Abstract
Background
Although FGF 19 gene aberrations are associated with carcinogenesis and progression in human cancers, the roles of FGF 19 genetic amplification and expression in Chinese patients with lung squamous cell carcinoma (LSCC) and FGF 19 amplification as a potential therapeutic target for LSCC are not well understood.
Methods
Fluorescence in situ hybridization analysis and quantitative real‐time‐PCR was used to detect FGF 19 genetic amplification and FGF 19 messenger RNA expression in LSCC tumor and paired adjacent samples. Small interfering RNA and short hairpin RNA were used to knockdown FGF19 in vitro and in vivo.
Results
FGF 19 amplification was identified in a subset of LSCC patients (37.5%, 15/40), and upregulation of FGF 19 expression was found in 60% (24/40) of tumor tissues compared to adjacent non‐tumorous tissues. Correlation analysis with clinicopathologic parameters showed that FGF 19 upregulation was significantly associated with heavy smoking. Small interfering RNA knockdown of FGF 19 led to the significant inhibition of cell growth and induced apoptosis in LSCC cells carrying the amplified FGF 19 gene, but these effects was not observed in non‐amplified LSCC cells. Interfering FGF 19 expression with short hairpin RNA also resulted in tumor growth inhibition and induced apoptosis in LSCC xenografts with amplified FGF 19 in tumor cells.
Conclusion
Our results suggested that FGF 19 signaling activation is required for cell growth and survival of FGF 19 amplified LSCC cells, both in vitro and in vivo. Intervention of FGF 19 activation could be a potential therapeutic strategy for LSCC patients with FGF 19 amplification.
Keywords: Apoptosis, cell proliferation, FGF19, genetic amplification, lung squamous cell carcinoma
Introduction
Lung cancer is the leading cause of cancer death worldwide.1, 2, 3 Lung squamous cell carcinoma (LSCC) is the second most common type of lung cancer, accounting for approximately 30% of lung cancer cases, and is significantly associated with cigarette smoking.4 No targeted therapeutic agent is available for the clinical treatment of LSCC. Currently, overall survival for LSCC patients with advanced disease remains unsatisfactory, with a five‐year survival rate for patients with late stage metastatic LSCC below 10%.5, 6, 7 Thus, further investigation of the molecular pathogenesis of LSCC and the development of better clinical management strategies are required to improve survival in LSCC patients.
Lung squamous cell carcinoma is a particularly heterogeneous disease. The specific gene aberrations, such as gene amplification, mutation, or deletion/insertion, that drive LSCC development and progression have not been well characterized. A number of candidates have been identified as potential clinical targets for cancer treatment, including amplification of SOX2, PDGFR2, FGFR1 and deletion of CDKN2A, and recurrent mutations in TP53, KLHL19, NRF2, CDC4, GPRC1H, MUC16, RUNX1T1, BAI3, LKB1, and HER4 genes.8, 9, 10, 11 However, no effective targeted agents have emerged based on these related targets/pathways. Therefore, identification of the novel molecular targets for LSCC is urgently required.
FGF19 is a hormone‐like enterokinase released postprandially.12 Previous studies have shown that FGF19 is widely expressed in human tissues and plays an important role in cell proliferation, morphogenesis, differentiation, tissue repair, and motility.13, 14 FGF19 is a high affinity, unique ligand that binds to FGFR4 specifically in a heparin dependent manner. The interaction of ligand and receptor mediates almost all related biological activities at both the N and C terminals of FGF19. 15 A previous study suggested that the FGF19‐FGFR4 signaling axis may be a crucial player, contributing to the development of a certain type of hepatocellular carcinoma (HCC), drawing great attention to therapeutic intervention of the FGF19/FGFR4 autocrine loop in this disease setting.16, 17 Small interfering RNA (siRNA)‐mediated silencing of FGF19 was also reported to decrease AKT phosphorylation, inhibit cancer cell growth, and sensitize doxorubicin treatment in both FGFR4 and FGF19 positive breast cancer cells. These data indicate that inhibition of the FGF19 pathway may potentially serve as a novel therapeutic strategy for the treatment of human cancers.18
In the current study, the genetic amplification of FGF19 was identified in a subset of Chinese patients with LSCC, and we explored the potential role of FGF19 in cancer cell proliferation and apoptotic response in human lung squamous cancer cells and in LSCC xenografts with FGF19 gene amplification in tumor cells.
Methods
Patients
To examine FGF19 amplification and upregulation of FGF19 expression in LSCC tumor tissues, we collected 40 fresh tumor tissues and paired adjacent non‐tumorous tissues from patients at The First Affiliated Hospital, Zhejiang University from April 2013 to December 2014. The median age of the patients was 60.0 years (range 32–83), 82.5% of patients were male (n = 33), and 85% of patients (n = 34) had a history of smoking.
Cell culture
Lung squamous cell carcinoma cell line EPLC‐272H and NCI‐H1703 were purchased from American Type Culture Collection (Rockville, MD, USA). The two cell lines were cultured in RPMI‐1640 medium and supplemented with 10% fetal bovine serum (FBS) (Gibco, Waltham, MA, USA). The cell lines were maintained in an incubator containing 5% CO2 at 37°C.
Tissue samples
All fresh tissue samples, including tumor and adjacent non‐tumorous tissues, were collected from LSCC patients after surgery at The First Affiliated Hospital, Zhejiang University, Zhejiang, China. Informed consent was obtained from all patients, and the Medical Ethics Committee of The First Affiliated Hospital, Zhejiang University approved all procedures in advance. All tissues were frozen immediately in liquid N2 and stored in a freezer at −80°C. Two independent pathologists evaluated the collected tissues before the following experiment was conducted.
Fluorescence in situ hybridization analysis
Briefly, the FGF19 fluorescence in situ hybridization (FISH) probe and the centromere of chromosome 11 (CEN11P) probe were labeled with Texas Red and
fluorescein isothiocyanate to identify gene amplification (FG0125, Abnova, Taibei, Taiwan). Tissue sections (4 μm) were pretreated with a Tissue Pretreatment Kit (KA2375, Abnova). The tissue samples and FGF19/CEN11P probes were hybridized at 37°C for 48 hours after denaturing at 80°C for five minutes. The samples were then washed with post hybridization wash buffer three times at 75.5°C, and the nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole.
Image analysis was conducted using a fluorescence microscope and Cyto‐Vision (Leica, Solms, Germany). We enumerated the FGF19 gene and chromosome 11 in 50 tumor nuclei for each sample and calculated a ratio of FGF19 to CEN11P. An amplified sample was defined by a FGF19 to CEN11P ratio >2 or the presence of a >10% gene cluster.
Messenger RNA expression analysis
Total RNAs extracted with Trizol and cDNAs were synthesized using a High Capacity RNA‐to‐cDNA Master Mix (Applied Biosystems, Foster City, CA, USA). Quantitative real‐time‐PCR was performed using FGF19 TaqMan assay Hs00192780_m1 and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) TaqMan assay Hs99999905_m1 (Invitrogen, Carlsbad, CA, USA) on an ABI 7500 instrument (Applied Biosystems). The messenger RNA (mRNA) levels were normalized to GAPDH as an internal control. To compare the FGF19 expression between tumor and paired adjacent samples, the mRNA levels of the tumor sample were normalized to the normal samples. A twofold change or greater was defined as upregulation.
Western blot analysis
Western blot analysis was conducted using 20 μg of cell lysates in radioimmunoprecipitation assay buffer and the proteins were examined with monoclonal antibodies directed against FGF19 (83 348), p‐FRS2 (3961), AKT (11 962), p‐AKT (9271), ERK (4695), p‐ERK (12 638), cleaved‐Caspase 3 (CC3; 9661) and anti‐GAPDH (5174, Cell Signaling Technology, Danvers, MA, USA), and FRS2 (ab10425), FGFR4 (ab5841), and p‐FGFR4 (ab192589, Abcam, Cambridge, UK).
Enzyme‐linked immunosorbent assay analysis
Supernatant collected from cultured 106 cells was centrifuged to remove cell debris. Standard protein was serial diluted for enzyme‐linked immunosorbent assay (ELISA) analysis as a standard curve. FGF19 concentrations in cell supernatant were determined using a Human FGF‐19 Quantikine ELISA Kit, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). FGF19 concentrations (pg/mL) were calculated according to a standard curve.
Small interfering RNA transfection
Small interfering RNA transfection cells were seeded at a density of 3 × 105 cells/well in six well plates. The cells were transfected with siRNA using Lipofectamine 2000 following the manufacturer’s instructions (Invitrogen). siRNA control and FGF19 siRNAs (Sigma‐Aldrich, https://en.wikipedia.org/wiki/St._Louis,_Missouri USA) were used. qPCR and Western blot analysis were used to verify the gene silencing efficiency after transfection. After 72 hours, cells were analyzed for cell growth inhibition.
In vitro and in vivo short hairpin RNA knockdown of FGF 19 expression
The constructs used for short hairpin RNA (shRNA) knockdown experiments contained FGF19 specific shRNA sequences under the control of the tetracycline promoter. The plasmid of FGF19 shRNA (sc‐39480‐V) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Briefly, LSCC EPLC‐272H cells with genetic FGF19 amplification were transfected with the FGF19 shRNA constructs, or a construct containing enhanced GFP (EGFP) shRNA sequence. We used puromycin to select the successfully transfected cells, in which the expression of FGF19 shRNA can be induced by doxycycline (Sigma‐Aldrich).
In vitro, 1 mg/mL doxycycline was used to induce the EPLC‐272H cells for three days, and cell proliferations analysis was performed by MTS assay (tetrazolium‐based CellTiter 96 AQueous One Solution Cell Proliferation assay, Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions. Expressions of FGF19 and secret FGF19 protein in the supernatant were measured by Western blot and ELISA assay.
In vivo, transfected EPLC‐272H cells and parental EPLC‐272H cells were inoculated subcutaneously into the right thigh of female Nu/Nu mice (Charles River, Beijing, China). When the tumor volumes reached approximately 200 mm3, the mice were randomized into four groups of 10 mice per group: (i) FGF19‐sh‐RNA transfected EPLC‐272H mice were treated with 2 mg/mL of doxycycline, (ii) FGF19‐sh‐RNA transfected EPLC‐272H mice were treated with sucrose solution as a control, (iii) EPLC‐272H mice were treated with 2 mg/mL of doxycycline, and (iv) EPLC‐272H mice were treated with sucrose solution. The doxycycline was administered orally for 14 days. The tumor size and body weight of the mice were measured every three days. Tumor volumes were determined according to the equation: V = (length × width2)/2 by measuring two perpendicular diameters with calipers. The tumor growth inhibition was calculated using the equation %TGI = 1 − (change of tumor volume in treatment group/change of tumor volume in the control group) × 100.
Immunohistochemistry
Xenograft tumor tissues were collected after 14 days of both doxycycline and corresponding control treatment. The tissues collected were first fixed in 4% formalin. After antigen retrieval with the retrieval buffer (DAKO, Glostrup, Denmark), formalin‐fixed paraffin‐embedded sections were washed in running water and then rinsed in tris‐buffered saline plus tween 20. Endogenous peroxidase blocker was used to incubate the tissue slides, followed by hybridization of the primary antibodies (FGF19, ab85042, 1:200, Abcam; p‐AKT, 4060, 1:500; CC3, 9661, 1:200, Cell Signaling Technology) for one hour at room temperature. DAKO EnVision and System‐HRP were used as a second antibody for staining, which was detected by using 3,3'‐diaminobenzidine‐tetrahydrochloride (DAKO). In Ki67 immunohistochemical analysis, slides were hybridized with biotinylated primary antibody (M7240, 1:100, DAKO) for 60 seconds at room temperature. After 10 minutes of streptavidin‐peroxidase treatment, slides were counterstained with 4′,6‐diamidino‐2‐phenylindole and visualized by chemiluminescence. Analysis of fluorescent signals was conducted using the Ariol system (Genetix, San Jose, CA, USA).
Statistical analysis
Statistical analyses were conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The association analysis was conducted using a χ2 likelihood ratio test. Data were presented as mean ± standard deviation from at least three independent experiments. Statistical significance was determined by Student’s t test. P < 0.05 was considered statically significant.
Results
Aberration of FGF 19 gene in tumor tissues of lung squamous cell carcinoma patients
Using qRT‐PCR analysis, 24 tumor tissues (60%) showed significantly upregulated FGF19 expression compared to adjacent non‐tumorous tissues (log2 transformed fold change >1), as shown in Figure 1a. The FGF19 protein level was further validated in available paired fresh tissues. Elevated FGF19 expression was observed in 79.2% (19/24) of cases with the mRNA upregulated (Fig 1b). However, there were three tumor tissues identified with lower FGF19 expression, with a log2 value below −1. Correlation analysis with clinicopathologic parameters showed that FGF19 upregulation is significantly associated with heavy smoking of more than 20 packs/year (P = 0.001, χ2 test). No obvious correlation was observed between FGF19 upregulation and patient gender, age, or tumor stage (Table 1).
Figure 1.
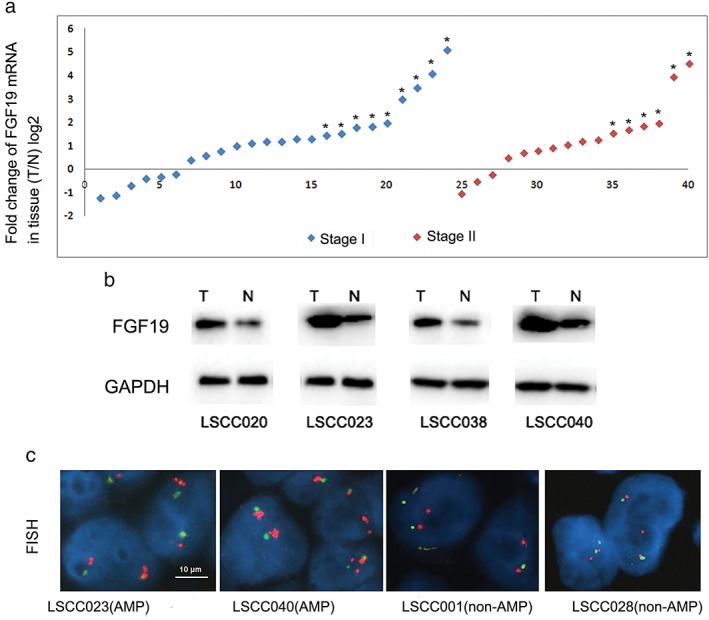
FGF 19 messenger RNA (mRNA) upregulation and gene amplification in lung squamous cell carcinoma LSCC tumors. (a) FGF19 mRNA expression levels were analyzed in paired tumor and adjacent non‐tumorous tissues. Values were presented as log2 transformed relative fold changes in mRNA expression level compared to the paired non‐tumorous tissue. A twofold change threshold was set to identify obvious changes to gene expression. T, tumor tissue; N, non‐tumorous tissue. * represents tumor samples with gene amplification. (b) Representative images of FGF19 protein expression in LSCC tumor samples LSCC020, SCC023, LSCC038, and LSCC040. (c) Representative images of FGF 19 gene amplification in LSCC tumor samples LSCC001, LSCC023, LSCC028, and LSCC040. Fluorescence in situ hybridization (FISH) analysis was performed in tissue sections using probes against FGF 19 (red) and CEP 11 (green). Scale bars represent 10 μm for FISH images. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Table 1.
Correlation analyses of FGF19 mRNA upregulation and clinicopathologic parameters
| FGF19 mRNA expression (T vs. N) | ||||
|---|---|---|---|---|
| Parameters | Median (range) | ≥ 2‐fold N (%) | < 2‐fold N (%) | P |
| Gender | ||||
| Male | 20 (83.3) | 13 (81.2) | 0.865 | |
| Female | 4 (16.7) | 3 (19.8) | NA | |
| Age (year) | ||||
| ≤60.0 | 60.0 (32–83) | 15 (62.5) | 8 (50.0) | 0.433 |
| >60.0 | 9 (37.5) | 8 (50.0) | NA | |
| Smoking status | ||||
| Heavy | 20 (83.3) | 5 (31.2) | 0.001 | |
| Light | 4 (16.7) | 5 (31.2) | NA | |
| Never | 0(0.0) | 6 (37.6) | NA | |
| Stage | ||||
| I | 15 (62.5) | 9 (62.5) | 0.693 | |
| II | 9 (37.5) | 7 (37.5) | NA | |
mRNA, messenger RNA; N, non‐tumorous tissue; NA, not available; T, tumor tissue.
We performed FISH analysis on paired tissue sections. FGF19 gene amplification (ratio of FGF19/CEP11 probes >2 or cluster signals >10%) was identified in 15 (37.5%) of 40 tumor samples, whereas non‐amplified FGF19 was observed in paired adjacent non‐tumorous tissues in the nucleus (Fig 1c). Importantly, we also observed that all 15 tumor tissues with amplified FGF19 genes had upregulated FGF19 mRNA expression.
siRNA knockdown of FGF19 expression inhibits cell proliferation in EPLC‐272H cells with amplified FGF 19 gene
To test the potential role of FGF19 on LSCC cell growth, we determined that EPLC‐272H lung squamous cancer cells carry FGF19 amplification (Fig 2a). In siRNA intervention experiments, we also included NCI‐H1703 cells as a control, which carried a normal copy of the FGF19 gene and a relatively moderate level of FGF19 mRNA expression (Fig 2b).
Figure 2.
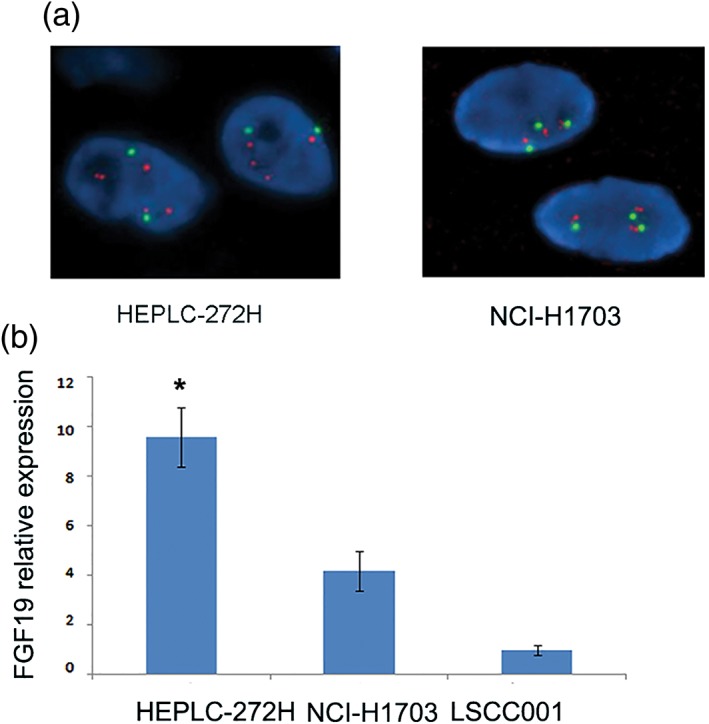
Characterization of lung squamous cell carcinoma (LSCC) tumor cells. (a) Representative images for fluorescence in situ hybridization (FISH) analysis staining with probes of FGF 19 (red) and CEP 11 (green) in EPLC‐272H and NCI‐H1703 cells. (b) FGF19 mRNA expression in EPLC‐272H, NCI‐H1703, and LSCC001 tumor tissues. Messenger RNA (mRNA) level of FGF19 was determined by quantitative real‐time‐PCR. The mRNA level of EPLC‐272H, and NCI‐H1703 was normalized to that of LSCC001 tumor tissue.
After transient transfection of these cells with siRNAs, the FGF19 expression in the cells greatly decreased. We observed that FGF19 expression was effectively knocked down by siRNAs, which led to significantly reduced protein levels secreted in supernatant and growth inhibition of EPLC‐272H cells in vitro. CC3 expression indicated that the cell apoptotic level was also elevated in EPLC‐272H with siRNA. However, no similar effects were observed in NCI‐H1703 cells with normal genetic copy numbers of the FGF19 gene and a moderate expression level of FGF19 (Fig 3).
Figure 3.
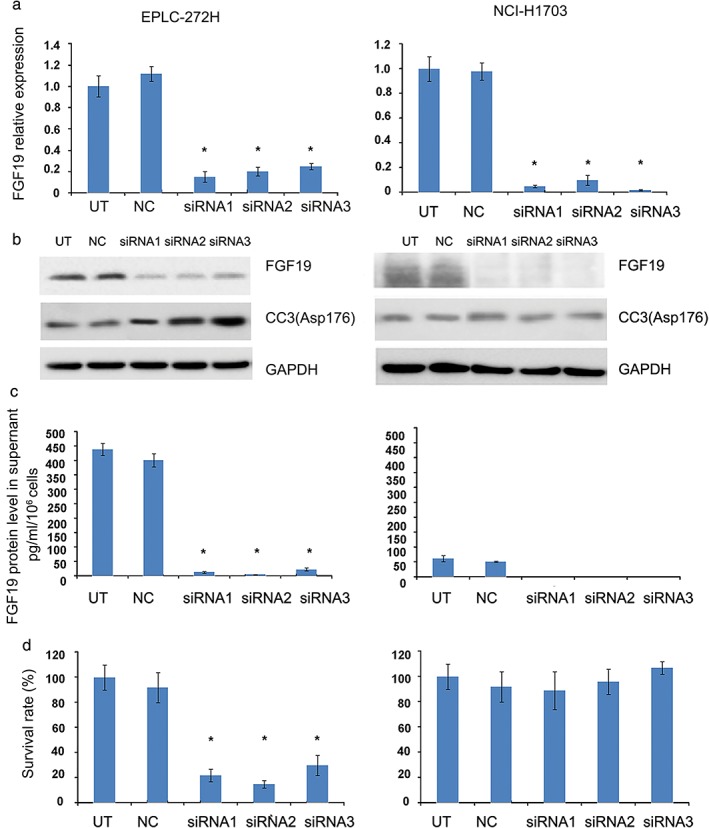
Effect of FGF19 knockdown by small interfering RNA (siRNA) on cell growth in EPLC‐272H cells. (a) Relative FGF19 messenger RNA (mRNA) expression in cells was assessed 72 hours after FGF19 siRNA knockdown. (b) Effects of FGF19 knockdown on FGF19 and CC3 protein levels. Cell lysates were collected 72 hours post treatment and analyzed by Western blot. (c) Secreted FGF19 protein level in supernatant was analyzed by enzyme‐linked immunosorbent assay 72 hours after siRNA transfection. (d) Effect of FGF19 knockdown on cell proliferation. EPLC‐272H cell proliferation was assessed with MTS proliferation assay 72 hours after transfection. Data represent the mean ± standard deviation; *P < 0.05. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; NC, a sample treated with scramble siRNA as control; siRNA1,2,3, a sample treated with siRNA1,2,3; UT, a sample without treatment.
Knockdown of FGF19 expression modulates FGF19 downstream signaling pathways in EPLC‐272H cells
We next determined the effects of FGF19 knockdown on the downstream signaling pathways in EPLC‐272H cells. We examined the phosphorylation level of FRS2, which is an important substrate of FGFR4, AKT, and ERK1/2. As expected, we found that phosphorylation levels of FRS2, FGFR4, AKT, and ERK1/2 were significantly reduced in cells with FGF19 knockdown compared to control cells that were not treated with siRNA (Fig 4).
Figure 4.
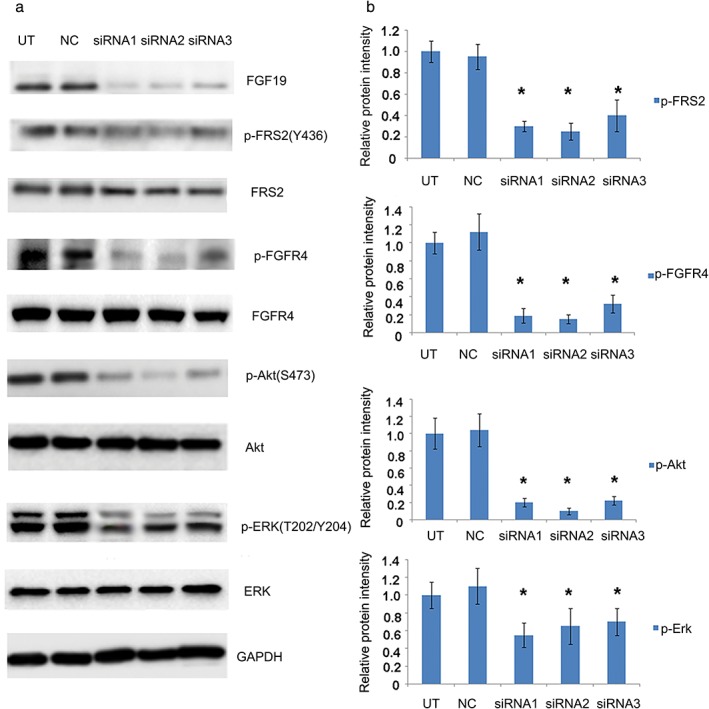
Effects of FGF19 knockdown by small interfering RNA (siRNA) on protein phosphorylation. (a) Total cell lysates were collected 72 hours after indicated treatments, and analyzed for phosphorylation of FGF19, FRS2, FGFR4, AKT and ERK proteins. Anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibody was included as a loading control. (b) Quantitative analysis of the changes in protein phosphorylation. Densitometry for Western blot signal was conducted, and intensity of the targeted protein/modification was normalized to corresponding GAPDH. Data represent the average results from three independent experiments. *P < 0.05. NC, sample treated with scramble siRNA as control; siRNA1,2,3, sample treated with siRNA1,2,3.
shRNA knockdown of FGF19 expression inhibits tumor growth in EPLC‐272H xenografts in vivo
To validate the role of FGF19 activity on tumor growth and apoptosis in vivo, we first used an shRNA experiment to modulate the FGF19 expression in EPLC‐272H cells. After successful transfection of inducible tetracycline‐controlled FGF19 shRNA, EPLC‐272H cells were treated with doxycycline to induce FGF19 shRNA expression. FGF19 expression can be modulated by shRNA in EPLC‐272H cells and an in vitro proliferation assay showed that shRNA knockdown of FGF19 expression significantly reduced the proliferation of EPLC‐272H cells (Fig 5). We then established a xenograft with EPLC‐272H cells expressing tetracycline‐controlled FGF19 shRNA. Mice were treated with 2 mg/mL of doxycycline or sucrose solution as a control for two weeks. Our data showed that obvious tumor growth inhibition of 44.8% was observed in the group of FGF19‐sh‐RNA mice treated with doxycycline, compared to the control group (P < 0.05) (Fig 6a). We also tested the toxicity of doxycycline treatment and shRNA transfection. The tumor growth curve showed no difference among the groups of EPLC‐272H xenografts treated with and without doxycycline, and xenografts with and without shRNA transfection.
Figure 5.
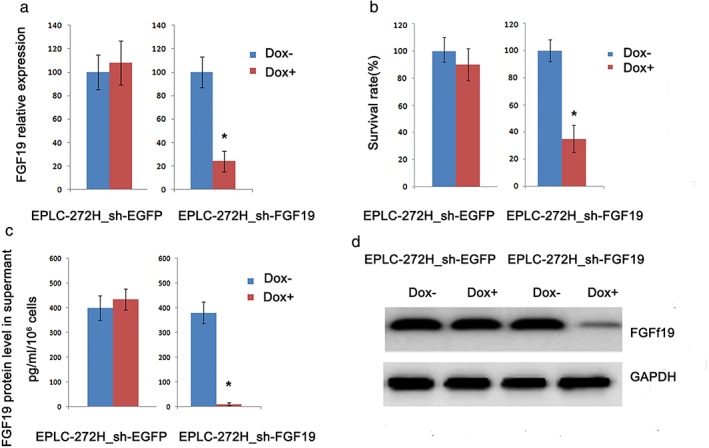
Effect of FGF19 knockdown by short hairpin RNA (shRNA) on cell growth in EPLC‐272H cells. (a) Relative FGF19 messenger RNA (mRNA) expression in cells was assessed 72 hours after FGF19 shRNA knockdown. EPLC‐272H cells engineered with inducible FGF19 shRNA and vector control (EGFP) were treated with doxycycline for 72 hours or left untreated. (b) Effect of FGF19 knockdown on cell proliferation. Cell proliferation was assessed with MTS proliferation assay. (c) Secreted FGF19 protein level in supernatant was analyzed by enzyme‐linked immunosorbent assay 72 hours after treatment with doxycycline or left untreated. Data represent the mean ± standard deviation; *P < 0.05. (d) Effects of FGF19 knockdown on FGF19 protein levels. Cells were treated with doxycycline or left untreated. Cell lysates were collected 72 hours post treatment and analyzed by Western blot.
Figure 6.
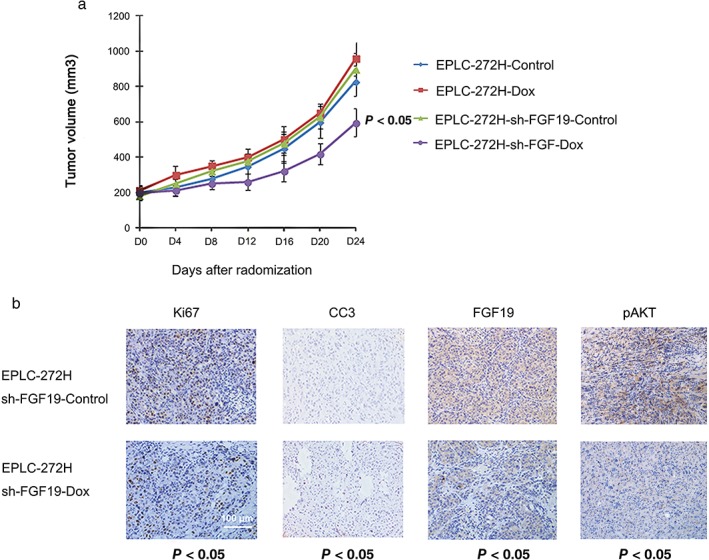
Inhibitory effect of FGF19 knockdown on the growth of EPLC‐272H xenografts in vivo. (a) EPLC‐272H xenografts developed from EPLC‐272H cells, or cells carrying inducible engineered expression of tet‐controlled FGF19 short hairpin RNA (shRNA) (EPLC‐272H‐sh‐FGF19), or EGFP control (EPLC‐272H‐sh‐FGF19‐control) were randomized into indicated groups and treated with/without doxycycline for 14 days. (b) Immunohistochemical analysis was performed using anti Ki67, anti CC3, anti‐FGF19, and pAKT in tumor samples excised from xenografts of EPLC‐272H‐sh‐FGF19 treated with or without doxycycline. Ki67, CC3, FGF19, and pAKT analysis was conducted after 14 days of treatment. Quantification of positive signals was conducted using the Ariol system. Data represent the mean ± standard deviation.
In a parallel immunohistochemistry assay, we also tested expression changes in FGF19, downstream signaling pAKT, cell proliferation marker Ki67, and apoptotic marker CC3 in tumor tissue from xenografts expressing FGF19 shRNA after treatment. Our data showed significant downregulation of FGF19 expression in tumor tissue from xenografts administrated with doxycycline (P < 0.05), but not in the group without doxycycline administration. The AKT phosphorylation level was obviously suppressed in the group with FGF19 knockdown, compared to the control group. FGF19 downregulation by doxycycline also increased the staining of CC3 and reduced staining of Ki67 (P < 0.05) (Fig 6b). These data show that silencing of FGF19 expression inhibits tumor growth and increases apoptosis in EPLC‐272H cells in vivo. In summary, our data suggest that FGF19 amplification is an oncogenic driver that plays an important role in cell proliferation and apoptosis in LSCC cells.
Discussion
FGF19 is a member of the fibroblast growth factor (FGF) family. Previous studies have demonstrated that FGF19 possesses many mitogenic and cell signaling activities, and is involved in several biological processes, including embryonic development, cell growth, differentiation, tumor growth, and invasion.12, 14 The aberrations of FGF19 and its high affinity, heparin‐dependent ligand for FGFR4 play an important role in the promotion of tumorigenesis and primary resistance to drug treatment.19 A recent clinical report showed that FGF19 expression is associated with cancer progression and poor prognosis in HCC.16, 20 Other preclinical studies have demonstrated that FGF19 may serve as a predictive biomarker for primary resistance to sorafenib in HCC, as FGF19 silencing in sorafenib‐resistant cells significantly increased the sensitivity to sorafenib.17 In breast cancer, neutralization of extracellular FGF19 by anti‐FGF19 antibody or siRNA‐mediated knockdown of FGF19 can decrease the p‐AKT level, suppress cancer cell growth, and enhance doxorubicin sensitivity.18
Recently, amplification of the FGF19 gene has been identified as a frequent genetic aberration in HCC,19 prostate,21, 22 breast,10 and lung squamous cancers.9, 23, 24 However, the clinical correlation of FGF19 gene amplification and the potential therapeutic effects of targeting FGF19 in LSCC are not yet clear. In this study, we defined FISH positive with a FGF19 to CEN11P ratio >2 or the presence of a >10% gene cluster. This standard referred to the National Comprehensive Cancer Network guidelines for Her2 scoring, which defined patients with a ratio greater than two HER2 gene copies per chromosome 17 and strong complete membrane staining in >10% of tumor cells as gene amplified, and, thus, eligible for trastuzumab treatment for breast cancer.25 Previous studies have explored the correlation of FGFR4 amplification to AZD4547 response,26 and Glo1 amplification as a potential therapeutic target in HCC also uses the same standard.27 The data show that FGF19 amplification is a genetic event in Chinese LSCC patients, with a frequency of 37.5% (15/40), which is consistent with a previously published study showing FGF19 gene copy number amplification in lung squamous cancer.23 Compared to paired adjacent non‐tumorous tissues, 60% (24/40) of LSCC tumors express elevated FGF19expression. Of note, we found that all tumor samples from LSCC patients carrying FGF19 amplifications also expressed high levels of FGF19 mRNA, which indicated that FGF19 amplification and upregulation of FGF19 expression may correlate closely in human LSCC. Interestingly, we also observed that high FGF19 expression has a clear association with cigarette smoking, while no other correlation was observed in this cohort. This finding is consistent with a previous study, which showed that amplification regions containing FGF19, FGF3, FGF4, and CCND1 were found five‐times more often in smoking LSCC than in non‐smoking LSCC patients.24
With siRNA intervention of the FGF19 gene, downregulation of FGF19 expression could significantly inhibit tumor cell growth and enhance apoptosis in LSCC cells with gene copy number amplification and a high expression of FGF19. FGF19 knockdown by inducible shRNA further demonstrated the tumor growth inhibitory effect in xenografts in vivo. siRNA interference of FGF19 expression in NCI‐H1703 cells with normal genetic copy and moderate expression levels had no obvious effects on cell proliferation and apoptosis. Therefore, these results suggest that FGF19 amplification could be a potential therapeutic target in clinical LSCC management.
Fibroblast growth factors interacting with FGFR result in receptor homodimerization and autophosphorylation to recruit downstream cytosolic adaptors, such as FRS2, the main mediator of multiple signaling pathways.28 Previous studies have suggested that anti‐FGF19 can abolish FGF19‐mediated activity in vitro and inhibit the growth of colon tumor xenografts in vivo with a monoclonal antibody, which selectively blocks the interaction of FGF19 with FGFR4, and the efficacy of the antibody in these models was linked to inhibition of FGF19‐dependent activation of FGFR4, FRS2, AKT and ERK.29 Liu et al. reported that hyperactivated FGF signaling can promote tumor angiogenesis and predicts a poor clinical outcome in prostate patients with overexpressed FRS2.23
In this study, we also investigated the phosphorylation level of downstream proteins. The results showed obvious inhibition of p‐FGFR4, FRS2, AKT, and ERK in LSCC cells with FGF19 knockdown interference, which indicated the potential therapeutic effect of targeting FGF19 may mainly depend on the downstream pathway of FGFR4/FRS2α‐ERK1/2 signaling.
This study showed that FGF19 amplification is a genetic event in Chinese LSCC patients, with a frequency of 37.5%. FGF19 amplified LSCC cells express relatively higher levels of FGF19 mRNA expression, and downregulation of FGF19 expression can induce significant cell killing effects in vitro and in vivo. These preclinical data strongly support the potential role of FGF19 as a therapeutic target and the development of molecular inhibitors targeting FGF19 amplification in LSCC patients. Considering our findings are preliminary, the clinical implications of targeting FGF19 genetic amplification for LSCC treatment require further investigation.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by grants from Projects of Medical and Health Technology in Zhejiang Province (N20090540), and the Project of Zhejiang Provincial Administration of Traditional Chinese Medicine (2016ZB069).
References
- 1. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013) 2015; 98: 25–8. [PubMed] [Google Scholar]
- 2. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e1S–e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 4. Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control 2008; 17: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuribayashi K, Funaguchi N, Nakano T. Chemotherapy for advanced non‐small cell lung cancer with a focus on squamous cell carcinoma. J Cancer Res Ther 2016; 12: 528–34. [DOI] [PubMed] [Google Scholar]
- 6. Cortinovis D, Gregorc V, Migliorino MR et al New perspectives in the second‐line treatment of non squamous NSCLC patients: Results from a large Italian Lung Cancer Working Group. Crit Rev Oncol Hematol 2017; 109: 35–41. [DOI] [PubMed] [Google Scholar]
- 7. Koutsoukos K, Mountzios G. Novel therapies for advanced squamous cell carcinoma of the lung. Future Oncol 2016; 12: 659–67. [DOI] [PubMed] [Google Scholar]
- 8. Zhou JX, Yang H, Deng Q et al Oncogenic driver mutations in patients with non‐small‐cell lung cancer at various clinical stages. Ann Oncol 2013; 24: 1319–25. [DOI] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. (Published erratum appears in Nature 2012; 491: 288.) Nature 2012; 489: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlsson E, Waltersson MA, Bostner J et al High‐resolution genomic analysis of the 11q13 amplicon in breast cancers identifies synergy with 8p12 amplification, involving the mTOR targets S6K2 and 4EBP1. Genes Chromosomes Cancer 2011; 50: 775–87. [DOI] [PubMed] [Google Scholar]
- 11. Govindan R, Ding L, Griffith M et al Genomic landscape of non‐small cell lung cancer in smokers and never‐smokers. Cell 2012; 150: 1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu X, Ge H, Lemon B et al Selective activation of FGFR4 by an FGF19 variant does not improve glucose metabolism in ob/ob mice. Proc Natl Acad Sci U S A 2009; 106: 14379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanley S, Buettner C. FGF19: How gut talks to brain to keep your sugar down. Mol Metab 2014; 3: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcelin G, Jo YH, Li X et al Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab 2014; 3: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mellor HR. Targeted inhibition of the FGF19‐FGFR4 pathway in hepatocellular carcinoma; translational safety considerations. Liver Int 2014; 34: e1–9. [DOI] [PubMed] [Google Scholar]
- 16. Hyeon J, Ahn S, Lee JJ, Song DH, Park CK. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci 2013; 58: 1916–22. [DOI] [PubMed] [Google Scholar]
- 17. Gao L, Wang X, Tang Y, Huang S, Hu CA, Teng Y. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res 2017; 36: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiong KH, Tan BS, Choo HL et al Fibroblast growth factor receptor 4 (FGFR4) and fibroblast growth factor 19 (FGF19) autocrine enhance breast cancer cells survival. Oncotarget 2016; 7: 57633–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Futami T, Okada H, Kihara R et al ASP5878, a novel inhibitor of FGFR1, 2, 3, and 4, inhibits the growth of FGF19‐expressing hepatocellular carcinoma. Mol Cancer Ther 2017; 16: 68–75. [DOI] [PubMed] [Google Scholar]
- 20. Miura S, Mitsuhashi N, Shimizu H et al Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 2012; 12: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagamatsu H, Teishima J, Goto K et al FGF19 promotes progression of prostate cancer. Prostate 2015; 75: 1092–101. [DOI] [PubMed] [Google Scholar]
- 22. Kwabi‐Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer 2004; 11: 709–24. [DOI] [PubMed] [Google Scholar]
- 23. Liu J, You P, Chen G et al Hyperactivated FRS2alpha‐mediated signaling in prostate cancer cells promotes tumor angiogenesis and predicts poor clinical outcome of patients. Oncogene 2016; 35: 1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan Q, Li F, Wang G et al Identification of FGF19 as a prognostic marker and potential driver gene of lung squamous cell carcinomas in Chinese smoking patients. Oncotarget 2016; 7: 18394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pathmanathan N, Bilous AM. HER2 testing in breast cancer: An overview of current techniques and recent developments. Pathology 2012; 44: 587–95. [DOI] [PubMed] [Google Scholar]
- 26. Xie L, Su X, Zhang L et al FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res 2013; 19: 2572–83. [DOI] [PubMed] [Google Scholar]
- 27. Zhang S, Liang X, Zheng X et al Glo1 genetic amplification as a potential therapeutic target in hepatocellular carcinoma. Int J Clin Exp Pathol 2014; 7: 2079–90. [PMC free article] [PubMed] [Google Scholar]
- 28. Ho HK, Yeo AH, Kang TS, Chua BT. Current strategies for inhibiting FGFR activities in clinical applications: Opportunities, challenges and toxicological considerations. Drug Discov Today 2014; 19: 51–62. [DOI] [PubMed] [Google Scholar]
- 29. Desnoyers LR, Pai R, Ferrando RE et al Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 2008; 27: 85–97. [DOI] [PubMed] [Google Scholar]


