Abstract
Insulin resistance is central to the development of type 2 diabetes and related metabolic disorders. Because skeletal muscle is responsible for the majority of whole body insulin-stimulated glucose uptake, regulation of glucose metabolism in this tissue is of particular importance. Although Rho GTPases and many of their affecters influence skeletal muscle metabolism, there is a paucity of information on the protein kinase N (PKN) family of serine/threonine protein kinases. We investigated the impact of PKN2 on insulin signaling and glucose metabolism in primary human skeletal muscle cells in vitro and mouse tibialis anterior muscle in vivo. PKN2 knockdown in vitro decreased insulin-stimulated glucose uptake, incorporation into glycogen, and oxidation. PKN2 siRNA increased 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling while stimulating fatty acid oxidation and incorporation into triglycerides and decreasing protein synthesis. At the transcriptional level, PKN2 knockdown increased expression of PGC-1α and SREBP-1c and their target genes. In mature skeletal muscle, in vivo PKN2 knockdown decreased glucose uptake and increased AMPK phosphorylation. Thus, PKN2 alters key signaling pathways and transcriptional networks to regulate glucose and lipid metabolism. Identification of PKN2 as a novel regulator of insulin and AMPK signaling may provide an avenue for manipulation of skeletal muscle metabolism.
Keywords: protein kinase N2, insulin resistance, skeletal muscle, AMP kinase, lipid metabolism
because skeletal muscle is the predominant site of insulin-stimulated glucose uptake, skeletal muscle insulin resistance is a major factor contributing to defective blood glucose disposal in type 2 diabetes (5, 6). The physiological role of novel and previously identified candidate genes/proteins that regulate inter- and intracellular signaling pathways controlling cellular and whole body glucose and lipid homeostasis is an active area of current research. Through the discovery of key regulatory proteins in glucose and energy homeostasis, new diabetes prevention and treatment targets may be identified.
The Rho family of guanosine triphosphatases (GTPases) comprised of Rho, Rac, and CDC42 is an essential regulator of diverse biological functions, including glucose metabolism. In particular, Rac1 is essential for both insulin-dependent and -independent glucose uptake in skeletal muscle (31, 32). Rho GTPases utilize protein kinases to elicit many of their downstream effects. Among the Ser/Thr kinases that function as Rho GTPase effector molecules are Rho- (ROCK1/2) and p21-activated (PAK1–PAK6) and protein kinase N (PKN1-PKN3) kinases (38). Although members of the ROCK and PAK family have well-established roles in glucose metabolism and insulin signaling, little is known regarding the function of PKNs in skeletal muscle metabolic regulation (12, 33, 34).
PKNs are members of the atypical protein kinase C subfamily known for regulating actin cytoskeletal rearrangement and cell migration. Although the three mammalian PKN family members share a large degree of homology in their COOH-terminal catalytic domain, variation in their regulatory domain results in selectivity to upstream signals (17, 21). Both PKN1 and PKN2 respond to Rho and Rac, but these isoforms display differential responsiveness to lipids and binding partner proteins (10, 11, 13, 21, 24, 26). Importantly, PKN2 represents the majority of Rho-associated autophosphorylation activity in all tissues tested (36). A high degree of isoform selectivity was confirmed by the finding that mice lacking PKN1, PKN3, or both are without overt phenotype, whereas loss of PKN2 is embryonically lethal (25).
In addition to Rho GTPases, phosphoinositide-dependent kinase-1 (PDK1), a key kinase in the insulin-signaling cascade, stimulates PKNs by phosphorylation of the activation loop (9). In adipocytes, insulin stimulates PKN activity, and PKN1 transmits the insulin signal to the actin cytoskeleton (9, 30). Conversely, PKNs may inhibit insulin signaling by directly interacting with PDK1 and Akt (8, 15, 35). In C2C12 cells, PKN2 contributes to cell adhesion-mediated activation of Akt (18). Moreover, phosphoproteomics of PKN2−/− mouse embryonic fibroblasts revealed elevations in the Akt pathway (25). Because PKN2 is the predominant PKN isoform in skeletal muscle, we investigated a potential role for PKN2 in metabolic regulation in this tissue (7). We found that PKN2 knockdown impairs insulin responsive glucose metabolism and, unexpectedly, activates 5′-adenosine monophosphate-activated protein kinase (AMPK) with downstream effects on lipid and protein metabolism.
MATERIALS AND METHODS
Cell culture and transfection.
Primary human skeletal muscle cell (HSMCs) cultures were established from vastus lateralis biopsies taken from healthy men, as described previously (1). Cells were grown and differentiated as previously described (19). On days 4 and 6 of differentiation, myotubes were transfected with 25 nM small-interfering RNA (siRNA) targeting PKN2 or scrambled (SCR) control (781 and negative control no. 2, respectively; Ambion) utilizing Lipofectamine RNAiMax (Invitrogen) according to the manufacturer’s instructions. All experiments were performed on day 8 of differentiation. HEK-293 cells were grown in DMEM (no. 31966; Thermofisher) supplemented with 10% FBS. Cells were cotransfected with siRNA (25 nM) and plasmid (1 μg/ml) utilizing Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions 48 h prior to harvest. The plasmid encoding constitutively active Fyn kinase was a gift from Dr. Jeffrey Pessin (37). All experiments were performed in technical triplicate, and results were normalized to protein content determined by the bicinchoninic acid assay (Pierce), with the exception of lipid fate. DNA content was quantified by the Qubit dsDNA HS assay (Thermofisher).
Glucose uptake, incorporation into glycogen, and glucose oxidation in HSMCs.
2-Deoxyglucose uptake was measured as described previously (19). Briefly, 4-h serum-starved HSMCs were incubated with 120 nM insulin or vehicle control for 1 h. Following a PBS wash, glucose-free medium with 2-[3H]deoxyglucose and 10 µM 2-deoxyglucose was added to the cells for 15 min. Cells were lysed in 0.03% SDS and the lysate analyzed for protein concentration and 3H content. Glucose incorporation into glycogen was determined as described previously (22). Transfected HSMCs were incubated in the absence or presence of 120 nM insulin for 2 h with an addition of [14C]glucose for the final 90 minutes. Glycogen was precipitated from cell lysate and analyzed for 14C content. Glucose oxidation was performed as described previously (2). Transfected HSMCs were incubated with [14C]glucose in the absence or presence of 120 nM insulin. Plates were sealed for 4 h to accumulate radioactive 14CO2, which was captured and analyzed in 2 M NaOH following acidification of the medium with 2 M HCl.
Fatty acid oxidation and lipid fate.
Fatty acid oxidation was measured as previously described (22). HSMCs were incubated in media with [3H]palmitate and 25 μM of unlabeled palmitate with or without 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR; 2 mM). Following 6 h of incubation, palmitate was stripped from the media by incubation with charcoal and 3H content in the palmitate-free media assessed. Lipid fate was measured as previously described (20). Transfected HSMCs were incubated with [14C]palmitate in the absence or presence of AICAR (2 mM) for 6 h. Total cellular lipids were extracted utilizing isopropanol-hexane-KCl (2:4:1), dried, reconstituted in chloroform-methanol (1:1), spotted on thin-layer chromatography (TLC) plates (Whatman), and separated in a hexane-diethylether-acetic acid (80:20:3) system. Lipid species were quantified by autoradiography.
Protein synthesis.
Transfected HSMCs were incubated with [3H]phenylalanine and 2 mM unlabeled phenylalanine for 6 h. Following four washes with ice-cold PBS, cells were lysed in 0.03% SDS. 3H content was determined in trichloroacetic acid precipitate of the cell lysate.
Animals and in vivo experimental protocol.
All animal procedures were approved by the Regional Animal Ethics Committee of Northern Stockholm. Male C57BL/6J mice (12–14 wk old) were purchased from Charles River (Sulzfeld, Germany) and acclimatized for ≥1 wk before use. Mice were housed in a humidity- and temperature-controlled environment with a 12:12- h light-darkness cycle and provided ad libitum access to water and standard rodent chow (4% fat, 16.5% protein, 58% carbohydrates, 3.5% fiber, 6% ash, and 12% water and 3.0 kcal/g; purchased from Lantmännen, Stockholm, Sweden). Tibialis anterior muscles of adult C57BL/6J mice were transfected with either Sure Silencing GFP negative control or a mixture of four plasmids encoding short-hairpin RNAs (shRNAs) targeting PKN2 (KM34588G; Qiagen) by electroporation, as described previously (16). One week after electroporation, mice were fasted for 4 h and subjected to a modified oral glucose tolerance test to assess glucose uptake into skeletal muscle as described (16). Glycogen content was determined as previously described (19). For insulin-signaling experiments, male C57BL/6J mice (12 wk old) were fasted for 4 h and treated intraperitoneally with insulin (5 U/kg) or saline for 15 min. Mice were anesthetized with Avertin and electroporated or quadriceps muscle removed and frozen immediately.
Western blot analysis.
Transfected cells were harvested, placed on Laemmli buffer, and subjected to Western blot analysis, as described previously (19). Ponceau staining was used to confirm equal protein loading. Membranes were also probed against β-actin to control for equal loading of proteins. Proteins were quantified by densitometry utilizing Quantity One Software (Bio-Rad). The quantifications displaying statistical significance or trends (P < 0.1) are presented in the in graphical format. Antibodies used are given in Table 1.
Table 1.
Antibodies used
| Target | Catalog No. | Company |
|---|---|---|
| PKN2 (cells) | 8697 | Cell Signaling Technology |
| PAX7 | 27–583 | Prosci |
| DES | 15200 | Abcam |
| β-Actin | A5541 | Sigma |
| p-PKN1/2 (Thr774/816) | 2611 | Cell Signaling Technology |
| p-Akt (Ser473) | 9271 | Cell Signaling Technology |
| p-Akt (Thr308) | 4056 | Cell Signaling Technology |
| Akt | 9272 | Cell Signaling Technology |
| Phospho-GSK-3α/β (Ser21/9) | 9331 | Cell Signaling Technology |
| GSK-3β | 9315 | Cell Signaling Technology |
| p-TBC1D4 | 8619 | Cell Signaling Technology |
| TBC1D4 | 07–741 | EMB Millipore |
| p-mTOR (Ser2448) | 5536 | Cell Signaling Technology |
| mTOR (7C10) | 2983 | Cell Signaling Technology |
| p-AMPK (Thr172) | 2531 | Cell Signaling Technology |
| AMPK | 2532 | Cell Signaling Technology |
| p-ACC (Ser79) | 3661 | Cell Signaling |
| ACC | 3676 | Cell Signaling Technology |
| Fyn | sc-16 | Santa Cruz Biotechnology |
| GAPDH | 25778 | Santa Cruz Biotechnology |
| p-S6 (Ser235/236) | 2211 | Cell Signaling Technology |
| S6 | 2317 | Cell Signaling Technology |
| PKN2 (mouse muscle) | 2612 | Cell Signaling Technology |
PKN2, protein kinase N2; DES, desmin; mTOR, mammalian target of rapamycin; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; p, phosphorylated.
RNA extraction and mRNA expression quantification.
mRNA was extracted from HSMC and skeletal muscle tissue with the RNeasy Mini Kit (Qiagen) and TRIzol reagent (Invitrogen), respectively, according to the manufacturer’s recommendations. cDNA synthesis and semiquantitative real-time PCR was performed as described previously (19). Primer sequences are presented in Table 2.
Table 2.
Primers used
| Forward | Reverse | |
|---|---|---|
| Human | ||
| rplp0 | TGGAGAAACTGCTGCCTCAT | GATTTCAATGGTGCCCCTGG |
| ppia | AGGGTTCCTGCTTTCACAGA | CAGGACCCGTATGCTTTAGG |
| pkn2 | ATTGTGGCTCGAGATGAAGT | TTTGGTTTGGAAACATGCAA |
| pax7 | GAGGACCAAGCTGACAGAGG | CTGGCAGAAGGTGGTTGAA |
| myog | GCTCAGCTCCCTCAACCA | GCTGTGAGAGCTGCATTCG |
| des | CTGGAGCGCAGAATTGAATC | GGCAGTGAGGTCTGGCTTAG |
| ppara | TTCGCAATCCATCGGCGAG | CCACAGGATAAGTCACCGAGG |
| ppard | CAGGGCTGACTGCAAACGA | CTGCCACAATGTCTCGATGTC |
| pgc1a | TCTGAGTCTGTATGGAGTGACAT | CCAAGTCGTTCACATCTAGTTCA |
| cpt1b | CATGTATCGCCGTAAACTGGAC | TGGTAGGAGCACATAGGCACT |
| fabp3 | TGGAGTTCGATGAGACAACAGC | CTCTTGCCCGTCCCATTTCTG |
| pdk4 | GGAAGCATTGATCCTAACTGTGA | GGTGAGAAGGAACATACACGATG |
| srebp1c | GTTGGCCCTACCCCTCC | CTTCAGCGAGGCGGCTT |
| fasn | CCACAACTCCAAGGACACAG | CTGCTCCACGAACTCAAACA |
| scd1 | CCTGCGGATCTTCCTTATCA | GCCCATTCGTACACGTCATT |
| acc2 | CTGAGAGTGCGGAGGACTTC | AGCGAGGATCTGAACTTCCA |
| dgat1 | GTCCCTCTGCGAATGTTCC | GCTATTGGCTGTCCGATGAT |
| gpat1 | AACACCAGATGGACGGAAAG | CCGAGCACAAGAGGTTTTTC |
| Mouse | ||
| pkn2 | CGACCAAAACTCCAAAGACA | GTCTTCCCCAAGTGGCAATA |
| 36b4 | CCCTGAAGTGCTCGACATCA | TGCGGACACCCTCCAGAA |
myog, Myogenin; rplp0, ribosomal protein lateral stalk subunit P0; ppia, peptidylpropyl isomerase A; des, desmin; ppara and -d, peroxisome proliferator-activated receptor-α and -δ, respectively; pgc1a, PPAR coactivator-1α; cpt1b, carnitine palmitoyltransferase-1β; fabp3, fatty acid-binding protein 3; pdk4, pyruvate dehydrogenase kinase 4; srebp1c, sterol regulatory element-binding protein-1c; fasn, fatty acid synthase; scd1, stearoyl-CoA desaturase-1; acc2, acetyl-CoA carboxylase 2; dgat1, diacylglycerol O-acyltransferase 1; gpat1, glycerol-3-phosphate acyltransferase 1; pkn2, protein kinase N2.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software. San Diego, CA). Two-way analysis of variance was performed on untransformed data to assess the effects of siRNA and compounds. Data are presented as fold change to remove intercell line variation for visualization purposes. Paired t-test analysis was utilized for single variable experiments. Significance was set at P < 0.05. Data are presented as means ± SE.
RESULTS
Gene silencing of PKN2 does not alter myotube differentiation.
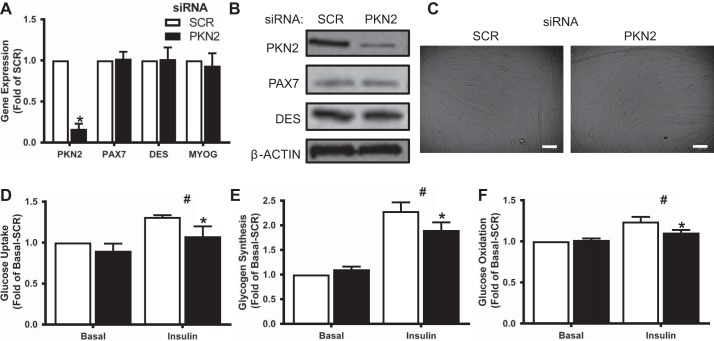
To assess the impact of PKN2 on skeletal muscle metabolism, primary human skeletal muscle cells were transfected with PKN2 siRNA on days 4 and 6 after the initiation of differentiation. This treatment achieved a robust knockdown of PKN2 mRNA and protein (Fig. 1, A and B). Because PKN2 regulates myotube differentiation in C2C12 cells (18), we sought to ensure that PKN2 knockdown did not alter the differentiation status of the human myotubes used here. Visual appearance of cultures as well as mRNA expression and protein abundance of myogenic (desmin) and proliferative markers (PAX7) were unchanged (Fig. 1, A–C). Myotube protein to DNA ratio was unchanged by siRNA treatment (SCR: 0.84 ± 0.07 mg protein/mg DNA; PKN2: 0.86 ± 0.05 mg protein/mg DNA; n = 5).
Fig. 1.
Protein kinase N2 (PKN2) knockdown decreases insulin responsiveness of glucose metabolism in skeletal muscle. A and B: mRNA levels of PKN2, PAX7, MYOG (myogenin), and DES (desmin) (A) and protein abundance of PKN2, PAX7, and DES (B) in small-interfering (si)RNA-treated primary HSMCs. C: representative bright-field images of siRNA-treated primary human skeletal muscle cells (HSMC). Scale bar, 100 µm. D–F: basal and insulin-stimulated (120 nM) glucose uptake (D), incorporation into glycogen (E), and oxidation (F) in siRNA-treated primary HSMC. Open bars, scrambled (SCR); black bars, small-interfering (si)PKN2. *PKN2 effect, P < 0.05; #insulin main effect, P < 0.05. Results are means ± SE for n = 5 biological replicates.
Role of PKN2 in glucose metabolism and insulin signaling.
Having established that PKN2 knockdown does not alter the differentiation status of HSMCs, we utilized radioactive tracer-based methods to assess glucose metabolism. PKN2 knockdown decreased insulin-stimulated glucose uptake and incorporation into glycogen without altering basal glucose metabolism (Fig. 1, D and E). Similarly, insulin-stimulated glucose oxidation was diminished in cells depleted of PKN2 (Fig. 1F).
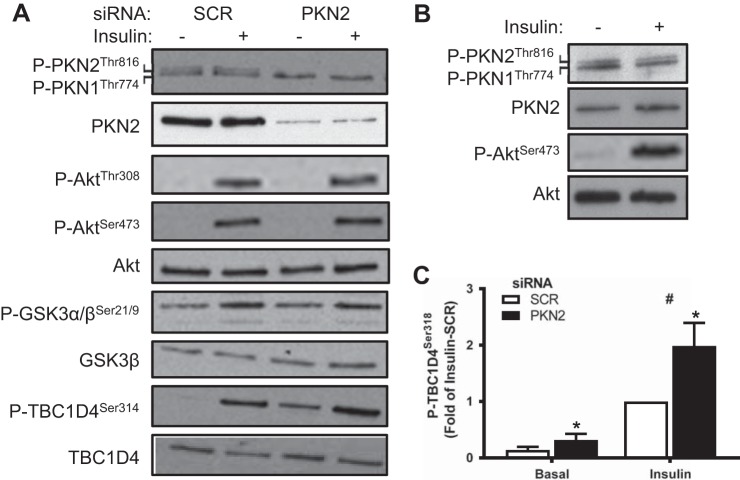
PKN2 has been reported to be phosphorylated by PDK1 (9). Given that PKN2 knockdown diminished insulin responsiveness of glucose metabolism, we sought to determine whether PKN2 constitutes a branch of or otherwise influences insulin signaling. Western blot analysis revealed that insulin treatment of either HSMC (Fig. 2A) or mouse quadriceps muscle (Fig. 2B) did not alter phosphorylation of the activation loop in either PKN2 or PKN1. PKN2 knockdown did not alter the phosphorylation of Akt or GSK-3α/β (Fig. 2A). However, PKN2 knockdown increased phosphorylation of TBC1D4 under both basal and insulin-stimulated conditions in HSMC (Fig. 2, A and C). Thus, the activation loop of PKN2 does not appear to be phosphorylated in response to insulin. Furthermore, decreased insulin-stimulated glucose metabolism in PKN2 knockdown cells cannot be explained by altered phosphorylation within the canonical insulin-signaling cascade.
Fig. 2.
PKN2 knockdown increases TBC1D4 phosphorylation in HSMCs. A:Western blot analysis of PKN2, Akt, GSK-3, and TBC1D4 protein and phosphorylation from basal and insulin-stimulated (120 nM; 15 min) primary HSMCs (representative immunoblot from n = 5 biological replicates). B: Western blot analysis of PKN2 and Akt protein and phosphorylation in mouse quadriceps muscle 15 min following saline or insulin (5 IU/kg ip) injection (representative immunoblot from n = 4 mice). C: quantification of phosphorylated (p)-TBC1D4 Ser318 abundance in basal and insulin-stimulated primary HSMCs. Open bars, SCR; black bars, siPKN2. *PKN2 effect, P < 0.05; #insulin main effect, P < 0.05. Results are means ± SE for n = 5 biological replicates.
PKN2 gene silencing increases AMPK signaling.
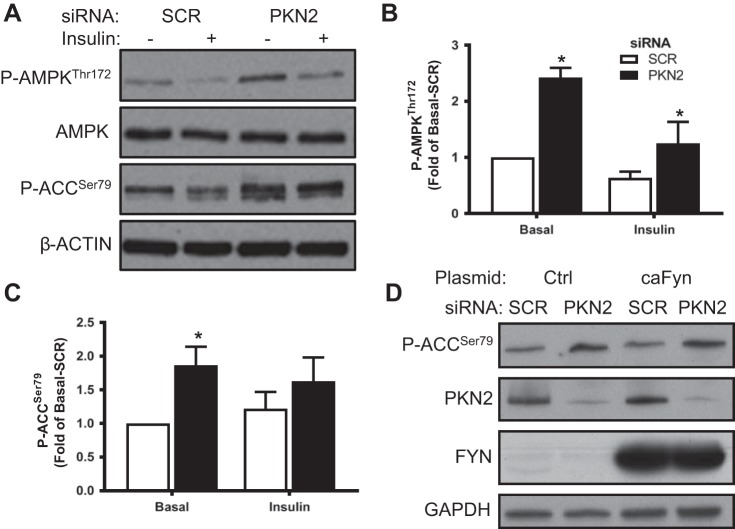
Because PKN2 knockdown impaired insulin responsiveness of glucose metabolism, we sought to examine whether PKN2 influences AMPK signaling. PKN2 knockdown increased the phosphorylation of AMPK and its substrate acetyl-CoA carboxylase (ACC; Fig. 3, A–C). Fyn kinase inhibits AMPK activity by sequestering LKB1 in the nucleus (37). Because PKN2 activates Fyn kinase, we determined whether PKN2 knockdown increases AMPK signaling by decreasing Fyn kinase activity using constitutively active Fyn kinase (caFyn) in HEK-293 cells (28, 37). Increased phosphorylation of ACC upon knockdown of PKN2 persisted irrespective of caFyn overexpression (Fig. 3D). Thus, PKN2 knockdown increases AMPK signaling independently of Fyn kinase.
Fig. 3.
PKN2 knockdown increases AMP-activated protein kinase (AMPK) signaling. A: Western blot analysis of p-AMPK Thr172, AMPK, and p-ACC Ser79 in primary HSMCs incubated in the absence or presence of insulin (120 nM; 15 min) (representative immunoblot from n = 5 biological replicates). B and C: quantification of p-AMPK Thr172 (B) and p-ACC Ser79 abundance (C). D: Western blot analysis of p-ACC Ser79 abundance in PKN2 siRNA-treated human embryonic kidney-293 cells overexpressing constitutively active Fyn kinase (caFyn; representative immunoblot from n = 3 biological replicates). Open bars, SCR; black bars, siPKN2. *PKN2 post hoc effect. Results are means ± SE for n = 5 biological replicates.
PKN2 gene silencing increases lipid metabolism and genes involved in lipid handling.
To determine whether PKN2 knockdown influences lipid metabolism, fatty acid oxidation and lipid fate were assessed in HSMC incubated in the absence or presence of the AMPK activator AICAR. PKN2 knockdown increased both basal and AICAR-stimulated fatty acid oxidation (Fig. 4A). Similar to AICAR treatment, PKN2 knockdown decreased palmitate incorporation into 1,3-diacylglycerol and the origin, which contains polar lipids (Fig. 4B). Interestingly, PKN2 markedly increased incorporation of palmitate into triglycerides (Fig. 4B). To gain insight into mechanisms by which PKN2 alters lipid metabolism, we performed quantitative PCR analysis of genes involved in lipid handling and synthesis. PKN2 knockdown increased expression of the transcriptional coactivator PGC-1α and several of its target genes (carnitine palmitoyl transferase-1β, PDK4, and fatty acid-binding protein-3; Fig. 4C). PKN2 silencing also increased expression of genes involved in fatty acid synthesis (stearoyl-CoA desaturase-1, fatty acid synthase, and sterol regulatory element-binding protein-1c) and, unexpectedly, decreased the expression of genes involved in triglyceride synthesis (diacylglycerol O-acyltransferase 1 and glycerol-3-phosphate acyltransferase 1) (Fig. 4D).
Fig. 4.
PKN2 knockdown increases fatty acid oxidation and incorporation into triglycerides (TAG). Palmitate oxidation (A) and incorporation (B) into lipid species in siRNA-treated primary HSMC incubated in the absence or presence of without 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR; 2 mM). mRNA level of peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α; C) and sterol regulatory element-binding protein-1c (SREBP-1c; D) target genes in siRNA-treated primary HSMCs. Open bars, SCR; black bars, siPKN2. *PKN2 post hoc effect, P < 0.05; #AICAR main effect, P < 0.05. Results are means ± SE for n = 5 biological replicates. CPT-1β, carnitine palmitoyltransferase-1β;FABP3, fatty acid-binding protein 3; PDK4, pyruvate dehydrogenase kinase 4; SCD1, stearoyl-CoA desaturase-1; ACC2, acetyl-CoA carboxylase 2; DGAT1, diacylglycerol O-acyltransferase 1; GPAT1, glycerol-3-phosphate acyltransferase 1.
PKN2 gene silencing decreased mammalian target of rapamycin signaling and protein synthesis.
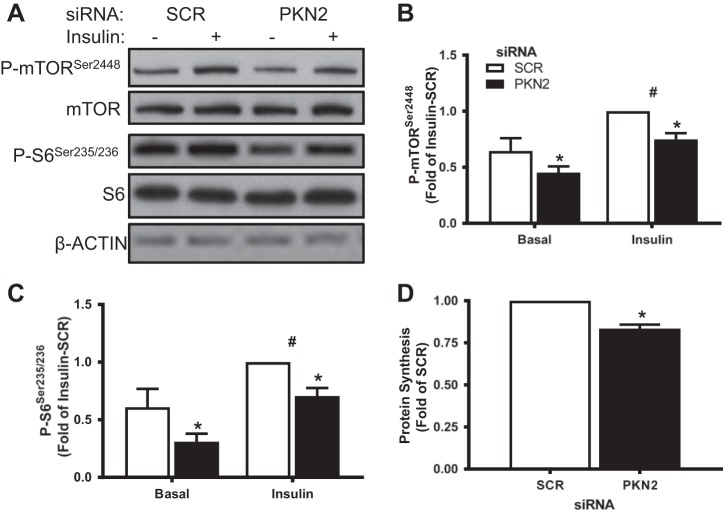
Because PKN2 knockdown led to increased AMPK signaling, we determined whether downstream targets involved in protein metabolism might also be altered. Consistent with AMPK activation, PKN2 knockdown decreased both basal and insulin-stimulated phosphorylation of mammalian target of rapamycin (mTOR) and S6 ribosomal protein (Fig. 5, A–C). To determine whether these changes were associated with alterations in protein metabolism, we performed a protein synthesis assay. PKN2 knockdown decreased incorporation of phenylalanine into protein (Fig. 5D). Consistent with AMPK activation, PKN2 knockdown decreased mTOR signaling and protein synthesis.
Fig. 5.
PKN2 knockdown decreases mammalian target of rapamycin (mTOR) signaling and protein synthesis. A:Western blot analysis of p-mTOR Ser2448, mTOR, p-S6 Ser235/236, and S6 in primary HSMCs incubated in the absence or presence of insulin (120 nM; 15 min) (representative immunoblot from n = 5 biological replicates). B and C: quantification of p-mTOR Ser2448 (B) and p-S6 Ser235/236 abundance (C). D: protein synthesis in siRNA-treated primary HSMCs. Open bars, SCR; black bars, siPKN2. *PKN2 effect, P < 0.05; #insulin main effect, P < 0.05. Results are means ± SE for n = 5 biological replicates.
PKN2 knockdown in mature skeletal muscle.
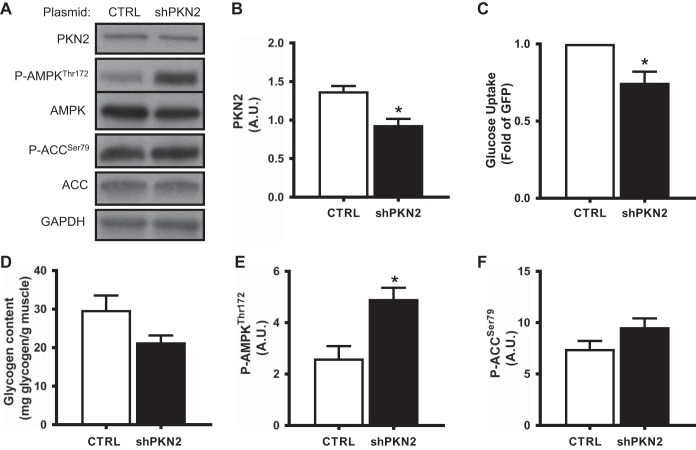
To assess the impact of PKN2 gene silencing in mature skeletal muscle in vivo, contralateral tibialis anterior muscles were electroporated with shRNA targeting PKN2 or a scrambled control sequence. PKN2 shRNA produced a modest decrease in both PKN2 mRNA expression (77 ± 11% of control leg) and protein abundance (Fig. 6, A and B). To determine whether PKN2 gene silencing affects glucose uptake in adult skeletal muscle in vivo, we performed a modified oral glucose tolerance test utilizing radiolabeled 2-deoxyglucose. PKN2 depletion reduced glucose uptake in tibialis anterior muscle (Fig. 6C). Similarly, PKN2 silencing was associated with a trend (P = 0.07) for decreased glycogen content in skeletal muscle (Fig. 6D). We next determined whether PKN2 silencing activates AMPK signaling by assessing phosphorylation of AMPK and its substrate ACC in adult skeletal muscle. Similar to our in vitro results, PKN2 silencing was associated with an increase in the phosphorylation of AMPK (Fig. 6, A and E) and its substrate ACC, although ACC phosphorylation did not reach statistical significance (Fig. 6, A and F). Thus, PKN2 knockdown in vivo inhibits glucose uptake during a glucose challenge and activates AMPK signaling in mature skeletal muscle.
Fig. 6.
PKN2 silencing in vivo decreases glucose uptake and activates AMPK. Contralateral tibialis anterior muscles were electroporated with shRNA targeting PKN2 or scrambled control. Seven days following electroporation, 4-h fasted mice were administered an oral glucose load (3 g/kg), followed by ip injection of [3H]deoxyglucose. Tibialis anterior muscle was harvested 2 h following the oral glucose challenge and analyzed for PKN2, P-AMPKThr172, AMPK, P-ACC Ser79, and ACC protein abundance (representative immunoblot from n = 7 mice) (A), quantification of PKN2 protein abundance (B), in vivo glucose uptake (C), intramuscular glycogen content (D), quantification of p-AMPK Thr172 abundance (E), and p-ACC Ser79 abundance (F) in PKN2 shRNA-treated mouse tibialis anterior muscle. *Paired t-test, P < 0.05. Results are means ± SE for n = 7 mice. AU, arbitrary units.
DISCUSSION
Insulin and AMPK are powerful regulators of metabolism in skeletal muscle. Insulin favors cell growth and energy storage, whereas AMPK signals energy stress within the cell to favor catabolic processes. Here, we provide evidence that PKN2 depletion in skeletal muscle impairs insulin responsiveness of glucose metabolism and augments AMPK signaling with concomitant effects on protein and lipid metabolism. The late initiation and duration of PKN2 knockdown utilized in the present study may explain the noneffects on myotube differentiation and hypotrophy despite previous findings in C2C12 cells and observed decreases in protein synthesis, respectively (18).
A complex network of insulin-regulated signals control glucose metabolism. These signals include Rho GTPases and their effector molecules. Because PKN2 silencing reduced insulin-simulated glucose uptake in HSMCs and glucose uptake during a glucose challenge in adult skeletal muscle, it may function as an effector protein in the insulin-signaling network. Given that PKN2 knockdown impairs insulin-stimulated glucose uptake despite stimulating two distinct signals, phosphorylation of TBC1D4 on Ser318 and activation of AMPK, that normally stimulate glucose uptake, PKN2 likely functions downstream of Rab GTPases to facilitate insulin-stimulated glucose metabolism. PKN2 is known to regulate the cytoskeleton (36). Thus, PKN2 may play a role in relaying the insulin signal to the cytoskeleton in skeletal muscle by a mechanism analogous to that of PKN1 in adipocytes (9). The exact nature of PKN2’s role in transducing the insulin signal to downstream targets remains unclear. Although we could not detect alterations in PKN2 phosphorylation in response to insulin, we cannot exclude the possibility that insulin treatment alters PKN2 activity or localization (30).
Aside from a potential role within the insulin-signaling cascade, PKN2 has been shown to influence Akt signaling by both directly binding to PDK1 and indirectly influencing its activity (8, 15, 35). Unbiased phosphoproteomic studies reveal that Akt signaling is decreased in PKN2−/− mouse embryonic fibroblasts (25). Although we did not detect changes in Akt or GSK-3α/β phosphorylation, we found that phosphorylation of Ser318 on TBC1D4 was increased upon PKN2 knockdown. Ser318 on TBC1D4 is phosphorylated by Akt in response to insulin but not by AMPK activation. Target- and context-specific activation of Akt signaling is supported by the finding that PKN2 functions in a complex with adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1), and cell adhesion molecule-related downregulated by oncogene (CDO) to increase Akt phosphorylation in differentiating but not proliferating myoblasts (18). Interestingly, APPL1 inhibition phenocopies the effect of PKN2 silencing on glucose uptake, glycogen content, and AMPK signaling (4). Thus, APPL1 and PKN2 may share common points of action in the regulation of glucose metabolism. Another member of the APPL family, APPL2, has been shown to interact with TBC1D1 and control its phosphorylation (3). The mechanism by which PKN2 influences TBC1D4 phosphorylation requires further study.
AMPK is a cellular energy sensor that influences lipid, glucose, and protein metabolism as well as gene expression. PKN2 depletion in vitro and in vivo augments AMPK signaling, but the mechanism is unclear. PKN2 activates Fyn kinase to regulate cell adhesion in keratinocytes (28). Notably, Fyn kinase-induced phosphorylation of LKB1 regulates AMPK activity by sequestering LKB1 in the nucleus (37). Thus, inhibition of Fyn kinase may be responsible for AMPK activation upon PKN2 knockdown. However, our finding of a persistent AMPK activation by PKN2 knockdown in the presence of constitutively active Fyn kinase demonstrates that Fyn kinase is dispensable. Interestingly, several Rho kinase inhibitors known to activate AMPK and influence obesity-related insulin resistance also inhibit PKN2 (14, 23).
AMPK signaling inhibits mTOR and ACC to decrease protein synthesis and increase lipid oxidation, respectively. Consistent with activation of AMPK, PKN2 knockdown decreased protein synthesis and stimulated fatty acid oxidation. Our findings that PKN2 knockdown decreased phosphorylation of mTOR and S6 ribosomal protein are consistent with decreased S6 kinase phosphorylation in PKN2−/− mouse embryonic fibroblasts (25). AMPK controls lipid metabolism by phosphorylating ACC as well as by activating transcriptional regulators. PKN2 knockdown increased expression of PGC-1α and several of its target genes (29). Despite decreased expression of genes involved in triglyceride synthesis, PKN2 knockdown increased palmitate incorporation into triglycerides. This altered partitioning of fatty acids toward oxidation and triglyceride synthesis and away from diacylglycerol also occurs upon AMPK activation and in response to exercise (27).
Taken together, our results demonstrate that PKN2 is a novel regulator of insulin-stimulated glucose metabolism and AMPK signaling in skeletal muscle. Additionally, our findings suggest that PKN2 knockdown phenocopies APPL1 inhibition, supporting the notion that these two proteins may function together in a signaling complex (4). Further understanding of the role of PKN2 in controlling key signaling and metabolic events in skeletal muscle could aid in the treatment of insulin resistance in type 2 diabetes.
GRANTS
This work was funded by the Novo Nordisk Foundation, the Strategic Research Program in Diabetes at Karolinska Institutet, the European Research Council Ideas Program (ICEBERG, ERC-2008-AdG23285), the Swedish Research Council (2011-3550), the Swedish Diabetes Foundation (DIA2012-082), and the Swedish Foundation for Strategic Research (SRL10-0027).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.R. and J.R.Z. conceived and designed research; M.A.R., I.R., J.M., and M.Å. performed experiments; M.A.R., I.R., J.M., M.Å., and J.R.Z. analyzed data; M.A.R., I.R., J.M., M.Å., and J.R.Z. interpreted results of experiments; M.A.R. prepared figures; M.A.R., J.M., and M.Å. drafted manuscript; M.A.R., J.M., and J.R.Z. edited and revised manuscript; M.A.R., I.R., J.M., M.Å., and J.R.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Arja Kants for administrative help and Dr. Jeffrey Pessin for the Fyn constitutively active construct.
REFERENCES
- 1.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- 2.Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR. Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57: 1508–1516, 2008. doi: 10.2337/db07-0583. [DOI] [PubMed] [Google Scholar]
- 3.Cheng KK, Zhu W, Chen B, Wang Y, Wu D, Sweeney G, Wang B, Lam KS, Xu A. The adaptor protein APPL2 inhibits insulin-stimulated glucose uptake by interacting with TBC1D1 in skeletal muscle. Diabetes 63: 3748–3758, 2014. doi: 10.2337/db14-0337. [DOI] [PubMed] [Google Scholar]
- 4.Cleasby ME, Lau Q, Polkinghorne E, Patel SA, Leslie SJ, Turner N, Cooney GJ, Xu A, Kraegen EW. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol 210: 81–92, 2011. doi: 10.1530/JOE-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh AS, Murgia M, Nagaraj N, Treebak JT, Cox J, Mann M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol Cell Proteomics 14: 841–853, 2015. doi: 10.1074/mcp.M114.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dettori R, Sonzogni S, Meyer L, Lopez-Garcia LA, Morrice NA, Zeuzem S, Engel M, Piiper A, Neimanis S, Frödin M, Biondi RM. Regulation of the interaction between protein kinase C-related protein kinase 2 (PRK2) and its upstream kinase, 3-phosphoinositide-dependent protein kinase 1 (PDK1). J Biol Chem 284: 30318–30327, 2009. doi: 10.1074/jbc.M109.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong LQ, Landa LR, Wick MJ, Zhu L, Mukai H, Ono Y, Liu F. Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc Natl Acad Sci USA 97: 5089–5094, 2000. doi: 10.1073/pnas.090491897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn P, Mellor H, Casamassima A, Parker PJ. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J Biol Chem 275: 11064–11070, 2000. doi: 10.1074/jbc.275.15.11064. [DOI] [PubMed] [Google Scholar]
- 11.Flynn P, Mellor H, Palmer R, Panayotou G, Parker PJ. Multiple interactions of PRK1 with RhoA. Functional assignment of the Hr1 repeat motif. J Biol Chem 273: 2698–2705, 1998. doi: 10.1074/jbc.273.5.2698. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2: 119–129, 2005. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Gross C, Heumann R, Erdmann KS. The protein kinase C-related kinase PRK2 interacts with the protein tyrosine phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Lett 496: 101–104, 2001. doi: 10.1016/S0014-5793(01)02401-2. [DOI] [PubMed] [Google Scholar]
- 14.Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20: 169–171, 2006. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 15.Koh H, Lee KH, Kim D, Kim S, Kim JW, Chung J. Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage. J Biol Chem 275: 34451–34458, 2000. doi: 10.1074/jbc.M001753200. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni SS, Karlsson HK, Szekeres F, Chibalin AV, Krook A, Zierath JR. Suppression of 5′-nucleotidase enzymes promotes AMP-activated protein kinase (AMPK) phosphorylation and metabolism in human and mouse skeletal muscle. J Biol Chem 286: 34567–34574, 2011. doi: 10.1074/jbc.M111.268292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachmann S, Jevons A, De Rycker M, Casamassima A, Radtke S, Collazos A, Parker PJ. Regulatory domain selectivity in the cell-type specific PKN-dependence of cell migration. PLoS One 6: e21732, 2011. doi: 10.1371/journal.pone.0021732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Hwang J, Jeong HJ, Yoo M, Go GY, Lee JR, Leem YE, Park JW, Seo DW, Kim YK, Hahn MJ, Han JW, Kang JS, Bae GU. PKN2 and Cdo interact to activate AKT and promote myoblast differentiation. Cell Death Dis 7: e2431, 2016. doi: 10.1038/cddis.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massart J, Sjögren RJO, Lundell LS, Mudry JM, Franck N, O’Gorman DJ, Egan B, Zierath JR, Krook A. Altered miR-29 Expression in Type 2 Diabetes Influences Glucose and Lipid Metabolism in Skeletal Muscle. Diabetes 66: 1807–1818, 2017. doi: 10.2337/db17-0141. [DOI] [PubMed] [Google Scholar]
- 20.Massart J, Zierath JR, Chibalin AV. A simple and rapid method to characterize lipid fate in skeletal muscle. BMC Res Notes 7: 391, 2014. doi: 10.1186/1756-0500-7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J Biochem 133: 17–27, 2003. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento EB, Riedl I, Jiang LQ, Kulkarni SS, Näslund E, Krook A. Enhanced glucose metabolism in cultured human skeletal muscle after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 11: 592–601, 2015. doi: 10.1016/j.soard.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Noda K, Nakajima S, Godo S, Saito H, Ikeda S, Shimizu T, Enkhjargal B, Fukumoto Y, Tsukita S, Yamada T, Katagiri H, Shimokawa H. Rho-kinase inhibition ameliorates metabolic disorders through activation of AMPK pathway in mice. PLoS One 9: e110446, 2014. doi: 10.1371/journal.pone.0110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen D, Lowe PN, Nietlispach D, Brosnan CE, Chirgadze DY, Parker PJ, Blundell TL, Mott HR. Molecular dissection of the interaction between the small G proteins Rac1 and RhoA and protein kinase C-related kinase 1 (PRK1). J Biol Chem 278: 50578–50587, 2003. doi: 10.1074/jbc.M304313200. [DOI] [PubMed] [Google Scholar]
- 25.Quétier I, Marshall JJ, Spencer-Dene B, Lachmann S, Casamassima A, Franco C, Escuin S, Worrall JT, Baskaran P, Rajeeve V, Howell M, Copp AJ, Stamp G, Rosewell I, Cutillas P, Gerhardt H, Parker PJ, Cameron AJ. Knockout of the PKN family of Rho effector kinases reveals a non-redundant role for PKN2 in developmental mesoderm expansion. Cell Rep 14: 440–448, 2016. doi: 10.1016/j.celrep.2015.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quilliam LA, Lambert QT, Mickelson-Young LA, Westwick JK, Sparks AB, Kay BK, Jenkins NA, Gilbert DJ, Copeland NG, Der CJ. Isolation of a NCK-associated kinase, PRK2, an SH3-binding protein and potential effector of Rho protein signaling. J Biol Chem 271: 28772–28776, 1996. doi: 10.1074/jbc.271.46.28772. [DOI] [PubMed] [Google Scholar]
- 27.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt A, Durgan J, Magalhaes A, Hall A. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J 26: 1624–1636, 2007. doi: 10.1038/sj.emboj.7601637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res 53: 2490–2514, 2012. doi: 10.1194/jlr.R025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standaert M, Bandyopadhyay G, Galloway L, Ono Y, Mukai H, Farese R. Comparative effects of GTPγS and insulin on the activation of Rho, phosphatidylinositol 3-kinase, and protein kinase N in rat adipocytes. Relationship to glucose transport. J Biol Chem 273: 7470–7477, 1998. doi: 10.1074/jbc.273.13.7470. [DOI] [PubMed] [Google Scholar]
- 31.Sylow L, Jensen TE, Kleinert M, Højlund K, Kiens B, Wojtaszewski J, Prats C, Schjerling P, Richter EA. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes 62: 1865–1875, 2013. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sylow L, Jensen TE, Kleinert M, Mouatt JR, Maarbjerg SJ, Jeppesen J, Prats C, Chiu TT, Boguslavsky S, Klip A, Schjerling P, Richter EA. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes 62: 1139–1151, 2013. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunduguru R, Chiu TT, Ramalingam L, Elmendorf JS, Klip A, Thurmond DC. Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells. Biochem Pharmacol 92: 380–388, 2014. doi: 10.1016/j.bcp.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 286: 41359–41367, 2011. doi: 10.1074/jbc.M111.291500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem 275: 40400–40406, 2000. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 36.Vincent S, Settleman J. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol Cell Biol 17: 2247–2256, 1997. doi: 10.1128/MCB.17.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC. Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab 11: 113–124, 2010. doi: 10.1016/j.cmet.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao ZS, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J 386: 201–214, 2005. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]