We develop a new method that allows targeted lesioning of vagal afferent neurons that innervate the upper gastrointestinal tract while sparing vagal efferent neurons. This reliable approach provides superior tissue specificity and selectivity for vagal afferent over efferent targeting than traditional approaches. It can be used to address questions about the role of gut to brain signaling in physiological and pathophysiological conditions.
Keywords: CCK, gut, gut-brain signaling, vagus nerve, deafferentation
Abstract
There is a lack of tools that selectively target vagal afferent neurons (VAN) innervating the gut. We use saporin (SAP), a potent neurotoxin, conjugated to the gastronintestinal (GI) hormone cholecystokinin (CCK-SAP) injected into the nodose ganglia (NG) of male Wistar rats to specifically ablate GI‐VAN. We report that CCK-SAP ablates a subpopulation of VAN in culture. In vivo, CCK-SAP injection into the NG reduces VAN innervating the mucosal and muscular layers of the stomach and small intestine but not the colon, while leaving vagal efferent neurons intact. CCK-SAP abolishes feeding-induced c-Fos in the NTS, as well as satiation by CCK or glucagon like peptide-1 (GLP-1). CCK-SAP in the NG of mice also abolishes CCK-induced satiation. Therefore, we provide multiple lines of evidence that injection of CCK-SAP in NG is a novel selective vagal deafferentation technique of the upper GI tract that works in multiple vertebrate models. This method provides improved tissue specificity and superior separation of afferent and efferent signaling compared with vagotomy, capsaicin, and subdiaphragmatic deafferentation.
NEW & NOTEWORTHY We develop a new method that allows targeted lesioning of vagal afferent neurons that innervate the upper GI tract while sparing vagal efferent neurons. This reliable approach provides superior tissue specificity and selectivity for vagal afferent over efferent targeting than traditional approaches. It can be used to address questions about the role of gut to brain signaling in physiological and pathophysiological conditions.
the vagus nerve is a crucial link between peripheral organs and the central nervous system. Afferent and efferent vagal fibers communicate bidirectional information that controls physiological functions necessary for survival (21). Vagal efferent neurons located centrally in the dorsal motor nucleus of the vagus (DMNV) signal motor control to visceral organs, while vagal afferent neurons (VAN) in the nodose ganglia (NG) convey sensory information from the periphery to the brain. A major constraint in this field of research has been the lack of tools to delineate vagal afferent and efferent signaling and selectively target the vagus nerve innervating specific organs. Given that vagally mediated gut-brain signaling has been implicated in a broad range of pathologies (7, 8, 19, 22, 26, 27, 29, 37), methods that selectively target VAN innervating the gastrointestinal tract (GI-VAN) would be particularly beneficial and could impact a number of different fields.
In developing a novel method for selective vagal deafferentation of the gut, four different challenges need to be overcome. First, the method should ablate afferent neurons while leaving efferent neurons intact. Second, this technique should predominantly target the afferent population that innervates the gut. This is complicated by the fact that vagal afferent neurons are intermingled in the NG with no clear topographic organization (2). Ideally, the approach would inhibit both mechanosensitive and chemosensitive gut vagal afferent terminals. Finally, to replace the current surgical techniques widely used to block gut-brain signaling, the novel method should be applicable in different vertebrate models. We hypothesized that a pharmacological agent injected in the NG that could target neurons based on their receptor expression would address all of these challenges.
The ribosomal inhibitor saporin is inert until transported into a cell (48), at which time it becomes a potent neurotoxin. One molecule of ribosomal inhibitor in a cell has been demonstrated to be sufficient to cause cell death (24). Saporin conjugated to ligands for G protein-coupled receptors enables targeted ablation by taking advantage of ligand-mediated receptor internalization. As a result of the stability (43) and the relative safety in handling saporin (1), saporin conjugation has been widely used to ablate select intermingled neuronal populations in a number of different central and peripheral sites (33). Since vagal afferent and efferent neurons are localized at different sites, injection of an appropriate ligand conjugated to saporin into NG should selectively ablate afferent neurons while leaving efferent neurons intact.
The GI hormone cholecystokinin (CCK) is an ideal substrate to target saporin-mediated ablation of vagal afferent neurons innervating the gut. CCK is secreted from endocrine I-cells dispersed throughout the small intestinal mucosa (31). The G protein-coupled CCK receptors are localized on afferent fibers in the gut (18, 32), and CCK-A receptor-expressing vagal afferent neurons have been demonstrated to extensively innervate the gut (4, 13). Importantly CCK activates both chemosensitive and/or mechanosensitive populations of vagal afferent neurons (44, 47). Single unit recordings from afferent vagal fibers in the cervical vagus (20), and from second order neurons in the nucleus of the solitary tract (NTS) where vagal afferent fibers terminate centrally (25), demonstrate that CCK depolarizes vagal afferent fibers and activates a gut-brain signaling pathway. The physiological effects of CCK includes gallbladder contraction, pancreatic secretion, gastric emptying, gastric acid secretion, and satiation (23). Studies using subdiaphragmatic vagotomy (45) and selective surgical transection of afferent vagal fibers (46) demonstrate the necessity of an intact vagus nerve to mediate the physiological effects of intraperitoneal CCK injections. Crucially, saporin conjugated to CCK (CCK-SAP) has been previously validated and successfully used to target CCK receptor-expressing cells in the rostral ventromedial medulla (50). Therefore, CCK-SAP was injected bilaterally into NG to determine whether this could be an effective novel approach for selective vagal deafferentation of the gut.
MATERIALS AND METHODS
Animals and Housing
All experiments were approved by the John B. Pierce Laboratory and University of California Davis Institutional Animal Care and Use Committees. Adult male Wistar rats (n = 56; initial weight 200 g; Harlan, San Diego, CA) or C57BL6/J mice (n = 6) were individually housed at 22°C under a 12-h:12-h light-dark cycle (light 4 am to 4 pm) with ad libitum access to water and chow (Teklad 2018; Envigo, Sommerset, NJ).
Peptides and Drugs
SAP and saporin conjugates were obtained from Advanced Targeting Systems (San Diego, CA). Cholera toxin B conjugated to Alexa555 (CTB-555) was obtained from ThermoFisher Scientific (Waltham, MA). Glucagon like peptide-1 (GLP-1) and CCK carboxyl terminal octapeptide (CCK8S) were obtained from Bachem BioScience (King of Prussia, PA).
In Vitro Studies
NG were dissected under aseptic conditions, desheathed, and digested for 120 min at 37°C in 3 ml of Ca2+- and Mg2+-free HBSS (ThermoFisher Scientific) containing 6 mg collagenase type A (Sigma-Aldrich Chemicals, St. Louis, MO). Cells were plated onto four-well chamber slides (EMD Millipore, Billerica, MA) and maintained in low-glucose DMEM (ThermoFisher Scientific) supplemented with 10% fetal calf serum (ThermoFisher Scientific) and 1% antibiotic-antimycotic (ThermoFisher Scientific) at 37°C in 5% CO2 with media changed every 48 h. Cells were maintained in culture for 72 h and stained with Hoechst 33342 (0.005 µg/ml; ThermoFisher) to count the total number of live VAN. After being counted, cells were returned to incubator for 1 h, and each well was treated with a different dose of saporin conjugates (0, 2.4, 24, or 240 ng) for 24 h. After treatment, cells were stained with Hoechst 33342 (0.005 µg/µl) and fixed with 4% paraformaldehyde (PFA) in PBS. Slides were prepared with ProLong Diamond Antifade Reagent (ThermoFisher) and coverslipped. Cells were imaged using an Olympus spinning disk confocal microscope (BX61 System; Olympus, Melville, NY) and counted by two individuals blinded to the experimental conditions.
NG injection.
The day before surgery rats were provided with a 15-ml sipper of condensed milk (300 ml mixed 570 ml water; 1.5 kCal/ml) in addition to ad libitum access to chow and water, then were fasted overnight. Twenty minutes before surgery, rats received a subcutaneous injection of anticholinergic atropine sulfate (0.05 mg/kg; Henry Schein, Wallingford, CT) and analgesic carprofen (5.0 mg/kg; Henry Schein). Rats were anesthetized with isoflurane (2% isoflurane in 100% oxygen; Henry Schein) or intraperitoneal injection of an anesthesia cocktail containing ketamine (65 mg/kg; Henry Schein), xylazine (5 mg/kg; Henry Schein), and acepromazine maleate (1.5 mg/kg; Henry Schein). Animals were shaved from the chin to thorax and placed supine on a heated pad (CMA 450; Harvard Apparatus, Holliston, MA). A midline incision was made with a scalpel along the length of the neck; salivary glands and lymph nodes were retracted. The sternohyoid and omohyoid muscles were separated to expose the trachea and carotid artery. The vagus nerve was separated from the carotid artery with Graefe forceps until the NG became accessible. A glass capillary (rat: 20-μm tip, beveled 30° angle; mouse: 15-µm tip, beveled 45° angle) attached to a micromanipulator was used to position and puncture the NG and an equal volume (rat: 1 µl; mouse: 0.5 µl) of CCK-SAP (250 ng/µl) or SAP (250 ng/µl) was injected with a Picospritzer III injector (Parker Hannifin, North Haven, CT) at two sites rostral and caudal to the laryngeal nerve branch. The same procedure was repeated on the other side before the skin was closed with sterile suture. Animals were allowed to recover under infrared heat until they chose to reside in the unheated side of the cage, at which point they were returned to their home cage deprived of water for 6 h and food overnight. Day 1 postoperation animals received carprofen (5.0 mg/kg sq) and were given ad libitum access to condensed milk; day 2 postoperation they received mash (10 g powdered chow mixed with 20 ml condensed milk diluted as above); day 3 postoperation they received mash and solid chow pellets; from day 4 onward, animals received ad libitum access to chow. CCK-SAP potency was determined for each lot by quantifying the percent reduction in cultured VAN after 24 h at 240 ng/µl compared with stimulation with vehicle for 24 h. All lots were determined to have approximately the same potency, based on the percent ablation of cultured VAN in response to 240 ng/µl CCK-SAP.
Retrograde tracing.
For all retrograde labeling studies, rats received CCK-SAP unilaterally into the left or right NG with SAP on the contralateral side.
fluorogold tracing.
To nonselectively label all abdominal fibers, we injected fluorogold (20 µg·ml saline−1·rat−1; Ref. 51) 7 days after NG surgery and euthanized animals after 5 days.
ctb-555 tracing.
After a 7-day recovery period from NG surgery, overnight food deprived rats were anesthetized with isoflurane. A midline abdominal incision was made and cholera toxin B conjugated to Alexafluor 555 (CTB-555) was injected into the 1) stomach and duodenum (n = 8), 2) stomach lumen (n = 6), or 3) colon (n = 8).
stomach and duodenum.
A glass capillary (30-µm tip) loaded with 10 µl CTB-555 (0.5% solution in 0.9% saline) was injected using 8-ms pulses (Picospritzer III; Parker) at various points in the wall of the stomach (0.5 µl at 12 different sites: 4 injections at the pyloric junction, antrum, and corpus) and duodenum (0.5 µl at 8 different sites, every 1 cm on the ventral and dorsal side). After each injection, the syringe was held in place for 30 s to prevent backflow. The organs were thoroughly rinsed with warm Ringers lactate solution before the peritoneal wall and skin was closed with sterile suture.
colon.
A glass capillary loaded with 5 µl CTB-555 was injected using 8-ms pulses into the ascending colon (0.5 µl at 10 different sites evenly divided over ventral and dorsal sides of the first 2 cm of the ascending colon). After each injection, the syringe was held in place for 30 s to prevent backflow.
mucosal tracing.
We modified a previously used approach for retrograde tracing of mucosal vagal afferent in mice (28). Rats were fasted 40 h to minimize the amount of food in the stomach. A 27-gauge needle was positioned into the lumen of the stomach of anesthetized rats. The mucolytic agent N-acetylcysteine (1 ml; 10% wt/vol; Sigma-Aldrich) was injected through this needle over 30 s and incubated 5 min before being aspirated with a syringe. This was followed by two saline rinses (1 ml each) each aspirated with a syringe. The needle was removed, and subsequently, 0.5% CTB-555 was injected into the lumen of the stomach (60 µl) using a glass capillary. The stomach was massaged to ensure optimal contact of CTB-555 with epithelial wall for 1 min. Animals recovered with no food or water for 2–4 h.
Seven days was allowed for retrograde transport to the NG (17), at which time the rats were euthanized with an intraperitoneal injection of euthasol (Henry Schein) and transcardially perfused with 10% sucrose solution followed by 4% PFA in 3% sucrose water. Perfused brains were extracted and postfixed for 24 h in 4% PFA in PBS at 4°C overnight followed by 25% sucrose in PBS at 4°C until sectioning. Left and right NG were collected in separate tubes with 25% sucrose in PBS and stored at 4°C until sectioning. Cryostat sections of fixed NG (9 µm) and of hindbrain (35 µm) were either mounted onto Superfrost/Plus Slides (Fisher Scientific, Pittsburgh, PA) or collected in free floating 10% glycerol solution and stored at −80C.
Quantification of CTB-555 and fluorogold positive NG neurons was performed by two experimenters who were blind to the experimental conditions. Images were captured using Zeiss LSM 710 Duo NLO confocal microscope equipped with 488- and 561-nm laser lines. With the use of ImageJ, the mean absorbance of outlined neurons was measured. The highest peak pixel absorbance of clearly negative neurons in each tissue section was used as the threshold. Any neuron with a peak pixel absorbance above threshold was considered positive. Three to five NG sections were used for quantification for each rat. This low threshold was used to ensure that all positive cells were quantified. This favors false positives of CCK-SAP-treated NG and possibly underrepresents the percent ablation of VAN by CCK-SAP.
c-FOS and α-choline acetyltransferase staining.
Rats received bilateral NG injection of CCK-SAP (250 ng/µl; n = 7) or SAP (250 ng/µl; n = 7). After at least 7 days for recovery rats were fasted overnight on wire bottom cages with ad libitum access to water before the day of experiment. On the experimental day, half of the fasted rats received 10 g of chow. Two hours after the onset of eating rats were euthanized and perfused as described above. Perfused brains were extracted and postfixed for 24 h in 4% PFA in PBS at 4°C overnight followed by 25% sucrose in PBS at 4°C until sectioning.
c-fos.
Free-floating brain sections (2 h ± food after overnight fast) were washed three times 10 min in 1 M Tris-buffered saline (TBS; 0.06 M Tris, 0.2 M NaCl pH 7.6) and incubated with 10% methanol, 3% H2O2 in TBS for 10 min. Incubation was followed by three 10-min washes in 1 M TBS and overnight incubation at 4°C with goat anti-Fos IgG [1:1,500; c-Fos (4):sc-52; Santa Cruz Biotechnology, Santa Cruz, CA] diluted in supermix (SUMI, 0.25% gelatin, 0.5% Triton X-100 in TBS pH 7.6). The next day, sections were rinsed three times for 10 min in 1 M TBS, followed by 1-h incubation in biotinylated horse anti-goat IgG (1:400 in SUMI; BA-5000; Vector Laboratories, Burlingame, CA), washed three times for 10 min in 1 M TBS, and incubated 1 h in avidin-biotin complex (ABC in SUMI; Vector Laboratories). Following incubation, sections were washed in TBS. Incubation in 1% diaminobenzidine (DAB) with 0.01% H2O2 for 7 min was used to visualize the reaction. As a control, 0.05% nickel ammonium sulfate was added to the DAB solution to ensure that the reaction product became dark. After incubation, sections were rinsed with water and stored in 1 M TBS at 4°C until mounting on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). After overnight drying, slides were run through ethanol (50, 60, 70, 90, 96, and 100%), and xylene and covered for observation by light microscopy. Bright-field images were collected using Zeiss Axio Imager 2 with AxioCam503.
α-choline acetyltransferase.
Free-floating brainstem slices were incubated with antibodies on a shaking platform at room temperature. After each incubation period, sections were rinsed in Tris-PBS (TPBS) three times for 20 min. We used rabbit-α-choline acetyltransferase primary antibody (ChAT; 1:5,000; AB143; EMD Millipore, Temecula, CA) and donkey-α-rabbit conjugated to Alexafluor 488 or Alexafluor555 secondary antibody (1:400; Jackson ImmunoResearch, West Grove, PA). Tissues were incubated in primary antibodies diluted in TPBS containing 2% DS for 48 h and in secondary antibodies diluted in TPBS containing 2% DS for 24 h before being coverslipped using Prolong (ThermoFisher Scientific).
immunohistochemistry quantification.
Images were captured using AxioImager2 with AxioCam 503. Quantification was performed by an experimenter blind to the experimental conditions. Positive cells and nuclear profiles were counted bilaterally on Adobe Photoshop CS4 of images taken at ×20 magnification. For each rat, three to four sections were quantified (from Bregma −12.72 to −16.86 mm). The mean count per section was calculated, followed by calculation of the mean per group.
Feeding studies.
rats.
Rats received bilateral NG injection of CCK-SAP (250 ng/µl, n = 8) or SAP (250 ng/µl; n = 8). After a couple of weeks, rats were fasted in the morning on wire-bottomed cages with ad libitum access to water. Feeding tests were performed in animals trained daily with intraperitoneal saline injections after a 6-h fast that spans into the dark phase, when the animals have the strongest drive to eat. The satiation tests were only performed once a steady baseline intake was achieved in each individual animal ensuring that the 60 min postinjection intake was within a 10% range. On experimental day, saline-treated animals in the counterbalanced groups were compared with baseline to ensure that there were no external influences on the behavior. Since we observed daily fluctuation of up to 10% in 60 min intake within subject, we set the inclusion threshold for CCK-SAP-treated rats at 10%. However, most CCK-SAP-treated rats ate more food after treatment compared with saline. Food weight and spill was continuously recorded in an automated episodic food intake monitoring system (BioDAQ; ResearchDiets, New Brunswick NJ).
glucagon like peptide 1.
A small premeal (3 g) was given for 40 min before the onset of the dark phase (41). GLP-1 (100 µg/kg ip) or physiological saline (400 µl ip) was injected at the start of the dark phase and food was immediately returned to the cage.
cck.
CCK (2.5µg/kg ip) or physiological saline (400 µl ip) was administered 30 min into the dark phase, and food was immediately returned to the cage.
mice.
Before surgery, wild-type C57BL6 mice (n = 6) were trained to lick in a MedAssociates behavioral chamber equipped with a contact lickometer controlled by MedPC. This system monitored the number and timing of individual licks with millisecond resolution. Mice were injected with omtraperitoneal saline daily, immediately before being placed in the chamber with access to 10% sucrose in a sipper tube. After 1 h of sucrose access, mice were returned to their home cage with 1 g of chow. This protocol was repeated daily until a steady baseline was achieved in each animal, ensuring that the 60-min postinjection lick number was within a 10% range. On experimental days, mice received CCK (2.5 µg/kg ip) or physiological saline (100 µl ip) and were immediately placed in the chamber with access to 10% sucrose for 1 h. The day after mice received a CCK injection, the protocol was repeated with a saline injections to ensure that the animals returned to baseline.
The following week, mice received bilateral NG injection of CCK-SAP (250 ng/µl, n = 6). One mouse did not survive the bilateral NG surgery. After a week-long recovery period, food-restricted (1 g/day) mice were again trained to lick sucrose in the chamber and were tested for CCK responsiveness once achieving baseline (n = 5).
Statistics
Comparisons between groups for the quantification of cultured neurons were made using a one-way ANOVA with a Bonferroni post hoc test. CTB-555 and fluorogold quantification in the NG and CTB-555 and ChAT in DMNV was tested using an unpaired Student’s t-test (*P < 0.05). Comparisons between groups for the CCK and GLP-1 feeding tests and quantification of c-Fos were made using a two-way ANOVA to test for effects of treatment (SAP vs CCK-SAP), nutritional state (fed vs. fasted), and treatment × nutritional state interaction. Data are means ± SE; P < 0.05. For all experiments in which ANOVA was performed.
RESULTS
CCK-SAP Ablates a Subpopulation of Vagal Afferent Neurons In Vitro
To determine the ability of CCK-SAP to ablate VAN, we administered increasing concentrations of conjugated SAP to cultured primary VAN. Cytotoxicity quantification was performed before and after CCK-SAP stimulation in 1,300–3,100 cells per condition in six biological replicates. CCK-SAP dose dependently ablated VAN [F(3,12) = 4.818, P = 0.019, Fig. 1, A and B]. The highest dose of CCK-SAP (240 ng) resulted in 36.9 ± 5.1% decrease in live cells (P < 0.05; Fig. 1B, n = 6). Unlike G protein-coupled receptors, leptin receptors are not internalized in response to ligand binding (3). Therefore, leptin was conjugated to SAP to demonstrate that VAN ablation is dependent on internalization of SAP. We observed no effect of increasing concentrations of leptin-SAP on VAN viability [F(3,40) = 0.5354, P = 0.6607; Fig. 1, C and D, n = 4]. Together these data demonstrate that CCK-SAP, but not leptin-SAP, is capable of selectively ablating a subpopulation of VAN in vitro.
Fig. 1.
In vitro validation of cholecystokinin-saporin (CCK-SAP) for targeted ablation of vagal afferent neurons (VAN). A–D: in vitro count of cultured VAN before and after treatment with increasing concentrations of CCK-SAP (n = 6) or leptin-SAP (n = 4). A: VAN numbers were dose dependently reduced after 24-h treatment of CCK-SAP (*P = 0.02). B: the greatest reduction in cultured VAN occurred at 240 ng. C and D: leptin-SAP had no effect on cell counts (P = 0.66). Two-way ANOVA; F(3, 9) = 7.059; P = 0.0097; Sidak's post hoc test, *P < 0.05, **P < 0.01, and ***P < 0.001.
CCK-SAP Selectively Ablates Vagal Afferent Neurons of the Upper GI Tract
To determine whether CCK-SAP selectively ablates GI-VAN in vivo, we injected CCK-SAP (250 ng/µl) unilaterally into the left (n = 26) or right (n = 26) NG with SAP (250 ng/ml) injected contralaterally. This approach provides a control for the inherent variability of CTB labeling of terminals by comparing the amount of back-labeled VAN between treatment within animal. Furthermore, counterbalancing the side that received CCK-SAP between animals provided a control for the different innervation patterns of left and right NG (5). Intraperitoneal injection of fluorogold (20 µg/ml saline/rat) to nondiscriminately label fibers innervating abdominal organs (Fig. 2, A–C; n = 6) labeled 41.2 ± 1.3% of VAN in SAP-treated NG. CCK-SAP-treated NG significantly reduced fluorogold labeling 54.9 ± 6.2% (P = 0.005). These data indicate that CCK-SAP ablates a population of VAN innervating the abdomen.
Fig. 2.
CCK-SAP nodose ganglia (NG) injection is selective to vagal afferent neurons innervating the upper gastrointestinal tract. A–D: retrograde labeling of NG following intraperitoneal fluorogold injection (20 µg/ml saline/rat). A and B: stitched image of VAN innervating the abdomen (ip; fluorogold) after NG injection of SAP (A; 250 ng/µl; n = 6) or CCK-SAP (B; 250 ng/µl; n = 6). C: percent fluorogold-positive VAN is reduced in CCK-SAP-treated NG (**P < 0.0046; n = 6). D–G: retrograde labeling of NG following injection of Cholera toxin B 555 (CTB-555) into the wall of the stomach and small intestine. D: reduced CTB-positive VAN back-labeled from the stomach and small intestine in CCK-SAP-injected NG (****P < 0.0001; n = 8). E: 80% reduction in gastrointestinal (GI)-VAN irrespective of the side CCK-SAP was injected (n = 4). F and G: stitched image of VAN back labeled from the stomach and small intestine (arrows) in the same rat after NG injection of SAP (F; n = 8) or CCK-SAP (G; n = 8). H: quantification of non-CTB labeled VAN were not significantly different between SAP and CCK-SAP-treated nodose ganglia. I: quantification of retrograde labeling of NG from the colon was not significantly different between SAP- and CCK-SAP-treated nodose ganglia. J–L: retrograde labeling of NG following injection of CTB-555 into the lumen of the stomach to label VAN innervating the gastric mucosa. J: reduced CTB-positive VAN back labeled from the gastric mucosa in CCK-SAP-injected NG (**P < 0.002; n = 6). K and L: stitched image of VAN innervating the gastric mucosa after NG injection of SAP (K; n = 6) or CCK-SAP (L; n = 6).
To determine whether CCK-SAP selectively targets VAN innervating the gut, the retrograde tracer cholera toxin B conjugated to Alexa555 (CTB-555) was injected 7 days after NG surgery into the wall of the stomach and small intestine (Fig. 2, D–G; n = 8/group), lumen of the gut (Fig. 2, J–L; n = 6/group), or colon (Fig. 2I; n = 6/group). Gastric and small intestine CTB-555 labeled 20–40% of GI-VAN in SAP-treated NG (Fig. 2, D and F), while CCK-SAP reduced CTB-labeled VAN 80% (P < 0.0001; Fig. 2, D and G) irrespective of the side injected (Fig. 2E). Retrograde labeled VAN in SAP-treated NG exhibits a similar pattern of expression as previous studies, with no clear organotypic organization (2). The number of unlabeled VAN in SAP and CCK-SAP-treated animals was not statistically different (P = 0.61; Fig. 2H), indicating that CCK-SAP has limited impact on VAN innervating non-GI organs.
Retrograde labeling of luminal CTB-555 injections labeled 11.9 ± 0.7% of VAN from SAP-treated NG (Fig. 2, J and L). CCK-SAP reduced CTB-labeling 61.2 ± 7.0% (P = 0.002; Fig. 2, J and L), demonstrating that CCK-SAP ablates VAN innervating both mucosal and muscular layers of the gut. CCK-SAP did not significantly reduce retrograde labeling from the colon compared with SAP-injected VAN (3% reduction; P = 0.480; Fig. 2I). Together these data demonstrate that CCK-SAP injection in NG preferentially ablates vagal afferent neurons innervating the upper gut.
CCK-SAP Spares Vagal Efferent Neurons
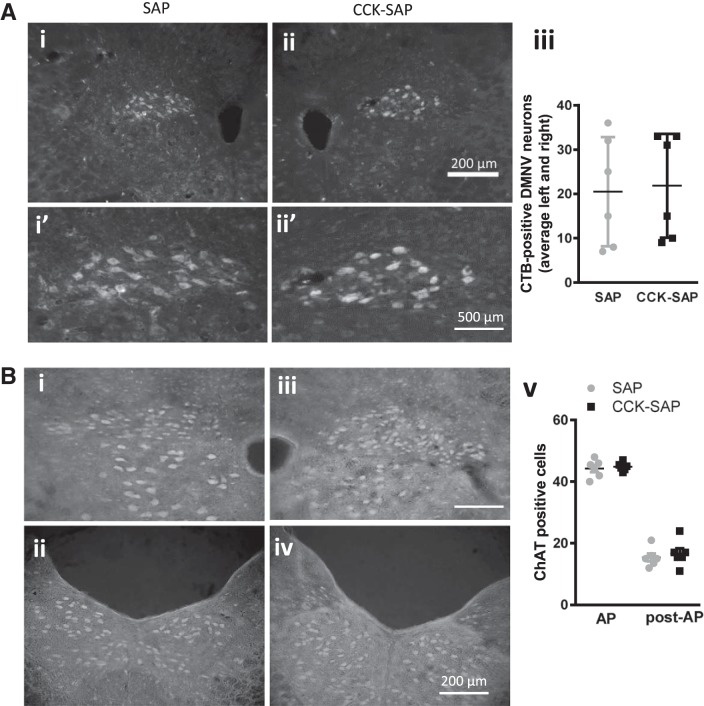
To determine the effect of NG injection of CCK-SAP on vagal efferent neurons innervating the gut, we quantified the number of gut-innervating vagal efferent neurons in the DMNV of rats after unilateral NG injection of SAP and contralateral CCK-SAP injection (Fig. 3A; n = 6). There was no observable or quantifiable reduction in CTB-555 labeling of vagal efferent neurons in the DMNV between the left and right sides (P = 0.85), indicating that vagal efferent neurons innervating the stomach and small intestine remain intact. Since retrograde tracing will only target a subset of vagal efferent neurons, we also stained the DMNV with a ChAT antibody to visualize the impact of CCK-SAP NG injection on all vagal efferent neurons. We found no statistical difference in vagal efferent neuron number at the level of the area postrema (AP) (between −13.56 and −14.04 mm from bregma; P = 0.66) or post-AP (14.2 to 14.6 mm from bregma; P = 0.57) in rats with bilateral injection of SAP or CCK-SAP (Fig. 3B; n = 5/group). Crucially, these data demonstrate that NG injection of CCK-SAP selectively ablates VAN over vagal efferent neurons.
Fig. 3.
CCK-SAP NG injection spare vagal efferent neurons. A: representative images of DMX from rats with NG injection of SAP (Ai: 250 ng/µl) or CCK-SAP (Aii; 250 ng/µl) labeled with retrograde tracer CTB-555 injection into the stomach and small intestine at ×10 magnification (Ai and Aii) and ×20 magnification (Ai′ and Aii′). Aiii: quantification showing no effect of CCK-SAP on vagal efferent neurons innervating the stomach and small intestine (P = 0.85). B: representative images of dorsal motor nucleus of the vagus (DMNV) of rats with NG injection of SAP (Ai and Aii; 250 ng/µl) or CCK-SAP (Aiii and Aiv; 250 ng/µl) stained with α-choline acetyltransferase (ChAT) antibody. Av: quantification showing no effect of CCK-SAP on ChAT neurons in the DMNV (P = 0.66).
CCK-SAP Blunts Postprandial NTS Activation
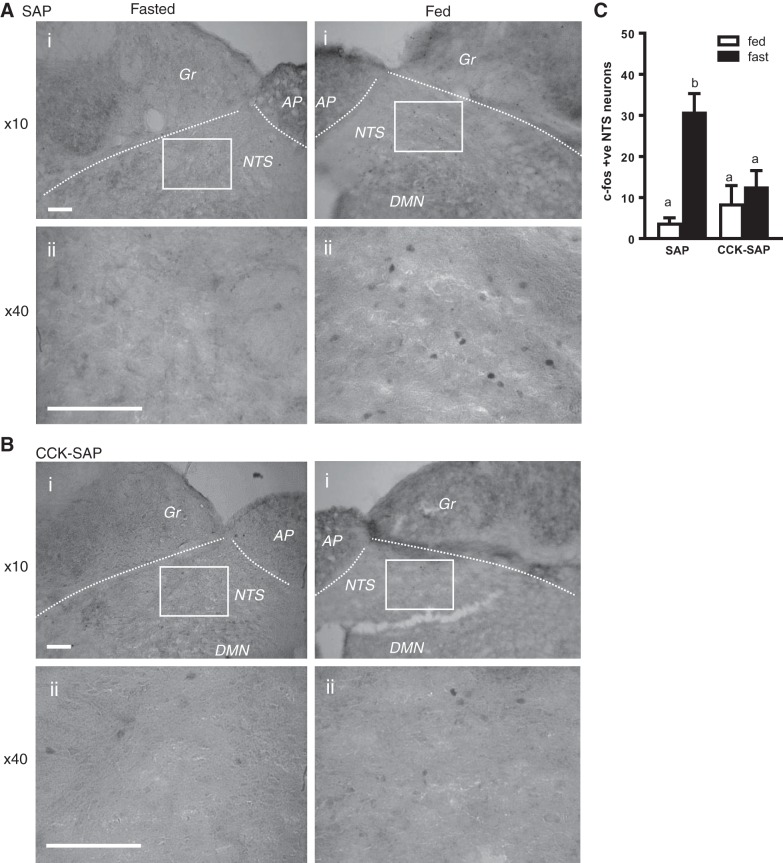
To functionally validate GI-VAN ablation in vivo, we injected CCK-SAP (250 ng/µl; n = 10) or SAP (250 ng/µl; n = 10) bilaterally into NG of rats and quantified meal-induced gut-brain signaling. There was no significant difference in food intake between groups re-fed after an overnight fast in wire bottom cages (P = 0.19). Using c-Fos as a marker of neuronal activity, we report that in SAP-treated control rats, feeding increased activation in neurons of NTS, the site of central vagal afferent termination (Fig. 4, A and C). Conversely, feeding failed to induce NTS activity in CCK-SAP-treated rats compared with fasting [nutritional state: F(1,16) = 22.12, P = 0.0002, injection: F(1,16) = 6.496, P = 0.021; interaction: F(1,16) = 13.63, P = 0.002; Fig. 4, B and C)] This provides evidence that CCK-SAP prevents a range of physiological cues to be communicated from the gut to the brain.
Fig. 4.
Feeding-induced neuronal activation and GI hormone response on feeding in SAP and CCK-SAP-injected rats. A and B: representative images of c-Fos staining in the medial NTS in SAP (A; 250 ng/µl)-treated and CCK-SAP (B; 250 ng/µl)-treated rats at ×10 (Ai and Bi) and ×40 (Aii and Bii) magnification. C: in the NTS c-Fos increases with 2-h refeeding in SAP-treated animals, but CCK-SAP blunts feeding induced c-Fos (n = 5). AP, area postrema; Gr, gracile nucleus; DMN, dorsal motor nucleus; NTS, nucleus tractus solitaries. Scale bar = 50 µm. a,bSignificant differences between group.
CCK-SAP Inhibits GI Hormone-Induced Satiation in Rats and Mice
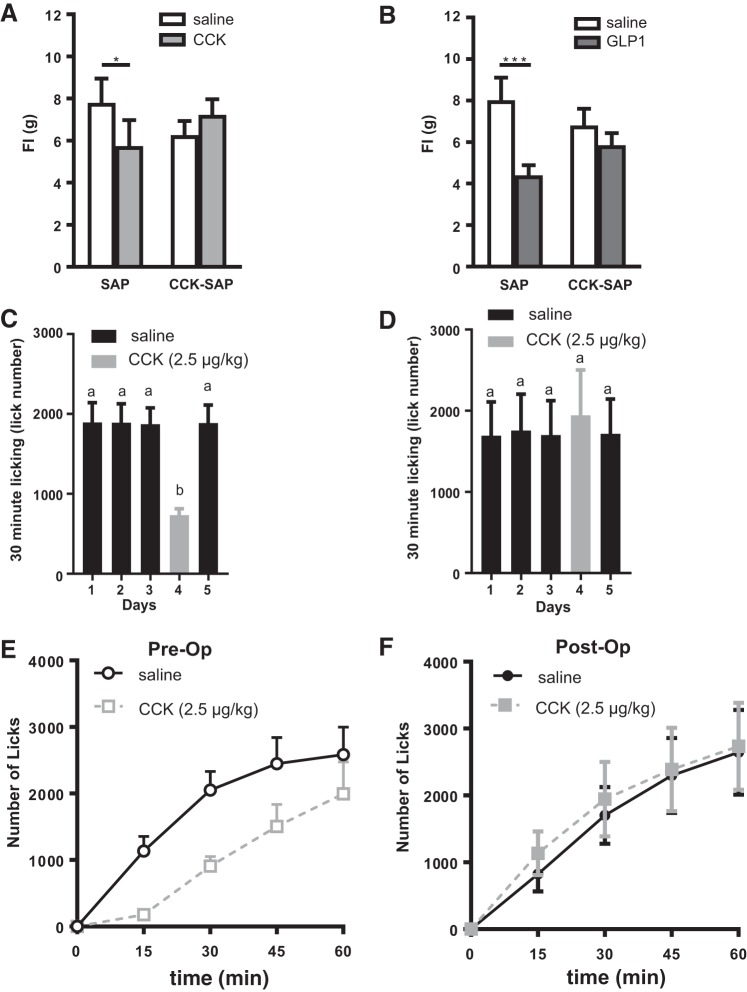
Exogenous administration of the gut satiety hormone CCK8S (2.5 μg/kg ip; Fig. 5A; n = 8) reduced 1-h food intake in rats with bilateral NG injection of SAP but not in rats with bilateral NG injection of CCK-SAP [interaction: F(1,13) = 6.591, P = 0.023]. In addition to blunting CCK-induced satiation, CCK-SAP treatment in rats also abolished satiation induced by exogenous GLP-1 [n = 6; 100 μg/kg ip; injection: F(1,18) = 8.85, P = 0.008; Fig. 5B]. These data confirm that CCK-SAP ablates chemosensitive vagal afferent neurons.
Fig. 5.
CCK-SAP NG injection inhibits GI hormone-induced satiation. A: intraperitoneal CCK significantly decreased food intake in rats treated with SAP (250 ng/µl; n = 7) compared with saline (P = 0.004), but CCK had no effect on satiation in rats treated with CCK-SAP (250 ng/µl; n = 8; *P = 0.15). B: after a short premeal, intraperitoneal glucagon like peptide-1 (GLP-1) significantly decreased food intake in SAP rats (***P = 0.0001; n = 5) compared with saline, but GLP-1 failed to reduce food intake in CCK-SAP rats (P = 0.13; n = 6). C–F: CCK satiation test in mice. C: upon reaching a baseline lick number, intraperitoneal CCK significantly decreased lick number in wild-type mice (n = 5, P = 0.006) and returned to baseline with saline treatment on following day. D: intraperitoneal CCK had no effect on satiation in mice treated with CCK-SAP (250 ng/µl; n = 5; P = 0.41). E: lick number over time is reduced in response to CCK preoperation. F: CCK-induced satiation is abolished postoperation. a,bSignificant differences between group.
To demonstrate feasibility of this approach to study the role of gut-brain signaling in mice, we performed CCK-induced satiation test in mice before and after bilateral NG injection of CCK-SAP (n = 5). Once a baseline lick rate was achieved, exogenous CCK administration (2.5 µg/kg ip) robustly reduced sipper lick number at 30 min in wild-type C57BL/6 mice (P = 0.006) (Fig. 5C). In the same mice, 1 wk after bilateral CCK-SAP injection into the NG, a similar baseline lick rate was achieved in response to saline; however CCK (2.5µg/kg ip) failed to reduce intake 30 min postinjection(Fig. 5D). Before the operation, mice reduced lick numbers at 15, 30 45, and 60 min (Fig. 5E). CCK had no effect on lick number at any time point postoperation (Fig. 5F). This provides a proof of principle that CCK-SAP injection into the NG can be used to block gut-brain signaling in multiple vertebrate models.
DISCUSSION
We provide multiple lines of evidence that bilateral injection of CCK-SAP into the NG is an effective method for selective deafferentation of the upper gut. CCK-SAP targets VAN that innervate the mucosa (chemosensitive) and muscular (mechanosensitive) layers of the upper GI tract without ablating vagal efferent neurons. NG injection of CCK-SAP severely blocks GI cues to the brain in response to a meal and the effect of humoral signals on feeding behavior in both rat and mouse models. Therefore, CCK-SAP injection into NG is a novel vagal deafferentation that significantly improves our ability to study gut to brain signaling. CCK-SAP injection into the NG is no more invasive than vagotomy, subdiaphragmatic deafferentation, or capsaicin but provides tissue specificity and superior separation between afferent and efferent signals than these traditional vagal-mediated treatments (Table 1).
Table 1.
Comparison of methods that target the vagus nerve
| Targets Vagal Afferent Neurons | Leaves Vagal Efferent Neurons Intact | Exclusively Targets the Vagus Nerve | Selectively Abolishes Gut-Brain Signaling | |
|---|---|---|---|---|
| Total subdiaphragmatic vagotomy | Yes | No | Yes | No |
| Capsaicin | Yes | No | No | No |
| Subdiaphragmatic deafferentation | Yes | Yes (50%) | Yes | No |
| NG shRNA viral injection | Yes | Yes | Yes | No |
| Mouse genetics (Nav1.8 Cre/Phox2b-Cre) | Yes | Yes | No | No |
| CCK-SAP | Yes | Yes | Yes | Yes |
NG, nodose ganglia; CCK, cholecystokinin; SAP, saporin.
In this study, we used SAP conjugated to the sulfated CCK carboxyl terminal octapeptide (CCK8S). Previous binding affinity studies indicate that CCK8S has high affinity for both CCKA and CCKB receptors and that conjugating SAP to CCK8S does not significantly alter binding (30, 50). In situ hybridization studies of NG indicate that 33% of VAN express the CCK-A receptor and 9% express the CCK-B receptor (9). The 36.9% ablation we observed in cultured VAN suggests that neurons expressing either form of the receptor are targeted for ablation. This effect is specific since SAP alone (data not shown) or SAP conjugated to leptin had no effect on the viability of cultured neurons. Importantly, leptin receptors are present on ~70% of NG neurons (14, 35) and VAN in culture are responsive to leptin (36). The absence of significant ablation after 24-h incubation of cultured cells with leptin-SAP is therefore not caused by a lack of leptin receptor expression. Instead, it is likely that SAP is not efficiently transported into the cells since leptin receptors are not internalized in a ligand-dependent manner (3). Conversely CCK receptors are internalized in response to ligand binding (40).
CCK-SAP causes neuronal damage and death as seen by the reduction in cultured neurons and the conspicuous neuronal shaped holes throughout the tissue in CCK-SAP-injected NG (Fig. 2G). This cell death is further substantiated in vivo by the 80% reduction in VAN back labeled from the stomach and small intestine. This was not due to the pressure injection procedure as pressure injection of unconjugated SAP into the NG caused little visible damage (Fig. 2, A and F). The consequence of this damage is reduced fibers associated with sensing both chemical and mechanical signals. We demonstrate anatomically that CCK-SAP-induced ablation inhibits retrograde tracing from both muscular and mucosal vagal afferent fibers and that bilateral NG injection of CCK-SAP blunts meal-induced activation of second order neurons in the NTS. Previous work has shown that CCK activates vagal mucosal fibers in the stomach and small intestine, and duodenal mechanosensitive vagal afferent fibers dose dependently respond to physiological doses of exogenous CCK administered intra-arterially (6), supporting the idea that CCK-SAP ablates both chemosensitive and mechanosensitive VAN. Importantly, the loss of vagal afferent signaling is achieved without altering vagal efferent neuron integrity. CCK-SAP injection in NG discriminately ablate vagal afferent cell bodies, while leaving intact the gut-innervating vagal efferent neurons that reside in the DMNV. Vagotomy and capsaicin treatment are subject to neuronal regeneration (38, 42). We have not directly tested the regeneration of CCK receptor-expressing neurons or vagal afferent innervation of the gut after CCK-SAP injection. However, we continue to observe blunted CCK response in rats 12 wk after surgery, suggesting that regeneration is incomplete at this time point. Histological studies will be required to validate this observation.
It is important to note that CCK-SAP will cause cell death of CCK receptor-expressing neurons rather than selectively knockdown CCK receptors in the neurons. Previous studies have observed extensive colocalization of Y2 receptor (11), GLP-1 receptor (49), vanilloid receptor 1 (16), leptin receptor (14), cannabinoid receptor 1 (13), ghrelin receptor 1a and 1b (16), melanin-concentrating hormone receptor 1 (15), and orexin receptor 1 (12) in VAN that express CCK-A receptors. This suggests that CCK-SAP ablation will inhibit a range of signals from the gut to the brain. In support of this, we report that in addition to the anticipated loss of CCK satiation, CCK-SAP deafferentation abolishes postprandial expression of the neuronal marker of activation c-Fos in NTS neurons. More specifically, we demonstrate that CCK-SAP-treated rats have blunted GLP-1 induced satiation, supporting recent findings indicating that CCK-A receptors extensively colocalize in VAN that encode GLP-1 receptor and that these neurons predominantly innervate the upper GI tract (49).
The most widely used methods to test the role of the vagus nerve in gut-brain signaling are vagotomy and capsaicin (Table 1). Subdiaphragmatic truncal vagotomy is the resection of both vagal trunks below the diaphragm resulting in the complete loss of vagal afferent and efferent signaling to all abdominal organs. This method is useful when attempting to determine a role of the vagus nerve but is not capable of distinguishing between afferent and efferent signaling. Capsaicin treatment is often referred to as a vagal deafferentation method; however, capsaicin only ablates transient receptor potential vanilloid 1 neurons (39), which make up ~70% of VAN. Furthermore, capsaicin treatment has been reported to damage vagal efferent neurons (10), and although topical application of capsaicin reduces the amount of off-target ablation, it is difficult to determine the extent of damage to other (nonvagal) sensory neurons. The gold standard to study vagal afferent signaling is subdiaphragmatic deafferentation surgery in which one of the subdiaphragmatic vagal trunks is severed along with the contralateral intracranial vagal afferent rootlets (34). The advantage of this approach is that it prevents all afferent signaling below the diaphragm, while leaving 50% of efferent fibers intact. However, the disadvantages of this approach are rarely discussed. The technical challenge of the surgery aside, this approach blocks afferent signals from most organs nonselectively (100% of signals from abdominal organs, and 50% from organs above the diaphragm). It remains unclear if vagal afferent fibers from different organs send competing signals to the brain; this type of nuanced effect would be missed with this approach. Furthermore, the loss of 50% of efferent signaling is likely to impact physiology. Finally, similar to vagotomy, circulating factors can still activate receptors expressed on vagal afferent cell bodies with intact connection to the hindbrain. Therefore, none of these three methods are ideal to study vagal afferent gut-brain signaling. CCK-SAP deafferentation resolves these issues by selectively ablating afferent neurons that innervate the upper gut and leaving efferent signaling intact. The main limitation of this approach is that it is not reversible. However this can be mitigated by testing animals before and after the surgery and/or having animals that receive unconjugated SAP injections as a control.
Verification
CCK satiation or feeding-induced c-Fos activation in the NTS provide effective functional tests to verify the loss of vagal afferent signaling. It is important to additionally include histological verification. Retrograde tracing from the wall of the gut provides optimal histological validation although intraperitoneal injection of fluorogold can also be used. Both of these tracing approaches allow validation of both deafferentation and sparing of efferent neurons of the DMNV. Since vagal efferent neurons were shown here to be spared in response to CCK-SAP injection in the NG, it may be possible in future to evaluate the effectiveness of the deafferentation by quantifying CCK receptor expression by quantitative PCR or in situ hybridization in NG. The advantage of combining a CCK satiation test (as opposed to c-Fos) with a post mortem histology test is that this can provide an indication of successful deafferentation before and after the experiment in each animal.
In conclusion, CCK-SAP injection into NG enhances the toolbox to study gut-to-brain signaling. The novel approach improves on previous methods by exclusively targeting afferent neurons that selectively target the upper gut. Vagal deafferentation of the gut can help define the importance of vagal afferents in communicating information about diet, microbiota, infection, and inflammation from the gut to the brain in a number of physiologic and pathophysiologic conditions (7, 8, 19, 22, 26, 27, 29, 37).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R00-DK-094871 and R21-DK-110511.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.d.L. conceived and designed research; G.d.L. drafted manuscript. C.D., D.Q., R.S., B.Z., S.A., A.P., and G.d.L. performed experiments; C.D., D.Q., R.S., B.Z., S.A., A.P., and G.d.L. analyzed data; C.D. and G.d.L. interpreted results of experiments; C.D., D.Q., B.Z., and G.d.L. prepared figures; C.D., D.Q., B.Z., and G.d.L. edited and revised manuscript; C.D., D.Q., R.S., B.Z., S.A., A.P., and G.d.L. approved final version of manuscript;
ACKNOWLEDGMENTS
We thank Myrtha Arnold for technical suggestions and Analise Rivero for help with quantification.
REFERENCES
- 1.Allen JW, Mantyh PW, Horais K, Tozier N, Rogers SD, Ghilardi JR, Cizkova D, Grafe MR, Richter P, Lappi DA, Yaksh TL. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci 91: 286–298, 2006. doi: 10.1093/toxsci/kfj143. [DOI] [PubMed] [Google Scholar]
- 2.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 3.Belouzard S, Delcroix D, Rouillé Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem 279: 28499–28508, 2004. doi: 10.1074/jbc.M400508200. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 6.Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst 31: 191–201, 1990. doi: 10.1016/0165-1838(90)90185-L. [DOI] [PubMed] [Google Scholar]
- 7.Boeckxstaens G. The clinical importance of the anti-inflammatory vagovagal reflex. Handb Clin Neurol 117: 119–134, 2013. doi: 10.1016/B978-0-444-53491-0.00011-0. [DOI] [PubMed] [Google Scholar]
- 8.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055, 2011. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903: 128–140, 2001. doi: 10.1016/S0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 10.Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 591: 1563–1580, 2013. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 28: 11583–11592, 2008. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, Saeed S, Grundy D, Varro A, Dimaline R, Dockray GJ. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology 124: 129–139, 2003. doi: 10.1053/gast.2003.50020. [DOI] [PubMed] [Google Scholar]
- 13.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24: 2708–2715, 2004. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience 109: 339–347, 2002. doi: 10.1016/S0306-4522(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 15.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience 137: 1405–1415, 2006. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 16.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290: G1289–G1297, 2006. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 17.Conte WL, Kamishina H, Reep RL. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat Protoc 4: 1157–1166, 2009. doi: 10.1038/nprot.2009.93. [DOI] [PubMed] [Google Scholar]
- 18.Corp ES, McQuade J, Moran TH, Smith GP. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res 623: 161–166, 1993. doi: 10.1016/0006-8993(93)90024-H. [DOI] [PubMed] [Google Scholar]
- 19.Daulatzai MA. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol Disord Drug Targets 14: 110–131, 2015. doi: 10.2174/1871527314666150202152436. [DOI] [PubMed] [Google Scholar]
- 20.Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol Gastrointest Liver Physiol 255: G55–G61, 1988. [DOI] [PubMed] [Google Scholar]
- 21.de Lartigue G. Putative roles of neuropeptides in vagal afferent signaling. Physiol Behav 136: 155–169, 2014. doi: 10.1016/j.physbeh.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lartigue G, Diepenbroek C. Novel developments in vagal afferent nutrient sensing and its role in energy homeostasis. Curr Opin Pharmacol 31: 38–43, 2016. doi: 10.1016/j.coph.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dockray GJ. The versatility of the vagus. Physiol Behav 97: 531–536, 2009. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Eiklid K, Olsnes S, Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res 126: 321–326, 1980. doi: 10.1016/0014-4827(80)90270-0. [DOI] [PubMed] [Google Scholar]
- 25.Ewart WR, Wingate DL. Cholecystokinin octapeptide and gastric mechanoreceptor activity in rat brain. Am J Physiol Gastrointest Liver Physiol 244: G613–G617, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817: 115–133, 2014. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 27.Gorky J, Schwaber J. The role of the gut-brain axis in alcohol use disorders. Prog Neuropsychopharmacol Biol Psychiatry 65: 234–241, 2016. doi: 10.1016/j.pnpbp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kentish SJ, O’Donnell TA, Isaacs NJ, Young RL, Li H, Harrington AM, Brierley SM, Wittert GA, Blackshaw LA, Page AJ. Gastric vagal afferent modulation by leptin is influenced by food intake status. J Physiol 591: 1921–1934, 2013. doi: 10.1113/jphysiol.2012.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol 11: 625–636, 2015. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 30.Lai J, Zhang W, Badghisi H, Hruby VJ, Porreca F. The biologically active cholecystokinin (26–33) peptide, [Tyr2-S03] CCK-8, retains high affinity for CCK2 receptors after covalent conjugation to saporin. Targeting Trends 7: 1, 2006. [Google Scholar]
- 31.Liddle RA. Cholecystokinin cells. Annu Rev Physiol 59: 221–242, 1997. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526: 95–102, 1990. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- 33.Ng TB, Wong JH, Wang H. Recent progress in research on ribosome inactivating proteins. Curr Protein Pept Sci 11: 37–53, 2010. doi: 10.2174/138920310790274662. [DOI] [PubMed] [Google Scholar]
- 34.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Peiser C, Springer J, Groneberg DA, McGregor GP, Fischer A, Lang RE. Leptin receptor expression in nodose ganglion cells projecting to the rat gastric fundus. Neurosci Lett 320: 41–44, 2002. doi: 10.1016/S0304-3940(02)00023-X. [DOI] [PubMed] [Google Scholar]
- 36.Peters JH, Ritter RC, Simasko SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 290: C427–C432, 2006. doi: 10.1152/ajpcell.00439.2005. [DOI] [PubMed] [Google Scholar]
- 37.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37: 984–995, 2015. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips RJ, Baronowsky EA, Powley TL. Long-term regeneration of abdominal vagus: efferents fail while afferents succeed. J Comp Neurol 455: 222–237, 2003. doi: 10.1002/cne.10470. [DOI] [PubMed] [Google Scholar]
- 39.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol 179: 155–171, 2007. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 40.Pohl M, Silvente-Poirot S, Pisegna JR, Tarasova NI, Wank SA. Ligand-induced internalization of cholecystokinin receptors. Demonstration of the importance of the carboxyl terminus for ligand-induced internalization of the rat cholecystokinin type B receptor but not the type A receptor. J Biol Chem 272: 18179–18184, 1997. doi: 10.1074/jbc.272.29.18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronveaux CC, de Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav 135: 222–229, 2014. doi: 10.1016/j.physbeh.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryu V, Gallaher Z, Czaja K. Plasticity of nodose ganglion neurons after capsaicin- and vagotomy-induced nerve damage in adult rats. Neuroscience 167: 1227–1238, 2010. doi: 10.1016/j.neuroscience.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 43.Santanché S, Bellelli A, Brunori M. The unusual stability of saporin, a candidate for the synthesis of immunotoxins. Biochem Biophys Res Commun 234: 129–132, 1997. doi: 10.1006/bbrc.1997.6597. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol 261: R64–R69, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Smith GP, Gibbs J, Jerome C, Pi-Sunyer FX, Kissileff HR, Thornton J. The satiety effect of cholecystokinin: a progress report. Peptides 2, Suppl 2: 57–59, 1981. doi: 10.1016/0196-9781(81)90011-5. [DOI] [PubMed] [Google Scholar]
- 46.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249: R638–R641, 1985. [DOI] [PubMed] [Google Scholar]
- 47.van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol 289: R695–R703, 2005. doi: 10.1152/ajpregu.00809.2004. [DOI] [PubMed] [Google Scholar]
- 48.Wiley RG, Lappi DA. Targeted toxins in pain. Adv Drug Deliv Rev 55: 1043–1054, 2003. doi: 10.1016/S0169-409X(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 49.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, Gardell S, Zhang D, Xie JY, Agnes RS, Badghisi H, Hruby VJ, Rance N, Ossipov MH, Vanderah TW, Porreca F, Lai J. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain 132: 778–787, 2009. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am J Physiol Gastrointest Liver Physiol 288: G1066–G1073, 2005. doi: 10.1152/ajpgi.00497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]