Abstract
Micronodular thymoma with lymphoid stroma (MTWLS) is a rare type of thymoma that shows a similar pattern but varied morphology and immunophenotype of tumor cells. Because of the extremely limited number of cases reported, the pathology and biology of MTWLS are equivocal. Herein, we report two cases located in the anterior mediastinum: Case 1: a 58‐year‐old woman with a cystic mass measuring 5 × 3.0 × 2.5 cm in Mosaoka stage I; and Case 2: a 50‐year‐old man with a solid mass measuring 2.5 × 2.5 × 2.0 cm in stage IIb. Both patients were treated by thymectomy and are alive without recurrence or metastasis 15 and 17 months after surgery, respectively. Regardless of the spectrum of pathology and stage of MTWLS, this unique type of thymoma has a homogeneously favorable prognosis.
Keywords: Immunophenotype, micronodular thymoma with lymphoid stroma, morphology, prognosis
Introduction
Thymoma, a neoplasm originating from the epithelial cells of the thymus, is a rare malignancy, although it is the most common mediastinal tumor to occur in adults. Incidence rates reported in population studies have ranged from 2.2 to 2.6 per million per year.1 In addition to the usual types of thymoma, that is, A, AB, B1, B2, and B3, the Word Health Organization (WHO) classification recognizes some unusual types, including metaplastic thymoma, microscopic thymoma, sclerosing thymoma, lipofibroadenoma, and micronodular thymoma with lymphoid stroma (MTWLS).2
MTWLS is a very rare type of thymoma. Seventy‐four cases have been reported in the English literature to date since Suster and Moran described the first case in 1999.3 The rare occurrence of MTWLS means that we have limited understanding of its pathology and biology. In this study, we report two cases of MTWLS with very distinct tumor cell features, one of which is in a multilocular thymic cyst.
Case reports
Clinical findings of Case 1
A mass was detected by computed tomography (CT) in the anterior mediastinum of a 58‐year‐old woman who did not present with any symptoms. The mass was cystic and measured 5 × 3.0 × 2.5 cm. Thymectomy was performed using video‐assisted thoracoscopy (VATS). No recurrence or metastasis was documented during follow‐up 15 months after surgery on 31 December 2016.
Pathological findings of Case 1
The tumor was encapsulated and multilocular cystic with a small solid nodule within the fibrous septa. A single layer of flattened or cuboidal epithelium lined the cyst. The small solid nodule was 1.5 cm in its greatest dimension with a soft, gray‐white appearance, and was characterized by multiple small tumor islands surrounded by abundant lymphoid stroma. The lymphoid stroma was free of epithelial cells and contained lymphoid follicles with germinal centers, while only a few lymphocytes were scattered within the tumor islands. The tumor islands were composed of bland, short spindle to oval cells without mitoses and with Ki67 averaging 1%. They were diffusely positive for CK (AE1/AE3) and completely negative for CD5 and CD117. Stromal lymphocytes were CD20+/CD3+/TDT− (Figs 1, 2).
Figure 1.
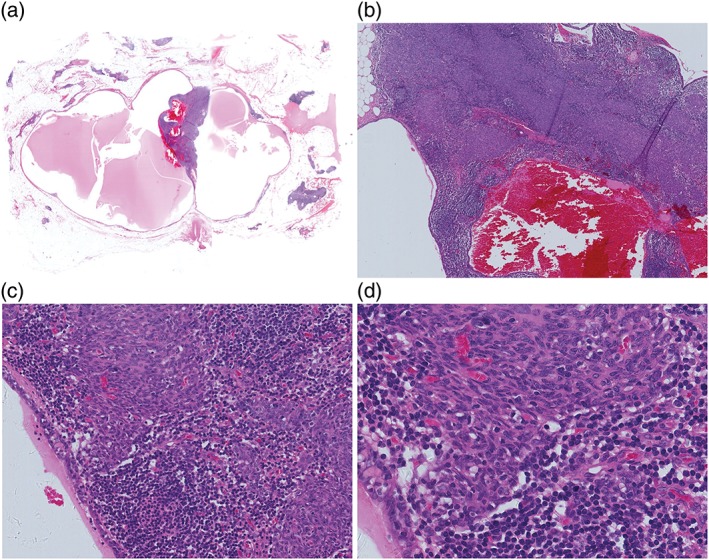
Case 1: Morphological features. (a) The tumor is multilocular cystic with a solid nodule within the fibrous septa. (b) The cyst is lined by a single layer of flattened or cuboidal epithelium; the solid nodule is characterized by multiple small tumor islands surrounded by abundant lymphoid stroma. (c) The lymphoid stroma is epithelial cell‐free and contains lymphoid follicles with germinal centers, with only a few lymphocytes scattered within tumor islands. (d) The tumor islands are composed of bland, short, spindle to oval cells without mitoses.
Figure 2.
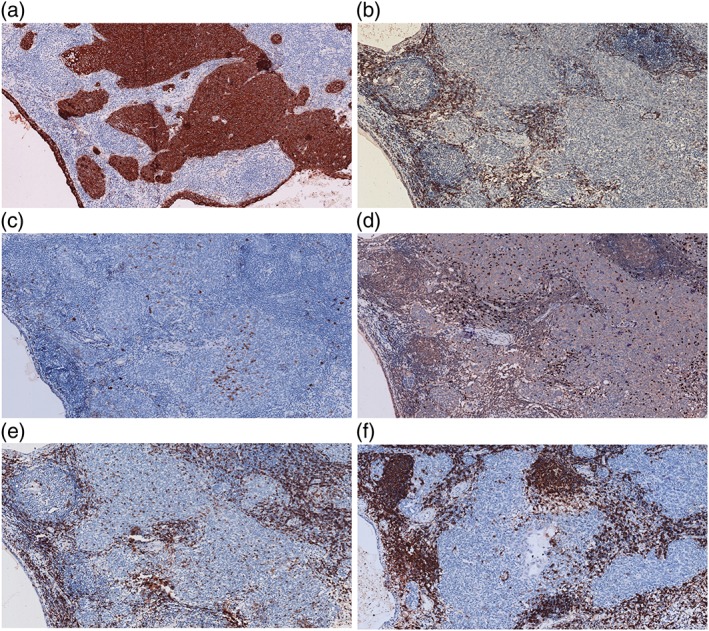
Case 1: Immunostaining pattern. The epithelial cells lining cysts and in tumor islands are diffusely positive for (a) CK (AE1/AE3) and are completely negative for (b) CD5 and (c) CD117. Stromal lymphocytes are negative for (d) TDT, and positive for (e) CD3 and (f) CD20.
Clinical findings of Case 2
A 50‐year‐old man without any symptoms was admitted to the hospital after a mass was detected in the anterior mediastinum via CT scan. The mass was solid and measured 2.5 × 2.5 × 2.0 cm. Thymectomy was performed using VATS. No recurrence or metastasis was documented during follow‐up 17 months after surgery on 31 December 2016.
Pathological findings of Case 2
The tumor was solid, soft, and gray‐white, on a cut surface, and was composed of several small nodules separated by fibrous, fatty thymic tissue. Each small nodule was characterized by multiple small tumor islands surrounded by abundant lymphoid stroma. The lymphoid stroma was free of epithelial cells and contained lymphoid follicles with germinal centers, while only a few lymphocytes were scattered within the tumor islands. The tumor islands were composed of round to polygonal cells with moderate atypia, mitoses up to 7/10 high‐power fields (HPFs) and Ki67 averaging 10%. They were diffusely positive for CK (AE1/AE3), as well as CD5 and CD117. Stromal lymphocytes were CD20+/CD3+/TDT− (Figs 3, 4).
Figure 3.
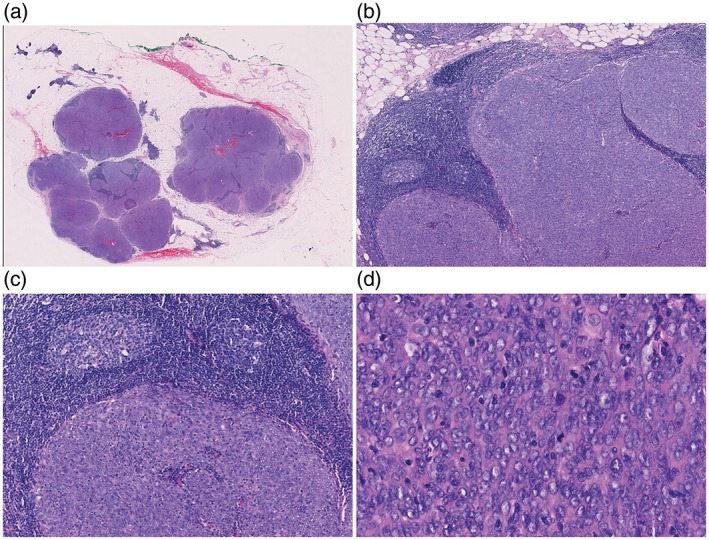
Case 2: Morphological features. (a) The tumor is composed of several small nodules separated by fibrous, fatty thymic tissue. (b) Each small nodule is characterized by multiple small tumor islands surrounded by abundant lymphoid stroma. (c) Lymphoid stroma is free of epithelial cells and contains lymphoid follicles with germinal centers, with only a few small lymphocytes scattered within tumor islands. (d) The tumor islands are composed of round to polygonal cells with moderate atypia and mitoses up to 7/10 high power fields.
Figure 4.
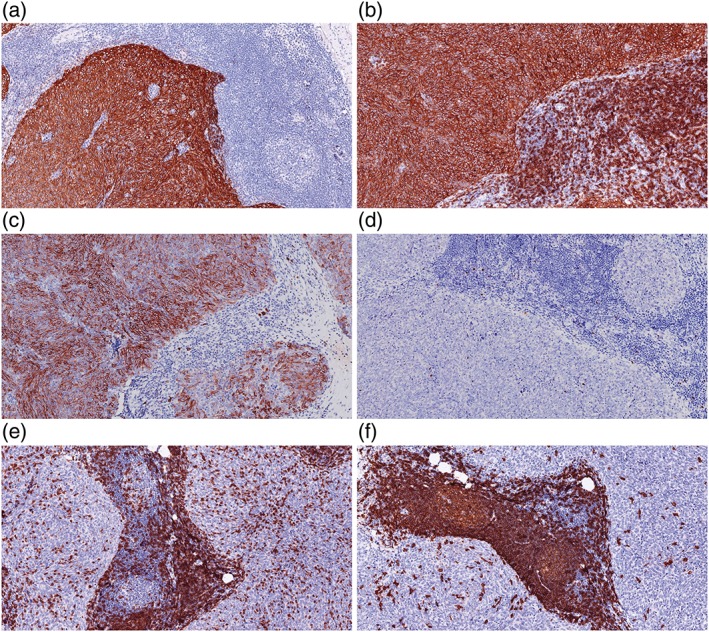
Case 2: Immunostaining pattern. The epithelial cells in tumor islands are diffusely positive for (a) CK (AE1/AE3), (b) CD5, and (c) CD117. Stromal lymphocytes are negative for (d) TDT and positive for (e) CD3 and (f) CD 20.
Discussion
Because of specific histological morphology and anatomic location, the most relevant prognostic factors for major thymoma are histological type and tumor stage.2, 4 However, as MTWLS is a rare type of thymoma, the correlation between prognosis and histological type/tumor stage is not well known.
Although thymoma is defined as a tumor of the thymic epithelium and only epithelial cells are recognized as tumor cells, a classification of thymoma is largely dependent on both epithelium and lymphocyte contents. In usual types of thymoma, including types A, AB, B1, B2, and B3, non‐neoplastic lymphocytes are distinctive immature T cells.2 MTWLS differs from these because of the noticeable lymphoid stroma consisting of mature B and T cells.2 Suster and Moran first described this unique type of thymoma in 1999.3 In their study, tumors in all 18 cases showed a characteristic pattern, that is, multiple small tumor islands surrounded by abundant epithelial cell‐free lymphoid stroma consisting of mature B and T cells, and the tumor cells were spindle‐shaped, containing oval nuclei devoid of atypia or mitoses.3 In subsequent studies, all tumors had the same pattern as the cases reported by Suster and Moran, but the morphology of the tumor cells varied.5, 6, 7, 8, 9, 10, 11, 12, 13 Eleven cases were spindle or oval cell predominant, and 14 cases were polygonal epithelial cell predominant.5, 6, 7, 8, 9, 10, 11, 12, 13 Thirty‐nine cases showed bland morphology and others showed various atypia, which were mild in 11 cases, moderate in 5, and severe in 9.6, 7, 8, 9, 10, 11, 12, 13 In 15 cases, no mitoses were detected, and in the others, mitoses varied from 1 to 16 per 10 HPFs.6, 7, 8, 9, 10, 11, 12, 13 In our study, both cases showed the characteristic pattern described in other cases of MTWLS reported in the literature, but the morphology of tumor cells was different. Case 1 was spindle and oval cell predominant, with bland morphology and absent mitoses, while Case 2 consisted predominantly of round to polygonal cells with moderate atypia and mitoses up to 7/10 HPFs. In addition to morphology, the immunophenotype of tumor cells in the cases reported in the literature was also varied. In 11 cases reported by Tateyama et al. CD5 immunostaining was diffusely positive in three, focally positive in four, and negative in four.8 In our study, CD5 and CD117 immunostaining were completely negative in Case 1 and diffusely positive in Case 2. It is well known that CD5 and CD117 are very helpful markers for differentiating thymoma from thymic carcinoma. The proportion of positive cases in thymoma versus thymic carcinoma is 3% versus 62% for CD5 and 3% versus 78% for CD117, respectively.2 This implies that stratifying tumors within the category of MTWLS is necessary. Tateyama et al. proposed the first histological grading system in 2000, in which MTWLS was graded into four groups: Grade 1: spindle cell predominant, bland cytology, no mitoses, CD5 negative; Grade 2: mixed spindle and polygonal cells, mild cytological atypia, no mitoses, CD5 negative; Grade 3: polygonal cell predominant, contained moderate cytological atypia, mitoses ≤ 2/10 HPF, focally CD5 positive; and Grade 4: polygonal cell predominant, with severe cytological atypia, mitoses >2/10 HPF, diffusely CD5 positive.8 According to Tateyama et al.'s criteria, Case 1 was grade 1 and Case 2 was grade 4. Based on our cases and the literature review, we draw the conclusion that MTWLS is a unique thymoma group showing a similar pattern but varied morphology and immunophenotype of tumor cells. The characteristic pattern of multiple small tumor islands surrounded by abundant epithelial cell‐free lymphoid stroma consisting of mature B and T cells is a key point to differentiate MTWLS from the usual thymoma types, while the pathology of tumor cells constitutes a spectrum from low to high grade.
In 68 cases available for Mosaoka staging in the literature, 37 cases (54%) were stage I, 25 (37%) were stage II, 4 (6%) were stage III, and 2 (3%) were stage IV.3, 6, 7, 8, 9, 10, 11, 12, 13 In our study, Case 1 was stage I and Case 2 was stage IIb.
In Tateyama's study, follow‐up data was available in 10 cases. Two patients died of another disease, while the other eight patients were alive with no evidence of disease during periods ranging from 1.5 to 15 years.8 In our study, both patients were alive without recurrence or metastasis during follow‐up periods of 15 and 17 months. In the literature, one case with a histological morphology of grade 4 died of MTWLS, 10 died of another disease, and the other 19 were alive without recurrence or metastasis during follow‐up periods of between 0.2 and 180 months, regardless of grade or stage.3, 5, 7, 8, 9, 10, 11, 12, 13 Based on this evidence, we drew a second conclusion that regardless of the spectrum of MTWLS pathology or stage, this unique type of thymoma shows a homogeneously favorable prognosis (Table 1).
Table 1.
Cases of micronodular thymoma with lymphoid stroma, including cases in the literature and from the current series
| Case | Age | Gender | Tumor cells | Atypia | Mitotic index | Masaoka staging | Follow‐up | Follow‐up duration |
|---|---|---|---|---|---|---|---|---|
| Suster and Moran 19993 | ||||||||
| 1 | 41 | F | S | No | No | 1 | LFU | LFU |
| 2 | 42 | M | S | No | No | 1 | LFU | LFU |
| 3 | 47 | M | S | No | No | 1 | LFU | LFU |
| 4 | 48 | F | S | No | No | 1 | A | 2Y |
| 5 | 48 | F | S | No | No | 1 | LFU | LFU |
| 6 | 55 | M | S | No | No | 1 | LFU | LFU |
| 7 | 57 | F | S | No | No | 1 | A | 3Y |
| 8 | 57 | M | S | No | No | 1 | LFU | LFU |
| 9 | 61 | F | S | No | No | 1 | LFU | LFU |
| 10 | 62 | M | S | No | No | 2 | A | 7Y |
| 11 | 63 | M | S | No | No | 4 | LFU | LFU |
| 12 | 66 | M | S | No | No | 1 | A | 1Y |
| 13 | 69 | F | S | No | No | 1 | A | 2Y |
| 14 | 70 | F | S | No | No | 1 | A | 1Y |
| 15 | 70 | M | S | No | No | 1 | DNON | 1Y |
| 16 | 72 | M | S | No | No | 1 | LFU | LFU |
| 17 | 76 | M | S | No | No | 1 | DNON | 1Y |
| 18 | 45 | M | S | No | No | Unavailable | LFU | LFU |
| Tateyama et al. 20018 | ||||||||
| 1 | 63 | M | S | No | 0 | 2 | DNON | 11Y 5M |
| 2 | 71 | F | S | No | 0 | 1 | A | 3Y 6M |
| 3 | 61 | F | SP | Mild | 0 | 1 | DNON | 6Y |
| 4 | 65 | F | SP | Mild | 0 | 1 | A | 3Y 6M |
| 5 | 58 | F | P | Mod | 2 | 1 | A | 14Y 7M |
| 6 | 73 | M | P | Mod | 2 | 2 | A | 3Y 7M |
| 7 | 61 | M | P | Mod | 1 | 3 | A | 1Y6M |
| 8 | 71 | F | P | Mod | 1 | 1 | A | 15Y |
| 9 | 72 | M | P | Mod | 1 | 1 | A | 3Y 6M |
| 10 | 56 | F | P | Severe | 5 | 3 | LFU | LFU |
| 11 | 59 | M | P | Severe | 6 | 4a | A | 39M |
| Thomas De Montpréville et al. 20026 | ||||||||
| 1 | 47 | M | S | No | No | 1 | A | 10Y5M |
| 2 | 73 | F | S | No | No | 2b | DNOD | 6D |
| 3 | 75 | M | S | No | No | 2b | A | 4Y |
| 4 | 59 | M | S | No | No | 2b | A | 3Y |
| 5 | 69 | F | S | No | No | 2b | A | Recent |
| 6 | 70 | M | S | No | No | 2a | A | Recent |
| Mende et al. 20049 | ||||||||
| 1 | 45 | M | S | No | No | 1 | A | 12M |
| Ströbel et al. 200510 | ||||||||
| 1–15 | 47–79 | 8M | S | No | No | Stage 1 in 5; | A | 24‐190M |
| 7F | Stage 2 in 7; | |||||||
| LFU in 3 | ||||||||
| 16 | 68 | M | S | No | No | 2 | A | 24M |
| 17 | 69 | M | S | No | No | 2 | LFU | LFU |
| 18 | 72 | M | S | No | No | 2 | A | 24M |
| Mourra et al. 200511 | ||||||||
| 1 | 68 | F | S | No | No | 1 | LFU | LFU |
| El et al. 200612 | ||||||||
| 1 | 62 | F | S | No | No | 2 | A | 2Y |
| 2 | 64 | M | S | No | No | 1 | A | 17M |
| Kim et al. 20137 | ||||||||
| 1 | 73 | M | S | No | No | 1 | A | 12M |
| Mneimneh et al. 20155 | ||||||||
| 1 | 79 | F | S | No | 2 to 8 | 1 | LFU | LFU |
| 2 | 83 | M | P | Mild | 3 | 1 | LFU | LFU |
| 3 | 70 | M | P | Mild | 11 | 2a | LFU | LFU |
| 4 | 64 | F | S | No | 1 | 2b | LFU | LFU |
| 5 | 61 | F | SP | No | 2 | 2a | LFU | LFU |
| 6 | 73 | F | SP | No | 2 | 2a | A | 6Y |
| 7 | 63 | M | P | Mild | 5 | 2a | A | 3Y |
| 8 | 51 | M | SP | Mild | 5 | 2a | A | Recent |
| 9 | 53 | F | S | No | 1 | 2a | A | 3Y |
| 10 | 75 | F | P | Severe | 8 | 1 | LFU | LFU |
| Weissferdt and Moran 201213 | ||||||||
| 1 | 61 | F | P | Severe | 5 to 16 | 1 in 3 3 in 2 |
A | 3M |
| 2 | 78 | M | P | Severe | A | 26M | ||
| 3 | 71 | M | P | Severe | A | 26M | ||
| 4 | 67 | F | P | Severe | DOD | 21M | ||
| 5 | 42 | M | P | Severe | A | 24M | ||
| Current case | ||||||||
| 1 | 58 | F | S | No | 0 | 1 | A | 15M |
| 2 | 50 | M | P | Mod | 7 | 2b | A | 17M |
A, alive; D, day; DNOD, died, not of disease; DOD, died of disease; LFU, lost to follow‐up; M, month; P, polygonal predominant; S, spindle predominant; SP, spindle and polygonal; Y, year.
In the newest WHO classification of thymoma, MTWLS is defined as benign. Compared to type A, AB, B1, B2, and B3, all of which are defined as malignant, the biological behavior of MTWLS is unique and may be related to histological pattern. The micronodular pattern of MTWLS is caused by overgrowth of lymphoid stroma, which divides sheets of tumor cells into small islands. It is well known that prosperous lymphoid stroma in tumors implies a strong host immune response to tumor antigens, which defend patients from tumors and leads to improved overall survival.6, 9, 11, 14
One of our cases developed in a multilocular thymic cyst (MTC). Thymic cysts, both congenital and acquired, are relatively uncommon and account for 3% to 5% of all mediastinal masses. A unilocular thymic cyst is usually congenital and thought to originate from sequestrated embryonal nests, whereas MTC is acquired and caused by the cystic transformation of medullary duct epithelium‐derived structures, as well as Hassall corpuscles.14 MTC has been associated with various inflammatory conditions, but is rare with thymoma; only 32 cases have been reported in the English literature, of which only one was MTWLS.14, 15, 16, 17 Therefore, we now report the second case of MTC with MTWLS. Based on data of 20 cases of MTC with thymoma, Nakamura et al. considered that thymoma that develops in MTC may exhibit better clinical behavior compared to a solid thymoma.17 Further investigation is necessary to confirm their results.
In conclusion, micronodular thymoma with lymphoid stroma is a very rare type of thymoma showing a similar pattern but varied morphology and immunophenotype of tumor cells. Despite the spectrum of pathology and stage of MTWLS, this unique type of thymoma shows a homogeneously favorable prognosis.
Disclosure
No authors report any conflict of interest.
References
- 1. Gadalla SM, Rajan A, Pfeiffer R et al A population‐based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer 2011; 128: 2688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 2015; 10: 1240–2. [DOI] [PubMed] [Google Scholar]
- 3. Suster S, Moran CA. Micronodular thymoma with lymphoid B‐cell hyperplasia: Clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol 1999; 23: 955–62. [DOI] [PubMed] [Google Scholar]
- 4. Weis CA, Yao X, Deng Y et al The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015; 10: 367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mneimneh WS, Gökmen‐Polar Y, Kesler KA, Loehrer PJ Sr, Badve S. Micronodular thymic neoplasms: Case series and literature review with emphasis on the spectrum of differentiation. Mod Pathol 2015; 28: 1415–27. [DOI] [PubMed] [Google Scholar]
- 6. Thomas De Montpréville V, Zemoura L, Dulmet E. [Thymoma with epithelial micronodules and lymphoid hyperplasia: Six cases of a rare and equivocal subtype.] Ann Pathol 2002; 22: 177–82. (In French.) [PubMed] [Google Scholar]
- 7. Kim NR, Lee JI, Ha SY. Micronodular thymoma with lymphoid stroma in a multilocular thymic cyst: A case study. Korean J Pathol 2013; 47: 392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tateyama H, Saito Y, Fujii Y et al The spectrum of micronodular thymic epithelial tumours with lymphoid B‐cell hyperplasia. Histopathology 2001; 38: 519–27. [DOI] [PubMed] [Google Scholar]
- 9. Mende S, Moschopulos M, Marx A, Laeng RH. Ectopic micronodular thymoma with lymphoid stroma. Virchows Arch 2004; 444: 397–9. [DOI] [PubMed] [Google Scholar]
- 10. Ströbel P, Marino M, Feuchtenberger M et al Micronodular thymoma: An epithelial tumour with abnormal chemokine expression setting the stage for lymphoma development. J Pathol 2005; 207: 72–82. [DOI] [PubMed] [Google Scholar]
- 11. Mourra N, Duron F, Parc R, Flejou JF. Cervical ectopic thymoma: A diagnostic pitfall on frozen section. Histopathology 2005; 46: 583–5. [DOI] [PubMed] [Google Scholar]
- 12. El MF, Braham E, Ayadi A, Ismail O, Kilani T. Micronodular thymoma with lymphoid stroma: Report of two cases and particular association with thymic lymphoid hyperplasia in one case. Pathology 2006; 38: 586–8. [DOI] [PubMed] [Google Scholar]
- 13. Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: A clinicopathological and immunohistochemical study of five cases. Mod Pathol 2012; 25: 993–9. [DOI] [PubMed] [Google Scholar]
- 14. Singhal M, Lal A, Srinivasan R, Duggal R, Khandelwal N. Thymic carcinoma developing in a multilocular thymic cyst. J Thorac Dis 2012; 4: 512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izumi H, Nobukawa B, Takahashi K et al Multilocular thymic cyst associated with follicular hyperplasia: Clinicopathologic study of 4 resected cases. Hum Pathol 2005; 36: 841–4. [DOI] [PubMed] [Google Scholar]
- 16. Weissferdt A, Moran CA. Thymic carcinoma associated with multilocular thymic cyst: A clinicopathologic study of 7 cases. Am J Surg Pathol 2011; 35: 1074–9. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura S, Tateyama H, Taniguchi T et al Multilocular thymic cyst associated with thymoma: A clinicopathologic study of 20 cases with an emphasis on the pathogenesis of cyst formation. Am J Surg Pathol 2012; 36: 1857–64. [DOI] [PubMed] [Google Scholar]


