Abstract
Dclk1-expressing tuft cells constitute a unique intestinal epithelial lineage that is distinct from enterocytes, Paneth cells, goblet cells, and enteroendocrine cells. Tuft cells express taste-related receptors and distinct transcription factors and interact closely with the enteric nervous system, suggesting a chemosensory cell lineage. In addition, recent work has shown that tuft cells interact closely with cells of the immune system, with a critical role in the cellular regulatory network governing responses to luminal parasites. Importantly, ablation of tuft cells severely impairs epithelial proliferation and tissue regeneration after injury, implicating tuft cells in the modulation of epithelial stem/progenitor function. Finally, tuft cells expand during chronic inflammation and in preneoplastic tissues, suggesting a possible early role in inflammation-associated tumorigenesis. Hence, we outline and discuss emerging evidence that strongly supports tuft cells as key regulatory cells in the complex network of the intestinal microenvironment.
Keywords: cancer initiation, tuft cell, niche cell, inflammation, intestinal stem cells
the intestinal epithelium is functionally compartmentalized into multipotent intestinal stem cells (ISCs) residing predominantly near the bottom of the crypts (15), transit-amplifying (TA) and lineage-primed progenitor cells, and differentiated cells such as enterocytes, goblet cells, Paneth cells, enteroendocrine (EE) cells, and tuft cells (3, 89). While much attention has been drawn to the regulation and function of the former epithelial cells, only quite recently have intestinal tuft cells emerged as an anatomically and functionally distinct epithelial cell entity.
Tuft cells in the intestine are found both neighboring the ISC zone within the crypt and scattered upward along the crypt-villus axis. The majority of tuft cells within the intestinal epithelium uniquely express Dclk1. Their unique marker signature in combination with their intimate physical contact to enteric nerves suggests a likely chemosensory role within the intestinal epithelium (10, 112). Importantly, recent observations demonstrate that by sensing luminal contents, tuft cells are able to precisely modulate stromal immune responses (38, 57, 142). Such responses may ultimately regulate ISC differentiation and intestinal homeostasis. Furthermore, ablation of epithelial tuft cells severely impairs regeneration and survival following acute injury and inflammation (146), supporting a role for tuft cells in governing postinjury epithelial proliferation and remodeling. Interestingly, Dclk1-expressing tuft cells have been shown to expand dramatically in response to chronic inflammation, particularly in the early stages of carcinogenesis, which has led to the notion that they might also function as cancer-initiating cells (32, 50, 78, 79).
Furthermore, given their unique appearance and cellular properties, it is becoming increasingly apparent that tuft cells likely serve an important role within the gastrointestinal (GI) niche. While the specific definition of niches in the GI tract, as well as the precise cellular composition of niches in general, remain both challenging and context-dependent, the best studied is certainly the ISC niche. In the intestine, stem cell characteristics and fate are determined by niche cells in close spatial proximity to the stem cells (125). These niche signals include paracrine epithelial signaling as well as mesenchymal signaling, originating from neighboring endothelial cells (vascular and lymphatic endothelium), pericytes, fibroblasts, neurons, microbiota, myofibroblasts, and immune cells (108, 128). This has also been referred to as the dual stem cell niche concept, which hypothesizes a role for both specialized surrounding epithelial cells (i.e., epithelial niche) and a nonspecialized niche with regional or systemic signaling predominantly involving mesenchymal cell types (i.e., mesenchymal niche) in the surrounding stroma (23). Hence, tuft cells would appear to be mostly a specialized niche cell for ISCs (23), but given their anatomical distribution along the intestinal crypt-villus axis and interactions with other cell types, tuft cells may also orchestrate regional or systemic niche signals. The exact definition of tuft cells, however, and their importance for intestinal homeostasis, are currently a matter of controversy. Thus we aim here to first define carefully tuft cells in light of recently emerging evidence, which clearly implicates an important role of tuft cells within the intestinal niche.
Tuft Cells Represent Unique Intestinal Epithelial Cells
Tuft cells differ both anatomically and functionally from the other better-characterized epithelial cell lineages (37). Detailed descriptions of the morphological appearance of tuft cells date back to the 1950s and have been reviewed in detail by Sato et al. (112, 113). Characteristically, tuft cells bear prominent long, blunt microvilli at the luminal surface, along with numerous vesicles in the apical cytoplasm, with microtubules and a tubulovesicular system along the long axis of the cell to the basal portion. In the alimentary tract, they exhibit a close proximity to nerve terminals, which are most likely efferent in nature (86, 112) (Fig. 1). During embryogenesis, tuft cells appear to arise in a distal to proximal gradient along the gastrointestinal tract, concurrent with its embryonic development. Thus, while from embroynic day E16.5 onwards tuft cells are found evenly distributed throughout intestinal crypts and villi, they are less abundant in the gastric mucosa at this time point. Beginning only at embroynic day E16.5, tuft cells first appear in the distal stomach and reach the adult distribution pattern throughout the stomach epithelium by postnatal week 3 (13, 37, 110). In the adult mouse, tuft cells have been detected at all levels of the gastrointestinal villus (19) and throughout the digestive tract (Fig. 2), including the pancreas, bile ducts, gall bladder, and liver. Moreover, they are present in many, if not most, epithelia of hollow organs, including the parotid and submandibular glands and the entire respiratory tract (37, 59, 103, 112, 113, 146). Of note, similar to the common bile and pancreatic ducts (112), the so-called limiting ridge or gastric cardia, the boundary between murine forestomach and oxyntic mucosa of gastric corpus, shows an abundance of tuft cells (48), consistent with a highly proliferative progenitor cell rich zone (Fig. 2), suggestive of a specific function for tuft cells in this region.
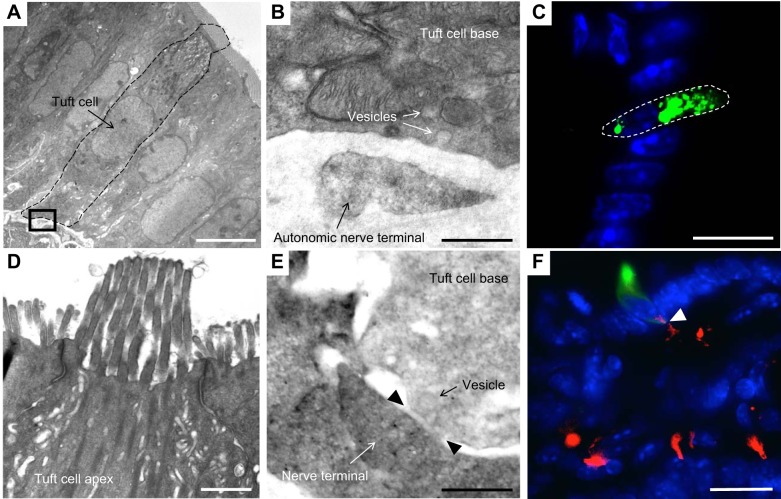
Fig. 1.
The ultrastructure of tuft cells and close interactions with neighboring neurons. A–D: electron-microscopic pictures of tuft cells and neighboring nerve terminals. The tissue was fixed with glutaraldehyde-formaldehyde and further processed specifically for immunoelectron microscopy. A: complete tuft cell body (dashed line) with adjacent, basolateral nerve terminal; bar graph = 5 µm. B: inset of A, vesicles in the tuft cell appear to be directed to and fuse with its basolateral membrane, which is opposed toward a characteristic autonomic nerve terminal; bar graph = 0.5 µm. C: tuft cell apex with tubule vesicles, microvilli, and elegant tight junctions; bar graph = 0.5 µm. D: tuft cell base of the same tuft cell as shown in C, demonstrating close proximity to a nerve terminal with formation of a junction containing typical synaptic cleft material and a vesicle in close proximity; bar graph = 0.5 µm. E: tuft cell labeled by ZSgreen in a Dclk1-DTR-ZSgreen reporter mouse; DCLK1 protein localization usually appears in the cytoplasmic area close to the apex of a tuft cell; bar graph = 25 µm. F: intestinal villus of an induced Wnt1-Cre; Rosa-TomatoRed mouse, which labels neural crest-derived cells and thus enteric neurons; demonstrating a close contact between epithelial tuft cell (stained with anti-DCLK1; green) and the stromal Wnt1 lineage (red); bar graph = 25 µm.
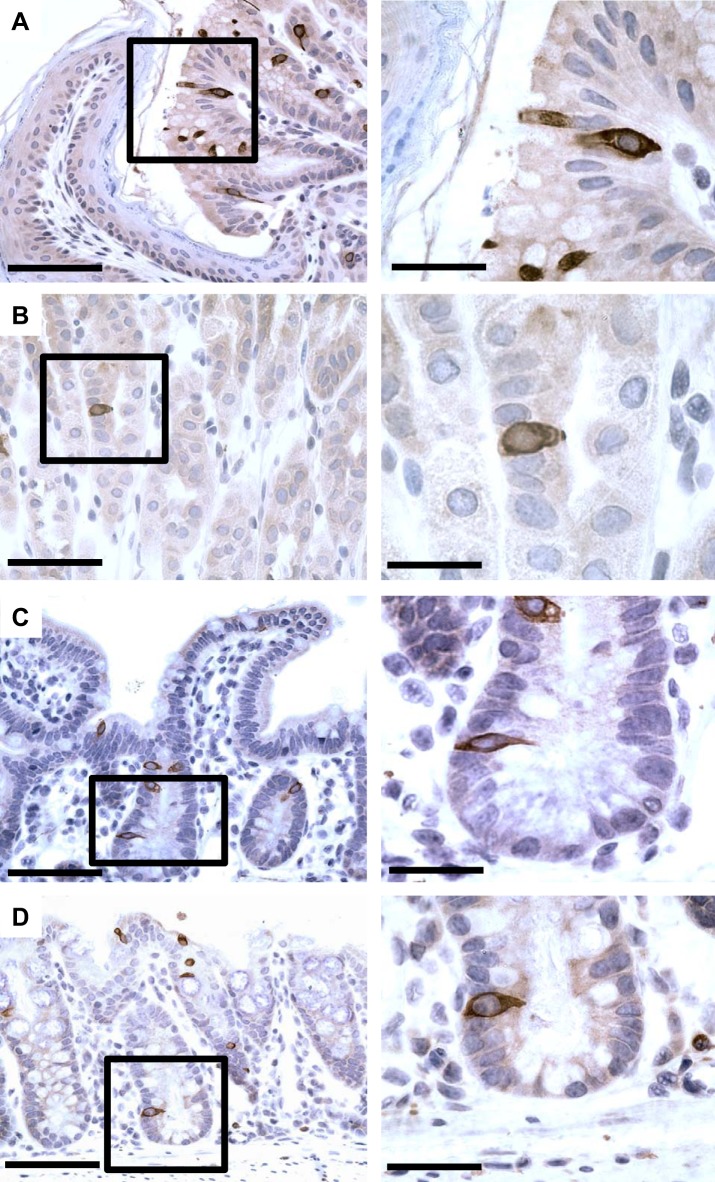
Fig. 2.
Tuft cell distribution and location within the gastrointestinal tract. Immunohistochemical staining for doublecortin-like kinase 1 protein (DCLK1; antibody 31704; Abcam) in the stomach and small and large intestine. Interestingly, the squamocolumnar junction between stomach and esophagus bears abundant tuft cells (A), which are less abundant in the corpus of the stomach mucosa (B). C: characteristically, tuft cells are found near +4 position in the crypts of small intestine as well as scattered along the crypt-villus axis. D: similarly, a scattered distribution of tuft cells can be found along colonic crypt epithelium. Bars at left are lower magnifications (×20) = 25 µm, and bars at right and insets are at higher magnifications (×40) = 12.5 µm.
While tuft cells have been recognized morphologically for decades, functional analysis has been limited by the absence of genetic tools and definitive markers. For example, tuft cells are known to be positive for villin and fimbrin, but these markers are not tuft-cell specific (116, 130). In 2006, DCAMKL-1 was proposed to label putative “epithelial progenitor” cells that were located adjacent to the TA cell population in the intestine (42). Indeed, in both small intestine and colon crypts, DCAMKL-1-expressing cells were often found near the +4 cell position, which is believed to harbor intestinal stem and progenitor cells (101) (Fig. 2). Studies from the laboratory of Houchen and colleagues (36) initially raised the possibility that DCAMKL-1-expressing cells might represent putative gastrointestinal and adenoma stem cells (78). Gerbe et al., however, were the first to show that doublecortin-like kinase 1 protein (DCLK1, previously referred to as DCAMKL-1) robustly marked differentiated tuft cells in the small intestine and colon epithelium. Dclk1 encodes a microtubule-associated protein with a COOH-terminal serine-threonine kinase domain, for which there exist at least three major splice variants (DCLK, DCLK DCX-like, and CPG16), with altered kinase activity and differential splicing of DCLK1 in embryonic compared with adult tissue (17). Interestingly, the embryonic forms DCLK and DCX-like are expressed in populations of migrating and postmitotic neurons that also express doublecortin (DCX) and in radial glia cells, which are multipotent neural progenitors (71, 143). In the developing mammalian brain, DCLK1 is also highly expressed in regions of active neurogenesis, particularly in the neocortex (in the SVZ/VZ) and cerebellum, but its expression is drastically diminished in adults (121). Long and short isoforms of DCLK1 (DCLK1-L and DCLK1-S) have been reported with important differences between the isoforms in both human and mouse tissues. In the context of human neoplasia, hypermethylation of DCLK1-L appears to cause a predominant switch to the short isoform, which confers a more invasive tumor phenotype (124). Thus the DCLK1 isoforms likely have distinct functions, which may be regulated through epigenetic silencing, orchestrated in part by β-catenin and NF-κB signaling pathways (90). Furthermore, expression of different DCLK1 isoforms may occur in different cellular compartments, which may not all represent tuft cells.
In 2008, Bezencon and colleagues (10) published the first gene expression signature for intestinal tuft cells from sorted Trpm5-eGFP-expressing cells, indicating that acetylated tubulins, α-gustducin, Trpm5, Ptgs1, and Ptgs2 were all relatively specific for intestinal tuft cells. Acetylated-α-tubulins are part of microtubule bundles that are abundant in tuft cells (39, 110, 146). α-Gustducin represents a taste cell-specific GTP-binding protein (54, 112), which in tuft cells appears to activate the nonselective cation channel Trpm5 downstream for sensation of luminal content (see below) (10, 13, 57, 118). Ptgs1 and Ptgs2 encode cyclooxygenase (COX)1 and COX2, which are enzymes well known to catalyze the formation of prostaglandins (e.g., PGE2) and are important targets for anti-inflammatory drugs (10, 28, 37, 142). Recently, choline acetyltransferase (ChAT), a rate-limiting enzyme in the production of acetylcholine, was shown to be expressed specifically in intestinal tuft cells (118) (see details in Table 1). In addition, Bjerknes and colleagues (13) used the tuft cell-specific marker Gfi1b to demonstrate that tuft cells differentiate during their upward migration along the crypt-villus axis, with weak DCLK1 staining near the crypt base but strong Dclk1 expression in the upper crypt compartment. Of note, in Dclk1-Cre-GFP mice, not all Dclk1-GFP-expressing cells are positive for DCLK1 protein by immunohistochemistry, although the cells appear morphologically identical. This discrepancy reinforces the overall conclusion of considerable heterogeneity within the tuft cell lineage. Despite the expression of some neural markers (e.g., DCLK1 and ChAT), intestinal tuft cells are clearly epithelial cells based on their consistent expression of both E-cadherin and cytokeratin-18 (10, 39, 53, 118) and their anatomical location within the epithelial cell monolayer (Fig. 2). Taken together, the expanding toolbox of tuft cell markers has in recent studies facilitated tuft cell identification, providing greater insight into their physiological roles in the gut. Nevertheless, we would recommend some caution and that the combination of two or more markers, or one marker with morphologic analysis, might represent a more rigorous standard. In any case, the expression of these diverse markers clearly identifies tuft cells as a distinct intestinal epithelial lineage, raising important questions regarding its function within the gastrointestinal tract.
Table 1.
Summary of characteristic tuft cell markers, defining their origin, sensing, and signaling functions in the niche
| Marker | Acronym | Function |
|---|---|---|
| Structural | ||
| Acetylated tubulin | Ac-tub | Posttranslationally modified form of tubulins; acetylation occurs in a tissue-specific manner and confers functional changes in tubulins (56) |
| Growth factor independent 1B transcription repressor | Gfi1b | TF, transcriptional repressor, important during hematopoietic development (13) |
| POU Class 2 homeobox 3 | Pou2f3 | TF, necessary for development of sweet, bitter, and umami taste cells (38, 76) |
| Sensing | ||
| α-Gustducin | Gα gust | GTP-binding subunit of heterotrimeric GPCR (54), triggering intracellular PLC2-IP3 cascade and Ca2+ release; sensing bitter, sweet (133), and eventually umami (51) |
| Transient receptor potential channel 5 | Trpm5 | Nonselective cation channel, downstream of taste-related GPCR signaling, activated by cytosolic Ca2+ increases and causing depolarization of the cell (70) |
| Signaling | ||
| Cyclooxygenase 1 | Ptgs1 | Enzyme catalyzing reduction of arachidonic acid to PGG2 and PGH2 (source of important downstream prostaglandins); homodimer; wide distribution, constitutively expressed (106) |
| Cyclooxygenase 2 | Ptgs2 | Enzyme similar to COX-1 but restrictedly expressed upon inflammatory and proliferative stimuli; eventually altered oxygenase activities (106) |
| Interleukin-25 | IL-25 (or IL-17E) | Member of IL-17 family; induces IL-4, IL-5, and IL-13 gene expression (30); induces ILC2 during helminth infection; only epithelial intestinal source are tuft cells (38, 57, 142) |
| Choline acetyltransferase | ChAT | Rate-limiting enzyme in the production of acetylcholine, the main cholinergic neurotransmitter (91) |
TF, transcription factor; GPCR, G protein-coupled receptors; COX, cyclooxygenase; IP3, inositol triphosphate.
Tuft Cells Are Not Stem Cells
Despite the demonstration that DCLK1 colocalized with other tuft cell markers, several studies suggested a potential contribution of Dclk1-expressing cells to the GI stem or progenitor cell pool. Dclk1-expressing cells were occasionally found in the +4 region and often colocalized in the intestinal crypts with the stem cell marker Musashi-1 (58, 78, 96). Since they rarely showed evidence of proliferation, it was proposed that DCLK1 might label a subset of quiescent ISCs (34). Many of their features were consistent with label-retaining quiescent epithelial cells, which were thought to comprise a subpopulation of Lgr5-expressing, lineage-committed progenitor cells, with the potential to acquire clonogenic potential in response to injury (16). This pool of “reserve stem cells” in the intestine appears to be heterogeneous and thus includes both quiescent cells, as well as terminally differentiated and lineage-committed progenitor cells (69).
Lineage tracing, commonly used to examine the in vivo stemness of specific cell types (4, 65, 123, 128), has been used to address the self-renewal properties of Dclk1-expressing tuft cells. Nakanishi et al. (88) were the first to report lineage tracing of Dclk1-expressing cells using a Dclk1-CreER knockin allele. Upon tamoxifen induction, they found scattered Dclk1-expressing epithelial cells, which subsequently migrated upwards along the crypt-villus axis, with decreased numbers over time (88). Furthermore, bromodeoxyuridine (BrdU)-labeling studies showed a complete absence of proliferation in Dclk1-expressing cells. Using BAC transgenic Dclk1-CreERT mice, our group similarly observed that the vast majority of Dclk1-expressing cells turned over within 7–10 days. However, we also found a subset (e.g., 5%) of Dclk1-expressing cells that were long lived and remained labeled up to 18 mo after tamoxifen induction (146). In line with a previous report (42), we observed no labeling with short-term (< 2 wk) BrdU treatment, while long-term BrdU treatment only rarely labeled tuft cells, indicating that this subset of Dclk1-expressing cells may indeed divide, albeit infrequently. While these observations are consistent with the known heterogeneity of Dclk1-expressing epithelial cells, the BrdU-positive Dclk1-expressing cells we observed resided predominantly near the crypt base, somewhat reminiscent of the label-retaining cells described above. Thus, this subset of long-lived Dclk1-expressing crypt cells could represent a subpopulation of reserve progenitors, originating from rapidly cycling or quiescent stem cells. However, since we did not observe robust lineage tracing under either resting conditions or with injury, similar to Nakanishi et al. (88), we conclude that epithelial Dclk1-expressing tuft cells do not represent active nor quiescent epithelial stem cells, with the vast majority comprising differentiated, postmitotic cells.
Tuft Cells Represent a Fifth Epithelial Cell Lineage
Analogous to other epithelial lineages, tuft cells derive from ISCs, both under resting conditions as well as in response to injury (142, 146). Gerbe et al. (39) initially concluded that tuft cells belonged primarily to a secretory lineage based on their findings that epithelial-specific deletion of Atoh1, a transcription factor (TF) downstream of the Notch pathway known to direct a secretory cell fate, caused an absence of tuft cells. In contrast to this finding, other studies reported on the presence of Dclk1-expressing cells in the setting of whole body Atoh1 deletion (13, 139) and also demonstrated that pharmacologic Notch inhibition led to a dramatic increase in tuft cells after Atoh1 deletion (13). Given that tuft cells probably originate at least in part from Lgr5-expressing stem cells (39), we analyzed Lgr5-CreERT2; Atoh1F/F mice and concluded that tuft cells develop largely independent of Atoh1 (146). However, a major limitation of these analyses is that, apart from the mosaicism of inducible mouse models, some tuft cells may originate outside the Lgr5-positive lineage (10, 13).
Growing evidence suggests that tuft cells differ in important ways from other presumptive secretory cell lineages. Bjerknes et al. (13) nicely demonstrated that tuft cells near the +4 cell position in the crypt expressing the tuft cell transcription factor Gfi1b are more often positive for proliferation markers and resemble “newborn” cells, with continuing differentiation and migration along the crypt-villus axis, gradually increasing their expression of Dclk1 while losing their proliferative potential, congruent with our observations of BrdU-positive Dclk1-expressing cells near the crypt base. Interestingly, there is also reported overlap of DCLK1 with EE cell markers (13, 34). Unlike EE cells, though, the presence of tuft cells is not dependent on the TF Neurog3 (39), and in contrast to Paneth and goblet cells, not dependent on the TF Sox9, Gfi1, or SPDEF for their development (39). The only transcription factor tuft cells appear to be highly dependent on is Pou2f3 (38), which has previously been shown to be required for the differentiation of taste receptor cells as well as Trpm5-expressing chemosensory cells (38). A recent study used single-cell RNA sequencing to analyze the heterogeneity of sorted crypt Bmi1-GFP-expressing epithelial cells that were presumed to mark a quiescent ISC population but in fact identified five distinct subsets of fully mature EE cells that differed in their expression of various EE developmental TFs secretory products. Some clusters of Bmi1-GFP-expressing cells expressed Prox1, a putative TF for EE lineage specification. Single-cell analysis of crypt Prox1-positive cells using a Prox1-GFP transgenic strain yielded distinct clusters of EE cells and a highly specific cluster expressing Dclk1, Trpm5, Ptgs1, Rgs13, and Nrgn (154), which resembled the microarray gene expression profile of sorted Trpm5-eGFP-positive cells reported by Bezencon et al. (10), consistent with a tuft cell population. A virtual lineage hierarchy, constructed using a computational lineage inference algorithm from the Bmi1-GFP and Prox1-GFP single-cell profiling data, supported the close lineage relationship between the tuft cell and diverse subsets of EE cells, which are indeed the most heterogeneous of all the epithelial cell lineages. In fact, the tuft cell cluster profile was much more closely related to EE than Paneth/goblet cells. Intriguingly, these Prox1/Dclk1-positive tuft cells also concurrently expressed modest levels of Lgr5 and the master transcription factor Ascl2 that enables Lgr5-positive ISC fate. However, they did not cluster with the Lgr5-eGFP-positive sorted population from Lgr5-eGFP-IRES-CreER mice nor did they express the crypt base columnar-associated surrogate marker Olfm4 mRNA. Thus single-cell transcriptomics reveal that tuft cells are quite distinct from Lgr5-expressing ISCs and other epithelial cellular subsets consistent with the hypothesis that tuft cells are indeed a distinct lineage.
Tuft Cells Are Chemosensory Cells in the Gut
Accumulating evidence suggests that tuft cells are indeed part of a diffuse chemosensory system, composed of solitary chemosensory cells along the respiratory and Gl tract, which function in the regulation of local homeostasis and postprandial chemosensation (95, 115, 142). For instance, pulmonary tuft cells sense hypoxia and regulate capillary resistance and perfusion during regeneration of injured lung tissue (115). Recent studies have elucidated the role of the taste cell-specific GTP-binding protein α-gustducin and the cation channel transient receptor potential melastatin subtype 5 (TRPM5) in tuft cells (10, 54, 112, 118) (Table 1). In the oral cavity, specific tastes activate G protein-coupled receptors, which trigger an intracellular Ca2+ increase that subsequently opens TRPM5 channels, causing depolarization of the taste receptor cell and thus taste sensation (67, 152). TRPM5 is essential for transduction of bitter, sweet, and umami tastes (70). Furthermore, TRPM5 has been functionally implicated in the sensation of smell, temperature, and mechanical forces, and together with α-gustducin represents the characteristic expression profile of solitary chemosensory cells (115). Strikingly, TRPM5-expressing tuft cells in murine colon are indeed activated upon luminal stimulation with bitter substrate, confirming their importance in chemosensory sensing (118) (see Fig. 4). TRPM5 receptors are completely blocked by acidic pH, but the ingestion of food leads to significant buffering effects, which are more pronounced in the proximal stomach (122). Thus, similar to G cells in the gastric antrum, the abundance of tuft cells in the cardia of the stomach might represent an early gastric regulatory mechanism designed to sense alterations in pH, nutrients or microbiota, thus registering key features of the luminal contents (48). Strikingly, both α-gustducin and TRPM5 have recently been found to be essential for sensing intestinal luminal microbiota (see below) (57). The sensing of luminal helminth infection induces an epithelial response, predominantly triggered by activation of tuft cells and release of IL-25, leading to the activation of stromal innate lymphoid cells type 2 (ILC2) and IL-13 secretion (38, 142). Nevertheless, the exact luminal contents that are recognized by Dclk1-expressing tuft cells remain to be defined but will be important to understand the maintenance of gut homeostasis.
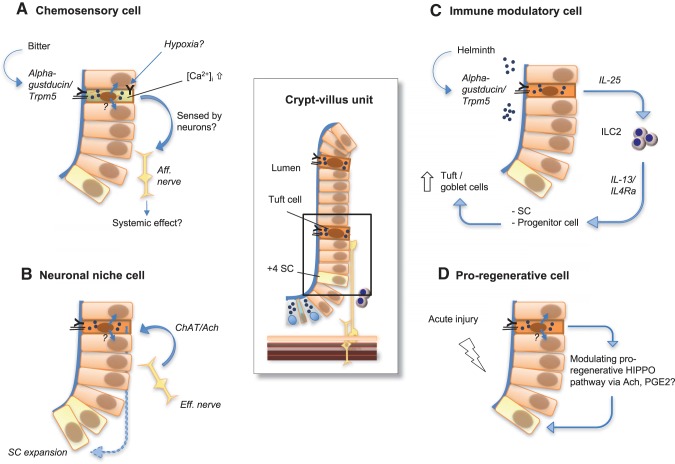
Fig. 4.
Tuft cells are key regulatory intestinal niche cells orchestrating both sensing and signaling. In the enlargements, we outline the 4 major roles of tuft cells within the epithelium elucidated to date: A: α-gustducin and transient receptor potential melastatin subtype 6 (TRPM6) receptors have been shown to enable tuft cells to sense bitter substance. This reportedly causes tuft cell activation (i.e., intracellular Ca2+ influx), with the further transmission of this signal remaining obscure, but eventually being forwarded to a neighboring epithelial cell via recently described cytospinules or afferent ( = aff.) nerve terminal. Also, additional stimuli (such as hypoxia) might activate similar sensing in tuft cells. B: locally, tuft cell survival depends on neuronal stimuli as well as epithelial proliferation depends on the presence of tuft cells to integrate this signal. This might be reflected by their expression of choline acetyltransferase (ChAT), which catalyzes the production of acetylcholine (Ach), which might then signal in a paracrine fashion to promote proliferation in the epithelial stem (SC) or progenitor cell compartment. C: additionally, upon luminal helminth infection sensed via α-gustducin and TRPM5 receptors, tuft cells release IL-25 to actively modulate innate lymphoid cells type 2 (ILC2), which in turn promote goblet and tuft cell expansion by IL-13/IL-4Ra signaling on epithelial stem or progenitor cells. D: ultimately, tuft cells appear to be essential for regeneration during acute tissue injury. This might involve their use of local paracrine signaling (such as Ach and PGE2) to create a noncrypt-base stem cell environment or to modulate the proregenerative Hippo pathway in ISC populations.
The Cross Talk Between Tuft Cells and Neurons
Studies from our group and others have also suggested a close relationship between tuft cells and nerves (10, 50, 118, 146). Using transgenic Dclk1-CreERT mice and analyzing electron microscopic images of tuft cells (Fig. 1), we frequently observed direct cell-to-cell contact between epithelial Dclk1-expressing tuft cells and neuronal structures in the lamina propria emanating from the submucosa, thereby forming a connection between tuft cells and enteric ganglia within the stomach, small intestine, and colon. In addition, we observed the presence of numerous synaptic vesicles near the basolateral membrane that appeared to be emerging from tuft cells and directed toward the adjacent autonomic nerve terminal. The combination of tuft cells and nerve terminals appeared roughly equivalent to a “directed synapse,” further evident by a narrow cleft separating tuft cell and nerve terminal containing material characteristic of such a synapse (Fig. 1). Bezencon et al. (10) provided additional evidence of an abundance of afferent and efferent synaptic molecules present in tuft cells. Furthermore, they showed a close anatomical relationship between nerve endings and tuft cells by eGFP and PGP9.5 costainings, the latter specifically labeling neurons (10). This anatomic proximity to neural structures supports a potential sensing function, as this may represent a sensing apparatus analogous to that of hair cells of the cochleovestibular organ or pulmonary neuroendocrine cells, both of which have been shown to sense and transmit signals to neighboring nerve fibers (14, 20, 31).
The enteric nervous system (ENS) physiologically regulates gut motility, as well as gut vascularization, and epithelial proliferation (21, 41, 46, 74, 75). While the ENS clearly modulates responses to injury, this function was thought to occur in a transepithelial manner, given the absence of nerve fibers entering the gastrointestinal lumen (40). Interestingly, coculture of epithelial colonic organoids with wild-type neurons led to an increase in both the number and size of the resulting organoids. Ablation of Dclk1-expressing tuft cells abrogated this stimulatory effect mediated by nerves, thus demonstrating a functional interaction between nerves and tuft cells, and arguing for a role of tuft cells as a signal transducer between nerves and epithelium (146) (see Fig. 4). Additionally, in denervated tissue, such as human intestinal transplants, Ret−/− mice, or vagotomized mouse stomach, a reduction in Dclk1-expressing tuft cell number could be observed, arguing that nerves in some way support the growth or survival of tuft cells, either through direct interactions or through effects on stem cell differentiation. Interestingly, the latter scenario has been supported by recent in vitro studies. In organoid cultures, enteric neurons are able to substitute for Wnt, most likely through stimulation of epithelial stem cells (94, 155). Furthermore, following vagotomy in the stomach, we observed persistent downregulation of both Wnt and Notch signaling, which correlated with a reduction in Lgr5-expressing gastric stem cells and Dclk1-expressing cells. Interestingly, these effects were mediated largely through stimulation of the muscarinic receptor 3 in Lgr5-expressing stem cells (155). The importance of cholinergic signaling in tuft cell regulation has recently been further supported by studies in EGFPChAT mice, which demonstrated in tuft cells the expression of ChAT, a rate-limiting enzyme in the production of the cholinergic agonist acetylcholine (118). Tuft cells have been reported to lack both vesicular acetylcholine transporter and high-affinity choline transporter, which are needed for synaptic release of acetylcholine. Nevertheless, while the mechanism for putative acetylcholine secretion from tuft cells requires further investigation, collectively these observations underscore tuft cells as central to neuronal-epithelial cross talk.
Intestinal Regeneration Requires Tuft Cells
Over 30 years ago, alveolar brush (or tuft) cells were reported to respond to tissue injury in interstitial pneumonitis (116). Since then, epithelial Dclk1 expression has been found to be highly important for homeostasis and tissue regeneration (32), and knocking out epithelial Dclk1 compromises intestinal barrier function after whole body irradiation injury (77) and exacerbates tissue injury in a mouse model of DSS-induced colitis (99). Following ablation of Dclk1-expressing tuft cells using Dclk1-CreERT; R26-iDTR mice, we observed a significant reduction in proliferating epithelial cells, but otherwise the absence of tuft cells appeared to be tolerated well under basal conditions. However, when mice were challenged with severe injury, such as whole body irradiation or chemically induced colitis, the absence of Dclk1-expressing tuft cells greatly impaired epithelial regeneration and led to significantly increased morbidity and mortality (146). Whether this is due to a deficiency in critical tuft cell-derived niche factors, or to some other contribution to the regenerative program by Dclk1-expressing epithelial cells, remains to be elucidated. Interestingly, since tuft cells express Cox2, one area of deficiency might be the reduction in secreted epithelial PGE2, which has recently been shown to govern intestinal epithelial regeneration (81). Furthermore, we found that the reduction in epithelial proliferation after tuft cell ablation could be rescued by simultaneous administration of a cholinergic agonist, suggesting that tuft cell-derived acetylcholine might represent another critical niche factor (50).
While the evidence suggests that tuft cells are important to the stem cell niche in regenerative states, it has yet to be established whether there is direct communication between tuft cells and gastrointestinal stem cells or whether tuft cells mediate responses indirectly via stromal cells. In theory, the absence of the sensing abilities of tuft cells could abrogate stromal signaling loops needed to guide regeneration. Along these lines, tuft cells produce the cytokine IL-17E, also known as IL-25 (10). Von Moltke et al. (142) reported a positive feedback mechanism, where upon luminal helminth infection, tuft cell-derived IL-25 activated lamina propria ILC2, which in turn promoted tuft and goblet cell differentiation in epithelial progenitors through IL-13/IL-4Rα signaling (see Fig. 4), thus altering the balance of Notch signaling. Two other studies independently defined tuft cells as the predominant epithelial source of IL-25 and highlighted their importance in initiating type 2 immune responses to luminal helminth infection through interactions with ILC2s (38, 57), which ultimately accelerated worm clearance. These findings support our previous observations that demonstrated a key role for ILC2 in the regulation of the gastric stem cell niche, with CXCR4-expressing ILC2s being modulated by endothelial cells that secrete CXCL12 (49).
Tuft Cells Expand During Chronic Injury and Early Tumorigenesis
Chronic inflammation differs mechanistically from acute inflammation, especially with respect to inflammation associated with carcinogenic insults. Accordingly, we observed a pronounced difference between acute and chronic inflammatory states, with a marked increase in the abundance of tuft cells in chronic inflammation. We previously reported on the accumulation of Dclk1-expressing tuft cells in the setting of chronic gastritis in transgenic mice (H-K-ATPase-IL1β) overexpressing IL-1β in gastric parietal cells, as well as in wild-type mice chronically infected with H. felis (135). Saqui-Salces and colleagues independently demonstrated an abundance of tuft cells in separate mouse models of gastric hyperplasia and metaplasia (110). We also found increased tuft cells in the gastric cardia of L2-IL1β mice, which develop esophagitis leading to Barrett’s-like metaplasia (100), and in the intestines of mice infected with Helicobacter hepaticus or Bacteroides fragilis (146). Tuft cell expansion has also been observed in human Barrett’s esophagus, an inflammatory condition caused by bile/acid reflux (100), and it has been reported in several additional studies on chronic injury models (63, 78, 93, 140). In early pancreatic tumorigenesis, Dclk1-expressing tuft cells also dramatically increase with metaplasia and PanIN formation (pancreatic intraepithelial neoplasia) (2). In addition, DCLK1 protein has been demonstrated to be important for early neoplastic growth and epithelial-to-mesenchymal transition (2, 88, 149) and been evaluated as a possible target therapy for therapeutic intervention (127).
While the tuft cell expansion in these studies supports the notion of their importance for regeneration, it remains to be elucidated where and how these cells arise, given that the majority of tuft cells are known to be postmitotic and nonproliferative. One would suppose that the expansion in tuft cells was due to both increased production along with decreased turnover. However, increased production of tuft cells from actively dividing stem or progenitor cells should result in BrdU-positive tuft cells, which is generally not seen even with prolonged labeling. Another possibility might be differentiation of other postmitotic cells into tuft cells following chronic injury, although this remains highly speculative at best. Furthermore, while tuft cells demonstrate a close relationship with stromal neurons, we could not find any evidence that these might actually arise from stromal neural crest-derived cells (147).
Long-lived tuft cells are thus ideally equipped to serve as cancer-initiating cells, harboring oncogenic mutations in a quiescent state but becoming activated and progressing to cancer in response to injury. Whether this represents true somatic reprogramming or “dedifferentiation” as suggested by others seems unlikely (80). While tuft cells are not highly proliferative, it appears more likely that mutations acquired by stem or progenitors can be passed on to tuft cells, which might then interconvert into tumor initiating cells under inflammatory or injurious conditions. Strikingly, the selective expansion of tuft cells appears to promote tumor formation, as their expansion during early neoplasia precedes neuronal cholinergic innervation, and cholinergic signaling has been shown to modulate gastric tumorigenesis via the Hippo pathway (50), which is involved in the tumorigenesis of many different organs (82). Interestingly, cholinergic signaling also upregulated epithelial nerve growth factor expression, which in turn promoted tumorigenesis, and tuft cell ablation in this model significantly inhibited tumor development, demonstrating their functional importance (62, 83). In addition, although a specific niche for cancer initiating cells or cancer stem cells has not been defined, cholinergic signaling also directly modulates Wnt signaling (155), which seems to be required by tumor initiating cells. Thus the capacity to foster or support tumorigenesis can be mediated by differentiated epithelial cells in the microenvironment, and is not limited to stromal cells and cancer-initiating cells (119). In addition to ChAT and other markers (Table 1), tuft cells are the major epithelial source of Ptgs1 and Ptgs2, and in colorectal cancer, Ptgs2 expression is often upregulated (10, 26, 87). COX1 and COX2 are the rate-limiting enzymes of prostaglandin production, which are closely linked to the regulation of stem cell homeostasis in the setting of inflammation (145). Inhibition of COX1 and COX2 appears to be useful for prevention of GI cancer and has even been proposed recently as a therapeutic approach for pancreatic carcinoma (102). Interestingly, PGE2 have recently also been shown to actively modulate Wnt signaling during homeostasis, inflammation, and colon cancer formation (117). The effect of COX inhibition on tuft cell function in these settings, however, has not been clarified.
Is There a More Direct Role for Tuft Cells in Tumorigenesis?
Chronic inflammation is known to precede many GI cancers (43) and foster malignant progression (47), and tuft cells may clearly participate in the initiation and formation of inflammation-associated cancer. Nevertheless, it remains unclear as to whether tuft cells can serve only as cancer-initiating cells or whether they might comprise actual cancer stem cells, thus persisting in tumors to sustain tumor growth (104). Nakanishi et al. (88) concluded that DCLK1 identifies tumor stem cells, as they observed complete Dclk1-lineage traced polyp formation in Apc/Min mice upon Cre-mediated inactivation of the Apc gene. In their model, Dclk1-expressing tumor cells demonstrated accumulation of β-catenin and expressed proliferation markers with sustained tumor growth, which recently has been supported by a study employing the same model (44). In contrast, we did not observe colonic tumor formation from Dclk1-expressing cells in our Dclk1-Cre-ERT BAC transgenic mouse model upon Apc deletion and concluded that Dclk1 does not identify cancer stem cells. However, in the setting of additional DSS-induced chronic inflammation, we did observe robust colorectal tumor formation in our Dclk1-CreERT; ApcF/F mice, months after induction, with Dclk1 lineage-traced colonic polyps (146) (Fig. 3). This indicated that long-lived tuft cells harboring Apc mutations can serve as colon cancer-initiating cells. The main difference between the two studies lies with the mouse model employed (Dclk1 knockin vs. Dclk1 BAC transgenic). The knockin approach in the former resulted in Dclk1 haploinsufficiency, while the latter maintains the integrity of both Dclk1 alleles. The presence of two functioning Dclk1 alleles appears to be crucial to maintain proper tuft cell function and longevity, as demonstrated by conditional knockout of Dclk1 in tuft cells (148).
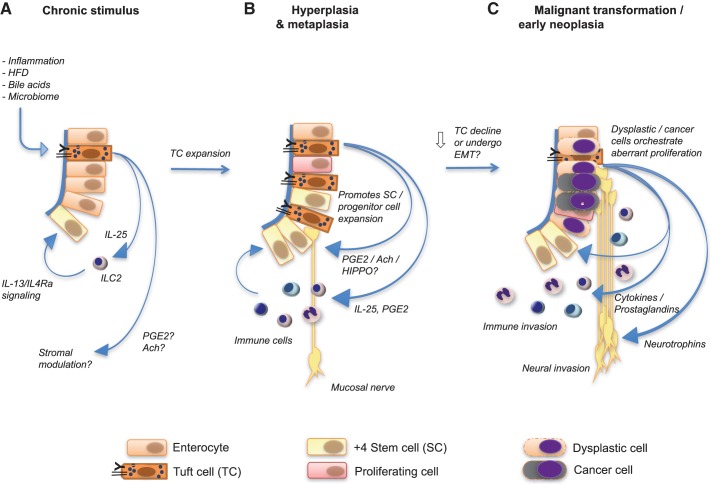
Fig. 3.
Tuft cell conduct during chronic inflammation, hyperplasia, and metaplasia and early neoplasia. A: the persisting stimulation by different substrates [high-fat diet (HFD)] or inflammatory stimuli creates a chronic inflammatory microenvironment, which is sensed by tuft cells and potentially stimulates these to signal to either stromal immune cells (IL-25), or modulates the stroma or the stem or progenitor cell compartment [prostaglandins E2 (PGE2), Ach?]. B: persisting inflammation, however, reportedly increases tuft cell count, thus further enhancing tuft cell signaling to adjacent stromal cells (immune cells, eventually also neurons) and stem and progenitor cells. In addition, this environment may also cause an increasing burden of mutations in susceptible cells. C: interestingly, during progression to early neoplasia, tuft cell count has been shown to decrease and even to be absent of more advanced neoplastic tissue. In this scenario, highly proliferative tumor cells appear to take over and orchestrate aberrant proliferation by directing immune cell invasion, neuronal invasion, or neoangiogenesis. Eventually, Dclk1-expressing tuft cells may also undergo epithelial-to-mesenchymal transition (EMT), thereby loosing Dclk1 expression but maintaining tumor growth as actual tumor stem cells.
While tuft cells are typically increased in preneoplastic states, they are less abundant in invasive adenocarcinomas. Thus, while tuft cells are increased in early PanINs, they are less abundant or almost absent with progression to invasive pancreatic cancer (2), and Dclk1 expression appears to be generally downregulated in high-grade dysplasia or cancer (100). The mechanisms involved leading to their decline and what fate tuft cells undergo with progression to cancer remain to be elucidated. However, it is possible that proliferating cancer cell clones somehow inhibit the normal differentiation of progenitors into tuft cells or perhaps they simply crowd out tuft cells during their expansion. Interestingly, while Dclk1 expression decreases during tumor progression, some Dclk1-expressing tumor cells actually persist, possibly providing some of the tuft cell-like niche factors, but nevertheless, they are likely at this point no longer true tuft cells or, alternatively, tumor cells that acquired Dclk1 expression. On the other hand, some tuft cells could also lose Dclk1 expression but still persist in tumors, possibly contributing to late-stage tumor growth and metastasis (22, 73), which, however, also remains to be elucidated.
Tuft Cells as Emerging Intestinal Niche Cells
Similar to stem cells in highly regenerative tissues such as bone marrow or skin, ISCs are surrounded by a microenvironment or niche, which modulates their proliferation and self-renewal (3, 23, 108). In the gastrointestinal epithelium, actively cycling and slow-cycling/quiescent ISC populations have been described (7, 29, 32, 58, 120, 137, 138), with Lgr5-expressing cells representing actively cycling stem cells located at the crypt base (5, 6, 8, 12, 101, 114, 144) and markers such as Hopx, Bmi1, mTert, and Lrig1 labeling a slow-cycling stem cell localized near the +4 position (3, 84, 97, 132, 153). Interestingly, although, loss of Lgr5-expressing cells has been demonstrated to be well tolerated, at least transiently, without major impact on crypt architecture or overall epithelial proliferation under basal conditions, with other stem cell populations presumably able to compensate for loss of this one stem cell (69, 111, 129, 132). Some additional plasticity has been described for secretory progenitor cells (136), TA cells, and even a few differentiated epithelial cell types (52, 129), pointing to the complexity of the intestinal stem cell pool. Some caution in interpreting these data is needed, as many of these markers are not highly specific for one cell type but instead mark a heterogeneous cell population near the crypt base (69), ultimately regulated by a complex niche (125).
Epithelial turnover is modulated by cytokines and growth factors and by signaling pathways such as Wnt, bone morphogenetic protein, Hedgehog, Notch, and Hippo (11, 45, 85). Epithelial Wnt signaling regulates progenitor cell fate, likely in conjunction with Notch signaling in Lgr5-expressing stem cells. Both Wnt and Notch inhibition have been demonstrated to affect epithelial proliferation (66, 131), and there is significant interplay among the Wnt, Notch, and Hippo pathways (9, 18, 131). Moreover, the Hippo pathway has been demonstrated to be essential for ISC proliferation and progenitor cell fate (18, 61) as well as regeneration in response to injury (9, 45, 61), although its precise interactions with Wnt pathway members remain controversial (82).
Intestinal sources of Wnt ligands have been found to be highly redundant (60, 109), and stromal rather than epithelial Wnt secretion has been demonstrated to contribute to the growth of intestinal epithelial progenitors (25). Examples of important stromal Wnt sources include Foxl1-expressing subepithelial myofibroblasts (1), nonmyofibroblastic CD34+gp38+ mesenchymal cells (126), innate lymphoid cells type 2 (49), and type 3 (72) as well as macrophages (107). Importantly, the ENS actively modulates intestinal progenitor activity by cholinergic signaling (46), which activates Wnt signaling through the muscarinic receptor subtype 3 (155), highlighting potential neuronal interactions with epithelial and stromal cells within the intestinal niche.
The contribution of epithelial cells to the ISC niche remains somewhat controversial. Within the small intestine, Paneth cells in close proximity to ISCs were first suggested to influence neighboring stem and progenitor cells in a paracrine fashion through Wnt or EGF signaling (24, 114). In the colonic epithelium, Paneth-like cKIT-positive crypt base goblet cells also appear to support in vitro growth of Lgr5-expressing stem cells (105). However, the overall importance of Paneth cells as critical ISC niche cells remains uncertain, given solid evidence that their depletion has minimal impact on ISCs (35, 64), thereby underscoring the high degree of redundancy of niche signaling. In contrast, Reg4-positive cells in the colon have recently been shown to constitute a local niche for Lgr5-expressing progenitors and Lgr5-expressing cells disappear after ablation of Reg4-positive cells (111).
Physiological regulation of ISCs would thus seem to require close communication with immune cells, nerves, and connective tissue cells surrounding the niche, and our data would support tuft cells as being particularly well positioned to coordinate such cross talk between stem cells and the rest of the niche. This would be in line with a concept of niches as not only central to stem cell maintenance but also as units of production of specific cellular outputs (92, 125). With a unique arrangement of apical cytoskeletal components mediating mechanical stability and tolerance to mechanical stress, tuft cells are well situated to function as mechanoreceptors (112). Tuft cells appear to have direct synaptic contact with nerve terminals and therefore can serve as chemosensory or reporting cells in the gut (Fig. 1), in close analogy to well-known sensing cells (20, 27, 151). Other morphological features such as the presence of small intracellular vesicles adjacent to microvilli, along with glycocalceal bodies, strongly suggest that tuft cells have a paracrine signaling function. Strikingly, this is supported by the recent finding of cytospinules emerging from tuft cells toward the nuclei of neighboring cells (55). These anatomic features of tuft cells are complemented by their unique marker signature (Table 1), with many of the secreted molecules well described to act on important downstream signaling pathways. Hence, it appears plausible that tuft cells located near the crypt base may rather have the capacity to support stem cells (92). Upon leaving the intestinal crypts, migrating along the villus axis tuft cells with perhaps increasing differentiation, they might lose this niche function but still execute sensing functions, thereby relegating the proliferative signals to stromal immune cells or other lineages (38, 142). In the setting of chronic injury or inflammation, tuft cells in the villi might also contribute to a noncrypt-base stem cell environment, generating proliferative zones outside of the usual anatomical niche (125). Thus we hypothesize that tuft cells may function to regulate niches at many locations throughout the gut, depending on the regional signals or insults that are present (Fig. 4). Many of these proposed niche functions, however, currently lack detailed mechanistic evidence that might clarify exactly how tuft cells integrate and regulate intestinal niche signals. Nevertheless, these cells likely play key roles in normal gut physiology and may represent valuable targets in inflammatory diseases or tumorigenesis (33, 50, 68, 88, 98, 127, 141, 149, 150).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1-U01-DK-103155-01 and R01-DK-097016-02 (to T. Wang), Deutsche Krebshilfe Grant 70111870 (to M. Middelhoff), and Max Eder Program Deutsche Krebshilfe Grants DFG SFB 824 and DFG GRK 1482 (to M. Quante).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Q. and T.C.W. conceived and designed research; M.M., C.B.W., and M.D.G. prepared figures; M.M., T.C.W., and M.Q. drafted manuscript; M.M., C.B.W., Y.H., K.S.Y., M.D.G., T.C.W., and M.Q. edited and revised manuscript; M.M., C.B.W., Y.H., K.S.Y., M.D.G., T.C.W., and M.Q. approved final version of manuscript.
REFERENCES
- 1.Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CVE, Kaestner KH. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol 2: 175–188, 2016. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, Maitra A, Leach SD. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 146: 245–256, 2014. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15: 19–33, 2014. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 4.Barker N, Clevers H. Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol 5: Unit5A.4, 2010. doi: 10.1002/9780470151808.sc05a04s13. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology 133: 1755–1760, 2007. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611, 2009. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 7.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev 22: 1856–1864, 2008. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 9.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110, 2013. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509: 514–525, 2008. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 11.Biswas S, Davis H, Irshad S, Sandberg T, Worthley D, Leedham S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J Pathol 237: 135–145, 2015. doi: 10.1002/path.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116: 7–14, 1999. doi: 10.1016/S0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 13.Bjerknes M, Khandanpour C, Möröy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martín MG, Cheng H. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol 362: 194–218, 2012. doi: 10.1016/j.ydbio.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351: 707–710, 2016. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brittan M, Wright NA. Gastrointestinal stem cells. J Pathol 197: 492–509, 2002. doi: 10.1002/path.1155. [DOI] [PubMed] [Google Scholar]
- 16.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 17.Burgess HA, Reiner O. Alternative splice variants of doublecortin-like kinase are differentially expressed and have different kinase activities. J Biol Chem 277: 17696–17705, 2002. doi: 10.1074/jbc.M111981200. [DOI] [PubMed] [Google Scholar]
- 18.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060, 2007. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Chase CC, Munn EA. Surface binding and uptake of polycationic ferritin by neonatal piglet intestinal epithelium. J Cell Sci 46: 235–252, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Corwin JT, Warchol ME. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci 14: 301–333, 1991. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- 21.Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, Wang Y, Rowley D, Younes M, Ayala GE. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol 38: 299–307, 2007. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, Wells JM, Crawford HC. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology 146: 233–44.e5, 2014. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ema H, Suda T. Two anatomically distinct niches regulate stem cell activity. Blood 120: 2174–2181, 2012. doi: 10.1182/blood-2012-04-424507. [DOI] [PubMed] [Google Scholar]
- 24.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530: 340–343, 2016. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 25.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143: 1518–1529.e7, 2012. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Ferrández A, Prescott S, Burt RW. COX-2 and colorectal cancer. Curr Pharm Des 9: 2229–2251, 2003. doi: 10.2174/1381612033454036. [DOI] [PubMed] [Google Scholar]
- 27.Finger TE. Cell types and lineages in taste buds. Chem Senses 30, Suppl 1: i54–i55, 2005. doi: 10.1093/chemse/bjh110. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des 10: 577–588, 2004. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- 29.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 296: G1108–G1118, 2009. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985–995, 2001. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 31.Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 138: 5403–5414, 2011. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagliardi G. DCLK1 expression in gastrointestinal stem cells and neoplasia. J Cancer Ther Res 1: 12, 2012. doi: 10.7243/2049-7962-1-12. [DOI] [Google Scholar]
- 33.Gagliardi G, Goswami M, Passera R, Bellows CF. DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol 5: 35–42, 2012. doi: 10.2147/CEG.S30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gagliardi G, Moroz K, Bellows CF. Immunolocalization of DCAMKL-1, a putative intestinal stem cell marker, in normal colonic tissue. Pathol Res Pract 208: 475–479, 2012. doi: 10.1016/j.prp.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem 272: 23729–23740, 1997. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 36.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 137: 2179–2180, 2009. doi: 10.1053/j.gastro.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 37.Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci 69: 2907–2917, 2012. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230, 2016. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192: 767–780, 2011. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 39, Suppl 3: S184–S193, 2005. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 41.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 281: 11292–11300, 2006. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 43.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle 8: 2005–2013, 2009. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 44.Goto N, Ueo T, Fukuda A, Kawada K, Sakai Y, Miyoshi H, Taketo MM, Chiba T, Seno H. Distinct roles of Hes1 in normal stem cells and tumor stem-like cells of the intestine. Cancer Res 77: 3442–3454, 2017. doi: 10.1158/0008-5472.CAN-16-3192. [DOI] [PubMed] [Google Scholar]
- 45.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526: 715–718, 2015. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 46.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143: 408–17.e2, 2012. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Hass N, Schwarzenbacher K, Breer H. A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol 128: 457–471, 2007. doi: 10.1007/s00418-007-0325-3. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28: 800–814, 2015. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31: 21–34, 2017. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24: 7674–7680, 2004. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henning SJ, von Furstenberg RJ. GI stem cells–new insights into roles in physiology and pathophysiology. J Physiol 594: 4769–4779, 2016. doi: 10.1113/JP271663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Höfer D, Drenckhahn D. Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol 105: 405–412, 1996. doi: 10.1007/BF01463662. [DOI] [PubMed] [Google Scholar]
- 54.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 110: 303–309, 1998. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 55.Hoover B, Baena V, Kaelberer MM, Getaneh F, Chinchilla S, Bohórquez DV. The intestinal tuft cell nanostructure in 3D. Sci Rep 7: 1652, 2017. doi: 10.1038/s41598-017-01520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howes SC, Alushin GM, Shida T, Nachury MV, Nogales E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol Biol Cell 25: 257–266, 2014. doi: 10.1091/mbc.E13-07-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351: 1329–1333, 2016. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 14: 106–114, 2011. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarvi O, Keyrilainen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand Suppl 39, Suppl 111: 72–73, 1956. doi: 10.1111/j.1600-0463.1956.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 60.Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141: 2206–2215, 2014. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 61.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137: 4135–4145, 2010. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiberstis PA. Cancer and nerves: a tuf(t) partnership. Science 355: 144–145, 2017. doi: 10.1126/science.355.6321.144-d. [DOI] [PubMed] [Google Scholar]
- 63.Kikuchi M, Nagata H, Watanabe N, Watanabe H, Tatemichi M, Hibi T. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol 10: 65, 2010. doi: 10.1186/1471-230X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 109: 3932–3937, 2012. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kretzschmar K, Watt FM. Lineage tracing. Cell 148: 33–45, 2012. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271, 2004. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusumakshi S, Voigt A, Hübner S, Hermans-Borgmeyer I, Ortalli A, Pyrski M, Dörr J, Zufall F, Flockerzi V, Meyerhof W, Montmayeur JP, Boehm U. A binary genetic approach to characterize TRPM5 cells in mice. Chem Senses 40: 413–425, 2015. doi: 10.1093/chemse/bjv023. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Bellows CF. Doublecortin-like kinase 1 exhibits cancer stem cell-like characteristics in a human colon cancer cell line. Chin J Cancer Res 25: 134–142, 2013. doi: 10.3978/j.issn.1000-9604.2013.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology 151: 298–310, 2016. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liman ER. TRPM5 and taste transduction. Handb Exp Pharmacol 179: 287–298, 2007. doi: 10.1007/978-3-540-34891-7_17. [DOI] [PubMed] [Google Scholar]
- 71.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci 20: 9152–9161, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MR, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564, 2015. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol 23: 675–699, 2007. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 74.Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One 6: e16295, 2011. doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mancino M, Ametller E, Gascón P, Almendro V. The neuronal influence on tumor progression. Biochim Biophys Acta 1816: 105–118, 2011. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci 14: 685–687, 2011. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Li L, Sureban SM, Houchen CW. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells 32: 822–827, 2014. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637, 2008. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 79.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 27: 2571–2579, 2009. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mills JC, Sansom OJ. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 8: re8, 2015. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J 36: 5–24, 2017. doi: 10.15252/embj.201694660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15: 642–656, 2014. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monje M. Settling a nervous stomach: the neural regulation of enteric cancer. Cancer Cell 31: 1–2, 2017. doi: 10.1016/j.ccell.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat 213: 52–58, 2008. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore KA, Lemischka IR. Stem cells and their niches. Science 311: 1880–1885, 2006. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 86.Morroni M, Cangiotti AM, Cinti S. Brush cells in the human duodenojejunal junction: an ultrastructural study. J Anat 211: 125–131, 2007. doi: 10.1111/j.1469-7580.2007.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutoh H, Sashikawa M, Sakamoto H, Tateno T. Cyclooxygenase 2 in gastric carcinoma is expressed in doublecortin- and CaM kinase-like-1-positive tuft cells. Gut Liver 8: 508–518, 2014. doi: 10.5009/gnl13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, Chiba T. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45: 98–103, 2013. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 89.Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res 317: 2702–2710, 2011. doi: 10.1016/j.yexcr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Connell MR, Sarkar S, Luthra GK, Okugawa Y, Toiyama Y, Gajjar AH, Qiu S, Goel A, Singh P. Epigenetic changes and alternate promoter usage by human colon cancers for expressing DCLK1-isoforms: clinical implications. Sci Rep 5: 14983, 2015. doi: 10.1038/srep14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49: 921–937, 1999. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 92.Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol 16: 693–699, 2004. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG, Rustgi AK, Wang TC. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res 70: 8435–8445, 2010. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, Quante M. Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem Cells Int 2016: 3710836, 2016. doi: 10.1155/2016/3710836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176, 2002. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 96.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71: 28–41, 2003. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 97.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qu D, Johnson J, Chandrakesan P, Weygant N, May R, Aiello N, Rhim A, Zhao L, Zheng W, Lightfoot S, Pant S, Irvan J, Postier R, Hocker J, Hanas JS, Ali N, Sureban SM, An G, Schlosser MJ, Stanger B, Houchen CW. Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS One 10: e0118933, 2015. doi: 10.1371/journal.pone.0118933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qu D, Weygant N, May R, Chandrakesan P, Madhoun M, Ali N, Sureban SM, An G, Schlosser MJ, Houchen CW. Ablation of doublecortin-like kinase 1 in the colonic epithelium exacerbates dextran sulfate sodium-induced colitis. PLoS One 10: e0134212, 2015. doi: 10.1371/journal.pone.0134212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, Mahmood U, Figueiredo JL, Kitajewski J, Shawber C, Lightdale CJ, Rustgi AK, Wang TC. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21: 36–51, 2012. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol 6: 724–737, 2009. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rao CV, Janakiram NB, Madka V, Devarkonda V, Brewer M, Biddick L, Lightfoot S, Steele VE, Mohammed A. Simultaneous targeting of 5-LOX-COX and EGFR blocks progression of pancreatic ductal adenocarcinoma. Oncotarget 6: 33290–33305, 2015. doi: 10.18632/oncotarget.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat 44: 345–412, 1956. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- 104.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 324: 1670–1673, 2009. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, Lobo N, Kulkarni S, Sim S, Qian D, Beachy PA, Pasricha PJ, Quake SR, Clarke MF. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 142: 1195–1205.e6, 2012. doi: 10.1053/j.gastro.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res 50, Suppl: S29–S34, 2009. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, Zhang J, Godwin AK, Tome WA, Wang TC, Guha C, Pollard JW. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 7: 13096, 2016. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sailaja BS, He XC, Li L. The regulatory niche of intestinal stem cells. J Physiol 594: 4827–4836, 2016. doi: 10.1113/JP271931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.San Roman AK, Jayewickreme CD, Murtaugh LC, Shivdasani RA. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports 2: 127–134, 2014. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 136: 191–204, 2011. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sasaki N, Sachs N, Wiebrands K, Ellenbroek SI, Fumagalli A, Lyubimova A, Begthel H, van den Born M, van Es JH, Karthaus WR, Li VS, Lopez-Iglesias C, Peters PJ, van Rheenen J, van Oudenaarden A, Clevers H. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA 113: E5399–5407. 2016. doi: 10.1073/pnas.1607327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato A. Tuft cells. Anat Sci Int 82: 187–199, 2007. doi: 10.1111/j.1447-073X.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 113.Sato A, Miyoshi S. Ultrastructure of the main excretory duct epithelia of the rat parotid and submandibular glands with a review of the literature. Anat Rec 220: 239–251, 1988. doi: 10.1002/ar.1092200304. [DOI] [PubMed] [Google Scholar]
- 114.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340: 1190–1194, 2013. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 114a.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sbarbati A, Bramanti P, Benati D, Merigo F. The diffuse chemosensory system: exploring the iceberg toward the definition of functional roles. Prog Neurobiol 91: 77–89, 2010. doi: 10.1016/j.pneurobio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 116.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat 206: 349–358, 2005. doi: 10.1111/j.1469-7580.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schewe M, Franken PF, Sacchetti A, Schmitt M, Joosten R, Böttcher R, van Royen ME, Jeammet L, Payré C, Scott PM, Webb NR, Gelb M, Cormier RT, Lambeau G, Fodde R. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell 19: 38–51, 2016. doi: 10.1016/j.stem.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 118.Schütz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, Gudermann T, Diener M, Kummer W, Krasteva-Christ G, Weihe E. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol 6: 87, 2015. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152: 25–38, 2013. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res 156: 180–187, 2010. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, Sanada K, Fischer A, Coquelle FM, Reiner O, Tsai LH. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 49: 25–39, 2006. doi: 10.1016/j.neuron.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 122.Simonian HP, Vo L, Doma S, Fisher RS, Parkman HP. Regional postprandial differences in pH within the stomach and gastroesophageal junction. Dig Dis Sci 50: 2276–2285, 2005. doi: 10.1007/s10620-005-3048-0. [DOI] [PubMed] [Google Scholar]
- 123.Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res 317: 2719–2724, 2011. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 124.Singh P, O’Connell M, Shubhashish S. Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications. Stem Cell Investig 3: 51, 2016. doi: 10.21037/sci.2016.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 414: 98–104, 2001. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 126.Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, and Peduto L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA 114: E506–E513, 2017. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology 137: 649–659, 2009. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol 107: 77–107, 2014. doi: 10.1016/B978-0-12-416022-4.00003-2. [DOI] [PubMed] [Google Scholar]
- 129.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kr etzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213, 2016. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Thurlbeck WM. A rose is a rose is a rose, but what is a brush cell? Pediatr Pulmonol 8: 3, 1990. doi: 10.1002/ppul.1950080104. [DOI] [PubMed] [Google Scholar]
- 131.Tian H, Biehs B, Chiu C, Siebel CW, Wu Y, Costa M, de Sauvage FJ, Klein OD. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Reports 11: 33–42, 2015. doi: 10.1016/j.celrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomonari H, Miura H, Nakayama A, Matsumura E, Ooki M, Ninomiya Y, Harada S. Gα-gustducin is extensively coexpressed with sweet and bitter taste receptors in both the soft palate and fungiform papillae but has a different functional significance. Chem Senses 37: 241–251, 2012. doi: 10.1093/chemse/bjr098. [DOI] [PubMed] [Google Scholar]
- 135.Tu SP, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD, Muthuplani S, Shibata W, Fox JG, Pritchard DM, Wang TC. IFN-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res 71: 4247–4259, 2011. doi: 10.1158/0008-5472.CAN-10-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Es JH, Sato T, van de Wetering M, Lyubimova A, Gregorieff A, Zeinstra L, van den Born M, Korving J, Martens AC, van den Oudenaarden A, Clevers H, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Landeghem L, Santoro MA, Mah AT, Krebs AE, Dehmer JJ, McNaughton KK, Helmrath MA, Magness ST, Lund PK. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J 29: 2828–2842, 2015. doi: 10.1096/fj.14-264010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139: 488–497, 2012. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, Reed A, Weygant N, Ramanujam R, Souza R, Madhoun M, Whorton J, Anant S, Meltzer SJ, Houchen CW. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol 27: 773–780, 2012. doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verissimo CS, Molenaar JJ, Meerman J, Puigvert JC, Lamers F, Koster J, Danen EH, van de Water B, Versteeg R, Fitzsimons CP, Vreugdenhil E. Silencing of the microtubule-associated proteins doublecortin-like and doublecortin-like kinase-long induces apoptosis in neuroblastoma cells. Endocr Relat Cancer 17: 399–414, 2010. doi: 10.1677/ERC-09-0301. [DOI] [PubMed] [Google Scholar]
- 142.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225, 2016. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vreugdenhil E, Kolk SM, Boekhoorn K, Fitzsimons CP, Schaaf M, Schouten T, Sarabdjitsingh A, Sibug R, Lucassen PJ. Doublecortin-like, a microtubule-associated protein expressed in radial glia, is crucial for neuronal precursor division and radial process stability. Eur J Neurosci 25: 635–648, 2007. doi: 10.1111/j.1460-9568.2007.05318.x. [DOI] [PubMed] [Google Scholar]
- 144.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell 116: 639–648, 2004. doi: 10.1016/S0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 145.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 10: 181–193, 2010. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 124: 1283–1295, 2014. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Westphalen CB, Middelhoff M, Quante M, Wang TC. Epithelial Dclk1+ cells are not neural crest derived. Stem Cell Investig 3: 60, 2016. doi: 10.21037/sci.2016.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Westphalen CB, Quante M, Wang TC. Functional implication of Dclk1 and Dclk1-expressing cells in cancer. Small GTPases 26: 1–8, 2016. doi: 10.1080/21541248.2016.1208792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weygant N, Qu D, May R, Tierney RM, Berry WL, Zhao L, Agarwal S, Chandrakesan P, Chinthalapally HR, Murphy NT, Li JD, Sureban SM, Schlosser MJ, Tomasek JJ, Houchen CW. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget 6: 2193–2205, 2015. doi: 10.18632/oncotarget.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whorton J, Sureban SM, May R, Qu D, Lightfoot SA, Madhoun M, Johnson M, Tierney WM, Maple JT, Vega KJ, Houchen CW. DCLK1 is detectable in plasma of patients with Barrett’s esophagus and esophageal adenocarcinoma. Dig Dis Sci 60: 509–513, 2015. doi: 10.1007/s10620-014-3347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamamoto K, Ishimaru Y. Oral and extra-oral taste perception. Semin Cell Dev Biol 24: 240–246, 2013. doi: 10.1016/j.semcdb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 153.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 6: 78–90, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D. Denervation suppresses gastric tumorigenesis. Sci Transl Med 6: 250ra115, 2014. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]