Abstract
During the early phase of ANG II-dependent hypertension, tubular PGE2 is increased. Renin synthesis and secretion in the collecting duct (CD) are upregulated by ANG II, contributing to further intratubular ANG II formation. However, what happens first and whether the triggering mechanism is independent of tubular ANG II remain unknown. PGE2 stimulates renin synthesis in juxtaglomerular cells via E-prostanoid (EP) receptors through the cAMP/cAMP-responsive element-binding (CREB) pathway. EP receptors are also expressed in the CD. Here, we tested the hypothesis that renin is upregulated by PGE2 in CD cells. The M-1 CD cell line expressed EP1, EP3, and EP4 but not EP2. Dose-response experiments, in the presence of ANG II type 1 receptor blockade with candesartan, demonstrated that 10−6 M PGE2 maximally increases renin mRNA (approximately 4-fold) and prorenin/renin protein levels (approximately 2-fold). This response was prevented by micromolar doses of SC-19220 (EP1 antagonist), attenuated by the EP4 antagonist, L-161982, and exacerbated by the highly selective EP3 antagonist, L-798106 (~10-fold increase). To evaluate further the signaling pathway involved, we used the PKC inhibitor calphostin C and transfections with PKCα dominant negative. Both strategies blunted the PGE2-induced increases in cAMP levels, CREB phosphorylation, and augmentation of renin. Knockdown of the EP1 receptor and CREB also prevented renin upregulation. These results indicate that PGE2 increases CD renin expression through the EP1 receptor via the PKC/cAMP/CREB pathway. Therefore, we conclude that during the early stages of ANG II-dependent hypertension, there is augmentation of PGE2 that stimulates renin in the CD, resulting in increased tubular ANG II formation and further stimulation of renin.
Keywords: kidney, prostaglandin E2, protein kinase C, CREB, gene expression
renin produced by the juxtaglomerular (JG) cells is the primary source of plasma renin activity and is the limiting step for the activation of the systemic renin-angiotensin system (RAS) (8). In conditions of volume and/or sodium depletion, the synthesis and secretion of renin are increased in JG cells (7, 10, 31, 32) by the activation of receptors coupled to stimulatory G proteins (Gαs). In turn, these activate adenylyl cyclase (AC), increasing intracellular cAMP levels (21). Accumulation of cAMP activates PKA and subsequent phosphorylation of cAMP response element-binding protein (CREB), turning on renin gene transcription (21). After systemic RAS activation, a negative-feedback mechanism, mediated by the ANG II type 1 (AT1) receptor, stimulates Ca2+ release (22) and PKC (29), which inhibit the stimulation of renin synthesis in the JG cells.
In contrast, ANG II increases renin synthesis in collecting duct (CD) cells (33, 34) via a mechanism mediated by the AT1 receptor (15) and signaling through the PKCα/Ca2+/cAMP/CREB pathway and is partially dependent on AC6 activity (14), which is opposite to what has been observed for AC6 in other tissues (38). Augmented CD renin synthesis and secretion, observed in ANG II-dependent hypertensive rats (15, 26), suggest that renin, locally produced in the CD segments, promotes intratubular ANG II formation, thereby contributing to enhanced distal tubular sodium reabsorption and blood pressure regulation. The demonstration that mice overexpressing renin in the CD exhibit elevated blood pressure (35) further supports the concept that CD renin contributes to the pool of intratubular ANG II and local actions mediated by the AT1 receptors. However, during ANG II-dependent hypertension, a mechanism, independent of the activation of tubular AT1 receptors, might be necessary to initiate the augmentation of renin to start the cascade, leading to increased intratubular ANG II levels.
During the early phase of ANG II-dependent hypertension, tubular PGE2 is increased (13). PGE2 is the most prominent PG in rat and mouse kidney. PGE2 exerts its effects through four types of E-prostanoid (EP) receptors: EP1, EP2, EP3, and EP4 (5). Along the nephron, EP4 receptors are predominantly expressed in the macula densa cells, whereas EP3 receptors are more abundant in the thick, ascending limb (16). The CD segment expresses EP1, EP3, and EP4 receptors (5). EP1 is a Gαq-coupled receptor that activates PKC and increases intracellular Ca2+, whereas the EP3 receptor is coupled to a Gαi protein that inhibits cAMP formation. The EP4 receptor is coupled to a Gαs protein, which enhances cAMP formation (3, 4). We have shown that the activation of the PKC/cAMP/CREB pathway increases renin synthesis in the CD cells; however, the role of PGE2 in the regulation of CD renin synthesis has not been delineated. With the use of the mouse cortical CD M-1 cell line, which maintains the phenotype of CD cells (39) and stable expression of renin, we tested the hypothesis that renin is upregulated by PGE2 in CD cells. Furthermore, with the use of pharmacological and molecular approaches, we explored the role of different EP receptors and most relevant intracellular pathways involved in the synthesis of renin.
METHODS
M-1 cell culture and reagents.
Mouse cortical CD M-1 cells, which maintain the phenotype of CD cells (39), were obtained from American Type Culture Collection (CRL-2038; Manassas, VA), grown in DMEM and Ham’s F-12 nutrient mixture (HyClone) with L-glutamine and HEPES, and used at passages 1–8. Mycoplasma contamination was previously ruled out by using the MycoFluor Mycoplasma Detection Kit (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. Cells were cultured, as previously described (12, 14). Before treatments, cells were fasted for 20 h in serum-free media in a 70–80% confluence. M-1 cells have been shown to express renin 1 transcripts and protein, mainly prorenin (14). To establish the dose needed to increase renin mRNA and prorenin and renin protein bands in vitro, we performed a dose-response curve with different concentrations (10−9–10−6 mol/l) of PGE2 (Cayman Chemical, Ann Arbor, MI). Additionally, to establish the receptor involved in renin regulation, we used the following EP antagonists at doses described in the literature (20). The EP1 antagonist SC-19220 (at 100 μM; Cayman Chemical) was assessed in a dose-response curve to verify its effect on PGE2-induced prorenin/renin protein abundances at doses of 6.7, 30, and 100 μM, described in the literature (6, 11, 20). L-798106 (Cayman Chemical), a highly selective EP3 receptor antagonist with Ki values of 0.3 µM, was used at 0.2 µM, which has been shown to block the agonist effect of sulprostone (1, 19, 23). Finally, we used L-161982 (Cayman Chemical), which is a potent and selective EP4 receptor antagonist that demonstrates selective binding to human EP4 receptors with a Ki value of 0.024 µM. We used a fourfold-higher concentration (100 nM) (41). Cells were harvested after 6 h of PGE2 treatments, with or without antagonists, or after 32 h of transfections. PGE2 and antagonists were dissolved in DMSO for stock and ethanol (vehicle) for dilutions. Controls were treated with vehicle with respective subsequent dilutions in cell culture media. Antagonists were added 20 min before PGE2. Treatments were performed in the presence of indomethacin (1 µM) to block endogenous cyclooxygenase activity and candesartan (1 µM) to rule out the possibility of AT1 receptor activation. Calphostin C was used at 500 nM to avoid inhibition of PKA (IC50 > 50 µM) and PKG (IC50 > 25 µM). PMA was used at 10−6 mol/l. The PKA inhibitor H89 (ATP-competitive inhibitor of PKA, Ki = 48 nM) was used at 100 nM, as described previously. Calphostin C, PMA, and H89 reagents were purchased from Sigma Chemical (St. Louis, MO) and used as described previously (14).
EP receptors and renin mRNA quantitation by real-time qRT-PCR.
Total RNA extracted (100 ng) was transcribed to cDNA and amplified using the TaqMan system. Primers used to amplify EP receptors and Renin-1c are listed in Table 1. Results are presented as a ratio between the levels of mRNA of the interest gene against β-actin as percentage of control in triplicates of n = 5–6. Gel electrophoresis of PCR products from quantitative (q)RT-PCR of EP receptors was also performed to confirm the quality of the primers used and the efficacy of the parameters used: cycle number, annealing temperature, and amount of starting RNA.
Table 1.
Primers used to amplify EP receptors and renin 1c gene
| Forward | Fluorogenic Probe | Reverse | Size, bp | |
|---|---|---|---|---|
| EP1 | 5′-CTACCCACACGGCCTTAAAA-3′ | 5′6-FAM-GCATGTTCCCATCTTGACCT-BHQ1-3′ | 5′-TCTGGACTGGAGTGTGCTTG-3′ | 156 |
| EP2 | 5′-GGTGGTGCTGGCTTCATATT-3′ | 5′6-FAM-CCTTCTTTAGTCTGGCCACG-BHQ1-3′ | 5′-CAGGGAACAGAAGAGCAAGG-3′ | 250 |
| EP3 | 5′-TACCTGTTTCCCTGGGTCTG-3′ | 5′6-FAM-CAAAGGTTCTGAGGCTGGAG-BHQ1-3′ | 5′-TTCTTCTCCCAGGGAAGGAT-3′ | 263 |
| EP4 | 5′-CCATCGCCACATACATGAAG-3′ | 5′6-FAM-CCACGCCTACTTCTACAGCC-BHQ1-3′ | 5′-TGCATAGATGGCGAAGAGTG-3′ | 209 |
| Renin 1 | 5′-AGTACTATGGCGAGATCGGCATT-3′ | 5′6-FAM-TTCAAAGTCATCTTTGACACGGGTTCAG-BHQ1-3 | 5′-TGATCCGTAGTGGATGGTGA-3′ | 200 |
| β-Actin | 5′-ATCATGAAGTGTGACGTTGA-3′ | 5′6-HEX-TCTATGCCAACACAGTGCTGTCTGGT-BHQ2-3′ | 5′-GATCTTCATGGTGCTAGGAGC-3′ | 138 |
qRT-PCR was normalized by using the β-actin housekeeping gene.
Western blot analysis of EP receptors, prorenin-renin, phospho-CREB, and total CREB.
After treatments, M-1 cells were homogenized in lysis buffer (in mmol/l: NaCl 150, Tris 50, DTT 100, 1% Nonidet P-40, and 1% protease inhibitor cocktail; Sigma Chemical) and a complete protease inhibitor mixture tablet with 1 mM EDTA. Lysates were sonicated for 10 s on ice and centrifuged at 5,000 rpm for 10 min. The supernatants were measured for protein using the Bradford Protein Assay (Bio-Rad, Hercules, CA). Twenty micrograms of total protein was separated by SDS-PAGE (10 or 12%), transferred to the nitrocellulose membrane (Amersham Biosciences Europe, Freiburg, Germany), and probed with EP receptors (EP1, Cat. 101740; EP2, Cat. 101750; EP3, Cat. 101760; EP4, Cat. 101775; Cayman Chemical); rabbit anti-renin epitope, corresponding to amino acids 116–220 and mapping to an internal region of renin (Cat. sc H-105; Santa Cruz Biotechnology, Dallas, TX); anti-phospho-CREB (Ser133 and activating transcription factor 1); and total CREB (obtained from Cell Signaling Technology, Danvers, MA). For prorenin and renin detection, IgG B-12 antibody raised against amino acids 116–220 of renin of human origin to detect the precursor, and mature renin of mouse was used (Santa Cruz Biotechnology). Identities of the bands are fully characterized in our previous publications (12, 14). Results were expressed as the ratio between the protein of interest and β-actin in percentage vs. control (100%).
Immunofluorescence studies.
M-1 cells were fixed in cold methanol. Then, they were blocked with BSA 0.1%–Triton solution, stained with either rabbit EP antibodies mentioned before or rabbit anti-renin (Cat. sc H-105; Santa Cruz Biotechnology), and detected with Alexa Fluor 594 (renin) or 488 (EP receptors), conjugated to anti-rabbit or anti-mouse IgG (Thermo Fisher Scientific) accordingly. Samples were counterstained with 4′6-diamidino-2-phenylindole (Thermo Fisher Scientific). Negative controls were obtained by omission of the specific primary antibody.
Transfection with PKCα dominant negative and shRNA for CREB and the EP1 receptor.
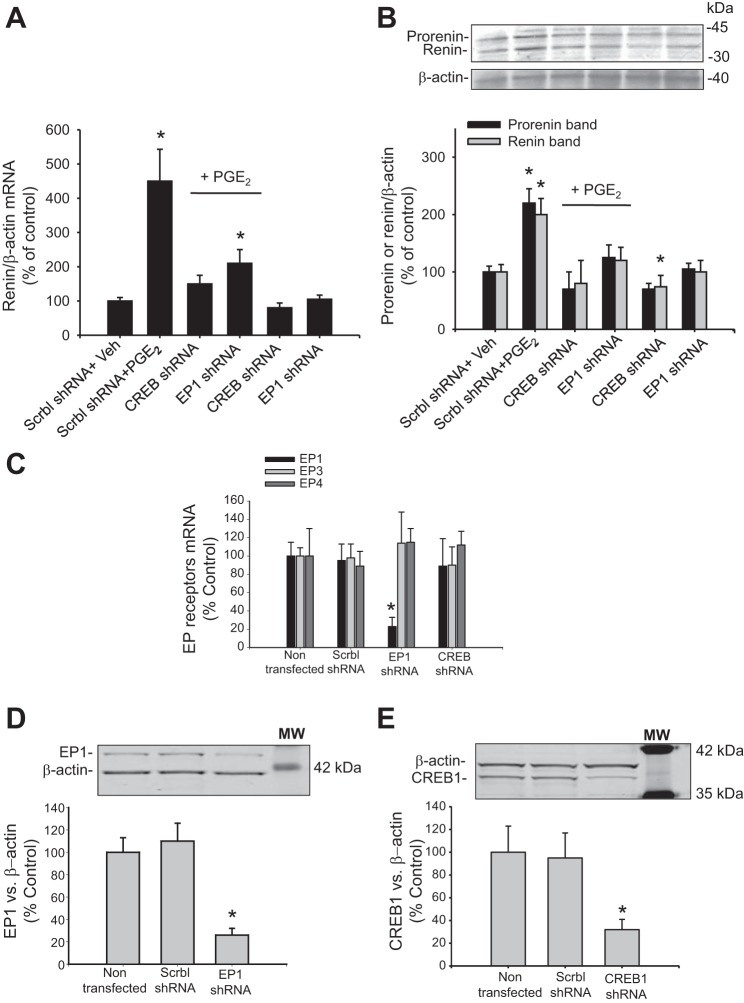
M-1 cells were transiently transfected with PKCα dominant-negative (DN) plasmids (K368R kinase dead mutation, plasmid 21235; Addgene, Cambridge, MA) using Lipofectamine (Thermo Fisher Scientific), as described previously (12). The short hairpin (sh)RNA, used for the EP1 receptor, was purchased from SABioscience (KM05159G; Qiagen, Germantown, MD). CREB shRNA plasmid was used and characterized previously (12). Cells were transfected during 32 h before PGE2 treatment. Transfection efficiency of the knockdown of EP1 and CREB is shown (see Fig. 7, C–E). Cell viability, assessed by alamarBlue Cell Viability Reagent (DAL 1025; Thermo Fisher Scientific), was not affected by transfections (data not shown).
Fig. 7.
PGE2 upregulates renin through EP1 via the CREB pathway. M-1 cells were transiently transfected with scrambled (Scrbl) shRNA, CREB shRNA, or EP1 receptor shRNA, 32 h before PGE2 treatment. A: CREB shRNA blunted the induction of renin mRNA mediated by PGE2; EP1 knockdown partially prevented this effect. B: representative Western blot of all groups showing similar effects observed for renin mRNA levels. *P < 0.05 vs. Veh, n = 6. C: semiquantitative qRT-PCR measurements of EP mRNAs (percentage of control) showing the effect of EP1 shRNA and CREB1 shRNA transfections (32 h). Scrambled shRNA was used as control. D: protein abundances of EP1 after transfections (32 h) with EP1 shRNA plasmids showing nearly 77% of reduction. E: protein abundances of CREB1 protein after transfections (32 h) with EP1 shRNA plasmids showing nearly 68% of reduction. *P < 0.05 vs. nontransfected cells, n = 4–6.
cAMP measurements.
The cAMP levels of M-1 cells were determined with cAMP ELISA 581001 (Cayman Chemical), according to the manufacturer’s instructions after 20 min of treatment.
Docking assay of CREB phosphorylation by PKCα.
Two models were generated for protein–protein docking: PKCα and CREB1. These models were constructed based on the amino acid sequence of PKCα (P20444) of Mus musculus and CREB1 (Q01147) of Mus musculus, obtained from UniProtKB (www.uniprot.org), using the web server Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index). Modeling gave a confidence level of 46% for CREB1 and 78% for PKCα. Both models were analyzed with PyMOL molecular graphics system software (https://pymol.org/). Protein–protein docking was performed using a web server, ClusPro (http://cluspro.bu.edu/home.php). Molecular graphics were created using PyMOL.
Statistics analyses.
All observations were made from multiple splits. For Western blot and mRNA levels, an average number of six independent observations were performed for each treatment and represented as percentage vs. controls (100%). Data were evaluated by the Grubbs’ test, followed, when appropriate, by paired and unpaired Student’s t-tests or by one-way ANOVA with a Tukey post-test. Significance was defined as P < 0.05. No significant differences are expressed as “NS.” Results are expressed as means ± SE.
RESULTS
M-1 cells express EP1, EP3, and EP4 receptors.
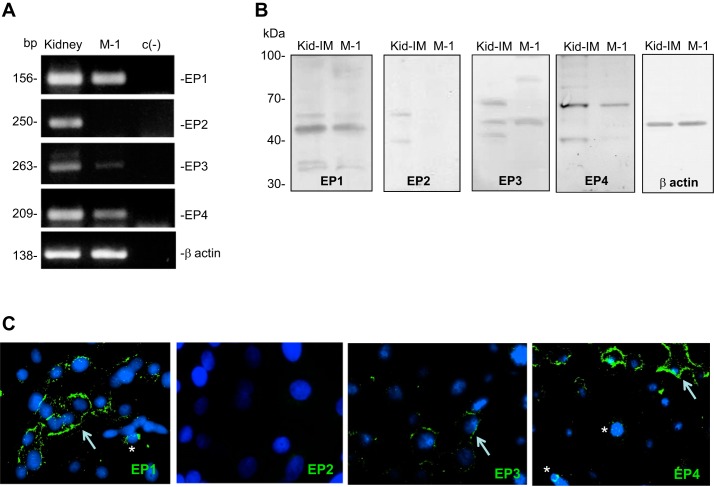
Results of qRT-PCR (n = 3) demonstrated a robust amount of the mRNA for EP1. EP3 and EP4 were also detected, whereas the EP2 receptor was not expressed in M-1 cells (Fig. 1A). Representative Western blot of EP receptors in M-1 cells and kidney demonstrated the expression of EP1, EP3, and EP4 receptors. Expected molecular size is shown in Fig. 1B. Immunofluorescence studies confirmed the presence of EP1, EP3, and EP4 receptors, whereas EP2 was not detectable. Positive labeling was detected in the plasma membrane and nuclei for EP1 and EP4 (Fig. 1C).
Fig. 1.
E-Prostanoid receptors in M-1 collecting duct cells. A: primers used for qRT-PCR were used in a conventional PCR to demonstrate the specificity of the band. These results also demonstrated the robust amplification of the mRNA for EP1 and EP4 receptors and EP3, to a lesser extent. The EP2 receptor was not detectable in M-1 cells. Housekeeping gene β-actin is also shown. PCR product size is shown in base pairs (bp). c(-), negative control without template. B: protein expression of EP receptors in the kidney inner medulla (Kid-IM) and M-1 cells detected by Western blot. Molecular mass is shown in kilodaltons (kDa). EP1 was detected at the expected molecular mass (42 kDa). For EP3, a second band was detected at 70 kDa. The expected band (52 kDa) for the EP4 receptor was abundantly expressed in whole mouse kidney but diffusely detected in M-1 cells. C: immunocytochemistry in M-1 cells showing the expression of EP receptors in the plasma membrane (arrows) and perinuclear pattern (asterisks). EP2 was not detected. Positive labeling, green.
PGE2 increases renin expression in M-1 cells.
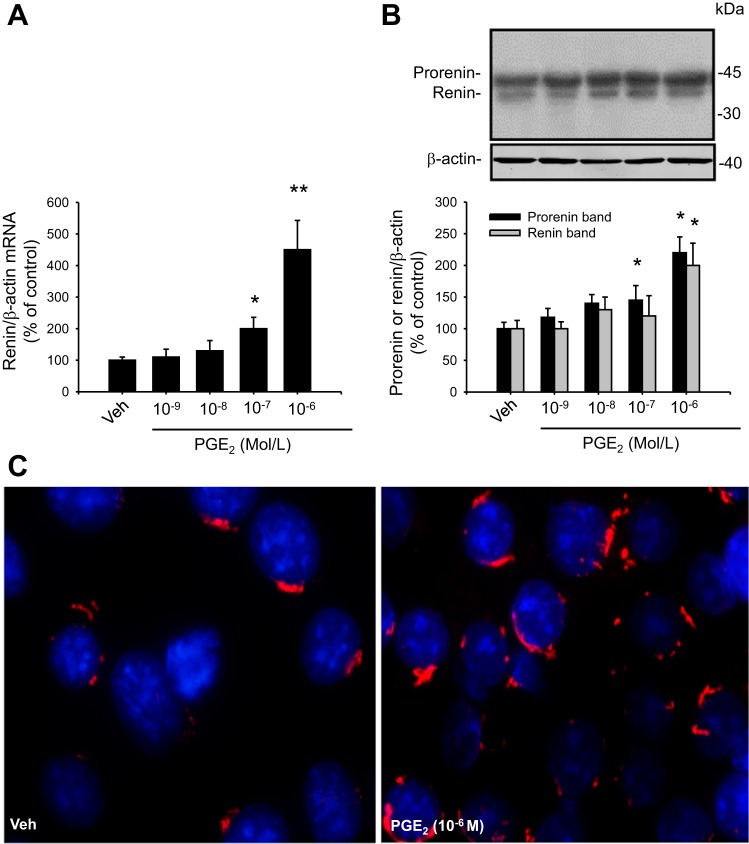
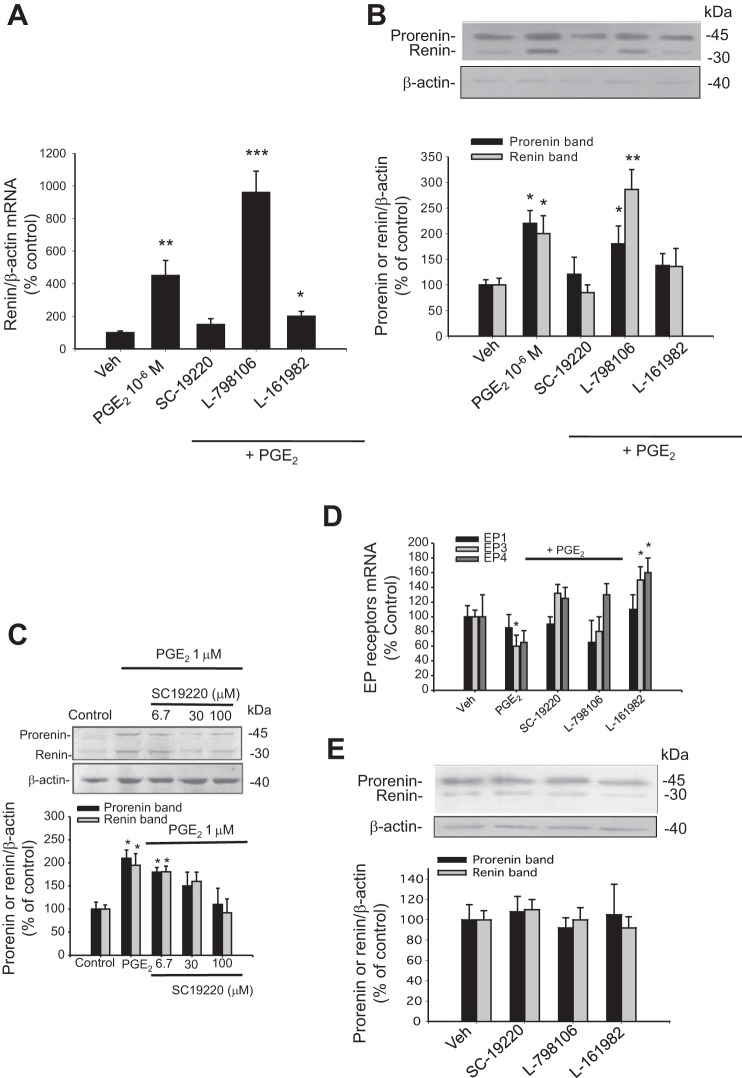
Dose-response curves for renin mRNA and prorenin and renin protein bands were performed using PGE2 concentrations from 10−9 to 10−6 mol/l. After 6 h of treatment, cell lysates were used for RNA isolation for qRT-PCR or Western blot analysis. As shown in Fig. 2A, PGE2 significantly increased renin mRNA at 10−7 mol/l (200 ± 36% vs. 100 ± 10%, P < 0.05) and was further increased at 10−6 mol/l (450 ± 93%, P < 0.001). Prorenin and renin protein bands were increased at 10−6 mol/l (220 ± 25% and 200 ± 28%, respectively, P < 0.05 vs. controls; Fig. 2B). Immunostaining experiments using prorenin/renin antibody demonstrated a perinuclear pattern. Labeling intensity was augmented after PGE2 treatment (Fig. 2C).
Fig. 2.
PGE2 increases renin mRNA and prorenin/renin protein levels in M-1 cells. A: dose-response curve from 10−9 to 10−6 M PGE2 for renin mRNA levels after 6 h treatment. B: dose-response curve from 10−9 to 10−6 M PGE2 for prorenin/renin protein levels after 6 h treatment. Band identity of prorenin and renin bands in collecting duct cells and medullary renal tissues is described previously (10, 12, 23). C: renin immunofluorescence in M-1 cells treated with vehicle (Veh; ethanol) or PGE2 at 10−6 M. *P < 0.05, **P < 0.01 vs. Veh, n = 6. Immunostaining demonstrated perinuclear pattern.
Effects of PGE2 and EP receptor antagonists on cAMP production in M-1 cells.
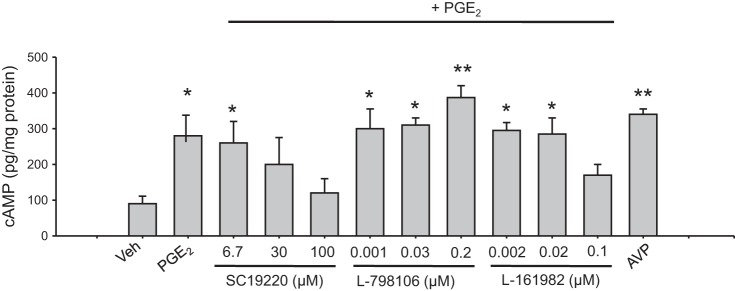
To determine the status of the PGE2 response on exposure to EP receptor antagonists, we measured cAMP levels in cells after stimulation with PGE2. Figure 3 shows cAMP levels after PGE2 treatment (1 μM), with or without EP antagonists SC-19220 (EP1), L-798106 (EP3), and L-161982 (EP4) at doses described in methods. As observed, PGE2 induced cAMP production in M-1 cells. SC-19220 was effective in blocking the PGE2-induced cAMP production at 100 μM. L-798106 was able to enhance cAMP production further after PGE2 treatment at 0.2 μM, whereas the EP4 antagonist partially decreased the PGE2-induced cAMP accumulation at 0.1 μM. As a positive control, we used arginine-vasopressin (AVP; 10−6 mol/l) to induce cAMP accumulation in M-1 cells (12).
Fig. 3.
Intracellular cAMP levels in M-1 cells treated with PGE2 or PGE2 plus different doses of EP antagonists described in the literature. Maximum responses were observed at 100 μM SC-19220, 0.2 μM L-798106, and 0.1 μM L-161982. Treatment with AVP was used as a positive control. *P < 0.05 vs. Veh, **P < 0.01 vs. Veh, n = 5.
EP1 antagonist prevented the PGE2-induced upregulation of renin, whereas EP3 antagonists enhanced the effect of PGE2.
We next measured the effect of each EP antagonist on the PGE2-mediated induction of renin mRNA and prorenin/renin protein levels. EP antagonists were added 20 min before addition of PGE2 (1 μM). As shown in Fig. 4, A and B, the EP1 antagonist SC-19220 prevented the effect of PGE2 on renin mRNA upregulation (150 ± 36 vs. 100 ± 10%, P = NS). Likewise, the effect of PGE2 to increase prorenin (121 ± 33 vs. 100 ± 10%, P = NS) and renin (85 ± 15 vs. 100 ± 13%, P = NS) protein levels was prevented. The EP4 antagonist attenuated the PGE2-induced expression of renin mRNA (200 ± 30%, P < 0.05 vs. controls), prorenin (138 ± 23, P < 0.05 vs. controls), and renin protein levels (136 ± 32%, P < 0.05 vs. controls). Interestingly, the EP3 antagonist exacerbated the PGE2-mediated increase in renin mRNA (960 ± 130%, P < 0.001 vs. controls), prorenin (180 ± 36%, P < 0.001 vs. controls), and renin (286 ± 39%, P < 0.001 vs. controls) proteins. To explore further if the preponderant inhibitory effect of EP1 antagonist on PGE2-mediated cAMP production correlates with the expression of prorenin/renin protein abundances, we performed a similar experiment at the three doses described above. The maximal inhibitory effect on prorenin/renin protein expression was observed at 100 μM (Fig. 4C). In addition, we explored the possibility that PGE2 and antagonists might influence EP receptor mRNA expression after 6 h of treatment. As shown in Fig. 4D, PGE2 caused a significant reduction in EP3, whereas EP1 was not altered. Interestingly, EP4 antagonist L-161982 caused an upregulation of EP3 (~150%) and EP4 (~160%). Treatment with antagonists alone had no effect on prorenin/renin levels or EP receptor abundances after 6 h (Fig. 4E).
Fig. 4.
A: EP1 antagonist prevented PGE2-induced renin expression. M-1 cells were incubated with PGE2 (10−6 M), alone or in combination with EP antagonists. PGE2-induced renin expression was abolished by EP1 antagonist SC-19220 and partially blunted by the EP4 antagonist L-161982. EP3 antagonist L-798106 exacerbated the increased expression of renin mediated by PGE2 at mRNA (A) and prorenin/renin protein levels (B). *P < 0.05 vs. Veh, **P < 0.01, ***P < 0.001 vs. Veh, n = 6. C: effect of different doses of SC-19220 in M-1 cells treated with 1 μM PGE2 on prorenin and renin protein levels. In accordance with the results observed for cAMP levels, 100 μM SC-19220 completely prevented the PGE2-induced prorenin and renin upregulation. D: effect of PGE2 and antagonist treatments on EP receptor mRNA expression after 4–6 h. PGE2 causes a significant reduction in EP3 and EP4, whereas EP1 was not altered. PGE2 plus EP4 antagonist L-161982 treatment causes the upregulation of EP3 (150%) and EP4 (160%). E: treatment with antagonists alone had no effect on prorenin/renin levels after 6 h. *P < 0.05 vs. Veh, n = 4.
Inhibition of PKC activity suppressed the PGE2-mediated increase in renin expression.
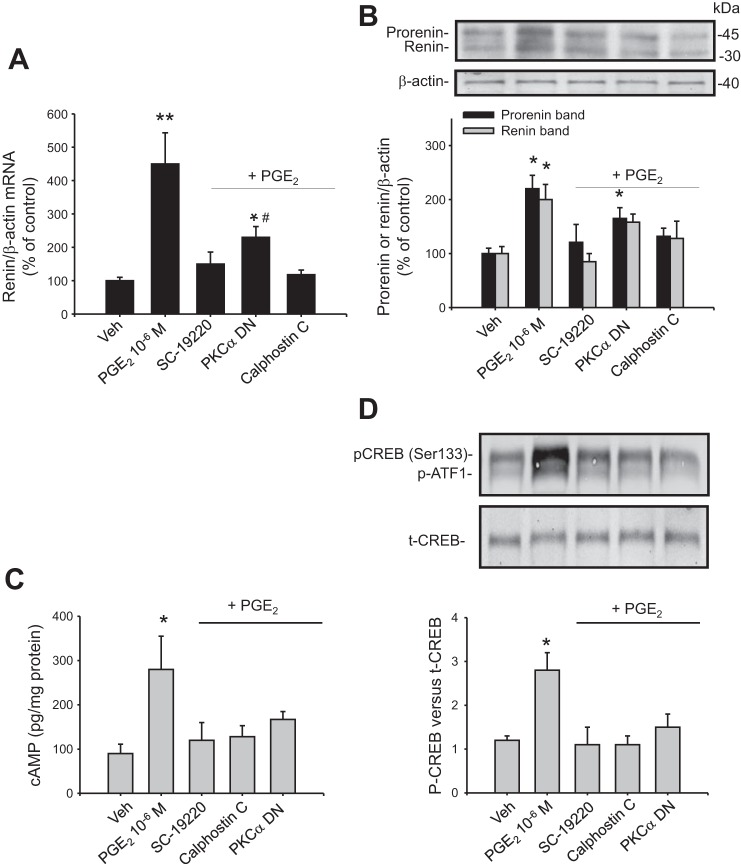
Since the EP1 antagonist blunted the PGE2-mediated renin upregulation, we evaluated the second messengers involved in the activation of the EP1 receptor. EP1 is a Gαq-coupled receptor that increases PKC activity and raises intracellular Ca2+. We used the broad-spectrum PKC inhibitor calphostin c to explore the possibility that EP1 may act through this pathway to increase renin expression. As shown in Fig. 5, A and B, calphostin C prevented the PGE2-induced renin mRNA (118 ± 14%, P = NS vs. controls) and prorenin (132 ± 15%, P = NS vs. control group) and renin (128 ± 32%, P = NS vs. control group) protein levels. In view of our recent evidence showing that PKCα is responsible for renin regulation in the CD (12), we tested the effect of a PKCα DN (kinase dead mutation) on PGE2-induced renin upregulation. The M-1 cells were transiently transfected with plasmids containing the aberrant PKCα. PKCα DN had a similar effect on renin mRNA, although the effect was not as strong as calphostin C, probably due to the efficiency of transfections (230 ± 32%, P < 0.05 vs. control and PGE2-induced group). The same effect was observed for prorenin and renin (PKCα DN: 165 ± 20 vs. PGE2-induced: 220 ± 25%, P < 0.05, and PKCα DN: 158 ± 15 vs. PGE2-induced: 200 ± 28%, P = NS vs. control group).
Fig. 5.
Inhibition of PKC activity and PKCα dominant negative (DN) suppressed the effect of PGE2 on renin expression. PGE2-mediated increase in renin mRNA (A) and prorenin/renin protein levels (B) were prevented by pretreatment with calphostin c (PKC inhibitor) and by previous transfection with PKCα DN. Cells were transfected 32 h before PGE2 treatment. Efficiency of transfections was assessed by green fluorescent protein fluorescence (see methods; *P < 0.05 vs. Veh, **P < 0.01, #P < 0.05 vs. PGE2-treated group, n = 6). We further examined the inhibition of PKC activity, and PKCα DN suppressed the effect of PGE2 on cAMP production and CREB phosphorylation (pCREB) in M-1 cells. Cells were treated with PGE2 or PGE2 plus pretreatment with the EP1 antagonist SC-19220 or PKC inhibitor calphostin C or were previously transfected with PKCα DN. The cAMP levels (C) and pCREB (D) were evaluated after 20 min of treatment. *P < 0.05 vs. Veh, n = 6. p-ATF1, activating transcription factor 1 phosphorylation; t-CREB, total CREB.
Inhibition of PKC suppressed the PGE2-mediated increase in cAMP and CREB phosphorylation.
Because cAMP/PKA/CREB is a central pathway in the regulation of renin gene expression, we evaluated if CREB phosphorylation is affected by EP1 antagonism or PKC inhibition. PGE2 caused a marked increase in cAMP compared with vehicle-treated cells (298 ± 85 vs. 75 ± 26 pg/mg protein, P < 0.05; Fig. 5C). Importantly, cAMP production was inhibited by SC-19220 (120 ± 40 pg/mg protein) and calphostin c (118 ± 25 pg/mg protein) and to a lesser extent, PKCα DN (167 ± 18 pg/mg protein, P = 0.078). As shown in Fig. 5D, the PGE2-mediated increase in the intensity of CREB phosphorylation was prevented by SC-19220, calphostin c, and PKCα DN (phospho-CREB vs. total CREB ratio: vehicle: 1.2 ± 0.1; PGE2: 2.8 ± 0.4; SC-19220 + PGE2: 1.1 ± 0.4; calphostin c + PGE2: 1.1 ± 0.2; PKCα DN + PGE2: 1.5 ± 0.3). Prorenin or renin expression and CREB phosphorylation levels were not different in PKC DN vs. green fluorescent protein-transfected cells or vehicle (data not shown).
Inhibition of PKA activity suppressed the PGE2-mediated increase in renin expression.
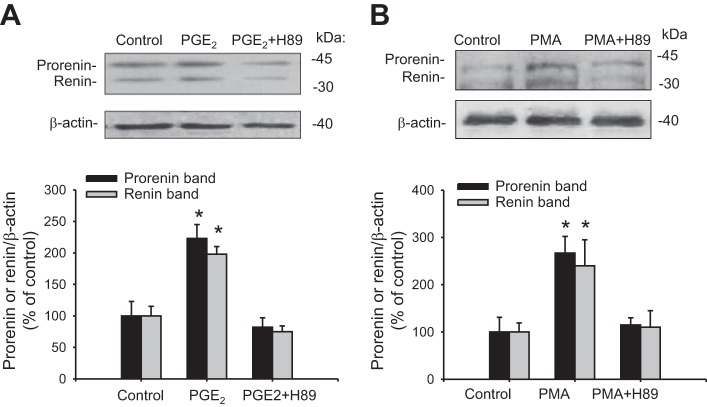
Because the central pathway for renin regulation in JG cells depends on PKA activity, we tested if PKA inhibition alters the stimulatory effect of PGE2 on renin protein expression. As shown in Fig. 6A, prorenin and renin protein levels were upregulated by PGE2 (223 ± 22 vs. 100 ± 23%, P < 0.05, and 19 ± 12 vs. 100 ± 15%, P < 0.05). PKA inhibition with H89 prevented the upregulation of prorenin (82 ± 15%, P = NS) and renin (75 ± 9, P = NS). To confirm further that PKC activation by itself increases prorenin and renin protein levels via PKA activation, we used PMA to activate PKC. As shown in Fig. 6B, PMA treatment for 6 h induced prorenin (267 ± 35%, P < 0.05 vs. controls) and renin (240 ± 55%, P < 0.05 vs. controls) expression, whereas PKA inhibition with H89 prevented these effects. Taken together, these results indicate that PGE2 mediates the PKC activation.
Fig. 6.
Augmented prorenin and renin expression mediated by PGE2 or PKC activation is blunted by PKA inhibition. M-1 cells were incubated during 6 h with PGE2 or PGE2 plus PKA inhibitor H89. A: to evaluate the effect of PKC activation and the possible role of PKA, M-1 cells were treated with PMA and prorenin and renin abundances evaluated after 6 h. B: PMA increases prorenin and renin protein expression, whereas PKA inhibition suppressed this effect. *P < 0.05 vs. Veh, n = 6.
Knockdown of CREB or EP1 prevented the PGE2-mediated upregulation of prorenin/renin.
To confirm further the role of CREB in the pathway for renin regulation in M-1 cells, we used shRNA technology to knock down its expression (Fig. 7). M-1 cells were transfected 32 h before PGE2 treatments with CREB shRNA plasmids, as previously described (12). As shown in Fig. 7A, the induction of renin mRNA caused by PGE2 (450 ± 9%) was prevented by CREB shRNA transfections (150 ± 25%, P = NS vs. control) and EP1 knockdown (210 ± 40%, P < 0.05 vs. control group and PGE2-induced group). EP1 knockdown also impairs increases in cAMP. No changes were observed in cAMP levels in M-1 cells treated with EP1 or CREB1 shRNA alone (Table 2). Similar results were observed for prorenin and renin protein levels (Fig. 7B). No effects on EP3 or EP4 were observed after EP1 knockdown (Fig. 7C). These data demonstrate a preponderant role of EP1/CREB in the pathway responsible for the PGE2-mediated renin upregulation. Protein abundances of CREB1 after transfections (32 h) with EP1 shRNA plasmids showed nearly 68% reduction (P < 0.05 vs. nontransfected cells, n = 4–6; Fig. 7, D and E).
Table 2.
Levels of cAMP in M-1 cells treated with EP1 or CREB1 shRNA
| Veh | shRNA EP1 | shRNA CREB1 | PGE2 | PGE2 + shRNA EP1 | PGE2 + shRNA CREB1 |
|---|---|---|---|---|---|
| 98 | 86 | 88 | 289 | 120 | 65 |
| 112 | 78 | 78 | 315 | 110 | 89 |
| 85 | 80 | 65 | 348 | 135 | 87 |
| 78 | 99 | 98 | 240 | 89 | 54 |
| 88 | 89 | 113 | 210 | 98 | 110 |
| 92 | 86 | 88 | 280* | 110 | 81 |
sh, Small hairpin. *P < 0.05 vs. vehicle (Veh).
DISCUSSION
Previous studies from our group have demonstrated that the PKC/Ca2+/PKA/CREB pathway is stimulated by the ANG II/AT1 receptor to regulate renin expression in the CD. However, in the absence of intratubular ANG II availability, other mechanisms stimulating CD renin synthesis and release are essential to initiate the generation of distal tubular ANG II. In ANG II-infused rats, high expression levels of cyclooxygenase-2 (COX-2) and increased urinary levels of PGE2 are observed during the early phase (13). Increased COX-2 expression in the renal medulla and kidney and urinary PGE2 might reach distal tubular segments, activating EP receptors in the CD. During the early phase of ANG II-induced hypertension, PGE2 might exert a buffer mechanism in the kidney but also might be the initial stimuli for CD renin-dependent intratubular ANG II formation. These previous animal studies (13) raised awareness of the need for some other factor to get the cascade of CD ANG II formation started. With this rationale, we evaluated the mechanisms involved on renin regulation mediated by PGE2 in the absence of AT1 receptor activation. We used a simple cellular model that maintains the phenotypic characteristics of the CD cells, and more importantly, these cells constitutively express stable levels of renin (12, 14). Although it is common that in vitro studies require much higher concentrations of the agonist agent (micromolar concentrations of PGE2 in our report), it is possible that PGE2 might have an effect at lower doses in vivo. Primary cultures of inner medullary CD cells also have been used; however, this model shows a rapid decrease in renin gene expression after 8–12 h. The mechanisms responsible for this phenomenon are under investigation.
In the present study, we demonstrate robust PGE2-induced renin mRNA and protein upregulation and strong perinuclear staining, suggesting a possible pathway for secretion and intracellular activity. We also observed the augmentation of cAMP levels and CREB phosphorylation, which are prevented by inhibition and knockdown of the EP1 receptor. The induction of renin expression by PGE2 was blunted by PKA inhibition and knockdown of CREB, supporting the involvement of the cAMP/PKA/CREB pathway in the upregulation of renin in M-1 cells. Interestingly, CREB shRNA resulted in low levels of renin protein, suggesting that in M-1 cells, CREB protein is essential for renin expression. Transient transfections with EP1 shRNA resulted in a partial but incomplete reduction of the stimulatory effect of PGE2. This might be due to the transfection efficiency, which might not be enough to have an effect in the full population of cells (Fig. 7, D and E). We also demonstrated that PKC and in particular, PKCα play a role in this pathway. Similarly, we showed that Ca2+-dependent PKC activity is necessary for the cAMP production in CD cells (14).
PGE2 exerts a variety of actions in the kidney, including the stimulation of renin synthesis in JG cells (17, 31, 32) and regulation of renal medullary blood flow and tubular sodium and water transport (2). Although PGs also exist as PGI2 and thromboxane A2, with roles in renal homeostasis and pathology, there is no evidence that they have an effect on CD renin regulation. A close relationship between ANG II and COX-2 has been proposed (18, 43, 44). Augmented COX-2, in response to high glucose or increased ANG II, is dependent on reactive oxygen species generation and increased PGI2 synthesis, supporting the concept that increased oxidative stress is a factor in transitioning COX-2 effects from beneficial to deleterious (18).
Recent studies also indicate the role of EP receptors in ANG II-dependent, hypertensive responses and urine concentration (43, 44). This wide range of actions of PGE2 can be explained by tissue-specific expression of four distinct EP receptors (EP1-4) that are coupled to different signaling pathways. In this study, we showed that M-1 CD cells express EP1, EP3, and EP4 but not EP2 (Fig. 1, A and B); this result agrees with previous studies, showing that M-1 cells do not express EP2 mRNA (30). As judged by the qRT-PCR approach, EP1 seems to be the most prominent EP receptor expressed in M-1 cells. EP1 was detected at 43 kDa, whereas EP3 was detected at 40, 70, and 90 kDa. Accordingly, the expected molecular mass was 40 kDa for EP3; EP4 was detected at 53 and 70 kDa (Fig. 1B). Whereas in the JG cells, the activation of EP4 receptors enhances cAMP formation and activates the cAMP/CREB pathway and renin synthesis (37), the present study shows that the EP1 receptor antagonism prevents the PGE2-dependent increases in cAMP and upregulation of renin expression (Fig. 3). Blockade of the EP4 in the presence of PGE2 only partially blunted this effect. These observations suggest that activation of EP4 receptors does not contribute substantially to the increase and potentiation of cAMP-dependent renin upregulation in the CD. In contrast, cAMP accumulation and augmentation of prorenin and renin protein abundances seem to be dependent on PKC. Consistent with the inhibitory effect of EP3 receptor activation on cAMP production through a Gαi protein, the EP3 antagonism allowed the potentiation of the effect of PGE2 on renin mRNA and prorenin/renin protein levels and cAMP accumulation (Fig. 3). It is probable that the EP3 agonist alone will impair cAMP formation in the cell, leading to no effect of PKC on cAMP formation. Because PGE2 binds to the EP3 receptor with high affinity (1, 20, 41), it is likely that PKC-mediated decreases of Gαi activity impair the interaction of the C1 domain of the AC6 with Gαi that is phosphorylated by PKC in Ser560 and Ser660, increasing cAMP formation (25). More recently, we reported that AVP-mediated activation of AC also requires the intact activity of PKC (12).
Although the signaling pathways in the regulation of renin expression are activated shortly after stimulation of EP receptors, there is a possibility that PGE2 and their specific antagonists can regulate the expression of EP receptors after 6 h of treatment. As shown in Fig. 4D, we only observed a reduction in the expression of EP3 mRNA after PGE2 treatment, which might explain the increase in cAMP and the potentiation of renin expression after EP3 blockade. On the other hand, EP4 receptor blockade caused EP3 and EP4 upregulation after 6 h treatment. The expression of the EP1 receptor was not significantly changed by any treatment, suggesting that any further modulation of the PGE2 signaling probably depends on EP3 and EP4. The silencing of EP1 did not cause changes in the expression of EP3 or EP4 receptors (Fig. 7C).
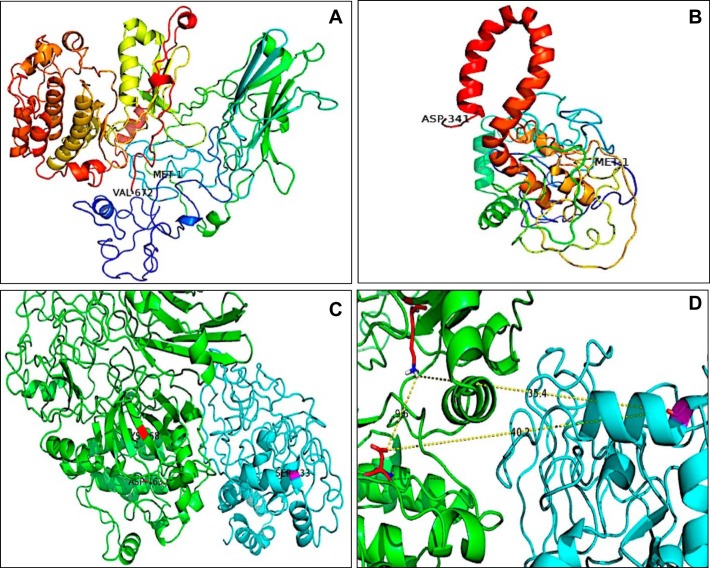
The EP1 receptor is coupled to PKC signaling. Among the PKC isoforms expressed in the CD, PKCα is the most important PKC isoform (9). Our results indicate that PKCα is responsible for the regulation of renin expression in the CD. ANG II increases renin synthesis in the CD through PKCα and augmentation of cAMP-dependent PKC activity (14). We have demonstrated that the increase of PKC activity with PMA leads to the upregulation of renin protein expression in M-1 cells, as previously described in rat inner-medullary CD cells (15). Although the role of Ca2+ was not examined in the present study, Ca2+ alone has no effects on prorenin/renin upregulation (14). Furthermore, we showed that inhibition of PKA blunted the EP1 receptor-mediated stimulation of renin in M-1 cells. These results agree with previous studies, showing that PKCα knockout mice are unable to concentrate urine (24, 42). Along the same line, it has been suggested that PKC potentiates PKA-mediated signals responsible for aquaporin 2 expression in the plasma membrane in CD cells (20). This evidence suggests that PKC acts on the regulatory pathways that enhance cAMP formation and aquaporin 2 expression. However, the mechanisms involved are not completely delineated. It is possible that PKCα also modulates the activity of AC, thus enhancing its activity, although it is not known whether PKC is capable of directly phosphorylating AC. The calcium-dependent isoform of AC6 is highly expressed in both the cortical and medullary segments of the CD and also in M-1 cells (40) and regulates urinary concentration (36). Because Ca2+ inactivates AC6, EP1-mediated Ca2+ elevations in the M-1 cell might therefore inhibit cAMP production; however, we have recently shown that the silencing of AC6 impairs ANG II-mediated renin upregulation and that ANG II treatments in M-1 cells increase intracellular Ca2+. The results from this work are supported by previous studies from our group and others showing ANG II-dependent, enhanced cAMP production (9, 14). Another possibility is a direct phosphorylation of phosphodiesterases, reducing its activity and promoting the accumulation of cAMP; however, this possibility was not explored in the present study. Some reports have shown a direct phosphorylation of CREB mediated by PKC (27, 28). Phosphorylation of CREB in Ser133 leads to the interaction with its coactivators CREB-binding protein and p300 to initiate the transcription of CREB-responsive genes (45). With the use of in silico modeling of mouse PKCα and mouse CREB1, we examined the possibility of a PKC–CREB interaction between the catalytic site of PKCα and the CREB1 Ser133 phosphorylation site in a protein–protein docking assay. Figure 8 displays the three-dimensional structures of PKCα and CREB1. The amino acids involved in the catalytic reaction in PKCα (LYS 368 and ASP 463) were in close proximity with Ser133 of CREB1 (35.4 and 40.2 Å, respectively). Although we did not consider the interaction of PKCα with diacylglycerol and Ca2+, the analysis suggests that the catalytic site of PKCα might interact with Ser133 of CREB, promoting its binding process to the cAMP response element region and CREB-binding protein, allowing the activation of gene transcription.
Fig. 8.
Three-dimensional in silico protein–protein docking of PKCα and CREB1. A and B: the 3-dimensional structures were built using the web server Phyre2. N-Terminal and C-terminal amino acids are shown in the images and correspond to MET 1 and VAL 672 for PKCα and MET 1 and ASP 341 for CREB1. C: analysis performed with ClusPro software. PKCα is shown in green, and CREB1 is shown in cyan. D: amino acids involved in the catalytic reaction in PKCα: LYS 368 and ASP 463, in close proximity with Ser133 of CREB1 (35.4 and 40.2 Å, respectively).
In conclusion, our results support the hypothesis that PGE2 serves as a central regulator of the Ren-1c gene in the principal cells of the CD via the PKCα/cAMP/CREB pathway. PGE2 plays a critical role in the stimulation of CD renin as an ANG II-independent mechanism able to initiate intratubular ANG II formation. Further studies using animal models are necessary to establish the role of EP1 in the CD under conditions of activated intratubular RAS on the equilibrium between antinatriuretic and natriuretic stages.
GRANTS
Funding for the present study was provided by the National Institutes of Health from the National Institute of General Medical Sciences (NIGMS; CoBRE P30GM103337; to L. G. Navar) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK104375) and NIGMS (1U54-GM104940; to M. C. Prieto) and the NIGMS Grant from Chile (No. 11121217; to A. A. Gonzalez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.G., L.G.N., and M.C.P. conceived and designed research; A.A.G., N.S-P., and D.L. performed experiments; A.A.G., N.S-P., D.L., and M.C.P. analyzed data; A.A.G., N.S-P., L.G.N., and M.C.P. interpreted results of experiments; A.A.G. and N.S-P. prepared figures; A.A.G. and M.C.P. drafted manuscript; A.A.G., L.G.N., and M.C.P. edited and revised manuscript; A.A.G., L.G.N., and M.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the molecular core of the Tulane Hypertension & Renal Center of Excellence.
REFERENCES
- 1.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyer MD. Regulation of water and salt transport in collecting duct through calcium-dependent signaling mechanisms. Am J Physiol Renal Physiol 260: F1–F11, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Breyer MD, Breyer RM. Prostaglandin receptors: their role in regulating renal function. Curr Opin Nephrol Hypertens 9: 23–29, 2000. doi: 10.1097/00041552-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol 7: 8–17, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco E, Casper D, Werner P. PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J Neurosci Res 85: 3109–3117, 2007. doi: 10.1002/jnr.21425. [DOI] [PubMed] [Google Scholar]
- 7.Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skøtt O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl- cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol 289: F1185–F1192, 2005. doi: 10.1152/ajprenal.00455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celio MR, Inagami T. Renin in the human kidney. Immunohistochemical localization. Histochemistry 72: 1–10, 1981. doi: 10.1007/BF00496773. [DOI] [PubMed] [Google Scholar]
- 9.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol 274: F564–F572, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Desch M, Harlander S, Neubauer B, Gerl M, Germain S, Castrop H, Todorov VT. cAMP target sequences enhCRE and CNRE sense low-salt intake to increase human renin gene expression in vivo. Pflugers Arch 461: 567–577, 2011. doi: 10.1007/s00424-011-0956-z. [DOI] [PubMed] [Google Scholar]
- 11.Funk CD, Furci L, FitzGerald GA, Grygorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem 268: 26767–26772, 1993. [PubMed] [Google Scholar]
- 12.Gonzalez AA, Cifuentes-Araneda F, Ibaceta-Gonzalez C, Gonzalez-Vergara A, Zamora L, Henriquez R, Rosales CB, Navar LG, Prieto MC. Vasopressin/V2 receptor stimulates renin synthesis in the collecting duct. Am J Physiol Renal Physiol 310: F284–F293, 2016. doi: 10.1152/ajprenal.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez AA, Green T, Luffman C, Bourgeois CR, Gabriel Navar L, Prieto MC. Renal medullary cyclooxygenase-2 and (pro)renin receptor expression during angiotensin II-dependent hypertension. Am J Physiol Renal Physiol 307: F962–F970, 2014. doi: 10.1152/ajprenal.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez AA, Liu L, Lara LS, Bourgeois CR, Ibaceta-Gonzalez C, Salinas-Parra N, Gogulamudi VR, Seth DM, Prieto MC. PKC-α-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am J Physiol Renal Physiol 309: F880–F888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao S, Hernandez A, Quiroz-Munoz M, Cespedes C, Vio CP, Ferreri NR. PGE(2) EP(3) receptor downregulates COX-2 expression in the medullary thick ascending limb induced by hypertonic NaCl. Am J Physiol Renal Physiol 307: F736–F746, 2014. doi: 10.1152/ajprenal.00204.2014. [DOI] [PubMed] [Google Scholar]
- 17.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol 298: F125–F132, 2010. doi: 10.1152/ajprenal.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol 158: 104–145, 2009. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 122: 217–224, 1997. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klar J, Sandner P, Müller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflügers Arch 444: 335–344, 2002. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- 22.Klar J, Sigl M, Obermayer B, Schweda F, Krämer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension 46: 1340–1346, 2005. doi: 10.1161/01.HYP.0000192025.86189.46. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol 660: 375–380, 2011. doi: 10.1016/j.ejphar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol 300: F1255–F1261, 2011. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin TH, Lai HL, Kao YY, Sun CN, Hwang MJ, Chern Y. Protein kinase C inhibits type VI adenylyl cyclase by phosphorylating the regulatory N domain and two catalytic C1 and C2 domains. J Biol Chem 277: 15721–15728, 2002. doi: 10.1074/jbc.M111537200. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol 301: F1195–F1201, 2011. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao LM, Tang Q, Wang JQ. Protein kinase C-regulated cAMP response element-binding protein phosphorylation in cultured rat striatal neurons. Brain Res Bull 72: 302–308, 2007. doi: 10.1016/j.brainresbull.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín F, Mora L, Laorden M, Milanés M. Protein kinase C phosphorylates the cAMP response element binding protein in the hypothalamic paraventricular nucleus during morphine withdrawal. Br J Pharmacol 163: 857–875, 2011. doi: 10.1111/j.1476-5381.2011.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller MW, Todorov V, Krämer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflügers Arch 444: 499–505, 2002. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 30.Nasrallah R, Laneuville O, Ferguson S, Hébert RL. Effect of COX-2 inhibitor NS-398 on expression of PGE2 receptor subtypes in M-1 mouse CCD cells. Am J Physiol Renal Physiol 281: F123–F132, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003. doi: 10.1172/JCI200318018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutiérrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens 26: 965–972, 2013. doi: 10.1093/ajh/hpt071. [DOI] [PubMed] [Google Scholar]
- 36.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE. Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012. doi: 10.1152/ajprenal.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweda F, Klar J, Narumiya S, Nüsing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 38.Stevens T, Nakahashi Y, Cornfield DN, McMurtry IF, Cooper DM, Rodman DM. Ca(2+)-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc Natl Acad Sci USA 92: 2696–2700, 1995. doi: 10.1073/pnas.92.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoos BA, Náray-Fejes-Tóth A, Carretero OA, Ito S, Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int 39: 1168–1175, 1991. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- 40.Strait KA, Stricklett PK, Chapman M, Kohan DE. Characterization of vasopressin-responsive collecting duct adenylyl cyclases in the mouse. Am J Physiol Renal Physiol 298: F859–F867, 2010. doi: 10.1152/ajprenal.00109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama K, García-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem 277: 44147–44154, 2002. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 42.Thai TL, Blount MA, Klein JD, Sands JM. Lack of protein kinase C-α leads to impaired urine concentrating ability and decreased aquaporin-2 in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 303: F37–F44, 2012. doi: 10.1152/ajprenal.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic action of collecting duct (pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol 185: 6413–6419, 2010. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]