Abstract
Chronic myeloid leukemia (CML)-study IV was designed to explore whether treatment with imatinib (IM) at 400 mg/day (n=400) could be optimized by doubling the dose (n=420), adding interferon (IFN) (n=430) or cytarabine (n=158) or using IM after IFN-failure (n=128). From July 2002 to March 2012, 1551 newly diagnosed patients in chronic phase were randomized into a 5-arm study. The study was powered to detect a survival difference of 5% at 5 years. After a median observation time of 9.5 years, 10-year overall survival was 82%, 10-year progression-free survival was 80% and 10-year relative survival was 92%. Survival between IM400 mg and any experimental arm was not different. In a multivariate analysis, risk group, major-route chromosomal aberrations, comorbidities, smoking and treatment center (academic vs other) influenced survival significantly, but not any form of treatment optimization. Patients reaching the molecular response milestones at 3, 6 and 12 months had a significant survival advantage. For responders, monotherapy with IM400 mg provides a close to normal life expectancy independent of the time to response. Survival is more determined by patients’ and disease factors than by initial treatment selection. Although improvements are also needed for refractory disease, more life-time can currently be gained by carefully addressing non-CML determinants of survival.
Introduction
Chronic myeloid leukemia (CML)-study IV was designed to explore whether treatment with imatinib (IM) at a dose of 400 mg/day as used in the International Randomized Study on Interferon (IFN) and STI571 (IRIS)1, 2 could be improved by doubling the dose or by combining IM with IFN or cytarabine. Primary goals were the comparative response and long-term survival analyses of the experimental arms vs IM400 mg. Molecular monitoring of all patients was an integral part of the study from the beginning. The study has generated new insights in the relevance of molecular monitoring,3, 4 of comorbidities,5 additional chromosomal aberrations6, 7 and deep molecular response.8 CML-study IV has also shown that IM at 800 mg results in significantly earlier cytogenetic and molecular responses than IM400 mg.3, 8 Various observational and randomized studies have tried to improve IM-treatment by combination with IFN, cytarabine or a dose increase to 600 or 800 mg9, 10, 11, 12, 13, 14, 15, 16 and have achieved earlier and deeper responses. In no instance a better survival was reported after median observation periods up to 3.5 years. Two studies have compared survival with IM400 mg and 2nd generation tyrosine kinase inhibitors (2G-TKI). After 5 years, 2G-TKI showed earlier and deeper responses than IM400 mg, but no survival advantage.17, 18 CML-study IV was powered to detect a 5% survival difference after 5 years. We here report survival outcome after a median observation time of close to 10 years.
Patients and methods
Study design and treatment strategy have been published previously.3, 8 In brief, newly diagnosed CML patients in chronic phase (CP) were randomized into a 5-arm study comparing IM400 mg/day vs IM400 mg/day in combination with IFN vs IM400 mg/day in combination with low-dose cytarabine vs IM400 mg/day after IFN-failure vs IM800 mg/day. Recruitment was from July 2002 through March 2012. There was no upper age limit. Exclusion criteria were pretreatment except with hydroxyurea or anagrelide, no consent, pregnancy, participation in another study, second neoplasia and serious illness that made per protocol participation a priori unlikely. Only low- and intermediate-risk patients were randomized to primary IFN and, during a pilot-phase of 3 years, only high-risk patients to IM800 mg/day. After 3 years, recruitment to IM plus cytarabine and IM after IFN-failure was terminated, and the IM800 mg/day arm started to include non-high-risk patients, too. Data lock was on 19 September 2016.
Initial treatment in all study arms except IM-after-IFN-failure was IM400 mg once daily. If no complete hematologic remission was reached after 2 months or no partial cytogenetic remission (PCyR) after 6 months, a dose increase was permitted. If IM-treatment failed, stem-cell transplantation or risk-adapted drug treatment (hydroxyurea, cytarabine, intensive chemotherapy) was recommended - depending on type of mutation and degree of proliferation or progression. After availability, either dasatinib or nilotinib was recommended. Participation of IM-resistant or intolerant patients in the dasatinib and nilotinib phase II studies was permitted. The first patient was switched to 2G-TKI (dasatinib) on 30 March, 2005.
IFN, subcutaneous cytarabine and the full 800 mg/day dose were administered after a 6-week run-in period with IM 400 mg/day to avoid cytopenias.8 The IM-dose could be reduced according to tolerability.
Initial primary goal of CML-study IV were comparative response probabilities. Long-term primary goal was comparative survival (study protocol in the Supplementary Appendix). The strategy was to give more intensive treatment early since this has improved outcome.19
Definitions and end points
Definitions followed the ELN (European LeukemiaNet) recommendations.20, 21 Risk assignment was made according to Euro-score.22 IFN-failure was defined as no complete hematologic remission after 6 months or not at least PCyR after 21 months, loss of complete hematologic remission or complete cytogenetic remission, or higher-grade AE. Overall survival (OS) was defined as the time between diagnosis and death resulting from any cause. Progression-free survival (PFS) considered the additional events accelerated phase and blast crisis (BC). Death unrelated to CML was defined as death without prior progression and unrelated to CML-therapy. Death due to CML was stratified according to the European treatment and outcome study (EUTOS)-long-term-survival (ELTS) score.23 All living patients were censored at the time of their last visit. When estimating the cumulative incidences of molecular remissions, patients were censored when they received a 2G-TKI. No patient was removed from the study except at patient’s request (n=14).
Cytogenetic and molecular analyses
Cytogenetic and molecular diagnostics were performed as described.6 Testing for residual BCR-ABL1 transcripts24, 25 was done in two standardized and accredited laboratories with defined conversion factors for equivalence of tests (Mannheim and MLL Munich). Confirmed MR4, MR4.5 and MR5 were defined as a reduction of residual BCR-ABL1 transcripts of ⩾4, ⩾4.5 and ⩾5 logs compared with the standardized baseline in two consecutive analyses.24, 25 Testing was restricted to patients expressing b2a2 and/or b3a2 transcripts. For a negative quantitative reverse-transcription polymerase chain reaction, the number of ABL1 transcripts used for nested PCR had to be ⩾10 000 for MR4, ⩾32 000 for MR4.5 and ⩾100 000 for MR5.
Mutation analysis was performed according to the ELN recommendations.26
Sample size estimation
At first, differences in probability of MMR at 12 months were investigated.3 If the null hypothesis of equal probabilities could be rejected, OS differences between IM400 mg and IM800 mg were examined. Assuming an alpha=0.05, a 5-year recruitment, and an additional 5-year follow-up, it would be possible to identify a survival difference with a power of at least 80%, if patients in the IM400 mg arm had a 5-year survival probability of 90% and in the IM800 mg arm of at least 95% or not more than 84%, and if n=400 patients were randomized to each arm. Exponential distribution was assumed and survival probabilities were compared with the log-rank test.27, 28
Statistical analyses
OS and PFS were analyzed using Kaplan–Meier curves and log-rank tests. To estimate relative survival, OS probabilities were adjusted by survival probabilities of matched German population data from the Human Mortality Database for each year of diagnosis in CML-study IV29 with regard to sex and individual age at diagnosis.30 Cumulative incidences were calculated under consideration of competing risks31 of death defined by accelerated phase, BC and death from any cause. Comparisons between cumulative incidences were performed by the Gray test32 and prognostic impact of remissions determined by landmark analyses.33 Besides the cumulative incidences of molecular responses, all analyses were by intention to treat. Level of significance was 0.05 two sided. For estimation of relative survival probabilities software R (version 3.0.3.3, GNU General Public License, R Foundation, Vienna, Austria) was applied.34 All other calculations were performed with SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Ethics
The protocol followed the Declaration of Helsinki and was approved by the ethics committees of the Medizinische Fakultät Mannheim and of participating centers. Written informed consent was obtained from all patients before randomization.
Results
Patients
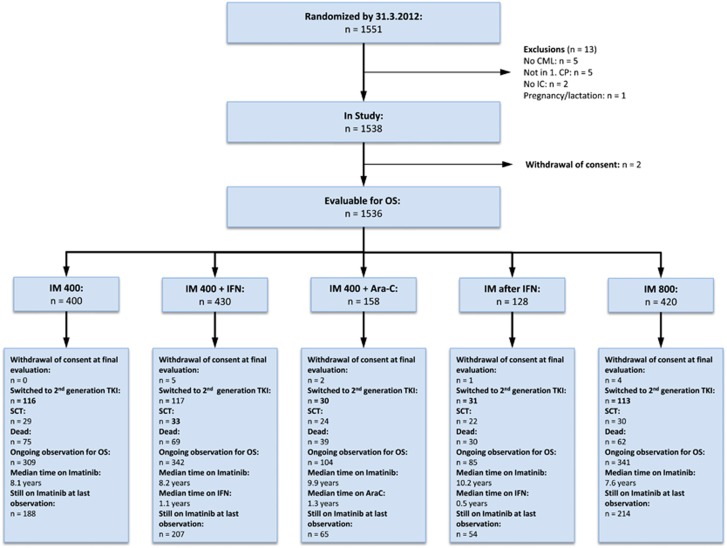
From July 2002 to March 2012, 1551 newly diagnosed CML patients in CP were randomized, 1536 were evaluable, 400 for IM400 mg, 430 for IM plus IFN, 158 for IM plus cytarabine, 128 for IM after IFN and 420 for IM800 mg. Patients were recruited by 210 centers in Germany, Switzerland and the Czech Republic. Patients’ characteristics are shown in Table 1. Median age was 53 years, 60% of patients were male. Euro score was low-risk in 36%, intermediate in 52% and high-risk in 12% of patients. In the arm IM plus IFN, IFN was added to IM400 mg for a median of 1.1 years. After 10 years, six patients still received IFN. In the IM after IFN-failure arm, the median time on IFN monotherapy was 0.5 years. After 10 years, one patient still continued in remission on IFN monotherapy. The median time on low-dose cytarabine was 1.3 years. The main reason for discontinuation of IFN and cytarabine was intolerance. In the IM800 mg arm, the dose could be reduced according to tolerability, the median IM-dose declined from a maximum of 645 mg/day in the 2nd quarter of year 1 to 400 (200–800) mg/day in year 4. The median dose in the IM400 mg arm was 400 (200–800) mg/day with a dose increase reported in 86 patients. Median observation time was 9.5 years (11.8 years for IM plus cytarabine and IM after IFN and 8.3 years for IM800 mg). The flow of patients in the five study arms is shown in Figure 1. At the last evaluation, at least 728 of 1181 patients under observation (62%) still received IM.
Table 1. Patients’ characteristics.
| n | Imatinib 400 | Imatinib+IFN | Imatinib+AraC | Imatinib after IFN | Imatinib 800 | Total | |
|---|---|---|---|---|---|---|---|
| Age (years), median (range) | 1538 | 53 (16–88) | 53 (16–83) | 52 (18–79) | 53 (18–87) | 51 (18–85) | 53 (16–88) |
| % Male | 1538 | 61% | 59% | 63% | 63% | 59% | 60% |
| % Smoker | 1326 | 21% | 16% | 21% | 20% | 20% | 19% |
| Karnofsky index (%), median (range) | 1394 | 100 (70–100) | 100 (50–100) | 100 (70–100) | 100 (70–100) | 100 (50–100) | 100 (50–100) |
| Hemoglobin (g/dl), median (range) | 1524 | 12.4 (4.9–17.5) | 12.2 (6.2–17.7) | 12.5 (6.7–15.9) | 12.9 (8.1–17.6) | 12.2 (4.7–19.1) | 12.3 (4.7–19.1) |
| WBC (× 109/l), median (range) | 1531 | 77 (5.7–582) | 89 (2.8–630) | 58 (2.9–529) | 56 (3.2–456) | 79 (2.6–570) | 76 (2.6–630) |
| Platelets (× 109/l), median (range) | 1533 | 382 (58–2419) | 343 (49–3020) | 403 (34–2799) | 390 (44–2205) | 386 (39–2716) | 374 (34–3020) |
| Eosinophils (%), median (range) | 1530 | 2 (0–20) | 2 (0–12) | 2 (0–14) | 2 (0–14) | 2 (0–16) | 2 (0–20) |
| Basophils (%), median (range) | 1526 | 3 (0–22)a | 3 (0–20) | 4 (0–21) | 3 (0–17) | 4 (0–26) | 3 (0–26) |
| Blasts in blood (%), median (range) | 1525 | 1 (0–17)b | 1 (0–16) | 1 (0–19) | 0 (0–16) | 1 (0–17) | 1 (0–19) |
| Spleen size (cm below costal margin), median (range) | 1529 | 2 (0–28) | 2 (0–38) | 0 (0–20) | 0 (0–19) | 2 (0–30) | 2 (0–38) |
| Euro score, n (%) | 1527 | ||||||
| Low | — | 142 (36) | 150 (35) | 55 (35) | 48 (38) | 159 (38) | 554 (36) |
| Intermediate | — | 205 (51) | 226 (53) | 81 (51) | 79 (62) | 202(48) | 793 (52) |
| High | — | 51 (13) | 49 (12) | 22 (14) | 1 (1) | 57 (14) | 180 (12) |
| Sokal score, n (%) | 1513 | ||||||
| Low | 140 (36) | 164 (39) | 62 (39) | 51 (40) | 153 (37) | 570 (38) | |
| Intermediate | 155 (40) | 164 (39) | 53 (34) | 58 (45) | 152 (37) | 582 (38) | |
| High | 97 (25) | 92 (22) | 42 (27) | 19 (15) | 111 (27) | 361 (24) | |
| EUTOS score, n (%) | 1523 | ||||||
| Low | 348 (88) | 384 (90) | 139 (88) | 118 (92) | 352 (85) | 1341 (88) | |
| High | 49 (12) | 44 (10) | 19 (12) | 10 (8) | 60 (15) | 182 (12) | |
| ELTS score, n (%) | 1521 | ||||||
| Low | 212 (54) | 236 (55) | 106 (67) | 80 (62) | 235 (57) | 869 (57) | |
| Intermediate | 123 (31) | 136 (32) | 35 (22) | 40 (31) | 116 (28) | 450 (30) | |
| High | 60 (15) | 55 (13) | 17 (11) | 9 (7) | 61 (15) | 202 (13) | |
| BCR-ABL1 transcript type, n (%) | 1506 | ||||||
| b2a2 | 147 (38) | 192 (46) | 54 (35) | 43 (34) | 160 (39) | 596 (40) | |
| b3a2 | 178 (46) | 167 (40) | 69 (45) | 57 (46) | 187 (45) | 658 (44) | |
| b2a2 and b3a2 | 54 (14) | 55 (13) | 29 (19) | 24 (19) | 61 (15) | 223 (15) | |
| Atypical transcripts | 10 (2) | 8 (1) | 3 (1) | 1 (1) | 7 (1) | 29 (1) |
Abbreviations: ELTS, European treatment and outcome study (EUTOS)-long-term-survival; IFN, interferon-α WBC, white blood cells.
There were no significant differences between the treatment groups.
One patient with 66% basophils (basophil leukemia).
One patient with ambivalent findings: 30% blasts in blood, 7% blasts in the marrow.
Figure 1.
Flow diagram of all 1551 randomized patients. Ara-C, cytarabine; CP, chronic phase; IC, informed consent; IFN, interferon-α IM, imatinib; OS, overall survival; SCT, stem cell transplantation; TKI, tyrosine kinase inhibitor.
Survival
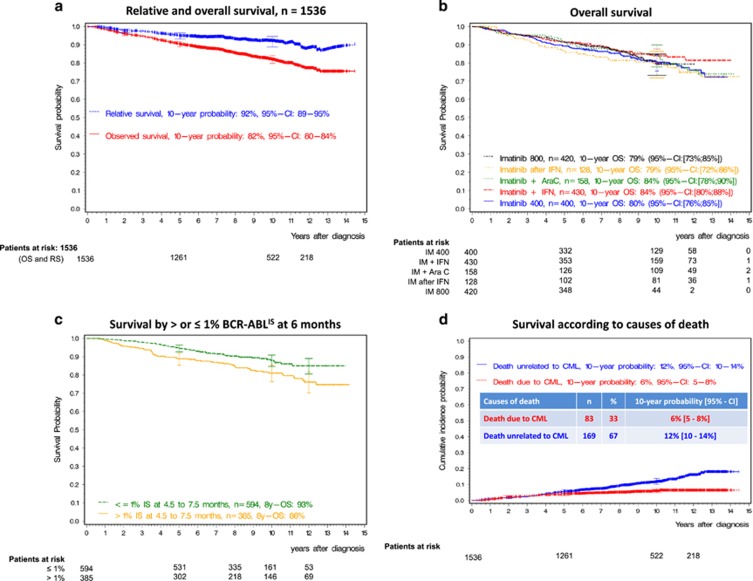
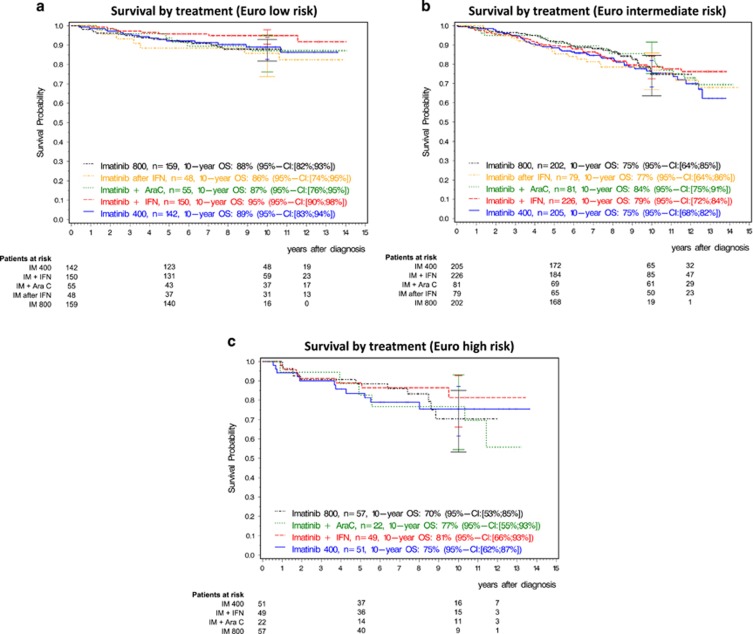
In all, 10-year OS of all patients was 82% (95% confidence interval (CI): 80; 84) (Figure 2a), 10-year PFS (95% CI: 78; 82) 80%. 10-year OS was 80% with IM400 mg, 84% with IM plus IFN, 84% with IM plus cytarabine, 79% with IM after IFN-failure and 79% with IM800 mg, (Figure 2b). In all, 10-year PFS was 80% with IM400 mg, 83% with IM plus IFN, 82% with IM plus cytarabine, 75% with IM after IFN and 77% with IM800 mg (Supplementary Figure 1). Adjusted for matched general population data, 10-year relative survival probability was 92% (95% CI: 89; 95) (Figure 2a; 91% for IM400 mg, 94% for IM plus IFN, 94% for IM plus cytarabine, 93% for IM after IFN and 87% for IM800 mg) and 96% (95% CI: 88; 99) for the 594 patients with BCR-ABL1 ⩽1% (Figure 2c). Two-hundred seventy five patients died, 23 after stem cell transplantation in first CP. Of patients not transplanted in first CP, more deaths were unrelated to CML (n=169, 67%) than due to CML (n=83, 33%). The 10-year probability of death due to CML was 6%, of death unrelated to CML 12% (Figure 2d). In all, 10-year OS and PFS according to Euro score and treatment are shown in Supplementary Table 1. Whereas Euro low-risk patients had significantly better survival than higher-risk patients, survival with any treatment was not significantly different from IM400 mg at any risk level (Figure 3) nor was a significant difference detectable by any other risk score.23, 35, 36 The non-CML causes of death correspond to those observed in the general population (Table 2). The cumulative incidences of death related and unrelated to CML were not different between the five treatment arms (Supplementary Figure 2), whether stratified for ELTS or not.23
Figure 2.
Long-term survival evaluation. (a) Overall survival and relative survival of all 1536 CML-patients. (b) Overall survival according to treatment groups over time. (c) Survival by landmark analysis at 6 months according to achieving and not achieving the milestone ⩽1% BCR-ABL1IS at 6 months. The 594 responders have a significantly better survival and show a 10-year relative survival of 96%. The 385 non-responders include slow responders with very good prognosis and high-risk patients requiring attention to patients’ and disease risk factors. (d) Survival according to causes of death defined as related or unrelated to CML. AraC, cytarabine; IFN, interferon-α OS, overall survival; RS, relative survival; IM, imatinib.
Figure 3.
Overall survival by disease risk (Euro-score). (a) Low, (b) intermediate, (c) high. AraC, cytarabine; IFN, interferon-α OS, overall survival; IM, imatinib.
Table 2. Causes of death.
| IM 400 mg | IM+IFN | IM+cytaribine | IM after IFN-failure | IM 800 mg | Total | |
|---|---|---|---|---|---|---|
| Total deaths (n) | 75 | 69 | 39 | 30 | 62 | 275 |
| Causes (n) | ||||||
| Progression to AP/BC | 17 | 15 | 9 | 6 | 20 | 67 |
| Transplantation related | 6 | 9 | 7 | 4 | 5 | 31 |
| Infection in CP | 7 | 6 | 1 | 2 | 4 | 20 |
| Secondary malignancy | 16 | 12 | 3 | 6 | 7 | 44 |
| Bleeding | 1 | 2 | 0 | 0 | 1 | 4 |
| Cardiopulmonary | 10 | 10 | 5 | 6 | 9 | 40 |
| Renal insufficiency | 2 | 1 | 1 | 1 | 2 | 7 |
| Thromboembolic/ischemic (not cardiac) | 1 | 1 | 2 | 1 | 3 | 8 |
| Suicide | 1 | 1 | 0 | 0 | 0 | 2 |
| Others | 3 | 4 | 2 | 1 | 2 | 12 |
| Unknown | 11 | 8 | 9 | 3 | 9 | 40 |
Abbreviations: AP, accelerated phase; BC, blast crisis; CP, chronic phase; IFN, interferon-α IM, imatinib.
n indicates number of patients.
Multivariate analysis for impact on survival of variables at diagnosis: risk score, comorbidities, major-route additional chromosomal aberrations, smoking and treatment center (academic vs others) influenced survival significantly, but not gender, transcript-type or initial treatment selection (Table 3).
Table 3. Multivariate analysis for impact on survival (n=1252).
| Variable | Regression coefficient | Standard error | P-value | Hazard ratio | Type-3-test | |
|---|---|---|---|---|---|---|
| Therapy | IM-after-IFN-failure vs IM 400 | 0.288 | 0.254 | 0.256 | 1.334 | 0.676 |
| IM 800 vs IM 400 | 0.033 | 0.207 | 0.875 | 1.033 | ||
| IM+cytarabine vs IM 400 | 0.157 | 0.244 | 0.519 | 1.170 | ||
| IM+IFN vs IM 400 | −0.069 | 0.199 | 0.727 | 0.933 | ||
| ELTS-score | Low vs high risk | −0.778 | 0.210 | <0.001 | 0.459 | <0.001 |
| Intermediate vs high risk | 0.061 | 0.208 | 0.770 | 1.062 | ||
| Treatment center | Academic center better than community hospital | 0.416 | 0.181 | 0.021 | 1.515 | 0.012 |
| Academic center better than private practice | 0.570 | 0.199 | 0.004 | 1.768 | 0.004 | |
| Comorbidity (Charlson index) | Per point (age not considered)a | 0.417 | 0.050 | <0.001 | 1.518 | <0.001 |
| Gender | Male vs female | 0.181 | 0.154 | 0.240 | 1.199 | 0.240 |
| Transcript type | b2a2 vs b3a2 | 0.088 | 0.157 | 0.574 | 1.092 | 0.713 |
| b2a2+b3a2 vs b3a2 | 0.158 | 0.208 | 0.447 | 1.171 | ||
| Smoking habit | Smoker vs non-smoker | 0.547 | 0.169 | 0.001 | 1.728 | 0.001 |
| Major-route ACA | Major-route ACA vs no major-route ACA at diagnosis | 1.814 | 0.392 | <0.001 | 6.137 | <0.001 |
Abbreviations: ACA, additional chromosomal aberration; ELTS, EUTOS-long-term survival; IM, imatinib, IFN, interferon-α.
Also better education (bachelor vs no bachelor) had an impact (P<0.001), but was not independent of smoking and selection of treatment center.
Age considered by ELTS-score.
Power
With n=400 randomized to IM400 mg and n=420 randomized to IM800 mg, an accrual time of 6.75 years across treatment arms and an additional follow-up of 4.25 years, the power would have been above 80% to observe OS differences, if the assumptions for the sample size estimation (see Methods) had been correct. In fact, survival probabilities at 5 years were 89% (95% CI: 86% 92%) and 92% (95% CI: 88% 94%), respectively. At 10 years, the difference in OS probability was only 1%. The hazard ratio of IM400 mg to IM800 mg was 1.091 (95% CI: 0.767; 1.550) instead of 2 or 0.61.
Switching to 2G-TKI
Four-hundred seven patients (26.5%) were switched to another TKI, mostly dasatinib or nilotinib, due to intolerance or resistance. Seven patients were switched to bosutinib, 5 to ponatinib, and 57 to more than one TKI. The median time to switching was 34 months. Switching was evenly distributed between treatment arms (Figure 1) arguing against an influence on comparative survival analyses. Censoring at the time of switching raised 10-year OS by 3% across treatment arms, indicating that predominantly poorer risk patients were switched.
Mutations and progressions
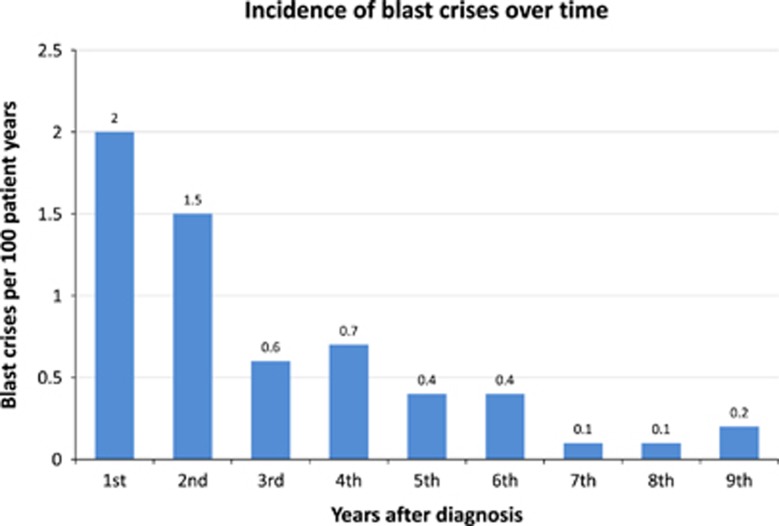
One-hundred ten of 541 analyzed patients (20,3%) had mutations of the BCR-ABL1-kinase domain, 70 (64%) had known resistance mutations (T315I (n=33), E255K (n=11), Y253H (n=11), F359C (n=8), G250E (n=4) and F486S (n=3)) and 73 (66%) were switched to 2G-TKI. More high-risk patients (31.5%) than low (16.9%) and intermediate risk patients (18.7%) had mutations. One-hundred fifteen patients fulfilled the criteria of progression to accelerated phase and BC, of which 89 had mutation analyses which were positive in 35 (39%). Eighty-seven patients progressed to BC. The 10-year cumulative incidence of BC was 5.8% (95% CI: 4.7% 7.1%). Most BC occurred in the first two years, but some continued to occur later during the entire observation time (Figure 4). Median survival after BC was 7.9 months across all treatment arms. Thirty-eight patients had myeloid, 28 lymphoid BC, in 21 patients the type was mixed or unknown.
Figure 4.
Incidence of blast crisis over time.
Transplantation
One-hundred thirty-eight patients were transplanted, 91 in first CP. Median age at transplantation was 41 (16–65) years, 94 (68%) were male. Eight-year survival after transplantation in first CP was 73%, of those transplanted not in first CP 38%.
Cytogenetic and molecular responses
By 10 years, the cumulative rates of complete cytogenetic remission were 77% (95% CI: 75; 79), of molecular response equivalent to complete cytogenetic remission37 (⩽1% BCR-ABL1IS) 91% (95% CI: 89; 94), of MMR 88% (95% CI: 86; 90), of MR4 83% (95% CI: 80; 85), and of MR4.5 70% (95% CI: 67; 73). The molecular responses according to treatment over time are shown in Table 4. Compared with IM400 mg, significantly faster responses were observed with IM800 mg for MR2-MR4, but not for MR5. A faster response was observed with IM800 mg also for MR4.5, but this was not significant (P=0.053). No patient who stopped IM in deep molecular remission or because of other reasons has died.
Table 4. Molecular response by response depth and treatment over time.
| BCR-ABL1⩽1% | n | Median time to response (mo) | Year 1 | 95% CI | Patients at risk | Year 3 | 95% CI | Patients at risk | Year 5 | 95% CI | Patients at risk | Year 10 | 95% CI | Patients at risk | P-value IM 400 vs IM 800 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imatinib 400 | 372 | 7.9 | 67.5% | (62.4;72.1) | 108 | 90.1% | (86.3;92.9) | 18 | 91.0% | (87.3;93.6) | 12 | 94.6% | (90.9;96.9) | 2 | 0.003 |
| Imatinib + IFN | 405 | 7.9 | 67.8% | (63.0;72.2) | 111 | 83.9% | (79.8;87.2) | 31 | 87.5% | (83.7;90.5) | 16 | 91.2% | (87.5;93.8) | 2 | |
| Imatinib + AraC | 150 | 11.0 | 53.6% | (45.2;61.2) | 59 | 87.6% | (81.2;91.9) | 8 | 89.8% | (84.0;93.6) | 3 | 91.0% | (85.9;94.3) | 1 | |
| Imatinib after IFN | 122 | 18.6 | 25.7% | (18.3;33.7) | 85 | 67.6% | (58.7;75.0) | 21 | 77.4% | (69.6;83.5) | 6 | 80.9% | (73.6;86.4) | 2 | |
| Imatinib 800 | 399 | 6.3 | 77.6% | (73.1;81.4) | 67 | 90.0% | (86.3;92.7) | 7 | 90.0% | (86.3;92.7) | 4 | ⩾ 91.4% | — | 0 | |
| MMR | |||||||||||||||
| Imatinib 400 | 372 | 14.9 | 36.7% | (31.8;41.7) | 216 | 80.6% | (76.1;84.4) | 43 | 86.3% | (82.3;89.5) | 19 | 92.2% | (88.2;94.9) | 2 | 0.003 |
| Imatinib + IFN | 405 | 13.5 | 43.1% | (38.9;48.7) | 198 | 76.3% | (71.7;80.4) | 48 | 83.5% | (79.0;87.1) | 20 | 87.9% | (83.6;91.2) | 3 | |
| Imatinib + AraC | 150 | 17.8 | 29.6% | (22.5;37.2) | 93 | 79.6% | (71.9;85.4) | 16 | 85.8% | (79.1;90.5) | 4 | 87.2% | (81.1;91.5) | 1 | |
| Imatinib after IFN | 122 | 29.9 | 10.0% | (5.5;16.2) | 103 | 57.8% | (48.6;65.9) | 31 | 69.1% | (60.4;76.3) | 12 | 74.7% | (66.6;81.1) | 3 | |
| Imatinib 800 | 399 | 10.3 | 55.6% | (50.5;60.4) | 147 | 83.2% | (78.8;86.7) | 24 | 86.8% | (82.7;90.0) | 11 | 89.1% | (85.0;92.0) | 1 | |
| MR4 | |||||||||||||||
| Imatinib 400 | 353 | 36.7 | 8.2% | (5.6;11.4) | 301 | 48.5% | (43.0;53.9) | 133 | 65.7% | (60.0;70.7) | 64 | 81.0% | (75.4;85.5) | 8 | 0.033 |
| Imatinib + IFN | 380 | 33.9 | 16.4% | (12.8;20.4) | 285 | 51.2% | (45.8;56.4) | 130 | 67.4% | (62.0;72.2) | 65 | 83.1% | (77.9;87.2) | 11 | |
| Imatinib + AraC | 141 | 36.8 | 5.9% | (2.8;10.7) | 123 | 49.4% | (40.4;57.8) | 55 | 67.5% | (58.5;75.0) | 27 | 85.5% | (79.0;90.1) | 5 | |
| Imatinib after IFN | 113 | 56.6 | 0.9% | (0.08;4.5) | 105 | 33.7% | (24.9;42.6) | 55 | 54.0% | (44.2;62.9) | 27 | 62.7% | (52.6;71.2) | 11 | |
| Imatinib 800 | 376 | 26.2 | 20.1% | (16.1;24.3) | 269 | 59.1% | (53.7;64.1) | 98 | 68.6% | (63.3;73.3) | 57 | 81.0% | (75.8;85.2) | 2 | |
| MR4.5 | |||||||||||||||
| Imatinib 400 | 346 | 60.6 | 4.8% | (2.8 ;7.4)] | 308 | 34.6% | (29.4;39.9) | 175 | 49.4% | (43.6;54.9) | 109 | 67.2% | (60.6;73.0) | 21 | 0.053 |
| Imatinib + IFN | 376 | 54.2 | 7.7% | (5.3;10.8) | 314 | 38.3% | (33.1;43.4) | 175 | 53.8% | (48.2;59.0) | 106 | 73.9% | (68.1;78.8) | 24 | |
| Imatinib + AraC | 138 | 61.8 | 3.8% | (1.4 ;8.0) | 124 | 31.1% | (23.1;39.3) | 77 | 49.8% | (40.6;58.4) | 47 | 69.6% | (60.5;76.9) | 18 | |
| Imatinib after IFN | 105 | 74.5 | 1.0% | (0.09;4.8) | 99 | 18.9% | (11.8;27.3) | 66 | 45.5% | (35.2;55.2) | 34 | 61.3% | (50.5;70.5) | 12 | |
| Imatinib 800 | 373 | 44.6 | 9.2% | (6.5 ;12.4) | 306 | 43.1% | (37.8;48.4) | 150 | 58.4% | (52.9;63.6) | 86 | 70.6% | (62.5;77.3) | 2 | |
| MR5 | |||||||||||||||
| Imatinib 400 | 318 | 8.8 | 2.3% | (1.0 ;4.4) | 290 | 16.5% | (12.5;21.1) | 216 | 32.9% | (27.3;38.5) | 146 | 53.5% | (46.5;60.0) | 37 | 0.933 |
| Imatinib + IFN | 356 | 10.3 | 3.2% | (1.7 ;5.5) | 316 | 18.6% | (14.6;23.1) | 230 | 28.7% | (23.7;33.8) | 173 | 48.9% | (42.3;55.1) | 54 | |
| Imatinib + AraC | 124 | 9.0 | 0.0% | — | 117 | 10.7% | (5.9;17.3) | 95 | 21.3% | (14.1;29.4) | 73 | 53.6% | (43.6;62.7) | 34 | |
| Imatinib after IFN | 94 | n.r. | 0.0% | — | 89 | 6.9% | (2.8;13.5) | 70 | 20.8% | (12.9;30.0) | 49 | 41.5% | (30.6;52.0) | 22 | |
| Imatinib 800 | 339 | 8.4 | 2.4% | (1.2;4.6)] | 300 | 16.2% | (12.3;20.6) | 216 | 31.5% | (26.2;36.9) | 153 | 49.8% | (43.2;56.1) | 8 | |
Abbreviations: CI, confidence interval;IFN, interferon- αmo, months;n.r., not reached.
Responses (confirmed) were defined as reductions of residual BCR-ABL1 transcripts of ⩾2, 3, 4, 4.5 and 5 logs compared with the standardized baseline in two consecutive analyses. Testing was restricted to patients expressing b2a2 and/or b3a2 transcripts. In case of a positive quantitative reverse-transcription polymerase chain reaction (qRT-PCR) for BCR-ABL1 transcripts, BCR-ABL1IS⩽1% was designated MR2 equivalent to complete cytogenetic remission, BCR-ABL1IS⩽0.1% MR3 or MMR, BCR-ABL1IS⩽0.01% MR4, BCR-ABL1IS⩽0.0032% MR4.5 and BCR-ABL1IS⩽0.001% MR5. For a negative qRT-PCR, the number of ABL1 transcripts used for nested PCR had to be ⩾10.000 for MR,4 ⩾32.000 for MR,4.5 and ⩾100.000 for MR5.
Survival by response milestones
One-thousand three-hundred and eleven patients had molecular tests at response milestones. Patients who reached ≤10% BCR-ABL1IS at 3 months (n=598 of 873 (68.5%)), ⩽1% BCR-ABL1IS (equivalent to complete cytogenetic remission) at 6 months (n=594 of 979 (61%)), or ⩽0.1% BCR-ABL1IS (MMR) at 12 months (n=469 of 914 (54.7%)) had significantly better survival than those who did not regardless of therapy. Supplementary Table 2 summarizes survival and response according to milestones at 3, 6 and 12 months. Figure 2c shows the landmark analysis at 6 months across treatment groups with a survival difference of 6.4% after 10 years. When patients reaching and not reaching milestones were analyzed by therapy, the faster response with one therapy (IM800 mg) did not translate into a detectable survival advantage.
Safety
A detailed safety analysis38 showed frequent, but mostly mild adverse drug reactions. Over the last 3 years, no new safety concerns have evolved. No serious late toxicity was observed. Observation time is still short, late effects in cancer survivors may well appear decades later. Continuous monitoring of patients under TKI-treatment appears mandatory.
Discussion
The data of this large randomized 5-arm treatment optimization study with the long median observation time of 9.5 years showed that high survival probabilities (82% at 10 years) can be achieved with IM-based therapy. This corresponds well to the 83.3% survival after 10 years in IRIS.2 The study further demonstrates that with regard to survival none of the experimental treatments is superior to IM400 mg. Interestingly, the faster and earlier cytogenetic and molecular responses of the experimental arm IM800 mg3, 8 did not translate into longer survival. The lack of survival differences between treatment arms may be explained by relatively few events attributable to CML, considering an overall 10-year relative survival of 92%, matching well with the 10-year CML mortality of just 6% and pointing to the relevance of non-CML causes of mortality. Similar observations have been reported with 2G-TKI after median observation times of 5 years.17, 18
CML-study IV was powered to detect an OS difference between the IM400 mg and IM800 mg arms of at least 5% 5 years after diagnosis, but due to survival probabilities of 89% and 92%, respectively, at 5 years, the difference was only 3%, and only 1% at 10 years. Any therapy aiming at improving survival above what is currently achieved with standard IM would have to further decrease the incidence and/or mortality of BC. The benefit of such therapy would have to be weighed against its toxicity.18, 39
Patients that reached response milestones of ⩽10% BCR-ABL1IS by 3 months, ⩽1% by 6 months or ⩽0.1% by 12 months had higher survival probabilities than those who did not—regardless of treatment. That this survival advantage was not detectable by analysis according to treatment group is probably due to the small survival difference (ca. 6% after 10 years) and the lower number of patients reaching milestones. Also the composition of the group not reaching the milestones has to be taken into account consisting of slow responders with very good survival as well as higher proportions of high-risk patients and progressions to BC. Given the excellent overall prognosis of CML, considerably larger patient numbers than in this already large study are required to detect the expected small survival difference.
Survival as shown by multivariate analysis was influenced more by disease biology, patients’ demographics and microeconomic elements than by initial treatment selection indicating that more attention has to be paid to non-CML factors in order to improve outcome of CML-patients. It is remarkable that a single tablet per day of a well tolerable drug reverses the course of a formerly uniformly fatal malignant disease and moves CML close to a potential cure. This is in line with reports of relapse-free survival following discontinuation of IM after deep and durable responses.40 The 10-year deep molecular remission rates of 70–80% in this study indicate that the majority of IM-treated patients are candidates for treatment discontinuation.
Our experience with the two IFN-arms might provide useful information. Neither IFN-arm achieved a survival advantage over IM400 mg, but OS in the IM-after-IFN-failure arm, which resembles the IFN-arm of IRIS, was not inferior to that of the IM400 mg arm (with the limitation that no Euro-high-risk patients were randomized to IM-after-IFN). It is also noteworthy that the simultaneous application of IM and IFN may have an advantage, since, by intention to treat analysis, it showed a significantly better PFS than the consecutive application of IM after IFN-failure. As favorable response results on IM in combination with IFN were reported by others,9, 10 it appears worthwhile to further follow this line of treatment.
Four-hundred seven patients (26.5%) were switched to one or more other TKI. As switching was evenly distributed between treatment arms an influence on survival comparisons is unlikely. We cannot determine to what extent survival was improved by switching to 2G-TKI in this study, as this was not planned prospectively. Censoring at the time of switching, however, improved survival by about 3% across treatment arms indicating that predominantly poorer risk patients had been switched. Also progression was distributed evenly. Eighty-seven patients (5.8%) developed BC of whom 67 died in spite of multiple lines of therapy including intensive chemotherapy and transplantation.
In conclusion, the results show that monotherapy with IM400 mg achieved a survival not much different from that of the general population, and that survival with CML is currently more determined by patients’ and disease factors than by initial treatment selection. More attention has to be paid to these factors in a personalized approach. Comorbidities should be addressed and smoking discouraged. Although improvements are also needed for the subgroups of refractory disease, more life-time can currently be gained by carefully addressing non-CML determinants of survival.
Acknowledgments
Supported by the German Government (BMBF 01GI0270); Deutsche Krebshilfe (Nr. 106642); Deutsche José-Carreras Leukämiestiftung (DJCLS H09/f, H06/04v, H03/01, R05/23, AH06.01); European Union (LSHC-CT-2004–503216); Novartis Oncology, Nürnberg (Drs G Gerhard, S Schaffert, A Jacob and U Haus); Roche, Grenzach-Wyhlen; and Essex, Munich, Germany. We thank E Matzat, R Pleil-Lösch, I Stalljann, G Bartsch, C Sodan-Boyer, M Meckesheimer, U Böhm and J Hehlmann for assistance.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
RH received research support from Novartis and honoraria from BMS, SS research support from Novartis, BMS, Ariad and Pfizer, MP honoraria from Novartis and BMS, SK honoraria from Novartis, GMB honoraria from Novartis, BMS and Pfizer, THB research support from Novartis, MCM grants and honoraria from Novartis, BMS, Ariad and Pfizer, AB honoraria from BMS, JM research support from Novartis and BMS, HL honoraria from Novartis, PS honoraria from Novartis, BMS, Pfizer and Ariad, CS honoraria from Novartis, AH research support from Novartis and honoraria from Novartis, BMS and Pfizer; all other authors reported no conflict of interest.
Supplementary Material
References
- O'Brien S, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 2017; 376: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Mueller MC, Pletsch N et al. Tolerability-adapted imatinib 800mg/d versus 400mg/d versus 400mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol 2011; 29: 1634–1642. [DOI] [PubMed] [Google Scholar]
- Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012; 26: 2096–2102. [DOI] [PubMed] [Google Scholar]
- Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML Study IV. Blood 2015; 126: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabarius A, Leitner A, Hochhaus A, Muller MC, Hanfstein B, Haferlach C et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood 2011; 118: 6760–6768. [DOI] [PubMed] [Google Scholar]
- Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C et al. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol 2015; 94: 2015–2024. [DOI] [PubMed] [Google Scholar]
- Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol 2014; 32: 415–423. [DOI] [PubMed] [Google Scholar]
- Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med 2010; 363: 2511–2521. [DOI] [PubMed] [Google Scholar]
- Simonsson B, Gedde-Dahl T, Markevarn B, Remes K, Stentoft J, Almqvist A et al. Combination of pegylated IFN-alpha 2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood 2011; 118: 3228–3235. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E et al. Comparison of imatinib 400mg and 800mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood 2009; 113: 4497–4504. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW et al. Phase III, randomized, open-label study of daily imatinib mesylate 400mg versus 800mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol 2010; 28: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarani M, Druker BJ, Branford S, Kim DW, Pane F, Mongay L et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol 2014; 99: 616–624. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Kopecky KJ, Radich JP, Kamel-Reid S, Stock W, Paietta E et al. Imatinib 800mg daily induces deeper molecular responses than imatinib 400mg daily: results of SWOG S0325, an intergroup randomized PHASE II trial in newly diagnosed chronic phase chronic myeloid leukaemia. Br J Haematol 2014; 164: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic- phase CML patients receiving 600mg/day of imatinib as initial therapy. Blood 2008; 112: 3965–3973. [DOI] [PubMed] [Google Scholar]
- Yeung DT, Osborn MP, White DL, Branford S, Braley J, Herschtal A et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood 2015; 125: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C et al. Final 5-Year Study Results of DASISION: The Dasatinib versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol 2016; 34: 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016; 30: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A, Pfirrmann M, Zander A, Kroger N, Beelen D, Novotny J et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia 2016; 30: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006; 108: 1809–1820. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013; 122: 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998; 90: 850–858. [DOI] [PubMed] [Google Scholar]
- Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016; 30: 48–56. [DOI] [PubMed] [Google Scholar]
- Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012; 26: 2172–2175. [DOI] [PubMed] [Google Scholar]
- Branford S, Fletcher L, Cross NCP, Müller MC, Hochhaus A, Kim D-W et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008; 112: 3330–3338. [DOI] [PubMed] [Google Scholar]
- Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2011; 118: 1208–1215. [DOI] [PubMed] [Google Scholar]
- Dupont WD. PS power and sample size program available for free on the internet. Controlled Clin Trial 1997; 18: 274. [Google Scholar]
- Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics 1982; 38: 163–170. [PubMed] [Google Scholar]
- Shkolnikov V, Barbieri M, Wilmoth J. The Human Mortality Databasehttp://www.mortality.org/.
- Pfirrmann M, Lauseker M, Hoffmann VS, Hasford J. Prognostic scores for patients with chronic myeloid leukemia under particular consideration of competing causes of death. Ann Hematol 2015; 94: S209–S218. [DOI] [PubMed] [Google Scholar]
- Pfirrmann M, Hochhaus A, Lauseker M, Sausele S, Hehlmann R, Hasford J. Recommendations to meet statistical challenges arising from endpoints beyond overall survival in clinical trials on chronic myeloid leukemia. Leukemia 2011; 25: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983; 1: 710–719. [DOI] [PubMed] [Google Scholar]
- Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed 2006; 81: 272–278. [DOI] [PubMed] [Google Scholar]
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE et al. Prognostic discrimination in ‘good-risk’ chronic granulocytic leukemia. Blood 1984; 63: 789–799. [PubMed] [Google Scholar]
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 2011; 118: 686–692. [DOI] [PubMed] [Google Scholar]
- Lauseker M, Hanfstein B, Haferlach C, Schnittger S, Pfirrmann M, Fabarius A et al. Equivalence of BCR-ABL transcript levels with complete cytogenetic remission in patients with chronic myeloid leukemia in chronic phase. J Cancer Res Clin Oncol 2014; 140: 1965–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmanti L, Saussele S, Lauseker M, Muller MC, Dietz CT, Heinrich L et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 2015; 29: 1123–1132. [DOI] [PubMed] [Google Scholar]
- Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 612–621. [DOI] [PubMed] [Google Scholar]
- Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.