Maintenance therapy after allogeneic hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) is conceptually attractive to prevent relapse, but has been hampered by the limited number of suitable anti-leukemic agents. The deacetylase inhibitor (DACi) panobinostat demonstrated moderate anti-leukemic activity in a small subset of patients with advanced AML and high-risk MDS in phase I/II trials.1, 2 It also displays immunomodulatory activity3 that may enhance leukemia-specific cytotoxicity4 and mitigate graft versus host disease (GvHD), but conversely could impair T- and NK cell function.5, 6 We conducted this open-label, multi-center phase I/II trial (NCT01451268) to assess the feasibility and preliminary efficacy of prolonged prophylactic administration of panobinostat after HSCT for AML or MDS. The study protocol was approved by an independent ethics committee and conducted in compliance with the Declaration of Helsinki. All patients provided written informed consent.
Patient eligibility and study design are summarized in Supplementary Figure S1. Briefly, between January 2011 and January 2015, 42 patients (37 AML, 5 MDS) were enrolled at a median of 96 days (60–147) post HSCT. Patients had to be in complete hematologic remission (CR) post HSCT, and fulfill one or more of the following criteria: (i) AML refractory or with delayed response to or relapsed after greater than or equal to one cycle of standard chemotherapy; (ii) adverse risk cytogenetics; (iii) secondary to MDS or radio/chemotherapy; (iv) MDS intermediate-2 or high risk according to international prognostic scoring system or MDS refractory anemia with excess blasts (WHO classification). At transplant, 67% of patients (n=28) had active disease (bone marrow blasts 8–80%, median 21%, 1 patient with isolated extramedullary AML), 9 were in CR1 (21%) and 5 in CR2 (12%).
Primary objective of the phase I part was determination of the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of panobinostat, given orally thrice weekly (TIW) in one of two sequentially tested administration schedules: weekly (schedule A; starting dose 10 mg) or every other week (schedule B; starting dose 20 mg) using a 3+3 design. DLT was determined separately in both schedules during the first 28 days of panobinostat treatment, which was scheduled for up to 1 year. In phase 2, patients were randomized 1:1 to schedule A or B at the respective MTD.
Patient and transplant characteristics were equally distributed between both schedules (Supplementary Table S1). Median age was 52 (21–71) years and eastern cooperative group performance status either 0 (57%) or 1 (43%). Patient disposition is outlined in Supplementary Figure S2. All 12 patients in the phase 1 part of schedule A and 11 of 12 patients in schedule B were evaluable for MTD. Five DLTs were observed, three in schedule A (fatigue G3 at 20 mg, colitis and nausea/emesis G3 at 30 mg in one patient each) and two in schedule B (diarrhea and headache G3 at 40 mg in one patient each). One patient discontinued study treatment after three doses of panobinostat (schedule B, 20 mg TIW) because of G2 electrocardiogram alterations; this patient was not evaluable for DLT and was replaced. The MTDs for schedules A (weekly) and B (every other week) were 20 mg and 30 mg TIW, respectively, and were selected as recommended phase 2 dose. These MTDs resemble those in patients with myeloma requiring prolonged therapy.7
All patients were analyzed for safety. Thirty-five of 42 patients (83%) experienced at least one G3/4 adverse event (AE), considered panobinostat-related in 22 patients (52%). Rates of G3/4 AEs did not differ significantly between schedules (A: n=12, 57% B: n=10, 48%). All panobinostat-related G3/4 AEs and G1/2 AEs that constituted DLTs or triggered dose reductions are listed in Table 1. Panobinostat-related AEs were fully and rapidly reversible after interrupting panobinostat. Thrombocytopenia G3/4 was observed in both treatment schedules (A: n=6, 28% B: n=4, 19%); in schedule B, platelet counts recovered to baseline values by day 15. Clinically relevant constitutional symptoms were observed only with schedule A. No patient died on treatment or within 28 days of the last panobinostat dose, and 10 patients died post study (relapse n=6, sepsis n=1, severe chronic GvHD n=1, relapse of pre-existing lung cancer n=1 and sudden death 3.5 months after study discontinuation n=1).
Table 1. Adverse events considered related to panobinostat by treatment schedule and initial dose cohort.
| Panobinostat-related toxicity |
Arm A, n (%) |
Arm B, n (%) |
||||
|---|---|---|---|---|---|---|
| G1 and 2 | G3 | G4 | G1 and 2 | G3 | G4 | |
| Blood/bone marrow | ||||||
| Leukocytopenia | 0 | 2 (10) | 0 | 0 | 2 (10) | 0 |
| Neutropenia | 0 | 1 (5) | 1 (5) | 0 | 4 (19) | 0 |
| Thrombocytopenia | 0 | 5 (24) | 1 (5) | 0 | 3 (14) | 1 (5) |
| Anemia | 0 | 2 (10) | 0 | 0 | 0 | 0 |
| Cardiac | 1 (5) | 0 | 0 | 1 (5) | 0 | 0 |
| Constitutional symptoms | ||||||
| Fatigue | 2 (10) | 4 (19) | 0 | 0 | 0 | 0 |
| Weight loss | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal symptoms | ||||||
| Nausea/vomiting | 4 (19) | 1 (5) | 0 | 3 (14) | 0 | 0 |
| Diarrhea | 3 (14) | 1 (5) | 0 | 1 (5) | 2 (10) | 0 |
| Colitis | 0 | 1 (5) | 0 | 0 | 0 | 0 |
| Anorexia | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Oral mucositis | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| Taste alteration | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| Pain | 1 (5) | 1 (5) | 0 | 1 (5) | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 1 (5) | 0 |
| Renal failure | 0 | 1 (5) | 0 | 0 | 0 | 0 |
| Rash | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| Sensory neuropathy | 0 | 0 | 0 | 0 | 1 (5) | 0 |
| Metabolic/laboratory | ||||||
| Elevated liver function tests | 0 | 0 | 0 | 2 (10) | 2 (10) | 0 |
| Creatinine increased | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Hyperuricemia | 0 | 1 (5) | 0 | 0 | 0 | 0 |
The most common G3/4 AEs irrespective of causality are listed in Supplementary Table S2. Twelve patients (29%) developed AEs that led to permanent discontinuation of panobinostat after a median of 30 days (7–293). Previous studies showed that tolerability and hematologic AEs differed by schedule of panobinostat administration and that the MTD was not necessarily compatible with prolonged administration.2, 8, 9 In our study, 22 patients (52%) received panobinostat for 1 year as scheduled (A: 10/21 patients, 52 vs B: 12/21, 57%), and seven of these (17%) required no dose interruptions or reductions. Reasons for premature discontinuation of study drug were AEs (n=12), relapse (n=5), patient decision (n=2) or prohibited co-medication (n=1). Of 27 patients treated at the MTD, 15 (55%) discontinued early after a median of 47 days (11–172); additional 4 patients (15%) required dose reductions. Median duration of treatment at the MTD was 52 days (range, 11–368) in schedule A versus 228 days (16–365) in schedule B (P=0.34). Alternating week administration resulted in delivery of a higher cumulative dose at the MTD and was more compatible with long-term administration. In comparison, in studies of post-transplant maintenance with hypomethylating agent, only 20–43% of patients received all scheduled cycles of hypomethylating agent (summarized in Brunner et al.10).
Donor lymphocyte infusions (DLIs) were permitted by the study protocol at the discretion of the treating physician. Eighteen patients (43% in schedule A and B) received a median of two DLIs (1–6), and initiated a median of 88 days (49–317) and 104 days (50–231) after the first panobinostat dose (median 0.2 × 106 and 0.9 × 106 CD3+ cells per kg body weight, respectively). Only 4/42 patients developed acute GvHD on study (G1, n=1; G3, n=3), all in schedule A. Of note, the proportion of regulatory T cells decreased with schedule A, while remaining stable in schedule B (Supplementary Figure S3). Cumulative incidence of moderate (n=10) or severe (n=2) chronic GvHD was 29% (95% confidence interval (CI), 16–42%) at 2 years after starting panobinostat and did not differ between schedules.
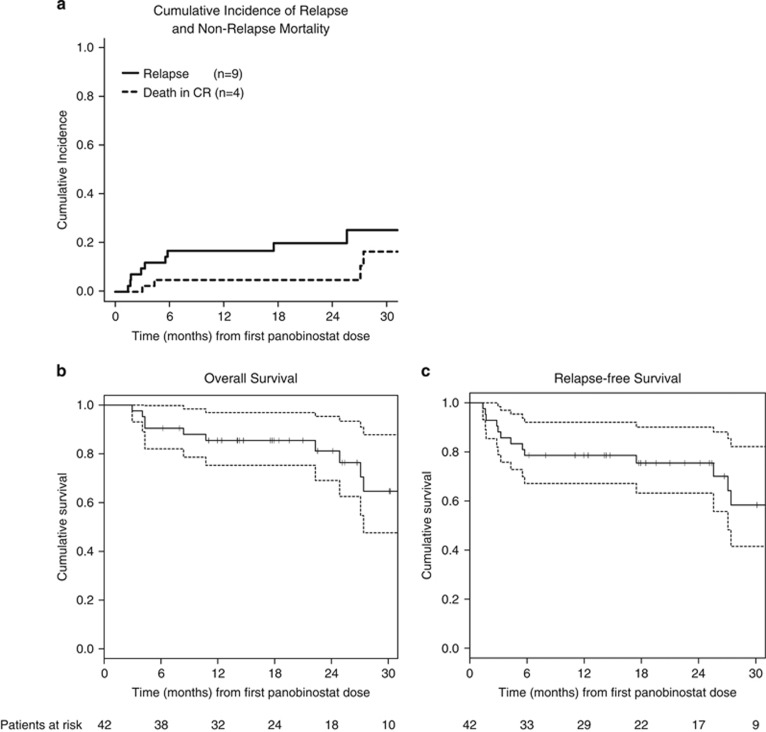
At 2 years after the first panobinostat dose, the cumulative incidence of relapse and non-relapse mortality across all dose levels was 20% (95% CI, 7–33%) and 5% (95% CI, 0–11%, Figure 1a). Thus, the low relapse rate observed in the PANOBEST trial was not offset by a higher than expected incidence of clinically significant chronic GvHD even among patients receiving additional DLI, suggesting that panobinostat does not impair development of peripheral tolerance and may actually mitigate GvHD. These data are consistent with a phase I/II study of short-term peri-transplant vorinostat showing a significantly lower incidence of acute GvHD greater than or equal to G2 compared to historical controls.11
Figure 1.
(a) Cumulative incidence of relapse and non-relapse mortality. (b) Overall survival. (c) Relapse-free survival. Kaplan–Meier curves are shown for all patients enrolled and calculated from the first dose of panobinostat. Symbols represent censoring times.
To date, median overall survival (OS) and relapse free survival have not been reached after a median follow-up of 22 months (range, 6–57) (Figures 1b and c). Probabilities of 2-year OS and relapse free survival are 81% (95% CI, 69–95%) and 75% (95% CI, 63–90%), respectively. In view of the median time to relapse (4–6 months after HSCT), the time from HSCT to starting panobinostat (median 96 days, 60–147) may have introduced a positive selection bias and led to under-representation of patients with very aggressive AML. Nevertheless, outcome in our high-risk AML and MDS population compares favorably with survival rates and cumulative incidence of relapse (exceeding 30–60% at 2–3 years) reported for similar patient cohorts.12, 13, 14, 15 The definite role of panobinostat maintenance after HSCT for high-risk myeloid malignancies will be determined in a large European randomized trial, using the alternate week dosing regimen found to be better tolerated in the present trial.
Acknowledgments
This study was supported by Novartis Oncology, the Alfred und Angelika-Gutermuth-Stiftung, the Verein Knochenmarktransplantation/Gentherapie Frankfurt (KGF), and the LOEWE Zentrum für Zell- und Gentherapie. OGO held a Stiftungsprofessur für Molekulare Therapieforschung der Deutschen José Carreras Leukämie-Stiftung (R09/11). Novartis Oncology also provided the study drug panobinostat.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
GB and OGO: honoraria for advisory board participation from Novartis; GB and NK: research support from Novartis; GB: travel grants from Novartis; the remaining authors declare no conflict of interest.
Supplementary Material
References
- Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 2006; 12: 4628–4635. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia 2013; 27: 1628–1636. [DOI] [PubMed] [Google Scholar]
- Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 2014; 13: 673–691. [DOI] [PubMed] [Google Scholar]
- West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014; 124: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 2007; 13: 1299–1307. [DOI] [PubMed] [Google Scholar]
- Hancock WW, Akimova T, Beier UH, Liu Y, Wang L. HDAC inhibitor therapy in autoimmunity and transplantation. Ann Rheum Dis 2012; 71 (Suppl 2): i46–i54. [DOI] [PubMed] [Google Scholar]
- San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol 2014; 15: 1195–1206. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol 2013; 162: 326–335. [DOI] [PubMed] [Google Scholar]
- Ocio EM, Herrera P, Olave MT, Castro N, Perez-Simon JA, Brunet S et al. Panobinostat as part of induction and maintenance for elderly patients with newly diagnosed acute myeloid leukemia: phase Ib/II panobidara study. Haematologica 2015; 100: 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Fathi AT, Chen YB. Life after transplant: are we becoming high maintenance in AML? Bone Marrow Transplant 2016; 51: 1423–1430. [DOI] [PubMed] [Google Scholar]
- Choi SW, Braun T, Chang L, Ferrara JL, Pawarode A, Magenau JM et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol 2014; 15: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from cancer and leukemia group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol 2015; 33: 4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25: 808–813. [DOI] [PubMed] [Google Scholar]
- Cornelissen JJ, Breems D, van Putten WL, Gratwohl AA, Passweg JR, Pabst T et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol 2012; 30: 2140–2146. [DOI] [PubMed] [Google Scholar]
- Middeke JM, Fang M, Cornelissen JJ, Mohr B, Appelbaum FR, Stadler M et al. Outcome of patients with abnl(17p) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Blood 2014; 123: 2960–2967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.