Abstract
Adult-onset autosomal-dominant polycystic kidney disease (ADPKD) is caused by mutations in either the PKD1 or PKD2 gene, leading to malfunction of their gene products, polycystin 1 or 2. Histone deacetylase 6 (HDAC6) expression and activity are increased in PKD1 mutant renal epithelial cells. Here we studied the effect of ACY-1215, a specific HDAC6 inhibitor, on cyst growth in ADPKD. Treatment with ACY-1215 slowed cyst growth in a mouse model of ADPKD that forms massive cysts within 3 wk after knockout of polycystin 1 function. It also prevented cyst formation in MDCK.2 cells, an in vitro model of cystogenesis, and in an ADPKD cell line derived from the proximal tubules from a pkd1−/−.mouse (PN cells). In PN cells ACY-1215 also reduced the size of already established cysts. We found that ACY-1215 lowered cAMP levels and protein expression of adenylyl cyclase 6. Our results suggest that HDAC6 could potentially serve as a therapeutic target in ADPKD.
Keywords: autosomal-dominant polycystic kidney disease; renal cyst growth; histone deacetylase 6 inhibitor; adenosine 3′,5′-cyclic monophosphate; polycystic kidney disease
autosomal-dominant polycystic kidney disease (ADPKD) is a common autosomal-dominant hereditary disorder characterized by the formation of fluid-filled cysts that arise from renal tubules in every nephron segment (42). These cysts progressively enlarge and destroy the normal renal tubules, leading to renal failure in nearly one-half of all ADPKD patients by the fifth decade of life (5). ADPKD is caused by mutations in either the PKD1 or PKD2 gene (14, 23). The gene products of the PKD1 and PKD2 genes, polycystin 1 and 2 (PC1 and 2), respectively, have been well characterized (24), and malfunction in each of these proteins leads to dysregulation of multiple signaling pathways that together establish and promote cyst growth (11). Currently there is no treatment that will prevent the development or growth of cysts in affected individuals.
Histone deacetylase 6 (HDAC6) inhibitors have been suggested as one possible strategy to inhibit cyst growth (3, 20, 43, 44). Unlike other HDACs that function in the nucleus, HDAC6 functions in the cytoplasm, where it regulates a number of important biological processes, including transcription, cell migration and proliferation, cell signaling, the immune response, and protein degradation (32). Furthermore, HDAC6 is an α-tubulin deacetylase that regulates the stability of α-tubulin (19). Despite its role in these processes, mice lacking HDAC6 are viable, fertile, and have no gross morphological abnormalities (45), suggesting that HDAC6 is not an absolutely essential protein. Conversely, increased HDAC6 protein expression and activity are upregulated in a wide range of disease states (7, 17, 28, 34). For example, increased HDAC6 activity has been noted in many cancer cells lines, and it plays a role in anchorage-independent proliferation (18). Thus, lowering HDAC6 activity is potentially a useful therapeutic goal, and a number of specific HDAC6 inhibitors (HDAC6i) have been developed thus far (32).
HDAC6 expression and activity are increased in PKD1 mutant renal epithelial cells, suggesting that it plays a role in cyst growth (21). Indeed, we have previously shown that cyst growth can be slowed in a mouse model of ADPKD and in vitro cell models by reducing the activity of HDAC6 using tubacin (3). We also found that tubacin reduced cyst growth and improved renal function in a PKD1 conditional mouse model. Further exploration showed that tubacin treatment inhibited proliferation in cyst-lining epithelial cells and also downregulated cAMP levels in the kidneys in a PKD1-conditional mouse model. We also found that HDAC6 inhibition downregulated cAMP levels, inhibited cell proliferation, and inhibited cAMP-activated CFTR chloride currents in MDCK cells, an in vitro model of cyst growth (2).
Based on previous studies showing that cystic cholangiocytes in polycystic liver disease have abnormal cell cycle profiles and malfunctioning cilia, Gradilone and collaborators (9) studied the role of HDAC6 in polycystic liver disease (PLD), which targets the epithelial lining of the biliary tree (41). They found that expression of the HDAC6 protein is six times higher in cystic liver tissue and in cultured cholangiocytes isolated from both PCK rats (an animal model of PLD) and humans with PLD. As in our studies of ADPKD, inhibition of HDAC6 activity by HDAC6 inhibitors decreased the proliferation of cystic cholangiocytes in a dose- and time-dependent manner and inhibited cyst growth in three-dimensional (3D) cultures.
In light of the recent studies suggesting that HDAC6 could play a role in cyst formation in ADPKD as well as PLD and could therefore serve as a potential therapeutic target, we have focused our current study on the selective HDAC6 inhibitor ACY-1215, a hydroxamic acid derivative that inhibits HDAC6 enzymatic activity with an IC50 of 5 nM. ACY-1215 was originally developed as a potential treatment for multiple myeloma in combination with bortezomib (39). It is selective for HDAC6, with ~10-fold lower activity against HDAC1, -2, and -3; minimal activity is seen against HDAC4, -5, -7, -8, -9, and -11 as well as Sirtuin1 and Sirtuin2. In PCK rats, a model of autosomal recessive polycystic kidney disease (9), ACY-1215 has been found to reduce liver and kidney cyst development and fibrosis.
We have now shown that ACY-1215 can indeed reduce cyst growth in vivo and in vitro and also reduce cAMP levels. We also discovered a novel effect of HDAC6 inhibition, namely a lowering of intracellular adenylate cyclase protein expression, which is abnormally increased in ADPKD (37). Thus, ACY-1215 lowers the abnormally high cAMP levels in ADPKD cystic cells.
METHODS
Mouse strains and treatment.
All animal use complied with the guiding principles of the Johns Hopkins University Institutional Animal Care and Use Committee, and the protocols for this work were approved by this committee. Pkd1fl/fl;Pax8rtTA;TetO-cre mice on a C57BL/6 background (22) were provided by the Baltimore PKD Center and used to test the effects of ACY-1215 on cyst growth as reported previously (3). Mice of both sexes were used in this study. Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected intraperitoneally with doxycycline resuspended in sterile water (4 µg of doxycycline/g body wt) on postnatal days 11, 12, and 13. This treatment produces very rapid and aggressive cyst growth (25, 38). Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected daily with ACY-1215 or DMSO from postnatal days 10 to 20. On postnatal day 21, the mice were euthanized. Serum was collected to measure blood urea nitrogen (BUN), and kidneys were harvested for histologic examination. BUN levels were measured by the Molecular and Comparative Pathobiology Laboratory of the Johns Hopkins University. These methods have been reported by us previously (3). ACY-1215 was provided by Acetylon.
Cell culture.
PKD1 null (PN) and heterozygous (PH) cells were cultured as previously described (16, 40). PN and PH cells were grown in 10-cm culture dishes at permissive conditions (33°C) with interferon-γ in the culture medium. The cells were then transferred to nonpermissive conditions at 37°C in interferon-γ-free culture medium and evaluated at full confluency. On day 5, the cells were treated for 16 h with ACY-1215 or with DMSO (vehicle) for control cells.
Cystic index.
Kidneys were fixed in 4% paraformaldehyde (PFA) in phosphate buffer (PBS) for 24 h and then paraffin embedded and sectioned. Sections were deparaffinized with xylene, rehydrated with a descending ethanol series in PBS, and then stained with hematoxylin and eosin. Whole kidney images were obtained with a Olympus BX51 microscope equipped with DP controller and manager. The total kidney area and total cystic area were measured using ImageJ [provided by the National Institutes of Health (NIH)]. Cystic index was described by the formula: cystic index = 100 × (total cystic area/total kidney area); the results are expressed as percentages.
cAMP assay.
Confluent cells were treated with ACY-1215 or DMSO for 16 h before they were harvested for assay. cAMP levels were measured with a direct cAMP Enzyme Immunoassay Kit (no. CA200; Sigma) based on the manufacturer’s protocol. Results are expressed as picomoles per milliliter. Forskolin (no.11018) was purchased from Sigma, and acetylated α-tubulin (SC23950), adenylate cyclase3 (SC588), and ezrin were purchased from Santa Cruz Biotech. Adenylate cyclase 6 (GTX47798) was purchased from GeneTex.
In vitro cystogenesis.
Canine renal epithelial MDCK.2 cells were split 1:10 in 10-cm dishes. After 24 h, the cells were split (~60–70% confluency), resuspended in 10 ml of medium, and pelleted. For MDCK2 cells, they were resuspended in 2 ml of medium, and 2 × 104 cells were mixed with growth factor-reduced Matrigel (1.5%, 354230; Corning) and collagen I (1.5%, A10483-01; ThermoFisher Scientific), minimal essential medium (×10 , 11430030; ThermoFisher Scientific), HEPES (20 μM, 15630–106; ThermoFisher Scientific), and NaHCO3 (0.24%, 25080-094; ThermoFisher Scientific). The Matrigel-collagen I-cell mixture was plated in 12-well plates (450 μl/well) and allowed to solidify for 30 min at 37°C before being overlaid with 500 μl of medium. Cells were treated with ACY-1215 or DMSO dissolved in medium on days 0, 2, 4, 6, 8, 10, 12, and 14 after old medium was removed. Images were taken with a Zeiss (Oberkochen, Germany) Axio microscope and Visiview software (version 3.2.02; Visitron Systems). Cystic areas were analyzed with ImageJ (provided by the NIH).
Confluent PN and PH cells were split 1:10 in 10-cm dishes as described previously (3). After 24 h, the cells were again split, resuspended in 10 ml of medium, and pelleted. They were resuspended in 2 ml of medium, and 2 × 104 cells were mixed with growth factor-reduced Matrigel (1.5%) and collagen I (1.5%), minimal essential medium (1×), HEPES (20 µM), and NaHCO3 (0.24%). The Matrgel-collagen I-cell mixture was plated in 24-well plates (450 µl/well) and allowed to solidify for 30 min at 37°C before being overlaid with 500 µl of medium. Cells were treated with ACY-1215 or DMSO dissolved in medium. Images were taken with a Zeiss Axio microscope. Cystic areas were analyzed with ImageJ (provided by the NIH).
Western blotting.
Cells cultured in six-well plates were treated with ACY-1215 for 7–17 h. Cells were harvested and processed as previously described (4). In brief, the cells were solubilized in lysis buffer [150 mM Tris·HCl (pH 7.4) with 50 mM NaCl, 1% Nonidet P-40, and Halt protease inhibitor] (no. 78438; Thermo Scientific). The cell lysates were centrifuged at 10,000 g for 10 min at 4°C to pellet insoluble material, and the supernatants were collected. The protein concentrations were measured with a Bio-Rad Protein Assay (no. 500-0006; Bio-Rad), and the supernatants were then denatured in 2× Laemmli buffer at 37°C for 20 min and run on 3–8% SDS-PAGE gels (no. EA03785; Thermo Scientific) before transfer to a polyvinylidene fluoride membrane (Bio-Rad). The membranes were incubated with primary antibodies against acetylated tubulin (T7451; Sigma-Aldrich) overnight and then washed with TBS-Tween 20 buffer. A horseradish peroxidase-conjugated secondary antibody from GE Healthcare (NA934V; 1:10,000) was incubated with the membranes for 1 h, and then ECL Prime (GE Healthcare) was used for detection on film from Denville Scientific (E3018). The membranes were then stripped using Restore Western blot buffer (VWR; Pierce) and reprobed with mouse anti-β-actin from Sigma-Aldrich (A5316, 1:50,000) or anti-ezrin from Santa Cruz Biotechnology (sc-58758, 1:5,000) and HDAC6 (SC-5258).
Statistical analyses.
Data were analyzed using GraphPad Prism 7 and presented as means ± SE. Differences between two groups were assessed by two-tailed Student’s t-test. Multiple groups were analyzed with a one-way analysis of variance (ANOVA). A P value <0.05 was considered statistically significant.
RESULTS
ACY-1215 slows renal cyst growth in Pkd1fl/fl;Pax8rtTA;TetO-cre mice.
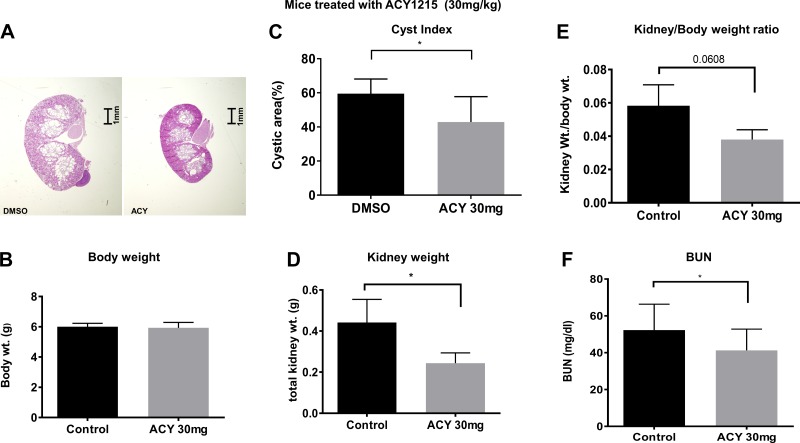
Renal cysts are the hallmark of ADPKD (35). Thus, any relevant therapy would have to be effective in reducing cyst growth. To address whether ACY-1215 can reduce cyst growth, we turned to a mouse model of ADPKD, the Pkd1fl/fl;Pax8rtTA;TetO-cre mouse, which is a PKD1−/−-inducible knockout mouse model that results in a very aggressive model of PKD, with numerous cysts appearing in both the kidney cortex and medulla at 3 wk of age (38). The mouse contains an inducible TetO-cre with a floxed PKD1 gene that, when treated with doxycycline, knocks out the functional product of the PKD1 gene. Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected intraperitoneally with doxycycline on postnatal days 11, 12, and 13. Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected daily from postnatal day 10 to 20 with ACY-1215 at either 30 or 100 mg/kg or with DMSO. Kidneys were harvested on postnatal day 21, and cystic kidneys were evaluated by measuring kidney-to-body weight ratio, cystic index (%cyst area), and kidney function by assaying for BUN.
Figure 1A shows that 30 mg ACY-1215 significantly reduced the kidney growth in Pkd1fl/fl;Pax8rtTA;TetO-cre mice. The total body weight of the mice did not differ between the treated and untreated animals (Fig. 1B), but the cyst index was reduced (Fig. 1C), and kidney weight was reduced by ~50% (Fig. 1D). The average kidney-to-body weight ratio was lowered by ~30% in the treated group compared with controls (Fig. 1E). These data suggest that the administration of ACY-1215 significantly decreased the renal cyst burden in the treated mice, with the biggest effect noted in kidney weight. Administration of ACY-1215 also lowered the BUN significantly (Fig. 1F). These reductions are indicative of improved glomerular function after ACY-1215 treatment.
Fig. 1.
ACY-1215 slows cyst growth and improves renal function in Pkd1−/− mice. A: representative images of postnatal day (PN) 21 kidney sections from DMSO- and ACY-1215-treated mice (30 mg/kg body wt). No differences were noted in overall body weight (B), but significant reductions occurred in cystic index (C), kidney weight (D), kidney-to-body weight ratio (E), and blood urea nitrogen (BUN, F). The total kidney area and total cystic area were measured with ImageJ (provided by the National Institutes of Health). Cystic index = 100 × (total cystic area/total kidney area) and is expressed as a percentage. Bars represent averages ± SE of DMSO (vehicle)-treated and ACY-1215-treated (n = 5–6) mice. *P < 0.05. Statistical analysis was performed using unpaired 2-tailed Student’s t-test.
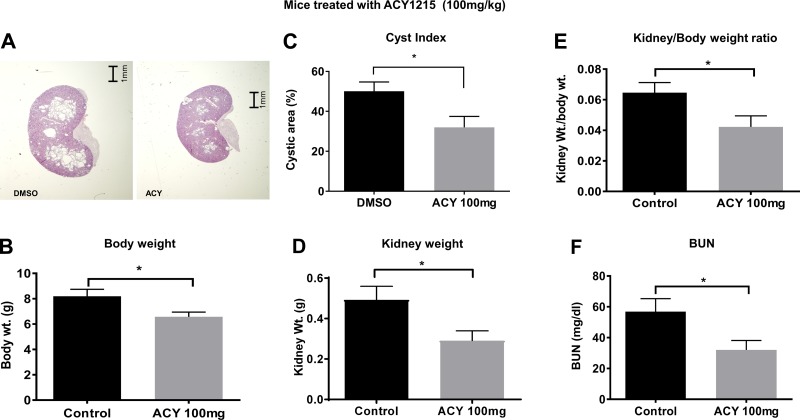
Higher dosing with ACY-1215 (100 mg/kg) had a similar effect (Fig. 2A); however, unlike those receiving the lower dose, the mice given 100 mg/kg weighed slightly less than did the untreated group (Fig. 2B). The higher dose also resulted in a reduction in cyst index of ~40% (Fig. 2C). The reduction in kidney weight was also ~40% (Fig. 2D), and the reduction in the kidney-to-body weight ratio was ~30% (Fig. 2E). As with the lower dose, the 100 mg/kg dose improved renal function, as evidenced by a reduction in BUN (Fig. 2F). Although we did not see a large dose-dependent effect, these data suggest that the administration of ACY-1215 significantly decreases the renal cyst burden in the treated mice, with the biggest effect noted in kidney weight, and it did improve kidney function, as noted by the reduction in BUN.
Fig. 2.
ACY-1215 slows cyst growth and improves renal function in Pkd1−/− mice. A: representative images of PN21 kidney sections from DMSO- and ACY-1215-treated mice (100 mg/kg body wt). A decrease in overall body weight was evident in the treated animals (B); significant reductions occurred in cystic area (C), kidney weight (D), kidney-to-body weight ratio (E), and BUN (F). Experimental details are in Fig. 1. *P < 0.05.
ACY-1215 prevents cyst formation in vitro.
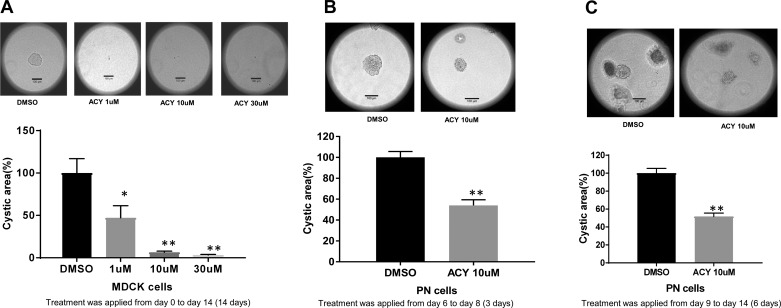
The animal studies discussed above involved injection of the drug in a mouse model of aggressive ADPKD. To obtain more direct information concerning the extent to which ACY-1215 inhibits cyst growth, we grew canine renal epithelial cells (MDCK.2 cells), which naturally form cysts in 3D Matrigel-collagen I gels. We treated the cells either with DMSO or ACY-1215 each day for 14 days. We used three doses of ACY-1215 ranging from 10 to 30 µM. Figure 3A shows that treatment with ACY-1215 did indeed inhibit cyst formation; at 10 and 30 µM, cyst formation was virtually abolished. To determine whether ACY-1215 could shrink already-established cysts, we took two approaches; however, this time we used PN cells grown in 3D culture. PN cells are immortalized mouse cells derived from the proximal tubules of PKD1 knockout (PN) mice, as described previously (10, 33). We showed previously and verified here again that MDCK.2 and PN cells behave similarly (3). In the first approach (Fig. 3B), we allowed the cysts to grow for 6 days and then treated them with ACY-1215 at an effective dose (10 µM) for 3 days (days 6–8). In the second approach (Fig. 3C), we grew the cysts for 9 days and applied ACY-1215 for 6 days (days 9–14). Importantly, treatment with ACY-1215 not only arrested cyst growth and but also shrank the cysts. Taken together, these data show convincingly that ACY-1215 very effectively reduces cyst growth in vitro.
Fig. 3.
A: ACY-1215 reduces cyst growth in MDCK.2 cells. Representative images of MDCK cells treated with either DMSO or ACY-1215. Treatment was applied to MDCK.2 cells grown in 3-dimensional (3D) culture from the beginning of the culture period. In the summary data for the in vitro cystogenesis experiment, bars represent averages ± SE. MDCK cells were treated with DMSO or ACY-1215 (1, 10, 30 µM) on days 0, 2, 4, 6, 8, 10, 12, and 14; images were taken on day 16. Bars represent means ± SE (n = 6). The average cyst from the DMSO group was considered 100%, and the rest of the cysts were compared with this cyst. B and C: ACY-1215 shrinks established cysts in MDCK.2 cells. B: representative images of PKD1 null (PN) cells treated with either DMSO or ACY-1215. Cysts were allowed to grow for 6 days. There were no statistically significant differences in cyst area among the groups. The cysts were then treated with DMSO or ACY-1215 on days 6, 7, and 8; images were taken on day 9. Note that the summary data show that ACY-1215 reduces the size of already-established cysts. C: representative images of PN cells treated with either DMSO or ACY-1215. Cysts were allowed to grow for 9 days and were statistically the same size for all groups. Cells were then treated with DMSO or ACY-1215 (10 µM) on days 9, 10, 11, 12, 13, and 14; images were taken on day 16. Note that the summary data show that ACY-1215 reduced the size of already-established cysts. Bars represent means ± SE (n = 5 for A and 6 for B). The mean size of the cysts from the DMSO group was considered 100%, and the size of the rest of the cysts was compared with this size. *P < 0.05 and **P < 0.01. One-way ANOVA and Student’s t-test were used for statistics.
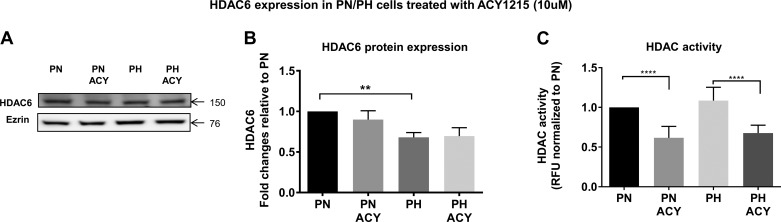
ACY-1215 increases tubulin acetylation and reduces HDAC6 activity in ADPKD mouse cells.
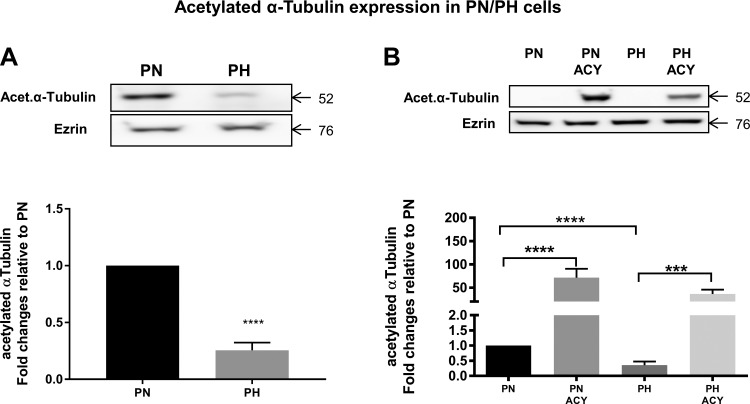
ACY-1215 was developed as an HDAC6 inhibitor (39). To verify that this compound indeed inhibited the enzyme, we measured HDAC6 activity. Although HDAC6 protein was higher in PN vs. PH cells, ACY-1215 did not change the protein expression of HDAC6 (Fig. 4, A and B). In contrast, ACY-1215 caused a significant drop in HDAC6 activity, consistent with it being an HDAC inhibitor. PH cells are heterozygous for pkd1 and as such are used as controls for PN. Tubulin deacetylation is one of the main downstream targets of HDAC6 (19); thus, we also measured the levels of α-tubulin, which showed a rather large increase following ACY-1215 treatment (Fig. 5). This result is a clear indication that ACY-1215, by virtue of its HDAC inhibitory activity, has a potent effect on α-tubulin.
Fig. 4.
Histone deacetylase 6 (HDAC6) inhibition decreases HDAC6 activity. A: Western blot showing expression of HDAC6 in treated or control cells. Ezrin was used as a loading control. B: summary data for Western blots show that the steady-state levels of HDAC6 are higher in PN cells than in PKD1 heterozygous (PH) cells. C: HDAC activity in PN/PH cells treated with ACY-1215 or DMSO for 16 h was measured using a HDAC chemiluminescent assay kit (no. 50076; BPS Bio Sciences). Fluorescence was measured at 360 nm excitation and 450 nm emission using a SpectraMax M3 microplate reader. Undiluted cell lysates were used in place of enzyme. Note that ACY-1215 reduces HDAC6 activity. Bars represent means ± SE of the HDAC6 expression. The experiment was repeated 3 times. ** P < 0.01 and ****P < 0.0001. Data were analyzed by nonparametric t-test.
Fig. 5.
HDAC6 inhibition increases acetylated α-tubulin. A: Western blot showing expression of acetylated α-tubulin in PN vs. PH cells. Ezrin was used as a loading control. Note that α-tubulin levels are higher in PN vs. PH cells. B: Western blot data for acetylated α-tubulin for cells treated with ACY-1215. Note the large increase in acetylated α-tubulin induced by ACY-1215. Bars represent means ± SE of the α-tubulin expression. The experiment was repeated 3 times. ***P < 0.001 and ****P < 0.0001. Data were analyzed by nonparametric t-test.
ACY-1215 downregulates cAMP levels by inhibiting adenylyl cyclase activity and protein expression.
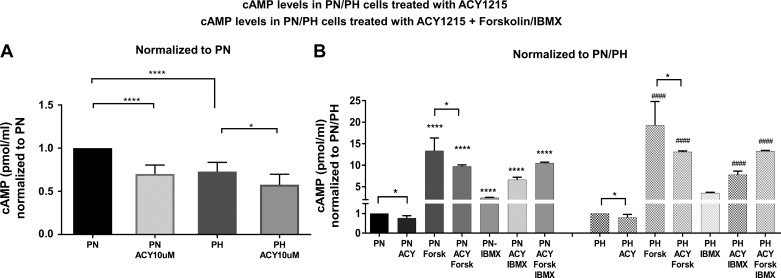
We have shown previously that HDAC inhibition reduces cAMP levels in PN cells (3). Thus, as a first step, we asked whether ACY-1215 had a similar effect on cAMP levels in PN and PH cells. As expected, ACY-1215 (Fig. 6A) did indeed reduce cAMP levels in these cells, consistent with our previous findings. To take this one step further, we treated the cells with forskolin, a direct activator (15) of adenylyl cyclase activity, and/or with isobutyl methylxanthine (IBMX), a phosphodiesterase inhibitor (13). As expected, forskolin treatment caused a large increase in cAMP, resulting from a large activation of AC activity (Fig. 6B). IBMX alone also increased the amounts of cAMP by inhibiting the degradation of cAMP. Interestingly, ACY-1215 inhibited the forskolin-dependent increase in cAMP, consistent with a decrease in overall AC activity. The similar effect of ACY-1215 on cAMP levels in the presence of forskolin or forskolin + IBMX suggests that phosphodiesterases are not the target of ACY-1215.
Fig. 6.
cAMP levels are reduced in PN/PH cells treated with ACY-1215: A: PN/PH confluent cells were treated with ACY-1215 (10 µM) or DMSO for 16 h. Note that cAMP levels are higher in PN cells than in PH cells and are reduced by ACY-1215, particularly in the case of the PN cells. B: PN/PH confluent cells were treated with ACY-1215 (10 µM) or DMSO for 16 h and then treated with adenylyl cyclase activator forskolin (100 µM) or the phosphodiesterase inhibitor isobutyl methylxanthine (IBMX, 100 µM) for 30 min before the cells were harvested for assay. Note that ACY-1215 reduces both resting cAMP levels and forskolin-dependent increases in cAMP. cAMP levels were measured with a direct cAMP Enzyme Immunoassay Kit (no. CA200; Sigma) based on the manufacturer’s protocol. Results are expressed as pmol/ml. Bars represent means ± SE. *P < 0.05, **** P < 0.0001, and ####P < 0.0001. Statistical analysis was performed using a 2-tailed Student’s t-test. Each set of data is from 6 individual wells for A and 5–10 wells for B. *Compared with PN cells. #Compared with PH cells.
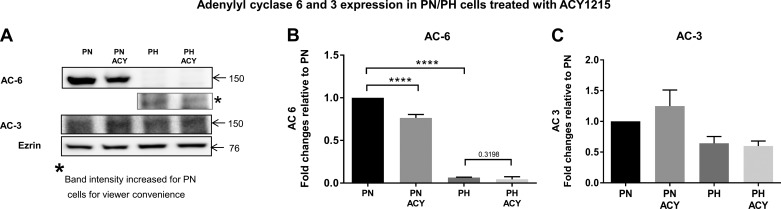
To determine whether ACY-1215 is affecting AC expression levels, we measured the steady-state levels of two Ca2+-dependent adenylyl cyclases in PN and PH cells (8). Depicted in Fig. 7 are the levels of AC-6 and AC-3 in PN vs. PH cells. Consistent with what others have found for cysts derived from collecting ducts, we also saw higher AC-6 levels in PN cells (27), which are derived primarily from proximal tubules, than in PH cells. Interestingly, ACY-1215 caused a reduction in the steady-state levels of AC-6, primarily in the PN cells. In contrast, the levels of AC-3 did not differ significantly between PN and PH cells and were not affected by ACY-1215.
Fig. 7.
Adenylyl cyclase 6 (AC-6), but not adenylyl cyclase 3 (AC-3), expression is higher in PN cells than in PH cells and is reduced by ACY-1215. A: Western blot showing the expression of AC-6 and AC-3 in 10 µM ACY-1215-treated or control cells. B: expression of AC-6 is much higher in PN than in PH cells and is reduced by ACY-1215 treatment. C: AC-3 is expressed to an equal extent in PN and PH cells and is not significantly affected by ACY-1215. Bars represent averages ± SE of the AC-6 and -3 expression. Data were analyzed by nonparametric t-test. The experiment was repeated 3 times. ****P < 0.0001. Note that the band intensity has been increased for PN cells for viewer convenience, since AC-6 is expressed much more abundantly in PN cells than in PH cells.
DISCUSSION
ACY-1215 reduces cyst growth.
Administration of ACY-1215 reduced the growth of renal cysts in the Pkd1fl/fl;Pax8rtTA;TetO-cre mice, as assessed by kidney-to-body weight ratio and kidney cystic index. Consistent with the reduction in cystic index was an improvement in renal function. Without treatment, aggressive renal cyst formation occurs, with the mice developing excessively large polycystic kidneys by 3 wk of age. Given that there is only a very short time to slow down cyst growth, the observation that ACY-1215 does indeed impede cyst growth is remarkable. In this mouse model, there are numerous small cysts within the cortex and fewer but larger cysts within the medullary region. ACY-1215 reduces both the cyst area as a ratio of total kidney area and, to a greater extent, kidney weight. These positive results are indicative of a reduction in cyst burden throughout the kidney that is accompanied by a relative preservation of renal function when compared with the untreated mice.
In our study, we did not observe much of a difference between animals treated with 30 vs. 100 mg/kg of ACY-1215. One possible explanation for this lack of difference is that the drug was injected in the intraperitoneal space, a particularly challenging technique in very young mice. Thus, to verify that ACY-1215 is indeed effective in reducing cyst growth, we also treated cultured MDCK.2 cells, which are well known to form cysts when grown in 3D culture (2). In these cells, we found a dose-dependent decrease in cyst formation after 14 days, when ACY-1215 had been applied at the same time that the cells were placed in the 3D culture medium and left to grow for 14 days. Importantly, ACY-1215 not only inhibited cyst growth when applied at initial time points, when cysts were just beginning to develop, but it was also able to shrink cysts that were already grown before drug application. These data strongly suggest that ACY-1215 is effective in reducing cyst growth.
ACY-1215 reduces HDAC6 activity.
As mentioned above ACY-1215 is an HDAC6 inhibitor (29). Indeed, we have confirmed this here by measuring a reduction in HDAC6 enzymatic activity following treatment with ACY-1215. One might ask, given the multiple roles that HDAC6 plays in the transport of defective proteins to the autophagosome for degradation and in the deacetylation of α-tubulin and 90-kDa heat shock protein (Hsp90), why would an HDAC6 inhibitor such as ACY-1215 be a viable treatment? The key to ACY-1215 as a potential treatment is that HDAC6 protein levels are elevated in the pkd−/− cells compared with those containing PC1. Because autophagy is inhibited in ADPKD cells because of the misregulation of the mammalian target of rapamycin signal transduction pathway (1), the upregulation of HDAC6 steady-state protein levels and activity would not have a large impact on HDAC6’s ability to transport proteins to the aggresome for degradation. Indeed we showed that in the absence of PC1 that PC2 protein expression is insensitive to HDAC6 inhibition, consistent with the reported malfunction in autophagy (3). With the autophagy pathway blocked in ADPKD, the elevated HDAC6 proteins levels in ADPKD are most likely facilitating cyst growth by reducing the acetylation of α-tubulin. In fostering the deacetylation of α-tubulin it promotes the cell cycle (19), a phenomenon observed in cancer cells. ADPKD is associated with slow proliferation of cells lining the cyst (12). Consistent with its role in the cell cycle, inhibition of HDAC6 activity with tubacin reduces proliferation of PN cells (3).
Another important target for HDAC6 is Hsp90. In certain cancers, elevated HDAC6 leads to the deacetylation of Hsp90, which upregulates its activity and a host of client proteins that HDAC6 regulates (30, 32). In ADPKD, Hsp90 is hyperactive in cysts, in turn activating its client proteins (26, 31). Likewise, inhibiting Hsp90 function with STA-2842 (31) will reduce cyst growth. Thus, a drug such as ACY-1215, which targets an overactive protein such as HDAC6 with the goal of reducing activity toward more normal levels, may be an important new potential treatment for ADPKD.
ACY-1215 reduces cAMP and adenylyl cyclase 6.
We have previously shown that treatment of the Pkd1fl/fl;Pax8rtTA;TetO-cre mice with tubacin reduces cyst growth and cAMP levels in vivo (3). To test whether ACY-1215 reduces cAMP, we used cells of proximal tubule origin that were isolated from single parental clones obtained from a pkdfl/− mouse manufactured in the ImmortoMouse containing the H-2Kb-tsA58 gene. The null cells (PN) stably express the Cre recombinase, and the control (PH) cells are from the original clone that is heterozygous for the expression of PC1 (16, 40). PN cells had higher resting levels of cAMP than did PH cells, and these higher levels were reduced by ACY-1215. Interestingly, ACY-1215 reduced both resting and forskolin-stimulated levels of cAMP, indicating a decrease in the overall levels of adenylyl cyclase. An increase in cAMP in the cells from the cystic kidneys could occur for several reasons (36). However, it has been suggested that adenylyl cyclase 6 is a major factor in generating cAMP in cysts derived from the collecting tubules (27). Indeed, we show here that adenylyl cyclase 6 protein levels are substantially higher in PN than in PH cells; interestingly, ACY-1215 decreased the steady-state levels of adenylyl cyclase protein in the PN cells.
In conclusion, we report that the specific HDAC6 inhibitor ACY-1215 reduces cyst growth in a mouse model of ADPKD and in MDCK.2 cells. This reduction likely occurs via a joint reduction in cAMP and adenylyl cyclase 6 steady-state protein levels. Our results provide compelling evidence that HDAC6 inhibitors offer a new avenue for ADPKD drug discovery.
GRANTS
We are grateful to S. Somlo for providing the PH2 and PN18 cells though the George M. O’Brien Kidney Center at Yale University, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant P30-DK-079310. These studies used resources provided by the NIDDK-sponsored Baltimore Polycystic Kidney Disease Research and Clinical Core Center, P30-DK-090868.
DISCLOSURES
LC received a contract from the Acetylon Corporation to conduct these experiments.
AUTHOR CONTRIBUTIONS
M.K.Y., Q.L., and L.C. performed experiments; M.K.Y., Q.L., and L.C. analyzed data; M.K.Y., Q.L., and L.C. prepared figures; M.K.Y., Q.L., and L.C. edited and revised manuscript; M.K.Y., Q.L., and L.C. approved final version of manuscript; L.C. conceived and designed research; L.C. interpreted results of experiments; L.C. drafted manuscript.
ACKNOWLEDGMENTS
We thank W.B. Guggino for help with the manuscript and Matt Jarpe for providing ACY-1215 and for supporting the research.
REFERENCES
- 1.Boletta A. Emerging evidence of a link between the polycystins and the mTOR pathways. PathoGenetics 2: 6, 2009. doi: 10.1186/1755-8417-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino GG. Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell 6: 1267–1273, 2000. doi: 10.1016/S1097-2765(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 3.Cebotaru L, Liu Q, Yanda MK, Boinot C, Outeda P, Huso DL, Watnick T, Guggino WB, Cebotaru V. Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int 90: 90–99, 2016. doi: 10.1016/j.kint.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebotaru V, Cebotaru L, Kim H, Chiaravalli M, Boletta A, Qian F, Guggino WB. Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway. J Biol Chem 289: 6404–6414, 2014. doi: 10.1074/jbc.M113.501205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czarnecki PG, Steinman TI. Polycystic kidney disease: new horizons and therapeutic frontiers. Minerva Urol Nefrol 65: 61–68, 2013. [PubMed] [Google Scholar]
- 7.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol 31: 2066–2078, 2011. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Gradilone SA, Habringer S, Masyuk TV, Howard BN, Masyuk AI, Larusso NF. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. Am J Pathol 184: 600–608, 2014. doi: 10.1016/j.ajpath.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm DH, Cai Y, Chauvet V, Rajendran V, Zeltner R, Geng L, Avner ED, Sweeney W, Somlo S, Caplan MJ. Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J Biol Chem 278: 36786–36793, 2003. doi: 10.1074/jbc.M306536200. [DOI] [PubMed] [Google Scholar]
- 11.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today 10: 1503–1519, 2005. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 14.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millán JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 15.Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem 274: 7599–7602, 1999. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- 16.Joly D, Ishibe S, Nickel C, Yu Z, Somlo S, Cantley LG. The polycystin 1-C-terminal fragment stimulates ERK-dependent spreading of renal epithelial cells. J Biol Chem 281: 26329–26339, 2006. doi: 10.1074/jbc.M601373200. [DOI] [PubMed] [Google Scholar]
- 17.Kalin JH, Bergman JA. Development and therapeutic implications of selective histone deacetylase 6 inhibitors. J Med Chem 56: 6297–6313, 2013. doi: 10.1021/jm4001659. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68: 7561–7569, 2008. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Yang XJ. Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cellular and molecular life sciences. Cell Mol Life Sci 72: 4237–4255, 2015. doi: 10.1007/s00018-015-2000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Qi N, Li L, Wu M, Mei C. Cytosolic HDAC6 is accumulated in cystic kidneys. Kidney Int 90: 705, 2016. doi: 10.1016/j.kint.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Fan LX, Zhou X, Sweeney WE Jr, Avner ED, Li X. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PLoS One 7: e49418, 2012. doi: 10.1371/journal.pone.0049418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 24.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int 88: 699–710, 2015. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proia D, Golemis E, Seeger-Nukpezah T. Treating Polycystic Kidney Disease with hsp90 Inhibitory Compounds. Patent US 20150283147 A1, 2013.
- 27.Rees S, Kittikulsuth W, Roos K, Strait KA, Van Hoek A, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 25: 232–237, 2014. doi: 10.1681/ASN.2013010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, Sakamoto KM. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res 68: 2557–2560, 2008. doi: 10.1158/0008-5472.CAN-07-5989. [DOI] [PubMed] [Google Scholar]
- 29.Santo L, Hideshima T, Kung AL, Tseng J-C, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, Cirstea D, Rodig S, Eda H, Scullen T, Canavese M, Bradner J, Anderson KC, Jones SS, Raje N. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood 119: 2579–2589, 2012. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell 25: 151–159, 2007. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeger-Nukpezah T, Proia DA, Egleston BL, Nikonova AS, Kent T, Cai KQ, Hensley HH, Ying W, Chimmanamada D, Serebriiskii IG, Golemis EA. Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc Natl Acad Sci USA 110: 12786–12791, 2013. doi: 10.1073/pnas.1301904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidel C, Schnekenburger M, Dicato M, Diederich M. Histone deacetylase 6 in health and disease. Epigenomics 7: 103–118, 2015. doi: 10.2217/epi.14.69. [DOI] [PubMed] [Google Scholar]
- 33.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung YM, Lee T, Yoon H, DiBattista AM, Song JM, Sohn Y, Moffat EI, Turner RS, Jung M, Kim J, Hoe HS. Mercaptoacetamide-based class II HDAC inhibitor lowers Aβ levels and improves learning and memory in a mouse model of Alzheimer’s disease. Exp Neurol 239: 192–201, 2013. doi: 10.1016/j.expneurol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812: 1314–1321, 2011. doi: 10.1016/j.bbadis.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 66: 1283–1285, 2004. doi: 10.1111/j.1523-1755.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 37.Torres VE. Therapies to slow polycystic kidney disease. Nephron, Exp Nephrol 98: e1–e7, 2004. doi: 10.1159/000079926. [DOI] [PubMed] [Google Scholar]
- 38.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogl DT, Hari PN, Jagannath S, Jones SS, Supko JG, Leone G, Wheeler C, Orlowski RZ, Richardson PG, Lonial S. ACY-1215, a selective histone deacetylase (HDAC) 6 inhibitor: interim results of combination therapy with bortezomib in patients with multiple myeloma (MM). Blood 122: 759–759, 2013. 23616623 [Google Scholar]
- 40.Wei F, Karihaloo A, Yu Z, Marlier A, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, Cantley LG. Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int 74: 1310–1318, 2008. doi: 10.1038/ki.2008.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wills ES, Cnossen WR, Veltman JA, Woestenenk R, Steehouwer M, Salomon J, Te Morsche RH, Huch M, Hehir-Kwa JY, Banning MJ, Pfundt R, Roepman R, Hoischen A, Drenth JP. Chromosomal abnormalities in hepatic cysts point to novel polycystic liver disease genes. Eur J Hum Genet 24: 1707–1714, 2016. doi: 10.1038/ejhg.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, Mei C. Histone deacetylases 6 increases the cyclic adenosine monophosphate level and promotes renal cyst growth. Kidney Int 90: 20–22, 2016. doi: 10.1016/j.kint.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 44.Yu F, Ran J, Zhou J. Ciliopathies: does HDAC6 represent a new therapeutic target? Trends Pharmacol Sci 37: 114–119, 2016. doi: 10.1016/j.tips.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28: 1688–1701, 2008. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]