Abstract
Hypertension is a complex, multifaceted disorder, affecting ~1 in 3 adults in the United States. Although hypertension occurs in both men and women, there are distinct sex differences in the way in which they develop hypertension, with women having a lower incidence of hypertension until the sixth decade of life. Despite observed sex differences in hypertension, little is known about the molecular mechanisms underlying the development of hypertension in females, primarily because of their underrepresentation in both clinical and experimental animal studies. The first goal of this review is to provide a concise overview of the participation of women in clinical trials, including a discussion of the importance of including females in basic science research, as recently mandated by the National Institutes of Health. The remaining portion of the review is dedicated to identifying clinical and experimental animal studies that concentrate on gender and sex differences in hypertensive kidney disease, ending with a proposed role for T cells in mediating sex differences in blood pressure.
Keywords: blood pressure, renin-angiotensin system, angiotensin type 2 receptor, T cells, T regulatory cells
hypertension affects ~33% of adults in the United States and is currently the second leading cause of kidney failure, although the rate of kidney failure due to high blood pressure (BP) is increasing (20). Although both men and women develop hypertension, distinct gender differences in the incidence and severity of hypertension are well established where men have a higher incidence of hypertension compared with women of the same age until the sixth decade of life (4). Despite the fact that gender differences in BP were first reported in 1947 (9), our understanding of the mechanisms underlying gender differences in BP control remains limited. Because of the central role of the kidney in the development of hypertension (18), gaining better understanding of fundamental differences in renal function in health and disease between the genders is important in gaining insight into the basis of BP control in both men and women. Our lack of knowledge regarding the molecular mechanisms responsible for observed sex and gender differences in BP control and renal function is likely due in large part to the scarcity of available data in females. As a result, the standard of care for most cardiovascular and renal diseases, including hypertension, has been designed on the basis of clinical studies conducted primarily in men, with the assumption that the treatment effects in women will be similar to those observed in men. This assumption, however, is becoming increasingly challenged as the scientific community learns more about how the gender of the individual impacts basic physiology, the pathophysiology of numerous diseases, and pharmacology. In particular, there is a robust literature base examining the impact of sex and gender on the development and progression of hypertension and hypertensive kidney disease (12, 30, 60). The goal of this review is to consider how sex and gender impact hypertensive kidney disease.
This review will use the terms “sex” and “gender” as defined by the Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences (40a). Sex refers to classification based on reproductive organs and functions assigned by the chromosomal complement, whereas gender includes the individual’s self-representation and presentation as male or female based on socially constructed characteristics. Therefore clinical studies will refer to gender differences, where participants self-report their gender, whereas experimental animal studies will refer to sex differences based on phenotype alone.
Historical Clinical Perspective
The so-called “gender gap in medical knowledge” began, in large part, following legislation and regulations in the mid-1970s based on Food and Drug Administration recommendations that women of child-bearing years be excluded from drug studies. These recommendations were put into effect to protect the unborn fetus from exposure to unknown drugs in light of the devastating birth defects in children of women that took thalidomide and diethylstilbestrol. However, it was soon realized that excluding women from research aimed at gaining better understanding of disease processes was negatively impacting women’s health (5, 32, 40). With this understanding came a redefining of women’s health from what was historically considered to refer only to women’s reproductive health, to now encompass not only diseases unique among women but also diseases with greater prevalence among women compared with men and diseases that present differently between men and women (58, 65), the latter of which include heart disease and hypertension.
Recognition of the fact that women were not being adequately represented in biomedical research efforts led to the formation of the Office of Research on Women’s Health at the National Institutes of Health (NIH) in 1990, establishment of the Women’s Health Initiative in 1991, and passage of the NIH Revitalization Act of 1993, which required that NIH-funded trials have sample sizes adequate to allow for statistical consideration of both gender and race. In November 1999, the Institute of Medicine formed the Committee on Understanding the Biology of Sex and Gender Differences with the goal of evaluating and considering the current level of understanding of sex differences and determinants at the biological level. The primary conclusion was that biological sex is a critical determinant of physiology, and sex differences extend all the way down to the level of the cell (40a). Unfortunately, 24 years following the NIH Revitalization Act, it remains common practice for women to be underrepresented in clinical trials for diseases that impact women’s health or for the data to be presented combined by sex, not allowing for sex-specific conclusions to be drawn. As a result, advances in health care and decreases in cardiovascular-related mortality rates lag in women compared with men (51).
Although women have historically been viewed as “protected” from hypertension, women account for up to 55% of individuals with hypertension (23); therefore it would follow that ~50% of clinical trial participants for hypertension studies should be female. A 2010 meta-analysis examining the representation of women in randomized clinical trials for cardiovascular disease prevention reported that representation of women in trials for hypertension at that time was 44%, which was increased from inclusion rates of 34% in 2006, yet still below the percentages reflected in the general population (52). Unfortunately, though, only 31% of cardiovascular clinical trials that included women reported the results by gender, therefore preventing any conclusions to be drawn regarding gender-specific effects. More effort needs to be put into recruiting women of all demographics for inclusion in clinical trials related to hypertension, and the data need to be made available and analyzed separately in men and women to allow for gender-specific conclusions to be drawn. On the basis of the prevalence and scope of established gender differences in hypertension, presenting the data combined by gender is likely obscuring important information in both genders.
Equally important in contributing to the overall poor understanding of the scientific and medical community of cardiovascular and renal health in females is the lack of experimental animal studies to directly examine disease presentation and progression in both sexes. A secondary conclusion of the Committee on Understanding the Biology of Sex and Gender Differences was that despite the breadth of available data characterizing and cataloging a wide variety of sex differences, until more effort was made to understand the mechanisms and fundamental differences between the sexes at all levels of physiology, simply including more women in clinical trials would have little value (40a). Experimental animal studies are necessary to provide the preclinical foundation to inform and direct clinical practice. However, the majority of experimental animal studies are performed almost exclusively using male animal models, despite growing evidence and acknowledgment that every cell in the body has a sex, suggesting that differences between males and females start at the cellular level and these sex differences manifest themselves at the tissue, organ, organ system, and organismal level (81).
To begin to address this critical gap and improve our understating of the basic physiology of the female, the NIH recently mandated that researchers study both males and females in all studies where applicable or provide a rationale for the inclusion of a single-sex study to allow for enhanced rigor, transparency, and generalizability of research findings (72, 81). Starting in fiscal year 2016 all research grant applications were required to include an accounting for sex as a biological variable. It is anticipated that this change in NIH policy will have a strong impact on basic science research, and it is expected that the number of studies to include both sexes will increase. However, this needs to be done in a thoughtful manner taking into account what is already known in each sex and gender (17). Simply including females in studies without any knowledge of how males and females differ has the potential to create even greater confusion in this field. One goal of this review is to identify key areas of renal physiology and hypertensive kidney disease where studies in females are lacking, with the hopes of identifying potential sex-specific targets from experimental animal research that could lead to important and necessary advances for the treatment of hypertensive kidney disease.
Gender Differences in Hypertensive Kidney Disease: Clinical Studies
Decreases in BP with antihypertensive therapy improve renal function and health (48, 79); however, there is evidence to suggest that the impact of BP on renal function in hypertension is gender dependent. Kidney function [reported as estimated glomerular filtration rate (eGFR)] was measured at baseline and at a 7-yr follow-up in a middle-aged rural Chinese cohort consisting of 2,379 participants (48% women) stratified by systolic BP ranging from <110 to ≥140 mmHg in 10-mmHg increments to examine the correlation between BP and eGFR in both genders. The authors found that in men, the higher the BP at baseline, the greater the annual eGFR decline over the course of the study and the higher the risk for rapid eGFR decline (77). In contrast, the risk of a rapid decline in eGFR among women was comparable among women with systolic BP <110 and among those with systolic BP above 140 mmHg, suggesting that men are more sensitive to hypertension-induced changes in renal function over time. In agreement with these findings, a meta-analysis of 68 studies including 11,345 patients with kidney disease due to multiple conditions evaluated the effect of gender on the progression of nondiabetic chronic renal disease and found that men with chronic renal disease show a more rapid decline in renal function compared with women (56). Although hypertensive kidney disease per se was not examined, in the majority of these studies, kidney function was only assessed by eGFR, and although eGFR measurements are widely used clinically, there are valid concerns regarding the sensitivity and accuracy of these methods particularly when being used to compare renal function in distinct patient cohorts, e.g., men vs. women (28, 69). A recent review on chronic kidney disease further noted that although sex and gender differences in the standard predictors of the decline in renal function are increasingly well accepted, little has been done to determine whether these factors can be sex-specifically modified or have the potential to be selectively targeted in one gender to provide therapeutic benefits (14).
To address some of these concerns, differences in the rate of progression of nondiabetic renal disease between men and women were examined, adjusting for baseline characteristics, using the Angiotensin-Converting Enzyme Inhibition in Progressive Renal Disease (AIPRD) database. The AIPRD database includes 1,860 patients (35% women) with nondiabetic renal disease in 11 randomized controlled trials for angiotensin-converting enzyme inhibitors (41). In this study, baseline systolic BP was greater in women (151 vs. 147 mmHg; P < 0.001) whereas baseline diastolic BP (91 mmHg) was similar between genders. Despite gender differences in BP, baseline serum creatinine was comparable between men and women whereas urinary protein was significantly lower in women despite a higher systolic BP (P < 0.001). After an average follow-up of 2.2 yr, systolic BP remained higher in women than in men (143 vs. 141 mmHg; P = 0.01) although diastolic BP was now lower in women (85 vs. 86 mmHg; P = 0.05) following treatment. Decreases in BP resulted in greater decreases in urinary protein excretion in men than in women, although absolute levels remained higher in men. Renal disease progression was measured by doubling of serum creatinine or onset of end-stage renal disease, and after adjusting for baseline variables, with gender differences in urinary protein excretion having the most profound effect, the authors reported that the rate of renal disease progression was faster in women with hypertension than in men. Consistent with these findings, in a cohort of 1,810 patients with hypertension (40.4% men) matched for BP (systolic BP, 146 ± 21 mmHg in men vs. 148 ± 23 mmHg in women), eGFR was lower in women (P < 0.001), and more women were diagnosed with chronic kidney disease than men (22.7 vs. 12.2%; 75). The Italy Developing Education and Awareness on Microalbuminuria in Patients with Hypertensive Disease (I-DEMAND) study included 3,558 hypertensive patients (46% women) with men and women matched for BP (55). Despite comparable BPs, women again had lower serum and urinary creatinine and urinary albumin excretion. Renal function was assessed by measuring eGFR and albuminuria. The authors reported a greater prevalence of reduced GFR among women and a higher prevalence of albuminuria in men. Women had a 2.1-fold-increased odds ratio of reduced eGFR with hypertension, whereas hypertensive men had a 1.9-fold-increased odds ratio of albuminuria compared with women.
These data underscore the importance of fully defining the characteristics of the individual cohorts and of gaining a better understanding of normal physiology in both genders. Is the greater increase in albuminuria in men because renal dysfunction with hypertension is more associated with loss of barrier function and therefore increased albumin in the urine in men, or do the genders simply handle urinary albumin and protein differently, since even at baseline in healthy individuals levels of urinary protein are much greater in men than in women? Similarly, is it appropriate to simply compare absolute eGFR values in men and women and make conclusions regarding how this relates to renal function? The evidence available suggests that there are sex-specific factors that are more or less important in determining eGFR in men vs. women (41, 55, 56, 75, 77), so can absolute values really be compared resulting in a meaningful conclusion regarding the progression of hypertensive renal disease? This question becomes even more relevant when individual human variation is taken into account since although sex as a variable is considered in some estimations of renal function, as noted in a review by Carrero (14), they do not differentiate on the basis of body type (i.e., an athletic woman or a small, lean man). It is now well acknowledged that there are distinct gender differences in the presentation, pathophysiological mechanisms, and outcomes in patients with acute myocardial infarction (51). Could the same not also be true with hypertensive kidney injury? More clinical studies are needed to determine whether comparing eGFR and albuminuria in men and women to determine whether there are gender differences in sensitivity to renal injury is even a valid comparison to make.
Sex Differences in Hypertensive Kidney Disease: Experimental Animal Studies
The majority of the early studies that laid the foundation for our current understanding of renal hemodynamics were performed in male animal models alone. However, as noted above, there is increasing evidence in the literature that gender differences exist in hypertensive kidney disease clinically, and these findings extend to experimental animal studies. Consistent with what is seen in men and women, male spontaneously hypertensive rats (SHR) have greater protein and albumin excretion than age-matched females (10, 70, 71, 74, 82). Our laboratory further showed that male SHR exhibit consistent increases in albumin excretion from 9 to 16 wk of age concomitant with increases in BP (71), whereas albumin excretion is not altered in female SHR during this same age range despite progressive increases in BP. Albumin excretion is also greater in hypertensive male Dahl salt-sensitive rats compared with females (50) and in male mice following a high-salt diet compared with female mice (54). Sex differences in protein and albumin excretion are complicated by sex differences in BP in SHR and Dahl salt-sensitive rats. However, protein excretion is also higher in normotensive male Wistar-Kyoto rats compared with females (7), and treatment with the nitric oxide synthase inhibitor nitro-l-arginine methyl ester (l-NAME) results in greater increases in BP in female SHR, yet protein excretion remains significantly lower in treated females vs. treated males (10).
The above data further call into question the notion that renal handling of protein is the same between the sexes. Indeed, direct assessment of protein handling in male and female rodents using fluorescently labeled ovalbumin have demonstrated that females have greater tubular reabsorption of protein and degrade the tracer at a higher rate compared with males (2). Studies of inulin clearance in male and female Munich-Wistar rats further showed that the calculated glomerular ultrafiltration coefficient is higher in males, resulting in the development of spontaneous proteinuria in males at much younger ages compared with females (61). These effects appear to be mediated by sex hormones, as castration increases protein reabsorption in males and decreases reabsorption in females (66) and treatment of castrated females with androgens increases glomerular ultrafiltration (8). More studies are needed to directly address the mechanisms regulating protein handling in both sexes. These findings also raise questions regarding the criterion used to assess the effectiveness of therapeutics used in women with renal disease. How can we assess optimal treatment regimens in women and prevent undertreatment or overtreatment if we do not understand the basic physiology? Are we even using the best treatments for women?
There are also sex differences in renal function in hypertension. Male SHR have a leftward shift in pressure-natriuresis (PN) curves relative to females, which is mediated by male sex hormones (59). GFR has been reported to be comparable in 17- to 19-wk-old male and female SHR (59), yet significantly higher in male SHR compared with female SHR at 12 wk of age (26); BP was higher in male SHR in both studies. Females have also been reported to have an attenuated decrease in renal function compared with males in another hypertensive model of kidney injury, the ANG II type 1 receptor antagonist during the nephrogenic period (ARAnp)-treated rat (63). In this model, Sprague-Dawley rats are treated with an angiotensin II type 1 receptor antagonist from postnatal days 1–14, resulting in adverse kidney development and the development of hypertension and kidney damage with age. Male ARAnp rats have a significant decline in GFR evident by 10–11 mo of age, whereas females maintain GFR until 16–17 mo of age. Importantly, this study further reported that it is not until after female rats reach senescence and the cessation of normal estrus cycling (~12 mo of age) that renal function begins to decline, suggesting a protective role for female sex hormones. Sex differences in renal function are not only apparent in hypertension, however. Normotensive male Sprague-Dawley rats also exhibit a leftward shift in PN compared with females (36, 44), although GFR was comparable between the sexes, again raising the notion that there are fundamental differences in renal function even in otherwise healthy animals. Without greater understanding of normal renal physiology in both sexes, it remains very difficult to interpret findings in different disease models and predict expected outcomes with disease progression in both sexes with confidence. The remaining portion of this review is focused on two potential mechanisms that may mediate the underlying sex differences in hypertensive kidney injury: the renin-angiotensin system and T regulatory cells.
Mechanisms Underlying Sex Differences in Hypertensive Kidney Injury: the Renin-Angiotensin System
On the basis of the central role of the renin-angiotensin system (RAS) in the control of BP and body fluid volume, most mechanistic studies focused on sex differences in renal function have centered on the role of the RAS. Angiotensin II (ANG II)-induced increases in BP result in greater increases in both BP and proteinuria in male SHR relative to female SHR (70). Male SHR also exhibit a decrease in GFR in response to acute low-dose (5 ng·kg−1·min−1) ANG II infusion independent of changes in BP, whereas GFR increases in female SHR in response to ANG II infusion. Sex differences in renal function have been linked to the angiotensin type 2 (AT2) receptor, and females have greater renal AT2 protein and mRNA expression compared with males (33, 54, 70, 71). Pharmacological blockade of the AT2 receptor in Sprague-Dawley rats results in a comparable downward shift in the PN relationship in both sexes; however, AT2 receptor blockade blunts autoregulation of renal blood flow only in females (36). These results suggest that although AT2 receptor activation is important in modulating sodium excretion in healthy males and females (36), females rely more on the AT2 receptor to control overall renal function than males. This latter conclusion is supported by the finding that normotensive female Sprague-Dawley rats exhibit also greater renal vasodilatory responses to the AT2 agonist Compound 21 than males (34). Additional studies in AT2 receptor knockout mice have further confirmed a greater role for the AT2 receptor in the regulation of renal function in females relative to males. Female AT2 receptor knockout mice exhibit a rightward shift in the PN curve relative to wild-type controls that is not seen in males, and only female mice exhibit a protective role for the AT2 receptor against subpressor ANG II-induced increases in tubuloglomerular feedback responses (11, 54).
The majority of the studies examining the role of the RAS in modulating sex differences in renal function have been conducted in normotensive animals; however, there are well-established sex differences in the RAS under hypertensive conditions as well (19, 30). On the basis of studies demonstrating a protective role for the AT2 receptor in hypertension in females, it is anticipated that the AT2 receptor would also offer protection against hypertension-induced alterations in renal function in a sex-dependent manner (19, 35). Indeed, treatment of male and female SHR with Compound 21 results in increases in renal blood flow with no change in BP, a greater reduction in filtration fraction, and increases in urine flow, sodium excretion, and fractional sodium excretion vs. vehicle controls only in females (33). Compound 21 had no effect on any parameter measured in male SHR. Previous studies by this same group reported similar increases in urine flow, sodium excretion, and fractional sodium excretion in male and female Sprague-Dawley rats relative to vehicle control, suggesting that in hypertension there is a loss of AT2 regulation of renal excretory function in males. The ability of females to maintain AT2 control of renal health and function may reflect greater AT2 receptor expression in the females and may be critical to the ability of female SHR to have a lower BP compared with young age-matched males. On the basis of these data, it is also tempting to speculate that women with renal disease in particular would benefit from AT2 receptor activation as a novel treatment option.
Mechanisms Underlying Sex Differences in Hypertensive Kidney Injury: T Regulatory Cells
Hypertension is now well accepted to be a state of inflammation, and there is accumulating evidence in both clinical and experimental studies that hypertension is associated with an increase in renal T cell infiltration and accumulation (13, 15, 21, 68, 76, 78). More specifically, we are interested in the potential protective role of cluster of differentiation 4 (CD4)+CD25+ T regulatory cells (Tregs) in hypertensive kidney injury. Tregs play a critical role in maintaining immune system homeostasis by suppressing the activation of conventional effector T cells. Tregs protect against Adriamycin-induced nephropathy (47), experimental antiglomerular basement membrane glomerulonephritis (80), kidney transplant rejection (39), acute kidney injury (29, 45), and diabetic nephropathy (25); therefore a direct protective role for Tregs in hypertensive kidney disease is quite plausible, although it should be noted that all of these experiments were conducted using exclusively male animals.
Moreover, Tregs are an attractive target for a potential therapeutic strategy in hypertensive kidney disease. Tregs are being developed as a potential therapy for a wide range of pathologies (37, 39, 62, 67), and there is evidence to support a protective role for Tregs in human hypertension. A recent small clinical study (20 men and 10 women) examined the association between CD4+CD25+ Tregs and the development of malignant hypertension-related kidney injury (38). Although the data were not presented separated by sex, Tregs are significantly lower in peripheral blood from patients with primary malignant hypertension-related kidney injury compared with healthy volunteers (38). In addition, CD4+CD25+ cell counts were positively correlated with eGFR, were negatively correlated with urinary protein excretion, and tended to decrease in parallel with increases in the degree of nephropathy. The authors concluded that the number of Tregs may reflect renal function and thereby provide clinical support for a protective role of Tregs against hypertensive kidney injury. Tregs are currently being employed in clinical trials for solid organ transplantation and type 1 diabetes mellitus. Might hypertensive kidney injury be among the next applications?
Experimental models of hypertension are associated with decreases in renal Tregs, and adoptive transfer of Tregs attenuates increases in BP (1, 3, 27, 43, 49, 53). On the basis of the close relationship between BP and renal injury, it is therefore probable that Tregs will similarly protect the kidney against hypertensive renal injury. Although a direct role for Tregs to modulate hypertensive kidney disease has not been examined, renal and aortic Tregs were assessed by measuring forkhead box P3 (FoxP3) expression by immunofluorescence (a marker for Tregs) in the aorta and kidneys in male mice following vehicle or ANG II infusion (3). Concomitant with an increase in BP, ANG II resulted in a 43% decrease in renal cortical FoxP3 expression, and injection of Tregs increased FoxP3+ cells twofold compared with control mice; aortic Tregs were not altered by either ANG II or adoptive transfer of Tregs. These findings suggest a role for renal Tregs in particular to modulate physiological responses to ANG II. Similarly, adoptive transfer of Tregs reverses aldosterone-induced decreases in FoxP3+ cells in the renal cortex and medulla of male C57BL/6 mice and prevents aldosterone-induced changes in BP (43). The authors suggest that on the basis of the central role of the kidney in controlling BP, the kidney may represent one of the sites of action of Tregs to impact BP, although this hypothesis has yet to be directly tested and how these cells impact renal function is largely unknown.
Our group was the first to report a sex difference in the renal T cell profile in hypertensive males and females (74). Male SHR have more CD4+ T cells and T helper 17 (Th17) cells, whereas females have more renal CD8+ T cells and Tregs. Moreover, greater numbers of Tregs in female SHR correspond to a lower BP and protein excretion compared with males at baseline. To gain further insight as to the relationship between BP and the renal T cell profile, renal T cells were measured in male and female SHR that were BP matched using hydrochlorothiazide and reserpine (73). Lowering BP and abolishing the sex difference in BP resulted in lower numbers of Tregs in treated female SHR compared with control female SHR and abolished the sex difference in renal Tregs; modulating BP had no effect on renal Tregs in male SHR. These data suggest that the development and maintenance of hypertension in female SHR results in an increase in Tregs that is not observed in males. Consistent with this interpretation of our data, hypertensive female SHR have more renal Tregs than normotensive female Wistar-Kyoto rats, whereas male and female Wistar-Kyoto rats have comparable BP and comparable numbers of renal Tregs (73). In addition, we recently assessed the renal T cell profile in 5-wk-old male and female SHR, which is before the development of hypertension and sex differences in BP. For this study, kidneys were isolated from 5-wk-old male and female SHR and processed for flow cytometry analysis of CD3+CD4+FoxP3+ Tregs and CD3+CD4+RORγ+ (ROR, retinoic acid-related orphan receptor) Th17 cells as previously described (73, 74). We found that the renal T cell profile is not different between 5-wk-old males and females (Fig. 1). On the basis of our studies we hypothesize that the ability of female SHR to maintain Tregs is a critical factor responsible for the lower BP and lesser degree of renal injury in hypertensive females relative to males. The following remain to be determined, however: 1) which comes first, the increase in BP or the increase in Tregs in females; 2) what the mechanism driving the increase in Tregs is; and 3) how Tregs modulate BP and renal function in either sex.
Fig. 1.
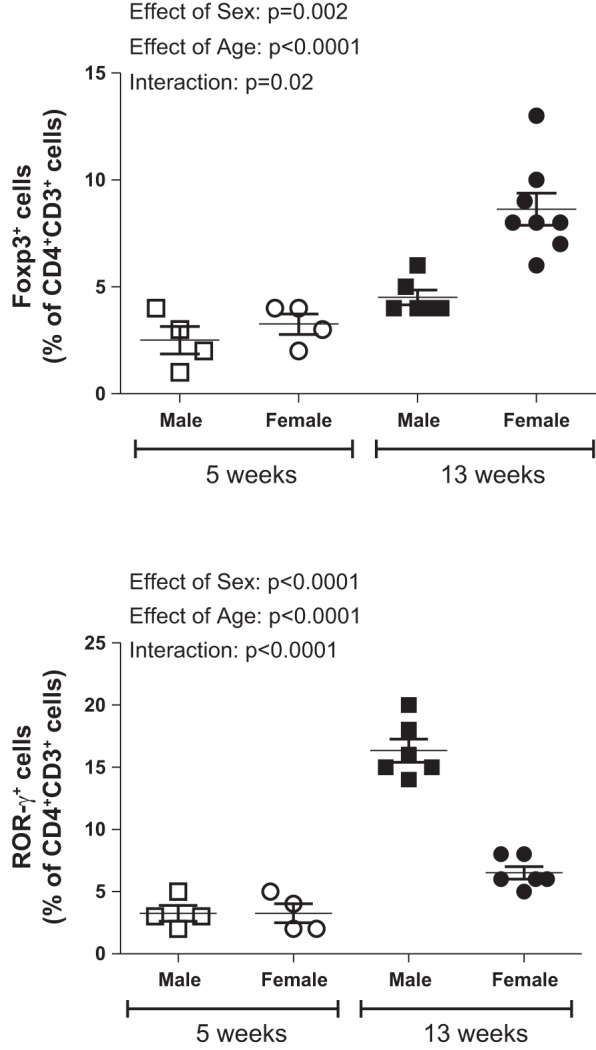
Renal T cells were measured by analytical flow cytometry of single-cell suspensions of kidneys from 5-wk-old (n = 4) and 13-wk-old (n = 6–8) male and female SHR as previously described (73, 74); these data have not been previously published. Tregs are expressed as CD3+CD4+FoxP3+ cells; Th17 cells are expressed as CD3+CD4+RORγ+ cells. All data are expressed as means ± SE, and data were compared using a two-way ANOVA (factor 1, sex; factor 2, age; GraphPad Prism; GraphPad Software, La Jolla, CA). FoxP3, forkhead box P3; ROR, retinoic acid-related orphan receptor.
Both the kidney and T cells have been demonstrated to be required for the full development of ANG II hypertension (22, 31), and recent studies employing male and female Rag1−/− mice, which lack mature B and T cells, have shown that T cells also mediate sex differences in ANG II hypertension (42, 57). Adoptive transfer of pan T cells from a wild-type male donor restores ANG II-induced increases in BP in male Rag1−/− recipients, although BP responses to ANG II are not augmented following adoptive transfer of pan T cells from a wild-type male donor to a female Rag1−/− recipient (57). Furthermore, adoptive transfer of pan T cells from a wild-type female donor fails to restore ANG II-induced increases in BP in male Rag1−/− recipients (42). Although renal function was not assessed in either of these studies, renal T cell infiltration and cytokines were measured. Male Rag1−/− mice had more T cells (total T cells and Tregs) both before adoptive transfer and following pan T cell adoptive transfer than females, although ANG II had no effect on renal T cell numbers in either sex (57). Despite the lack of a change in the numbers of renal T cells, ANG II increased proinflammatory cytokines in the kidneys of male mice only, suggesting that females are resistant to the proinflammatory effects of ANG II. Although the mechanism accounting for sex differences in the inflammatory profile in the kidney following ANG II was not determined, enhanced Treg activity and release of IL-10 independent of a change in Treg number could account for suppression of effector T cell activation and inflammatory cytokine release in the females relative to males, thereby offering protection against hypertensive renal injury. In support of this hypothesis, adoptive transfer of T cells from a female wild-type donor into male Rag1−/− mice resulted in greater renal FoxP3 and IL-10 mRNA expression as measured by flow cytometry, whereas there was not a difference in renal Treg infiltration in male Rag1−/− mice following adoptive transfer of T cells from a male donor (42). More studies are needed to directly assess the impact of Tregs on renal function in hypertension in both sexes.
Perspectives: Are AT2 and Tregs Working Together?
It is tempting to speculate that Tregs and AT2 receptors are working together to contribute to the lower levels of BP and renal injury in hypertensive females relative to males. Although this hypothesis has not yet been directly tested, there is evidence in the literature for a link between the AT2 receptor and Tregs in females. AT2 receptor activation is anti-inflammatory in cultured cells and in male animal models of obesity, atherosclerosis, and diabetes (16, 24, 46, 64); therefore greater AT2 receptor expression in hypertensive females may be linked to the increased anti-inflammatory profile relative to males. In addition, adoptive transfer of pan T cells from a wild-type female, but not male, donor to a male Rag1−/− mouse increases AT2 mRNA in the kidney concomitant with increases in anti-inflammatory IL-10 and FoxP3 (42). Studies designed to better understand the mechanisms underlying reported phenotypical and biochemical differences between the sexes are needed. A significant hurdle to this, however, remains the fact that our understanding of basic renal physiology in healthy females, let alone under different disease conditions, lags far behind our understanding in males, which becomes a confounding variable when data sets between the sexes are compared. Before studies can compare protein excretion, GFR, or renal blood flow between the sexes, studies need to be performed to better explore how all of these different parameters are controlled in the female as opposed to taking our understanding of male physiology and simply applying it to females.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 127091-02 and 134604-01 (to J. C. Sullivan) and American Heart Association Grant 17EIA33410565 (to J. C. Sullivan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.S. conceived and designed research; J.C.S. analyzed data; J.C.S. prepared figures; J.C.S. and E.E.G. drafted manuscript; J.C.S. and E.E.G. edited and revised manuscript; J.C.S. and E.E.G. approved final version of manuscript.
REFERENCES
- 1.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63: 797–803, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 2.Asan E, Kugler P, Schiebler TH. Sex-related differences in the handling of fluorescent ovalbumin by the proximal tubule of the rat kidney. Histochemistry 84: 408–417, 1986. doi: 10.1007/BF00482971. [DOI] [PubMed] [Google Scholar]
- 3.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum. Circulation 135: e646, 2017.] doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin JA, Ellenberg SS. Inclusion of women in clinical trials. BMC Med 7: 56, 2009. doi: 10.1186/1741-7015-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia K, Zimmerman MA, Sullivan JC. Sex differences in angiotensin-converting enzyme modulation of Ang (1-7) levels in normotensive WKY rats. Am J Hypertens 26: 591–598, 2013. doi: 10.1093/ajh/hps088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blantz RC, Peterson OW, Blantz ER, Wilson CB. Sexual differences in glomerular ultrafiltration: effect of androgen administration in ovariectomized rats. Endocrinology 122: 767–773, 1988. doi: 10.1210/endo-122-3-767. [DOI] [PubMed] [Google Scholar]
- 9.Boynton RE, Todd RL. Blood pressure readings of 75,258 university students. Arch Intern Med (Chic) 80: 454–462, 1947. doi: 10.1001/archinte.1947.00220160033003. [DOI] [PubMed] [Google Scholar]
- 10.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol 305: R701–R710, 2013. doi: 10.1152/ajpregu.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 59: 129–135, 2012. doi: 10.1161/HYPERTENSIONAHA.111.178715. [DOI] [PubMed] [Google Scholar]
- 12.Cadeddu C, Franconi F, Cassisa L, Campesi I, Pepe A, Cugusi L, Maffei S, Gallina S, Sciomer S, Mercuro G; Working Group of Gender Medicine of Italian Society of Cardiology . Arterial hypertension in the female world: pathophysiology and therapy. J Cardiovasc Med (Hagerstown) 17: 229–236, 2016. doi: 10.2459/JCM.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 13.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 18: 21, 2016. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 14.Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 33: 383–392, 2010. doi: 10.1159/000320389. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Agrawal DK. Dysregulation of T cell subsets in the pathogenesis of hypertension. Curr Hypertens Rep 17: 8, 2015. doi: 10.1007/s11906-014-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow BS, Koulis C, Krishnaswamy P, Steckelings UM, Unger T, Cooper ME, Jandeleit-Dahm KA, Allen TJ. The angiotensin II type 2 receptor agonist compound 21 is protective in experimental diabetes-associated atherosclerosis. Diabetologia 59: 1778–1790, 2016. doi: 10.1007/s00125-016-3977-5. [DOI] [PubMed] [Google Scholar]
- 17.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J 30: 519–524, 2016. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colafella KM, Hilliard LM, Denton KM. Epochs in the depressor/pressor balance of the renin-angiotensin system. Clin Sci (Lond) 130: 761–771, 2016. doi: 10.1042/CS20150939. [DOI] [PubMed] [Google Scholar]
- 20.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Am J Kidney Dis 59, Suppl 1: A7, 2012. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Crislip GR, Sullivan JC. T-cell involvement in sex differences in blood pressure control. Clin Sci (Lond) 130: 773–783, 2016. doi: 10.1042/CS20150620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daugherty SL, Masoudi FA, Zeng C, Ho PM, Margolis KL, O’Connor PJ, Go AS, Magid DJ. Sex differences in cardiovascular outcomes in patients with incident hypertension. J Hypertens 31: 271–277, 2013. doi: 10.1097/HJH.0b013e32835bdc44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhande I, Ali Q, Hussain T. Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: role in renoprotection in obese rats. Hypertension 61: 1218–1226, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes 60: 2954–2962, 2011. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmarakby AA, Bhatia K, Crislip R, Sullivan JC. Hemodynamic responses to acute angiotensin II infusion are exacerbated in male versus female spontaneously hypertensive rats. Physiol Rep 4: e12677, 2016. doi: 10.14814/phy2.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbiano S, Menacho-Márquez M, Robles-Valero J, Pericacho M, Matesanz-Marín A, García-Macías C, Sevilla MA, Montero MJ, Alarcón B, López-Novoa JM, Martín P, Bustelo XR. Immunosuppression-independent role of regulatory T cells against hypertension-driven renal dysfunctions. Mol Cell Biol 35: 3528–3546, 2015. doi: 10.1128/MCB.00518-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florkowski CM, Chew-Harris JS. Methods of estimating GFR: different equations including CKD-EPI. Clin Biochem Rev 32: 75–79, 2011. [PMC free article] [PubMed] [Google Scholar]
- 29.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 30.Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension 68: 1322–1327, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healy B. The Yentl syndrome. N Engl J Med 325: 274–276, 1991. doi: 10.1056/NEJM199107253250408. [DOI] [PubMed] [Google Scholar]
- 33.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension 64: 378–383, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02809. [DOI] [PubMed] [Google Scholar]
- 34.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension 59: 409–414, 2012. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 35.Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol 40: 542–550, 2013. doi: 10.1111/1440-1681.12067. [DOI] [PubMed] [Google Scholar]
- 36.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the angiotensin type 2 receptor. Hypertension 57: 275–282, 2011. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 37.Hu M, Wang YM, Wang Y, Zhang GY, Zheng G, Yi S, O’Connell PJ, Harris DC, Alexander SI. Regulatory T cells in kidney disease and transplantation. Kidney Int 90: 502–514, 2016. doi: 10.1016/j.kint.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Luo Y, Liang Y, Long X, Peng Y, Liu Z, Wen X, Jia M, Tian R, Bai C, Li C, He F, Lin Q, Wang X, Dong X. CD4+CD25+ T cells in primary malignant hypertension related kidney injury. Sci Rep 6: 27659, 2016. doi: 10.1038/srep27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney Int 87: 1116–1124, 2015. doi: 10.1038/ki.2015.50. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine Committee on Ethical and Legal Issues Relating to the Inclusion of Women in Clinical Studies Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies, edited by Mastroianni AC, Faden R, and Federman D. Washington, DC: National Academies Press, 1994, vol I. [PubMed] [Google Scholar]
- 40a.Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences Exploring the Biological Contributions to Human Health: Does Sex Matter?, edited by Wizemann TM and Pardue ML. Washington, DC: National Academies Press, 2001. [PubMed] [Google Scholar]
- 41.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, Marcantoni C, Becker G, Shahinfar S, De Jong PE, De Zeeuw D, Kamper AL, Strangaard S, Levey AS. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003. doi: 10.1093/ndt/gfg317. [DOI] [PubMed] [Google Scholar]
- 42.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 44.Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 14: 893–896, 2001. doi: 10.1016/S0895-7061(01)02164-1. [DOI] [PubMed] [Google Scholar]
- 45.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koulis C, Chow BS, McKelvey M, Steckelings UM, Unger T, Thallas-Bonke V, Thomas MC, Cooper ME, Jandeleit-Dahm KA, Allen TJ. AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension 65: 1073–1081, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05204. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DC. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol 17: 2731–2741, 2006. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 48.Marcantoni C, Jafar TH, Oldrizzi L, Levey AS, Maschio G. The role of systemic hypertension in the progression of nondiabetic renal disease. Kidney Int Suppl 57: S44–S48, 2000. doi: 10.1046/j.1523-1755.2000.07508.x. [DOI] [PubMed] [Google Scholar]
- 49.Matrougui K, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 178: 434–441, 2011. [Erratum. Am J Pathol 178: 1406, 2011.] doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 133: 916–947, 2016. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 52.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 3: 135–142, 2010. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 53.Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 34: 97–108, 2016. doi: 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 54.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 307: F901–F907, 2014. doi: 10.1152/ajprenal.00288.2014. [DOI] [PubMed] [Google Scholar]
- 55.Muiesan ML, Ambrosioni E, Costa FV, Leonetti G, Pessina AC, Salvetti M, Trimarco B, Volpe M, Pontremoli R, Deferrari G, Rosei EA. Sex differences in hypertension-related renal and cardiovascular diseases in Italy: the I-DEMAND study. J Hypertens 30: 2378–2386, 2012. doi: 10.1097/HJH.0b013e328359b6a9. [DOI] [PubMed] [Google Scholar]
- 56.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11: 319–329, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Public Health Service Task Force on Women’s Health Issues Women’s health. Report of the Public Health Service Task Force on Women’s Health Issues. Public Health Rep 100: 73–106, 1985. [PMC free article] [PubMed] [Google Scholar]
- 59.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. doi: 10.1161/01.HYP.31.1.435. [DOI] [PubMed] [Google Scholar]
- 60.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 61.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988. doi: 10.1038/ki.1988.206. [DOI] [PubMed] [Google Scholar]
- 62.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30: 656–665, 2009. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saez F, Reverte V, Paliege A, Moreno JM, Llinás MT, Bachmann S, Salazar FJ. Sex-dependent hypertension and renal changes in aged rats with altered renal development. Am J Physiol Renal Physiol 307: F461–F470, 2014. doi: 10.1152/ajprenal.00198.2014. [DOI] [PubMed] [Google Scholar]
- 64.Sampson AK, Irvine JC, Shihata WA, Dragoljevic D, Lumsden N, Huet O, Barnes T, Unger T, Steckelings UM, Jennings GL, Widdop RE, Chin-Dusting JP. Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br J Pharmacol 173: 729–740, 2016. doi: 10.1111/bph.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiebinger L. Women’s health and clinical trials. J Clin Invest 112: 973–977, 2003. doi: 10.1172/JCI19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sievers L, Kugler P. Reabsorption of fluorescein-isothiocyanate-labelled-ovalbumin in the kidney of normal and castrated male and female rats. Histochemistry 86: 215–220, 1986. doi: 10.1007/BF00493391. [DOI] [PubMed] [Google Scholar]
- 67.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol 5: 46, 2014. doi: 10.3389/fimmu.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39: 567–573, 2016. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 69.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 72.Tannenbaum C, Schwarz JM, Clayton JA, de Vries GJ, Sullivan C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ 7: 13, 2016. doi: 10.1186/s13293-016-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tziomalos K, Giampatzis V, Baltatzi M, Efthymiou E, Psianou K, Papastergiou N, Magkou D, Bougatsa V, Savopoulos C, Hatzitolios AI. Sex-specific differences in cardiovascular risk factors and blood pressure control in hypertensive patients. J Clin Hypertens (Greenwich) 16: 309–312, 2014. doi: 10.1111/jch.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens 25: 22–27, 2016. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q, Xie D, Xu X, Qin X, Tang G, Wang B, Wang Y, Hou F, Xu X, Wang X. Blood pressure and renal function decline: a 7-year prospective cohort study in middle-aged rural Chinese men and women. J Hypertens 33: 136–143, 2015. doi: 10.1097/HJH.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 78.Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol 27: 677–686, 2016. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whelton PK, Perneger TV, He J, Klag MJ. The role of blood pressure as a risk factor for renal disease: a review of the epidemiologic evidence. J Hum Hypertens 10: 683–689, 1996. [PubMed] [Google Scholar]
- 80.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, Mayer G, Gunsilius E, Rosenkranz AR. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 16: 1360–1370, 2005. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 81.Zakiniaeiz Y, Cosgrove KP, Potenza MN, Mazure CM. Balance of the sexes: addressing sex differences in preclinical research. Yale J Biol Med 89: 255–259, 2016. [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmerman MA, Harris RA, Sullivan JC. Female spontaneously hypertensive rats are more dependent on ANG (1-7) to mediate effects of low-dose AT1 receptor blockade than males. Am J Physiol Renal Physiol 306: F1136–F1142, 2014. doi: 10.1152/ajprenal.00677.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


