Abstract
Epithelioid hemangioendothelioma is a very rare, vascular, low‐grade malignant tumor found in the lungs, liver, bone, and other soft tissues. Most patients with pulmonary epithelioid hemangioendothelioma (PEH) are asymptomatic but usually present with multiple bilateral nodular lesions in the lungs. Currently, surgical lung biopsy, histology, and immunohistochemical methods are essential for diagnosis. However, there is no standard therapy for the treatment for PEH.
Our paper describes the clinico‐radiologic features and genomics of PEH based on next‐generation sequencing (NGS) in a 43‐year‐old male we encountered. The patient came to the hospital with right chest pain. After investigation, a lesion in the middle lobe of the right lung was found, together with smaller multiple lesions in both lungs. After resection of the lesion, histopathological analysis showed positive findings for PEH. The patient's blood and tumor tissue were sent for NGS analysis for further investigation. Results from the analysis revealed mutations of multiple genes. The information obtained from the genomic analysis of PEH using NGS may be significant for the planning and monitoring of treatment for this disease.
Keywords: Intravascular bronchoalveolar tumor, lobectomy, next‐generation sequencing, pulmonary epithelioid hemangioendothelioma, wedge resection
Introduction
Epithelioid hemangioendothelioma (EH), known as intravascular bronchoalveolar neoplasm, is a rare disease first described in 1975.1 Since then, more than 100 cases have been reported in the literature.2, 3 Pulmonary EH (PEH) originates from endothelial cells and has a characteristic epithelioid histological appearance.4 Currently, it remains a low‐grade malignant vascular tumor with no standard therapy and an undefined prognosis.2 Most previous case reports have discussed the clinical presentation, radiological and pathological findings, and prognosis, but very few have described the genomics of this tumor. Therefore, this report presents the clinico‐radiological features of PEH and its genomics based on next‐generation sequencing (NGS), a technique that provides clues of molecular markers in cancer.
Case Report
A 43‐year‐old man attended the hospital complaining of sharp right chest pain that had lasted for 10 days during exercise. He also complained of sleep deprivation as a result of worrying about his illness. He had no cough, sputum, hemoptysis, dyspnea, or weight loss. He had not been an active smoker for the past two decades, but drank about two units of alcohol per day. He had no history of malignancy or genetic disease but had suffered a puncture in his right chest 20 years before, which had been managed. Moreover, he had undergone high right groin hernia repair five years earlier.
At the time of admission, the physical examination revealed normal vital signs. Nothing abnormal was detected on auscultation.
A laboratory examination revealed white blood cell, red blood cell, hemoglobin, and platelet counts of 7130/mm3, 4 910 000/mm3, 158.0 g/L, and 3310 mm3, respectively. The prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), and hepatic and renal function tests were all normal. However, a positive test for hepatitis B was detected in the blood, showing an HBsAg level of 55.34 IU/mL.
A chest radiograph showed a mass lesion in the right middle lobe (Fig 1), while a computed tomography (CT) scan showed another nodule in the medial segment of the same right middle lobe. A smaller nodule was observed in the lingular segment of the left upper lobe, together with multiple smaller lesions throughout both lungs (Fig 2). Positron emission tomography‐CT showed 4.4 and 6.8 of the standard uptake value (SUV) of the lesions in the lingular segment of the left upper lobe and the medial segment of the right middle lobe, respectively. Bronchoscopy was performed and yielded normal tissue with fragments of the bronchial wall only. The preoperative forced expiratory volume in one second (FEV1) was 3.48 L (102.8% of the predicted volume) without an obstructive pattern. After assessing that the patient was stable, we decided to perform a palliative thoracotomy. The right middle lobe was resected and a wedge resection of the right lower lung was performed. The interlobular and lobar nodes were also resected. The smaller nodule observed in the lingular segment of the left upper lobe was left unresected.
Figure 1.

Chest radiograph showing a mass lesion in the right middle lobe (indicated by arrow).
Figure 2.

Chest computed tomography scan showing (a) a nodule in the medial segment of the right middle lobe and (b) a smaller nodule in the lingular segment of the left upper lobe and several smaller lesions throughout both lungs.
The resection from the right middle lobe revealed a whitish, 2.2 cm in diameter hard nodule, which had infiltrated to the visceral pleura. A red grey nodule of medium hardness without visceral pleura infiltration and measuring 1.0 cm in diameter was also resected from the same side. A nodule without visceral pleura infiltration measuring 0.2 cm in diameter was resected from the right lower lobe.
Histopathological study of the larger nodule sections from the right middle lobe showed tumor tissue. The tumor cells were either round, polygonal, or short spindle‐shaped, with an abundant eosinophilic cytoplasm in which vacuoles were observed (Fig 3). The cells were arranged in nests and cords with interstitial hyaline degeneration. The nucleoli of the tumor cells were not prominent and were accompanied by little mitosis. Histopathological examination of the sections from the right lower lobe and the nodule of the right middle lobe revealed non‐tumoral tissue. The interlobular node was infiltrated with tumor cells, which were not observed in the lobar node. After immunohistochemical staining, the tumor cells were immunoreactive for CD31, CD34, Vimentin, and Bcl‐2, and partially reactive for CD99. The cells were negative for cytokeratin‐7, CD56, thyroid transcription factor 1, and Syn. The proliferative index (Ki‐67) was about 1% (Fig 3). All of these positive findings suggested a diagnosis of PEH.
Figure 3.
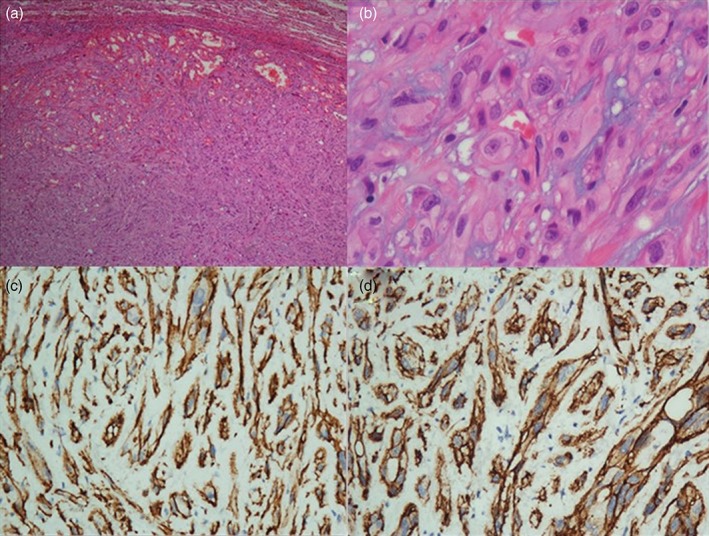
Surgical lung biopsy of the right middle lobe. (a) Low‐power view showing the lung tumor tissue surrounded by lung parenchyma (hematoxylin & eosin × 40 magnification). (b) Higher magnification of the lung tumor tissue showing epithelioid cells arranged in a nest and cords with interstitial hyaline degeneration (hematoxylin & eosin × 400 magnification). Immunohistochemical stain positive for (c) CD31 (× 400 magnification) and (d) CD34 (× 400 magnification).
After diagnosis, the patient's blood and tumor tissue were sent to a GENESEEQ agent (Nanjing, China) for genetic analysis using NSG to provide a guide for treatment. Results were obtained by detecting the exons of 416 genes containing their 1 463 540 bases. Eighteen mutated genes related to soft tissue sarcoma were found and the eight potential chemotherapeutic drug options were discussed.
Discussion
Epithelioid hemangioendothelioma was initially considered an aggressive form of bronchoalveolar cell carcinoma that invaded adjacent blood vessels and was thus named intravascular bronchoalveolar tumor.1 The development of the electron microscope and immunohistochemical techniques confirmed the endothelial lineage of the tumor cells and the term epithelioid hemangioendothelioma was coined in 1986.4 Clinically, EH is a rare vascular tumor with low to borderline malignancy between a benign hemangioma and a malignant angiosarcoma.5, 6 It is reported to arise from many organ systems, including the liver, bone, soft tissue, mediastinum, and lungs.7, 8
The mortality rate associated with EH varies according to the location of the tumor, reported as 13% in soft tissue, 35% in the liver, and 65% in lung tumors.9 EH arising from the lung and liver has a tendency to be multifocal.10 However, it is difficult in all cases to confirm whether the tumor is a primary, multicentric, or metastatic lesion.
Pulmonary EH is often diagnosed by chance because in most cases patients do not present with obvious symptoms.6, 7, 11, 12 Nevertheless, in most cases, patients complain of chest problems, such as dyspnea, cough, chest pain, and hemoptysis.13, 14, 15 Previous studies have found out that women tend to suffer from PEH more often than men and the mean age of patients suffering from PEH is around 40 years.8, 11, 16 In our case, the patient was male but was almost the same age at which patients usually present with PEH.
Pulmonary EH is mainly characterized by multiple nodules with well or ill‐defined margins in both lungs, as observed in this case. The nodules are usually less than 1 cm in diameter, although larger nodules have been reported. Radiographically, PEH can initially present as bilateral or unilateral multiple nodular opacities and are nearly always mistaken for metastatic carcinoma.17 PEH can also include pleural involvement and pleural effusion because diagnosis is difficult and often delayed.18 According to previous reports, bronchoalveolar lavage and transbronchial biopsies are often inadequate to make a definitive diagnosis.19 A diagnosis is always made based on histological features and immunohistochemistry results of pulmonary biopsy.20, 21 The immunoreactivity of PEH to vascular endothelial makers distinguishes it from mesothelial, epithelial, and muscular tissue differentiation.22 In this study, immunohistochemical staining for malignant cells with CD31 and CD34 were all positive and indicative of PEH.
Although the prognosis of PEH is difficult to predict, studies have reported that patients can survive from 10 to 20 years.4, 21, 23 However, cases with a poor prognosis have been reported.7, 24, 25 Horek et al. found that PEH patients with pleural effusion died within a year, while those without pleural effusion survived for longer than a year.26 In the same study, partial spontaneous regression of PEH was found in three asymptomatic patients who lived for five, 13, and 15 years after diagnosis. In a study of male patients, symptoms such as the presence of a cough, hemoptysis, chest pain, multiple unilateral nodules, and pleural effusion were all significant risk factors for PEH.8 The symptoms and the presence of pleural effusion were two independent predictors of survival in these patients.
Because of insufficient data, there is currently no standard treatment for PEH;22 however, NGS analysis of our patient's blood and tumor tissue revealed important information to guide treatment. CYP2D6*10 (C100T) and NQO1 gene mutations may indicate that a tumor is not sensitive to tamoxifen or mitomycin, respectively. CYP3A5*3 gene mutations may cause a patient to suffer greater toxicity when administered sunitinib. Fluoropyimidine can also cause greater incidence of side affects as a result of DPYD*2B and DPYP*9A gene mutations. UGT1A1*6 and CYP2B6*6 gene mutations may cause greater side effects in patients treated with irinotecan/etoposide and cyclophosphamide, respectively. The results indicated that our patient could benefit from platinum anti‐neoplastic drugs for ERCC1, GSTT1, and XRCC1 gene mutations. NGS can detect disease‐associated genomic alterations and might help to predict response to targeted therapies.26
According to previous published literature, partial spontaneous regression of PEH can occur and careful monitoring is considered an acceptable option, especially in asymptomatic patients.27 Surgical resection seems to provide remission in cases of solitary or multiple lesions. Currently, there is no sufficient evidence to recommend a specific drug regimen, although chemotherapeutic agents have been attempted.24 Complete remission after six courses of carboplatin plus etoposide chemotherapy has been reported,28 but our patient only underwent surgical resection. After almost a year of follow‐up, the patient is stable.
Mediastinal EH (MEH) is very rarely reported, with an approximate incidence of 8% in the literature.29 The clinical symptoms of MEH are non‐specific, and mainly depend upon local compression and stimulation of adjacent organs, such as chest pain, cough, dyspnea, and hoarseness of the voice. MEH is discovered in asymptomatic patients by physical examination.30 In nearly 65% of MEH patients, the tumor is located in the anterior mediastinum, and more than half of the MEH originates from blood vessels, especially from veins, including the brachiocephalic vein, superior vena cava, and azygos vein.31 Occasionally, the tumor involves multiple blood vessels and blocks the lumen.32 MEH is typically characterized by a soft‐tissue mass with a well‐defined margin on CT or magnetic resonance imaging.30 Because like PEH, MEH follows a relatively indolent clinical course, it is easily misdiagnosed as a benign tumor, such as a teratoma. For mediastinal tumors, poor preoperative prognostic factors include size >3 cm, malignant pleural effusion, and clinical symptoms related to the compression or obstruction of vascular structures.33 A complete resection of the tumor followed by vein reconstruction (if the vascular involved) is recommended. However, adjuvant therapy should be taken into account to avoid recurrence, particularly in cases that are not completely surgically resected.34
In conclusion, we described a case of PEH, a rare vascular tumor with intermediate malignancy. To our knowledge, our patient's genetic findings represent the first report explaining a possible relationship between mutated genes and drug treatment. Our discussion of the diagnosis of this tumor and therapeutic options may improve management strategies for PEH.
Disclosure
No authors report any conflict of interest.
References
- 1. Dail DH, Liebow AA. Intravascular bronchioloalveolar tumor. Am J Pathol 1975; 78: 6a–7a. [Google Scholar]
- 2. Semenisty V, Naroditsky I, Keidar Z, Bar‐Sela G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma‐‐a suitable treatment option: Case report and review of anti‐angiogenic treatment options. BMC Cancer 2015; 15: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakata KK, Gotway MB, Smith ML et al. Pulmonary epithelioid hemangioendothelioma diagnosed with endobronchial biopsies: A case report and literature review. J Bronchology Interv Pulmonol 2016; 23: 168–73. [DOI] [PubMed] [Google Scholar]
- 4. Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol 1986; 3: 259–87. [PubMed] [Google Scholar]
- 5. Ciliberti MP, Caponio R, Pascali A, Matichecchia G, Lioce M. A rare case of intravascular epithelioid hemangioendothelioma of the cephalic vein treated with surgery and postoperative radiation therapy: A case report and review of the literature. J Med Case Rep 2015; 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kundu S, Misra S, Biswas D, Mitra R, Naskar BG. Common presentation with uncommon diagnosis: Multifocal epithelioid hemangioendothelioma. Oman Med J 2015; 30: 477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cronin P, Arenberg D. Pulmonary epithelioid hemangioendothelioma: An unusual case and a review of the literature. Chest 2004; 125: 789–93. [DOI] [PubMed] [Google Scholar]
- 8. Amin RM, Hiroshima K, Kokubo T et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology 2006; 11: 818–25. [DOI] [PubMed] [Google Scholar]
- 9. Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: An overview and update on a rare vascular tumor. Oncol Rev 2014; 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: A vascular tumor often mistaken for a carcinoma. Cancer 1982; 50: 970–81. [DOI] [PubMed] [Google Scholar]
- 11. Mehta SR, Das A, Barnard N, Marcus A. Metastatic pulmonary epithelioid hemangioendothelioma: A case report and review of the literature. Respir Med Case Rep 2012; 7: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sugunaraj JP, Pedroso C, Kethireddy S, Mogri M. An unusual presentation of pulmonary epitheloid hemangioendothelioma. Respir Med Case Rep 2015; 16: 38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye B, Li W, Feng J, Shi JX, Chen Y, Han BH. Treatment of pulmonary epithelioid hemangioendothelioma with combination chemotherapy: Report of three cases and review of the literature. Oncol Lett 2013; 5: 1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shao J, Zhang J. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: A report of four cases and review of the literature. Oncol Lett 2014; 8: 2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim M, Chang J, Choi H et al. Pulmonary epithelioid hemangioendothelioma misdiagnosed as a benign nodule. World J Surg Oncol 2015; 13: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dail DH, Liebow AA, Gmelich JT et al. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer 1983; 51: 452–64. [DOI] [PubMed] [Google Scholar]
- 17. Mukundan G, Urban BA, Askin FB, Fishman EK. Pulmonary epithelioid hemangioendothelioma: Atypical radiologic findings of a rare tumor with pathologic correlation. J Comput Assist Tomogr 2000; 24: 719–20. [DOI] [PubMed] [Google Scholar]
- 18. Fan Y, Wang F, Li S, Ye C, Ying Y, Mao H. Pleural epithelioid hemangioendothelioma: A case report and literature review. J Natl Med Assoc 2016; 108: 124–9. [DOI] [PubMed] [Google Scholar]
- 19. Geramizadeh B, Ziyaian B, Dehghani M, Tahmasebi K. Prolonged hemoptysis caused by primary pulmonary epithelioid hemangioendothelioma; a case report and review of the literature. Iran J Med Sci 2014; 39 (2 Suppl.): 223–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Ouadnouni Y, Bouchikh M, Achir A et al. Pulmonary epithelioid hemangioendothelioma: A case report. Cases J 2009; 2: 8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jinghong X, Lirong C. Pulmonary epithelioid hemangioendothelioma accompanied by bilateral multiple calcified nodules in lung. Diagn Pathol 2011; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi L, Cheng D, Shi H et al. Pulmonary epithelioid hemangioendothelioma coexisting with pulmonary nodular amyloidosis: Case discussion and review of the literature. Int J Clin Exp Med 2014; 7: 1891–7. [PMC free article] [PubMed] [Google Scholar]
- 23. Okamura K, Ohshima T, Nakano R, Ouchi H, Takayama K, Nakanishi Y. A case of pulmonary epithelioid hemangioendothelioma surviving 10 years without treatment. Ann Thorac Cardiovasc Surg 2010; 16: 432–5. [PubMed] [Google Scholar]
- 24. Rosengarten D, Kramer MR, Amir G, Fuks L, Berkman N. Pulmonary epithelioid hemangioendothelioma. Isr Med Assoc J 2011; 13: 676–9. [PubMed] [Google Scholar]
- 25. Soo CI, Ng BH, Tan EL, Abdul Hamid F. Ambiguous presentations of pulmonary epithelioid hemangioendothelioma: Two case reports of a rare pulmonary malignancy. SAGE Open Med Case Rep 2016; 4: 2050313X16650323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horak P, Fröhling S, Glimm H. Integrating next‐generation sequencing into clinical oncology: Strategies, promises and pitfalls. ESMO Open 2016; 1: e000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitaichi M, Nagai S, Nishimura K et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J 1998; 12: 89–96. [DOI] [PubMed] [Google Scholar]
- 28. Pinet C, Magnan A, Garbe L, Payan MJ, Vervloet D. Aggressive form of pleural epithelioid haemangioendothelioma: Complete response after chemotherapy. Eur Respir J 1999; 14: 237–8. [DOI] [PubMed] [Google Scholar]
- 29. Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008; 32: 924–7. [DOI] [PubMed] [Google Scholar]
- 30. Wan Q, Zhou J, Yu Y et al. Epithelioid hemangioendothelioma of right innominate vein mimics a teratoma: A case report. Medicine (Baltimore) 2017; 96: e6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferretti GR, Chiles C, Woodruff RD, Choplin RH. Epithelioid hemangioendothelioma of the superior vena cava: Computed tomography demonstration and review of the literature. J Thorac Imaging 1998; 13: 45–8. [DOI] [PubMed] [Google Scholar]
- 32. Long K, Skinner S, Martin J. Epithelioid hemangioendothelioma encasing the left brachiocephalic vein. J Surg Case Rep 2014; 2014: pii: rju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brill JB, Schwartz IE, Prescher LM, Pratt TC. A case of an epithelioid hemangioendothelioma arising from the innominate vein mimicking cervical metastatic lymphadenopathy. Case Rep Surg 2016; 2016: 4238575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suster S, Moran CA, Koss MN. Epithelioid hemangioendothelioma of the anterior mediastinum. Clinicopathologic, immunohistochemical, and ultrastructural analysis of 12 cases. Am J Surg Pathol 1994; 18: 871–81. [DOI] [PubMed] [Google Scholar]


