Abstract
With the increasing global epidemic of obesity, the clinical importance of non‐alcoholic fatty pancreas disease (NAFPD) has grown. Even though the pancreas might be more susceptible to ectopic fat deposition compared with the liver, NAFPD is rarely discussed because of the limitation of detection techniques. In the past, NAFPD was considered as an innocent condition or just part of clinical manifestations during the course of obesity. Recently, a growing body of research suggests that NAFPD might be associated with β‐cell dysfunction, insulin resistance and inflammation, which possibly lead to the development of diabetes and metabolic syndrome. The present review summarized the current literature on the epidemiology, potential pathophysiology, diagnostic techniques, impact of NAFPD on β‐cell function and insulin resistance, and the clinical relevance of the interplay between NAFPD and glucometabolic disorders.
Keywords: Diabetes mellitus, Metabolic syndrome, Non‐alcoholic fatty pancreas disease
Introduction
Obesity has emerged as a major health problem worldwide, as it is linked to several metabolic complications, including type 2 diabetes, metabolic syndrome, non‐alcoholic fatty liver disease (NAFLD) and cardiovascular disease. In the past two decades, there has been a growing awareness that obesity is not a homogeneous condition, and the regional distribution of adipose tissue plays a pivotal role in obesity‐associated disturbances of glucose and lipid metabolism1, 2, 3. When the circulating levels of triglycerides and free fatty acids (FFAs) exceed the metabolic capacity of adipose tissue needs, they are accumulated as ectopic fat in non‐adipose tissues, such as skeletal muscle, the liver, heart and pancreas4, 5, 6. Fat infiltration of the liver in the absence of significant alcohol consumption and other chronic conditions of the liver is termed as NAFLD. It can progress to local liver injury, cirrhosis and hepatocellular carcinoma7. In addition, NAFLD has been shown to be associated with insulin resistance, type 2 diabetes, metabolic syndrome and atherosclerosis8, 9, 10.
Lipid deposition in the pancreas has recently gained more attention. Nowadays, a growing body of evidence has shown that non‐alcoholic fatty pancreas disease (NAFPD) might be associated with lipotoxicity, insulin resistance and inflammation, which possibly leads to the development of glucometabolic disorders. In 1933, Ogilvie first used the term ‘pancreatic lipomatosis’ to represent the pathological process of excessive fat storage in the pancreas11. Owing to the lack of distinction between the accumulation of triglycerides in acinar cells, β‐cells or intrapancreatic adipocyte infiltration, so far many synonymous (such as pancreatic steatosis, pancreatic lipomatosis or fatty pancreas) have been used for all forms of pancreatic fat accumulation. According to a current article by Smits et al., NAFPD is specifically defined as pancreatic fat accumulation in association with obesity in the absence of significant alcohol consumption12. However, the precise definition of ‘significant alcohol consumption’ in this context is uncertain. In general, most published literature regarding NAFPD has stated that ‘significant alcohol consumption’ is defined as >2 drinks (~10 g of alcohol per one drink unit) per day, and some studies have used sex‐specific definitions: >3 drinks on average per day in men and >2 drinks on average per day in women. Unlike NAFLD, the pathophysiology and clinical implications of NAFPD have not been well established.
Therefore, the aim of the present literature review was to summarize the impact of NAFPD on glucose metabolism. In particular, we will focus on the current knowledge regarding the possible pathophysiology and diagnostic techniques, effects on β‐cell function and insulin resistance, and the clinical relevance of the interplay between NAFPD and glucometabolic disorders (Table 1).
Table 1.
Summary of human studies investigating the association between pancreatic steatosis and glucometabolic disorders
| First author (reference) | Study design and population | Assessment of pancreatic steatosis | No. patients | Prevalence of fatty pancreas | Assessment of β‐cell function | Results |
|---|---|---|---|---|---|---|
| Tushuizen et al. (2007)43 | Case‐control study; Caucasian men; aged 35–65 years; alcohol intake <20 units/week | 1H‐MRS |
Total: 36 Subgroup: 12/24 (type 2 diabetes/non‐type 2 diabetes) |
– | OGTT‐derived insulinogenic index |
Patients with type 2 diabetes have higher pancreatic fat content than those with non‐type 2 diabetes. Pancreatic fat content negatively correlates with β‐cell function only in non‐type 2 diabetes group. Pancreatic fat content is not associated with BMI, WC, TG, FFAs, VAT, hepatic fat content |
| Al‐Haddad et al. (2009)53 | Case–control study in USA; patients who underwent EUS; median age 65 years | EUS |
Total: 120 Subgroup: 60/60 (hyperechogenic pancreas/healthy control) |
– | – | Hyperechogenic pancreas is associated with BMI, hepatic steatosis, alcohol use |
| Lee et al. (2009)15 | Cross‐sectional study in South Korea; adults visiting an obesity clinic; mean age 44.9 ± 9.5 years; alcohol intake <20 g/day in women and <40 g/day in men | Abdominal US |
Total: 293 Subgroup: 180/113 (NAFPD/non‐NAFPD) |
61.4% | – |
HOMA‐IR, TG and VAT tend to increase with the degree of NAFPD. The incidence of metabolic syndrome and the number of metabolic syndrome parameters are higher in NAFPD group compared with in the control group |
| Lingvay et al. (2009)30 | Cross‐sectional study in USA; volunteer with normal or abnormal glucose tolerance; alcohol intake <2 units/day | MRS |
Total: 79 Subgroup: 15/30/23/11 (NGT plus BMI <25/NGT plus BMI ≥25/IGT or IFG/type 2 diabetes) |
– | – |
In normoglycemic population, overweight or obese subjects have higher pancreatic fat content compared with lean subjects. Subjects with similar BMI, but with abnormal glucose tolerance (IGT or IFG, or type 2 diabetes) have even higher pancreatic fat content. Post‐challenge blood glucose level has the strongest relationship to pancreatic fat content. Pancreatic and hepatic fat contents are only weakly correlated |
| van Greenen et al. (2010)14 | Retrospective study in the Netherlands; deceased adults who underwent autopsy; mean age of death 68 ± 14 years; alcohol intake <14 units/week in women and <21 units/week in men | Pathology | Total: 80 | – | – |
Intralobular pancreatic fat is associated with non‐alcoholic steatohepatitis. Total pancreatic fat is a significant predictor for NAFLD |
| Choi, et al. (2010)18 | Cross‐sectional study in South Korea; subjects who underwent EUS; mean age 52.1 ± 12.2 years | EUS |
Total: 284 Subgroup: 110/174 (hyperechogenic pancreas/non‐ hyperechogenic pancreas) |
38.7% | – | Hyperechogenic pancreas is associated with fatty liver, male, aged >60 years, hypertension, and VAT |
| Heni et al. (2010)19 | Cross‐sectional study in Germany; healthy Caucasian subjects with increased risk of type 2 diabetes | MRI |
Total: 51 Subgroup: 23/28 (IFG and/or IGT/NGT) |
– | OGTT‐derived insulinogenic index |
Pancreatic fat content negatively correlates with insulin secretion only in subjects with IGT and/or IFG. Pancreatic fat content positively correlates with BMI, VAT, and WC. No association of pancreatic fat contents with age, sex and hepatic fat content |
| Rossi et al. (2011)20 | Cross‐sectional study in Italy; obese subjects without diabetes; mean age 49.1 ± 13.0 years; alcohol intake <20 g/day in women and <30 g/day in men | MRI |
Total: 38 Subgroup: 18/20 (men/women) |
– | – |
Obese subjects had higher pancreatic fat content than lean subjects. Obese men had higher pancratic fat content than obese women despite same BMI. Pancreatic fat content is positively related with WC, VAT, TG and fat intake, and negatively related to adiponectin. No association between pancreatic fat content and insulin resistance |
| Lê et al. (2011)44 | Cross‐sectional study; young obese African Americans or Hispanics; aged 13–25 years | MRI |
Total: 138 Subgroup: 74/64 (Hispanic/African American) |
– | IVGTT‐derived disposition index |
Hispanics had higher pancreatic fat content than African Americans, and the ethnic difference becomes greater with increasing age. Pancreatic fat content is positively associated with VAT, hepatic fat content and FFAs. No association between pancreatic fat content and insulin sensitivity or β‐cell function |
| Sepe et al. (2011)26 | Cross‐sectional study in USA; patients who are referred for EUS; mean age 62.9 ± 13.9 years | EUS |
Total: 230 Subgroup: 64/166 (fatty pancreas/non‐fatty pancreas) |
27.8% | – |
The presence of fatty pancreas is associated with BMI, fatty liver, hyperlipidemia and metabolic syndrome. Subjects with increasing number of metabolic syndrome parameters have a higher risk of fatty pancreas. No association between fatty pancreas and chronic pancreatitis or pancreatic adenocarcinoma |
| van der Zijl et al. (2011)45 | Case–control study; overweight Caucasian subjects with a family history of type 2 diabetes; alcohol intake <20 units/week | 1H‐MRS |
Total: 64 Subgroup: 16/29/19 (NGT/IFG/IFG and/or IGT) |
– | Hyperglycemic hyperinsulinemic clamp‐derived disposition index |
Pancreatic fat content gradually increases between NGT, IFG and IFG/IGT. Pancreatic fat content is positively associated with age, BMI and 2‐h plasma glucose, and negatively related to insulin sensitivty and e‐cholesterol. No association between pancreatic fat and β‐cell function |
| Ou et al. (2013)23 | Cross‐sectional study in Taiwan; adults who underwent health check‐up; alcohol intake <20 g/day | Abdominal US |
Total: 7,464 Subgroup: 5,756/1,225/483 (NGT/prediabetes/diabetes) |
– | – |
The prevalence of NAFPD gradually increases between NGT, prediabets and diabetes. NAFPD is associated with an increased risk for diabetes. NAFPD is related to prediabetes only in men, but not in women |
| Wu et al. (2013)24 | Cross‐sectional study in Taiwan; adults who underwent health check‐up; mean age 50.8 ± 12.4 years | Abdominal US |
Total: 557 Subgroup: 72/485 (fatty pancreas/non‐fatty pancreas) |
12.9% | – |
Subjects with fatty pancreas have a greater proportion of obesity, hypertension, dyslipidemia (e.g., high TG, low HDL‐cholesterol) and hyperglycemia than those without fatty pancreas. The incidence of metabolic syndrome and the numbers of metabolic syndrome parameters are higher in subjects with fatty pancreas compared with in the control group |
| Wong et al. (2014)32 | Cross‐sectional study in Hong Kong; healthy adults; mean age 48 ± 10 years; alcohol intake <10 g/day (<70 g/week) in women and <20 g/day (<140 g/week) in men | MRI |
Total: 685 Subgroup: 110/575 (NAFPD/non‐NAFPD) |
16.1% | HOMA‐β |
NAFPD is more common in men and in postmenopausal women. Subjects with both NAFPD and NAFLD had higher HOMA‐IR than did those with either condition alone NAFPD is associated with central obesity, hypertriglyceridemia, hyperferritinemia and insulin resistance. No association between NAFPD and β‐cell function |
| Wang et al. (2014)16 | Cross‐sectional study in Taiwan; adults who underwent health check‐up; alcohol intake <20 g/day | Abdominal US |
Total: 8,097 Subgroup: 1,297/6,800 (NAFPD/non‐NAFPD) |
16.0% | – |
Subjects with NAFPD have a greater proportion of diabetes and NAFLD than those without NAFPD. NAFPD is associated with age, obesity, diabetes and NAFLD |
| Lesmana et al. (2015)25 | Cross‐sectional study in Indonesia; adults who underwent routine medical check‐up; mean age 43.1 ± 12.19 years; alcohol intake <20 g/day | Abdominal US |
Total: 901 Subgroup: 315/586 (NAFPD/non‐NAFPD) |
35% | – | NAFPD is associated with age >35 years, male sex, obesity, hyperglycemia, higher blood pressure, dyslipidemia and NAFLD |
| Della Corte et al. (2015)17 | Cross‐sectional study in Italy; consecutive children and adolescents with NAFLD; mean age 13.16 ± 2.69 years | Abdominal US |
Total: 121 Subgroup: 58/63 (NAFPD/non‐NAFPD) |
48% | – |
Subjects with NAFPD have a higher BMI, inflammatory cytokine (e.g., TNF‐a, IL‐1β), fasting insulin, insulin resistance, and lower insulin sensitivity index compared with those without NAFPD. NAFPD is positively associated with hepatic fibrosis, steatosis and NAFLD activity score. No association between NAFPD and IL‐6 |
| Begovatz et al. (2015)29 | Cross‐sectional study in Germany; Caucasian subjects; alcohol intake <10 g/day in women and <20 g/day in men | MRI and 1H‐MRS |
Total: 56 Subgroup: 28/14/14 (NGT/IFG and/or IGT/type 2 diabetes) |
– | OGTT‐derived insulinogenic index |
Pancreatic fat consists of an inhomogenous distribution of adipose tissue infiltration instead of uniform pancreatic steatosis. Pancreatic fat infiltration increased with age and decreasing glucose tolerance. No association of pancreatic fat infiltration with β‐cell function regardless of glucose tolerance status |
| Yamazaki, et al. (2016)28 | 5‐year retrospective cohort study in Japan; volunteer who underwent a health check; mean age 51.8 ± 9.8 years | CT scan |
Total: 813 New‐onset type 2 diabetes during follow‐up: 62 (7.6%) |
– | – |
Pancreatic steatosis at baseline is positively associated with incident type 2 diabetes in a univariate analysis; however, the association disappears after adjustment for potential confounders (i.e., age, sex, BMI, liver attenuation and alcohol intake). Pancreatic steatosis is not independently associated with future type 2 diabetes |
BMI, body mass index; CT, computed tomography; EUS, endoscopic ultrasonography; FFA, free fatty acid; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐β, the homeostasis model assessment of β‐cell function; HOMA‐IR, the homeostasis model assessment of insulin resistance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IL‐1β, interleukin‐1β; IL‐6, interleukin‐6; IVGTT, intravenous glucose tolerance test; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAFLD, non‐alcoholic fatty liver disease; NAFPD, non‐alcoholic fatty pancreas disease; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; TG, triglycerides; TNF‐α, tumor necrosis factor‐α; US, ultrasonography; VAT, visceral adipose tissue; WC, waist circumference.
Pathophysiology
To date, the pathophysiology of NAFPD remains unclear. Obesity leads to adipocyte infiltration in the pancreas. In addition, there are two potential mechanisms for pancreatic fat accumulation: (i) death of acinar cells, followed by the replacement of adipose tissue; and (ii) intracellular triglyceride accumulation associated with excessive energy balance12.
Human and animal studies have shown that NAFPD frequently coexists with NAFLD13, 14, 15. Individuals with NAFPD have a higher prevalence of NAFLD compared with those without NAFPD14, 16, 17. Both NAFLD and NAPFD are strongly associated with obesity and visceral adipose tissue (VAT)15, 18, 19, 20. Of note, the pancreas seems to be more susceptible to fat deposition compared with the liver. In mice, during the period of 3–15 weeks on a high‐fat diet, there was an increase in pancreatic, fat but not hepatic fat21. Based on the viewpoint of ectopic fat deposition, it is conceivable that some possible common mechanisms might exist between NAFPD and NAFLD.
Fat accumulation in the liver or pancreas associated with obesity might result from a mismatch between energy supply, formation, consumption, β‐oxidation or disposal of triglycerides10. The potential sources of lipids in the liver or pancreas might come from circulating FFAs, de novo lipogenesis and dietary fat intakes. There is a relationship between overfeeding, an increase in VAT and subsequent ectopic fat deposition. Mice fed with a high‐fat diet to induce obesity have an increase in VAT, adipocyte hypertrophy, hepatopancreatic steatosis and glucose intolerance13. VAT appears to be a pathogenic factor in the development of hepatic and pancreatic steatosis. It can release greater amounts of adipokines and pro‐inflammatory cytokines, promoting insulin resistance, enhancing triglyceride lipolysis and thus releasing FFAs into the circulation. Increasing the availability of FFAs to all tissues leads to self‐reinforcing cycles that interact to bring excess adipocytes and ectopic fat deposition in the liver and pancreas.
Technology of Assessing Pancreatic Steatosis
Histology and biochemical measurements are the most direct and straightforward way to assess pancreatic steatosis. In contrast with the liver, where triglycerides accumulate in hepatocytes, pancreatic steatosis is histologically characterized by adipocyte infiltration and intracellular fat deposition in both acinar and islet cells22. However, because of the difficulties of obtaining adequate pancreatic specimens and rapid autolysis encountered in autopsy, no dichotomous histopathological cut‐off is used to define ‘fatty pancreas.’ Recently, several imaging techniques, including ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) have been used to detect pancreatic steatosis. Nevertheless, there is no consensus over the ‘gold standard’ for in vivo quantification of pancreatic fat content.
Abdominal US is a non‐invasive and less‐costly method in the diagnosis of NAFPD. In most studies, the diagnostic criteria for NAFPD using abdominal US are an increase in echogenicity of the pancreatic body over that of the kidney15, 16, 17, 23, 24, 25, which is metabolically more stable than the liver. As the pancreas cannot be compared directly with the kidney in the same acoustic window, the examiner needs to compare the echogenicity differences between the liver and the kidney, and between the liver and the pancreas, to obtain an objective pancreas–kidney echogenicity contrast. However, as the pancreas is located in the retroperitoneal space, overlying bowel gas or obesity can obscure the pancreas. The evaluation of the pancreas by abdominal US is highly dependent on the skill of the operators as well as the quality of the machine.
Endoscopic ultrasonography (EUS) can provide detailed images of the entire pancreas and simultaneously compare the echogenicity of the pancreas with adjacent organs in real time. Some grading systems using EUS to classify the intensity of NAFPD have been reported based on the echogenicity of pancreatic parenchyma and pancreatic duct margins26. Although abdominal US and EUS are cost‐effective modalities to screen NAFPD, they cannot accurately quantify the degree of pancreatic steatosis.
CT scan is an operator independent and simple procedure that can be carried out with a short acquisition time. The amount of pancreatic steatosis on CT scans can be assessed using Hounsfield Units. CT scans show the fatty pancreas as a decrease in attenuation compared with the spleen. However, the clinical value of CT scan in the diagnosis of NAFPD remains controversial. Some studies have proposed that CT scan is a less valuable technique for judgment of pancreatic steatosis compared with other imaging15, whereas others have not27, 28. To compare the echogenicity on abdominal US with objective Hounsfield Units on CT scan, Lee et al.15 have found there was no statistically significant difference in clinical and biochemical parameters between individuals with and without NAFPD, defined by CT scan. This discrepant finding in abdominal US and CT scan might be due to an inhomogenous distribution of pancreatic adipose tissue infiltration29, resulting in heterogeneous patterns with a large difference in Hounsfield Units on CT scan. In contrast, Kim et al.27 have reported that pancreatic attenuation on CT scan had a good negative correlation with the histological pancreatic fat fraction, considering CT scan as a reliable modality for quantifying pancreatic fat content.
Currently, there is strong evidence emerging for the use of MRI and MRS in detection and quantification of pancreatic steatosis. Like CT scan, MRI and MRS are non‐invasive and reproducible techniques to measure the fat content of the whole pancreas. MRI is based on the signal differences between fat and water, whereas MRS is based on the differences in resonance frequencies of protons. Pancreatic fat content measured by MRS correlated well with biochemical determination of intra‐islet triglyceride concentrations, considering MRS‐measured fat content in the whole pancreas as a useful surrogate marker for islet fat content30, 31. However, MRI and MRS still have some limitations, such as high cost, long scanning duration and susceptibility to MR chemical shift artifact as a result of the surrounding visceral fat.
Epidemiology of NAFPD
There are limited data on the prevalence of NAFPD in the general population because of the lack of standard screening tools. The prevalence of NAFPD varies widely depending on the ethnicity of the population and diagnostic methodology applied. Among 230 individuals referred for EUS examination in the USA, 27.8% were found to have fatty pancreas26. A study in Indonesia reported that 35% of 901 adults who underwent a routine medical check‐up had NAPFD detected by abdominal US25, whereas the other study in South Korea showed the prevalence of NAFPD increased to 61.4% among 293 individuals visiting an obesity clinic15. By contrast, a large cohort study in Taiwan involving 8,097 individuals who underwent a health check‐up reported 16% prevalence of NAFPD detected by abdominal US16. The population prevalence in Taiwan was similar to that reported in a Hong Kong study, which used MRI to quantify pancreatic fat content32. In addition, NAFPD can even occur in children. A retrospective single‐center study in the USA showed pancreatic steatosis was identified in ~10% of 232 pediatric patients aged 2–18 years who underwent abdominal CT scan33.
Like NAFLD, the risk of NAFPD increases with age, and it occurs more often in men than in women16, 18, 19, 20. Men have a higher pancreatic fat content compared with women of comparable body mass index (BMI)20. Saisho et al.34 have found that pancreatic fat content increased linearly with age throughout childhood and reached a plateau until the age of ~50 years. Wong et al.32 have shown that men had the highest prevalence of NAFPD at the age of 40–49 years, whereas the prevalence of NAFPD in women was very low in early life, but rapidly increased after menopause. These studies imply that sex might reflect the difference in propensity for ectopic fat deposition in the pancreas. Aging and hormonal changes appear to have relevance to the development of NAFPD, but more studies are required to examine this speculation.
NAFPD and β‐cell Dysfunction
Several studies have suggested a potential interplay between dysglycemia, NAFPD and β‐cell dysfunction (Figure 1). The explanation most commonly offered for the relationship of NAFPD to β‐cell dysfunction is based on glucolipotoxicity. In β‐cells, hyperglycemia inhibits carnitinine‐palmitoyl transferase‐1 through increasing malonyl coenzyme A, decreasing mitochondria β‐oxidation, and thereby promoting intracellular triglyceride accumulation. In addition, insulin resistance decreases the inhibitory action of insulin on peripheral lipolysis, thus increasing circulating FFAs. Chronic exposure of β‐cells to elevated FFAs results in increased triacylglycerol content, decreased insulin gene expression, blunted glucose‐stimulated insulin secretion and increased risk of apoptosis35, 36. The adverse effect of glucolipotoxicity contributes to β‐cell dysfunction and causes a vicious cycle of continuous deterioration of the glucometabolic state.
Figure 1.
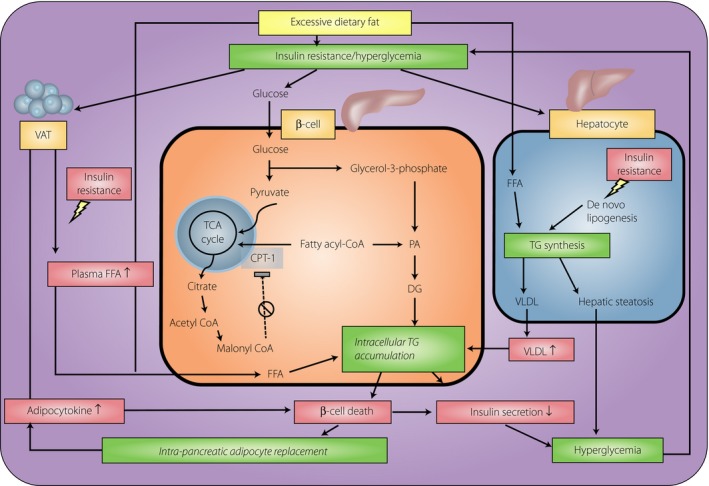
A potential interplay between dysglycemia, non‐alcoholic fatty pancreas disease (NAFPD) and β‐cell dysfunction. During long‐term intake of excessive calories, dietary fatty acids and hyperinsulinemia stimulate hepatic steatosis, leading to increased export of very‐low‐density lipoprotein (VLDL), which will increase fat delivery to the islets. In β‐cells, hyperglycemia inhibits carnitinine‐palmitoyl transferase‐1 (CPT‐1) through increasing malonyl coenzyme A (malonyl CoA), decreasing mitochondria β‐oxidation and further promoting intracellular triglyceride (TG) accumulation. In contrast, insulin resistance enhances triglyceride lipolysis and free fatty acid (FFA) release from visceral adipose tissue (VAT), thus increasing circulating FFAs. Chronic exposure of β‐cell to elevated FFAs results in increased intracellular triacylglycerol content, decreased insulin gene expression and blunted glucose‐stimulated insulin secretion. In addition, adipocyte‐derived cytokines and FFAs also contribute to β‐cell destruction, which further blunts insulin secretion as well as promotes intrapancreatic replacement by adipocytes. When fat deposition in the pancreas exceeds the tolerance threshold, hyperglycemia will supervene and causes a vicious cycle of continuous deterioration of glucometabolic state. DG, diglycerides; PA, phosphatidic acid; TCA, tricarboxylic acid.
Several in vitro and animal studies have shown a link between pancreatic steatosis and β‐cell dysfunction. A reduced insulinogenic signaling on pancreatic acinar cells, as occurs in diabetes, might have an influence on viability and growth of cells, apoptosis, and subsequent fat replacement37, 38. In rats, chronic high‐fat diet can induce an increase in pancreatic FFAs, acute inflammatory response, resulting in the damage of acinar cells and islets, as well as fatty infiltration in the pancreas39. However, the relationship between pancreatic steatosis and β‐cell dysfunction in humans remains inconsistent. Previous studies have found pancreatic volume reduction and steatosis in diabetes patients40, 41. In non‐diabetic non‐obese children with a mutation in carboxyl‐ester lipase, pancreatic steatosis reflects early events in the pathogenesis of diabetes42. Otherwise, several studies have shown that pancreatic fat content is inversely correlated with insulin secretion in individuals with impaired fasting glucose or impaired glucose tolerance, but not in individuals with normoglycemia or type 2 diabetes19, 43. Similarly, in young obese normoglycemic individuals, there is no significant association between pancreatic fat content and β‐cell function44. These results show the concept of pancreatic steatosis being crucial in the deterioration of glucose homeostasis. Once diabetes develops, other factors superimposing the effect of pancreatic steatosis might contribute to a progressive decline in β‐cell function.
In contrast, some studies have found no association of NAFPD with β‐cell function. A community cohort study in Hong Kong showed no significant correlation between NAFPD and the homeostasis model assessment of β‐cell function after adjusting for hepatic fat content and BMI32. Using hyperglycemic clamp as the gold standard measurement of β‐cell function, van der Zijl et al.45 could not establish an association between pancreatic fat content and β‐cell function despite the fact that the impairments in β‐cell function in individuals with impaired glucose metabolism were accompanied by pancreatic fat deposition. Similar findings were also obtained in a study by Begovatz et al.29, who found no association between pancreatic adipose tissue infiltration and the first‐phase insulin response to oral glucose challenge, regardless of glucose tolerance status. These contradictory findings about the relationship between NAFPD and β‐cell dysfunction might possibly arise from methodology differences (including techniques to measure pancreatic fat content and methods to assess β‐cell function) or from differences in age and ethnicity of the population.
Although many studies have pointed to an upward trend in pancreatic steatosis on the progression of the diabetic state, there is no clear evidence of a causal link between pancreatic steatosis and β‐cell dysfunction in humans. The inconsistent results cast doubt on whether pancreatic steatosis might cause lipotoxicity to β‐cells, or whether its presence is merely a marker of β‐cell dysfunction46. Additional large‐scale longitudinal studies are warranted to investigate the contributing role of pancreatic steatosis during the progressive β‐cell failure.
NAFPD and Insulin Resistance
The association between NAFPD and insulin resistance is still controversial. In healthy monozygotic twins, pancreatic fat content was associated with insulin sensitivity index and plasma adiponectin, which plays a unique role in maintaining insulin sensitivity47. In addition, Della Corte et al.17 found obese children with NAFLD complicated with NAFPD had a higher insulin resistance and circulating levels of tumor necrosis factor‐α and interleukin‐1β than those without NAFPD. Similarly, a community cohort study also proved that adults with both NAFPD and NAFLD had a higher homeostasis model assessment of insulin resistance (HOMA‐IR) than those with either condition alone. Pancreatic fat content was associated with HOMA‐IR, even after adjusting for hepatic fat content and BMI32. A study involving patients with impaired fasting glucose and/or impaired glucose tolerance that used hyperglycemic clamp to assess insulin sensitivity showed an inverse correlation between pancreatic fat content and insulin sensitivity45. Furthermore, Lee et al.15 found HOMA‐IR tended to increase with the severity of NAFPD. In multivariate logistic regression analysis, HOMA‐IR was correlated with NAFPD after adjustment for age, BMI and lipid profiles. However, the significant association between NAFPD and HOMA‐IR disappeared after further adjustment for VAT, suggesting that VAT might be a much stronger relational factor or mediate the association between NAFPD and insulin resistance.
In contrast, Lê et al.44 did not find any relationship between pancreatic fat content and markers of insulin resistance in obese young individuals. Similar findings were also obtained in a study by Rossi et al.20, who found that insulin resistance was related with hepatic fat instead of pancreatic fat in obese adults. Because of the inconsistent results of the available studies, whether NAFPD is a causal factor of insulin resistance or is just part of a cluster of abnormalities during the course of obesity remains a matter for speculation.
Clinical Implications of NAFPD
NAFPD and metabolic syndrome
NAFPD has been shown to be associated with obesity and the features of metabolic syndrome. Ogilvie11 found that the degree of adiposity in the pancreas was higher in obese cadavers than in lean ones (17 vs 9%, respectively). In C57BL/6 mice, diet‐induced obesity developed common features of metabolic syndrome, elevated insulin resistance and NAFPD13. Most human studies have shown pancreatic fat content increased with BMI and waist circumference16, 17, 19, 20, 25, 26, 30, 32, 45. The association between NAFPD and VAT is found in some studies15, 19, 20, 44, but not others43. Ethnicity has an influence on the association between pancreatic steatosis and VAT, resulting in a stronger relationship between pancreatic fat content and VAT in Hispanics than African Americans44.
In contrast, several studies have shown that individuals with fatty pancreas have an increased prevalence of metabolic syndrome compared with those without fatty pancreas. The number of metabolic syndrome components in fatty pancreas groups was significantly higher than in normal groups15, 24, 26. Triglycerides and VAT gradually increased with the severity of pancreatic steatosis15. Sepe et al.26 found a higher prevalence of fatty pancreas in patients with metabolic syndrome than those without metabolic syndrome. The presence of any metabolic syndrome components increased the prevalence of fatty pancreas by 37%. Hyperlipidemia was related with fatty pancreas in univariate analysis, but not in multivariate analysis. Hypertension showed a trend toward an association with fatty pancreas despite no statistical significance26. Wu et al.24 reported that compared with healthy controls, those with fatty pancreas had higher levels of several metabolic risk factors (including BMI, waist circumference, triglycerides, fasting plasma glucose, hemoglobin A1c and systolic blood pressure), as well as lower levels of high‐density lipoprotein cholesterol. These aforementioned studies show that NAFPD might be the pancreatic manifestation of metabolic syndrome. Insulin resistance probably represents as a link between NAFPD and metabolic syndrome. However, all previous studies are cross‐sectional in design, which cannot clearly clarify cause and effect relationships. Future longitudinal studies are warranted to investigate the association between NAFPD and the development of metabolic syndrome.
NAFPD and diabetes
Insulin resistance is a common pathway for the development of NAFLD, NAFPD and diabetes. The consequence of pancreatic fat infiltration might provoke a decrease in β‐cell number and function, leading to more rapid progression to diabetes. To date, some studies have shown that NAFPD is associated with diabetes independently of NAFLD, suggesting that possible mechanisms (e.g., β‐cell failure) other than insulin resistance link NAFPD to diabetes23. In contrast, Della Corte et al.17 found that approximately 50% of pediatric patients with NAFLD and 80% of biopsy‐proven non‐alcoholic steatohepatitis concurrently had NAFPD. Compared with those without NAFPD, children with NAFLD complicated by NAFPD had a higher insulin resistance and a more advanced form of liver disease17. This result is in agreement with a study carried out in adults, showing that individuals with non‐alcoholic steatohepatitis and NAFPD had higher glucose parameters as well as prevalence of prediabetes and diabetes than those with non‐alcoholic steatohepatitis alone48. In addition, Lee et al.15 showed that most individuals with NAFLD (96.6%) concurrently had NAFPD. The positive predictive value of NAFLD in NAFPD was 69.4%, whereas the negative predictive value of NAFLD in normal pancreas was 96.4%. Taken together, these studies imply that NAFPD might be used as an initial indicator of ectopic fat deposition and as an additional factor, other than NAFLD, able to deteriorate gluco‐insulinemic disarray.
So far, whether NAFPD contributes to the development of type 2 diabetes remains inconclusive. In obese Zucker diabetic fatty rats, a rapid increase in pancreatic fat preceded the onset of hyperglycemia49. Wang et al.16 reported that the NAFPD group had a higher proportion of type 2 diabetes than the non‐NAFPD group. Some human studies have shown that individuals with type 2 diabetes had an increased MRS‐ or MRI‐measured pancreatic fat content compared with their non‐diabetes group30, 43, 50, whereas the other study observed no difference in pancreatic fat content measured by CT scans or histology at autopsy34. This discrepancy might be attributed to different methods for assessing pancreatic fat content.
Although a growing body of evidence has linked NAFPD to diabetes through insulin resistance and β‐cell dysfunction, most studies were cross‐sectional in design. To our best knowledge, there is so far only one 5‐year retrospective cohort study by Yamazaki et al.28 that investigated longitudinal effects of pancreatic steatosis on incident diabetes. In that study, pancreatic steatosis at baseline was associated with an increased incidence of type 2 diabetes in univariate analysis. The association disappeared after adjustment for potential confounders (e.g., age, sex, BMI, liver attenuation and alcohol intake), suggesting that pancreatic steatosis seems not to be an independent risk factor for future type 2 diabetes28. However, that study still had some limitations. First, it remains to be elucidated whether the findings could be observed in populations other than the Japanese population, because the pathogenesis of type 2 diabetes and adipose tissue distribution can vary in different ethnicities. Second, the study participants were mainly middle‐aged adults (mean age 51 ± 9.8 years), and the follow‐up period of 5 years is relatively short. Thus, statistical power might not be sufficient to detect an association in this relatively low‐risk subgroup. Therefore, additional studies with longer follow up in other ethnic groups are warranted to verify the clinical relevance of NAFPD in type 2 diabetes.
Drain Fat Out of the Pancreas: Normalization of β‐cell Function and Reversal of Type 2 Diabetes
Type 2 diabetes has been considered as an inevitably progressive process, but it is now understood as a potentially reversible metabolic state precipitated by chronic excess ectopic fat deposition51. A current study used MRI to compare pancreatic fat change before and after bariatric surgery between participants with type 2 diabetes and the normoglycemic group. Participants with type 2 diabetes were found to have an attenuated first‐phase insulin response and increased pancreatic fat content compared with the BMI‐matched normoglycemic group. Of note, 8 weeks after bariatric surgery, first‐phase insulin response and pancreatic fat content both returned to normal uniquely in the type 2 diabetes group, but not in the normoglycemic group50. These findings are consistent with a previous study, which showed that acute restriction of dietary energy intake can normalize β‐cell function in step with decreasing pancreatic fat content52. Even though a cause‐and‐effect relationship between pancreatic steatosis and type 2 diabetes has not been clearly clarified, the time‐course data suggest that pancreatic steatosis might play a pivotal role in glucose metabolism. The various thresholds of susceptibility to the adverse metabolic effects of excess fat deposition in the pancreas could be a crucial point to determine whether or not β‐cell failure occurs. In addition, the varying degree of liposusceptibility in relation to ethnic difference could explain the discrepancy of results on the relationship between NAFPD and β‐cell function in previous studies.
Conclusion
With the rise in epidemic of obesity, NAFPD has become a growing health problem that deserves greater attention. Emerging studies suggest that NAFPD should not only be considered as an inert accumulation of fat, but also as an early marker of glucometabolic disturbance. In vitro and animal studies have shown that NAFPD might contribute to glucometabolic disorders through effects on insulin resistance and β‐cell dysfunction. However, the data in humans remain inconclusive. To date, evidence to support the long‐term effects of NAFPD on glucose homeostasis is insufficient, and much remains unknown about NAFPD. Additional longitudinal research is required to explore the detailed mechanism and validate the clinical implications of NAFPD. In contrast, most past studies on NAFPD merely focus on pancreatic fat content at a single point in time and dismiss the influence of individual degree of liposusceptibility. Future studies must focus on tracing the time sequence of pathophysiological events in the dynamic change of pancreatic steatosis, and hence to unravel the role of NAFPD in the development of diabetes and related metabolic disorders.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2017; 8: 735–747
References
- 1. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697–738. [DOI] [PubMed] [Google Scholar]
- 2. Vague J. La différenciation sexuelle; facteur déterminant des formes de l'obésité. Presse Med 1947; 55: 339. [PubMed] [Google Scholar]
- 3. Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 1993; 14: 72–93. [DOI] [PubMed] [Google Scholar]
- 4. Pitt HA. Hepato‐pancreato‐biliary fat: the good, the bad and the ugly. HPB (Oxford) 2007; 9: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeon KJ, Lee O, Kim HK, et al Comparison of the dietary intake and clinical characteristics of obese and normal weight adults. Nutr Res Pract 2011; 5: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin W, Liao D, Kusunoki M, et al NO‐1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet‐induced diabetic swine. J Endocrinol 2004; 180: 399–408. [DOI] [PubMed] [Google Scholar]
- 7. Adams LA, Lymp JF, St Sauver J, et al The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005; 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 8. Seppala‐Lindroos A, Vehkavaara S, Hakkinen AM, et al Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002; 87: 3023–3028. [DOI] [PubMed] [Google Scholar]
- 9. Targher G, Bertolini L, Poli F, et al Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005; 54: 3541–3546. [DOI] [PubMed] [Google Scholar]
- 10. Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009; 13: 9–19. [PMC free article] [PubMed] [Google Scholar]
- 11. Ogilvie RF. The islands of langerhans in 19 cases of obesity. J Pathol Bacteriol 1933; 37: 473–481. [Google Scholar]
- 12. Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol 2011; 8: 169–177. [DOI] [PubMed] [Google Scholar]
- 13. Fraulob JC, Ogg‐Diamantino R, Fernandes‐Santos C, et al A Mouse Model of Metabolic Syndrome: insulin Resistance, Fatty Liver and Non‐Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr 2010; 46: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Geenen EJ, Smits MM, Schreuder TC, et al Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 2010; 39: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Kim SH, Jun DW, et al Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol 2009; 15: 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang CY, Ou HY, Chen MF, et al Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc 2014; 3: e000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Della Corte C, Mosca A, Majo F, et al Nonalcoholic fatty pancreas disease and Nonalcoholic fatty liver disease: more than ectopic fat. Clin Endocrinol (Oxf) 2015; 83: 656–662. [DOI] [PubMed] [Google Scholar]
- 18. Choi CW, Kim GH, Kang DH, et al Associated factors for a hyperechogenic pancreas on endoscopic ultrasound. World J Gastroenterol 2010; 16: 4329–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heni M, Machann J, Staiger H, et al Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev 2010; 26: 200–205. [DOI] [PubMed] [Google Scholar]
- 20. Rossi AP, Fantin F, Zamboni GA, et al Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity (Silver Spring) 2011; 19: 1747–1754. [DOI] [PubMed] [Google Scholar]
- 21. Pinnick KE, Collins SC, Londos C, et al Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring) 2008; 16: 522–530. [DOI] [PubMed] [Google Scholar]
- 22. Catanzaro R, Cuffari B, Italia A, et al Exploring the metabolic syndrome: nonalcoholic fatty pancreas disease. World J Gastroenterol 2016; 22: 7660–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ou HY, Wang CY, Yang YC, et al The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One 2013; 8: e62561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu WC, Wang CY. Association between non‐alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case‐control retrospective study. Cardiovasc Diabetol 2013; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lesmana CR, Pakasi LS, Inggriani S, et al Prevalence of Non‐Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check‐up patients in a private hospital: a large cross sectional study. BMC Gastroenterol 2015; 15: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sepe PS, Ohri A, Sanaka S, et al A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc 2011; 73: 987–993. [DOI] [PubMed] [Google Scholar]
- 27. Kim SY, Kim H, Cho JY, et al Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology 2014; 271: 104–112. [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki H, Tsuboya T, Katanuma A, et al Lack of independent association between fatty pancreas and incidence of type 2 diabetes mellitus: 5‐year Japanese cohort study. Diabetes Care 2016; 39: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 29. Begovatz P, Koliaki C, Weber K, et al Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia 2015; 58: 1646–1655. [DOI] [PubMed] [Google Scholar]
- 30. Lingvay I, Esser V, Legendre JL, et al Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab 2009; 94: 4070–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee Y, Lingvay I, Szczepaniak LS, et al Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond) 2010; 34: 396–400. [DOI] [PubMed] [Google Scholar]
- 32. Wong VW, Wong GL, Yeung DK, et al Fatty pancreas, insulin resistance, and beta‐cell function: a population study using fat‐water magnetic resonance imaging. Am J Gastroenterol 2014; 109: 589–597. [DOI] [PubMed] [Google Scholar]
- 33. Pham YH, Bingham BA, Bell CS, et al Prevalence of Pancreatic Steatosis at a Pediatric Tertiary Care Center. South Med J 2016; 109: 196–198. [DOI] [PubMed] [Google Scholar]
- 34. Saisho Y, Butler AE, Meier JJ, et al Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type‐2 diabetes. Clin Anat 2007; 20: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harmon JS, Gleason CE, Tanaka Y, et al Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes 2001; 50: 2481–2486. [DOI] [PubMed] [Google Scholar]
- 36. Poitout V, Amyot J, Semache M, et al Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta 2010; 1801: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korc M, Owerbach D, Quinto C, et al Pancreatic islet‐acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science 1981; 213: 351–353. [DOI] [PubMed] [Google Scholar]
- 38. Williams JA, Goldfine ID. The insulin‐pancreatic acinar axis. Diabetes 1985; 34: 980–986. [DOI] [PubMed] [Google Scholar]
- 39. Zhang X, Cui Y, Fang L, et al Chronic high‐fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas 2008; 37: e31–e38. [DOI] [PubMed] [Google Scholar]
- 40. Walters MN. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol 1966; 92: 547–557. [DOI] [PubMed] [Google Scholar]
- 41. Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol 1984; 15: 677–683. [DOI] [PubMed] [Google Scholar]
- 42. Raeder H, Haldorsen IS, Ersland L, et al Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl‐ester lipase. Diabetes 2007; 56: 444–449. [DOI] [PubMed] [Google Scholar]
- 43. Tushuizen ME, Bunck MC, Pouwels PJ, et al Pancreatic fat content and beta‐cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916–2921. [DOI] [PubMed] [Google Scholar]
- 44. Le KA, Ventura EE, Fisher JQ, et al Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 2011; 34: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Zijl NJ, Goossens GH, Moors CC, et al Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta‐cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011; 96: 459–467. [DOI] [PubMed] [Google Scholar]
- 46. van Raalte DH, van der Zijl NJ, Diamant M. Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care 2010; 13: 478–485. [DOI] [PubMed] [Google Scholar]
- 47. Hannukainen JC, Borra R, Linderborg K, et al Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol 2011; 54: 545–552. [DOI] [PubMed] [Google Scholar]
- 48. Uygun A, Kadayifci A, Demirci H, et al The effect of fatty pancreas on serum glucose parameters in patients with nonalcoholic steatohepatitis. Eur J Intern Med 2015; 26: 37–41. [DOI] [PubMed] [Google Scholar]
- 49. Lee Y, Hirose H, Ohneda M, et al Beta‐cell lipotoxicity in the pathogenesis of non‐insulin‐dependent diabetes mellitus of obese rats: impairment in adipocyte‐beta‐cell relationships. Proc Natl Acad Sci USA 1994; 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steven S, Hollingsworth KG, Small PK, et al Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care 2016; 39: 158–165. [DOI] [PubMed] [Google Scholar]
- 51. Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care 2013; 36: 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lim EL, Hollingsworth KG, Aribisala BS, et al Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011; 54: 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Al‐Haddad M, Khashab M, Zyromski N, et al Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case‐control study. Pancreas 2009; 38: 672–675. [DOI] [PubMed] [Google Scholar]


