Summary
Inflammatory bowel disease comprises a group of heterogeneous diseases characterized by chronic and relapsing mucosal inflammation. Alterations in microbiota composition have been proposed to contribute to disease development, but no uniform signatures have yet been identified. Here, we compare the ability of a diverse set of microbial communities to exacerbate intestinal inflammation after chemical damage to the intestinal barrier. Strikingly, genetically identical wild-type mice differing only in their microbiota composition varied strongly in their colitis susceptibility. Transfer of distinct colitogenic communities in gene-deficient mice revealed that they triggered disease via opposing pathways either independent or dependent on adaptive immunity, specifically requiring antigen-specific CD4+ T cells. Our data provide evidence for the concept that microbial communities may alter disease susceptibility via different immune pathways despite eventually resulting in similar host pathology. This suggests a potential benefit for personalizing IBD therapies according to patient-specific microbiota signatures.
Keywords: gut microbiota, IBD, innate colitis, adaptive colitis, colitis pathogenesis, DSS colitis
Graphical Abstract

Highlights
-
•
Gut microbiota composition modulates colitis severity in immunocompetent hosts
-
•
Colitogenic microbiota drive colitis via innate or adaptive immunity
-
•
Distinct microbiota members induce pathogenic CD4+ T cells to drive colitis
Alterations in the microbiota contribute to the development of intestinal inflammation. Roy et al. demonstrate that distinct intestinal microbial communities cause colitis via opposing effector mechanisms independent or dependent on adaptive immunity. Their findings suggest that personalized immunomodulatory treatment according to distinct microbial signatures may be beneficial for IBD patients.
Introduction
Inflammatory bowel disease (IBD) consists of a complex group of incurable inflammatory disorders comprising Crohn’s disease (CD) and ulcerative colitis (UC). Although the etiopathogenesis of IBD development is not fully understood, numerous studies support the hypothesis of IBD as a pathological immune response against microbial and environmental antigens in genetically predisposed individuals (Imhann et al., 2016, Jostins et al., 2012). The relative contribution of innate and adaptive immune cells and various cytokines to the development of IBD has been controversially debated (Neurath, 2014). Nonetheless, an imbalanced interaction between the host immune system and gut microbiota is thought to play a pivotal role in disease manifestation and maintenance (Cho, 2008, Gevers et al., 2014, Honda and Littman, 2012).
Notably, various human disease conditions have been associated with imbalances in the composition of the gut microbiota, so-called dysbiosis; however, whether these changes contribute directly to the development of the disease or reflect an altered physiology of the host remains debated in many instances (Kamada et al., 2013, Ley et al., 2005, Turnbaugh et al., 2008). In various mouse models of IBD, the microbiota and, in some cases, specific members have been shown to influence disease outcome (Saleh and Elson, 2011). Examples of IBD mouse models that lack disease development in the absence of any microbiota are the Il10−/− model of colitis and the TNFdeltaARE model of ileitis (Keubler et al., 2015, Schaubeck et al., 2016). Furthermore, disease development in these models is impaired or delayed under specific pathogen-free (SPF) conditions compared with conventional housing conditions, which potentially contain pathogenic bacteria, demonstrating that particular microbiota members or distinct communities only present in conventionally housed mice modulate disease onset (Laukens et al., 2016). Specifically, Enterobacteriaceae in Tbet−/−Rag2−/− mice (Garrett et al., 2010) as well as Bacteroides spp. (Bloom et al., 2011), Helicobacter spp. (Fox et al., 2011), and Bilophila wadsworthia (Devkota et al., 2012) in Il10−/− have been shown to enhance intestinal inflammation.
The acute dextran sulfate sodium (DSS) colitis model of human UC is considered to be largely dependent on innate immunity (Chassaing et al., 2014). We previously demonstrated that the dysbiotic microbiota of Nlrp6 inflammasome-deficient mice has the ability to directly enhance DSS colitis severity, but the effector mechanism remained unknown (Elinav et al., 2011). Notably, a recent study identified that specific metabolites of this dysbiotic community actively modulate innate immune signaling and, subsequently, the host-microbiota interface (Levy et al., 2015). Subsequently, similar dysbiotic communities with the ability to modulate the severity of DSS colitis have been described in other gene-deficient mice (Couturier-Maillard et al., 2013, Hu et al., 2015, Roberts et al., 2014). However, it remains to be examined whether different colitogenic communities trigger intestinal pathologies via shared or distinct immune pathways. This knowledge could potentially explain the variable roles that have been suggested for various immune effectors and pathways for IBD pathogenesis.
In the present study, we have characterized the susceptibility of mouse lines differing only in their microbiota composition toward DSS colitis. Besides the dysbiotic community (DysN6) from Nlrp6−/− mice, interestingly, also certain but not all SPF communities demonstrated the ability to cause severe intestinal inflammation in immunocompetent mice. Strikingly, mice displayed different inflammatory responses depending on their intestinal microbiota composition, either characterized by infiltration of neutrophils or the presence of proinflammatory CD4+ T cells. By utilizing gene-deficient mice and antibody-mediated depletion of T cell subsets, we demonstrated that the DysN6 community, but not another colitogenic community, depends on CD4+ T cells to exacerbate DSS colitis severity. Our data identify that specific interactions between colitogenic communities and host immune pathways drive colitis development via distinct mechanisms.
Results
DSS Colitis Severity Is Strongly Influenced by Microbiota Composition in SPF Mice
Distinct differences in microbiota composition between isogenic mice from commercial vendors—e.g., the presence of segmented filamentous bacteria (SFB)—have been found to influence the outcome of disease models in mice (Ivanov et al., 2009). To investigate whether C57BL/6N mice differ in their susceptibility to intestinal inflammation after chemically induced damage to the intestinal barrier, we induced DSS colitis in SPF mouse lines obtained from vendors or bred in-house (Figure 1A; Table S1). The severity of disease was compared within lines of SPF mice and with previously described dysbiotic Nlrp6−/− mice that were obtained from the original vivarium and subsequently bred in our animal facility without rederivation (Figure 1B; Figure S1A; Elinav et al., 2011). SPF-1, SPF-5, and SPF-6 mice were characterized by mild colitis with moderate weight loss and no mortality, but SPF-2, SPF-3, and SPF-4 mice as well as dysbiotic Nlrp6−/− mice developed a similar severe colitis with profound loss of body mass and mortality (Figure 1B; Figure S1A). Colitis severity in each representative isogenic mouse line from different commercial or in-house sources (SPF-1, SPF-2, SPF-4, SPF-6, and DysN6) was also illustrated by measuring colon shortening and supported by histological characterization of tissue damage (Figures S1C and S1D). Next we investigated fecal microbiota composition before induction of DSS colitis using 16S rRNA gene sequencing. Analysis of β diversity using principle coordinates analysis (PCoA) showed that mice with mild colitis severity (SPF-1, SPF-5, and SPF-6) clustered separately from mice featuring a high severity of colitis (SPF-2, SPF-3, SPF-4, and DysN6). We noted a high similarity between SPF-2, SPF-3 (both from different barriers of the same vendor), and SPF-4 mice as well as between SPF-5 and SPF-6 mice (both from different barriers of the same vendor), respectively, whereas SPF-1 and DysN6 mice clustered distinctly (Figure 1C). A more detailed analysis revealed that species richness (Chao index) was lower in SPF-1 mice but that the complexity of the community structure (Shannon index) was not significantly different between mouse lines (Figure S1B). Global changes in the composition of microbiota have been associated with IBD (Gevers et al., 2014), such as a decrease in the level of resident Firmicutes and/or Bacteroides and an overabundance of Proteobacteria (Frank et al., 2007). We observed a significant expansion of Bacteroides over Firmicutes in colitogenic SPF-2, SPF-3, SPF-4, and DysN6 mice compared with SPF-1, SPF-5, and SPF-6 mice (Figure 1D). Overgrowth in Proteobacteria was highest in DysN6 mice, followed by SPF-2, SPF-3, SPF-4, and SPF-5 mice, and was mostly absent in SPF-1 and SPF-6 mice (Figure 1D; Table S2).
Figure 1.
Differences in Microbiota Composition Regulate the Severity of Acute DSS Colitis
(A) DSS colitis was induced in SPF WT (SPF-1–SPF-6) and in-house bred dysbiotic Nlrp6−/− (DysN6) mice by administering 2% DSS (w/v) for 7 days. Body weight and survival of mice were examined daily for 10 days.
(B) Body weight and survival of the mice described in (A). DSS severity is depicted as “o” being mild and “+” being severe. n = 9–21 mice/group.
(C and D) Analysis of fecal microbiota composition of the mice described in (A) before DSS colitis induction using 16S rRNA sequencing. Shown is analysis of β-diversity (PCoA) (C) and the ratio of relative abundances between Firmicutes to Bacteroides and Firmicutes to Proteobacteria (D). n = 15–33 mice/group.
(E) Germ-free C57BL/6N mice were cohoused with donor SPF WT (SPF-1, SPF-2) and Nlrp6−/− (DysN6) mice, followed by induction of DSS colitis. Shown is analysis of β-diversity (PCoA) before and disease severity (body weight and survival) upon induction of DSS colitis. n = 7–8 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S1.
To exclude the effect of genetic drift in inbred mice from different sources, we performed cohousing experiments with microbiota donor and germ-free recipient mice. We focused on SPF-1 (low susceptibility, higher Firmicutes), SPF-2 (high susceptibility, higher Bacteroides), and DysN6 mice (high susceptibility, higher Bacteroides, and higher Proteobacteria) representing the different colitis outcomes and microbiota compositions. Transfer of the donor microbiota into germ-free (GF) recipient (exGF) mice was confirmed by 16S rRNA gene sequencing (Figure 1E). Upon induction of DSS colitis, exGF mice phenocopied the respective donor mice, supporting that the differences in colitis severity were dependent on the microbiota (Figure 1E). Similar microbiota-driven phenotypes were confirmed for the SPF-5 and SPF-6 communities (data not shown). These data demonstrate that distinct types of microbial communities that are stably maintained in wild-type (WT) mice are able to alter the host’s susceptibility to DSS colitis.
Transfer of Colitogenic Microbial Communities into an Immunocompetent Host Induces Distinct Patterns of Host Gene Expression and Alters Colitis Susceptibility
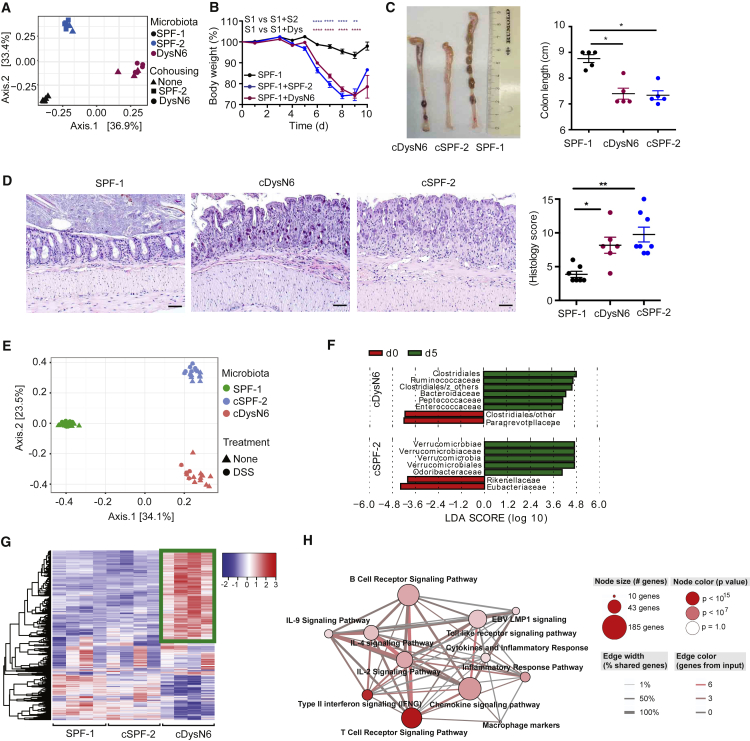
Next we investigated whether the degree of colitis severity was also transferable between SPF mice with variable DSS colitis susceptibility, similar to what has been observed for Nlrp6 inflammasome-deficient mice (DysN6) (Elinav et al., 2011). Therefore, we performed cohousing experiments of mice featuring mild colitis (SPF-1) with mice having high colitis severity (SPF-2 and DysN6). Cohousing for 4 weeks resulted in reshaping of the microbiota in SPF-1 mice cohoused with SPF-2 mice (SPF-1 + SPF-2) and DysN6 mice (SPF-1 + DysN6) compared with SPF-1 control mice, respectively (Figure 2A). Moreover, cohousing also transferred colitis susceptibility (Figure 2B; Figure S2A). Because SPF-1 + SPF-2 and SPF-1 + DysN6 mice behaved like SPF-2 and DysN6 mice, we refer to them hereafter as cSPF-2 and cDysN6 (cohoused SPF-2 or DysN6), respectively. A similar transfer of colitis severity was also achieved by cohousing SPF-2 with SPF-6 mice (Figure S2C) and after fecal transplantation (FT) from SPF-2 and DysN6 mice into SPF-1 mice (data not shown). Increased colitis severity in cSPF-2 and cDysN6 mice was also illustrated by enhanced colon shortening and corroborated by histological characterization of tissue damage as well as endoscopy (Figures 2C and 2D; Figure S2B). These data demonstrate that distinct types of microbial communities are able to alter the host’s susceptibility to DSS colitis even in already colonized immunocompetent recipients.
Figure 2.
Alteration of Colitis Susceptibility and Distinct Host Responses by Colitogenic Microbiota
(A–C) SPF-1 WT mice were cohoused with either SPF-2 WT or DysN6 Nlrp6−/− (SPF-1 + DysN6) mice, resulting in SPF-1 + SPF-2 and SPF-1 + DysN6 mice, respectively.
(A) Analysis of β-diversity (PCoA) of donor and recipient mice before induction of DSS colitis. n = 5–16 mice/group.
(B–D) Acute DSS colitis was induced, and the weight of microbiota recipient mice was monitored for 10 days (B). Colon length was measured 5 days after induction of DSS colitis. Shown is a representative image of excised colons (C). Histological analysis of distal colon was performed 5 days after induction of DSS colitis (D). Representative pictures of H&E-stained colon sections are shown. The scale bars represent ∼50 μm. n = 5–16 mice/group.
(E and F) 16S rRNA sequencing of fecal microbiota from WT SPF-1, cSPF-2, and cDysN6 on day 0 and day 5 of DSS colitis. Shown are analysis of β-diversity (PCoA) (E) and analysis of differentially abundant microbial families in cDysN6 and cSPF-2 mice on day 0 and day 5 of DSS by LEfSe (Kruskal-Wallis test, p < 0.05, LDA 4.0) (F). n = 8–12 mice/group.
(G and H) RNA-seq analysis from total colonic tissue of WT mice colonized with SPF-1, cSPF-2, or cDysN6. The heatmap shows quantification of RNA reads (G). Also shown is a pathway analysis based on gene ontology (GO) terms of genes significantly upregulated (2-fold) in cDysN6 mice compared with SPF-1 (H). n = 4 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test (B) and nonparametric Kruskal-Wallis test (C and D): ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S2.
Induction of DSS colitis has been shown to alter the composition of the intestinal microbiota (Schwab et al., 2014). To identify whether a shared group of commensals alters their abundance during DSS colitis in SPF-1 mice as well as in cSPF-2 and cDysN6 mice, we compared their fecal microbial communities before and after induction of DSS colitis (day 5). Strikingly, β-diversity analysis (PCoA) as well as an analysis of relative abundances of different bacterial families revealed minor differences between the two time points for each community, respectively (Figure 2E; Figure S2D). Minor alterations included an increase in Verrucomicrobiaceae in cSPF-2 and an increase in abundance of some Bacteroidaceae in cDysN6 (Figure 2F; Figure S2D), but no unified changes were observed between the cSPF-2 and cDysN6 communities despite a similar induction of colitis at this time point. Hence, we hypothesized that colitogenic communities already modulate host immunity before disease induction, which, in turn, results in enhancement of colitis severity. Thus, global gene expression in colonic tissues of mice harboring either SPF-1, cSPF-2, or cDysN6 was compared using RNA sequencing (RNA-seq). Interestingly, SPF-1 and cSPF-2 mice clustered together and separately from cDysN6 mice with a distinct gene expression signature (Figure 2G; Figure S2E). Specifically, pathway enrichment analysis showed that many upregulated genes in cDysN6 were involved in T cell and B cell signaling as well as cytokine and chemokine signaling (Figure 2H). In contrast, despite the fact that a similar colitis severity outcome was observed in cDysN6 mice, SPF-2 colonization of SPF-1 mice did not result in significant alterations in the host transcriptome (Figure 2G; Figure S2E). These data together suggest that alteration of the SPF-1 community by colonizing it with colitogenic SPF-2 or DysN6 triggers a very different response at the host transcriptional level.
DysN6, but Not SPF-2, Microbiota Depends on Adaptive Immune Cells to Develop Colitis
Because transfer of the colitogenic SPF-2 community, unlike the DysN6 community, did not trigger large changes in the host transcriptome in the intestine, we hypothesized that the mere presence of the SPF-2 community may be sufficient to trigger more severe colitis upon damage to the intestinal barrier. Therefore, we assessed disease severity in SPF-1 mice that received FT of the SPF-2 or DysN6 community 2 or 28 days prior to disease induction, respectively. Despite minor but detectable differences in communities of mice receiving FT for 2 or 28 days (Figures 3A and 3C; Figure S3A), brief colonization with the SPF-2 microbiota was sufficient to transfer exacerbated disease severity that was comparable with the result following extended colonization (Figure 3B). In contrast, brief colonization with the DysN6 microbiota did not transfer heightened disease susceptibility (Figure 3D). This inability of the DysN6 microbiota to transfer colitis severity potentially results from incomplete microbiota transfer, a requirement for extended immunomodulation or priming of adaptive immune responses. Comparison of the communities in mice receiving the DysN6 FT for 2 or 28 days by linear discriminant analysis (LDA) effect size (LEfSe) analysis revealed very minute differences (Figure 3E), including a higher abundance of SFB as well as Odoribacteriaceae 28 days after the transfer. Notably, despite successful transfer of colitis severity, communities differed stronger in the case of SPF-2 FT (Figure 3F). Interestingly, similar to the DysN6 FT, SFB and Odoribacteriaceae displayed higher abundances 28 days after SPF-2 transfer. This suggests that these bacteria may not be involved in modulating DSS colitis severity. Next, to test whether DysN6 requires priming of adaptive immunity, we compared the severity of DSS colitis between Rag2−/− mice harboring either the SPF-1, cSPF-2, or cDysN6 communities. Strikingly, unlike in WT mice, cDysN6 could not enhance colitis severity in Rag2−/− mice, as indicated by similar weight loss (Figure 3G) and colon length (Figure S3D) between Rag2−/− mice with SPF-1 and cDysN6. In contrast, cSPF-2 also induced severe colitis in Rag2−/− mice, as indicated by increased weight loss and mortality (Figure 3H; Figure S3E). Importantly, we confirmed comparable transfer of the donor communities into WT and Rag2−/− mice (Figures S3B and S3C). We used permutational multivariate analysis of variance (ADONIS) (Anderson, 2001), considering the variables “genotype,” “microbiota,” and “cage” to evaluate their relative contribution to variability within the groups (Figures S3B and S3C). This analysis revealed that genotype contributed only 3% of variability, whereas microbiota contributed around 60%. Together, these data demonstrate that extended immunomodulation and priming of adaptive immunity by DysN6, but not SPF-2, are required to exacerbate colitis severity.
Figure 3.
The Adaptive Immune System Is Important for DysN6-Mediated, but Not SPF-2-Mediated, Colitis
(A–F) SPF-1 mice were mock-transferred or received a fecal transplant from Nlrp6−/− DysN6 or WT SPF-2 donor mice 2 days or 28 days prior to colitis induction, respectively.
(A and C) PCoA plot of fecal microbiota composition at steady state of WT SPF-1 mice receiving SPF-2 (A) or DysN6 (C) microbiota for different time periods. n = 3–11 mice/group.
(B and D) Body weight and survival of WT SPF-1 mice receiving SPF-2 (B) or DysN6 (D) microbiota for different time periods during DSS colitis. n = 12–15 mice/group.
(E and F) Analysis of differentially abundant microbial families in mice with short (2 days) and prolonged (28 days) exposure to DysN6 (E) and SPF-2 (F) were analyzed by LEfSe (Kruskal-Wallis test, p < 0.05, LDA 2.0) before induction of DSS colitis. n = 3–11 mice/group.
(G and H) SPF-1 WT and SPF-1 Rag2−/− recipients were cohoused with donor SPF-2 or DysN6. Body weight was monitored upon colitis induction. n = 8–12 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S3.
Colitis Development Is Characterized by the Presence of Distinct Immune Signatures in DysN6 and SPF-2 Mice
Despite similar disease severity in DysN6 and SPF-2 mice upon DSS colitis induction, our initial results corroborated the hypothesis that distinct colitogenic communities contribute to disease development via different pathways. To further compare intestinal inflammation induced in cDysN6 compared with cSPF-2 mice, the presence of cytokines and chemokines was measured in tissue homogenates on day 7 of DSS colitis. The levels of the pro-inflammatory cytokines interleukin-6 (IL-6) and IL-17A were significantly higher in the distal colon of both cDysN6 and cSPF-2 mice compared with SPF-1 mice (Figure S4A). Compared with SPF-1 and cDysN6, colitis induced in cSPF-2 mice was distinctively characterized by higher levels of interferon γ (IFN-γ), IL-22, and tumor necrosis factor alpha (TNF-α) as well as lower levels of IL-18, mainly in the distal colon (Figure S4A). No changes were observed in IL-2, IL-4, IL-5, IL-10, and IL-13 between the three microbiota communities (data not shown). In line with our previous observations (Elinav et al., 2011), higher levels of the chemokine CCL5 were detected in the proximal colon of cDysN6 mice compared with SPF-1 and cSPF-2 mice (Figure S4B). In contrast, several other chemokines, including LIX and KC, which recruit and activate neutrophils, along with MIP-1a and MIP-1b, were significantly increased during colitis induced by cSPF-2 (Figure S4B). In parallel, we analyzed lamina propria leukocytes (LPLs) from colonic tissue by flow cytometry to identify whether distinct immune cell subsets are associated with disease induced by SPF-1, cDysN6, and cSPF-2 communities. Indeed, 2-fold increased numbers of CD45+ cells were observed in cDysN6 WT mice compared with SPF-1 and cSPF-2 WT mice both before and 5 days after induction of DSS colitis (Figure 4A). In line with the enhanced levels of neutrophil-attracting chemokines, colitis in cSPF-2 mice was associated with a specific increase in the relative abundance and total number of neutrophils (Figures 4B–4D). However, all SPF-1-, cSPF-2-, and cDysN6-colonized mice did not demonstrate any significant difference in disease outcome while being treated with antibody against Ly6G compared with the isotype control (data not shown). This might indicate a complex interaction among different components of the innate immune system to enhance microbiota-mediated colitis severity. Despite similar frequencies of immune cell subsets of the adaptive immune system (Figures S4C and S4D), significant increases in the numbers of B220+ B cells and CD3+ T cells were observed before and after induction of DSS colitis in cDysN6 mice (Figures 4E and 4F). Increases in the numbers of CD4+ and CD8+ T cells, but not γδ T cells, contributed to this difference (Figure 4F). During, but not before DSS colitis, a higher frequency of CD4+ T cells in the colon of cDysN6 and cSPF-2 mice displayed an activated phenotype (Figure S4D). Notably, the absolute numbers of activated CD4+ T cells were only increased in cDysN6 mice, both before and after induction of DSS colitis (Figure 4F). These analyses show that two colitogenic communities trigger distinct inflammatory immune pathways—i.e., enhanced neutrophil recruitment and pathogenic adaptive immune cell responses—during DSS colitis.
Figure 4.
Colitis Driven by DysN6 and SPF-2 Is Characterized by Distinct Infiltration of Innate and Adaptive Immune Cells
(A–F) Colonic lamina propria leukocytes (cLPLs) were isolated from WT mice harboring SPF-1, cDysN6, or cSPF-2 microbiota during the steady state (day 0) and on day 5 after DSS induction and analyzed by fluorescence-activated cell sorting (FACS).
(A) Total number of CD45+ cells in cLPLs.
(B–D) Analysis of neutrophil infiltration upon DSS induction. Representative FACS plots show frequencies of neutrophils (B). Also shown are frequencies (C) and total numbers (D) of neutrophils on day 0 and day 5 of DSS.
(E and F) Analysis of adaptive immune cells upon DSS induction. Representative FACS plots show CD4 and CD8 frequencies gated on CD3+ cells and frequencies of naive and activated CD4+ T cells during the steady state (E). Also shown are total numbers of the indicated immune cell subsets on day 0 and day 5 of DSS (F).
Data represent 5–17 mice/group as mean ± SEM from at least two independent experiments. The indicated p values represent nonparametric Kruskal-Wallis test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S4.
αβ T Cells Trigger DysN6-Mediated, but Not SPF-2-Mediated, Colitis Development
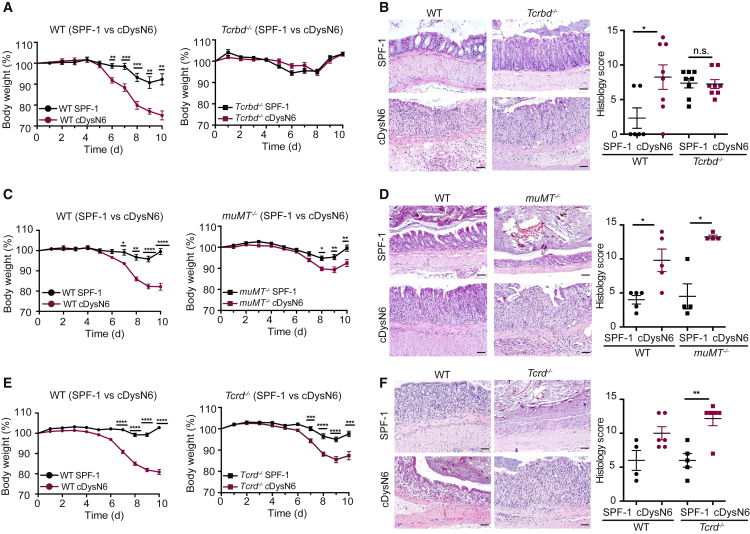
To investigate which type of pathogenic adaptive immune responses contribute to disease exacerbation after colonization with the DysN6 community, we decided to compare the severity of DSS colitis in WT as well as B or T cell-deficient mice under SPF-1 and cDysN6 conditions. To assure comparable microbiota composition in WT and gene-deficient mice at baseline, all gene-deficient mouse lines were initially rederived into SPF-1 conditions using embryo transfer. To then generate experimental cohorts of WT and gene-deficient mice, the DysN6 microbiota was transferred into SPF-1 recipients using FT or cohousing, and the composition of the fecal microbiota was recorded before induction of disease. To investigate an involvement of T and B cells, we studied SPF-1 and cDysN6 Tcrbd−/− and muMT−/− mice, respectively. Comparison of microbiota composition and multi-variate analysis before the start of DSS colitis revealed that mice clustered according to SPF-1 and cDysN6 communities, with genotype contributing only little (i.e., 3%) to differences in microbiome composition (Figures S5A and S5B). Despite a similar transfer of DysN6 into WT and Tcrbd−/− mice, strikingly, no difference in the severity of DSS colitis was observed between SPF-1 and cDysN6 Tcrbd−/− mice, as indicated by similar weight loss, unlike in WT mice, which showed microbiota-modulated disease severity (Figure 5A). An involvement of T cells in transferring exacerbated disease severity was further corroborated by analyzing intestinal inflammation using histology (Figure 5B) and endoscopy (Figure S5C) as well as quantifying colon shortening (Figure S5D) of WT and Tcrbd−/− mice. In contrast to WT mice, deficiency in T cells resulted in no detectable differences in these parameters between SPF-1 and cDysN6 Tcrbd−/− mice. Transfer of the DysN6 community into SPF-1 muMT−/− mice resulted in exacerbation of DSS colitis severity, as indicated by significantly enhanced weight loss, colon shortening, and heightened intestinal inflammation compared with SPF-1 muMT−/− mice, suggesting limited involvement of B cells in colitis exacerbation (Figures 5C and 5D; Figure S5D). To investigate whether T cells are also required for disease exacerbation by the colitogenic SPF-2 microbiota, we introduced the SPF-2 community into SPF-1 WT, Tcrbd−/−, and muMT−/− mice. After confirming that the fecal microbiota of mice clustered according to their microbial communities and not by genotype (Figure S5E and S5F), we induced DSS colitis. As expected from the results with Rag2−/− mice, deficiency in B or T cells alone did not affect the transfer of heightened disease severity by cSPF-2 (Figure S5G). γδ T cells have been implicated in colonic tissue repair (Chen et al., 2002); hence, we next characterized DSS colitis severity in SPF-1 and cDysN6 Tcrd−/− mice. Notably, characterization of fecal microbiota demonstrated that mice clustered according to SPF-1 and cDysN6 microbiota (Figure S5H). Similar to what we observed in WT mice, transfer of DysN6 microbiota induced in Tcrd−/− mice enhanced weight loss, colon shortening, and heightened intestinal inflammation (Figures 5E and 5F; Figure S5I). From these results we concluded that T cells are essential for DysN6- but not SPF-2-induced exacerbation of disease. Specifically, our data suggest that modulation of αβ T cells by members of the DysN6 community is important. Finally, we exclude a major contribution of B cells to DysN6-mediated colitis.
Figure 5.
αβ T Cells Are Required for DysN6-Mediated Colitis
(A–F) SPF-1 WT and SPF-1 gene-deficient mice were cohoused with a DysN6 donor. Body weight was monitored upon induction of DSS colitis (A, C, and E). Histological analysis of the distal colon was performed 5 days after induction of DSS colitis (B, D, and F). Shown are representative pictures of H&E-stained colon sections. Scale bars represent ∼50 μm (B, D, and F).
(A and B) Body weight (A) and histological analysis (B) of SPF-1 and cDysN6 WT and Tcrbd−/− mice. n = 6–16 mice/group.
(C and D) Body weight (C) and histological analysis (D) of SPF-1 and cDysN6 WT and muMT−/− mice. n = 5–18 mice/group.
(E and F) Body weight (E) and histological analysis (F) of SPF-1 and cDysN6 WT and Tcrd−/− mice. n = 5–26 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S5.
Pathogenic CD4+ T Cells Are Crucial to Induce DysN6-Mediated Colitis
CD4+ but also CD8+ T cells contribute to different aspects of intestinal homeostasis and inflammation (Honda and Littman, 2012). Hence, we compared DSS colitis severity in SPF-1 and cDysN6 CD4−/− and CD8−/− mice. Fecal microbiota of mice clustered again according to SPF-1 and cDysN6 but not according to genotype (Figures S6A and S6B). After DSS induction, CD8−/− but not CD4−/− mice showed enhanced weight loss and colitis severity after DysN6 transfer, comparable with WT mice (Figures 6A and 6B; Figure S6C). Furthermore, analysis of intestinal inflammation using histology and quantification of colon shortening (Figures 6C and 6D) in SPF-1 and cDysN6 WT and CD4−/− mice corroborated that CD4+ but not CD8+ T cells are required for DysN6-induced exacerbation of disease. To evaluate whether CD4+ T cells contribute to enhanced colitis severity by the colitogenic SPF-2 microbiota, we introduced the SPF-2 community into SPF-1 WT and CD4−/− mice. Analysis of fecal microbiota of mice confirmed clustering according to microbiota and not by genotype (Figure S6D). Deficiency in CD4+ T cells did not affect the transfer of heightened disease severity (Figure S6E), further supporting that SPF-2 drives colitis severity irrespective of T cells.
Figure 6.
Pathogenic CD4+ T Cells Are Crucial to Develop DysN6-Mediated Colitis
(A) SPF-1 WT and CD8−/− mice were cohoused with DysN6 donor mice, and DSS colitis was induced.
(B–D) SPF-1 WT and CD4−/− mice were cohoused with DysN6 donor mice, and DSS colitis was induced. Body weight (B) during DSS colitis as well as colon shortening (C) and intestinal inflammation (D) on day 5 of DSS colitis were monitored. Shown are representative pictures of H&E-stained colon sections. Scale bars represent ∼50 μm (D). n = 5–20 mice/group.
(E) SPF-1 and cDysN6 WT mice were injected with isotype control, anti-CD8, or anti- CD4 antibodies on day 1, day 3, and day 7 of DSS colitis, and body weight was compared. n = 10–12 mice/group.
(F) Total numbers of CD4+ T cells producing IFN-γ and/or IL-17A from isolated cLPLs from IL-17AGFP IFN-γKatushka FoxP3RFP triple reporter mice with different microbiota. n = 6–14 mice/group.
(G) cLPLs were isolated from SPF-1, cDysN6, and cSPF-2 mice during the steady state and on day 5 after DSS colitis induction and restimulated with α-CD3/CD28 for 3 days. Cytokine levels were measured from supernatant. n = 5 mice/group.
(H–K) T cell transfer colitis was induced by injecting CD4+Foxp3−CD45RB(high) T cells into SPF-1, cDysN6, or cSPF-2 Rag2−/− recipients. Body weight was measured after T cell transfer (H). Shown is the colonoscopy severity score on day 14 after transfer (I). Colon weight/length ratio (J) and total numbers of CD4+ cells in cLPLs on day 16 after injection were monitored by FACS (K). n = 7–14 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test (A–E) and nonparametric Kruskal-Wallis test (F–K): ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S6.
To investigate whether pathogenic CD4+ T cells are required during DysN6-enhanced DSS colitis, we treated SPF-1 and cDysN6 WT mice during DSS colitis with an isotype control antibody or depleting antibodies against CD4 or CD8, respectively. Depletion of CD4-expressing cells, but not CD8-expressing cells, resulted in failure of the DysN6 community to exacerbate DSS colitis (Figure 6E), highlighting that CD4+ T cells are required during the development of DysN6-enhanced colitis.
Consequently, we extended our immunophenotyping and analyzed the production of proinflammatory cytokines in CD4+ T cells before and during DSS colitis. We initially focused on IFN-γ and IL-17 and, hence, isolated colonic LPLs (cLPLs) from SPF-1, cSPF-2, and cDysN6 IL-17AGFP IFN-γKatushka FoxP3RFP triple reporter mice allowing the in situ monitoring of cytokine production (Gagliani et al., 2015). Transfer of the DysN6 but not SPF-2 community resulted in enhanced numbers of IL-17A and IFN-γ single and IL-17A/IFN-γ double cytokine-producing CD4+ T cells already before induction of DSS colitis (Figure 6F). After induction of DSS colitis, enhanced numbers of cytokine-producing CD4+ T cells were observed in mice with both colitogenic communities (Figure 6F). In addition to monitoring cytokine production in situ, we isolated cLPLs from SPF-1, cSPF-2, and cDysN6 mice before and after induction of DSS colitis and stimulated them with αCD3 and αCD28 to quantify cytokine production from T cells. Strikingly, T cells from cDysN6 mice produced larger amounts of IL-17A and IFN-γ than T cells from SPF-1 and cSPF-2 mice (Figure 6G), both during the steady state and colitis. Notably, TNF-α production after restimulation of T cells was highest during colitis in mice colonized with SPF-2 (Figure 6G).
To further investigate the ability of the DysN6 to drive T cell-mediated intestinal inflammation, we transferred CD45RB(high)Foxp3-CD4+ T cells from IL-17AGFPIFN-γKatushka FoxP3RFP triple reporter mice into SPF-1, cSPF-2, and cDysN6 Rag2−/− mice. After 2 weeks, when mice differed only mildly in their weight loss (Figure 6H), we already observed higher intestinal inflammation, as quantified by colonoscopy in cDysN6 compared with SPF-1 and cSPF-2 recipients (Figure 6I). cDysN6 mice displayed an enhanced colon weight to length ratio and cellular infiltration (Figures 6J and 6K). Specifically, IFN-γ+ CD4+ T cells numbers were significantly increased (Figure S6G). Although the numbers of IL-17A+ and double cytokine-producing T cells were also significantly enhanced in cDysN6-colonized mice, their total numbers were much lower than those of IFN-γ+ CD4+ T cells (Figure S6G). Taken together, this demonstrates that DysN6 induces pathogenic CD4+ T cells producing high levels of proinflammatory cytokines. Moreover, these microbiota-induced cells are essential to drive disease in two distinct colitis models.
Recognition of Antigens from Dominant Microbial Members by CD4+ T Cells Drives DSS Colitis Severity in DysN6 Mice
To investigate whether recognition of microbial antigens by CD4+ T cells is required for exacerbation of colitis in DysN6 mice, DSS colitis was induced in OTII transgenic mice colonized with the SPF-1, cSPF-2, or cDysN6 communities (Figure S7A). Strikingly, cDysN6 OTII mice did not display exacerbation of DSS colitis severity, as indicated by the lack of DysN6 transfer-induced changes in body weight loss, intestinal inflammation, and colon shortening (Figures 7A and 7B; Figure S7B). In contrast, cSPF-2 OTII mice were characterized by similar weight loss compared with cSPF-2 WT mice (Figures S7C and S7D). This shows that antigen specificity of CD4+ T cells is required for modulation of disease severity by the DysN6 but not SPF-2 community.
Figure 7.
CD4+ T Cells Drive DSS Colitis Severity in DysN6 Mice by Recognizing Antigens from Dominant Microbial Members
(A and B) SPF-1 WT and OTII transgenic mice were cohoused with DysN6 donor mice, and DSS colitis was induced. Body weight (A) and intestinal inflammation (B) on day 5 of DSS colitis were monitored. Shown are representative pictures of H&E-stained colon sections. Scale bars represent ∼50 μm. n = 5–10 mice/group.
(C–E) SPF-1 WT mice were cohoused with DysN6, SPF-2, or both DysN6 and SPF-2 donor mice, respectively. Shown is β-diversity analysis (PCoA) of fecal microbiota (C) and pathway analysis based on GO terms of genes significantly upregulated (2-fold), as determined by RNA-seq in cDysN6+SPF-2 compared with SPF-1 mice (D). Also shown are body weight loss and survival after induction of DSS colitis (E).
(F) SPF-1 CD4−/− mice were cohoused with DysN6 and SPF-2 donor mice, and DSS colitis was induced. n = 5–12 mice/group.
Data are displayed as mean ± SEM from at least two independent experiments. The indicated p values represent unpaired Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S6.
Although the DysN6 and SPF-2 communities both trigger severe colitis in the host, the mechanisms of pathogenesis are completely opposing. Consequently, we wanted to understand whether triggering of innate or adaptive immunity by the SPF-2 or DysN6 community dominate over each other when cotransferring them into SPF-1 recipients. Analysis of microbiota composition in recipient mice after cohousing of SPF-1 recipient as well as SPF-2 and DysN6 donor mice showed that the resulting community largely resembled the cDysN6 community (Figure 7C). Accordingly, the host gene expression signatures in cDysN6+SPF-2 mice were similar to the ones observed in cDysN6 mice, including upregulation of genes associated with T cell, B cell, cytokine, and chemokine signaling as well as upregulation of Cd4 (Figure 7D; Figures S7E and S7F). Moreover, mice with cDysN6+SPF-2 displayed high weight loss, intestinal inflammation, colon shortening, and mortality compared with SPF-1 mice (Figure 7E; Figures S7G and S7H). Strikingly, the cDysN6+SPF-2 community failed to induce severe colitis in CD4−/− mice (Figure 7F; data not shown). These results demonstrate that the DysN6 community and its pathogenesis mechanism (i.e., the induction of pathogenic antigen-specific CD4+ T cell responses) dominate over SPF-2-induced changes during colitis induction.
Discussion
Alterations in the microbiome have been hypothesized to contribute to the development of IBD, and patient data suggest the existence of microbial signatures associated with specific disease entities such as CD (Frank et al., 2007, Gevers et al., 2014). However, it remains in question whether those changes are causal and can be potentially used to improve the selection of IBD therapy or, rather, are the result of ongoing inflammation that alters the intestinal microenvironment (Börnigen et al., 2013). Here we identified and then characterized distinct types of microbial communities that directly affect the severity of intestinal inflammation in an immunocompetent host. We showed that these communities alter disease susceptibility via opposing mechanisms, one requiring antigen-specific CD4+ T cell responses and the other being mediated by innate immune cells.
Healthy human individuals can differ greatly in the composition of their intestinal microbiota, but despite this variability, alterations in the microbiota of patients have been associated with different types of human diseases (Clemente et al., 2012, Falony et al., 2016). Germ-free mice have been fundamental to address the causal role of alterations of the microbiome in mouse models of human disease. Likewise, conventionally housed laboratory mice that feature tremendous differences in the microbiome represent a valuable resource to study the contribution of diverse microbial ecosystems to disease development (Stappenbeck and Virgin, 2016). In a first effort to reduce experimental variability, the concept of SPF housing conditions was introduced to exclude unwanted influences imposed by the presence of potential pathogens, such as Helicobacter spp. or mouse norovirus, commonly present in wild and conventionally housed mice (Stappenbeck and Virgin, 2016). However, microbiota composition differs greatly between SPF mice from different commercial breeders and academic institutions (Rausch et al., 2016), and those differences influence host responses; e.g., the presence of Th17 cells in SFB-colonized mice (Ivanov et al., 2009) or lowered susceptibility to malaria infection as a consequence of an increased abundance of Lactobacillaceae and Bifidobacterium spp. (Villarino et al., 2016). These observations also make genetically identical SPF mice a versatile experimental model to explore diverse microbial communities and to study host-microbiota interactions in health and disease.
A common feature in IBD, particularly in UC, is impairment of the intestinal barrier, resulting in enhanced exposure to luminal microbes. By employing a mouse model of damage to the intestinal barrier, DSS colitis, we demonstrate that isogenic SPF mice with differences in microbiome composition feature altered susceptibility to intestinal inflammation. Specifically, we noted that transfer of colitogenic communities into mice relatively resistant to induction of DSS colitis is sufficient to alter disease susceptibility even in immunocompetent mice. Upon induction of disease, the DysN6 community as well as the SPF-2 community induced severe colitis compared with the relatively resistant SPF-1 community, but the mechanisms of pathogenesis differed strongly. Inflammation in SPF-2 mice was characterized by high levels of TNF-α and neutrophil-attracting chemokines coinciding with significant higher infiltration of neutrophils into the inflamed tissue. In line with previous findings, DysN6 mice featured higher levels of the chemokine CCL5, known to attract innate and adaptive immune cells carrying CCR1, CCR3, CCR4, and CCR5 (Elinav et al., 2011). Here we identified high infiltration of activated CD4+ T cells in DysN6 mice, hinting toward a potential involvement of these cells in intestinal pathogenesis. The hypothesis of adaptive immune cells being involved in DysN6 mice was further corroborated by the observation that extended colonization with the DysN6 but not SPF-2 community was required to transfer disease susceptibility. Subsequently, we evaluated the effect of the transfer of the two colitogenic communities in mice lacking specific subsets of adaptive immune cells. For these comparisons we employed WT and gene-deficient mice that were embryo-transferred into our vivarium using SPF-1 foster mothers, resulting in a standardized microbiota (E.J.C.G, unpublished data). Moreover, we included cohousing of WT and gene-deficient mice to further reduce microbiota variability within experiments and documented, for all experiments, microbiota composition using 16S rRNA gene sequencing. Using this carefully controlled approach, we observed significant increases in IL-17A and IFN-γ secretion by CD4+ T cells during DysN6- and SPF-2 driven colitis. Notably, this is in line with an association of CD4+ T cells and proinflammatory cytokines, including IL-17, IFN-γ, and IL-23, with human IBD (Kaser et al., 2010). Strikingly, our experiments demonstrated that CD4+ T cells are only essential to mediate the exacerbation of DSS colitis in DysN6 but not SPF-2 mice. In contrast, despite measurable CD4+ T cell activation during DSS colitis, SPF-2 modulated disease severity independent of adaptive immune cells. T cell receptor (TCR)-mediated recognition of cognate antigens is required for proper T cell function, and recognition of microbial antigens has been suggested to significantly contribute to the development of colitis (Feng et al., 2010). Using OTII transgenic mice, we could show that DysN6-driven but not SPF-2-driven colitis development strongly depended on the presence of antigen-specific CD4+ T cells. The presence of in vivo cytokine-secreting CD4+ T cells before induction of DSS colitis in DysN6 mice suggests that colonic CD4+ T cells already recognize cognate microbial antigens during this phase, similar to what has been observed for SFB-specific CD4+ T cells in the small intestine (Yang et al., 2014). Importantly, antibody-mediated depletion of CD4+ T cells during colitis resulted in failure to transfer enhanced colitis susceptibility. This demonstrated that, to enhance colitis, modulation of the mucosal barrier by CD4+ T cells in the steady state was not sufficient and, rather, required the presence and, presumably, the effector functions of CD4+ T cells during colitis. The distinct property of the DysN6 community to prime and activate pathogenic CD4+ T cell responses was further corroborated using a model for CD4+ T cell-mediated colitis. Specifically, transfer of CD4+ T cells in Rag2−/− mice harboring the DysN6 but not the SPF-2 microbiota enhanced intestinal inflammation and cytokine production by CD4+ T cells. Whether these different communities also cause different disease susceptibility or pathogenesis via shared or distinct pathways in other inbred mouse strains or IBD models such as the Il10−/− model of colitis and the TNFdeltaARE model of ileitis, remains to be tested (Keubler et al., 2015, Schaubeck et al., 2016). This shows that colitogenic communities exert their pathogenic effects in the same disease model by opposing mechanisms.
Detailed characterization of the colitogenic communities using 16S rRNA gene sequencing revealed the varying presence of potential pathobionts such as SFB, Prevotella spp., Helicobacter spp., Enterobacteriaceae, and Verrucomicrobiaceae in DysN6 and SPF-2 mice. SFB have been shown to modulate intestinal T cell immunity and systemic autoimmunity (Ivanov et al., 2009). However, based on their presence in both SPF-2 and DysN6 mice, a role in driving the differential requirement for CD4+ T cells can be excluded. Similarly, members of the genus Prevotella, previously found to be enriched in the colitogenic microbiota of Nlrp6−/− mice (Elinav et al., 2011), were present in both colitogenic communities, indicating that they are not involved in regulating the different pathogenicity modes. Helicobacteraceae have been demonstrated to induce the development of colitis in Il10−/− mice in cooperation with other members of the microbiota (Keubler et al., 2015). Although Helicobacteraceae were absent in SPF-2 mice, DysN6 mice harbored different members of this family, including H. typhlonius, H. rodentium, and H. muridarum, but did not harbor H. hepaticus. Finally, both Enterobacteriaceae and Verrucomicrobiaceae, specifically Akkermansia muciphilia, bloomed during induction of DSS colitis in SPF-2 mice, but it is being debated whether expansion during disease suggests a contribution to disease development or, rather, a consequence of the ability to utilize inflammation-induced metabolites. In contrast to the “one microbe one disease” model, the concept of dysbiosis, an imbalance of the community, has been proposed for microbiome-mediated modulation of diseases (Petersen and Round, 2014). One characteristic of dysbiotic communities, including those in IBD patients, has been suggested to be an imbalance between Bacteroides, Firmicutes, and Proteobacteria, with an overexpansion of Bacteroides and Proteobacteria over Firmicutes (Frank et al., 2007). Lowered Firmicutes/Bacteroides ratios were noted in all colitogenic communities, including SPF-2 and DysN6, whereas the ratios between Firmicutes and Proteobacteria (F/P) was not consistently different between susceptible and resistant groups. Notably, the F/P ratio was the lowest in DysN6 mice, and according to our data, this is associated with a distinct mode of pathogenicity. Whether, in the cases of the SPF-2 and DysN6 community, specific pathobionts or a general dysbiosis are responsible for driving distinct pathogenicity requires further investigation because dysbiotic communities have also been reported in other gene-deficient mouse lines (Couturier-Maillard et al., 2013, Hu et al., 2015, Roberts et al., 2014).
Finally, it remains debated how these dysbiotic communities arise in gene-deficient mice and how similar they are in regard to their composition in different vivariums, taking into account that a large variability in the composition and function of microbial ecosystems in WT mice already exist. In this study, we employed Nlrp6−/− mice with a similar microbiome compared with what has been reported previously (Elinav et al., 2011). However, it remains to be tested whether the pathological mechanism of dysbiotic communities occurring in unrelated lines of Nlrp6 inflammasome-deficient mice causes exacerbated pathology via the same or different mechanisms or does not cause any pathology at all. Along these lines, a recent study has suggested that the intestinal microbiomes of WT and Nlrp6−/− mice raised under SPF conditions did not differ in their composition, suggesting that the development of dysbiotic communities reflects a complex interplay between genetic and environmental factors (Mamantopoulos et al., 2017).
In summary, our data show how distinct microbial communities drive the development of intestinal inflammation in immunocompetent hosts by modulating opposing arms of the immune system. Our study suggests the concept that triggering of different immune pathways by microbial communities can alter disease susceptibility, eventually resulting in similar host pathophysiology. This implies that personalized immunomodulatory treatment according to distinct microbial signatures may be beneficial for IBD patients.
Experimental Procedures
Mice
Wild-type and all transgenic mice, Rag2−/−, Tcrbd−/−, muMT−/−, Tcrd−/−, OTII, CD4−/−, CD8−/−, and IL-17AGFP IFN-γKatushka FoxP3RFP reporter mice used in the study were on the C57BL/6N background, rederived into SPF-1 microbiota by embryo transfer, and bred at the SPF animal facilities of the Helmholtz Centre for Infection Research (HZI). Nlrp6−/− mice were obtained from Yale University and subsequently bred under conventional housing conditions at the HZI without rederivation. Other donor microbiota for different composition were purchased from different commercial vendors (Janvier, Taconic, and Harlan) (Table S1). Germ-free C57BL/6NTac mice were bred in isolators (Getinge) in the germ-free facility of the HZI. All experiments were performed with 10- to 14-week-old age-matched and gender-matched animals. Both male and female animals were used for every experiment to exclude influence of gender.
DNA Isolation and 16S rRNA Microbial Community Analysis
Fresh stool samples of mice were collected and immediately stored at −20°C. DNA was extracted according to established protocols using a method combining mechanical disruption (bead-beating) and phenol/chloroform-based purification (Turnbaugh et al., 2009). Briefly, a sample was suspended in a solution containing 500 μL of extraction buffer (200 mM Tris, 20 mM EDTA, and 200 mM NaCl [pH 8.0]), 200 μL of 20% SDS, 500 μL of phenol:chloroform:isoamyl alcohol (24:24:1), and 100 μL of 0.1 mM zirconia/silica. Samples were homogenized twice with a bead beater (BioSpec) for 2 min. After precipitation of DNA, crude DNA extracts were resuspended in Tris, EDTA (TE) buffer with 100 μg/mL RNase and column-purified to remove PCR inhibitors (BioBasic). Amplification of the V4 region (F515/R806) of the 16S rRNA gene was performed according to previously described protocols (Caporaso et al., 2011). Samples were sequenced on an Illumina MiSeq platform (PE250). Filtering of sequences for low-quality reads (q > = 30) and barcode-based binning were performed by using QIIME v1.8.0 (Caporaso et al., 2010). Reads were clustered into operational taxonomical units (OTUs) based on 97% nucleotide identity of the amplicon sequences using UCLUST reference OTU picking, followed by taxonomic classification using the Ribosomal Database Project (RDP) classifier executed at 80% bootstrap confidence cutoff (Edgar, 2010, Wang et al., 2007). Sequences without a matching reference dataset were grouped as de novo using UCLUST. The OTU absolute abundance table and mapping file were used for statistical analyses and data visualization in the R statistical programming environment package phyloseq (McMurdie and Holmes, 2013). To determine bacterial OTUs that explained differences between microbiota settings, the LEfSe method was used (Segata et al., 2011). OTUs with Kruskal-Wallis test < 0.05 and LDA scores > 3.5 were considered informative. Raw data are available in the Sequence Read Archive (SRA): PRJNA407363.
DSS-Induced Colitis
To induce acute colitis, mice were provided 2% (w/v) DSS (molecular mass = 36–50 kDa, MP Biomedicals) in drinking water for 7 days, followed by 7 days of access to regular drinking water. Daily clinical assessment of DSS-treated animals included body weight loss measurement, stool consistency, and detection of blood in the stool. Experimental samples were collected on days 0, 5, and 7 of DSS treatment.
CD45Rbhi Colitis
CD4+Foxp3−CD45RB(high) cells were transferred adoptively into Rag2−/− mice according to the protocol described by Ostanin et al. (2009). Briefly, splenic lymphocytes were isolated from IL-17AGFP IFN-γKatushka FoxP3RFP triple reporter mice. CD4 enrichment was performed according to the manufacturer’s instructions using CD4 (L3T4) microbeads (Miltenyi Biotec). CD4-enriched cells were then stained with antibodies against CD45RB and CD4. Cells were sorted in a BD FACSAria II cell sorter by gating CD45RB(high), CD4+Foxp3− cells. Antibodies used for staining were anti-CD45RB (C363-16A) and anti-CD4 (GK1.5). A total of 500,000 cells were injected intraperitoneally (i.p.) per mouse. Disease development was monitored by weighing animals 3 times a week and performing colonoscopies.
Statistical Analyses
Statistical analysis was performed using the GraphPad Prism program (GraphPad). Data are expressed as mean ± SEM. Differences were analyzed by Student’s t test and ANOVA. The indicated p values represent non-parametric Mann-Whitney U test or Kruskal-Wallis test comparison between groups. p Values ≤ 0.05 were considered significant: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Author Contributions
U.R. and T.S. designed the experiments and wrote the manuscript with input from co-authors. U.R., A.I., and A.J.B. performed and analyzed the experiments. E.J.C.G. analyzed the RNA sequencing data. E.J.C.G. and T.R.L. designed and supported the analysis of the 16S rRNA sequencing data. M.C.P. and U.H. performed histological evaluation and analysis. R.A.F. contributed essential reagents. S.H., R.A.F., and T.S. supervised the study.
Acknowledgments
We thank the members of the Strowig, Huber, and Flavell laboratories, as well as Nicola Gagliani, for valuable discussions. We thank Achim Gronow, Annett Kluge, the staff of the animal unit, and the genome analytics core facility at the Helmholtz Centre for Infection Research for excellent technical support. The project was supported by the Helmholtz Association (VH-NG-933 to T.S.), by the DFG (STR-1343/1 and STR-1343/2 to T.S.), and the EU (MCCIG618925 to T.S. and StG337251 to S.H.).
Published: October 24, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.09.097.
Data and Software Availability
The accession number for the RNA-seq data reported in this paper is Sequence Read Archive (SRA): SRP118483. The accession number for the 16S rRNA gene sequencing data reported in this paper is SRA: SRP119278.
Supplemental Information
References
- Anderson M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001;26:32–46. [Google Scholar]
- Bloom S.M., Bijanki V.N., Nava G.M., Sun L., Malvin N.P., Donermeyer D.L., Dunne W.M., Jr., Allen P.M., Stappenbeck T.S. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börnigen D., Morgan X.C., Franzosa E.A., Ren B., Xavier R.J., Garrett W.S., Huttenhower C. Functional profiling of the gut microbiome in disease-associated inflammation. Genome Med. 2013;5:65. doi: 10.1186/gm469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., Fierer N., Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014;104 doi: 10.1002/0471142735.im1525s104. Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chou K., Fuchs E., Havran W.L., Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler R., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J., Peaper D.R., Bertin J., Eisenbarth S.C., Gordon J.I., Flavell R.A. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- Feng T., Wang L., Schoeb T.R., Elson C.O., Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.G., Ge Z., Whary M.T., Erdman S.E., Horwitz B.H. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W., de Zoete M.R., Licona-Limón P., Paiva R.S., Ching T. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett W.S., Gallini C.A., Yatsunenko T., Michaud M., DuBois A., Delaney M.L., Punit S., Karlsson M., Bry L., Glickman J.N. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Peng L., Kwak Y.T., Tekippe E.M., Pasare C., Malter J.S., Hooper L.V., Zaki M.H. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Rep. 2015;13:1922–1936. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhann F., Vich Vila A., Bonder M.J., Fu J., Gevers D., Visschedijk M.C., Spekhorst L.M., Alberts R., Franke L., van Dullemen H.M. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2016 doi: 10.1136/gutjnl-2016-312135. Published online October 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I.I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V.S. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., International IBD Genetics Consortium (IIBDGC) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Seo S.-U., Chen G.Y., Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keubler L.M., Buettner M., Häger C., Bleich A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm. Bowel Dis. 2015;21:1967–1975. doi: 10.1097/MIB.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukens D., Brinkman B.M., Raes J., De Vos M., Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Thaiss C.A., Zeevi D., Dohnalová L., Zilberman-Schapira G., Mahdi J.A., David E., Savidor A., Korem T., Herzig Y. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamantopoulos M., Ronchi F., Van Hauwermeiren F., Vieira-Silva S., Yilmaz B., Martens L., Saeys Y., Drexler S.K., Yazdi A.S., Raes J. Nlrp6- and ASC-Dependent Inflammasomes Do Not Shape the Commensal Gut Microbiota Composition. Immunity. 2017;47:339–348.e4. doi: 10.1016/j.immuni.2017.07.011. [DOI] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Ostanin D.V., Bao J., Koboziev I., Gray L., Robinson-Jackson S.A., Kosloski-Davidson M., Price V.H., Grisham M.B. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C., Round J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P., Basic M., Batra A., Bischoff S.C., Blaut M., Clavel T., Gläsner J., Gopalakrishnan S., Grassl G.A., Günther C. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int. J. Med. Microbiol. 2016;306:343–355. doi: 10.1016/j.ijmm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Roberts M.E., Bishop J.L., Fan X., Beer J.L., Kum W.W.S., Krebs D.L., Huang M., Gill N., Priatel J.J., Finlay B.B., Harder K.W. Lyn deficiency leads to increased microbiota-dependent intestinal inflammation and susceptibility to enteric pathogens. J. Immunol. 2014;193:5249–5263. doi: 10.4049/jimmunol.1302832. [DOI] [PubMed] [Google Scholar]
- Saleh M., Elson C.O. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaubeck M., Clavel T., Calasan J., Lagkouvardos I., Haange S.B., Jehmlich N., Basic M., Dupont A., Hornef M., Von Bergen M. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225–237. doi: 10.1136/gutjnl-2015-309333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C., Berry D., Rauch I., Rennisch I., Ramesmayer J., Hainzl E., Heider S., Decker T., Kenner L., Müller M. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 2014;8:1101–1114. doi: 10.1038/ismej.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck T.S., Virgin H.W. Accounting for reciprocal host-microbiome interactions in experimental science. Nature. 2016;534:191–199. doi: 10.1038/nature18285. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009;1 doi: 10.1126/scitranslmed.3000322. 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino N.F., LeCleir G.R., Denny J.E., Dearth S.P., Harding C.L., Sloan S.S., Gribble J.L., Campagna S.R., Wilhelm S.W., Schmidt N.W. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl. Acad. Sci. USA. 2016;113:2235–2240. doi: 10.1073/pnas.1504887113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Torchinsky M.B., Gobert M., Xiong H., Xu M., Linehan J.L., Alonzo F., Ng C., Chen A., Lin X. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.