Abstract
Background
Treatments that prevent the motility of breast cancer cells and inhibit formation of new capillary vessels are urgently needed. FSTL1 is a secreted protein that has been implicated in maintaining the normal physiological function of the cardiovascular system, in addition to a variety of other biological functions. We investigated the role of FSTL1 in the proliferation and migration of breast cancer and vascular endothelial cells.
Methods
Human umbilical vein endothelial cells and human breast cancer BT‐549 cells were used to test the effects of FSTL1 and the N‐terminal domain of FSTL1. Immunofluorescence microscopy and 3‐(4, 5‐dimethylthiazolyl‐2)‐2,5‐diphenyltetrazolium bromide, transwell invasion, and wound healing assays were conducted.
Results
Different doses of the N‐terminal fragment of FSTL1 (FSTL‐N) have variable effects on the migration of these cells. However, FSTL1 does not significantly affect tube formation in vitro from vascular endothelial cells. FSTL1‐FL and FSTL1‐N have modest effects on the invasion of breast cancer and vascular endothelial cells. Interestingly, FSTL1‐FL, but not FSTL‐N, modulates vascular endothelial cell polarization.
Conclusion
FSTL1 modestly affects the proliferation of breast cancer cells and vascular endothelial cells. Our findings improve our understanding of the functions of FSTL1 in breast cancer development and angiogenesis.
Keywords: Breast cancer, cell motility, cell proliferation, FSTL1, vascular endothelial cell
Introduction
Breast cancer is one of the most prevalent cancer types in women worldwide. Because of its high mortality rate and frequent metastasis, new diagnostics and more effective treatments for metastatic breast cancer are needed. Breast cancer metastasis is a complex process comprising multiple steps and mechanisms.1, 2 Metastasis occurs when unstable cancer cells adapt to and colonize a tissue microenvironment distant from the primary tumor. In order to prevent tumor invasion, it is necessary to inhibit tumor cell migration and angiogenesis, a tightly orchestrated process that requires cell proliferation, cell migration, and tubular morphogenesis.3, 4, 5 Both positive and negative regulators of angiogenesis contribute to a variety of physiological and pathogenic mechanisms, including embryonic development and tumor growth.6, 7 In the context of cancer, inhibition of tumor angiogenesis is an effective way to prevent tumor invasion.4, 8
FSTL1 is a secreted protein that belongs to the follistatin family.9 FSTL1 is widely expressed in mammalian tissues and is produced mainly by cells of mesenchymal origin. It has also been detected in medium collected from endothelial cells. However, the precise function of FSTL1 in endothelial cells is not fully understood. FSTL1 appears to play a key regulatory role in maintaining the normal physiological function of the cardiovascular system, where it is highly expressed in the blood vessels of the developing lung.10, 11, 12 FSTL1 is induced in response to cardiovascular stress.12 In addition, a recent study showed that FSTL1 expression preserved the viability of endothelial cells subjected to systemic vascular damage.13 However, little is known about the role of FSTL1 in cancer. In this study, we investigated the role of FSTL1 in the proliferation and migration of breast cancer cells and vascular endothelial cells. Our results provide a deeper understanding of the function and mechanism of FSTL1 in breast cancer development and angiogenesis.
Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) and human breast cancer BT‐549 cells were purchased from the American Type Culture Collection. HUVECs and BT‐549 cells were cultured in RPMI‐1640 and Dulbecco's modified Eagle's medium, respectively, containing 10% fetal bovine serum. Cells were maintained in a humidified incubator containing 5% CO2.
Drugs, antibodies, and reagents
Dr. Wen Ning of Nankai University kindly provided purified full‐length FSTL1 (FSTL1‐FL) and the N‐terminal domain of FSTL1 (FSTL1‐N). Antibodies against γ‐tubulin and α‐tubulin were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Fluorescein isothiocyanate and tetramethylrhodamine‐conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA, USA), 4′,6‐diamidino‐2‐phenylindole (DAPI) was obtained from Sigma‐Aldrich, and 3‐(4,5‐dimethylthiazolyl‐2)‐2,5‐diphenyltetrazolium bromide (MTT) from Songon Biotech (Shanghai, China).
Tube formation
Human umbilical vein endothelial cells were plated in 24‐well plates pre‐coated with Matrigel (diluted 1:1 with serum‐free media). To examine tube formation, images were captured using an Axio Observer A1 fluorescence microscope (Carl Zeiss, Baden‐Wuerttemberg, Germany). The degree of tube formation was quantified by measuring the cumulative tube length using imagej software (NIH, Bethesda, MD, USA), as previously described.5
Cell proliferation
Cells were seeded in 96‐well plates, and the density of cells was determined by MTT assay, as previously described.14
Fluorescence microscopy
For immunofluorescence microscopy, cells were grown on coverslips, fixed with methanol at −20°C, and blocked with 2% bovine serine albumin in phosphate buffered saline. Cells were incubated with primary antibodies for two hours and then with secondary antibodies for two hours.15, 16 DAPI was used for DNA staining, and coverslips were mounted in 90% glycerol.17 Images were captured using an Axio Observer A1 fluorescence microscope (Carl Zeiss).
Transwell invasion
For the invasion assay, chambers were assembled in 24‐well plates using 8 μm pore transwell inserts (BD Falcon, Franklin Lakes, NJ, USA). Inserts were coated with 50 μL Matrigel (diluted 1:4 in serum‐free media). HUVECs (1 × 105) were placed in the upper chamber. Invaded cells on the underside of the inserts were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Images were captured using a stereo microscope (Leica, Wetzlar, Germany).
Wound healing
Human umbilical vein endothelial cells treated with the indicated drugs for 48 hours were plated on coverslips and allowed to reach confluence. A 10 μL pipette tip was then used to create a scratch in the monolayer. The cells were washed twice with phosphate‐buffered saline to remove the cell debris and cultured in serum‐free media for 12 hours. Images of the wound were then captured, and the extent of wound repair was determined using imagej software.
Cell polarization
Human umbilical vein endothelial cells were plated onto coverslips and allowed to reach confluence. The monolayer was then scratched with a pipette tip to stimulate directed cell migration. Microtubules, centrosomes, and nuclei were stained using α‐tubulin and γ‐tubulin antibodies and DAPI, respectively. Coverslips were mounted in 90% glycerol and examined under the fluorescence microscope (Carl Zeiss). Border cells that exhibited a centrosome situated between the nucleus and the leading edge were considered as polarized.
Results
Domain organization of full‐length FSTL1 and an N‐terminal fragment
The FSTL1 protein contains 308 amino acids and has an N‐terminal signal peptide of 20 amino acids (Fig 1a). The N‐terminal domain of FSTL1 is critical for proper localization of the protein and has been shown to be required for various biological functions. FSTL1 also contains an FST‐like domain containing 10 conserved cysteine residues and an extracellular calcium‐binding domain that binds two calcium ions through a pair of canonical EF hand motifs. The EF hand is the most common calcium‐binding motif found in proteins. Other conserved domains in FSTL1 include a KAZAL‐like domain and a Von Willebrand factor type C domain. To characterize the role of FSTL1 in cancer development and angiogenesis, we used both full‐length FSTL1 (FSTL1‐FL) and the N‐terminal fragment of FSTL1 (FSTL1‐N) (Fig 1a).
Figure 1.
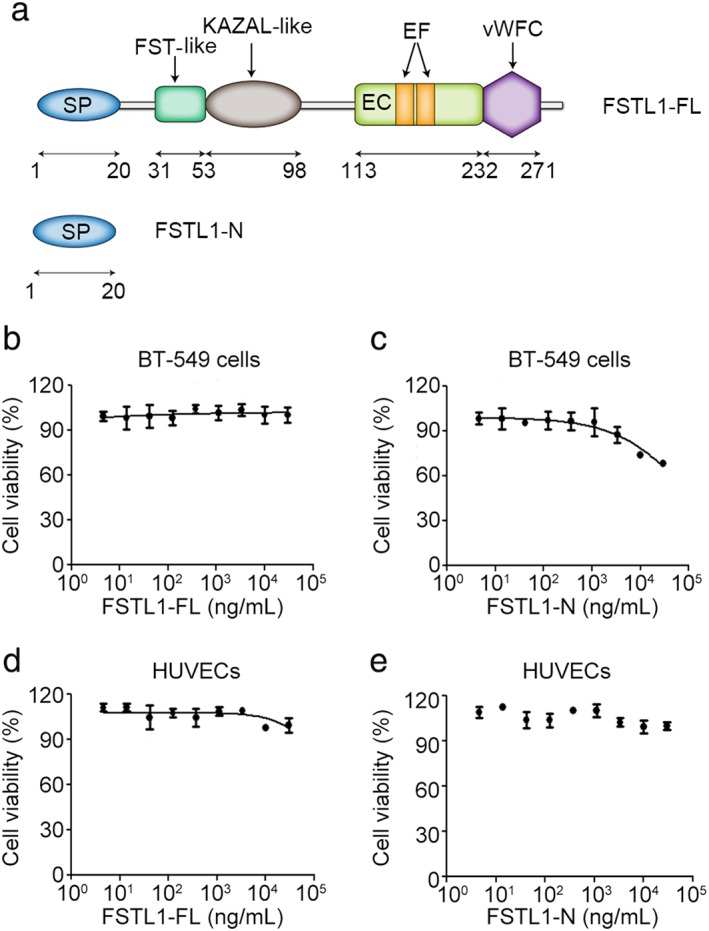
Domain organization and effects of full‐length FSTL1 (FSTL1‐FL) and its N‐terminal fragment (FSTL1‐N) on the proliferation of breast cancer and vascular endothelial cells. (a) Diagram showing domain organization of FSTL1‐FL and FSTL1‐N. EC, extracellular calcium‐binding domain; EF, EF hands; SP, signal peptide; vWFC, Von Willebrand factor type C domain. (b–e) 3‐(4, 5‐dimethylthiazolyl‐2)‐2,5‐diphenyltetrazolium bromide assays were used to measure the proliferation of (b,c) BT‐549 cells or (d,e) human umbilical vein endothelial cells (HUVECs) treated with the indicated doses of (b,d) FSTL1‐FL or (c,e) FSTL1‐N.
FSTL1 has a modest effect on the proliferation of breast cancer and vascular endothelial cells
Tumor cell proliferation is necessary for tumorigenesis, and vascular endothelial cell proliferation is a key factor influencing tumor‐associated angiogenesis. Therefore, we investigated the effects of FSTL1‐FL and FSTL1‐N on the proliferation of BT‐549 breast cancer cells and HUVECs using MTT assays. We observed modest differences in the effects of treatment with low and high doses of FSTL1‐FL in HUVECs, but not BT‐549 cells (Fig 1b, d). The viability of BT‐549 cells was slightly decreased after treatment with high doses of FSTL1‐N compared to the vehicle (Fig 1c), but FSTL1‐N treatment had no significant effect on HUVEC proliferation (Fig 1e).
Different doses of FSTL1‐N have variable effects on breast cancer cell migration
To further explore the role of FSTL1 in cancer, we investigated the effects of FSTL1 on breast cancer cell migration, a critical step in cancer progression.18, 19 Because FSTL1‐N plays a key role in the biological function of FSTL1, we analyzed the effects of FSTL1‐N on the migration of BT‐549 cells. Wound healing assays revealed that the wounded area fully recovered after 12 hours in control monolayers as a consequence of directed cell migration (Fig 2a). Treatment of BT‐549 cells with different doses of FSTL1‐N had variable effects on the extent of wound closure (Fig 2b).
Figure 2.
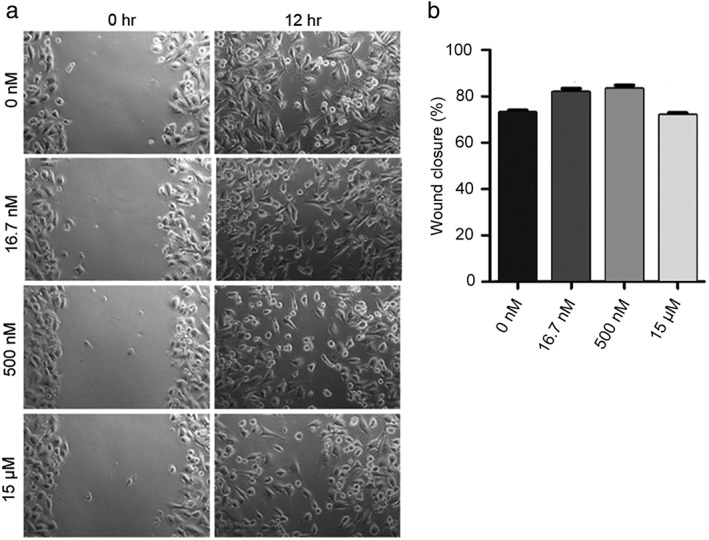
Effect of the N‐terminal fragment of FSTL1 (FSTL1‐N) on the migration of BT‐549 cells. (a) BT‐549 monolayers treated with control or FSTL1‐N were scratched, and wound margins were imaged after 0 and 12 hours. (b) Experiments were performed as in panel a, and the extent of wound closure was quantified by measuring the wound area at 12 hours compared to the initial wound area.
FSTL1 does not significantly affect tube formation in vitro from vascular endothelial cells
Angiogenesis plays a pivotal role in cancer progression, and because FSTL1 is a secreted protein, we hypothesized that it might regulate vascular endothelial cell function. To investigate the potential role of FSTL1 in the regulation of angiogenesis, we examined the effects of FSTL1‐FL and FSTL1‐N on vascular endothelial tube formation in vitro. Three hours after plating the cells, a tubular network of interconnecting branches was observed in the control cells. Measurement of cumulative tube length revealed no significant differences in comparison to FSTL1‐FL or FSTL1‐N‐treated groups (Fig 3), indicating that FSTL1 does not directly affect tubular morphogenesis.
Figure 3.
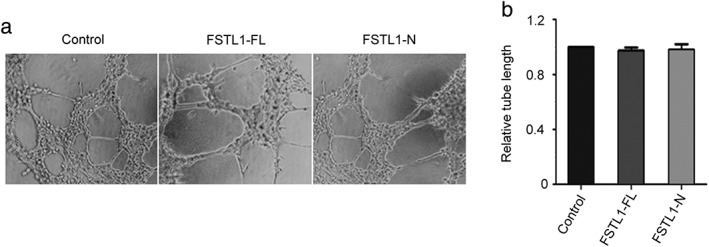
Effects of full‐length FSTL1 (FSTL1‐FL) and its N‐terminal fragment (FSTL1‐N) on tube formation in vitro from human umbilical vein endothelial cells (HUVECs). (a) HUVECs treated with the vehicle, FSTL1‐FL, or FSTL1‐N were plated on Matrigel, and images were captured after three hours. (b) Experiments were performed as described in panel a, and the cumulative tube lengths were measured.
Different doses of FSTL1‐N have variable effects on vascular endothelial cell migration
We next sought to examine the effects of FSTL1‐N on vascular endothelial cell migration, another critical step in the process of angiogenesis. Results from wound healing assays showed that the wounded area in the control group was fully recovered after 12 hours as a result of directed cell migration (Fig 4a). Similar to the findings in BT‐549 cells, treatment of HUVECs with different doses of FSTL1‐N had variable effects on the extent of wound closure (Fig 4b).
Figure 4.
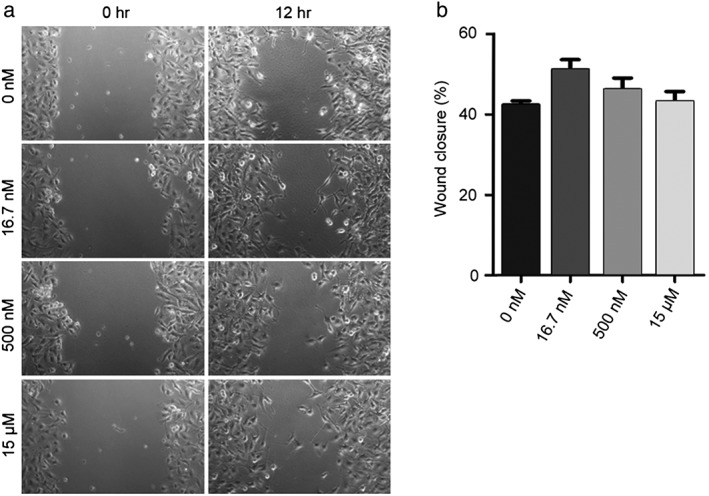
Effect of the N‐terminal fragment of FSTL1 (FSTL1‐N) on the migration of human umbilical vein endothelial cells (HUVECs). (a) Monolayers of HUVECs treated with FSTL1‐N or the vehicle were scratched, and wound margins were imaged at 0 and 12 hours. (b) Experiments were performed as in panel a, and the extent of wound closure was quantified by measuring the wound area at 12 hours compared to the initial wound area.
FSTL1‐FL and FSTL1‐N have modest effects on the invasion of breast cancer and vascular endothelial cells
To further explore the potential role of FSTL1 in cell motility, we conducted transwell invasion assays in the presence and absence of FSTL1‐FL and FSTL1‐N. As shown in Figure 5a, treatment with FSTL1‐FL or FSTL1‐N had modest effects on the invasion of HUVECs and BT‐549 cells through the porous membrane. We also quantified the effects of FSTL1‐FL and FSTL1‐N on the invasion of HUVECs or BT‐549 cells by staining cells (Fig 5b,c) or counting cell numbers (Fig 5d,e), confirming that FSTL1‐FL and FSTL1‐N had modest effects on cell invasion.
Figure 5.
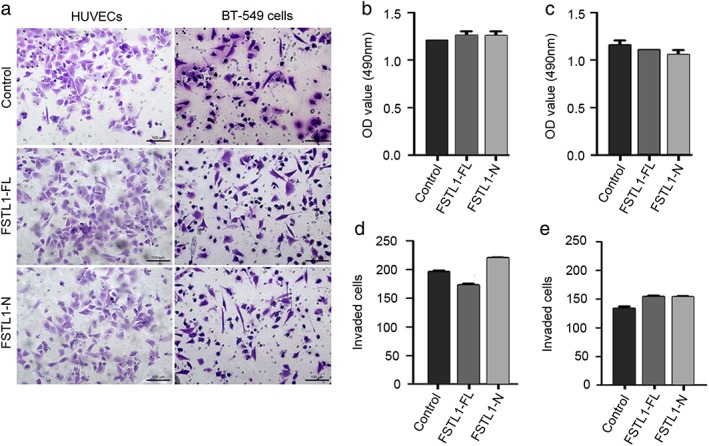
Effects of full‐length FSTL1 (FSTL1‐FL) and its N‐terminal fragment (FSTL1‐N) on the invasion of human umbilical vein endothelial cells (HUVECs) and BT‐549 cells. (a) Equal numbers of HUVECs and BT‐549 cells treated with the vehicle, FSTL1‐FL, or FSTL1‐N were plated in transwell inserts. After 20 hours, cells that invaded to the underside of the inserts were stained with crystal violet and photographed. (b,c) Measurement of the absorbance produced by crystal violet of the stained (b) HUVECs and (c) BT‐549 cells. (d,e) Quantification of the number of invaded (d) HUVECs and (e) BT‐549 cells. OD, optical density.
FSTL1‐FL, but not FSTL‐N, modulates vascular endothelial cell polarization
Polarization is a critical step in cell migration that involves the rearrangement of microtubules and reorientation of the centrosome toward the leading edge of cells.3, 20, 21 Therefore, we performed polarization assays to characterize any potential involvement of FSTL1 in HUVEC motility. In the control group, cells at the wound margin demonstrated a typical polarized orientation, with the centrosome positioned between the nucleus and the leading edge (Fig 6a). This polarized morphology was modestly affected in cells treated with FSTL1‐FL, but not FSTL1‐N (Fig 6b). These data indicate that FSTL1‐FL, but not FSTL‐N, modulates vascular endothelial cell polarization.
Figure 6.
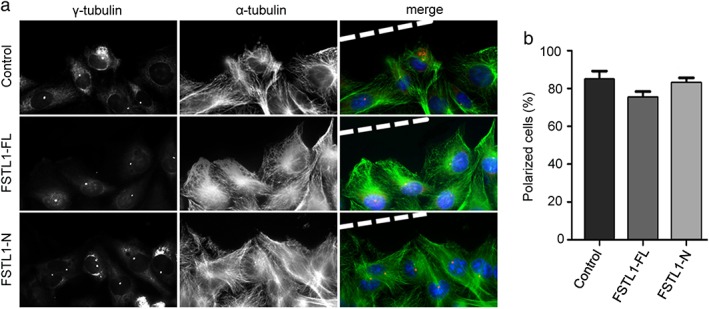
Effects of full‐length FSTL1 (FSTL1‐FL) and its N‐terminal fragment (FSTL1‐N) on the polarization of human umbilical vein endothelial cells (HUVECs). (a) Immunofluorescence microscopy images of control, FSTL1‐FL‐treated, and FSTL1‐N‐treated HUVECs at the margins of wounded monolayers. Staining of α‐tubulin (green), γ‐tubulin (red), and DNA (blue) was used to visualize cell polarization. (b) Quantification of the percentage of polarized cells from experiments performed as in panel a.
Discussion
An increasing number of studies have indicated that the secreted protein FSTL1 plays key roles in cardiovascular diseases and might be involved in the pathogenesis of cancer.22, 23, 24, 25 However, it is not clear whether FSTL1 is involved in breast cancer development and progression. Excessive proliferation plays a critical role in tumor growth; therefore, in this study we tested the effects of FSTL1 on proliferation of BT‐549 breast cancer cells. We also tested the influence of FSTL1 on proliferation of HUVECs. Our results demonstrate that FSTL1 modestly affects the proliferation of these cell types.
Tumor cell migration is an important function associated with tumor invasion and metastasis.26 Similarly, endothelial cell migration is required for neovascularization.3, 27, 28 Because the N‐terminal domain of FSTL1 plays a central role in the regulation of its cellular functions, we examined the effects of FSTL‐N on cell migration. We found that different doses of FSTL‐N had variable effects on the migration of breast cancer and vascular endothelial cells.
Angiogenesis plays a critical role in tumor development by supplying solid tumors with necessary nutrients.4 Therefore, we explored additional roles of FSTL1 in key processes associated with angiogenesis, including tube formation and polarization of endothelial cells. Cell polarization is the earliest step in cell migration, in which cells rearrange their cytoskeletons to prepare for migration.3, 20 Using HUVECs, we have shown that FSTL1 does not significantly affect tube formation in vitro from vascular endothelial cells and that FSTL1‐FL, but not FSTL‐N, modulates vascular endothelial cell polarization.
Our results indicate that FSTL1 plays a modest role in cell motility and does not significantly affect angiogenesis in vitro. However, it is still possible that FSTL1 could play an indirect role in these physiological processes together with other regulatory factors. Additional studies are needed to explore the impact of this protein on cell proliferation and motility to better understand the role of FSTL1 in breast cancer development and progression.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank Dr. Wen Ning for providing purified FSTL1‐FL and FSTL1‐N. This work was supported by grants from the National Natural Science Foundation of China (31371382), the Natural Science Foundation of Tianjin City (15JCYBJC49300), and the 111 Project of the Ministry of Education of China (B08011).
Contributor Information
Min Liu, Email: minliu@sdnu.edu.cn.
Dengwen Li, Email: dwli@nankai.edu.cn.
References
- 1. Karagiannis GS, Goswami S, Jones JG, Oktay MH, Condeelis JS. Signatures of breast cancer metastasis at a glance. J Cell Sci 2016; 129: 1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tala, Xie S, Sun X et al Microtubule‐associated protein Mdp3 promotes breast cancer growth and metastasis. Theranostics 2014; 4: 1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao J, Sun L, Huo L, Liu M, Li D, Zhou J. CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood 2010; 115: 4130–7. [DOI] [PubMed] [Google Scholar]
- 4. Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev 2017. https://doi.org/10.1002/med.21452 [DOI] [PubMed] [Google Scholar]
- 5. Sun X, Li F, Dong B et al Regulation of tumor angiogenesis by the microtubule‐binding protein CLIP‐170. Protein Cell 2013; 4: 266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bentley K, Chakravartula S. The temporal basis of angiogenesis. Philos Trans R Soc Lond B Biol Sci 2017; 372: pii: 20150522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Xie S, Ren Y et al Microtubule‐associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1‐dependent manner. Protein Cell 2011; 2: 150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie S, Zhou J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front Plant Sci 2017; 8: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sylva M, Moorman AF, van den Hoff MJ. Follistatin‐like 1 in vertebrate development. Birth Defects Res C Embryo Today 2013; 99: 61–9. [DOI] [PubMed] [Google Scholar]
- 10. Geng Y, Dong Y, Yu M et al Follistatin‐like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A 2011; 108: 7058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Liu Y, Li X, Zhao J, Geng Y, Ning W. Follistatin like‐1 (Fstl1) is required for the normal formation of lung airway and vascular smooth muscle at birth. PLoS ONE 2017; 12: e0177899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei K, Serpooshan V, Hurtado C et al Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015; 525: 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka K, Valero‐Muñoz M, Wilson RM et al Follistatin like 1 regulates hypertrophy in heart failure with preserved ejection fraction. JACC Basic Transl Sci 2016; 1: 207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu M, Wang X, Yang Y et al Ectopic expression of the microtubule‐dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol 2010; 221: 221–8. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Liu M, Li D et al CYLD regulates spindle orientation by stabilizing astral microtubules and promoting dishevelled‐NuMA‐dynein/dynactin complex formation. Proc Natl Acad Sci U S A 2014; 111: 2158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Ran J, Liu M et al CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res 2014; 24: 1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo Y, Ran J, Xie S et al ASK1 controls spindle orientation and positioning by phosphorylating EB1 and stabilizing astral microtubules. Cell Discov 2016; 2: 16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Luo Y, Lyu R et al Proto‐oncogenic Src phosphorylates EB1 to regulate the microtubule‐focal adhesion crosstalk and stimulate cell migration. Theranostics 2016; 6: 2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li D, Sun X, Zhang L et al Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014; 5: 214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi X, Liu M, Li D, Wang J, Aneja R, Zhou J. Cep70 contributes to angiogenesis by modulating microtubule rearrangement and stimulating cell polarization and migration. Cell Cycle 2012; 11: 1554–63. [DOI] [PubMed] [Google Scholar]
- 21. He X, Liu Z, He Q et al Identification of novel microtubule‐binding proteins by taxol‐mediated microtubule stabilization and mass spectrometry analysis. Thorac Cancer 2015; 6: 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchis‐Gomar F, Perez‐Quilis C, Lucia A. Overexpressing FSTL1 for heart repair. Trends Mol Med 2016; 22: 353–4. [DOI] [PubMed] [Google Scholar]
- 23. Su S, Parris AB, Grossman G, Mohler JL, Wang Z, Wilson EM. Up‐regulation of follistatin‐like 1 by the androgen receptor and melanoma antigen‐A11 in prostate cancer. Prostate 2017; 77: 505–16. [DOI] [PubMed] [Google Scholar]
- 24. Bae K, Park KE, Han J, Kim J, Kim K, Yoon KA. Mitotic cell death caused by follistatin‐like 1 inhibition is associated with up‐regulated Bim by inactivated Erk1/2 in human lung cancer cells. Oncotarget 2016; 7: 18076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao W, Han HB, Zhang ZQ. Suppression of lung cancer cell invasion and metastasis by connexin43 involves the secretion of follistatin‐like 1 mediated via histone acetylation. Int J Biochem Cell Biol 2011; 43: 1459–68. [DOI] [PubMed] [Google Scholar]
- 26. Qin J, Li D, Zhou Y et al Apoptosis‐linked gene 2 promotes breast cancer growth and metastasis by regulating the cytoskeleton. Oncotarget 2017; 8: 2745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li D, Gao J, Yang Y et al CYLD coordinates with EB1 to regulate microtubule dynamics and cell migration. Cell Cycle 2014; 13: 974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie S, Dong B, Sun X et al Identification of a cytoplasmic linker protein as a potential target for neovascularization. Atherosclerosis 2014; 233: 403–9. [DOI] [PubMed] [Google Scholar]


