, Ebersole JS. SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia 51: 2344–2347, 2010. doi: 10.1111/j.1528-1167.2010.02719.x. [DOI] [PubMed] [Google Scholar]
- Tao JX, Sandra R, Wu S, Ebersole JS. Should the “Back to Sleep” campaign be advocated for SUDEP prevention? Epilepsy Behav 45: 79–80, 2015. doi: 10.1016/j.yebeh.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed 113: 210–220, 2014. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Wolf S, Hainz N, Beckmann A, Maack C, Menger MD, Tschernig T, Meier C. Brain damage resulting from postnatal hypoxic-ischemic brain injury is reduced in C57BL/6J mice as compared to C57BL/6N mice. Brain Res 1650: 224–231, 2016. doi: 10.1016/j.brainres.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Wysocki M, Cracco C, Teixeira A, Mercat A, Diehl JL, Lefort Y, Derenne JP, Similowski T. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med 34: 2076–2083, 2006. doi: 10.1097/01.CCM.0000227175.83575.E9. [DOI] [PubMed] [Google Scholar]
- Yannielli P, Harrington ME. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog Neurobiol 74: 59–76, 2004. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Zarowski M, Loddenkemper T, Vendrame M, Alexopoulos AV, Wyllie E, Kothare SV. Circadian distribution and sleep/wake patterns of generalized seizures in children. Epilepsia 52: 1076–1083, 2011. doi: 10.1111/j.1528-1167.2011.03023.x. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW IV, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26: 12781–12788, 2006. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]