Abstract
Exosomes are nano-sized vesicles produced and secreted by cells to mediate intercellular communication. The production and function of exosomes in kidney tissues and cells remain largely unclear. Hypoxia is a common pathophysiological condition in kidneys. This study was designed to characterize exosome production during hypoxia of rat renal proximal tubular cells (RPTCs), investigate the regulation by hypoxia-inducible factor-1 (HIF-1), and determine the effect of the exosomes on ATP-depletion-induced tubular cell injury. Hypoxia did not change the average sizes of exosomes secreted by RPTCs, but it significantly increased exosome production in a time-dependent manner. HIF-1 induction with dimethyloxalylglycine also promoted exosome secretion, whereas pharmacological and genetic suppression of HIF-1 abrogated the increase of exosome secretion under hypoxia. The exosomes from hypoxic RPTCs had inhibitory effects on apoptosis of RPTCs following ATP depletion. The protective effects were lost in the exosomes from HIF-1α knockdown cells. It is concluded that hypoxia stimulates exosome production and secretion in renal tubular cells. The exosomes from hypoxic cells are protective against renal tubular cell injury. HIF-1 mediates exosome production during hypoxia and contributes to the cytoprotective effect of the exosomes.
Keywords: exosome, HIF-1, hypoxia, kidney injury, ATP depletion
exosomes are small, membrane-bound vesicles of 50–150 nm in diameter that are produced by cells and secreted to extracellular space. The contents of exosomes include a variety of proteins, RNAs, DNAs, and other types of molecules. After secretion, exosomes may traffic to other cells to release their contents and thus act as an important mean of cell-to-cell communication. The biogenesis, secretion, and cargo contents of exosomes depend on cellular context and are regulated under various pathophysiological conditions (6, 20). Recent research has implicated exosomes in renal pathophysiology (3, 7, 29). However, the role and regulation of exosomes in kidney cells and tissues remain poorly understood.
Hypoxia is an important pathophysiological condition encountered by cells and tissues. For example, hypoxia occurs in ischemic diseases such as myocardial infarction, stroke, and acute kidney injury. Hypoxia is also known to be a feature of solid tumors that may contribute to tumorigenesis, therapy resistance, and cancer metastasis. In response to hypoxia, cells may activate various signaling pathways and a myriad of transcription factors for cell survival and adaptation, and when hypoxic stress becomes overwhelming, cell death ensues. Hypoxia-inducible factors (HIFs) are a family of transcription factors that are responsive to hypoxia and mainly responsible for cellular adaptation (22). As such, HIFs are known to regulate energy use, metabolism, oxidative stress, and cell death and survival. Functional or transcriptionally active HIF consists of a heterodimer of α- and β-subunits. Whereas HIF-β is constitutively expressed, HIF-α is stabilized and expressed under hypoxia. Therefore, HIF activity is largely dependent on the level of HIF-α expression. Under normoxia or normal oxygen (21% O2) conditions, HIF-α is hydroxylated at specific proline residues by prolyl hydroxylases, resulting in its association with the E3 ligase von Hippel-Lindau (VHL) and consequent proteasomal degradation. In hypoxia or low-oxygen conditions, proline hydroxylation in HIF-α is blocked, leading to HIF-α accumulation, dimerization with HIF-β, and then translocation to the nucleus for hypoxic gene transcription.
In the field of cancer research, exosome secretion was reported to increase under hypoxia, and this increase appeared HIF-1α dependent (11). Hypoxic exosomes were further shown to be a mediator that aggravates tumor progression by transmission of particular content that may promote tumor cell survival and invasion (23). In kidney, increased exosome secretion was detected in ureteral obstructed kidney as well as MCT and HK2 cell lines under hypoxic conditions (2). However, the regulatory mechanism and function of exosome secretion in renal tubular cells under hypoxia remain largely unclear. In this study, we characterized exosome production during hypoxia of renal tubular cells, determined the role of HIF-1, and analyzed the effect of exosomes on ATP-depletion-associated injury.
MATERIALS AND METHODS
Antibodies and special reagents.
Antibodies and reagents were purchased from the following sources: monoclonal anti-CD63 and anti-tumor susceptibility gene 101 (anti-TSG101) antibodies from System Biosciences (Palo Alto, CA); anti-caspase-3, anti-HIF-1α, and anti-GAPDH antibodies from Cell Signaling Technology (Beverly, MA); secondary antibodies for immunoblot analysis from Jackson ImmunoResearch (West Grove, PA); dimethyloxalylglycine (DMOG) from Sigma (St. Louis, MO); YC-1 from Cayman Chemical; and Exosome-Depleted FBS from GIBCO (Thermo Fisher Scientific).
Cell culture.
The renal proximal tubular cell (RPTC) line was originally obtained from Dr. Ulrich Hopfer (Case Western Reserve University, Cleveland, OH) and cultured in DMEM/F-12 media containing 10% FBS for experiments. Wild-type and HIF-1α-null mouse embryonic fibroblasts (MEFs) were obtained from Dr. Gregg Semenza (Johns Hopkins University, Baltimore, MD) and grown in DMEM with 15% FBS. HIF-1α knockdown RPTCs were generated as described in our previous work (19). All of the cells were maintained at 37°C in an atmosphere of 5% CO2. Two percent Exosome-Depleted FBS (instead of normal FBS) was used for cell culture during experimental treatment.
Hypoxic incubation.
Hypoxia treatment was conducted in a hypoxic chamber as described previously (26). Briefly, dishes with cells were transferred into a hypoxic chamber (Coy Laboratory Products, Ann Arbor, MI) containing 1, 0.2, 2, 4, or 8% oxygen, respectively, according to the experimental design. The oxygen tension in the chamber was monitored and maintained by a computerized sensor probe. Humidity was maintained with a humidifier. All of the medium used for hypoxia treatment was preincubated in the chamber for over 6 h to reach a balanced oxygen concentration.
Exosome isolation and identification.
Cell culture medium was collected and subjected to a series of centrifugation (800 g, 10 min; 2,000 g, 20 min; 10,000 g, 30 min). The resultant supernatant was finally subjected to ultracentrifugation (Rotor SW 28 and SW 55, Optima L-100 XP; Beckman Coulter) at 100,000 g for 90 min to collect exosomes in pellet, which was lysed for protein analysis and resuspended in phosphate-buffered saline (PBS) for quantification or −80°C storage.
Nanoparticle tracking analysis.
We completed nanoparticle tracking analysis (NTA) with ZetaView (Particle Metrix) to analyze the size distribution and concentration of the exosome preparations as described recently (9, 30). Isolated exosomes were diluted to 1:500 or 1:1,000 in particle-free PBS and resuspended before being injected into the sample cell chamber. Size distributions and particle concentrations were assessed with NTA software. Exosome concentration analysis was normalized with the total number of cells from the corresponding dish. To quantify the cell number, the cells in each dish were harvested at the end of treatment and digested into suspension by trypsin for quantification with a TC20 Automated Cell Counter.
Transmission electron microscopy.
Transmission electron microscopy (TEM) was conducted by Electron Microscopy Core of Augusta University as described previously (9, 30). Three microliters of exosomes pellet solution was applied on Formvar/carbon-coated 200-mesh copper electron microscopy grids, incubated at room temperature for 5 min, and then subjected to standard uranyl acetate staining. The grid was washed with PBS three times and allowed to semidry at room temperature before observation in transmission electron microscope (Hitachi H7500 TEM; Tokyo, Japan).
Western blot analysis.
Cell lysate and exosomal proteins were extracted with 2% SDS buffer. Protein concentration was quantified with a Pierce BCA Protein Assay Kit (Thermo Scientific). Total exosomal protein loading for Western blot was normalized with total cell number of the corresponding dishes as described above. Protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% milk for 1 h at room temperature and then immunoblotted with primary antibody at 4°C overnight. The blot membrane was then washed three times and incubated with horseradish peroxidase-conjugated secondary antibodies. The blot signal was revealed with a chemiluminescence kit (Bio-Rad).
Statistical analysis.
All values are expressed as means ± SD. Statistical analysis was conducted using GraphPad Prism software (San Diego, CA). Comparisons between two groups were performed by Student's t-test. For multiple comparisons, ANOVA was used. P < 0.05 was considered reflecting significant differences. Each experiment was conducted independently at least three times.
RESULTS
Isolation and characterization of exosomes produced by RPTCs.
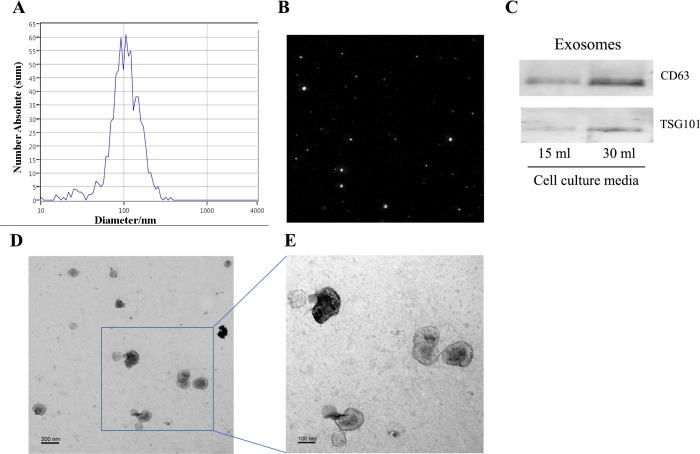
Exosomes isolated from cultured media of RPTCs by serial ultracentrifugation by nanoparticle tracking analysis (NTA) of the isolated samples indicated that most of the particles had a size of 50–150 nm in diameter with a peak at ~100 nm (Fig. 1, A and B). Immunoblot analysis (Fig. 1C) further detected CD63 and TSG101, two widely accepted markers of exosomes, in the isolated samples. As expected, the exosome sample from 30-ml cell culture medium showed higher content of CD63 and TSG101 than that from 15-ml medium (Fig. 1C). In addition, electron microscopy revealed the presence of exosomes within the expected size range of exosomes (50–150 nm) and bilayer cup-shaped morphology (Fig. 1, D and E). The results verify our exosome isolation protocol and indicate that renal tubular cells produce and secrete typical exosomes.
Fig. 1.
Isolation and characterization of exosomes produced by RPTCs. RPTCs were cultured for 24 h with exosome-free FBS to collect medium for exosome isolation by serial ultracentrifugation. A: size distribution of the isolated particles analyzed by NTA. B: representative potential image of the particles during NTA ZetaView quantification. C: immunoblot analysis of exosomal proteins CD63 and TSG101 in the exosome preparations from 15- and 30-ml cell culture media. D and E: examination of isolated exosomes by transmission electron microscopy. Scale bars are 200 and 100 nm in D and E, respectively.
Enhanced exosome production during hypoxia of RPTCs.
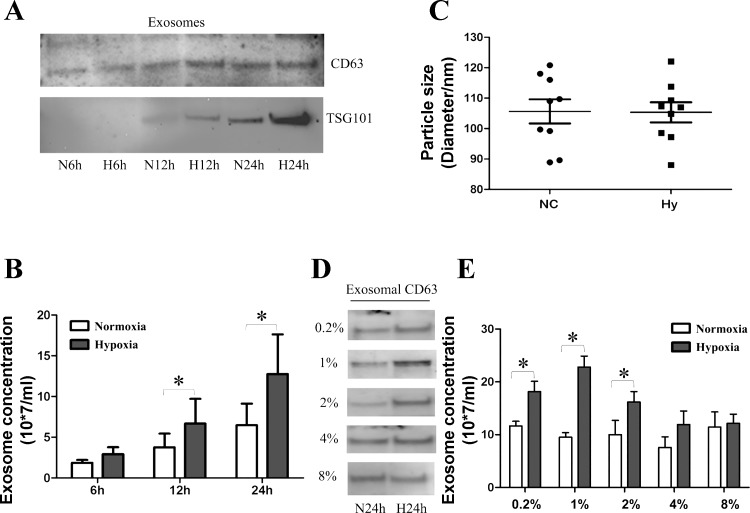
After characterizing isolated exosomes, we examined the impact of hypoxia on exosome secretion in RPTCs. The cells were exposed to normoxia or hypoxia (1% oxygen) for 6, 12, and 24 h. Western blot analysis (Fig. 2A) showed a time-dependent increase of exosomal protein CD63 and TSG101 under both normoxic and hypoxic conditions. This time dependence was expected because more exosomes were secreted into culture medium as the incubation time increased. Interestingly, further comparison indicated consistently higher CD63 and TSG101 expression in hypoxic group than normoxic group at all time points. NTA analysis (Fig. 2B) also confirmed that more exosomes were produced by hypoxic cells than normoxic cells after normalization with equal cell numbers, particularly at 12 h (1.5-fold higher than normoxia; P < 0.05) and 24 h (1.9-fold higher than normoxia; P < 0.05). In addition, we evaluated the sizes of the exosomes produced by the cells under hypoxic and normoxic conditions by NTA. The average diameter of the exosomes from normoxic cells was 108.2 ± 4.1 nm, which was not different from that of hypoxic exosomes (105.6 ± 3.4 nm; Fig. 2C). Having observed the time-dependent increase of exosome secretion in 1% hypoxic condition, we checked the effect of different hypoxic concentrations on exosome production. As shown in Fig. 2, D and E, by Western blot and NTA analyses, 0.2, 1, and 2% oxygen hypoxic concentrations could significantly increase exosome secretion compared with the corresponding control condition. However, moderate hypoxia of 4 and 8% oxygen did not induce exosome production.
Fig. 2.
Enhanced exosome production during hypoxia of RPTCs. RPTCs were cultured in normoxia (21% oxygen) or hypoxia (1% oxygen) for 6, 12, or 24 h to collect medium for exosome isolation by serial ultracentrifugation. A: immunoblot analysis of CD63 and TSG101 in exosomal preparations from normoxic (N6h, N12h, and N24h) and hypoxic (H6h, H12h, and H24h) RPTCs. The loading volume of exosome lysis for each condition was normalized with corresponding cell lysate protein. B: exosome concentrations analyzed by NTA. Data are means ± SD; *P < 0.05 vs. normoxic 12-h group; n = 3. C: size distribution analysis by NTA for exosomes from normoxic (NC) and hypoxic (Hy) cells. n = 9. D and E: RPTCs were cultured in different hypoxic conditions of 0.2, 1, 2, 4, or 8% oxygen for 24 h, whereas corresponding control cells were in normoxia (21% oxygen). D: immunoblot analysis of CD63 in exosomal preparations from normoxia (N24h) and hypoxia (H24h) of different oxygen concentrations. E: exosome concentrations from the corresponding groups as in D analyzed by NTA. Data are means ± SD; *P < 0.05 vs. indicated normoxia group; n = 3.
Effects of DMOG and YC-1 on exosome production in RPTCs.
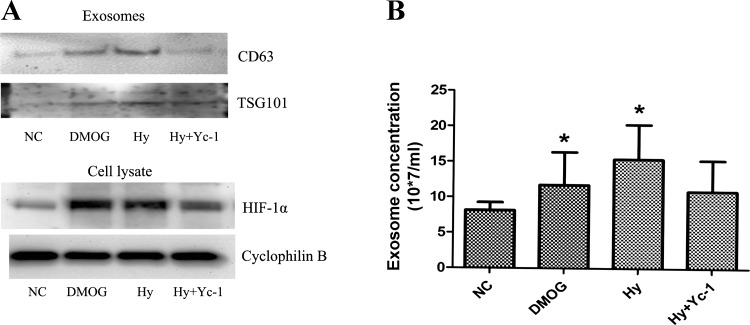
HIFs are the major transcription factors that are responsive to hypoxia in mammalian cells (22). Thus we examined whether HIF was involved in the increased exosome production during hypoxia of RPTCs. We initially tested the effects of DMOG (pharmacological inducer of HIF) and YC-1 (pharmacological inhibitor of HIF), respectively, used with or without hypoxia treatment for 24 h. DMOG increased CD63 and TSG101 expression (Fig. 3A) as well as exosome production (Fig. 3B) in normoxic cells. Moreover, YC-1 reduced exosome production from hypoxic cells. These data suggested the involvement of HIF in the production and secretion of exosomes in hypoxic renal tubular cells.
Fig. 3.
Effects of DMOG and YC-1 on exosome production in RPTCs. RPTCs were cultured in normoxia (21% oxygen) with or without 1 mM DMOG or in hypoxia (1% oxygen) for 24 h with or without 20 μM YC-1 to collect medium for exosome isolation by serial ultracentrifugation. A: immunoblot analysis of CD63 and TSG101 in exosomal preparations and HIF-1α in cell lysate from each condition. B: NTA quantification of exosomes. NC, normoxic control; DMOG, normoxia with DMOG; Hy, hypoxia; Hy+Yc-1, hypoxia with YC-1. Data are means ± SD; n = 3; *P < 0.05 vs. NC group.
Hypoxia-induced exosome production is suppressed in HIF-1-deficient cells.
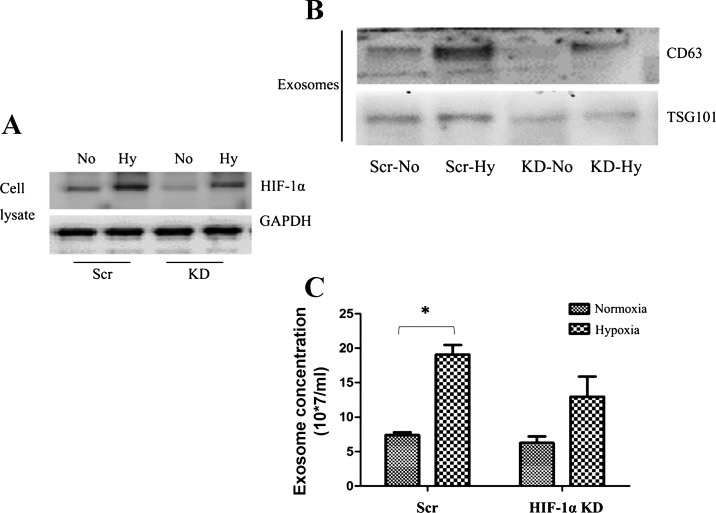
To prove further the role of HIF in hypoxia-induced exosome production, we turned to HIF-deficient cells. In kidneys, renal tubular cells mainly express HIF-1, whereas vascular endothelial cells express HIF-2 (21). Therefore, we established HIF-1α knockdown renal tubular cell lines by stably transfecting HIF-1α short hairpin RNAs (shRNAs). Compared with scrambled sequence-transfected cells (Scr), HIF-1α shRNA-transfected cell lines had lower HIF-1α expression under both normoxic and hypoxic conditions (Fig. 4A). In normoxia, these cells produced similar amounts of exosomes, but on hypoxia exposure HIF-1α knockdown cells produced much less exosomes than scrambled sequence-transfected cells (Fig. 4B). Consistently, hypoxia-induced expression of exosomal proteins CD63 and TSG101 was also suppressed in HIF-1α knockdown cells (Fig. 4C).
Fig. 4.
Hypoxia-induced exosome production is suppressed in HIF-1-deficient cells. A: immunoblot analysis of HIF-1α in scrambled sequence-transfected cells (Scr) and HIF-1α knockdown cells (KD) after 6 h of normoxic (No) or hypoxic incubation. GAPDH was blotted as the internal control. B: immunoblot analysis of CD63 and TSG101 in exosome preparations from Scr and HIF-1α KD after 24 h of normoxic or hypoxic incubation. C: NTA quantification of exosomes from Scr and HIF-1α KD after 24 h of normoxic or hypoxic incubation. Data are means ± SD; n = 3; *P < 0.05 vs. Scr Normoxia group.
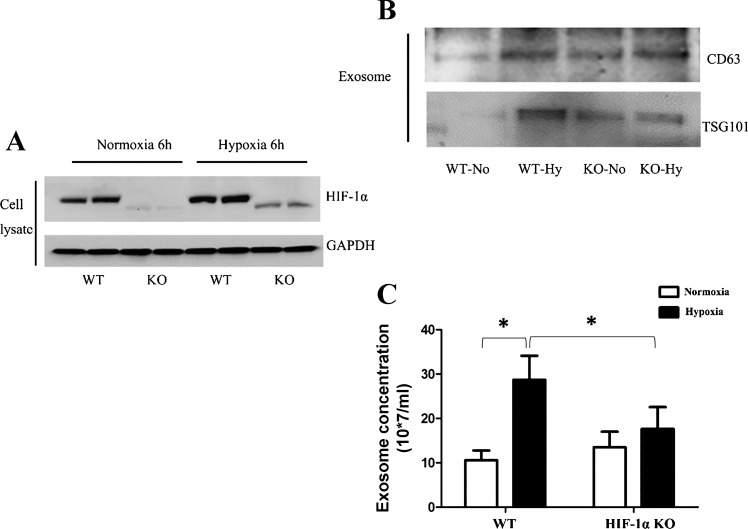
We further compared HIF-1α knockout (KO) and wild-type (WT) mouse embryonic fibroblasts for exosome production. HIF-1α expression was markedly lower in KO cells under both normoxic and hypoxic conditions, verifying HIF-1α knockout in KO cells (Fig. 5A). Hypoxia significantly increased exosome production in WT cells, but this inductive response was largely attenuated in KO cells as shown by exosomal protein and NTA analyses (Fig. 5, B and C).
Fig. 5.
Exosome production in MEF cells with HIF-1α knockout. A: immunoblot analysis of HIF-1α in wild-type MEF cells (WT) and HIF-1α knockout MEF cells (KO) after 6 h of normoxic or hypoxic incubation. B: immunoblot analysis of CD63 and TSG101 in exosome preparations from wild-type cells and HIF-1α knockout cells after 24 h of normoxic or hypoxic incubation. C: NTA test for exosome quantity from wild-type cells and HIF-1α knockout after 24 h of normoxic or hypoxic incubation. Data are means ± SD (n = 3); *P < 0.05 vs. indicated group.
Cytoprotective effect of the exosomes produced by hypoxic renal tubular cells.
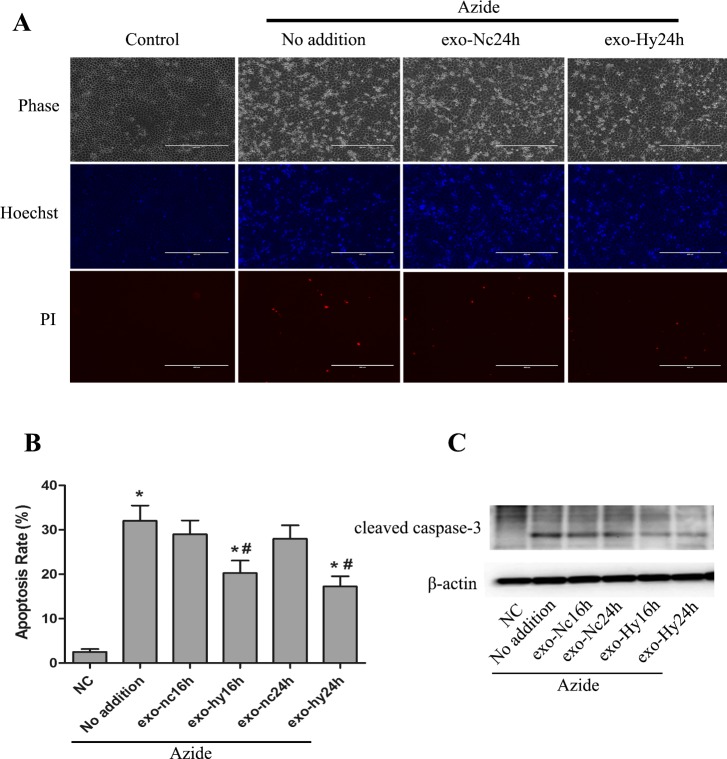
We wondered about the function of the exosomes produced by renal tubular cells, especially those induced to produce under hypoxia. Considering that hypoxia triggers cell stress, we hypothesized that the exosomes produced under hypoxia may help the cells cope with the stress. To test this possibility, we observed the effect of the exosomes isolated from normoxic or hypoxic cells on ATP-depletion-associated cell injury in RPTCs. ATP depletion was induced by inclusion of azide (inhibitor of cytochrome oxidase, complex IV of mitochondrial respiratory chain) in cell incubation buffer. In this model, apoptosis developed within 1–3 h after ATP-depleted cells were returned to normal culture medium. Preincubation with exosomes derived from normoxic cells had marginal effects on apoptosis, but the exosomes from hypoxic cells significantly suppressed apoptosis (Fig. 6). The effect of hypoxic exosomes was confirmed by morphological assessment (Fig. 6, A and B) and immunoblot analysis of caspase-3 cleavage or activation (Fig. 6C).
Fig. 6.
Cytoprotective effect of the exosomes produced by hypoxic RPTCs. RPTCs were cultured in normoxia or hypoxia for 16–24 h to collect medium for exosome isolation. exo-Nc24h, exosomes from cells with 24 h of normoxia; exo-Hy24h, exosomes from cells with 24 h of hypoxia. The exosomes were then added to another group of RPTCs at 5 × 1011 particles per milliliter for 12 h of pretreatment, followed by ATP depletion with azide and recovery to show apoptosis. A: representative images of cell and nuclear morphologies. After treatment, the cells were stained with Hoechst 33342 to record cellular morphology by phase-contrast microscopy and nuclear morphology by fluorescence microscopy. The cells were also stained with propidium iodide (PI) to show necrosis. Scale bars are 200 μm. B: percentage of apoptosis determined by counting the cells with typical apoptotic morphology. Data are means ± SD (n = 3); *P < 0.05 vs. NC group, #P < 0.05 vs. Azide (No addition) group. C: immunoblot analysis of cleaved caspase-3. β-Actin was probed as internal control.
Role of HIF-1 in producing cytoprotective exosomes by RPTCs under hypoxia.
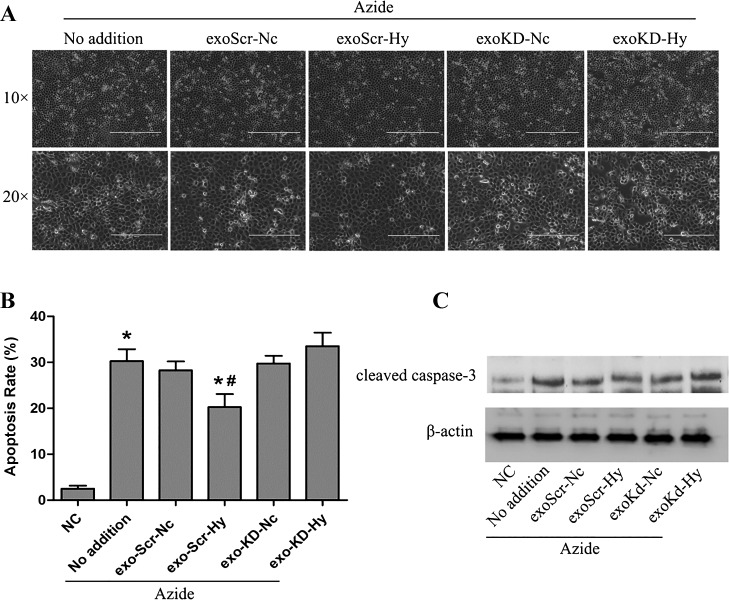
Finally, we determined whether HIF-1α was involved in the protective effect of exosomes from hypoxic cells. To this end, we collected exosomes from HIF-1α knockdown cells and scrambled sequence-transfected cells to test their effects on apoptosis following azide-induced ATP depletion. Representative cell morphology is shown in Fig. 7A, and the percentage of apoptosis is presented in Fig. 7B. ATP depletion induced ~30% apoptosis in RPTCs, which was reduced to 17% by the exosomes isolated from scrambled sequence-transfected cells under hypoxia, whereas the exosomes isolated from normoxic, scrambled sequence-transfected cells did not have significant effects. Notably, the exosomes isolated from hypoxic, HIF-1α knockdown cells did not reduce apoptosis either. The morphological results were confirmed by immunoblot analysis of active/cleaved caspase-3, a biochemical hallmark of apoptosis (Fig. 7C). These results support a critical role of HIF-1 in producing protective exosomes during hypoxia of renal tubular cells.
Fig. 7.
Role of HIF-1 in producing cytoprotective exosomes by RPTCs under hypoxia. HIF-1 knockdown RPTCs and scrambled sequence-transfected RPTCs were cultured in normoxia or hypoxia for 24 h to collect medium for exosome isolation. exo-Scr-Nc, exosomes from scrambled sequence-transfected cells with 24 h of normoxia incubation; exo-KD-Hy, exosomes from HIF-1 knockdown cells with 24 h of hypoxia. The exosomes were then added to another group of RPTCs at 5 × 1011 particles per milliliter for 12 h of pretreatment, followed by ATP depletion with azide and recovery to show apoptosis. A: representative images of cell morphology recorded by phase-contrast microscopy. Scale bars are 200 μm. B: percentage of apoptosis determined by counting the cells with typical apoptotic morphology. Data are means ± SD (n = 3); *P < 0.05 vs. NC group, #P < 0.05 vs. Azide (No addition) group. C: immunoblot analysis of cleaved caspase-3. β-Actin was probed as internal control.
DISCUSSION
The diagnostic potential and bioeffects of exosomes have drawn tremendous attention in recent years in biomedical research (29). In the current study, we have presented the results about the regulation of exosome production by hypoxia in renal tubular cells. We have further shown evidence that the exosomes from hypoxic cells are cytoprotective for renal tubular cells. Hypoxia is a feature of both acute and chronic kidney diseases in various clinical and experimental conditions (4, 13, 26). Exosomes have recently been implicated in physiological as well as pathophysiological processes in kidney (3, 7, 25). Our data demonstrate that exosome production in renal tubular cell is remarkably increased during hypoxia, which is consistent with previous work in cancer cells (14). Consistently, Borges et al. (2) showed the increase of exosomal protein CD63 production in kidney tissues of unilateral ureteral obstruction (UUO) mice and during hypoxia of renal tubular cells, suggesting the induction of exosomes under these conditions. In our study, exosomes were evaluated by complementary techniques, including electron microscopy visualization, nanoparticle tracking assay, and immunoblot analysis of exosomal proteins CD63 and TSG101.
Our study has further demonstrated an important role of HIF-1 in exosome production during hypoxia of renal tubular cells. In normoxic cells, activation of HIF by DMOG induced exosome production from RPTCs and inhibition of HIF by YC-1 blocked exosome production during hypoxia (Fig. 4). In addition, hypoxia-induced exosome production was suppressed in HIF-1α-deficient cells (Fig. 5). Together, these results support the role of HIF-1 in mediating exosome biogenesis and secretion in hypoxic renal tubular cells. However, the mechanism whereby HIF-1 regulates exosomes under hypoxia is unclear. In this regard, HIF has been reported to mediate the induction of Rab22 and RAB20 (8, 27), which may be associated with extracellular vesicle formation and secretion (6, 18). In addition, hypoxia may induce ceramide production by the activation of nSMase, another important regulator of exosome secretion (15).
Functionally, exosomes derived from hypoxic cells were demonstrated to facilitate tumor invasion and metastasis in cancer research and were also reported to be profibrotic during kidney fibrosis by their transforming growth factor-β mRNA content (2, 12, 23). Our current results show that exosomes from hypoxic RPTCs can protect against renal tubular cell apoptosis following ATP depletion. The effect, although not complete, suggests a promising, exosome-based approach of cell protection. There are reports about the cytoprotective effects of the exosomes derived from stem or progenitor cells (5, 17), but, to our knowledge, the current work is the first to demonstrate the protective effect of renal tubular cell-derived exosomes. Interestingly, our work showed a time window for RPTCs to produce cytoprotective exosomes under hypoxia. Specifically, the exosomes collected during 16 and 24 h of hypoxia were protective, but those collected from over 30 h of hypoxia were not (data not shown). In addition, we noticed that the cells needed serum (Exosome-Depleted FBS in our study) to produce cytoprotective exosomes during hypoxia (data not shown).
How do the exosomes from hypoxic cells protect renal tubular cells? Our current work does not provide a clear answer to this question. Nonetheless, hypoxic stimulation may change the content of exosomes that are believed to be the main mediators affecting recipient cells (1). The content of exosomes may change dynamically in response to time duration of cell culture as well as the cellular context (16). This appears relevant because in our study RPTCs started developing apoptosis after >24 h of hypoxia, a time point when produced exosomes were no more protective (data not shown). In our previous studies, hypoxia and HIF-1 induced cytoprotective genes or factors in renal tubular cells, including microRNAs (miRNAs; Refs. 26, 28). In the current study, we have further provided evidence for a role of HIF-1 in the production of cytoprotective exosomes by renal tubular cells under hypoxia (Fig. 7).
Hypoxic tumor-derived extracellular vesicles negatively regulated natural killer (NK) cell function by a mechanism involving transforming growth factor-β and miRNA transfer (1). Also, exosomes derived from hypoxic oral squamous cell carcinoma cells increased the migration and invasion of these cells by delivering miRNAs in a HIF-1α- and HIF-2α-dependent manner (16). Exosomes secreted by human myeloid leukemia cell line K562 also enhanced angiogenesis under normoxic and hypoxic conditions. With chronic hypoxia, exosomes could also enhance angiogenesis by targeting factor-inhibiting hypoxia-inducible factor-1 (24). Therefore, HIF-1 and associated miRNAs might be the main effectors in the protection by hypoxic exosomes. However, it is still hard to say the protective effect of hypoxic exosomes completely depends on HIF-1 because numerous transcription factors and cellular signaling pathways are activated during hypoxia that may mediate the protective effect. Further work should identify the molecules in the exosomes from hypoxic renal tubular cells that are responsible for the cytoprotective effect.
GRANTS
The study was supported in part by grants from National Natural Science Foundation of China (81430017 and 81470961) and the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (DK-058831 and DK-087843) and Department of Veterans Affairs (5I01 BX-000319).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Z., X.Z., H.Z., and Z.D. conceived and designed research; W.Z. and X.Z. performed experiments; W.Z., X.Z., H.Z., and Z.D. analyzed data; W.Z., X.Z., Q.Y., Y.L., H.Z., and Z.D. interpreted results of experiments; W.Z. prepared figures; W.Z. drafted manuscript; W.Z., X.Z., Q.Y., Y.L., H.Z., and Z.D. edited and revised manuscript; W.Z., X.Z., Q.Y., Y.L., H.Z., and Z.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gregg Semenza at Johns Hopkins University School of Medicine for providing HIF-1-null MEF cells.
REFERENCES
- 1.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, Chouaib S. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 5: e1062968, 2016. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24: 385–392, 2013. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res 46: 824–830, 2013. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brukamp K, Jim B, Moeller MJ, Haase VH. Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. Am J Physiol Renal Physiol 293: F1397–F1407, 2007. doi: 10.1152/ajprenal.00133.2007. [DOI] [PubMed] [Google Scholar]
- 5.Burger D, Viñas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol 185: 2309–2323, 2015. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 7.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackenbeck T, Huber R, Schietke R, Knaup KX, Monti J, Wu X, Klanke B, Frey B, Gaipl U, Wullich B, Ferbus D, Goubin G, Warnecke C, Eckardt KU, Wiesener MS. The GTPase RAB20 is a HIF target with mitochondrial localization mediating apoptosis in hypoxia. Biochim Biophys Acta 1813: 1–13, 2011. doi: 10.1016/j.bbamcr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12: e0170628, 2017. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol 309: C775–C782, 2015. doi: 10.1152/ajpcell.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khade PK, Giannakakou P. RABbing cancer the wrong way. Proc Natl Acad Sci USA 111: 11230–11231, 2014. doi: 10.1073/pnas.1410788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12: 421, 2012. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288: 10849–10859, 2013. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, Li X, Chen J, Liu K, Li C, Zhu G. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res 76: 1770–1780, 2016. doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- 17.Lindoso RS, Collino F, Bruno S, Araujo DS, Sant’Anna JF, Tetta C, Provero P, Quesenberry PJ, Vieyra A, Einicker-Lamas M, Camussi G. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev 23: 1809–1819, 2014. doi: 10.1089/scd.2013.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12: 19–30, 2010. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 19.Peng J, Ramesh G, Sun L, Dong Z. Impaired wound healing in hypoxic renal tubular cells: roles of hypoxia-inducible factor-1 and glycogen synthase kinase 3β/β-catenin signaling. J Pharmacol Exp Ther 340: 176–184, 2012. doi: 10.1124/jpet.111.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200: 373–383, 2013. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1α and -2α in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. doi: 10.1097/01.ASN.0000017223.49823.2A. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol 44: 170–181, 2017. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124: 3748–3757, 2014. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int 80: 1138–1145, 2011. doi: 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Biju MP, Wang MH, Haase VH, Dong Z. Cytoprotective effects of hypoxia against cisplatin-induced tubular cell apoptosis: involvement of mitochondrial inhibition and p53 suppression. J Am Soc Nephrol 17: 1875–1885, 2006. doi: 10.1681/ASN.2005121371. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA 111: E3234–E3242, 2014. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Q, Liu Y, Liu P, Hao J, Liang M, Mi QS, Chen JK, Dong Z. MicroRNA-489 induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. J Am Soc Nephrol 27: 2784–2796, 2016. doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311: F844–F851, 2016. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Zhang W, Yao Q, Zhang H, Dong G, Zhang M, Liu Y, Chen JK, Dong Z. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Physiol Renal Physiol 312: F963–F970, 2017. doi: 10.1152/ajprenal.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]