Abstract
Cell-cell adhesion molecules play key roles in maintaining quiescence or promoting activation of various stem cells in their niche. Muscle stem cells called satellite cells (SC) are critical for skeletal muscle regeneration after injury, but little is known about the role of adhesion molecules in regulating the behavior of these stem cells. Vascular cell adhesion molecule-1 (VCAM-1) is a cell-cell adhesion protein expressed on quiescent and activated SC whose function is unknown in this context. We deleted Vcam1 from SC using an inducible Cre recombinase in young mice. In the injured niche, Vcam1−/− SC underwent premature lineage progression to a more differentiated state as well as apoptosis leading to a transient delay in myofiber growth during regeneration. Apoptosis was also increased in Vcam1−/− SC in vitro concomitant with decreased levels of phosphorylated Akt, a prosurvival signal activated by VCAM-1 signaling in other cell types. During muscle regeneration, we observed an influx of immune cells expressing α4 integrin, a component of the major, high-affinity VCAM-1 ligand, α4β1 integrin. Furthermore, α4 integrin mRNA and protein were induced in SC 2 days after injury. These results suggest that SC interact with other SC as well as immune cells through α4β1 integrin in the injured niche to promote expansion of SC. In the uninjured niche, multiple cell types also expressed α4 integrin. However, only basal fusion of Vcam1−/− SC with myofibers was decreased, contributing to decreased myofiber growth. These studies define differential roles for VCAM-1 in SC depending on the state of their niche.
Keywords: apoptosis, fusion, Akt, VLA-4, muscle
tissue resident stem cells are typically located in a defined stem cell niche. Within the niche, stem cells interact with other cell types to properly function and respond to the needs of the tissue. The communication between the stem cells and the niche cells occurs both through secreted factors and intercellular interactions mediated through cell-cell adhesion molecules. In various stem cell systems, cell-cell adhesion molecules have been shown to play pivotal roles in maintaining quiescence or promoting activation (45).
Skeletal muscle is the site of frequent injury from trauma, surgery, or disease but possesses an immense capability to repair and regenerate after injury to restore tissue function. The ability of muscle to regenerate relies on tissue-specific stem cells called satellite cells (SC) (31). Similar to other stem cell populations, SC have a well-defined stem cell niche between the sacrolemma of the myofiber and the encapsulating basement membrane. SC are quiescent until activated by muscle injury, upon which they undergo proliferation, followed by differentiation and fusion to form new myofibers to restore muscle structure and function. While SC are the primary cells required to regenerate damaged myofibers (20, 31), numerous other cell types present in skeletal muscle regulate SC function throughout the regenerative process (2). These cell types include damaged myofibers (12), fibro/adipogenic progenitors (FAPs) (25), endothelial cells (11), and immune cells such as macrophages (49).
The role of immune cells during muscle regeneration has been extensively studied (48, 55). After injury, the initial degenerative phase is marked by a recruitment of proinflammatory immune cells, including neutrophils and M1 macrophages (7, 34, 51, 54). These cells, along with others, facilitate the activation and proliferation of SC as well as removal of the damaged tissue (7, 34, 49, 51, 54). Numerous studies have examined the secreted factors produced by these cells in regulating SC proliferation (55), but little is known how these cells can affect SC activation and proliferation through direct cell-cell contact.
Cell-cell adhesion molecules are highly expressed on satellite cells (38) and often utilized as markers to identify or isolate SC (14, 17, 23, 46). However, the majority of studies conducted on cell-cell adhesion molecules during muscle regeneration have focused on the later stages of cell fusion between differentiated muscle cells (1). Therefore, the role of cell-cell interactions between SC and the other cell types at early stages of regeneration remains largely unknown despite numerous studies highlighting the dynamic regulation of these genes in SC (17, 38).
Vascular cell adhesion molecule 1 (VCAM-1, CD106) is a transmembrane protein of the immunoglobulin (Ig) superfamily that is expressed on various cell types including SC (17, 28, 37, 43, 50), where VCAM-1 is routinely used as a marker to isolate SC by fluorescence-activated cell sorting (5, 9). The most widely studied and highest-affinity ligand for VCAM-1 is α4β1 integrin (VLA-4) as this interaction is critical for leukocyte diapedesis and inflammation (16, 39). VCAM-1 can also regulate stem cell behavior, including adhesion of hematopoietic stem cells to the bone marrow niche (56) and maintenance of quiescence in neural stem cells of the subventricular zone in the brain (28). Previous studies examining the role of VCAM-1 during myogenesis utilized the C2C12 mouse muscle cell line and showed that VCAM-1 is present on proliferating myoblasts while the VLA-4 subunit α4 integrin is present on multinucleated myotubes (46). Furthermore, culturing C2C12 cells in the presence of a blocking antibody to VCAM-1 or to α4 integrin reduced the number of nuclei within myotubes compared with controls, suggesting inhibition of the fusion process (46). However, antibody blocking studies can be misleading due to cross-reaction with or steric hindrance of other molecules. Indeed, muscle formation in vivo and in vitro was normal in chimeric mice generated from α4 integrin-deficient embryonic stem cells in which the embryonic stem cells contributed to adult skeletal muscle in high proportion (58). To date, the effects of genetic deletion of VCAM-1 on any aspect of muscle cell biology, including SC function, have not been performed. Therefore, the role of VCAM-1 during myogenesis remains ambiguous with no known function for VCAM-1 in SC.
Here, we specifically deleted Vcam1 from SC using an inducible Cre recombinase in young mice. In the injured niche, Vcam1−/− SC undergo premature lineage progression and apoptosis, leading to a transient delay in myofiber growth during regeneration. In the uninjured niche, only basal fusion of Vcam1−/− satellite cells with myofibers is decreased, which contributes to decreased myofiber growth. These studies define differential roles for VCAM-1 in SC depending on the state of the niche.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Charles River (Wilmington, MA). R26RtdTomato (35) and Vcam1flox/flox (29) mice were purchased from Jackson (Bar Harbor, ME). Pax7creERTM/creERTM mice were obtained from C. Keller (27). Vcam1flox/flox were bred to R26RtdTomato to obtain Vcam1flox/flox;R26RtdTomato mice. These mice were bred to Pax7creERTM/creERTM to generate the following experimental genotypes: Pax7creERTM/+;Vcam1wt/wt;R26RtdTomato and Pax7creERTM/+;Vcam1flox/flox;R26RtdTomato, referred to as Vcam1wt/wt and Vcam1flox/flox, respectively. To induce recombination, tamoxifen (Sigma-Aldrich, St. Louis, MO) was injected intraperitoneally at 1 mg per 10 g of body weight once daily for five consecutive days. After tamoxifen treatment, the genotypes Vcam1+/+ and Vcam1−/− signify the removal of Vcam1 in Pax7-expressing cells. Muscle injury experiments were conducted using same-sex cohorts (6–8 wk old). All other experiments were conducted using a combination of male and female mice (6–8 wk old).
Muscle injuries.
Muscle injuries were conducted as described previously (41). Briefly, mice were anesthetized and given analgesia pre– and post–muscle injury. Anesthetized mice were injected with 1.2% BaCl2 in sterile phosphate-buffered saline (PBS) into either the gastrocnemius muscle (40 µl) or the tibialis anterior (TA) muscle (20 µl). All experiments were performed in accordance with approved guidelines and ethical approval from Emory University’s Institutional Animal Care and Use Committee and in compliance with the National Institutes of Health.
Satellite cell isolation and flow cytometry.
For analyses by flow cytometry, hindlimb muscles (gastrocnemius and rectus femoris from uninjured animals or injured gastrocnemius) were harvested and rinsed in PBS. Muscles were mechanically minced in Dulbecco’s modified Eagle’s medium (DMEM; Corning) containing 1,000 U/ml collagenase type II (Life Technologies, Carlsbad, CA) and incubated for 1.5 h at 37°C with gentle rocking. The suspension was diluted in Ham’s F10 media (HyClone, South Logan, UT) with 10% fetal bovine serum (FBS; HyClone, South Logan, UT) plus 100 U/ml penicillin and 100 µg/ml streptomycin (P/S) (Life Technologies) (wash buffer), triturated, and further digested with 100 U/ml collagenase type II plus 1 U/ml dispase (Life Technologies) for 30 min at 37°C with gentle rocking. Subsequently, the suspension was triturated, diluted in wash buffer, and filtered through a 100-µm pore vacuum filtration system (Millipore, Peachtree Corners, GA). The cell pellet was resuspended in cold PBS containing 0.5% bovine serum albumin (BSA; Sigma-Aldrich) (FACS buffer). Cells were incubated with primary antibodies on ice for 20 min, washed in FACS buffer, incubated with fluorescently labeled streptavidin for 20 min for detection of biotinylated antibodies, washed again, and analyzed by a BD LSR II flow cytometry (BD Biosciences, San Jose, CA). Analyses of flow cytometry data were performed using FACSDiva (BD version 8.0.1) and FlowJo (FlowJo version 10.0.7).
For fluorescence-activated cell sorting, hindlimb muscle samples were prepared as described above and isolated using a BD FACSAria II (BD Biosciences). The isolated cells were washed in DMEM and cytospun (190 g for 4 min; Shandon Cytospin 3) onto charged glass slides. The cells were labeled by immunofluorescence for Pax7 and MyoD as described below.
The following primary antibodies were used: rat anti-VCAM-1-biotin (2.5 µg/ml–10 µg/ml; BD Biosciences), rat anti-CD45-FITC/PE/PECy7 (500 ng/ml; eBioscience, San Diego, CA), rat anti-α4 integrin-FITC (500 ng/ml; BioLegend, San Diego, CA), rat anti-F4/80-V450/PE (2 µg/ml/125 ng/ml; eBioscience), rat anti-Ly6G/C-eFluor660 (200 ng/ml; eBioscience), rat anti-CD31-FITC/PE/PECy7 (500 ng/ml; eBioscience), rat anti-Sca1-PECy7 (50 ng/ml; BD Biosciences), and rat anti-α7 integrin-AF647/APC (1 µg/ml; AbLab/1 µg/ml; R&D Systems, Minneapolis, MN). Streptavidin-V450 (5 µg/ml; BD Biosciences) or streptavidin-PE (1.25 µg/ml; Jackson ImmunoResearch, West Grove, PA) was used to detect biotin labeling. Appropriate rat isotype control antibodies (BD Bioscience and eBioscience) were utilized.
Cell proliferation and apoptosis assays by flow cytometry.
To analyze in vivo satellite cell proliferation, 5-bromo-2′-deoxyuridine (BrdU; 100 mg/g body weight; Sigma-Aldrich) was injected intraperitoneally twice a day for 2 days. Muscles were dissected and digested as described above. Isolated mononucleated cells were immunostained for BrdU using an FITC-BrdU flow kit in accordance with the manufacturer’s instructions (BD PharMingen, San Diego, CA). Proliferating satellite cells were identified as BrdU+ and tdTomato+ by flow cytometry.
To analyze in vivo satellite cell apoptosis, isolated mononucleated cells from hindlimb muscle were labeled with propidium iodide (PI) and 1:40 Annexin V-FITC (Biolegend) in 10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2 (pH 7.4) (10). Apoptotic cells were defined as Annexin V+/PI− from the tdTomato+ satellite cell population (10).
Myofiber isolation.
Single myofiber isolation was performed as described previously (41) with some modifications. Gastrocnemius muscles were gently dissected and cut into three longitudinal pieces and placed into a tube containing DMEM, 25 mM HEPES, and 400 U/ml collagenase type I (Worthington Biochemical, Lakewood, NJ.). The muscles were digested for 90 min at 37°C in an Enviro-Genie incubator (Scientific Industries, Biohemia, NY) with the tube rocked at 26 rpm. After digestion, single myofibers and the remaining muscle pieces were washed three times to remove debris and transferred to a horse serum (GIBCO, Grand Island, NY)-coated petri dish. Single myofibers were transferred with a fire-polished pasture pipette two times to horse serum-coated petri dishes containing DMEM plus P/S. After the final wash step, myofibers were transferred to a 24-well plate coated with Matrigel (BD PharMingen) containing DMEM plus P/S. A minimum of 24 myofibers were isolated per animal (n = 3–6 per genotype). The isolated myofibers were incubated for 30 min at 37°C, and then the plates were centrifuged at 1,100 g for 20 min to adhere the myofibers to the Matrigel. The myofibers were fixed with 4% paraformaldehyde for 10 min at room temperature.
Histology and immunofluorescence.
To analyze muscle regeneration, TA muscles were collected at 7 and 30 days postinjury (DPI) and frozen in Tissue Freezing Media (Triangle Biomedical Research, Cincinnati, OH) (n = 3–5 per genotype). Tissue cross sections of 14-µm thickness were collected every 200 µm using a Leica CM1850 cryostat and stained with hematoxylin and eosin. Analyses of regenerated myofiber cross-sectional area were performed using similar anatomical regions of each TA muscle with a total of 354–696 myofibers analyzed per genotype for each time point. Myofiber cross-sectional area was measured using ImageJ 1.43u (National Institutes of Health, Bethesda, MD).
For measurements of myofiber tdTomato intensity in tissue cross sections, TA muscles were collected, fixed with 4% formaldehyde for 2 h on ice, and incubated in 30% sucrose overnight (40). Subsequently, TA muscles were frozen and sectioned as described above. ImageJ 1.43u was utilized to measure mean fluorescent intensity in the center of the myofiber. Approximately 1,500–2,000 myofibers were analyzed per animal (n = 3 per genotype).
Immunofluorescent labeling of Pax7 and laminin on sections was performed as previously described (44). All incubations were performed at room temperature unless otherwise noted. Sections were fixed in 4% paraformaldehyde followed by antigen retrieval in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) heated with a pressure cooker (Cuisinart model CPC-600) for 6 min. The tissue sections were blocked with the M.O.M. Kit (Vector, Burlingame, CA) followed by a 1 h incubation with 5% donkey serum, 0.5% BSA, and 0.25% Triton X-100 in PBS (blocking buffer). Sections were labeled with mouse anti-Pax7 (3.8 µg/ml, Developmental Studies Hybridoma Bank, Iowa City, IA) and rabbit anti-laminin (2 µg/ml, Sigma-Aldrich) in 0.5% donkey serum in PBS plus 0.05% Tween 20 (PBST) overnight at 4°C. After washes in PBST, sections were incubated with a biotinylated donkey anti-mouse IgG (1 µg/ml, Jackson ImmunoResearch) in blocking buffer for 1 h. A tyramide signal amplification green kit (TSA; Perkin Elmer, Waltham, MA) was utilized to visualize Pax7. The sections were incubated with Tris-NaCl blocking buffer (TNB) for 30 min followed by incubation with horseradish peroxidase (HRP)-conjugated streptavidin (1:100) in TNB for 20 min. The sections were incubated with the TSA Green reagent (1:300) in amplification buffer for 8 min, washed in PBST, and incubated with a AF594-conjugated donkey anti-rabbit IgG (1 µg/ml, Jackson ImmunoResearch) or a AF647-conjugated donkey anti-rabbit IgG (5 µg/ml, Jackson ImmunoResearch) in blocking buffer for 40 min. After washes, nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) and sections were mounted with VectaShield (Vector) for imaging.
To analyze lineage progression of FACS isolated satellite cells, the cells were fixed with 2% formaldehyde for 10 min and blocked with the M.O.M. Kit (Vector) followed by a 1 h incubation with blocking buffer. Cells were labeled with mouse anti-Pax7 (3.8 µg/ml, Developmental Studies Hybridoma Bank) and rabbit anti-MyoD [1 µg/ml, Santa Cruz Biotechnology (C-20), Santa Cruz, CA] in 0.5% donkey serum in PBS plus 0.05% Tween 20 (PBST) overnight at 4°C. After washes in PBST, cells were incubated with a biotinylated donkey anti-mouse IgG (1 µg/ml, Jackson ImmunoResearch) in blocking buffer for 1 h. A tyramide signal amplification green kit (TSA; Perkin Elmer) was utilized to visualize Pax7 as described above for Pax7 immune-labeling on sections. After the TSA reaction, the cells were incubated with AF594-conjugated donkey anti-rabbit IgG (1 µg/ml, Jackson ImmunoResearch) or AF647-conjugated donkey anti-rabbit IgG (5 µg/ml, Jackson ImmunoResearch) in blocking buffer for 40 min. After washes, nuclei were labeled with DAPI and cells were mounted with VectaShield (Vector) for imaging.
For detection of Pax7+ cells on single myofibers, fixed myofibers were immunolabeled as described above for FACS isolated cells. Nuclei were labeled with DAPI and imaged. For detection of VCAM-1 on tdTomato-positive satellite cells on myofibers, fixed myofibers were incubated in blocking buffer (0.3% BSA with 0.1% Triton X-100 in PBS) and immunolabeled with anti-VCAM-1 antibody (2 µg/ml, Abcam, Cambridge, MA) in 0.5× blocking buffer overnight at 4°C. After washes in 0.5× blocking buffer, myofibers were incubated in FITC-conjugated anti-rabbit IgG (1.5 µg/ml, Jackson ImmunoResearch) in 0.5× blocking buffer for 1 h. After washing, nuclei were counterstained with DAPI and myofibers were mounted with VectaShield for imaging.
Satellite cell isolation for culture.
To isolate satellite cells for in vitro culture experiments, a magnetic bead cell isolation kit was utilized (Miltenyi Biotec, Auburn, CA). Hindlimb muscles were dissected, mechanically minced in DMEM and digested with 0.1% pronase (Calbiochem, San Diego, CA) plus 25 mM HEPES at 37°C in an Enviro-Genie incubator with the stir setting at 150 rpm. The suspension was triturated, diluted in DMEM containing 10% FBS, and filtered through a 100-µm pore vacuum filtration system. Red blood cells were lysed with ACK Lysing Buffer (Lonza, Basel, Switzerland), and the cells were incubated on ice with the magnetic bead-conjugated Satellite Cell Isolation Kit antibodies (microbead conjugated-CD31, -CD45, and -Sca-1 for negative selection) (Miltenyi Biotec) for 15 min. The samples were loaded onto a magnetic separation column and the resulting elute containing satellite cells was collected. Cells were resuspended in growth media [Ham’s F10, 20% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, 5 ng/ml basic fibroblast growth factor (Peprotech, Rocky Hill, NJ)] and plated on collagen type I (Invitrogen)-coated culture dishes. Cultures were maintained for 7 days (<3 cell passages) at 37°C in 5% CO2 before in vitro experiments.
Immunoblotting.
Cultured satellite cells were lysed in RIPA-2 buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitors [Complete protease inhibitor tablet (Roche, Indianapolis, IN), 10 mM EDTA, 10 µM leupeptin, 5 µg/ml pepstatin A, 200 µM phenylmethylsulfonyl fluoride, 50 mM sodium fluoride, 2 mM sodium orthovanate, and 15 mM sodium pyrophosphate] followed by centrifugation at 21,000 g for 15 min at 4°C. The resulting supernatant was subjected to SDS-PAGE. Protein concentrations were determined by a Bradford assay (3) followed by equal amounts of protein from each sample loaded onto a gradient polyacrylamide gel (Any kD TGX mini-precast gels; Bio-Rad, Richmond, CA) and transferred to a 0.2-µm nitrocellulose membrane (Bio-Rad). Total transferred protein was stained with Ponceau solution (Sigma-Aldrich). The membranes were blocked in 10% milk in Tris-buffered saline (20 mM Tris·HCl pH 7.6, 150 mM NaCl) with 0.05% Tween 20 (TBST) for 30 min at room temperature and then incubated overnight in rabbit anti-VCAM-1 (400 ng/ml; Abcam) or mouse ant-Akt (1 μg/ml, Cell Signaling Technology, Danvers, MA) in TBST containing 5% milk or rabbit anti-phospho-Akt Ser473 (1 μg/ml; Covance, San Diego, CA) in 5% bovine serum albumin in TBST at 4°C. Membranes were washed in TBST, incubated with appropriate HRP-conjugated secondary antibodies (Jackson ImmunoResearch), and visualized using enhanced chemiluminescence (Sigma-Aldrich). Subsequently, membranes were washed and stripped in stripping buffer (62.5 mM Tris·HCl pH 6.8, 2% SDS, 100 mM β-mercaptoethanol) at 55°C for 30 min. After washing the membranes, they were blocked and reprobed with mouse anti-HSP90 (40 ng/ml; Santa Cruz Biotechnology) as described above. Band intensities were quantified by ImageJ 1.43u.
PCR.
Quantitative RT-PCR (RT-qPCR) was performed using primers to Itga1, Itgb1, and Gapdh (QIAGEN, Germantown, MD) on cDNA from satellite cells isolated from uninjured or injured muscle. Relative mRNA levels were determined using the comparative Ct method (33). All samples were normalized to Gapdh. Semiquantitative PCR was performed on cDNA isolated from myofibers using the same primers used for RT-qPCR.
Image acquisition.
Images of tissue sections and FACS isolated cells were captured on a microscope (Axioplan; Carl Zeiss MicroImaging, Thornwood, NY) with either a 0.3 numerical aperture (NA) ×10 Plan-Neofluar objective or a 0.5 NA ×20 Plan-Neofluar objective (Carl Zeiss MicroImaging) equipped with a CCD camera (Carl Zeiss MicroImaging) using Scion Image 1.63 (Scion Software, Brooklyn, NY). Myofibers were imaged using an Axiovert 200M microscope (Carl Zeiss MicroImaging) with a ×10 or ×20 Plan-Neofluar objective (Carl Zeiss MicroImaging) and camera (QImaging, Surrey, CA) with OpenLab 5.50 software (Perkin Elmer). For confocal imaging, an Olympus IX81 inverted microscope (Olympus, Center Valley, PA) with a 0.75 NA ×20 objective equipped with a CCD camera (Hamamatsu Orca ER, Bridgewater, NJ) with Olympus Fluoview software (version 1.7, Olympus) was utilized. All images were assembled using Adobe Photoshop CS5.1 for Macintosh (Adobe, San Jose, CA) and equally processed for size, color levels, brightness, and contrast.
Statistical analyses.
Data were analyzed for statistical significance using GraphPad Prism version 5 for Macintosh (GraphPad Software, San Diego, CA). For all statistical tests, a 0.05 level of confidence was considered statistically significant. When comparing two groups, data were analyzed by unpaired Student’s t test. To determine significance among multiple groups, data were analyzed using one-way analysis of variance with Tukey’s posttest. Nonparametric data were analyzed using the Kruskal-Wallis test with Dunn’s posttest to identify statistical differences between sample distributions.
RESULTS
Vcam1 is efficiently deleted in satellite cells.
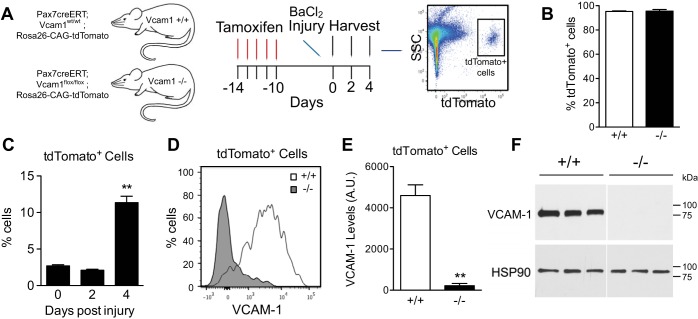
To define the function of VCAM-1 in mouse SC, we utilized a Pax7creERTM driver (22) for tamoxifen-inducible gene inactivation of a Vcam1 conditional allele (Vcam1fl/fl) (24). The Cre reporter allele R26RtdTomato was included for assessing the degree of recombination efficiency and for lineage marking (27, 29, 35). After tamoxifen treatment to generate Pax7creERTM/+;Vcam1wt/wt;R26RtdTomato (Vcam1+/+) and Pax7creERTM/+;Vcam1fl/fl;R26RtdTomato (Vcam1−/−) mice, SC were easily discernable by flow cytometry based on tdTomato fluorescence (Fig. 1A). To assess the degree of tamoxifen-mediated recombination in SC, we performed magnetic activated cell sorting (MACS) to isolate SC after negative selection with cell surface markers (Sca1, CD31, CD45). MACS isolated cells were >98% tdTomato+ by flow cytometry (Fig. 1B). By flow cytometry, tdTomato+ cells behaved as previously described for SC during the early stages of in vivo regeneration (58), including a delay in activation followed by a rapid proliferation phase resulting in a significant increase in the percentage of tdTomato+ cells 4 days postinjury (DPI) (Fig. 1C). Furthermore, surface levels of VCAM-1 on tdTomato+ SC were negligible in Vcam1−/− mice (Figs. 1, D and E). Total levels of VCAM-1 were also significantly decreased in immunoblots of Vcam1−/− SC (Fig. 1F). These results confirm that tamoxifen treatment results in efficient elimination of VCAM-1 in satellite cells compared with control.
Fig. 1.
Efficient deletion of Vcam1 in satellite cells. A: schematic of Vcam1 deletion and tdTomato activation by tamoxifen treatment and analysis of tdTomato-positive (tdTomato+) cells by flow cytometry. SSC, side scatter. B: satellite cells isolated from tamoxifen-treated mice were >95% tdTomato+. Satellite cells were isolated by magnetic activated cell sorting (MACS) after negative selection for CD31, CD45, and Sca-1. C: the percentage of tdTomato+ cells increased during regeneration in gastrocnemius muscles. D: representative flow cytometry plot of VCAM-1 on tdTomato+ cells 2 days postinjury. E: quantification of average VCAM-1 surface level intensity on tdTomato+ cells. AU, artificial units. F: satellite cells were isolated by MACS, cultured for 7 days, and analyzed by immunoblotting for total cellular levels of VCAM-1. Immunoblot depicts that total VCAM-1 was efficiently eliminated in Vcam1−/− satellite cells. Heat shock protein 90 (HSP90) was utilized as a loading control. **P < 0.01; n = 3.
Removal of VCAM-1 from satellite cells causes defects in early regeneration.
To determine whether the loss of VCAM-1 in SC alters muscle regeneration, we injured the TA muscles of Vcam1+/+ and Vcam1−/− mice with barium chloride injection and analyzed muscle sections at 7 and 30 days postinjury (DPI) for early- and late-stage regeneration defects, respectively (Fig. 2A). Histologic analyses of muscle sections suggested smaller myofibers in Vcam1−/− muscles at 7 DPI but not at 30 DPI (Fig. 2B). Indeed, the frequency distribution of myofiber cross-sectional areas (CSA) at 7 DPI was shifted to smaller myofibers in Vcam1−/− muscle compared with control (Fig. 2C). Furthermore, mean myofiber CSA at 7 DPI was significantly decreased by ~25% in Vcam1−/− mice compared with Vcam1+/+ controls (Fig. 2D). In contrast, at 30 DPI no difference in myofiber CSA between Vcam1−/− and Vcam1+/+ muscles was observed (Figs. 2, E and F). Taken together, these results suggest that VCAM-1 in SC plays a role during the early stages of muscle regeneration but is either compensated for or not necessary during later stages of regeneration.
Fig. 2.
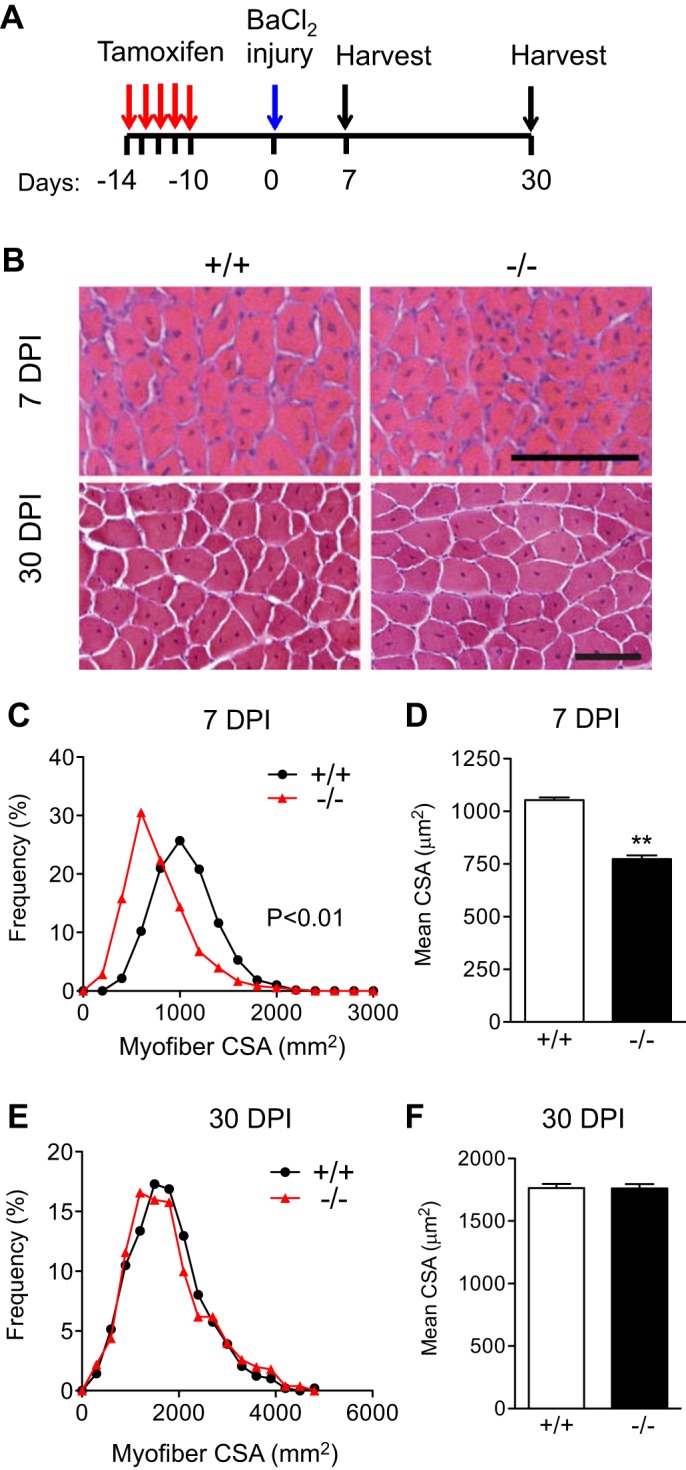
Loss of VCAM-1 in satellite cells results in early regeneration defects. A: schematic of tamoxifen treatment and muscle injury. B: representative images of tibialis anterior muscles 7 or 30 days postinjury (DPI). C and D: histogram shows shift to smaller myofiber cross-sectional area (CSA) in Vcam1−/− muscle compared with Vcam1+/+ resulting in decreased mean myofiber CSA at 7 DPI. E and F: no difference between Vcam1+/+ and Vcam1−/− muscles in either frequency distribution of myofiber CSA or mean myofiber CSA at 30 DPI. Scale bar, 100 µm. **P < 0.01; n = 3 for 7 DPI, n = 5 for 30 DPI.
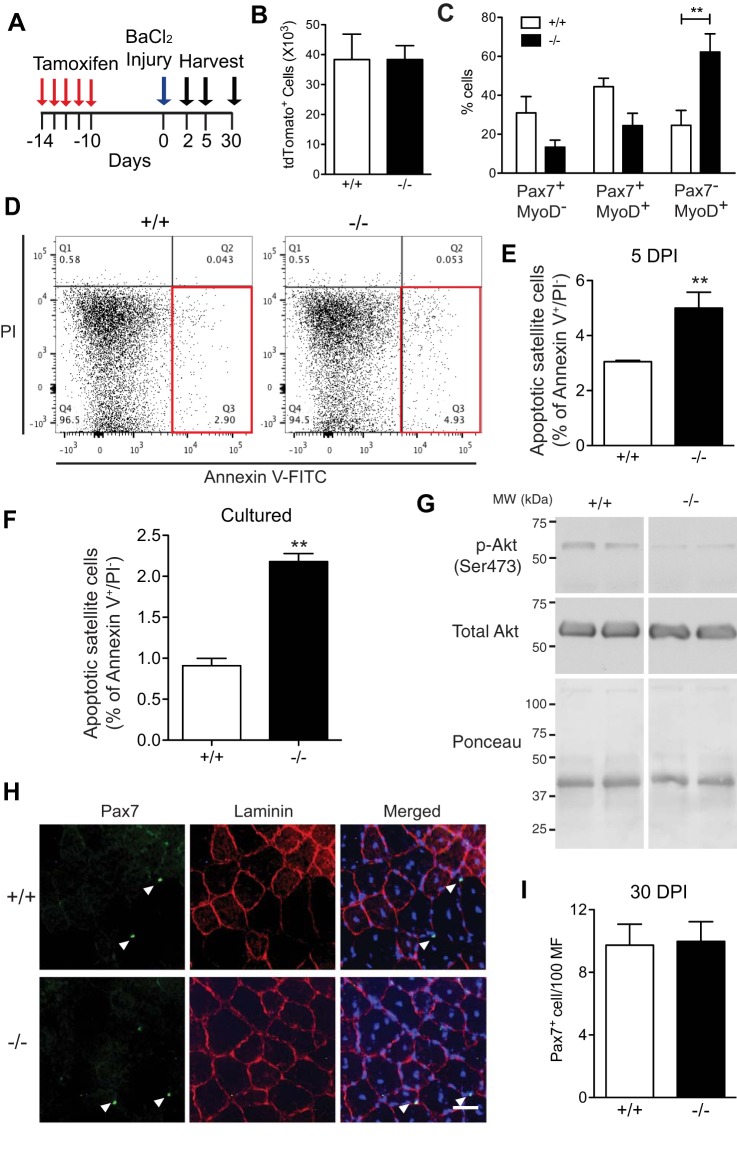
VCAM-1 deficiency alters SC lineage progression and survival but not self-renewal during regeneration.
To determine what changes occurred in Vcam1−/− SC that could explain the transient defect in the early stages of muscle regeneration, we injured muscles with barium chloride and harvested at either 2, 5, or 30 DPI (Fig. 3A). Multiple cellular parameters were analyzed including SC numbers, lineage progression, and apoptosis at early stages of muscle regeneration in gastrocnemius muscles. To analyze SC numbers during the early expansion phase after injury, the number of tdTomato+ cells in the mononucleated cell population was analyzed by flow cytometry at 2 DPI in Vcam1+/+ and Vcam1−/− mice but no significant difference was observed (Fig. 3B). Subsequently, to determine if myogenic lineage progression was disrupted upon VCAM-1 removal, SC were isolated by flow cytometry 2 DPI from Vcam1+/+ and Vcam1−/− mice followed by immediate immunofluorescent labeling for Pax7 and MyoD in vitro. The percentage of Pax7−/MyoD+ SC was significantly increased approximately threefold in Vcam1−/− SC compared with Vcam1+/+ (Fig. 3C), indicating premature lineage progression towards more differentiated SC. To further investigate the fate of Vcam1−/− SC, apoptosis was measured by flow cytometry at 5 DPI. Mononucleated cells were isolated from muscles and labeled with antibodies against Annexin V and PI to detect apoptotic cells (Fig. 3D), a method previously shown to correlate well with caspase 3 analyses of apoptotic SC in histologic sections (10). The percentage of early apoptotic (Annexin V+/PI−) tdTomato+ Vcam1−/− SC was approximately twofold higher than Vcam1+/+ (Fig. 3, D and E). To study the signaling pathways that may be involved in apoptosis of Vcam1−/− SC, further experiments were performed using Vcam1+/+ and Vcam1−/− myoblasts in vitro. Pure cultures of primary myoblasts were immunolabeled for Annexin V and stained with PI to detect apoptotic cells, as done on freshly isolated SC (Fig. 3D). Vcam1−/− myoblasts contained approximately twofold higher percentage of Annexin V+/PI− cells compared with Vcam1+/+ myoblasts (Fig. 3F) as previously observed in freshly isolated SC (Fig. 3E). Subsequently, we analyzed the levels of phospho-Akt, a prosurvival molecule downstream of VCAM-1 signaling in breast cancer cells (8). We observed that phospho-Akt was much decreased in Vcam1+/+ myoblasts (Fig. 3G) consistent with the increased apoptosis in these cells.
Fig. 3.
Loss of VCAM-1 alters satellite cell lineage progression and survival but not self-renewal during regeneration. A: schematic of tamoxifen treatment and muscle injury. Gastrocnemius muscles were used for B–G and TA muscles for H and I. B: no difference in the number of tdTomato+ cells 2 days postinjury was observed between the two genotypes. C: tdTomato+ cells were isolated by flow cytometry 2 days postinjury and immediately immunolabeled for Pax7 and MyoD. The percentage of Pax7−MyoD+ cells was significantly increased in Vcam1−/− satellite cells compared with Vcam1+/+. D: representative flow cytometry plots showing increased apoptotic tdTomato+ satellite cells (Annexin V+/PI−) in Vcam1−/− relative to Vcam1+/+ (red boxes). PI, propidium iodide. E: the percentage of apoptotic satellite cells was increased ~1.7-fold in Vcam1−/− relative to Vcam1+/+ muscles. F: apoptotic cells (Annexin V+/PI−) were increased ~2-fold in cultured Vcam1−/− myoblasts relative to Vcam1+/+ myoblasts. G: phospho (p)-AKT levels were decreased in cultured Vcam1−/− myoblasts relative to Vcam1+/+. H: representative images of Pax7 immunolabeling (green) with laminin immunostaining (red) 30 days postinjury. Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm. I: the number of Pax7+ cells/100 myofibers (MF) was not significantly different between genotypes 30 days postinjury (DPI). **P < 0.01; n = 3–5 per genotype.
Additional experiments were performed at late stages of muscle regeneration to determine whether loss of VCAM-1 altered self-renewal of SC. TA muscles were collected from Vcam1+/+ and Vcam1−/− mice 30 DPI, a time when self-renewal of satellite cells is complete, and then sectioned and immunostained for Pax7 to detect SC (Fig. 3H). No significant differences in the number of SC or their location underneath the basal lamina was observed between genotypes (Fig. 3I). Collectively, these results suggest that regeneration defects in Vcam1−/− muscles may result from a combination of premature SC differentiation and apoptosis.
Multiple cell types express the VCAM-1 ligand, VLA-4, within regenerating muscle.
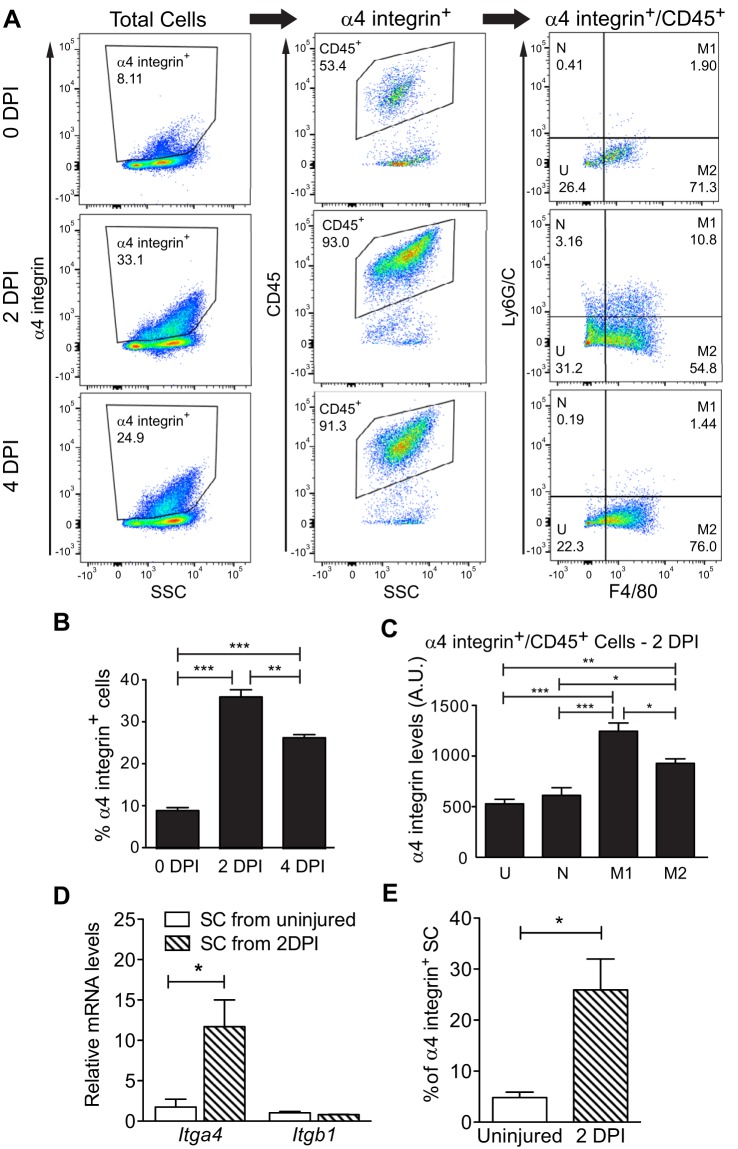
Given defects observed in Vcam1−/− SC during the early phases of muscle regeneration, we hypothesized that critical interactions between SC and cells expressing the major, high-affinity VCAM-1 ligand, α4β1 integrin (also referred to as VLA-4) (22), may be disrupted. Multiple types of immune cells express VLA-4 including neutrophils (13, 36) and macrophages (52), which are both cell types that infiltrate regenerating muscle and play important roles during regeneration (48, 55). To analyze VLA-4-expressing cells during regeneration, we assayed wild-type muscle at different times postinjury for α4 integrin by flow cytometry and noted that α4 integrin+ cells significantly increased at 2 and 4 DPI compared with uninjured muscle (Fig. 4, A and B), mirroring the expansion of satellite cells (Fig. 1B). The highest percentage of α4 integrin+ cells were CD45+, a marker of immune cells, with >90% of the α4 integrin+ cells positive for CD45+ after injury (Fig. 4A). Further characterization of the CD45+ cells using antibodies against Ly6G/C and F4/80 (30) showed that the majority of the α4 integrin+ cells were M1 and M2 macrophages (Fig. 4A, right panels). While α4 integrin was present on other immune cell types, M1 macrophages expressed the highest surface levels of α4 integrin (Fig. 4C). These findings suggest that VCAM-1/VLA-4-mediated interactions between SC and macrophages may play a functional role during regeneration.
Fig. 4.
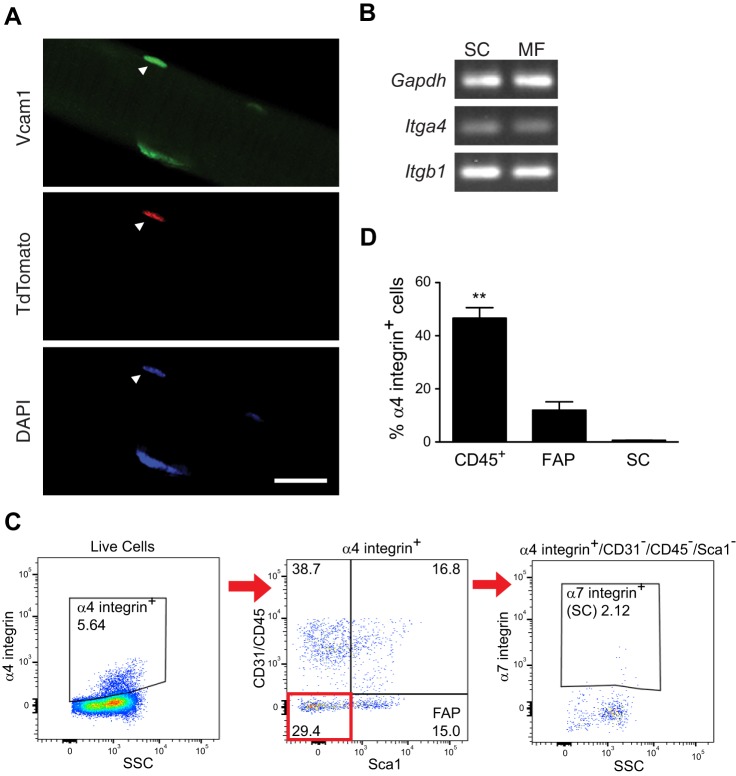
Multiple cell types express α4 integrin within regenerating wild-type muscles. A: uninjured hindlimb muscles (0 DPI) or injured gastrocnemius muscles (2 and 4 DPI) were analyzed by flow cytometry for surface levels of α4 integrin, CD45, Ly6G/C, and F4/80. B: the percentage of α4 integrin+ cells increased postinjury. C: M1 macrophages displayed the highest surface levels of α4 integrin compared with other CD45+ cells. DPI, days postinjury; N, neutrophils; M1, M1 macrophages; M2, M2 macrophages; U, uncharacterized CD45+ cells; AU, artificial units; n = 3. D: increased levels of Itga4 (encodes α4 integrin) but not Itgb1 (encodes β1 integrin) mRNA in satellite cells (SC) from injured muscle as detected by RT-qPCR and normalized to Gapdh; n = 3. E: increased percentage of α4 integrin-positive satellite cells after injury as measured by flow cytometry. ***P < 0.001, **P < 0.01, *P < 0.05; n = 4.
To determine if SC also express the VCAM-1 ligand VLA-4, we isolated SC from uninjured or 2 DPI wild-type muscles and used RT-qPCR to demonstrate that steady-state levels of Itga4 mRNA, which encodes α4 integrin, and surface levels of α4 integrin increase on SC isolated from 2 DPI muscle relative to SC isolated from uninjured muscle (Fig. 4, D and E). SC also express Itgb1, which encodes β1 integrin, but no change in expression was noted upon injury (Fig. 4D). Given that the percentage of apoptotic SC increases after injury in Vcam1−/− mice, together these results are consistent with a prosurvival interaction among SC and with immune cells via VCAM-1/VLA-4.
Loss of VCAM-1 differentially alters SC behavior in uninjured muscles compared with injured muscles.
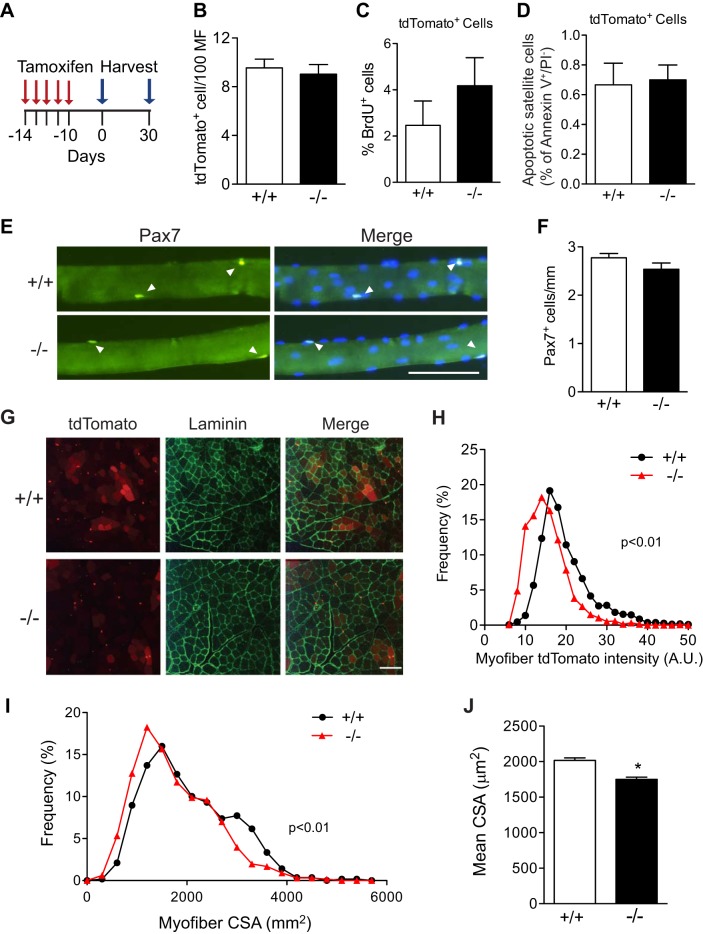
Given the dramatic differences that occur in the SC niche between the basal and regenerating states, we hypothesized that the loss of VCAM-1 in SC may differentially effect SC behavior in uninjured muscles compared with regenerating muscles. To address this question, muscles of 6- to 8-wk-old Vcam1−/− and Vcam1+/+mice were analyzed 10 and 40 days after tamoxifen treatment (Fig. 5A) using various assays. We observed no significant difference in the number of tdTomato+ cells per 100 myofibers on sections of TA muscles between genotypes 10 days after tamoxifen treatment (Fig. 5B). Similarly, no differences were noted in the percentage of proliferating BrdU+ SC (Fig. 5C) or of apoptotic SC (Fig. 5D) as analyzed by flow cytometry in Vcam1−/− and Vcam1+/+ mice. This latter result contrasts with the increase in apoptotic SC which was observed in regenerating muscles (Figs. 3, D and E) and isolated myoblasts (Fig. 3F) of Vcam1−/− mice. Further experiments were performed to determine if adhesion of SC to the myofiber niche is altered given reports that loss of VCAM-1 in hematopoietic stem cells decreases adhesion to their bone marrow niche (56). Single myofibers 10 days after tamoxifen injection were isolated from gastrocnemius muscles, and the number of associated Pax7+ SC was determined in vitro (Fig. 5E). No significant difference was noted in the number of Pax7+ SC between genotypes (Fig. 5F), indicating that loss of VCAM-1 does not affect the adhesion of SC to myofibers during the extensive washing steps of myofiber isolation. Recent reports indicate a basal level of SC fusion with myofibers occurs in uninjured muscle (26, 40). Myofibers in sections of TA muscles from uninjured Vcam1+/+ mice 10 days after tamoxifen injection displayed a range of tdTomato fluorescence intensities, indicating SC fusion with myofibers (Fig. 5G). However, myofiber tdTomato intensity was decreased in Vcam1−/− TA sections, indicating decreased SC fusion compared with Vcam1+/+ (Fig. 5H). To determine the consequences of decreased SC fusion in Vcam1−/− muscles on myofiber growth in these young adult mice, myofiber CSA was analyzed on TA muscle sections 40 days after tamoxifen treatment. The frequency distribution of myofiber CSA was shifted leftward, indicating smaller myofibers in Vcam1−/− muscle compared with Vcam1+/+ (Fig. 5I). Furthermore, mean myofiber CSA was significantly decreased by 10% in Vcam1−/− mice compared with Vcam1+/+ controls (Fig. 5J). Taken together, these results indicate that numerous SC parameters (number, proliferation, apoptosis, adhesion) are normal in the absence of VCAM-1 in uninjured muscle. However, SC fusion is decreased in uninjured Vcam1−/− muscle compared with Vcam1+/+ and likely contributed to decreased myofiber growth observed in these mice. The differences in apoptotic SC between uninjured and injured muscles may arise from differences in VCAM-1 ligand-expressing cells.
Fig. 5.
Loss of VCAM-1 only alters satellite cell fusion in uninjured muscles. A: schematic of tamoxifen treatment and cell harvest from uninjured muscles. B: the number of tdTomato+ cells per 100 myofibers in sections of tibialis anterior muscles 10 days after the end of tamoxifen treatment did not differ between genotypes. C: by flow cytometry, the percentage of BrdU+ tdTomato+ cells in hindlimb muscles 10 days after the end of tamoxifen treatment did not differ significantly between genotypes. D: by flow cytometry, the percentage of apoptotic tdTomato+ cells in hindlimb muscles 10 days after tamoxifen injection did not differ between genotypes. E: myofibers were isolated from gastrocnemius muscles 10 days after the end of tamoxifen treatment and immunolabeled for Pax7 (green). Pax7+ cells are indicated by arrowheads. Nuclei were counterstained with DAPI (blue). Scale bar, 100 µm. F: no significant difference in the number of Pax7+ cells on isolated myofibers between genotypes; n = 3 mice with >24 myofibers/mouse. G: representative sections of tibialis anterior muscles 10 days after the end of tamoxifen treatment showing tdTomato (red) immunofluorescence and laminin (green) immunostaining. Scale bar, 200 µm. H: histogram depicting frequency distribution of myofiber tdTomato intensity in sections. Vcam1−/− myofibers displayed significantly less tdTomato intensity than Vcam1+/+. AU, artificial units. I: histogram depicting shift to smaller myofiber cross-sectional area (CSA) in Vcam1−/− muscles compared with Vcam1+/+ 40 days after the end of tamoxifen treatment. J: mean myofiber cross-sectional area (CSA) was significantly decreased in Vcam1−/− muscles compared with Vcam1+/+ 40 days after tamoxifen treatment. *P < 0.05.
Differential expression of VCAM-1 ligands in uninjured muscles.
To determine which VCAM-1 interactions might be most relevant in the basal state, we first immunostained tdTomato+ SC cells on isolated myofibers for VCAM-1 to determine the localization of VCAM-1. If VCAM-1 is most relevant to interaction with the myofiber, VCAM-1 would be expected to be concentrated on the interface between SC and myofiber. If it were more important for interaction with other cell types, VCAM-1 would be expected to be localized to the external facing portion of the SC. We detected even VCAM-1 staining on all areas of SC (Fig. 6A), suggesting that VCAM-1 interactions with multiple cell types is important for function in the basal state. Semiquantitative PCR revealed the presence of both Itga4 and Itgb1 mRNA in isolated myofibers, demonstrating that myofibers express VLA-4 (Fig. 6B). In addition to myofibers, flow cytometry data showed a significant percentage of cell-surface α4 integrin-expressing cells was made up of CD45+ cells and lower percentages of cell surface α4 integrin-expressing cells were FAPs or satellite cells (Fig. 6, C and D). Taken together, these results suggest that interactions between satellite cells and multiple cells types may contribute to function in the basal niche.
Fig. 6.
Expression of VCAM-1 and its ligands in uninjured wild-type muscles. A: VCAM-1 (green) immunofluorescent labeling of tdTomato+ satellite cell (red) on isolated myofiber. Nuclei were counterstained with DAPI (blue). B: semiquantitative RT-PCR demonstrating that Itga4 (encodes α4 integrin) and Itgb1 (encodes β1 integrin) mRNAs are detected in myofibers. Shown is a representative gel of n = 3 experiments per cell type. C: flow cytometry analysis of α4 integrin+ cells for markers of fibro/adipogenic progenitors (FAP; CD31−/CD45−/Sca1+) and satellite cells (SC; CD31−/CD45−/Sca1−/α7 integrin+). D: the predominant α4 integrin+ mononucleated cell population in uninjured hindlimb muscle was CD45+. **P < 0.01, n = 3.
DISCUSSION
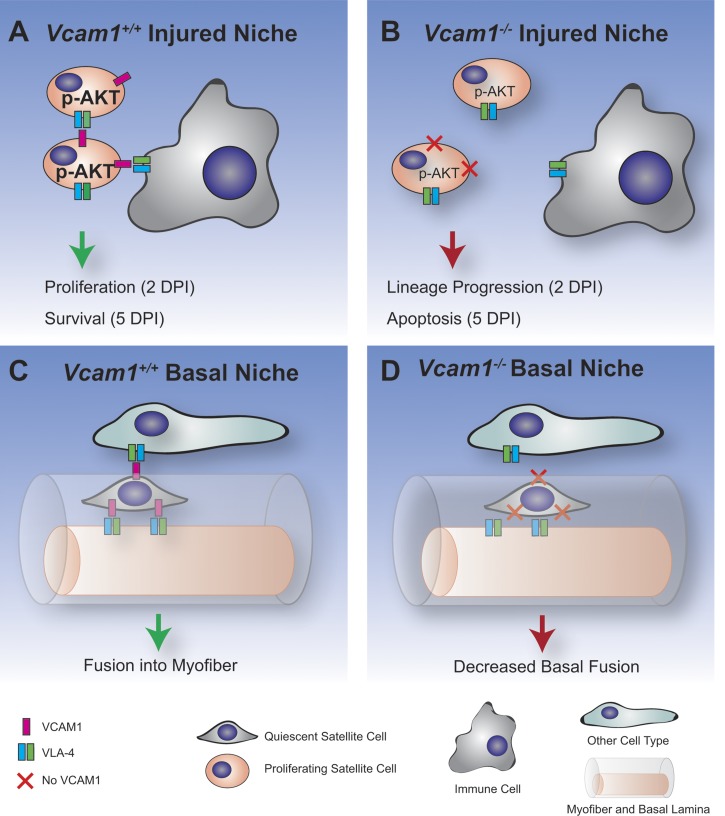
Multiple types of cell-cell adhesion molecules are expressed on SC including cadherins, integrins, claudins, and immunoglobulin (Ig) superfamily molecules, although most of these have only been identified at the mRNA level (4, 15, 19, 42, 46). Although the in vitro roles for some of these molecules have been studied using muscle cell lines and primary myoblasts (42, 46, 57), the in vivo roles on SC are unknown as mice with SC-specific deletion of these cell-cell adhesion molecules have not been generated. Here we studied the role of VCAM-1, a member of the Ig superfamily of molecules, which is routinely used to isolate quiescent and activated SC from mouse muscle by flow cytometry (5, 9, 17). Using SC-specific deletion of Vcam1, we analyzed the cellular behavior of SC under both basal and regenerating conditions and observed differences depending on the state of the SC niche (Fig. 7).
Fig. 7.
Model of niche-specific roles of VCAM-1 on satellite cells. A: in the injured niche, VCAM-1 on satellite cells interacts with the VLA-4 ligand on immune cells and other satellite cells to increase phospho-Akt and promote proliferation and survival. B: in the absence of VCAM-1 (Vcam1−/−), satellite cells in the injured niche undergo premature lineage progression and apoptosis. C: in the basal niche, VCAM-1 on satellite cells interacts with the VLA-4 ligand to mediate fusion into the myofiber. D: in the absence of VCAM-1, basal fusion of satellite cells is decreased.
The upregulation of VCAM-1 on SC during muscle regeneration (32) suggested that it may play a critical role in regulating the behavior of these cells. We observed enhanced lineage progression of Vcam1−/− SC as evidenced by an increased percentage of Pax7−MyoD+ satellite cells 2 DPI (Fig. 7, A and B). Furthermore, apoptotic SC also increased twofold during the early phases of muscle regeneration in the absence of VCAM-1 (Fig. 7, A and B). Increased apoptotic SC were similarly increased in cultures of Vcam1−/− myoblasts in vitro. Blocking antibodies against VCAM-1 have previously been shown to abrogate the prosurvival effect of macrophages on cultured human myoblasts and myotubes (53). Furthermore, we observed decreased levels of phospho-Akt in cultured Vcam1−/− myoblasts consistent with studies showing activation of VCAM-1 promotes cell survival in breast cancer cells through phospho-Akt (8). Both enhanced SC lineage progression and apoptosis would be predicted to alter the kinetics of muscle regeneration. Indeed, myofiber cross-sectional area was decreased 7 days after injury in the absence of VCAM-1 in SC. No defects in myofiber cross-sectional area were noted 30 days after injury in these mice, suggesting that either VCAM-1 is not required at later stages of regeneration or compensation occurs due to other cell-cell adhesion molecules. Of note, SC self-renewal was normal in Vcam1−/− SC as the number of Pax7+ SC did not differ from control 30 days after injury. These are the first studies to define the role of VCAM-1 in SC during muscle regeneration.
In contrast to our data in regenerating muscles, different phenotypes were observed for Vcam1−/− SC in uninjured muscles of young mice (Fig. 7, C and D). Loss of VCAM-1 in SC did not affect SC numbers, quiescence, proliferation, apoptosis, or adhesion to myofibers. These results were surprising as previous studies have suggested that loss of VCAM-1 in other stem cell niches led to changes in some of these parameters. For example, VCAM-1 in hematopoietic and endothelial cells is required for adhesion of immature lymphocytes in the bone marrow as increased numbers of progenitors were observed in the circulation upon VCAM-1 deletion (29, 56). Loss of VCAM-1 in neural stem cells of the subventricular zone in the brain leads to loss of quiescence and enhanced differentiation (28). These results indicate that VCAM-1 plays stem cell-specific roles likely due in part to the combination of cell-cell adhesion molecules expressed by different types of stem cells. We did observe that basal SC fusion with myofibers was decreased in Vcam1−/− SC, leading to decreased myofiber cross-sectional area (Fig. 7, C and D). Given this identified role of VCAM-1 in SC fusion, we cannot rule out a role for VCAM-1-mediated cell fusion during muscle regeneration. These effects of VCAM-1 on SC fusion in vivo extend previous studies performed in vitro in which blocking antibodies against VCAM-1 decreased myoblast fusion (46). VCAM-1 not only appears to regulate fusion of muscle cells to themselves but also fusion of other cell types. For example, VCAM-1 is required for fusion of non-muscle cells such as epicardium-derived cells mesenchymal stromal cells with preexisting myotubes in vitro (18). Furthermore, global knockout of Vcam1 in mice leads to defects in choriallantoic fusion and placentation resulting in early embryonic death (21). Our results, together with previous studies, indicate both cell-specific and niche-specific functions of VCAM-1.
What could account for these differences in Vcam1−/− SC behavior in the basal vs. regenerating niche? During muscle regeneration, dynamic changes occur in the SC niche including time-dependent influxes of other cell types including neutrophils, macrophages, and fibro/adipogenic progenitors (30). Immune cells such as neutrophils and macrophages are well known to control multiple aspects of satellite cell biology during regeneration through secreted molecules (6), but far less is known about interactions between SC and immune cells via cell-cell adhesion molecules (24). The major high-affinity ligand of VCAM-1 is VLA-4, which is a heterodimer of α4 integrin and β1 integrin (16, 39). We observed an influx of multiple immune cell types expressing α4 integrin during the early phases of muscle regeneration. These included neutrophils, M1 and M2 macrophages as well as other unidentified CD45+ immune cells. Immune cells were not the only cell type to express the VLA-4. Indeed, RT-qPCR revealed that both α4 and β1 integrins are expressed by SC. Furthermore, α4 integrin was approximately fivefold higher on SC at early stages of muscle regeneration compared with SC from uninjured muscles. These data suggest that VCAM-1 may regulate apoptosis of SC both through interactions with immune cells such as macrophages as well as with other SC through VLA-4. Under basal uninjured conditions, VLA-4 was expressed both by the myofiber as well as by SC, FAPs, and unidentified CD45+ cells. The effects of VCAM-1 on SC fusion are likely mediated by interactions between SC and myofibers. Together, these results indicate that multiple cell types in both uninjured and regenerating muscle express VLA-4 and have the potential to modulate the function of VCAM-1+ SC.
In summary, these studies are a first step towards a global understanding of the role of various cell-cell adhesion molecules in SC in vivo. Further experiments are needed to determine the degree of functional overlap between VCAM-1 and other cell-cell adhesion molecules expressed by SC. These studies underscore the complexity of molecules by which SC interact with their niche and integrate multiple signals to regulate SC behavior in basal and regenerating conditions.
GRANTS
This work was supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants 5F32AR068207 (to K. E. Vest) and AR061267 (to G. K. Pavlath).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.-J.C., J.P.C., K.E.V., and G.K.P. conceived and designed research; H.-J.C., J.P.C., K.E.V., and Z.T. performed experiments; H.-J.C., J.P.C., K.E.V., Z.T., and G.K.P. analyzed data; H.-J.C., J.P.C., K.E.V., Z.T., and G.K.P. interpreted results of experiments; H.-J.C., J.P.C., and K.E.V. prepared figures; H.-J.C., J.P.C., K.E.V., and G.K.P. drafted manuscript; H.-J.C., J.P.C., K.E.V., and G.K.P. edited and revised manuscript; H.-J.C., J.P.C., K.E.V., Z.T., and G.K.P. approved final version of manuscript.
REFERENCES
- 1.Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development 139: 641–656, 2012. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentzinger CF, Wang YX, von Maltzahn J, Rudnicki MA. The emerging biology of muscle stem cells: implications for cell-based therapies. BioEssays 35: 231–241, 2013. doi: 10.1002/bies.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Capkovic KL, Stevenson S, Johnson MC, Thelen JJ, Cornelison DD. Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp Cell Res 314: 1553–1565, 2008. doi: 10.1016/j.yexcr.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A, Brack AS. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development 141: 1649–1659, 2014. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37: 18–22, 2009. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 7.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20: 538–549, 2011. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482: 524–528, 2012. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choo HJ, Cutler A, Rother F, Bader M, Pavlath GK. Karyopherin alpha 1 regulates satellite cell proliferation and survival by modulating nuclear import. Stem Cells 34: 2784–2797, 2016. doi: 10.1002/stem.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science 302: 1575–1577, 2003. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 13.Conran N, Gambero A, Ferreira HH, Antunes E, de Nucci G. Nitric oxide has a role in regulating VLA-4-integrin expression on the human neutrophil cell surface. Biochem Pharmacol 66: 43–50, 2003. doi: 10.1016/S0006-2952(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 14.Covault J, Sanes JR. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol 102: 716–730, 1986. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dearth CL, Goh Q, Marino JS, Cicinelli PA, Torres-Palsa MJ, Pierre P, Worth RG, Pizza FX. Skeletal muscle cells express ICAM-1 after muscle overload and ICAM-1 contributes to the ensuing hypertrophic response. PLoS One 8: e58486, 2013. doi: 10.1371/journal.pone.0058486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 60: 577–584, 1990. doi: 10.1016/0092-8674(90)90661-W. [DOI] [PubMed] [Google Scholar]
- 17.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25: 2448–2459, 2007. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 18.Gentile A, Toietta G, Pazzano V, Tsiopoulos VD, Giglio AF, Crea F, Pompilio G, Capogrossi MC, Di Rocco G. Human epicardium-derived cells fuse with high efficiency with skeletal myotubes and differentiate toward the skeletal muscle phenotype: a comparison study with stromal and endothelial cells. Mol Biol Cell 22: 581–592, 2011. doi: 10.1091/mbc.E10-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassot V, Da Silva A, Saliba J, Maftah A, Dupuy F, Petit JM. Highlights of glycosylation and adhesion related genes involved in myogenesis. BMC Genomics 15: 621, 2014. doi: 10.1186/1471-2164-15-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Günther S, Kim J, Kostin S, Lepper C, Fan CM, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 13: 590–601, 2013. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev 9: 1–14, 1995. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev 114: 45–65, 1990. doi: 10.1111/j.1600-065X.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 23.Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199: 326–337, 1994. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- 24.Jesse TL, LaChance R, Iademarco MF, Dean DC. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J Cell Biol 140: 1265–1276, 1998. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6: 7087, 2015. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller C, Hansen MS, Coffin CM, Capecchi MR. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev 18: 2608–2613, 2004. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, Shen Q, Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11: 220–230, 2012. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med 193: 741–754, 2001. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 31.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc 10: 1612–1624, 2015. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 25: 358–369, 2011. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massena S, Christoffersson G, Vågesjö E, Seignez C, Gustafsson K, Binet F, Herrera Hidalgo C, Giraud A, Lomei J, Weström S, Shibuya M, Claesson-Welsh L, Gerwins P, Welsh M, Kreuger J, Phillipson M. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 126: 2016–2026, 2015. doi: 10.1182/blood-2015-03-631572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59: 1203–1211, 1989. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 38.Pallafacchina G, François S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res (Amst) 4: 77–91, 2010. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 91: 2231–2239, 1998. [PubMed] [Google Scholar]
- 40.Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin BB. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5: 42, 2015. doi: 10.1186/s13395-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichavant C, Pavlath GK. Incidence and severity of myofiber branching with regeneration and aging. Skelet Muscle 4: 9, 2014. doi: 10.1186/2044-5040-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przewǒzniak M, Czaplicka I, Czerwińska AM, Markowska-Zagrajek A, Moraczewski J, Stremińska W, Jańczyk-Ilach K, Ciemerych MA, Brzoska E. Adhesion proteins–an impact on skeletal myoblast differentiation. PLoS One 8: e61760, 2013. doi: 10.1371/journal.pone.0061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem 287: 25795–25807, 2012. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randolph ME, Phillips BL, Choo HJ, Vest KE, Vera Y, Pavlath GK. Pharyngeal satellite cells undergo myogenesis under basal conditions and are required for pharyngeal muscle maintenance. Stem Cells 33: 3581–3595, 2015. doi: 10.1002/stem.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raymond K, Deugnier MA, Faraldo MM, Glukhova MA. Adhesion within the stem cell niches. Curr Opin Cell Biol 21: 623–629, 2009. doi: 10.1016/j.ceb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69: 1107–1119, 1992. doi: 10.1016/0092-8674(92)90633-N. [DOI] [PubMed] [Google Scholar]
- 48.Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J 280: 4118–4130, 2013. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 49.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31: 384–396, 2013. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 50.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)–an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer 36: 2504–2514, 2015 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 51.Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res 314: 3232–3244, 2008. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Shima M, Teitelbaum SL, Holers VM, Ruzicka C, Osmack P, Ross FP. Macrophage-colony-stimulating factor regulates expression of the integrins alpha 4 beta 1 and alpha 5 beta 1 by murine bone marrow macrophages. Proc Natl Acad Sci USA 92: 5179–5183, 1995. doi: 10.1073/pnas.92.11.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonnet C, Lafuste P, Arnold L, Brigitte M, Poron F, Authier FJ, Chrétien F, Gherardi RK, Chazaud B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci 119: 2497–2507, 2006. doi: 10.1242/jcs.02988. [DOI] [PubMed] [Google Scholar]
- 54.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol 290: R1488–R1495, 2006. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 55.Tidball JG, Dorshkind K, Wehling-Henricks M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 141: 1184–1196, 2014. doi: 10.1242/dev.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 106: 86–94, 2005. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Hao Y, Alway SE. Suppression of GSK-3β activation by M-cadherin protects myoblasts against mitochondria-associated apoptosis during myogenic differentiation. J Cell Sci 124: 3835–3847, 2011. doi: 10.1242/jcs.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang JT, Rando TA, Mohler WA, Rayburn H, Blau HM, Hynes RO. Genetic analysis of alpha 4 integrin functions in the development of mouse skeletal muscle. J Cell Biol 135: 829–835, 1996. doi: 10.1083/jcb.135.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]