Abstract
Prostaglandins (PG), the products of cyclooxygenase-mediated conversion of arachidonic acid, become upregulated in many situations including allergic response, inflammation, and injury, and exhibit a variety of biological activities. Previous studies described barrier-enhancing and anti-inflammatory effects of PGE2 and PGI2 on vascular endothelial cells (EC). Yet, the effects of other PG members on EC barrier and inflammatory activation have not been systematically analyzed. This study compared effects of PGE2, PGI2, PGF2α, PGA2, PGJ2, and PGD2 on human pulmonary EC. EC permeability was assessed by measurements of transendothelial electrical resistance and cell monolayer permeability for FITC-labeled tracer. Anti-inflammatory effects of PGs were evaluated by analysis of expression of adhesion molecule ICAM1 and secretion of soluble ICAM1 and cytokines by EC. PGE2, PGI2, and PGA2 exhibited the most potent barrier-enhancing effects and most efficient attenuation of thrombin-induced EC permeability and contractile response, whereas PGI2 effectively suppressed thrombin-induced permeability but was less efficient in the attenuation of prolonged EC hyperpermeability caused by interleukin-6 or bacterial wall lipopolysaccharide, LPS. PGD2 showed a modest protective effect on the EC inflammatory response, whereas PGF2α and PGJ2 were without effect on agonist-induced EC barrier dysfunction. In vivo, PGE2, PGI2, and PGA2 attenuated LPS-induced lung inflammation, whereas PGF2α and PGJ2 were without effect. Interestingly, PGD2 exhibited a protective effect in the in vivo model of LPS-induced lung injury. This study provides a comprehensive analysis of barrier-protective and anti-inflammatory effects of different prostaglandins on lung EC in vitro and in vivo and identifies PGE2, PGI2, and PGA2 as prostaglandins with the most potent protective properties.
Keywords: pulmonary endothelial cells, permeability, inflammation, prostaglandins, acute lung injury
preservation of the endothelial cell (EC) vascular barrier integrity is important for the maintenance of homeostasis and lung fluid balance. In pathological settings, additional stimulation of vascular barrier-enhancing mechanisms is a key mechanism to prevent catastrophic consequences of uncontrolled vascular leakiness in the lung or other organs caused by bacterial pathogens, excessive mechanical forces, or cytokine storm associated with infection, sepsis, or trauma (3, 29).
Prostaglandins (PG) are products of the arachidonic acid metabolic pathway and are synthesized by many tissues, including vascular endothelial cells. The first step involves intracellular release of arachidonic acid from plasma membrane phospholipids via the action of phospholipase A2. Arachidonic acid is then converted sequentially to PGG2 and PGH2 by the cyclooxygenase and peroxidase activities of a single enzyme, PGH synthase (also known as cyclooxygenase or COX). There are two forms of COX: a constitutive form (COX-1) and an inducible form (COX-2), whose expression is induced by proinflammatory cytokines. Specific prostaglandins are then produced via the action of specific synthases including PGE2, PGD2, and PGF2α synthase. Alternatively, PGH2 may be converted to thromboxane (TxA2) or prostacyclin (PGI2). The cyclopentenone prostaglandins PGA2 and PGJ2 are formed by dehydration within the cyclopentane ring of PGE2 and PGD2, respectively (reviewed in 28).
Prostaglandins exert their biological activities by interacting with specific receptors. PGE2 activates four distinct G protein-coupled receptors, EP1–EP4. Of them, EP1, couples to Gq and simulates phospholipase C; EP2 and EP4 are coupled with Gs and stimulate adenylate cyclase (AC), leading to an increase in intracellular cAMP; and EP3 is coupled with Gi and inhibits AC, which suppresses the cAMP formation. PGI2 activates the IP receptor, which is coupled to Gs similarly to EP2 and EP4. PGF2α acts via receptor FP, which is coupled to Gq similarly to EP1. PGD2 receptors, DP1 and DP2, activate and inhibit AC via Gs and Gi, respectively (reviewed in 32). 15d-PGJ2 has been shown to activate two receptors: a membrane receptor, chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) (18) and a nuclear receptor, peroxisome proliferator-activated receptor γ (PPARγ) (14). The primary receptor for PGA2 is not well defined.
The role of PGs and their receptors in inflammation and regulation of vascular permeability is complicated. Previous studies by our and other groups described potent anti-inflammatory and barrier-protective effects of PGE2 and PGI2 on pulmonary vascular endothelium in vitro and in animal models of lung injury caused by bacterial pathogens and excessive mechanical ventilation (6, 7, 21). Barrier-enhancing effects of PGE2 and PGI2 are mediated by PG-induced elevation of intracellular cAMP levels, activation of cAMP-dependent protein kinase A (PKA), and the PKA-independent signaling pathway, which involves cAMP-activated guanine nucleotide exchange factor Epac1, its effector GTPase Rap1, and downstream GTPase Rac1 (16). Anti-inflammatory effects by PGI2 and PGE2 are in large part driven by cAMP-mediated inhibition of RhoA GTPase activity and suppression of the NFkB inflammatory signaling cascade (7). However, effects of many other PG products generated under inflammatory and other pathological conditions remain to be precisely characterized.
This study performed a comprehensive analysis of functional effects of PGA2, PGE2, PGI2, PGD2, PGF2α, and PGJ2 on lung endothelial cell barrier under basal conditions, in EC stimulated with barrier disruptive agonist thrombin, and in cells exposed to proinflammatory agents: IL-6 and Gram-negative bacterial wall lipopolysaccharide (LPS). Functional effects of PGs in these models were assessed by analysis of EC barrier function and associated Rho signaling, expression of EC adhesion molecule ICAM1, and IL-8 and soluble ICAM1 release as parameters of EC inflammatory activation. Protective effects of PGs were further tested in the mouse model of acute lung injury.
MATERIALS AND METHODS
Reagents and cell culture.
PGE2, PGI2, PGA2, PGD2, PGF2α, and PGJ2 were obtained from Cayman Chemical (Ann Arbor, MI). Human IL-6 and IL-6 soluble receptor were obtained from R&D Systems (Minneapolis, MN); human thrombin was obtained from Sigma (St. Louis, MI). Phospho-MLC antibody was obtained from Cell Signaling (Beverly, MA); ICAM1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Unless otherwise specified, all biochemical reagents including antibody to β-actin and bacterial wall lipopolysaccharide (LPS, Escherichia coli O55:B5) were obtained from Sigma (St. Louis, MO). Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (East Rutherford, NJ) and used for experiments at passages 5–7.
Endothelial permeability assays.
Measurements of transendothelial electrical resistance (TER) across human lung EC monolayers were performed using the electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as described (2, 4). Endothelial permeability to macromolecules was monitored by permeability visualization assay (XPerT) (12) available from Millipore (cat. no. 17–10398). Binding of FITC-avidin fluorescent tracer to the matrix-coated culture dish bottoms increases if the EC barrier is compromised. After cell stimulation with agonists, FITC-avidin solution at a final concentration of 25 µg/ml was added directly to the culture medium for 3 min before termination of the experiment. Unbound FITC-avidin was washed out with PBS, pH 7.4, 37°C (2 cycles, 10 s each). Visualization of FITC-avidin labeled permeability sites in EC monolayers was performed using inverted microscope Nikon Eclipse TE300 with epifluorescence module and SPOT RT monochrome digital camera and image processor (Diagnostic Instruments, Sterling Heights, MI). For the 96-well format assays, the fluorescence of matrix-bound FITC-avidin was measured on Victor X5 Multilabel Plate Reader (Perkin Elmer, Waltham, MA) using an excitation wavelength of 485 nm and emission wavelength of 535 nm.
RT-PCR analysis of PG receptors.
HPAEC and SAEC cell monolayers grown in 35-mm dishes were lysed in 350 µl RLT Plus Buffer (Qiagen) containing beta mercaptoethanol. The RNA was isolated using the RNeasy plus mini kit (Qiagen) as per manufacturer’s instructions. Five-hundred nanograms of RNA was reverse transcribed to cDNA using iScript cDNA synthesis kit (Bio-Rad). The expression levels of the different prostaglandin receptors were quantified by qRT-PCR using PerfeCTa SYBRGreen Fastmix (Quanta Bio cat. no. 95072-012) on a Bio-Rad CFX96 Real-Time PCR System as per the manufacturer’s instructions. The following primer sequences were used for RT-PCR analysis: EP1 receptor, forward 5′-ACCTTCTTTGGCGGCTCTC-3′, reverse 5′-GCACGACACCACCATGATAC-3′; EP2 receptor, forward 5′-CAGTCTCCCTGCTCTTCTGC-3′, reverse 5′-GCACCGAGACAATGAGAAGC-3′; EP3 receptor, 5′-TCATCGTCGTGTACCTGTCC-3′, reverse 5′-CGATGAACAACGAGGAGAGC-3′; EP4 receptor, forward 5′-TGCTCTTCTTCAGCCTGTCC-3′, reverse 5′-AGACTGCAAAGAGCGTGAGG-3′; DP1 receptor, forward 5′-TGATGACCGTGCTCTTCACT-3′, reverse 5′-CCA AGG GTC CAC AATTGAAA-3′, DP2 receptor, forward 5′-TCTGCGCGCTACCTTTCATG-3′, reverse 5′-TCCTCGTGGACCATCTGGATA-3′; FP receptor, forward 5′-GCACATTGATGGGCAACTAGAA-3′, reverse 5′-GCACCTATCATTGGCATGTAGCT-3′; IP receptor, forward 5′-GCCGATCAGCTGCTGTTTCT-3′, reverse 5′-TTTCCTCTGTCCCTCACTCTCTTC-3′. Ct values were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the delta-delta method.
Western blot analysis.
Immunoblot detection of proteins of interest was performed as described previously (5, 8). In brief, protein extracts were subjected to 15% or 7.5% SDS-PAGE, transferred to nitrocellulose membrane in semidry transfer apparatus (Bio-Rad, Hercules, CA) 10 V for 1 h, and probed with the antibody of interest. Immunoreactive proteins were visualized by SuperSignal West Dura chemiluminescence reagent according to the manufacturer’s protocol (Pierce, Rockford, IL).
Measurement of cytokines and chemokines.
For cytokine measurements in preconditioned medium of human pulmonary EC cultures, supernatants from treated EC were collected and centrifuged to remove debris. Levels of IL-8 and soluble ICAM1 (sICAM1) levels were determined by ELISA (R&D Systems, Minneapolis, MN) following manufacturer’s protocol. Absorbance was read at 450 nm within 30 min in a Victor X5 Multilabel Plate Reader (Perkin Elmer, Waltham, MA).
Animal studies.
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee. Animals were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Bacterial lipopolysaccharide (LPS, 1 mg/kg body wt; Escherichia coli O55:B5) or sterile water was injected intratracheally in a small volume (20–30 µl) using a 20-gauge catheter (Exelint International, Los Angeles, CA). Prostaglandins of choice (20 µg/kg) or sterile saline solution was injected concurrently with LPS instillation and 5 h after LPS challenge by intravenous injection in the external jugular vein. After 24 h of LPS challenge, animals were euthanized by exsanguination under anesthesia. BAL was performed using 1 ml of sterile Hanks’ balanced salt buffer and measurements of cell count and protein concentration were conducted as previously described (15).
In vivo optical imaging.
Control and agonist-treated mice were injected with 100 µl of 2 nmol vascular fluorescent blood pool imaging agent Angiosense 680 EX (Perkin Elmer, Boston, MA), intravenously via tail vein 24 h before imaging sessions. Fluorescent optical imaging was performed in the Integrated Small Animal Imaging Research Resource (iSAIRR) at the University of Chicago using Xenogen IVIS 200 Spectrum (Caliper Life Sciences. Alameda, CA). Mice were exposed to isoflurane anesthesia with O2 through the gas anesthesia manifold and placed on the imaging stage. Acquisition and image analysis were performed with Living Image 4.3.1 Software.
Statistical analysis.
Results are expressed as means ± SD of three to eight independent experiments. Stimulated samples were compared with controls by unpaired Student’s t-test. For multiple-group comparisons, one-way ANOVA and Tukey’s post hoc multiple-comparison test were used. P < 0.05 was considered statistically significant.
RESULTS
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on lung endothelial cell barrier.
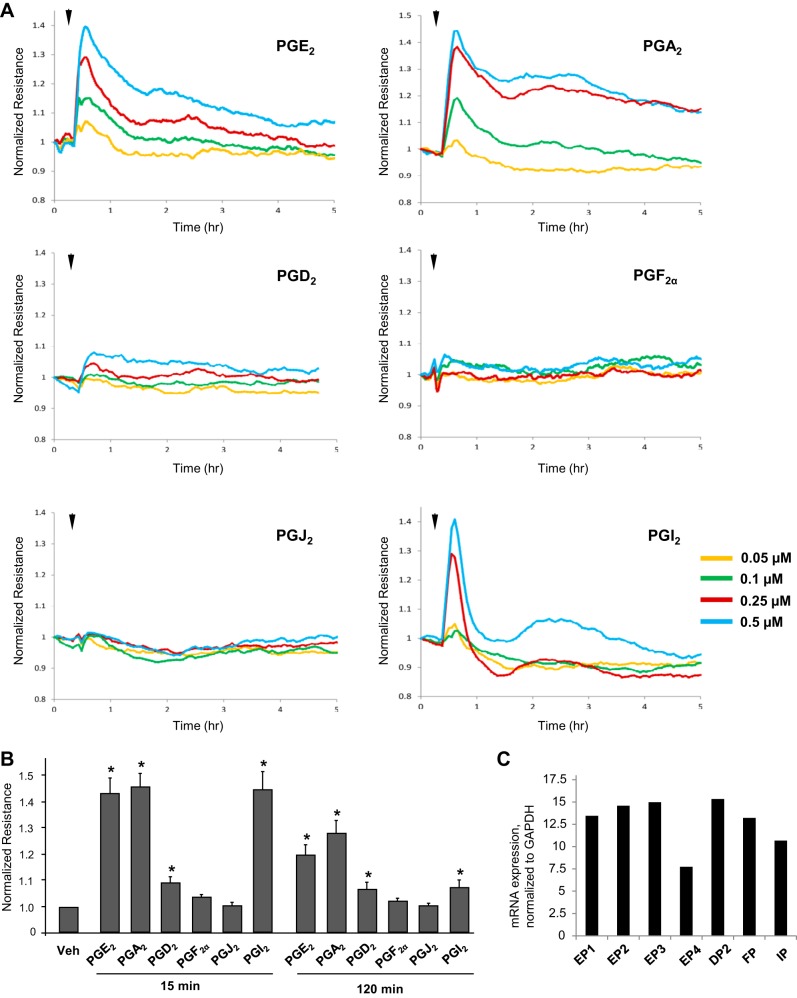
Effects of PGs on human pulmonary EC barrier properties were examined by the measurements of the changes in transendothelial electrical resistance (TER) of cell monolayers grown on gold microelectrodes and stimulated with various concentrations of PGs. Among six PG types, PGA2, PGE2, and PGI2 caused the most pronounced dose-dependent TER increases with maximal response to 0.5 µM of each PG compound (Fig. 1A). Maximal barrier-protective response was observed by 15 min of stimulation. TER levels remained elevated for up to 4–6 h in PGA2- and PGE2-stimulated cells, but returned to baseline in PGI2-stimulated cells by 1 h after treatment. In turn, stimulation with PGD2 caused a modest TER increase at 15 min which gradually declined over time, whereas PGF2α and PGJ2 did not affect TER levels in EC monolayers (Fig. 1, A and B). An RT-PCR analysis showed expression of all tested prostaglandin receptors in HPAEC (Fig. 1C).
Fig. 1.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on basal lung endothelial cell barrier properties. A: pulmonary EC grown on microelectrodes were stimulated with vehicle or 0.05 µM, 0.1 µM, 0.25 µM, or 0.5 µM of PGE2, PGA2, PGD2, PGF2α, PGJ2, or PGI2. Changes in transendothelial resistance (TER) were monitored over 5-h time period. B: summary of TER measurements performed at 15 min and 120 min after agonist stimulation; n = 5. *P < 0.05 vs. vehicle. C: RT-PCR analysis of prostaglandin receptor mRNA levels in human pulmonary EC. Receptor expression was normalized to GAPDH levels.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on thrombin-induced EC permeability.
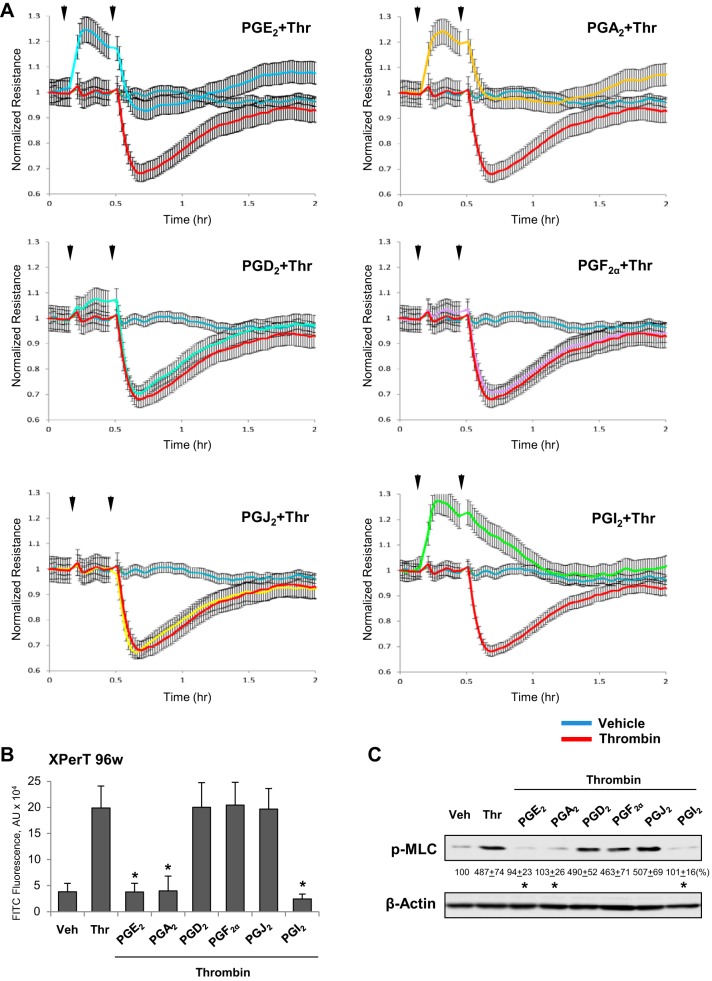
We evaluated the protective effects of PGs on thrombin-induced human lung EC hyperpermeability using TER measurements. First, EC pretreated with various PGs were challenged with low thrombin dose (0.2 U/ml), which causes a submaximal permeability response. Pretreatment with PGA2, PGE2, and PGI2 suppressed thrombin-induced TER decline. PGI2 completely abolished the TER decrease caused by thrombin, whereas PGA2 and PGE2 only partially attenuated the rapid phase of thrombin-induced EC permeability (Fig. 2A). Overall, all these three prostaglandins exhibited barrier-protective effects. In contrast, pretreatment with PGD2 PGF2α, or PGJ2 was without effect on thrombin-induced permeability. Similar results were obtained in quantitative assays of FITC-avidin permeability using a 96-well plate format (Fig. 2B).
Fig. 2.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on EC permeability caused by low dose of thrombin. A: pulmonary EC were treated with indicated PG (0.5 µM, first arrow) for 15 min followed by addition of low dose of thrombin (Thr; 0.2 U/ml, second arrow). Changes in TER were monitored over 2-h time period. B: analysis of EC permeability for macromolecules using XPert assay. Bar graph depicts permeability changes after 5 min of thrombin treatment; n = 4. *P < 0.05 vs. thrombin. C: cells treated with indicated PG (0.5 µM, 15 min) or vehicle were stimulated with thrombin for 30 min, and the levels of MLC phosphorylation were evaluated by Western blot with diphospho-MLC antibody. Probing with β-actin antibody was used as a normalization control. Results of densitometry are shown as means ± SD; n = 3. *P < 0.05.
Thrombin-induced EC barrier dysfunction is associated with activation of RhoA GTPase pathway leading to increased phosphorylation of regulatory myosin light chains (MLC) and actomyosin contraction of pulmonary EC (31). We next examined effects of PGs on the thrombin-induced MLC phosphorylation. The extent of PG-induced attenuation of thrombin-induced EC permeability directly correlated with inhibition of thrombin-induced MLC phosphorylation caused by each PG compound (Fig. 2C).
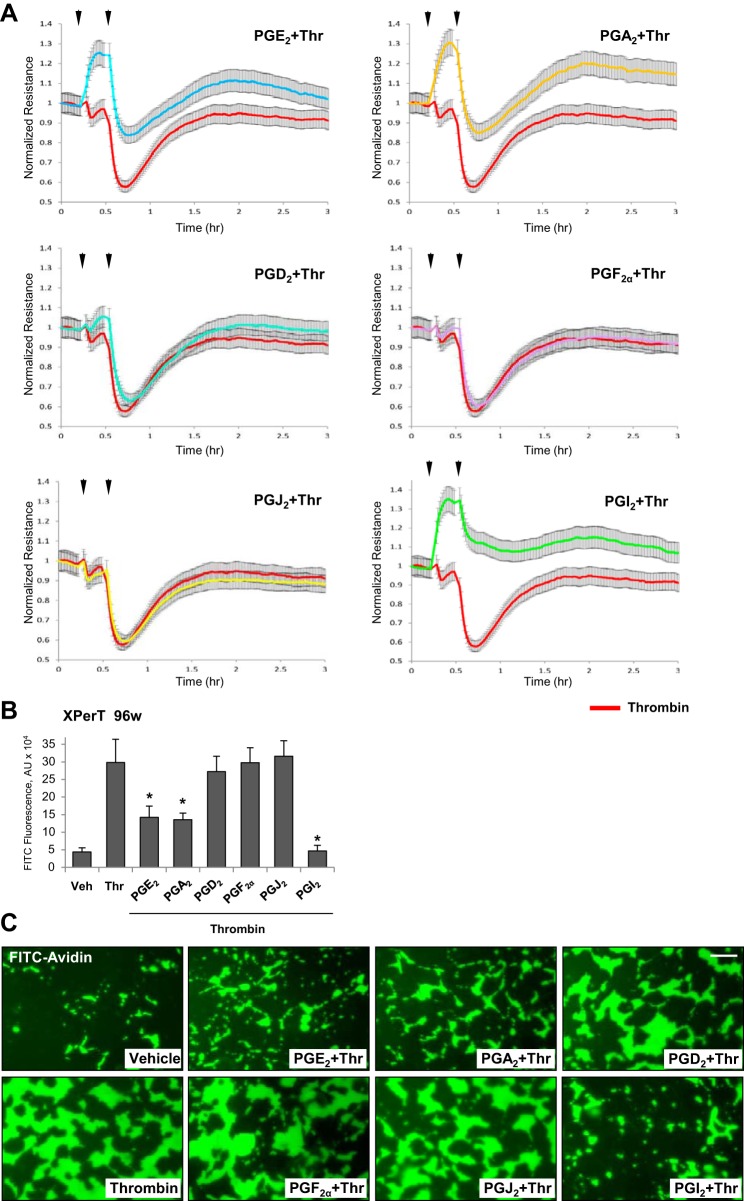
PGs also showed similar relative potency to attenuate EC permeability caused by high dose of thrombin (0.5 U/ml). PGI2 exhibited the most pronounced barrier-protective effect followed by PGE2 and PGA2 effects, whereas PGD2, PGF2, and PGJ2 were without effect at all (Fig. 3A).
Fig. 3.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on EC permeability caused by high dose of thrombin. A: pulmonary EC were treated with indicated PG (0.5 µM, first arrow) for 15 min followed by addition of thrombin (0.5 U/ml, second arrow). Changes in TER were monitored over 3-h time period. B: analysis of EC permeability for macromolecules using XPert assay. Bar graph depicts permeability changes after 5 min of thrombin treatment; n = 5. *P < 0.05 vs. thrombin. C: visualization of agonist-induced permeability in endothelial monolayers. HPAEC monolayers grown on coverslips coated with biotinylated gelatin were treated with corresponding PG (0.5 µM, 15 min) or vehicle followed by stimulation with thrombin (0.5 U/ml, 5 min) and addition of FITC-avidin tracer as described materials and methods. Green fluorescence depicts areas permeable for FITC-labeled avidin; bar = 20 µm.
In addition to TER measurements, PG’s protective effects against thrombin-induced EC hyperpermeability were examined using fluorimetry-based quantitative permeability assay and permeability visualization by microscopy. The bar graph provides comparative analysis of the potency of all six PGs in attenuation of thrombin-induced EC permeability (Fig. 3B). In visualization assays, thrombin caused a massive disruption of the EC barrier manifested by a robust accumulation of FITC-labeled tracer on the substrate under EC monolayers (Fig. 3C). Experiments using endothelial permeability imaging assay showed that pretreatment with PGA2, PGE2, and PGI2 (15 min) strongly attenuated thrombin-induced accumulation of fluorescent FITC-avidin tracer on the biotinylated gelatin coating of culture plates underneath EC monolayers after 5 min of thrombin stimulation. These results further substantiated protective effects of PGA2, PGE2, and PGI2 against thrombin-induced EC permeability. In contrast, PGD2, PGF2α, and PGJ2 did not affect thrombin-induced permeability for FITC-avidin tracer (Fig. 3B).
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on EC barrier dysfunction caused by inflammatory agonists.
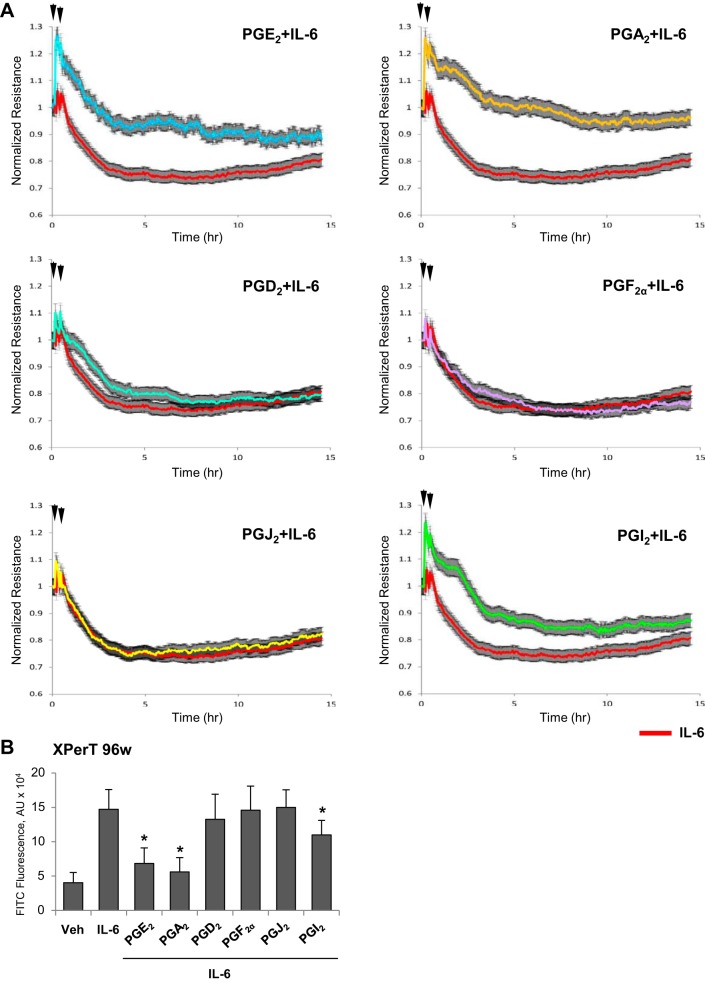
Because increased vascular leak is a distinctive feature of vascular response to bacterial pathogens and inflammatory cytokines, we evaluated effects of PGs on EC permeability caused by IL-6. Treatment with PGA2 and PGE2 caused significant and sustained attenuation of TER decline caused by IL-6 challenge. Protective effects of PGI2 were less expressed and markedly diminished after 5 h of treatment, although TER levels did not reach TER levels registered in EC stimulated with IL-6 alone (Fig. 4A). Treatment with PGD2, PGF2α, and PGJ2 did not protect human pulmonary EC from IL-6-induced barrier compromise (Fig. 4A). The results of quantitative fluorimetric permeability assay are presented in Fig. 4B.
Fig. 4.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on EC barrier dysfunction caused by IL-6. A: pulmonary EC pretreated with indicated PG (0.5 µM, first arrow) were stimulated with a combination of IL-6 (20 ng/ml) and IL-6 soluble receptor (SR, 40 ng/ml) as shown by second arrow. Changes in TER were monitored over 15-h time period. B: analysis of EC permeability using XPert assay. Bar graph depicts permeability changes after 6 h of IL-6/SR treatment; n = 5. *P < 0.05 vs. IL-6/SR.
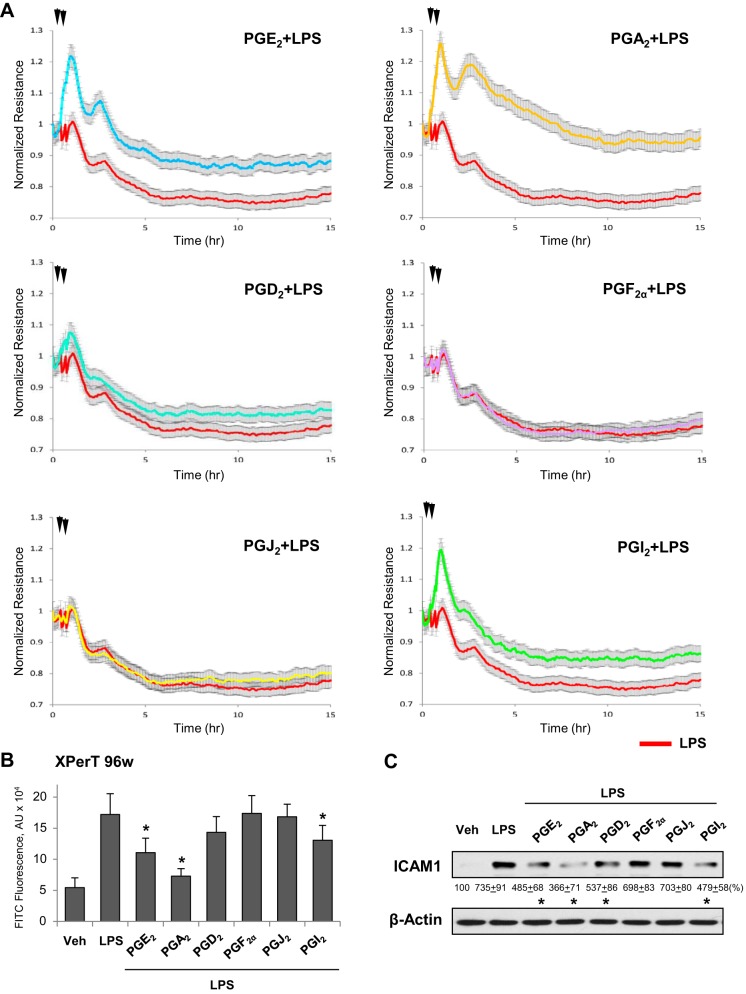
This group of PGs also exhibited similar effects on EC barrier disruption caused by another inflammatory agent, bacterial wall lipopolysaccharide (LPS). PGA2, PGE2, and PGI2 attenuated the LPS-induced TER decline (Fig. 5A) and FITC-avidin penetration through EC monolayer (Fig. 5B). PGA2 exhibited the most pronounced protective effect and PGI2 showed the weakest protective effect among these three PG products. Interestingly, whereas PGJ2 and PGF2α did not affect LPS-induced barrier dysfunction, PGD2 showed modest improvement of LPS-induced EC barrier compromise (Fig. 5, A and B); this effect was not observed in thrombin- or IL-6-challenged EC.
Fig. 5.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on LPS-induced EC permeability and ICAM1 expression. A: pulmonary EC were treated with indicated PG (0.5 µM, first arrow) followed by addition of LPS (300 ng/ml, second arrow). Changes in TER were monitored over 15-h time period. B: analysis of EC permeability using XPert assay. Bar graph depicts permeability changes after 6 h of LPS treatment; n = 5. *P < 0.05 vs LPS alone. C: cells treated with indicated PG (0.5 µM, 15 min) or vehicle were stimulated with LPS (300 ng/ml, 6 h), and ICAM1 protein expression was evaluated by Western blot. Probing with β-actin antibody was used as a normalization control. Results of densitometry are shown as means ± SD; n = 4. *P < 0.05.
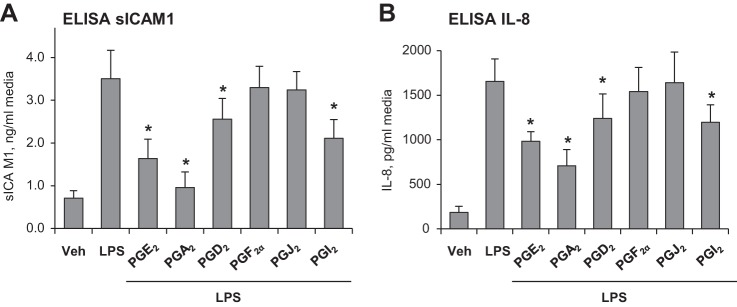
In addition to disruption of EC monolayer integrity, exposure of vascular EC to bacterial compounds activates an inflammatory response characterized by upregulation of proinflammatory cell adhesion molecules ICAM1 and VCAM1 required for leukocyte adhesion and PMN infiltration into inflamed organs, and by increased production of soluble ICAM1 and inflammatory cytokines including TNFα and IL-8 (7, 25). Treatment of LPS-challenged EC with PGA2, PGE2, or PGI2 suppressed the LPS-induced ICAM1 expression, and PGD2 exhibited a modest effect, whereas PGF2α and PGJ2 were without effect (Fig. 5C). Analysis of soluble ICAM1 and IL-8 levels in conditioned medium from LPS-challenged human pulmonary EC showed marked elevation of these markers of inflammation upon LPS challenge, which was significantly attenuated by PGA2, PGE2, and PGI2 (Fig. 6). In addition, LPS-induced elevation of cytokines and sICAM1 was partially attenuated by PGD2. In agreement with other results described above, PGF2α and PGJ2 did not significantly affect the pulmonary EC inflammatory response to LPS.
Fig. 6.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on LPS-induced sICAM1 and IL-8 production. Pulmonary EC were treated with indicated PG (0.5 µM, 15 min) followed by addition of LPS (300 ng/ml, 6 h). Secretion of inflammation markers into the culture medium was detected by ELISA assays. A: sICAM1. B: IL-8. n = 3. *P < 0.05 vs. LPS.
Protective effects of PGs in mouse models of acute lung injury.
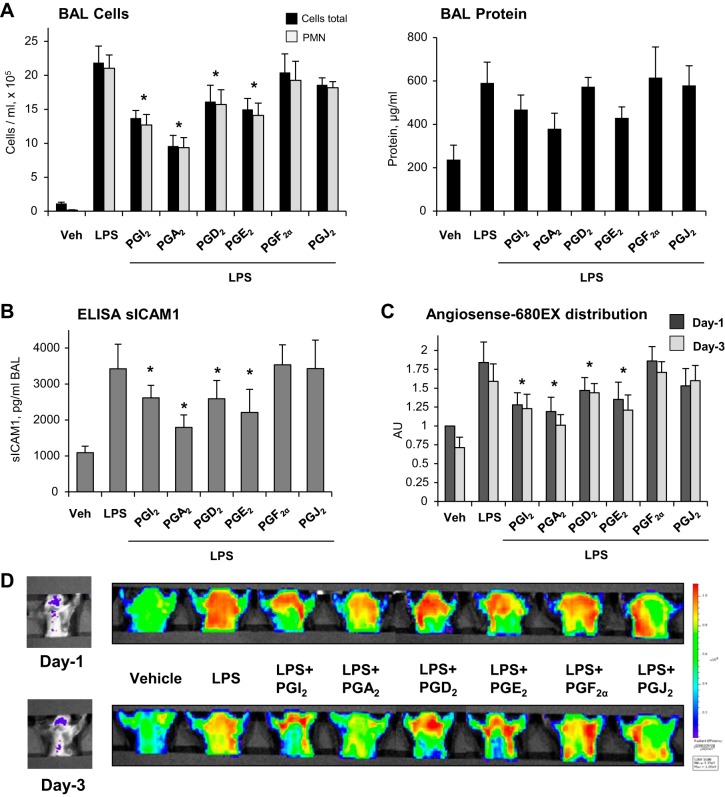
Effects of different prostaglandins were further tested in the murine model of acute lung injury (ALI) caused by intratracheal administration of LPS. Experimental animals were treated with PGA2, PGE2, PGD2, PGF2α, PGJ2 twice, concurrently with LPS administration and 5 h later. After 24 h, bronchoalveolar lavage was performed, and protein concentration and cell counts were analyzed as described in materials and methods. LPS induced a prominent acute inflammatory response, characterized by increase in BAL cell counts and BAL protein (Fig. 7A). Treatment with different PGs caused different levels of attenuation of parameters of LPS-induced ALI. PGA2, PGE2, and PGI2 significantly inhibited the LPS-induced increase in cell counts and protein content in BAL. PGD2 partially attenuated the LPS-induced increase in BAL cell count and protein content, whereas treatment with PGJ2 or PGF2α failed to significantly attenuate parameters of LPS-induced lung dysfunction (Fig. 7A). There was no significant difference in BAL cell counts and BAL protein between PG-treated animals and controls in the absence of LPS challenge (data not shown). In agreement with cell count and protein content data, PGA2, PGE2, PGI2, and PGD2 attenuated the LPS-induced increase of soluble ICAM1 detected in BAL samples (Fig. 7B).
Fig. 7.
Effects of PGA2, PGE2, PGD2, PGF2α, PGJ2, and PGI2 on LPS-induced ALI. Indicated PGs (20 µg/kg iv) were injected with indicated PG intravenously twice, concurrently and 5 h after LPS challenge (0.7 mg/kg it). Shown are cumulative data from 3 independent experiments, with 2 animals per experimental condition in each experiment. A: analyses of protein concentration and total cell and PNM counts were performed in BAL samples obtained from control and experimental groups 24 h after LPS challenge. B: levels of soluble ICAM1 in BAL samples were measured using ELISA assay; n = 4. *P < 0.05. C: live imaging analysis of lung vascular barrier dysfunction after LPS intratracheal injection with and without indicated PGs. LPS-induced accumulation of fluorescent Angiosense 680 EX imaging agent in the lungs of same animals was detected and quantified using Xenogen IVIS 200 Spectrum imaging system on day 1 and day 3 after LPS challenge; n = 3. *P < 0.05. D: visualization of fluorescent tracer intensity and distribution in the injured lungs is presented in arbitrary colors.
Lung vascular leak in mice challenged with LPS with and without PG treatment was further monitored using a noninvasive fluorescence optical imaging approach described in materials and methods. Lung accumulation of the fluorescent tracer Angiosense 680 EX tracer injected intravenously reflects lung vascular barrier dysfunction and lung injury. Effects of PG treatment on LPS-induced lung injury were analyzed 24 and 72 h after the treatment. LPS-induced lung dysfunction was significantly reduced in mice treated with PGA2, PGE2, or PGI2 and PGD2 during LPS challenge (Fig. 7C). In contrast, treatment with PGJ2 and PGF2α the compounds which did not exhibit protective effects in EC culture, did not show significant improvement of lung function in the animal model of LPS-induced ALI.
DISCUSSION
Beneficial effects of prostaglandins extend beyond their vasodilating effects and regulation of local circulation and involve direct protective effects on the vascular endothelium (1, 9, 16). However, the complexity of tissue-specific patterns of PG receptor expression and PG functional effects highlights the importance of understanding the role of specific PG in control of pulmonary vascular endothelium as a critical therapeutic target in ALI. This study performed a systematic analysis of barrier-enhancing, barrier-protective, and anti-inflammatory effects exhibited by different members of prostaglandin family on human pulmonary endothelial cells.
Our results show that PGE2 and PGA2 exhibited the most pronounced and sustained barrier enhancing effects in pulmonary EC. PGI2 also caused EC barrier enhancement response, which was more transient and diminished by 1–2 h of treatment. Other PGs—PGD2, PGF2α and PGJ2—did not considerably affect the EC barrier.
PGE2, PGA2, and PGI2 were also protective against thrombin-induced EC hyperpermeability, whereas three other PGs—PGD2, PGF2α, and PGJ2—did not affect barrier-disruptive effects of thrombin. PG barrier-protective effect against thrombin was directly associated with an extent of PG-induced inhibition of thrombin-induced RhoA GTPase signaling, which leads to increased MLC phosphorylation (13). This trend also remained in the ability of PGs to attenuate a more sustained EC barrier dysfunction and inflammatory response caused by inflammatory mediators, IL-6 and LPS, which caused development of EC barrier dysfunction within 5 h. PGA2 and PGE2 were the most efficient in protecting EC barrier and suppressing the LPS-induced EC inflammatory activation monitored by increased expression of ICAM1 and secretion of soluble ICAM1 and IL-8 into the culture medium. In contrast to potent protective effects on thrombin-induced permeability, PGI2 exhibited a less potent effect on LPS-induced TER decline. Modest, although statistically significant, attenuation of IL-6- and LPS-induced TER decline and inflammatory molecules expression was also observed in EC treated with PGD2, whereas PGF2α and PGJ2 were without effect in this model as well.
One potential explanation of the less potent PGI2 effects is relatively short PGI2 half-life [6–8 min (24)] vs. PGE2 [8–11 h (20)], which reduces its EC protective potential in settings of more sustained EC barrier dysfunction caused by inflammatory agents. In contrast, PGI2’s more stable analog, iloprost [half-life 30–40 min (19)], exhibited more potent and sustained protective and anti-inflammatory effects in vitro and in an animal model of LPS-induced ALI (7).
The differences in the PGs effects on pulmonary vascular inflammatory and permeability responses were consistent with PG effects on parameters of lung inflammation and vascular leak in vivo, as reflected by analysis of BAL samples, cytokine levels, and optical imaging of lung barrier dysfunction in live animals.
Despite the fact that PGA2, PGE2, and PGI2 engage different receptors, they all exhibited a protective effect on vascular EC in the settings of agonist-induced barrier disruption or inflammation. These findings suggest common protective mechanisms activated by these PGs in pulmonary EC. We have previously reported that activation of protein kinase A and Epac-Rap1-Rac1 signaling cascades by PGE2 and PGI2 stimulated peripheral actin cytoskeletal dynamics leading to enhancement of endothelial barrier and inhibition of RhoA GTPase-mediated cell contraction and EC barrier compromise (6, 9). The results of this study suggest that this signaling may be a unifying mechanism of barrier-protective effects observed in PGA2-, PGE2-, and PGI2-treated pulmonary EC. In addition to Epac- and PKA-dependent signaling, each receptor stimulates additional pathways (10, 17) which may affect the magnitude and duration of protective response to specific PG observed in this study. It is important to note that, in contrast to better understood mechanisms of PG barrier-protective effects, specific anti-inflammatory signaling cascades triggered by particular PG remain to be more precisely elucidated.
Interestingly, PGD2 did not noticeably affect thrombin-induced EC permeability but caused modest attenuation of LPS-induced EC inflammation and parameters of lung injury. In addition, PGJ2 did not affect the inflammatory response in EC experiments but showed a trend to decrease of LPS-induced ALI, although it did not reach statistical significance. This result is somewhat surprising given the anti-inflammatory effects of 15d-PGJ2, which prevented the release of proinflammatory cytokines by macrophages (26), neutrophils (30), and monocytes (22). The effects of 15d-PGJ2 were attributed to activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) (23). Taken together with published studies, our results suggest that EC barrier-protective responses mediated by PGs are less dependent on PPAR-γ than inflammatory signaling in myeloid cells. These mechanisms may also explain the low efficiency of PGJ2 on lung vascular barrier dysfunction in vivo. Based on these results, it is tempting to speculate that cell type-specific or even vascular bed-specific differences in PG receptors expression patterns may define the overall response to specific PG in particular pathological conditions.
In summary, this study performed a comprehensive analysis of functional effects of prostaglandin family members on pulmonary vascular endothelial EC barrier properties and inflammatory response to clinically relevant insults. We identified PGE2, PGA2, and PGI2 as prostaglandins with the most pronounced barrier-protective and anti-inflammatory effects on lung vascular endothelium and potential to attenuate lung injury caused by bacterial pathogens and vasoactive agonists.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-076259, HL-087823, and HL-107920 and National Institute of General Medical Sciences Grant GM-114171.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.K., O.O., N.S., Y.T., A.S., M.E.T., and S.S. performed experiments; Y.K., O.O., Y.T., A.S., M.E.T., A.A.B., and K.G.B. analyzed data; Y.K., O.O., A.S., M.E.T., A.A.B., and K.G.B. interpreted results of experiments; Y.K. and K.G.B. drafted manuscript; Y.K., O.O., N.S., Y.T., A.S., M.E.T., S.S., A.A.B., and K.G.B. approved final version of manuscript; O.O., A.A.B., and K.G.B. conceived and designed research; N.S., Y.T., S.S., and A.A.B. prepared figures; A.A.B. and K.G.B. edited and revised manuscript.
REFERENCES
- 1.Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol 129: 765–778, 2008. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- 2.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 3.Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema. Compr Physiol 3: 429–484, 2013. doi: 10.1002/cphy.c100066. [DOI] [PubMed] [Google Scholar]
- 4.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator-induced lung injury. J Appl Physiol (1985) 107: 1900–1910, 2009. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birukova AA, Meng F, Tian Y, Meliton A, Sarich N, Quilliam LA, Birukov KG. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim Biophys Acta 1852: 778–791, 2015. doi: 10.1016/j.bbadis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 11.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 273: 21867–21874, 1998. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 14.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803–812, 1995. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 15.Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612–624, 2009. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103: 147–166, 2004. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Hata AN, Zent R, Breyer MD, Breyer RM. Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther 306: 463–470, 2003. doi: 10.1124/jpet.103.050955. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand M. Pharmacokinetics and tolerability of oral iloprost in thromboangiitis obliterans patients. Eur J Clin Pharmacol 53: 51–56, 1997. doi: 10.1007/s002280050336. [DOI] [PubMed] [Google Scholar]
- 20.Ishihara O, Sullivan MH, Elder MG. Differences of metabolism of prostaglandin E2 and F2 alpha by decidual stromal cells and macrophages in culture. Eicosanoids 4: 203–207, 1991. [PubMed] [Google Scholar]
- 21.Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol 179: 6193–6203, 2007. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–86, 1998. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 23.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 83: 813–819, 1995. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 24.Lucas FV, Skrinska VA, Chisolm GM, Hesse BL. Stability of prostacyclin in human and rabbit whole blood and plasma. Thromb Res 43: 379–387, 1986. doi: 10.1016/0049-3848(86)90082-4. [DOI] [PubMed] [Google Scholar]
- 25.Meliton A, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, and Birukov KG. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 308: L550–L562, 2015. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391: 79–82, 1998. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel N, Waschke J. cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res 355: 587–596, 2014. doi: 10.1007/s00441-013-1755-y. [DOI] [PubMed] [Google Scholar]
- 28.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev 21: 185–210, 2001. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32: 24–33, 2006. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya S, Somers EP, Wright SD, Detmers PA, Bansal VS. 15-Deoxy-Delta12,1412,14-prostaglandin J2 inhibits the beta2 integrin-dependent oxidative burst: involvement of a mechanism distinct from peroxisome proliferator-activated receptor gamma ligation. J Immunol 163: 6187–6192, 1999. [PubMed] [Google Scholar]
- 31.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. doi: 10.1161/01.RES.87.4.335. [DOI] [PubMed] [Google Scholar]
- 32.Yagami T, Koma H, Yamamoto Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol 53: 4754–4771, 2016. doi: 10.1007/s12035-015-9355-3. [DOI] [PubMed] [Google Scholar]