Abstract
Infusion reaction is an adverse event of therapeutic monoclonal antibodies. Nivolumab, an anti‐programmed death‐1 antibody, directly activates T cells, which could probably interact with endothelial cells. The etiology of infusion reaction induced by nivolumab may differ from that of other antibodies; however, the detailed clinical features are unknown. We report a case of lung cancer treated with nivolumab, in which the infusion reaction manifested as plantar erythema, followed by a transient local pulmonary infiltrate around the tumor. Physicians should be aware that an infusion reaction induced by anti‐programmed death‐1 antibodies could appear as local cutaneous and pulmonary adverse events.
Keywords: Hand‐foot skin reaction, immunotherapy, infusion reaction, pulmonary infiltrate
Introduction
Nivolumab, an anti‐programmed death‐1 (PD‐1) antibody, induces an infusion reaction, which usually occurs after the administration of monoclonal antibodies.1 The mechanism of a monoclonal antibody‐induced infusion reaction has been proposed to be a result of cytokine release precipitated by an antibody–antigen interaction.2, 3 Although nivolumab belongs to the monoclonal antibody class, this anti‐PD1 antibody directly activates T‐cells, which could probably interact with endothelial cells.4, 5 The clinical features of a nivolumab‐induced infusion reaction may differ from those seen in other monoclonal antibodies. However, no studies have examined the clinical features of nivolumab‐induced infusion reactions. Herein, we report a lung cancer case in which a nivolumab‐induced infusion reaction unusually presented as plantar erythema and local pulmonary infiltrate.
Case report
A 68‐year‐old male current smoker was referred to our hospital for investigation of a lung nodule on chest radiography. A chest computed tomography scan showed a 9 cm solid nodule in the right upper lobe, with right mediastinal lymphadenopathy and right‐sided pleural effusion. He was diagnosed with stage IV lung squamous cell carcinoma by systemic survey and transbronchial biopsy (Fig 1a). Immunohistochemical examination showed that 10% of the tumor cells were positive for PD‐ligand 1 (PD‐L1) (Fig 1b), and that tumor‐infiltrating mononuclear cells expressing PD‐1 were scattered in the stroma and within the tumor (Fig 1c). SP142 and SP269 clones were used for staining as anti‐PD‐L1 and anti‐PD‐1 antibodies (Spring Bioscience, Pleasanton, CA, USA), respectively. The patient received cytotoxic chemotherapy with cisplatin/gemcitabine followed by docetaxel and S‐1.
Figure 1.
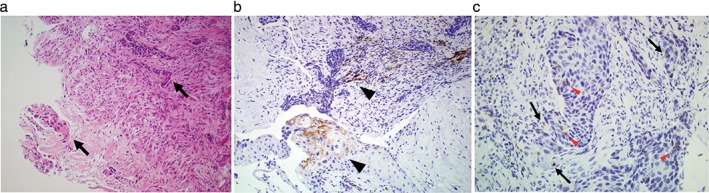
Histologic findings of the transbronchial biopsy of the lung tumor. (a) Cancer cell nest and fascicular invasion of squamous‐cell carcinoma (arrows) are seen in the stroma (hematoxylin & eosin stain, original magnification 200×). Immunohistochemical analysis revealed that (b) 10% of the cancer cells (arrowheads) were heterogeneously positive for programmed cell death ligand 1 (PD‐L1) (SP142 clone stain, original magnification 200×) and (c) tumor‐infiltrating mononuclear cells expressing programmed cell death‐1 (PD‐1) are scattered in the stroma (arrows) and within the tumor (arrowheads; SP269 clone stain, original magnification 400×).
Fourteen months after the lung cancer diagnosis, the tumor progressed and treatment was revised to 3 mg/kg nivolumab. After a few days, the patient complained of a skin rash and painful itching on both soles. Examination by a dermatologist revealed erythema on the bilateral soles and small bullous lesions on the sides of the feet (Fig 2a), which were clinically diagnosed as a hand‐foot skin reaction. Seven days after topical corticosteroid treatment, the skin erythema resolved and the bullous lesions erupted (Fig 2b).
Figure 2.
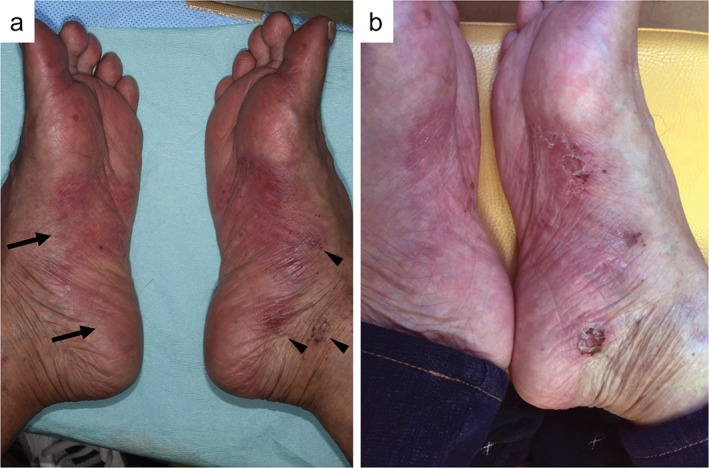
(a) Five days after the first nivolumab infusion, erythema (arrows) with small bullous lesions (arrowheads) were observed in both soles. (b) Seven days after treatment with topical corticosteroid, the erythema improved and the bullous lesions erupted.
On the 16th day, the patient was scheduled to receive a second infusion of nivolumab. Unexpectedly, 15 minutes after the injection, he noticed skin itching on the back of his head and skin flushing that immediately spread all over his body. Oxygen saturation decreased from 97% to 92%. Nivolumab infusion was interrupted and nasal oxygen inhalation, chlorpheniramine, and methylprednisolone were administered. Chest radiography revealed new infiltrates in the right upper lung field adjacent to the cancer lesions (Fig 3a,b). Two hours later, the skin rash had almost resolved. The next day, the focal pulmonary infiltrate had disappeared on chest radiography (Fig 3c) and oxygen saturation at room air was restored to 97%. There were no indications of infectious disease or pneumonitis.
Figure 3.
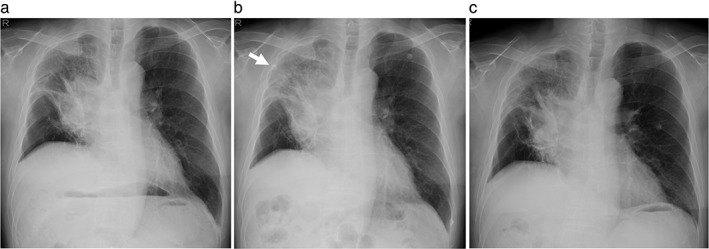
Chest radiograph images during the second nivolumab infusion. (a) Before nivolumab treatment, a lung tumor in the hilar portion of the right upper lobe is observed. (b) One hour after the nivolumab injection, an acute pulmonary infiltrate (arrow) in the upper lung field appeared adjacent to the lung cancer lesion. (c) The pulmonary infiltrate is no longer seen the day after nivolumab administration.
The patient underwent stepwise skin prick, scratch, and intradermal tests for nivolumab. All skin tests for nivolumab were negative. Therefore, anaphylaxis was ruled out and the patient was diagnosed as having had an infusion reaction. Nivolumab treatment was discontinued and three weeks after the second administration of nivolumab, carboplatin and albumin‐bound paclitaxel were commenced. The tumor rapidly regressed and a partial response was achieved two months after treatment (Fig 4). His lung cancer remained progression‐free for five months. Written informed consent for the publication of this case report was obtained from the patient.
Figure 4.
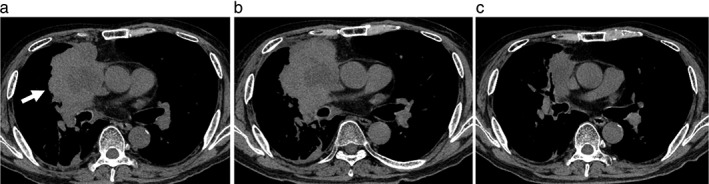
Chest computed tomography scans during nivolumab treatment. (a) Before treatment with nivolumab, a 90 mm lung tumor (arrow) is observed in the right upper lobe. (b) The lung tumor enlarged after two courses of nivolumab therapy. (c) After two courses of carboplatin and albumin‐bound paclitaxel, the lung tumor regressed to 55 mm in diameter.
Discussion
Our case highlights two important issues. The first issue was that a nivolumab‐induced infusion reaction may initially manifest as plantar erythema as a result of a hand‐foot‐skin reaction. We can assume some possible mechanisms of a nivolumab‐induced hand‐foot skin reaction, which is frequently observed during treatment with multi‐kinase inhibitors such as sorafenib. Multi‐kinase inhibitors have been reported to inhibit platelet‐derived growth factor receptor and vascular endothelial growth factor receptor on endothelial cells.6 Nivolumab possibly involves a similar mechanism and has a predilection for endothelial cells in the small vessels in the soles, which are damaged by frequent pressure or friction. Impaired endothelial cells can present antigens to T cells and express PD‐L1 on the surface in order to evade the immune response.4, 5, 7, 8 The anti‐PD‐L1 antibody overcomes this immune evasion and the activated lymphocytes recognize and target endothelial antigens.4
The second issue this case presented was the possibility of transient local pulmonary infiltrates adjacent to the cancer lesions after a second administration of nivolumab. These localized infiltrates were consistent with the manifestations of pulmonary edema. PD‐1 knockout mice had increased permeability of the damaged capillaries, resulting in pulmonary edema.9 In our patient, the pulmonary infiltrate was limited in the field adjacent to the lung cancer lesions. Because PD‐L1 was expressed on the lung cancer cells in this patient, nivolumab may have gathered the activated lymphocytes in the lung lesions. In this patient, lung cancer showed a drastic response to chemotherapy following nivolumab. The outcome suggested that activated lymphocytes may induce the appearance of local pulmonary infiltrates as an infusion reaction, as well as maximizing the efficacy of chemotherapy.10
One limitation regarding the clinical course of our patient needs to be considered. The reason for the hand‐foot skin reaction preceding the systemic reaction remains undetermined. A study of a cancer patient receiving sorafenib reported a similar pattern of hand‐foot skin reaction as an initial manifestation of Stevens–Johnson syndrome with diffuse pulmonary ground‐glass opacities.11 To explain these results, further studies that examine the interaction between T‐cell activation and endothelial function are required.
In conclusion, physicians should be aware that infusion reactions induced by anti‐PD‐1 antibodies potentially appear as local cutaneous and pulmonary adverse events, in addition to systemic reactions.
Disclosure
No authors report any conflict of interest.
References
- 1. Eigentler TK, Hassel JC, Berking C et al Diagnosis, monitoring and management of immune‐related adverse drug reactions of anti‐PD‐1 antibody therapy. Cancer Treat Rev 2016; 45: 7–18. [DOI] [PubMed] [Google Scholar]
- 2. Dillman RO. Infusion reactions associated with the therapeutic use of monoclonal antibodies in the treatment of malignancy. Cancer Metastasis Rev 1999; 18: 465–71. [DOI] [PubMed] [Google Scholar]
- 3. Doessegger L, Banholzer ML. Clinical development methodology for infusion‐related reactions with monoclonal antibodies. Clin Transl Immunology 2015; 4: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodig N, Ryan T, Allen JA et al Endothelial expression of PD‐L1 and PD‐L2 down‐regulates CD8+ T cell activation and cytolysis. Eur J Immunol 2003; 33: 3117–26. [DOI] [PubMed] [Google Scholar]
- 5. Rouhani SJ, Eccles JD, Riccardi P et al Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T‐cell tolerance induction. Nat Commun 2015; 6: 6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008; 19: 1955–61. [DOI] [PubMed] [Google Scholar]
- 7. Eppihimer MJ, Gunn J, Freeman GJ et al Expression and regulation of the PD‐L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002; 9: 133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tewalt EF, Cohen JN, Rouhani SJ et al Lymphatic endothelial cells induce tolerance via PD‐L1 and lack of costimulation leading to high‐level PD‐1 expression on CD8 T cells. Blood 2012; 120: 4772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frebel H, Nindl V, Schuepbach RA et al Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med 2012; 209: 2485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daste A, de Mones E, Digue L et al Immunotherapy in head and neck cancer: Need for a new strategy? Rapid progression with nivolumab then unexpected response with next treatment. Oral Oncol 2017; 64: e1–3. [DOI] [PubMed] [Google Scholar]
- 11. Ohtake S, Onuki T, Kato Y, Ohta J, Moriyama M. [A patient of progressive renal cell carcinoma with interstitial pneumonia and Stevens‐Johnson syndrome in the course of sorafenib treatment.] Hinyoki Geka 2013; 26: 387–91. (In Japanese.) [Google Scholar]


