Abstract
Female spontaneously hypertensive rats (SHR) have more renal regulatory T cells (Tregs) than males, and greater levels of Tregs in female SHR are dependent on blood pressure (BP). However, the molecular mechanism responsible for greater Tregs in female SHR is unknown. Transforming growth factor (TGF)-β is a pleiotropic cytokine critical in the differentiation of naïve T cells into Tregs, and female SHR have higher TGF-β excretion than male SHR. The goals of the current study were to test the hypotheses that 1) female SHR have greater renal TGF-β expression than male SHR, which is dependent on BP and 2) neutralizing TGF-β will decrease renal Tregs in female SHR. Renal cortices were isolated from 5- and 13-wk-old male and female SHR, and TGF-β levels were measured via Western blot and ELISA. Adult female SHR have more free, active TGF-β1 than 5-wk-old female SHR (46% more) or male SHR (44% more than 5-wk-old males and 56% more than 13-wk-old male SHR). We confirmed greater TGF-β1 in adult female SHR was due to increases in BP and not sexual maturation by measuring TGF-β1 levels following treatment with BP-lowering drugs or ovariectomy. Separate female SHR were treated with an antibody to TGF-β1,2,3; BP was measured, and T cells were assessed in whole blood and the kidney. Neutralizing TGF-β had no effect on BP, although circulating Tregs decreased by 32%, while Th17 cells increased by 64%. Renal Tregs were not altered by antibody treatment, although Th17 cells were decreased by 61%. In conclusion, although TGF-β promotes circulating Tregs in female SHR, it does not account for the sex difference in renal Tregs in SHR.
Keywords: sex, cytokines, hypertension, lymphocytes
It is now well accepted that T cells play a central role in the development and maintenance of elevated blood pressure (BP) in male animal models of hypertension (3, 17, 23). Similarly, there is growing acceptance that there are sex differences in immune cell activation in animal models of hypertension (5, 10). Lymphocytes contribute to hypertension in both male and female spontaneously hypertensive rats (SHR) (29), although we recently published that female SHR have a BP-dependent increase in renal T-regulatory cells (Tregs) that is not seen in males (28). We speculate that this increase in Tregs is important in the ability of female SHR to maintain a lower BP relative to age-matched males; however, the molecular mechanism(s) that regulates the T-cell profile in hypertension in either sex has not been elucidated.
Transforming growth factor (TGF)-β is traditionally viewed as a profibrotic cytokine that has been implicated in the pathogenesis of hypertension and end-organ damage in male experimental animals (16, 20). However, TGF-β also has a central role in T-cell differentiation, activation, survival, and proliferation (8, 11). In particular, TGF-β is critical in the generation and immunosuppressive function of Tregs (30). We previously published that female SHR excrete more urinary TGF-β than male SHR (26); therefore, the first goal of this study was to test the hypotheses that female SHR have greater renal TGF-β expression than male SHR and that higher levels of TGF-β in female SHR are dependent on BP. Once we confirmed greater expression of TGF-β in the kidneys of adult, hypertensive female SHR compared with males, additional studies tested the hypothesis that TGF-β is required to maintain Tregs in females. There remains a scarcity of data in the literature examining the immune system in female experimental models of hypertension; our study provides novel insight into the regulation of TGF-β in hypertensive females.
MATERIALS AND METHODS
Animals.
Five-week and 13-wk-old male and female SHR were used in this study (Augusta University colony). All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and use was approved and monitored by the Augusta University Institutional Animal Care and Use Committee. Animals were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. All rats were given free access to rat chow and tap water. At the end of all studies, rats were anesthetized with an injection of ketamine/xylazine (50 mg/kg and 6 mg/kg ip, respectively; Phoenix Pharmaceuticals, St. Joseph, MO), and the kidneys were removed. The renal cortex was dissected from each kidney and snap-frozen in liquid nitrogen.
To elucidate the relative contribution of increases in BP vs. sexual maturation on TGF-β levels, renal cortices were isolated from a subset of female rats (n = 5–7/group) randomized to receive vehicle or titrated doses of hydrochlorothiazide (HCTZ; 10–55 mg·kg−1·day−1) and reserpine (0.6–4.5 mg·kg−1·day−1) in drinking water 1) beginning at 6 wk of age until 12 wk of age to attenuate age-related increases in BP or 2) from 11 to 13 wk of age to reverse established hypertension. To see the full BP tracings from these rats, refer to the study by Tipton et al. (28). Rats were individually housed throughout the study. Water intake and body weights were measured every 3 days, and the doses of drugs were adjusted as needed to maintain consistent lowering of BP. BP was measured weekly in the 6–12-wk HCTZ/reserpine-treated SHR by tail-cuff plethysmography (IITC Life Sciences, Woodland Hills, CA), as previously described (21); all rats were trained to the tail-cuff system for 1 wk before recording initial BP readings. BP was measured by telemetry [Data Sciences International (DSI), St. Paul, MN] in the 11–13 wk HCTZ/reserpine study, according to the manufacturer's instructions, as previously described (24). To assess the impact of female sex hormones on TGF-β levels, additional female SHR (n = 9) underwent ovariectomy (OVX) at 5 wk of age, as previously described (25). Animals were allowed to recover, and kidneys were isolated at 13 wk of age. Successful OVX was confirmed at the time of sacrifice by increased body weight and decreased uterine weight compared with age-matched controls (4).
To determine the impact of TGF-β on BP and the T-cell profile, additional female SHR were implanted with telemetry transmitters at 10 wk of age, as previously described (24). Female SHR were randomized to receive either vehicle or TGF-β-neutralizing antibody. The neutralizing Ab (monoclonal anti-TGF-β1,2,3, clone 1D11 cat. no. MAB1835; R&D Systems, Minneapolis, MN) was given at a dose of 0.5 mg/kg every other day for 3 wk by intraperitoneal injection. This dosing regimen has previously been shown to inhibit TGF-β-induced alterations in renal health and BP in Dahl salt-sensitive (DSS) rats (6, 18). Whole blood and kidneys were isolated for flow cytometric analysis of T cells.
Immunohistochemical analysis.
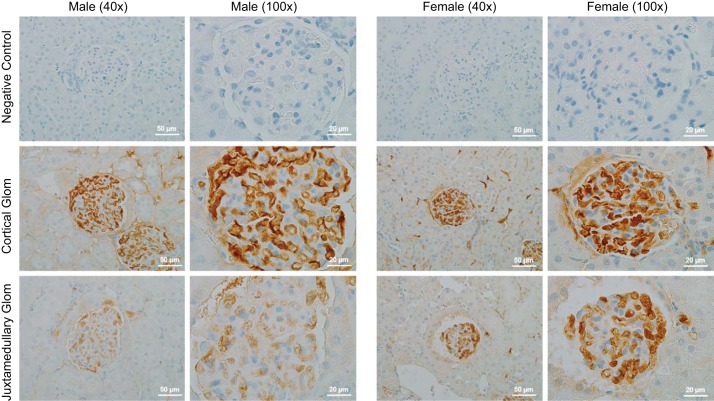
Kidneys from 13-wk-old control male and female SHR (n = 3) were isolated, and a single ~5-mm sagittal section was made for each kidney. The tissue was then fixed with formalin, paraffin embedded, and sectioned at a thickness of 4 μm onto Superfrost plus slides. Slides were incubated overnight in the absence or presence of primary monoclonal antibody to TGF-β1,2,3 (cat. no. MAB1835; R&D Systems) in humidity chambers at 4°C. The stained sections were viewed with an Olympus BX40 optical microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP70; Olympus America). Slides were labeled 1–6, providing a unique identifier to each animal and removing group identifiers. On each slide beginning at one pole of the kidney, each glomeruli was manually counted and noted as either having 1) a dark stain or 2) light to no stain by an individual blinded to the hypothesis, the individual rat identifiers, and the sex of the animal. Quantification continued until the opposite pole was reached to avoid double counting. The percentage for both stain intensities relative to the total number of glomeruli was determined for each sample, and then the average was calculated for each sex. Representative images are shown in Fig. 1. Antibody-negative controls were also performed by incubation of slides with antibody diluent, but no primary antibody followed by incubation with secondary antibodies and detection reagents to ensure signal specificity.
Fig. 1.
TGF-β expression and localization was assessed in the kidneys of 13-wk-old control male and female spontaneously hypertensive rats (SHR); n = 3. Brown staining indicates immunoreactive TGF-β; representative images are shown from the renal cortex.
Western blot analysis.
The renal cortex was homogenized, and the homogenate was used for Western blot protocols, as previously described using 50 μg total protein/lane (28). Briefly, following transfer of protein onto polyvinylidene difluoride (PVDF), membranes were blocked in 3% BSA (Fisher Scientific, Pittsburgh, PA) in Tris-buffered saline and 0.1% Tween-20 (TBST). Two-color immunoblots were performed using primary antibodies to TGF-β1, TGF-β2, (cat. nos. sc-146 and sc-90, respectively; Santa Cruz Biotechnology, Santa Cruz, CA) and TGF-β1,2,3 (cat. no. MAB1835; R&D Systems), and a monoclonal antibody to actin (A1978, 1:10,000; Sigma, St. Louis, MO). For all Western blots, protein was normalized to actin to verify equal protein loading. Specific bands were detected using the Odyssey infrared imager (LI-COR Biosciences). Protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using BSA as the standard. The selection of antibodies for the detection of TGF-β isoforms was based on a previous publication using rat kidneys (18). These antibodies resulted in a band at the appropriate molecular weight, as determined by the inclusion of a molecular weight marker, which is shown in each representative blot. In addition, the sex and age differences measured with the TGF-β1 antibody were confirmed using an ELISA, providing additional confidence in the selectivity of the antibody.
Free active TGF-β1 quantification.
TGF-β is synthesized as a homodimer with a propeptide region. The homodimer then attaches to a latency-associated peptide (LAP) to form the TGF-β-LAP complex and following a conformational change, the structure forms the small latent complex (SLC). The SLC then interacts with the latent TGF-β binding protein (LTBP) to form the large latent complex, which is then secreted out of the cell. However, it is free active TGF-β that binds to its receptor to induce biological effects (13). To measure free active TGF-β1, renal cortices were weighed and homogenized in lysis buffer (20 mM HEPES, 10 mM NaCl, 1 mM sodium orthovandate, at pH = 10, 10 mM NaF, and 10 mM EDTA), according to the weight in a 1 to 10 weight-to-volume ratio in the presence of a protease (Halt protease inhibitor cocktail, EDTA-free, cat. no. 87785; Thermo Scientific, Rockford, IL) and phosphatase inhibitor cocktails (phosphatase inhibitor cocktail 2, cat. no. P5726; Sigma Aldrich). The homogenates were centrifuged at 3,000 g for 5 min, and the supernatant was collected. Protein concentrations were determined by standard Bradford assay using BSA as the standard. Homogenates were diluted 1:250 in homogenization buffer and then used in the free active TGF-β1 ELISA, according to the manufacturers’ directions (Biolegend, San Diego, CA). Concentrations were normalized to the amount of protein in each sample (mg/ml) to obtain the amount of TGF-β1 per mg of homogenate (pg/mg).
Flow cytometric analysis.
Single-cell suspensions were generated as previously described for flow cytometric analysis of T cells in whole blood and the kidney (28, 29). Cells were incubated with antibodies for surface markers, including CD3 and CD4 (BD Biosciences, San Jose, CA). Cells were then washed, fixed, and permeabilized for intracellular staining of Foxp3 (to identify Tregs) or RORγt (to identify Th17 cells; BD Biosciences, San Jose, CA). Cells were then washed, fixed, and run through a four-color flow cytometer (FACS Calibur, BD Biosciences, San Diego, CA), and data were collected using CellQuest software, as previously described (28, 29).
Statistical analysis.
All data are expressed as means ± SE. TGF-β expression between 5- and 13-wk-old male and female SHR were compared using a two-way ANOVA followed by a Bonferroni post hoc analysis; factor 1 was sex of the animal and factor 2 was age. One-way ANOVA with a Tukey post hoc test was used to compare free active TGF-β1 levels in vehicle control and HCTZ/reserpine-treated SHR. BP was analyzed using repeated-measures ANOVA. Free active TGF-β1 levels in gonad-intact and OVX female SHR and flow cytometric data were compared using a Student’s t-test. Analyses were performed using GraphPad Prism version 5.0 software (GraphPad Software, La Jolla, CA).
RESULTS
Female SHR express more renal TGF-β1 than male SHR.
Initial studies examined total TGF-β expression by immunohistochemical analysis in kidneys of 13-wk-old male and female SHR (Fig. 1). Approximately half of the total glomeruli in male SHR stained positive for high levels of TGF-β (46 ± 0.05%), while 80 ± 0.04% of total glomeruli in kidneys of female SHR stained positive for TGF-β. In particular, females exhibited greater TGF-β expression in juxtamedullary glomeruli. Similarly, females had greater TGF-β staining in and around the arcuate arteries compared with males.
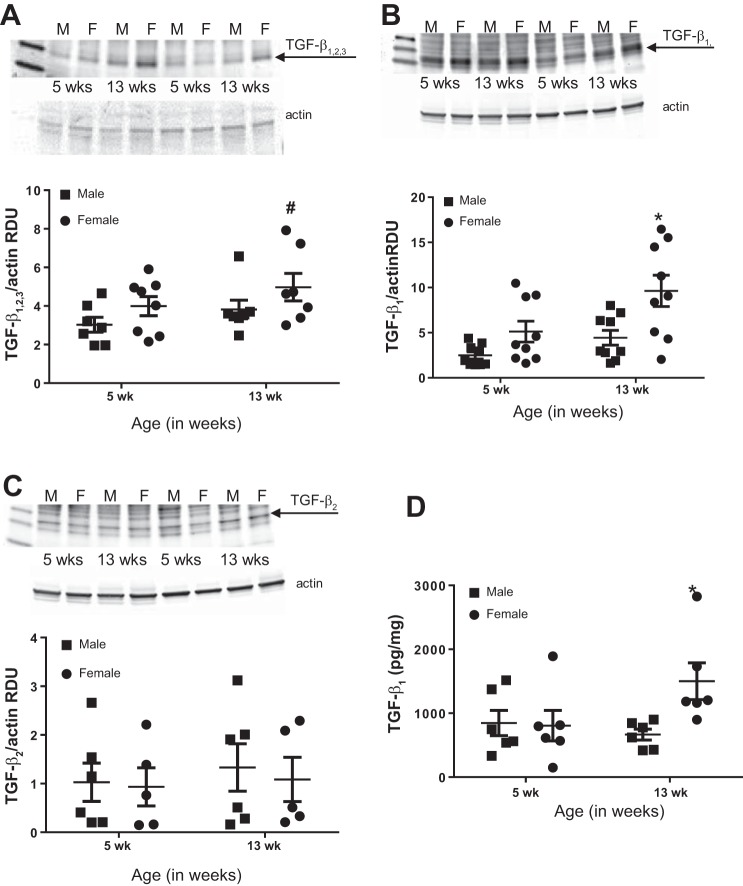
To begin to determine whether renal TGF-β expression was dependent on the sex of the animal, BP, or sex hormones, we measured renal TGF-β expression in 5-wk-old (before sexual maturation and increases occur in BP; 28) and 13-wk-old SHR. Total TGF-β1,2,3 protein expression was greater in female SHR compared with males (effect of sex, P = 0.058; Fig. 2A), although there was no significant effect of age (P = 0.11; interaction, P = 0.86). In contrast, TGF-β1 protein expression was significantly greater in 13-wk-old female SHR compared with both male SHR groups (effect of sex, P = 0.006; Fig. 2B) and 5-wk-old females (effect of age, P = 0.008; interaction, P = 0.27). TGF-β2 protein levels were comparable among all groups (effect of sex, P = 0.61; effect of age, P = 0.70; interaction, P = 0.87 Fig. 2C). Free active TGF-β1 levels were then measured by ELISA, which showed that 13-wk-old female SHR have greater renal cortical free active TGF-β1 levels compared with both male SHR and young female SHR (effect of sex, P = 0.080; effect of age, P = 0.24; interaction, P = 0.055; Fig. 2D). Free active TGF-β1 is the form of TGF-β1 that binds to the receptor to induce biological effects (13). Because our Western blot data indicated an increase in TGF-β1, which was confirmed by ELISA, additional studies below only measured free active TGF-β1.
Fig. 2.
TGF-β1,2,3 (A), TGF-β1 (B), and TGF-β2 (C) levels were quantified in the kidneys of 5- and 13-wk-old male and female SHR via Western blot analysis (n = 6–9) and ELISA (D; n = 6). A–C: representative blots and average TGF-β protein expression normalized to actin. Data were compared by a two-way ANOVA. *P < 0.05 indicates significant difference from male SHR and 5-wk female SHR based on Bonferroni post hoc analysis. #P = 0.058 vs. all other groups.
Renal cortical TGF-β1 expression is decreased in female SHR on antihypertensive therapy.
Maturation from 5 to 13 wk of age in female SHR is not only associated with increases in sex hormones, but also the development of hypertension (28). To determine the relative contribution of sex hormones vs. increases in BP on increases in TGF-β1 in female SHR, additional studies measured free active TGF-β1 in female SHR ovariectomized at 5 wk of age and studied in adulthood at 13 wk. Loss of female sex hormones significantly increased renal cortical TGF-β1 levels (P = 0.045; Fig. 3A).
Fig. 3.
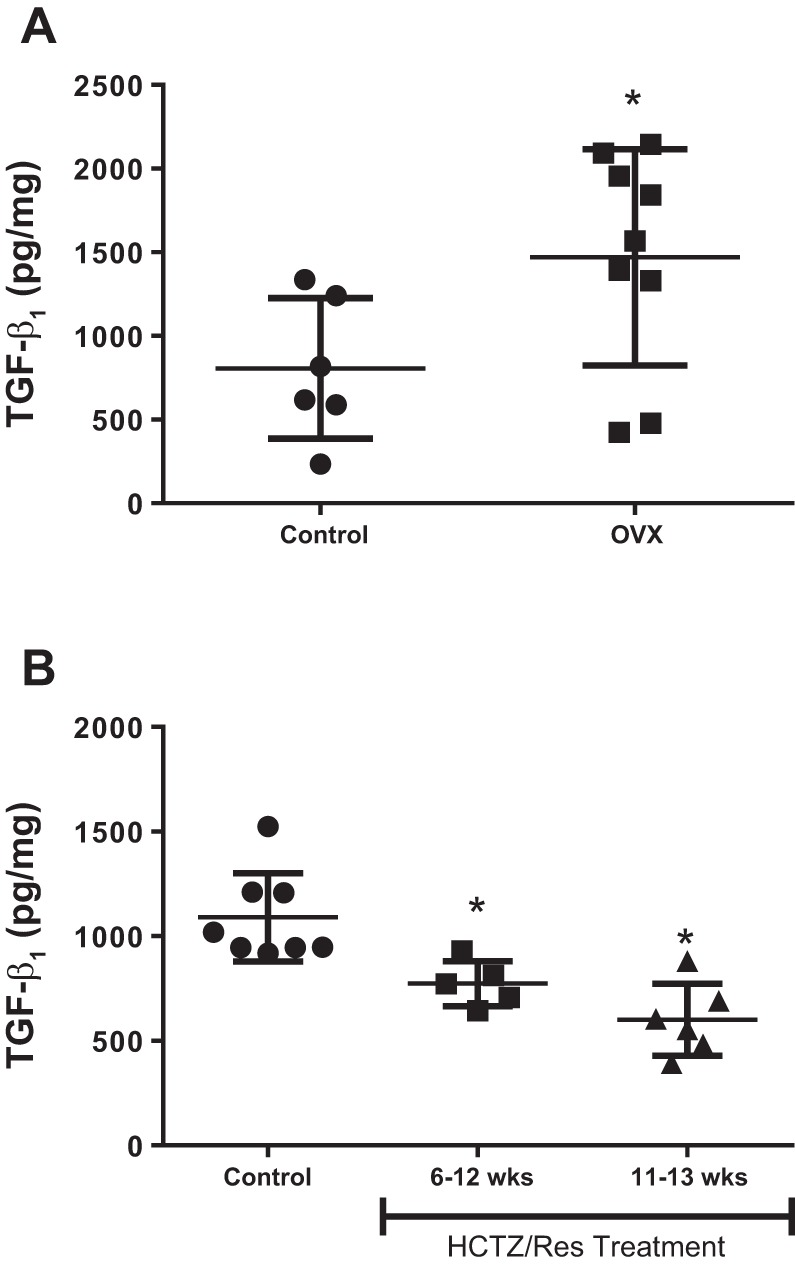
Active TGF-β1 levels were quantified by ELISA in female SHR. A: gonad-intact females (n = 6) and females ovariectomized (OVX) at 5 wk of age (n = 9); all females in A were studied at 13 wk of age. *Significant difference from control based on Student’s t-test. B: vehicle control females (n = 8) and females treated with hydrochlorothiazide (HCTZ) and reserpine from either 6–12 wk of age (n = 5) or 11–13 wk of age (n = 6) to lower blood pressure (BP). Data were compared by a one-way ANOVA. *Significant difference from vehicle control based on Tukey post hoc analysis.
To elucidate the relative contribution of increases in BP on the sexual dimorphism in TGF-β1, renal cortices were isolated from male and female SHR treated with vehicle or HCTZ/reserpine from either 6 until 12 wk of age to prevent age-related increases in BP or from 11 to 13 wk of age to reverse established hypertension. The increase in BP over this time and the full BP curves for the effect of the HCTZ/reserpine treatment in these same animals were recently published (28). HCTZ/reserpine treatment from 6 to 12 wk of age significantly attenuated the age‐dependent increase in systolic BP in male and female SHR (P < 0.05) and abolished the sex difference in BP observed in 12‐wk‐old vehicle‐treated SHRs (control male: 174 ± 4 mmHg; male HCTZ/reserpine: 137 ± 5 mmHg; control female: 161 ± 1 mmHg; female HCTZ/reserpine: 132 ± 4 mmHg). HCTZ/reserpine treatment from 11 to 13 wk of age also abolished the hypertension, as well as the sex difference in BP (control male: 151 ± 2 mmHg; male HCTZ/reserpine: 122 ± 3 mmHg; control female: 132 ± 2 mmHg; female HCTZ/reserpine: 116 ± 3 mmHg). Either preventing the age-related increases in BP or reversing established hypertension in female SHR resulted in significantly lower levels of TGF-β1 compared with vehicle-treated females (P = 0.0003; Fig. 3B).
Neutralizing TGF-β decreased circulating Tregs in female SHR, with no effect on BP or renal Tregs.
To test the hypothesis that greater levels of renal TGF-β contributed to maintaining Tregs in female SHR, additional rats were treated with a TGF-β-neutralizing antibody. BP was measured by telemetry, and 3 wk of treatment with the neutralizing antibody had no effect on BP (Fig. 4).
Fig. 4.
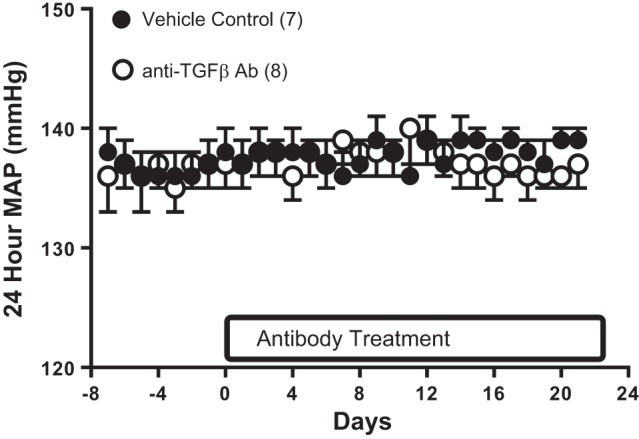
Twenty-four-hour mean arterial pressure (MAP) measured by telemetry in female SHR treated with control antibody (n = 7; ●) or a monoclonal antibody against TGF-β1,2,3 (n = 8; ○). Data were compared by a repeated-measures ANOVA.
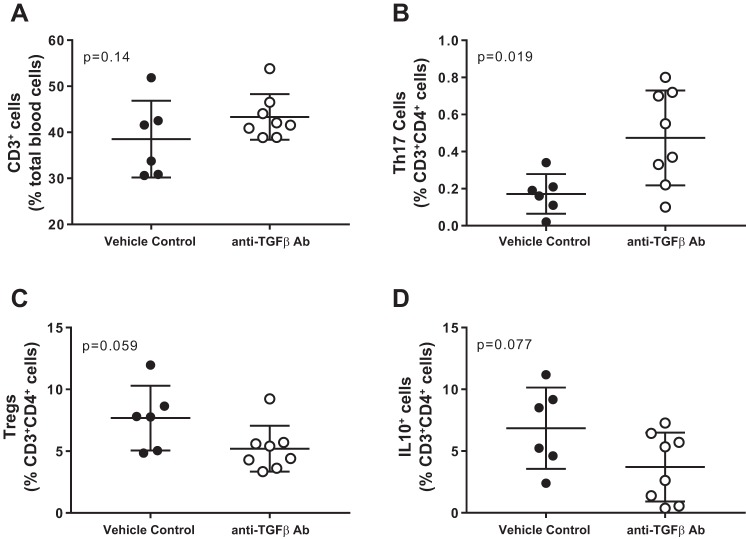
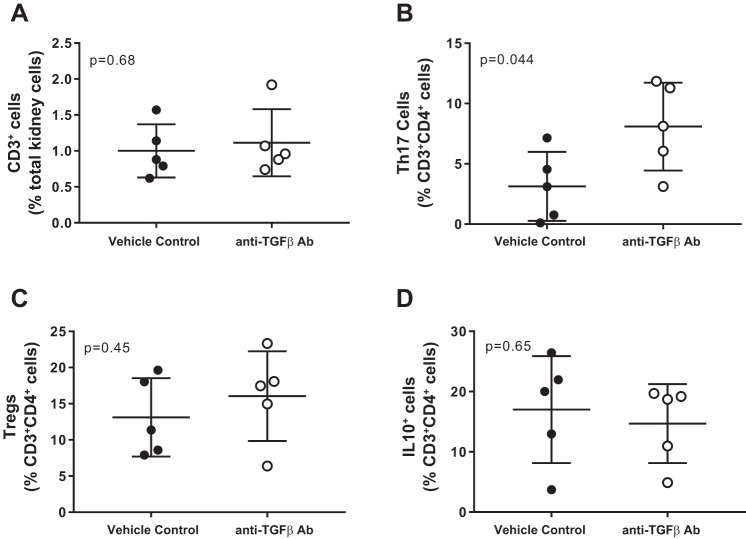
T cells were measured in whole blood and kidneys of control and TGF-β1,2,3-neutralizing antibody-treated female SHR. While neutralizing antibody had no effect on total circulating CD3+ T cells (P = 0.14; Fig. 5A), there was a significant increase in proinflammatory Th17 (P = 0.019; Fig. 5B) cells and decreases in anti-inflammatory Tregs and IL-10+ cells, which approached, but did not reach statistical significance (P = 0.059 and P = 0.077, respectively; Fig. 5, C and D). Similarly, total CD3+ T cells in the kidney were not impacted by treatment with the TGF-β-neutralizing antibody (P = 0.68; Fig. 6A), and Th17 cells were significantly increased (P = 0.044; Fig. 6B). However, there were no alterations in renal Tregs or IL-10+ cells (P = 0.45 and P = 0.65, respectively; Figs. 6, C and D).
Fig. 5.
T-cell profile in whole blood from female SHR-treated with vehicle (n = 7; ●) or a monoclonal antibody against TGF-β1,2,3 for 21 days (n = 8; ○). Shown are the percent of CD3+ T cells (A), ROR-γt+ Th17 cells (B), Foxp3+ Tregs (C), and IL-10+ T cells (D). Data were compared via Studentʼs t-test.
Fig. 6.
T-cell profile in kidneys from female SHR treated with vehicle (n = 7; ●) or a monoclonal antibody against TGF-β1,2,3 for 21 days (n = 8; ○). Shown are the percentage of CD3+ T cells (A), ROR-γt+ Th17 cells (B), Foxp3+ Tregs (C), and IL-10+ T cells (D). Data were compared via Student's t-test.
DISCUSSION
On the basis of the central role for TGF-β in the differentiation of naïve T cells into Tregs, the current study was designed to test the hypothesis that TGF-β mediates the sex difference in renal Tregs. We previously published that young adult female SHR have greater anti-inflammatory, anti-hypertensive Tregs compared with age-matched males (29) and that higher numbers of Tregs in the female SHR are dependent on increases in BP (28). The primary novel finding of the current study is that while changes in renal Tregs are paralleled by changes in renal TGF-β1 in female SHR, neutralization of TGF-β1,2,3 did not decrease renal T cells in females, suggesting that the sex difference in renal Tregs is not mediated by TGF-β1.
We previously reported that 12-wk-old female SHR excrete more urinary TGF-β than age-matched male SHR (26), and in the current study, we extend this finding to the kidney. In contrast to our findings, others have suggested that 12-wk-old male SHR express more TGF-β in the left ventricle than age-matched female SHR when measured by immunohistochemical staining of Smad protein, which is a downstream target of TGF-β (22). Similarly, male DSS rats express more TGF-β in the kidney cortex than female DSS rats under both baseline conditions and following a high-salt diet when measured by Western blot analysis (18). The conflicting reports might reflect the different methods used to measure TGF-β, suggest that sex differences in TGF-β expression is tissue specific, or be dependent on the type of hypertension studied. Regardless, our studies employed three different methods to assess TGF-β (histology, Western blot, and ELISA), and in each case, levels were greater in female SHR.
Maturation in SHR is associated with both increases in sex hormones and BP, both of which could potentially impact renal TGF-β expression. To gain additional mechanistic insight on renal TGF-β expression in female SHR, additional studies assessed the relative contribution of female sex hormones vs. hypertension on free active TGF-β1 levels. Female sex hormones, in particular, estrogen, have been shown to inhibit TGF-β production and signaling, both in vitro and in vivo (12, 19), and estrogen replacement attenuates TGF-β expression in the kidney of diabetic rats (7). More specifically, estrogen inhibits TGF-β activity by inhibiting the TGF-β/Smad pathway and by increasing production of the inhibitory Smad7, leading to decreases in the production of TGF-β (9, 14, 15). Consistent with this finding, OVX increased TGF-β1 levels in female SHR. Therefore, female sex hormones are unlikely to be the sole mechanism to account for why gonad-intact female SHR have greater renal TGF-β expression than males, since TGF-β1 levels were not decreased to levels comparable to intact males following OVX. However, there are many differences between males and OVX females that could affect baseline levels of proteins, including TGF-β1; as such, we cannot fully rule out an important effect of gonadal hormones in contributing to sex differences in renal TGF-β1 expression.
In contrast, when female SHR were treated with BP-lowering drugs to either prevent the development of hypertension or reverse established hypertension, TGF-β1 levels were significantly decreased to levels comparable to those in males, suggesting that increases in BP modulate increased renal TGF-β expression in female SHR. Although there are numerous reports of enhanced TGF-β expression in hypertension (1, 2, 6, 18), the mechanisms responsible have not been examined, and nothing is known in female SHR. In addition, the majority of studies in the literature examining TGF-β in hypertension have focused on the profibrotic, injurious properties of TGF-β, and greater TGF-β expression in the kidneys of female SHR does not correspond with higher levels of renal fibrosis or injury relative to males (27). We further measured collagen deposition in the current study and found no significant differences in collagen deposition between the sexes (data not shown). Future studies are planned to determine how BP increases TGF-β exclusively in females.
The finding that elevated BP drives TGF-β1 expression levels in adult female SHR parallels our previous report of greater BP-dependent Tregs in female vs. male SHR (28). To determine whether these two findings were related, female SHR were treated with TGF-β-neutralizing antibody using the same dose and route of administration that has previously been shown to attenuate salt-induced increases in both BP and renal injury in male and female DSS rats (6, 18). In contrast to what was observed in female DSS rats, neutralizing TGF-β had no effect on BP in female SHR. This may reflect the fact that hypertension was already established in female SHR when treatment began, whereas TGF-β neutralization attenuated salt-induced increases in BP when the high-salt diet and the antibody were administered concurrently in female DSS. However, despite no impact on BP, TGF-β neutralization significantly decreased circulating Tregs with a corresponding increase in Th17 cells, supporting a key role for TGF-β in the formation of Tregs in female SHR and effective neutralization of circulating TGF-β with the antibody treatment. This same decrease in Tregs was not apparent in the kidney, although there was an increase in Th17 cells, which may reflect a decrease in renal Treg function that would not be apparent from measuring levels exclusively. A potential limitation of the current study is that we cannot conclusively state that renal TGF-β was completely neutralized by the treatment protocol that we employed. However, since naïve Tregs are generated in the thymus and activated primarily in secondary lymphoid organs, the Tregs found in the kidney are likely coming from the circulation and not being generated within the kidney (31). Therefore, neutralization of renal TGF-β may be less important than other sources. Regardless, our findings suggest that greater circulating TGF-β levels in female SHR cannot account for the sex difference in renal Tregs.
Tregs are antihypertensive and young adult female SHR have more Tregs and a lower BP than age-matched males. On the basis of recent studies supporting a critical role for T cells in BP control in both sexes, studies are needed to increase our understanding of how the T-cell profile is regulated in both males and females. In particular, identifying the mechanism(s) by which female SHR maintain greater numbers of Tregs relative to males could be used to selectively increase Tregs, thereby lowering BP. Our studies support the conclusion that TGF-β is important in regulating circulating Tregs in female SHR; however, neutralizing TGF-β did not impact renal Tregs. However, whether the neutralizing Ab was less effective in the kidney or whether the kidney is able to efficiently recruit similar numbers of Tregs despite lower numbers of circulating Tregs remains to be determined. Regardless, our studies provide novel insight into how sex of the animal impacts the immune profile in hypertension with the potential to begin to explain the sex differences in the T-cell profile.
GRANTS
This work was supported by the National Institutes of Health (Grant R01 HL-127091-02 and to J. C. Sullivan) and the American Heart Association (17EIA33410565 to J. C. Sullivan; 13PRE16110000 to A. J. Tipton).
DISCLAIMERS
This article was prepared while Dr. Tipton was at Augusta University. The opinions expressed in this article are the author's own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.T. and J.C.S. conceived and designed research; A.J.T., J.B.M., and G.R.C. performed experiments; A.J.T., J.B.M., G.R.C., and J.C.S. analyzed data; A.J.T., G.R.C., and J.C.S. interpreted results of experiments; A.J.T., G.R.C., and J.C.S. prepared figures; A.J.T. and J.C.S. drafted manuscript; A.J.T., J.B.M., G.R.C., and J.C.S. edited and revised manuscript; A.J.T., J.B.M., G.R.C., and J.C.S. approved final version of manuscript.
REFERENCES
- 1.Bae EH, Kim IJ, Joo SY, Kim EY, Kim CS, Choi JS, Ma SK, Kim SH, Lee JU, Kim SW. Renoprotective effects of sildenafil in DOCA-salt hypertensive rats. Kidney Blood Press Res 36: 248–257, 2012. doi: 10.1159/000343414. [DOI] [PubMed] [Google Scholar]
- 2.Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res 33: 255–262, 2010. doi: 10.1038/hr.2009.217. [DOI] [PubMed] [Google Scholar]
- 3.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: Recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 18: 21, 2016. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 4.Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther 291: 524–530, 1999. [PubMed] [Google Scholar]
- 5.Crislip GR, Sullivan JC. T-cell involvement in sex differences in blood pressure control. Clin Sci (Lond) 130: 773–783, 2016. doi: 10.1042/CS20150620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 7.Dixon A, Maric C. 17β-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol 293: F1678–F1690, 2007. doi: 10.1152/ajprenal.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujio K, Komai T, Inoue M, Morita K, Okamura T, Yamamoto K. Revisiting the regulatory roles of the TGF-β family of cytokines. Autoimmun Rev 15: 917–922, 2016. doi: 10.1016/j.autrev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Ganai AA, Husain M. Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-β/Smad signaling pathways. Chem Biol Interact 261: 80–85, 2017. doi: 10.1016/j.cbi.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Gillis EE, Sullivan JC. Sex differences in hypertension: Recent advances. Hypertension 68: 1322–1327, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadaschik EN, Enk AH. TGF-β1-induced regulatory T cells. Hum Immunol 76: 561–564, 2015. doi: 10.1016/j.humimm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Hering S, Sürig D, Freystadt D, Schatz H, Pfeiffer A. Regulation of transforming growth factor beta by sex steroids. Horm Metab Res 27: 345–351, 1995. doi: 10.1055/s-2007-979976. [DOI] [PubMed] [Google Scholar]
- 13.Khalil N. TGF-beta: from latent to active. Microbes Infect 1: 1255–1263, 1999. doi: 10.1016/S1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Lee AS, Jung YJ, Yang KH, Lee S, Park SK, Kim W, Kang KP. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway. Nephrol Dial Transplant 29: 2043–2053, 2014. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
- 15.Li YC, Ding XS, Li HM, Zhang Y, Bao J. Role of G protein-coupled estrogen receptor 1 in modulating transforming growth factor-β stimulated mesangial cell extracellular matrix synthesis and migration. Mol Cell Endocrinol 391: 50–59, 2014. doi: 10.1016/j.mce.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Matsuki K, Hathaway CK, Lawrence MG, Smithies O, Kakoki M. The role of transforming growth factor β1 in the regulation of blood pressure. Curr Hypertens Rev 10: 223–238, 2014. doi: 10.2174/157340211004150319123313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ. Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 303: R57–R69, 2012. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis 20: 390–395, 2013. doi: 10.1053/j.ackd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Pardali E, Ten Dijke P. TGFβ signaling and cardiovascular diseases. Int J Biol Sci 8: 195–213, 2012. doi: 10.7150/ijbs.8.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol 275: R1719–R1723, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Romero M, Caniffi C, Bouchet G, Elesgaray R, Laughlin MM, Tomat A, Arranz C, Costa MA. Sex differences in the beneficial cardiac effects of chronic treatment with atrial natriuretic peptide in spontaneously hypertensive rats. PLoS One 8: e71992, 2013. doi: 10.1371/journal.pone.0071992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39: 567–573, 2016. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan JC, Pardieck JL, Brinson K, Kang KT. Effects of estradiol on renal cyclic guanosine monophosphate and oxidative stress in spontaneously hypertensive rats. Gend Med 6: 498–510, 2009. doi: 10.1016/j.genm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan JC, Pardieck JL, Doran D, Zhang Y, She JX, Pollock JS. Greater fractalkine expression in mesenteric arteries of female spontaneously hypertensive rats compared with males. Am J Physiol Heart Circ Physiol 296: H1080–H1088, 2009. doi: 10.1152/ajpheart.01093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 28.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol 4: 29–37, 2012. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 31.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood 108: 426–431, 2006. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]