Abstract
A 57‐year‐old man was admitted to our department 10 years ago, diagnosed with tracheal adenoid cystic carcinoma. After discontinuing chemotherapy and radiotherapy, the disease recurred in December 2016. Apatinib mesylate (500 mg/day) was administered and computed tomography revealed that his symptoms were significantly relieved. Treatment with apatinib mesylate represents a novel method of treatment for tracheal adenoid cystic carcinoma.
Keywords: Adenoid cystic carcinoma, apatinib, main bronchus, trachea
Introduction
Tracheal adenoid cystic carcinoma (ACC) is a rare low‐grade malignant cancer primarily originating in the trachea and bronchi.1 It has a low incidence, with a ratio of 10–20% of all tracheal primary cancers and grows slowly.2, 3 Surgical resection is the preferred and most effective treatment because ACC is resistant to radiotherapy and chemotherapy.
Moreover, targeting therapy and immunotherapy against ACC are still at the experimental stage and have not been generally applied to patients with ACC until now. Apatinib is a novel multiple kinase inhibitor targeting multiple sites, including VEGFR‐2 which correlates with angiogenesis, and it can be used in many types of cancer, such as advanced gastric cancer, non‐small‐cell lung carcinoma (NSCLC), and hepatocellular carcinoma.4
Herein, we report an unusual case of tracheal ACC treated with apatinib mesylate because of the patient's unwillingness to undergo surgical resection. The treatment achieved a good effect.
Case report
A 57‐year‐old man was admitted to our department on 16 July 2007 with a cough, expectoration, and pressure and suffocation in the chest, which had been all present for over six months, but were exacerbated over the last month. He had smoked 10 cigarettes every day for more than 30 years. Chest computed tomography (CT) performed at Jixian County People's Hospital showed space‐occupying lesions in the middle lobe of the right lung and lung cancer was suspected, with mediastinal lymph node metastasis (data not shown). Furthermore, slight intumescentia was found on the right wall of the trachea near the carina and cauliflower‐like neoplasms were detected on both the right main bronchial wall and the middle lobe of the right lung by fibrobronchoscope. Neoplasms also obstructed the bronchial lumen. Tracheal ACC was diagnosed by means of pathological hematoxylin and eosin (H&E) staining and immunohistochemistry (Fig 1). The tumor was classified as T4N3M0, stage IIIB.
Figure 1.

Pathological analysis. (a) Pathological hematoxylin and eosin staining; (b) immunohistochemistry/specific staining: CK(AE1)(−) CK(AE3)(+) CK34(+) CK5/6(+) P63(+) CgA(−) Syn(−) Calcitotin(−) 5‐HT(−) S‐100(−) NSE(−) TTF(+).
Because the patient and his family were unwilling to proceed with surgical resection, he was treated with cisplatin (60 mg days 1–2, 30 mg day 3) and paclitaxel (270 mg day 2) in a cycle. Treatment for hepatoprotection, acid inhibitors, and antiemetics were simultaneously administered to reduce adverse effects. However, because of economic reasons, the patient did not undergo regular examinations or continuous treatment, and was treated with four rounds of chemotherapy on July 25, August 15, and November 6 in 2007 and June 3 in 2008. Upon chest CT reexamination in November 2007 (after two cycles of chemotherapy) (Fig 2), the patient's pulmonary lesions had improved slightly and the therapeutic evaluation was stable disease (SD).
Figure 2.
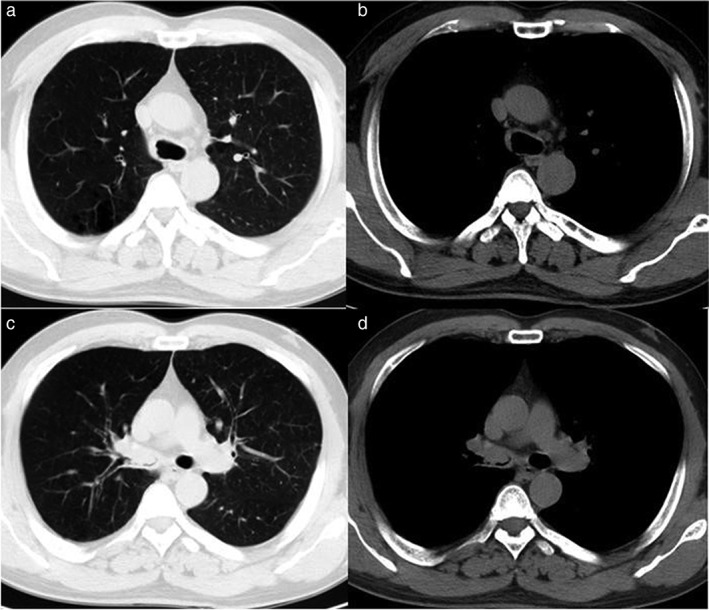
Chest computed tomography images after two cycles of chemotherapy. (a,b) The right wall of the trachea was thickened and lymph nodes near the trachea were slightly enlarged; (c,d) the right main bronchus and right superior lobar bronchus were filled with parenchyma and the lumen was narrow. (7 November 2007).
Nevertheless, in June 2008, CT reexamination showed that the pulmonary lesions had progressed. Although the patient was still evaluated as having SD, further treatment was required. Therefore, the patient visited the local county hospital for primary pulmonary tumor lesion and metastatic lymph node radiotherapy in July 2008 and was treated with 60 Gy in 30 fractions (2 Gy per fraction). Thereafter, his symptoms of cough and suffocation improved and another chest CT revealed that the tumors had shrunk.
The patient had not received any type of therapy for five years after the first course of radiotherapy until March 2014. As a result of disease progression, the patient visited the local county hospital once again for a second round of pulmonary tumor radiotherapy with a dose of 60 Gy in 30 fractions (2 Gy per fraction). Symptoms of suffocation and cough thereafter improved and chest CT revealed that the tumors had once again shrunk.
A severe cough, and pressure and suffocation in the chest had once again worsened by December 2016. Chest CT results showed space‐occupying lesions in the trachea and the right main bronchus with left pulmonary metastasis (Fig 3a,b), and the tumor was T4NxM1, stage IV. In February 2017, the patient was administered 500 mg/day apatinib mesylate. Symptoms including the cough, and pressure and suffocation in the chest gradually decreased. However, after administration of apatinib mesylate, the patient experienced a headache and his blood pressure increased to a maximum of 160/100 mm/Hg. Valsartan was administered and as a result his blood pressure returned to a normal level. Other adverse reactions included loss of appetite and desquamation of fingers and toes. Chest CT reexamination in April and June 2017 showed that the tumors in the trachea and right main bronchus were reduced. The metastatic foci in the left lung also shrank and cavitation developed (Fig 3c–f).
Figure 3.
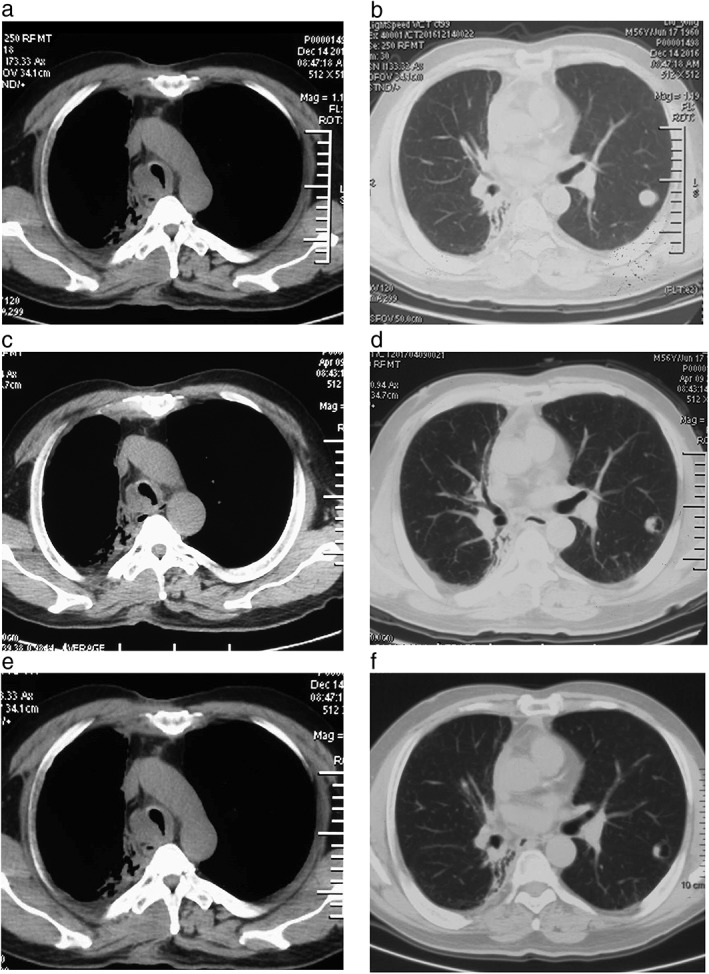
Chest computed tomography images after two cycles of radiotherapy and apatinib treatment. (a) The right wall of the trachea had thickened. Neoplasms in the parenchyma grew along the bronchial wall in the right main bronchus, extruded into lumen, and made the lumen narrow and (b) nodule metastasis occurred in the left upper lobe (14 December 2016). (c) The tumor in the trachea decreased and the stenosis of the lumen recovered, and (d) metastasis loci in the left upper lobe decreased slightly and porosis formed (9 April 2017). (e) The tumor in the trachea decreased slightly, (f) the nodule in the inner wall of the left upper lobe decreased, and porosis increased (5 June 2017).
Discussion
Tracheal ACC is a rare low‐grade malignant neoplasm and primarily occurs in the trachea and bronchi, originating from ducts in the mucous glands. Tracheal ACC accounts for 0.04–0.2% of lung cancer cases5 and is distributed equally between genders.6 The age of onset ranges from 40 to 59 years; our patient was 59. Lung ACC is characterized by slow disease progression and a long duration. The course of the disease is usually two to three years, and in some cases it can last more than 10 years;7our patient had the disease for 10 years. Treatments for lung ACC include surgical resection, radiotherapy, and chemotherapy.6 In some cases, fiberoptic bronchoscopic laser, flucticuli, electrotomy, and stent implantation have also been reported to be effective.8 Lung ACC is clinically rare and as such there is no uniform standard therapy. Surgical resection is the main method of radical treatment, and although some patients display benefits, overall, radiotherapy is ineffective for lung ACC patients. In our case, the evaluation was stable disease after chemotherapy, and radiotherapy had a positive effect with five‐year PFS after the first radiotherapy course. However, after that period, the patient's condition progressed severely. Considering the unsatisfactory effect of the previous chemotherapy, and that radiotherapy could not be administered, the patient received 500 mg per day of apatinib mesylate for treatment. His symptoms, such as the cough, and pressure and suffocation in the chest gradually improved. Chest CT reexamination showed that the neoplasm in the right main bronchus diminished, metastatic foci decreased in the left lung after two months, and the porosis expanded after four months. The patient experienced minor adverse effects, including hand‐foot syndrome, blood pressure elevation, and a slight loss of appetite.
Apatinib is a novel oral small molecular angiogenesis inhibitor that effectively inhibits tumor‐induced angiogenesis and decreases tumor growth by targeting VEGFR2.9, 10 Apatinib selectively competes highly with ATP binding sites on VEGFR2; blocks downstream signal transduction by targeting c‐Kit, RET and c‐Src; and plays an anti‐angiogenic role in in tumor tissue.11 Although little clinical research on the use of apatinib mesylate has been conducted, most cases have indicated a certain potential curative effect on advanced various tumors.12 There are currently two clinical trials of apatinib ongoing in head and neck ACC. We expect these trials to produce elicitations for the treatment of tracheal ACC.
Case reports of primary tracheal and bronchial ACC are rare, and no case report has previously documented the use of apatinib mesylate treatment in this tumor. In our case, the patient achieved positive effects and sustainable adverse effects with apatinib treatment. Apatinib has great therapeutic potential in tracheal and bronchial ACC treatment; however, a determination of whether this tumor has some target genes of apatinib, such as VEGFR‐2 or c‐Kit (CD117),13 requires further research.
Disclosure
The author has no conflict of interest.
References
- 1. Qing S, Zhou K, Liu X, Li X, Deng F, Ma Y. Primary pulmonary adenoid cystic carcinoma: Clinicopathological analyses of 12 cases. Int J Clin Exp Pathol 2015; 8: 7619–26. [PMC free article] [PubMed] [Google Scholar]
- 2. Kurul IC, Demiroz SM, Celik A et al Primary pulmonary adenoid cystic carcinoma: Report of two cases. Asian Cardiovasc Thorac Ann 2012; 20: 604–6. [DOI] [PubMed] [Google Scholar]
- 3. Huo Z, Meng Y, Wu H et al Adenoid cystic carcinoma of the tracheobronchial tree: Clinicopathologic and immunohistochemical studies of 21 cases. Int J Clin Exp Pathol 2014; 7: 7527–35. [PMC free article] [PubMed] [Google Scholar]
- 4. Langer CJ, Mok T, Postmus PE. Targeted agents in the third−/fourth‐line treatment of patients with advanced (stage III/IV) non‐small cell lung cancer (NSCLC). Cancer Treat Rev 2013; 39: 252–60. [DOI] [PubMed] [Google Scholar]
- 5. Shinohara S, Hanagiri T, Takenaka M et al Primary adenoid cystic carcinoma of the peripheral lungs. J UOEH 2015; 37: 121–5. [DOI] [PubMed] [Google Scholar]
- 6. Hu MM, Hu Y, He JB, Li BL. Primary adenoid cystic carcinoma of the lung: Clinicopathological features, treatment and results. Oncol Lett 2015; 9: 1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qin M, Fu Y, Yu D, Xu S, Han M, Wang Z. [Diagnosis and treatment of tracheal or bronchuotracheal adenoid cystic carcinoma.] Chin J Lung Cancer 2010; 13: 628–31. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shure D, Fedullo PF, Plummer M. Carinal forceps biopsy via the fiberoptic bronchoscope in the routine staging of lung cancer. West J Med 1985; 142: 511–3. [PMC free article] [PubMed] [Google Scholar]
- 9. Ding J, Chen X, Dai X, Zhong D. Simultaneous determination of apatinib and its four major metabolites in human plasma using liquid chromatography‐tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 895–896: 108–15. [DOI] [PubMed] [Google Scholar]
- 10. Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015; 9: 6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian S, Quan H, Xie C et al YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor‐2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin SK. Phase III study of Apatinib in advanced gastric cancer: A randomized, double‐blind, placebo‐controlled trial[C]. J Clin Oncol 2014; 32 (Suppl. 15): Abstract 4003. [Google Scholar]
- 13. Jiang MJ, Weng SS, Cao Y et al Metachronous primary adenocarcinoma of lung during adjuvant imatinib mesylate therapy for gastrointestinal stromal tumor of stomach: A case report. Medicine (Baltimore) 2015; 94 (36): e1484. [DOI] [PMC free article] [PubMed] [Google Scholar]


