Abstract
Background
The aim of this study was to investigate the patterns and influencing factors of local‐regional recurrence of lower thoracic esophageal squamous cell carcinoma (TESCC) after curative resection and to delineate the clinical target volume (CTV) of postoperative radiotherapy (PORT).
Methods
From January 2009 to December 2013, the clinical data of patients who experienced local‐regional recurrence after curative esophagectomy were collected and analyzed to determine local‐regional recurrence patterns and to evaluate whether a proposed T‐shaped PORT CTV could cover regions of local‐regional failure.
Results
A total of 108 patients were eligible for this study. All patients experienced postoperative recurrence of lower TESCC. The time to local‐regional failure varied from one to 52 months (average 13.4 ± 11.0). Among the 108 patients, 127 recurrence sites were detected as the first recurrence event: 37 cases in the bilateral supraclavicular region, 56 in the upper mediastinum, 14 in the middle mediastinum, 15 in the upper abdominal lymph nodes, and five cases of anastomotic recurrence. The proposed PORT CTV could successfully cover 89 (82.4%) out of the 108 recurrences and 84.2% of the sites (107/127) of recurrence in our sample.
Conclusion
Local‐regional recurrence of lower TESCC is mainly distributed in the supraclavicular, upper‐middle mediastinum, anastomotic stoma, and upper abdominal lymph node regions. The proposed T‐shaped PORT CTV field could cover over 80% of local‐regional failure in our sample; therefore, we suggest that PORT should focus on this area.
Keywords: Esophageal carcinoma, radiotherapy, recurrence, target region
Introduction
The prevalence of esophageal carcinoma is high in East Asia, especially in China. The incidence and mortality rates account for more than half of the globally reported cases, and by 2012, the annual death rate had reached 15/100 000.1 In China, 90% of esophageal carcinoma patients are diagnosed with esophageal squamous cell carcinoma (ESCC).2 Esophagectomy is the primary treatment for thoracic ESCC (TECSS), but prognosis is poor, with a low five‐year survival rate.3 Local‐regional recurrence and distant metastasis are the two main causes of treatment failure.
Recently, a series of studies indicated that postoperative local‐regional recurrences of TESCC were mainly distributed in the bilateral supraclavicular and upper‐middle mediastinum.4, 5 Lower, middle, and upper TESCC exhibited different prognosis after surgery. It has been reported that the metastatic route in lower TESCC differs from upper TESCC.6 Although a comparison of relapses between upper/middle and lower TESCC has been conducted,5 a comprehensive study of recurrence patterns focusing on lower TESCC is lacking. Therefore, this study retrospectively investigated local‐regional recurrence patterns in patients with lower thoracic ESCC after esophagectomy and its potential impacts on postoperative radiotherapy (PORT).
Methods
From January 2009 to December 2013, the clinical data of patients who experienced local‐regional recurrences (limited to the first recurrence) after curative esophagectomy at the Fudan University Shanghai Cancer Center and Shanghai Chest Hospital were retrieved and retrospectively analyzed. Surgery was performed using thoracotomy and videothoracoscopy.
Inclusion and exclusion criteria
To be eligible for the study, patients were required to have: received curative esophagectomy with two‐field or three‐field lymphadenectomy; pathologically confirmed SCC; undergone R0 resection with more than 12 lymph nodes dissected (according to the 7th edition American Joint Committee on Cancer/International Union Against Cancer [AJCC/UICC] staging system); complete and clear operational and clinical records; not received radiochemotherapy treatment either prior or postoperatively; postoperative local‐regional recurrence located within the bilateral supraclavicular region, mediastinum, anastomoses and the left upper gastric abdominal zone. “Lower esophagus” was defined as inferior to the pulmonary vein and superior to the cardia. The supraclavicular nodes in our study covered the areas lateral to the common carotid artery, up to the trapezius.
Methods
Classification and pathological tumor node metastasis (pTNM) staging of TESCC was based on 2009 AJCC staging guidelines.7 Thoracic lymph node mapping was catalogued as proposed by the American Thoracic Academy.8 Local‐regional recurrences, as well as primary tumor bed, anastomosis and cervical, thoracic, and abdominal lymph nodes, have been defined previously.5 The tumor draining lymph nodes were classified into five regions: (i) cervical: bilateral supraclavicular nodes; (ii) superior mediastinum; (iii) middle mediastinum; (iv) inferior mediastinum; and (v) left gastric abdominal zone.
Diagnostic approaches for supraclavicular lymph metastasis included physical examinations, B‐mode ultrasound of the supraclavicular region, computed tomography (CT) of the cervical region, and histological confirmation through biopsy. Thoracic and abdominal CT, and positron emission tomography (PET)‐CT were used to diagnose local recurrence in mediastinal zones and abdominal lymph nodes; thoracic CT, PET‐CT, and esophagoscopy were used for recurrences in anastomoses. Physical examination, CT scan, and ultrasonography were routinely performed after surgery. If patients reported symptoms, bone emission CT, magnetic resonance imaging, and PET‐CT were conducted to examine metastasis.
Statistical analysis
Clinical data were reported as the mean standard deviation or percentage. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. Local‐regional failure was calculated from surgery to the time of first recurrence.
Results
Local‐regional failure
Local‐regional failure varied from one to 52 months. The average time to local recurrence was 13.4 ± 11.0 months.
Summary of clinical records
A total of 108 patients with postoperative local recurrences at lower TESCC were recruited, including 100 men and eight women, with an average age of 59.1 ± 7.6 years. The general clinical data of patients is summarized in Table 1.
Table 1.
Clinical characteristics of lower TESCC patients with local‐regional recurrence
| Characteristic | N |
|---|---|
| Gender | |
| Male | 100 |
| Female | 8 |
| pT stage | |
| T1b | 13 |
| T2 | 31 |
| T3 | 61 |
| T4 | 3 |
| Lymph node metastasis | |
| N0 | 38 |
| N1 | 30 |
| N2 | 21 |
| N3 | 19 |
| Pathological differentiation degree | |
| G1 | 12 |
| G2 | 62 |
| G3 | 34 |
| TNM stage | |
| IA | 13 |
| IB | 6 |
| II | 11 |
| IIB | 15 |
| IIIA | 28 |
| IIIB | 14 |
| IIIC | 21 |
TESCC, thoracic esophageal squamous cell carcinoma; TNM, tumor node metastasis.
Distribution of recurrence sites based on lesions
In total, 127 lesions were identified among the 108 patients: 92 patients had one lesion, 13 had two, and three patients had three lesions. Recurrence occurred in the: bilateral supraclavicular (37 lesions), superior mediastinum (56 lesions), middle mediastinum (14 lesions), and anastomosis (5 lesions) regions, and abdominal lymph node metastasis (15 lesions). No recurrence was observed in the inferior mediastinum (Fig 1).
Figure 1.
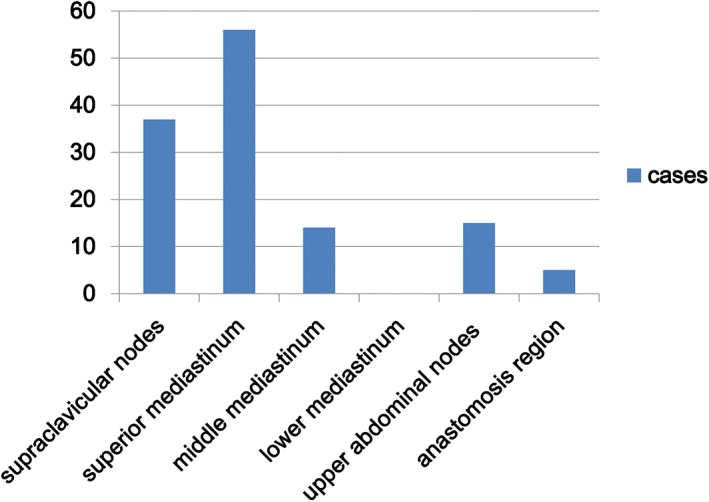
Locations of local‐regional recurrence after lower thoracic esophageal squamous cell carcinoma resection.
Analysis of T‐field coverage
The proposed T‐field successfully covered the recurrent sites in 89 patients (82.4%), but failed in 19 (17.6%) (Table 2). Fifteen patients developed abdominal lymph node metastasis, five developed recurrences at the anastomosis, and one patient developed recurrence in both of these sites. The existing atlas mainly focused on the target volume for radiation therapy without surgery.9
Table 2.
Distribution and ratio of the LRFs that could be covered by the T‐field
| Category | No. of patients (%) | Covered by T‐ field |
|---|---|---|
| Sup. | 27 (25) | 27 |
| SM. | 42 (38.9) | 42 |
| MM. | 11 (10.2) | 11 |
| Abd. | 10 (9.3) | 0 |
| Ana. | 2 (2.8) | 0 |
| Sup. + SM. | 6 (5.6) | 6 |
| SM. + MM. | 2 (2.8) | 2 |
| Sup. + Abd. | 1 (0.9) | 0 |
| SM. + Abd. | 1 (0.9) | 0 |
| Ana. + Abd. | 1 (0.9) | 0 |
| Ana. + SM. | 2 (2.8) | 0 |
| Sup. + SM. + MM | 1 (0.9) | 1 |
| Sup. + SM. + Abd. | 2 (2.8) | 0 |
| Total | 108 | 89 |
Abd., upper abdominal nodes; Ana., anastomotic recurrences; LRF, local‐regional failure; MM, middle mediastinum; SM, superior mediastinum; Sup, bilateral supraclavicular region.
Of the total 127 lesions, the proposed T‐field covered 107 lesions found in patients, with a coverage rate of 84.2%.
Discussion
Treatment failures in TESCC are mainly attributed to local recurrence and distant metastasis. The recurrence rate is 41.5~49.0% in esophageal carcinoma after curative esophagectomy with lymphadenetomy.10, 11 The 2016 National Comprehensive Cancer Network (NCCN) Guidelines do not recommend adjuvant treatment for patients after R0 resection, regardless of cancer stage. Nonetheless, a retrospective study showed that local‐regional recurrence was the main pattern of TESCC recurrence. Our retrospective analysis indicated that TESCC recurrence predominantly occurred at the supraclavicular and upper‐middle mediastinum regions, accounting for 80–90% of the total recurrences.4, 5
Distinct from the metastatic pattern of upper TESCC, lower TESCC transits regionally and bidirectionally along the longitudinal line. However, patterns of recurrence in lower TESCC after esophagectomy are not clear.
Our results showed that recurrence in lower TESCC mainly occurred in the bilateral supraclavicular region, upper‐middle mediastinum, and upper left gastric abdominal cavity. Eighty percent of the recurrences were distributed at the supraclavicular region and upper‐middle mediastinum. Nevertheless, 15 out of the 108 patients experienced a recurrent tumor in the upper left gastric abdominal cavity, a higher portion than in the previously documented recurrent ratio in this region in TESCC patients.
The recurrent sites of lower TESCC were also centered at the supraclavicular and upper‐middle mediastinum. Anatomically, the cervicothoracic junction adjacently contains bilateral recurrent laryngeal nerves, main blood vessels, and the trachea and enriches the lymph vessels. This complicates surgery, making it more difficult to radically dissect contaminated lymph nodes and fat tissues and resisting subclinical lesions. The higher recurrence rates in the upper left gastric abdominal cavity in lower TESCC patients, compared to those with upper and middle TESCC, may be attributed to the tendency of lower TESCC to transition to the upper left gastric abdominal cavity. Lymph node dissection cannot completely eliminate subclinical residuals, which further progress into metastasis in the upper left gastric abdominal cavity.
Radiotherapy and other adjuvant treatments should be considered in patients at a high risk of recurrence. Several studies have proven that PORT decreases local‐regional recurrence and extends long‐term survival rates. Schreiber et al. published a retrospective study of 1046 patients, which indicated that PORT prolonged the survival period of patients with stage III (T3N1 or T4N0–1) esophageal cancers, regardless of type.12 Postoperative chemoradiotherapy with adjuvant surgery has been reported to prolong survival rates in certain stages of the disease.13 However, some studies have indicated that neither neoadjuvant nor adjuvant therapy is beneficial.14, 15 Nevertheless, controversy remains over the application of adjuvant radiotherapy after esophagectomy, mainly focusing on the indications and volumes of PORT. One purpose of this study was to identify the proper volume of PORT by analyzing the local‐regional recurrent patterns of lower TESCC.
The proposed T‐shape field, consisting of the supraclavicular region and upper‐middle mediastinum, covered over 80% of the local‐regional recurrent sites in the patients in our sample. This result suggests that attention should be focused on the T‐shape field for the postoperative adjuvant treatment of patients with lower TESCC. Further evaluation needs to be conducted regarding patient tolerability and the accompanied benefits of including the upper left gastric abdominal cavity in the PORT clinical target volume in lower TESCC patients. From the perspective of tolerability, if the target volume including the bilateral supraclavicular region and the entire mediastinum is apparently larger than the proposed T‐shaped field, the patient may not be able to tolerate the PORT dose. Moreover, because of the change to postoperative anatomical position, it is relatively difficult to define the left upper gastric abdominal zone after surgery, which further diminishes the potential benefit presented by adjuvant treatment. The recurrent rate was not improved in this area, even when accompanied by adjuvant radiotherapy; therefore, the target volume for PORT of lower TESCC should focus on the T‐shape field.
The limitations of this study include the analysis of retrospective data, and the use of postoperative recurrent patient data from two hospitals, which did not include all postoperative patients in these two hospitals, and thus may have led to selective bias.
Further lower TESCC clinical data needs to be analyzed and rationality of the radiotherapy target field requires confirmation by prospective research.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was funded by grants from the Shanghai Chest Hospital (YZ2015‐ZX14) and the Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine (20161433).
References
- 1. Zhang SW, Zheng RS, Zuo TT, Zeng HM, Chen WQ, He J. [Mortality and survival analysis of esophageal cancer in China.] Chin J Oncol 2016; 38: 709–15. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 3. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009; 27: 5062–7. [DOI] [PubMed] [Google Scholar]
- 4. Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: Implications for the clinical target volume design of postoperative radiotherapy. PLoS ONE 2014; 9: e97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol 2010; 96: 104–7. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Zhu K, Zheng X et al [Prognostic analysis of cervical lymph node metastasis in patients with thoracic esophageal squamous cell carcinoma.] Zhonghua Zhong Liu Za Zhi 2014; 36: 612–6. (In Chinese.) [PubMed] [Google Scholar]
- 7. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Ann Surg Oncol 2010; 17: 1721–4. [DOI] [PubMed] [Google Scholar]
- 8. Casson AG, Rusch VW, Ginsberg RJ, Zankowicz N, Finley RJ. Lymph node mapping of esophageal cancer. Ann Thorac Surg 1994; 58: 1569–70. [DOI] [PubMed] [Google Scholar]
- 9. Wu AJ, Bosch WR, Chang DT et al Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys 2015; 92: 911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three‐field lymphadenectomy. J Am Coll Surg 2004; 198: 205–11. [DOI] [PubMed] [Google Scholar]
- 11. Kyriazanos ID, Tachibana M, Shibakita M et al Pattern of recurrence after extended esophagectomy for squamous cell carcinoma of the esophagus. Hepatogastroenterology 2003; 50: 115–20. [PubMed] [Google Scholar]
- 12. Schreiber D, Rineer J, Vongtama D et al Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol 2010; 5: 244–50. [DOI] [PubMed] [Google Scholar]
- 13. Zou B, Li T, Zhou Q et al Adjuvant therapeutic modalities in primary small cell carcinoma of esophagus patients: A retrospective cohort study of multicenter clinical outcomes. Medicine (Baltimore) 2016; 95: e3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malthaner RA, Wong RK, Rumble RB, Zuraw L, Members of the Gastrointestinal Cancer Diseases Site Group of Cancer Care Ontario's Program in Evidence‐based Care . Neoadjuvant or adjuvant therapy for resectable esophageal cancer: A systematic review and meta‐analysis. BMC Med 2004; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zieren HU, Müller JM, Jacobi CA, Pichlmaier H, Müller RP, Staar S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: A prospective randomized study. World J Surg 1995; 19: 444–9. [DOI] [PubMed] [Google Scholar]


