Abstract
Females are typically more insulin sensitive than males, which may be partly attributed to greater brown adipose tissue (BAT) activity and uncoupling protein 1 (UCP1) content. Accordingly, we tested the hypothesis that UCP1 deletion would abolish sex differences in insulin sensitivity and that whitening of thoracic periaortic BAT caused by UCP1 loss would be accompanied with impaired thoracic aortic function. Furthermore, because UCP1 exerts antioxidant effects, we examined whether UCP1 deficiency-induced metabolic dysfunction was mediated by oxidative stress. Compared with males, female mice had lower HOMA- and AT-insulin resistance (IR) despite no significant differences in BAT UCP1 content. UCP1 ablation increased HOMA-IR, AT-IR, and whitening of BAT in both sexes. Expression of UCP1 in thoracic aorta was greater in wild-type females compared with males. Importantly, deletion of UCP1 enhanced aortic vasomotor function in females only. UCP1 ablation did not promote oxidative stress in interscapular BAT. Furthermore, daily administration of the free radical scavenger tempol for 8 wk did not abrogate UCP1 deficiency-induced increases in adiposity, hyperinsulinemia, or liver steatosis. Collectively, we report that 1) in normal chow-fed mice housed at 25°C, aortic UCP1 content was greater in females than males and its deletion improved ex vivo aortic vasomotor function in females only; 2) constitutive UCP1 content in BAT was similar between females and males and loss of UCP1 did not abolish sex differences in insulin sensitivity; and 3) the metabolic disruptions caused by UCP1 ablation did not appear to be contingent upon increased oxidative stress in mice under normal dietary conditions.
Keywords: brown adipose tissue, oxidative stress, insulin resistance, vascular function
for a given body mass index females are more insulin sensitive than males (25, 34, 61) and this sex difference in insulin sensitivity is also true in rodents (1, 37, 64). Although differences in adipose tissue (AT) distribution and sex hormones are important factors contributing to sex differences in insulin sensitivity, additional mechanisms are likely involved. Brown AT (BAT) and uncoupling protein 1 (UCP1) content, the essential protein required for nonshivering thermogenesis (7) and an important regulator of BAT metabolic activity, are positively associated with insulin sensitivity (3, 11, 35, 66). Indeed, whole body deletion of UCP1 causes insulin resistance (IR) (67), whereas AT overexpression of UCP1 or sympathetically mediated (i.e., cold/pharmacological) induction of UCP1 is protective against diet-induced IR (8, 31, 58, 68). Given previous work in rodents indicating that BAT expression of UCP1 is greater in females than males (47, 49–51, 60), it is conceivable that increased insulin sensitivity in females may be partly attributable to their higher BAT activity. In addition, loss of UCP1 not only instigates IR but it also causes whitening of BAT depots including thoracic periaortic BAT (67), a fat depot strongly implicated in the regulation of adjacent aortic function (20, 23, 42). Indeed, thoracic periaortic BAT has been shown to exert anticontractile effects, whereas such anticontractile effects are lost with obesity and in the abdominal aorta which is surrounded by white AT (reviewed in 5). Thus, given that loss of UCP1 causes a shift from brown to white periaortic AT phenotype (67), it is possible that this transition contributes to impaired aortic function. Moreover, since female rodents have a greater percentage of brown-like adipocytes in periaortic fat compared with males (13), we surmised that aortic vasomotor dysfunction caused by deletion of UCP1 would be most pronounced in females.
The underlying mechanisms by which UCP1 confers systemic metabolic protection are not completely understood. One hypothesis is that UCP1 constitutes an adaptive mechanism to alleviate oxidative stress serving as an endogenous antioxidant (9, 38, 69). Given the causal link between oxidative stress and IR (28, 43, 55), it is plausible that metabolic dysfunction caused by loss of UCP1 is mediated by increased oxidative stress, particularly in BAT.
Herein we tested the hypothesis that increased insulin sensitivity in females is partly attributable to their greater BAT function. Specifically, we postulated that systemic ablation of UCP1 would normalize the sex differences in insulin sensitivity, as assessed by HOMA-IR and AT-IR. Furthermore, we hypothesized that lack of UCP1 would be accompanied with impaired aortic function. Last, we reasoned that UCP1 deficiency-induced metabolic dysfunction would be mediated by oxidative stress.
MATERIALS AND METHODS
Ethical approval.
All animal husbandry and experimental procedures were performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Missouri Institutional Animal Care and Use Committee.
Animals and experimental design.
Heterozygote male and female UCP1−/+ mice on a C57BL/6J background were purchased from Jackson Laboratory (stock no. 003124, Bar Harbor, ME) and bred at the University of Missouri Transgenic Animal Core (Columbia, MO) facility to produce homozygote UCP1 knockout (KO; n = 20 males, n = 20 females) and littermate wild-type (WT; n = 24 males, n = 22 females) mice. All mice were fed a standard chow diet (3.3 kcal/g of food, 13% kcal fat, 57% kcal carbohydrate, and 30% kcal protein, 5001, LabDiet, St. Louis, MO) and were housed two to three per cage (within group) in a light cycle from 0700 to 1900. Recent evidence suggests that when housing multiple animals per cage, using animal housing temperatures between 23 and 25°C may be best to mimic human physiology (56, 57), although this is debated (8, 24). Thus, in an effort to translate our findings to human physiology, we studied mice housed at 25°C which poses less thermostress than standard housing conditions (e.g., 20–22°C). At 18 wk of age, a subset of male UCP1 KO (n = 10) and WT (n = 11) animals were treated with the membrane-permeable free radical scavenger tempol (2 mmol/l of drinking water, Sigma-Aldrich no. 176141, lot no. BCBQ3465V) for 8 wk. Tempol has been demonstrated to quench reactive oxygen species (ROS) (17, 19, 21, 22, 39, 48). All postmortem procedures were conducted in 26-wk-old mice. Mice were euthanized following a 5-h fast and tissues were harvested and either fixed in 10% formalin, snap-frozen in liquid nitrogen and stored at −80°C until analysis, or immediately used in ex vivo experiments.
Body composition and tissue weights.
The percent body fat was measured by a nuclear magnetic resonance imaging whole body composition analyzer (EchoMRI 4in1/1100; Echo Medical Systems, Houston, TX). This noninvasive measure was performed on conscious mice within 1 wk before euthanasia. Upon euthanasia, interscapular BAT, thoracic periaortic BAT, subcutaneous (inguinal) white AT, visceral (gonadal) white AT, and liver were extracted and tissue weights were collected.
Fasting blood parameters.
Plasma glucose, cholesterol, triglycerides, and non-esterified fatty acids (NEFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) using assays as per manufacturer’s guidelines. Plasma insulin concentrations were determined using a commercially available, mouse-specific ELISA (Alpco Diagnostics, Salem, NH). The homeostasis model assessment of insulin resistance (HOMA-IR) was used as a surrogate measure of hepatic insulin resistance [(fasting insulin (μU/l) × fasting glucose (mg/dl)/405.1) (36)] and indexes of adipose tissue insulin resistance (AT-IR) were calculated as the product of fasting insulin (μU/l) and fasting NEFAs (mmol/l) (33).
Histological assessments.
Formalin-fixed samples were processed through paraffin embedment, sectioned at 5 µm, and stained with hematoxylin and eosin (interscapular BAT, thoracic periaortic BAT, visceral white AT, and liver). Sections were evaluated via an Olympus BX34 photomicroscope (Olympus, Melville, NY) and images were taken via an Olympus SC30 Optical Microscope Accessory CMOS color camera. Adipocyte size was calculated from three independent regions of the same 20× and 40× objective fields for visceral white AT (100 adipocytes/animal) and BAT depots (45 adipocytes/animal; interscapular and thoracic periaortic), respectively. Briefly, cross-sectional areas of the adipocytes were obtained from perimeter tracings using ImageJ software as performed previously (65). All procedures were performed by an investigator who was blinded to the groups.
RNA extraction and quantitative real-time RT-PCR.
Interscapular and thoracic periaortic BAT samples were homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA). Total RNA was isolated according to the Qiagen’s RNeasy lipid tissue protocol and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed as previously described using the ABI StepOne Plus sequence detection system (Applied Biosystems) (41, 52). Primer sequences were designed using the NCBI Primer Design tool. All primers were purchased from IDT (Coralville, IA). GAPDH was used as housekeeping control gene. GAPDH cycle threshold (CT) was not different among the groups of animals. mRNA expression was calculated by 2ΔCT where ΔCT = GAPDH CT − gene of interest CT and presented as fold difference. mRNA levels were normalized to the female WT group which was set at 1.
Western blotting.
Protein content was measured as previously described (67). Briefly, protein samples (10 µg/lane or 4 μg/lane) were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary antibodies, including UCP1 (no. U6382, 1:1,000; Sigma-Aldrich), UCP2 (no. 89326, 1:1,000; Cell Signaling), endothelial nitric oxide synthase (eNOS; no. 610296, 1:500; BD Biosciences), and β-tubulin (no. 2146, 1:1,000; Cell Signaling). Intensity of individual protein bands were quantified using FluoroChem HD2 (AlphaView, version 3.4.0.0) and expressed as ratio to control band β-tubulin unless otherwise stated.
Liver triglycerides.
Hepatic triacylglycerol (TG) concentration was determined using a commercially available kit (Wako L-Type TG M; Wako Pure Chemical Industries, Osaka, Japan). A BioTek μQUANT microplate spectrophotometer (Biotek Instruments, Winooski, VT) was used to analyze the absorbance set at a wavelength of 600 nm with a reference wavelength at 700 nm. Data are expressed as milligrams TG per gram of liver (wet wt), as described previously (65, 67).
Ex vivo vasomotor function experiments in thoracic aortic rings.
The thoracic aorta was harvested and immediately placed in cold (4°C) Krebs physiological salt solution (PSS). Using a dissecting scope, the thoracic aorta above the diaphragm was carefully stripped of peri-adventitial BAT and sectioned into 2-mm rings. Aortic rings were then mounted in a myograph chamber (model 620M, Danish Myo Technology, Aarhus, Denmark) containing PSS gassed with 95% O2-5% CO2 at 37°C, as previously described (63). After a 30-min equilibration period, an optimal tension (12 mN) was applied and then another 30 min of equilibration followed. Dilatory responses to acetylcholine (ACh, 1 nmol/l to 10 μmol/l) and sodium nitroprusside (SNP, 1 nmol/l to 100 μmol/l) were assessed in U46619-precontracted aortic rings, as previously described (40, 63). Relaxation at each concentration was measured and expressed as percent maximum relaxation, where 100% is equivalent to loss of all tension developed in response to U-46619 (20 nmol/l).
Tissue and plasma markers of oxidative and antioxidative stress.
Interscapular BAT oxidative stress was assessed via two independent commercial kits: hydrogen peroxide (H2O2) generation (no. 102500, lot GR275597–3, Abcam) and protein carbonyls (OxiSelect protein carbonyl fluorometric assay, no. STA-307, Cell Biolabs). Superoxide oxide dismutase (SOD) activity was determined in interscapular BAT and plasma using a commercial kit (SOD assay kit, no. 706002, Caymen Chemical). All kits were performed per manufacturer’s instructions. Data are presented as the fold difference relative to WT males.
Assessment of circulating tempol levels via electron paramagnetic resonance.
To confirm that tempol administration in drinking water increased circulating levels of tempol, a subset of plasma samples was submitted to the University of Iowa Electron Spin Resonance Facility for measurement of tempol concentrations. Electron paramagnetic resonance (EPR) spectra were obtained with a Bruker EMX EPR spectrometer (Bruker BioSpin; Billerica, MA). An aqueous solution of 3-carboxy-PROXYL [3-CxP, 3-(carboxy)-2,2,5,5-tetramethyl-1-pyrrolidinyloxy; Sigma-Aldrich; CAS No. 2154–68–9}, ε234 = 2,370 M−1·cm−1 (62), was used as a standard. The samples of plasma [27 μl plasma + 3 μl of K3Fe(CN)6, final concentration 0.1 mM] were placed into a capillary tube [Hirschmann melting point tube (100 mm × 0.8 mm × 1 mm) (Sigma-Aldrich)] supported in quartz EPR sample tubes (4 mm OD, Wilmad-LabGlass, Vineland, NJ) in an ER 4119HS cavity.
To quantitate the amount of tempol in the plasma samples, only spectra of the low-field line of the nitroxide triplet were gathered. For this, EPR instrument settings were center field, 3493 G; 1,024 points with a sweep rate, 10.0 G/20.97 s; modulation amplitude, 1.5 G; microwave frequency, 9.855 GHz; incident microwave power, 20 mW; modulation frequency, 100.0 kHz; conversion time, 20.48 ms; time constant, 327.68 ms. Spectra are the sum of 25 scans. To gather the complete 3-line EPR spectra, instrument settings were center field, 3510 G; 1,024 points with a sweep rate, 60.0 G/20.97 s; modulation amplitude, 2.0 G; microwave frequency, 9.855 GHz; and incident microwave power, 20 mW; modulation frequency, 100.0 kHz; conversion time, 20.48 ms; time constant, 163.84 ms. Spectra are the result of 25 scans.
Nitroxides are readily reduced by ascorbate and other reducing agents in plasma and tissues to the EPR-silent hydroxylamine (14, 44). The oxidizing agent potassium ferricyanide (final concentration 0.1 mM) was added to the plasma samples to ensure that total tempol was measured (27).
The nitroxide 3-CxP was used as a standard. Traditional double-integration of standard and samples was employed to arrive at the concentration of tempol in the plasma. A proportionality constant to convert line height to concentration was determined for the tempol spectra and used estimate concentrations (6).
Statistical analysis.
A 2 × 2 (sex × genotype) analysis of variance (ANOVA) was used to evaluate the effects of sex and UCP1 deficiency on all dependent variables. The effects of tempol and UCP1 deficiency were also determined via 2 × 2 ANOVA with treatment (i.e., tempol vs. control) and genotype as factors. LSD post hoc tests were utilized for pairwise comparisons. All data are presented as means ± standard error (SE). For all statistical tests, significance was accepted at P ≤ 0.05. All statistical analyses were performed with SPSS V20.0.
RESULTS
Body weight and adiposity.
Upon euthanasia, 26-wk-old WT male mice were heavier, exhibited greater fat mass, white AT mass, lean mass, and liver weight compared with female mice (P < 0.05) (Table 1). Loss of UCP1 similarly increased body weight and adiposity in both sexes, whereas liver weight tended to be increased to a greater extent by UCP1 deletion in male mice (sex × genotype interaction, P = 0.057, Table 1). When expressed relative to total body mass, sex differences in subcutaneous AT and liver weight were not observed. In addition, loss of UCP1 produced a greater increase in visceral AT per gram of body weight in female relative to male mice (sex × genotype interaction, P < 0.05, Table 1).
Table 1.
Effects of sex and UCP1 ablation on body weight, body composition, and tissue weights
| Females |
Males |
|||
|---|---|---|---|---|
| Variable | WT | UCP1 KO | WT | UCP1 KO |
| Body wt, g | 22.5 ± 0.4 | 24.1 ± 0.5† | 28.8 ± 0.7* | 32.4 ± 0.6*† |
| Fat mass, g | 2.7 ± 0.2 | 3.6 ± 0.1† | 3.5 ± 0.4* | 4.3 ± 0.3*† |
| Lean mass, g | 18.5 ± 0.3 | 19.0 ± 0.3† | 23.8 ± 0.5* | 26.2 ± 0.5*†‡ |
| Body fat, % | 11.9 ± 0.6 | 15.0 ± 0.3† | 12.0 ± 0.7 | 13.2 ± 1.1† |
| Subcutaneous AT, g | 0.23 ± 0.01 | 0.29 ± 0.03† | 0.29 ± 0.01* | 0.38 ± 0.02*† |
| Subcutaneous AT/body wt, g/g | 0.010 ± 0.0004 | 0.011 ± 0.001† | 0.010 ± 0.0005 | 0.011 ± 0.0005† |
| Visceral white AT, g | 0.30 ± 0.03 | 0.41 ± 0.03† | 0.54 ± 0.04* | 0.59 ± 0.05*† |
| Visceral white AT/body wt, g/g | 0.012 ± 0.0009 | 0.017 ± 0.001 | 0.018 ± 0.001* | 0.017 ± 0.001*‡ |
| Liver weight, g | 1.03 ± 0.03 | 1.06 ± 0.03† | 1.35 ± 0.03* | 1.50 ± 0.04*† |
| Liver weight/body wt, g/g | 0.045 ± 0.0007 | 0.044 ± 0.001 | 0.047 ± 0.001 | 0.046 ± 0.001 |
Values are means ± SE.
P < 0.05, main effect of sex;
P < 0.05, main effect of genotype;
P < 0.05, sex × genotype interaction.
Markers of insulin sensitivity and blood chemistry.
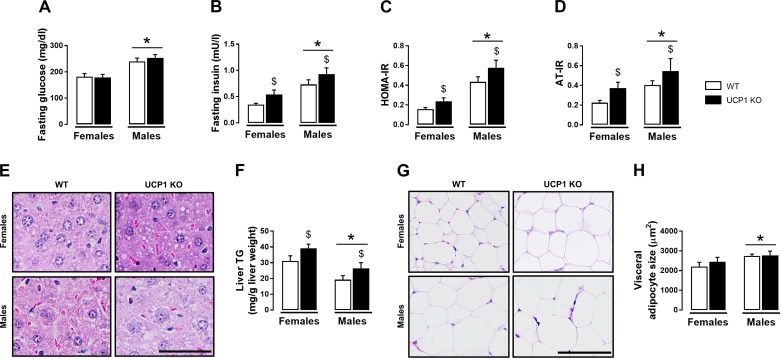
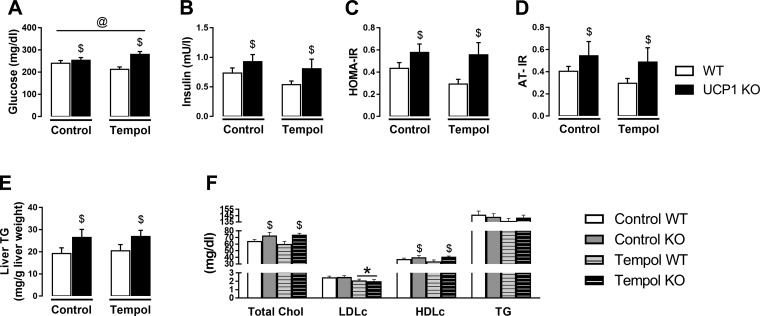
Compared with females, male mice had lower insulin sensitivity as evidenced by higher insulin levels, HOMA-IR, and AT-IR (Fig. 1, B–D). UCP1 KO mice were hyperinsulinemic and demonstrated increased HOMA-IR and AT-IR relative to WT mice (Fig. 1, B–D). However, UCP1 KO mice did not exhibit elevated fasting glucose (Fig. 1A). Total cholesterol and HDLc were elevated in male mice compared with female mice, whereas LDLc was lower in males (Table 2). A sex × genotype interaction was found for total cholesterol and HDLc such that loss of UCP1 decreased total cholesterol and HDLc in female but not male mice (Table 2). Compared with WTs, NEFA were higher in UCP1 KO mice but were not different between males and females (Table 2). Plasma TG levels did not differ by sex or genotype. However, liver TGs were higher in female mice and were similarly increased by UCP1 KO, supported by histological evidence (Fig. 1, E and F). In addition, histological examination of visceral white AT revealed increased adipocyte cell size in male mice with no main effect of genotype (Fig. 1, G and H).
Fig. 1.
Effects of sex and UCP1 ablation on whole body indexes of insulin resistance, glycemia, and liver and visceral white adiposity in WT and UCP1 KO female and male mice. A: fasting glucose. B: fasting insulin. C: HOMA-IR. D: AT-IR. E: representative H&E histology images (40× objective) of liver., F: liver TG. G: representative H&E histology images (20× objective) of visceral white AT. H: cell size. Data are expressed as means ± SE. AT, adipose tissue; WT, wild-type; HOMA-IR, homeostasis model assessment of insulin resistance; AT-IR, adipose tissue insulin resistance; TG, triglycerides. *P < 0.05, main effect of sex; $P < 0.05, main effect of genotype. No significant sex × genotype interactions were present. n = 9–13/group.
Table 2.
Effects of sex and UCP1 ablation blood lipids
| Females |
Males |
|||
|---|---|---|---|---|
| Variable | WT | UCP1 KO | WT | UCP1 KO |
| Total cholesterol, mg/dl | 50.4 ± 1.5 | 41.1 ± 1.9 | 64.6 ± 2.2* | 73.0 ± 4.4*‡ |
| HDLc, mg/dl | 28.5 ± 1.0 | 22.9 ± 1.1 | 37.4 ± 1.3* | 40.2 ± 2.4*‡ |
| LDLc, mg/dl | 3.6 ± 0.3 | 3.0 ± 0.1 | 2.4 ± 0.1* | 2.5 ± 0.1* |
| Triglycerides, mg/dl | 134.8 ± 8.8 | 131.3 ± 4.3 | 146.4 ± 5.7 | 143.0 ± 5.1 |
| NEFA, mmol/l | 0.63 ± 0.04 | 0.68 ± 0.04† | 0.56 ± 0.03 | 0.63 ± 0.03† |
Values are means ± SE.
P < 0.05, main effect of sex;
P < 0.05, main effect of genotype;
P < 0.05, sex × genotype interaction.
Characterization of BAT phenotype.
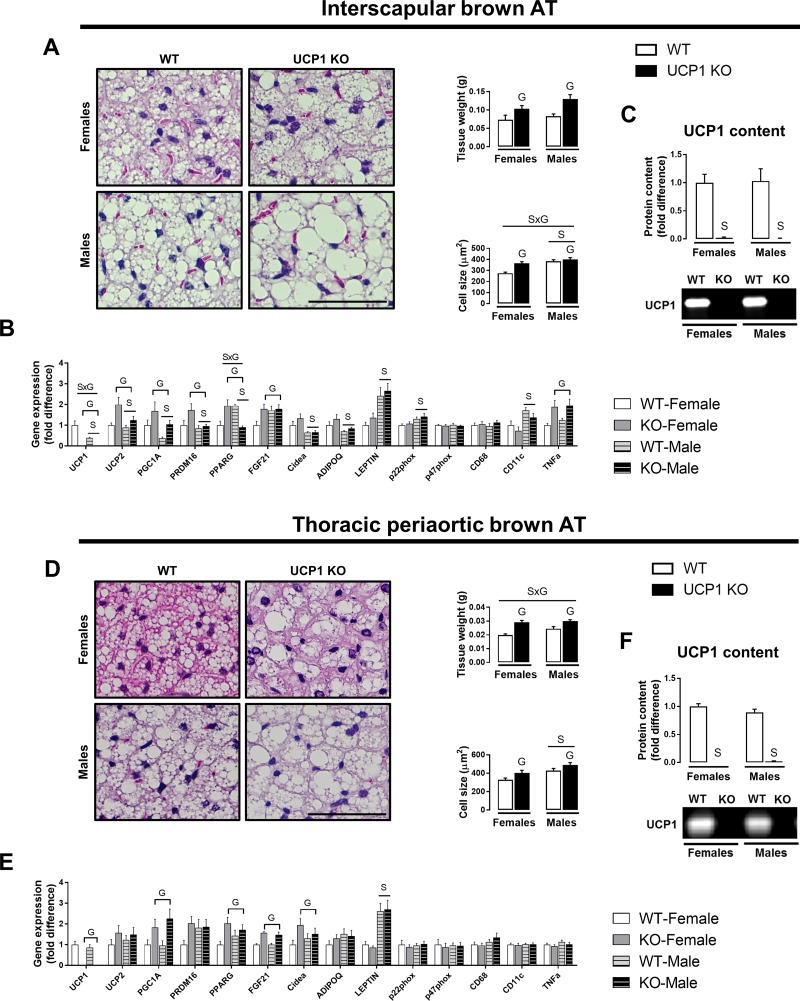
On average, compared with males, female mice exhibited smaller adipocyte size in interscapular and thoracic periaortic BAT, despite no differences in interscapular BAT and thoracic periaortic BAT weights (Fig. 2, A and D). UCP1 ablation increased thoracic periaortic BAT weight by 46.7% in females and 22.4% in males (sex × genotype interaction, P < 0.05), whereas the increase in interscapular BAT weight was similar between females and males (Fig. 2, A and D). The significant sex × genotype interaction in periaortic BAT weight and the main effect of genotype in interscapular BAT weight were also present when data were expressed relative to body mass. However, UCP1 deficiency caused a greater increase in interscapular BAT cell size in females compared with males (sex × genotype interaction, Fig. 2A). In addition, UCP1 ablation caused whitening of these respective fat pads in both sexes (Fig. 2, A and D). Compared with males, female mice demonstrated higher expression of multiple browning-related genes (i.e., UCP1, UCP2, PGC1α, PRDM16, Cidea) and higher adiponectin expression. In contrast, relative to females, male mice had higher expression of leptin in both BAT and thoracic periaortic BAT, whereas p22phox was elevated in BAT only (Fig. 2, B and E). Importantly, despite higher UCP1 mRNA expression in female vs. male interscapular BAT, we did not find significant sex differences in interscapular or thoracic periaortic BAT UCP1 protein content (Fig. 2, C and F).
Fig. 2.
Effects of sex and UCP1 ablation on interscapular and thoracic periaortic BAT phenotype in WT and UCP1 KO female and male mice. A: representative H&E histology images (20× objective) with AT weight and cell size. B: gene expression. C: UCP1 protein content. D: representative H&E histology images (20× objective) with AT weight and cell size. E: gene expression. F: UCP1 protein content; 10 μg of protein was loaded per lane. Data are expressed as means ± SE. AT, adipose tissue; WT, wild-type; KO, knockout. SP < 0.05, main effect of sex. GP < 0.05, main effect of genotype; S×GP < 0.05, sex × genotype interaction. n = 9–13/group.
Aortic function and thoracic aortic protein content.
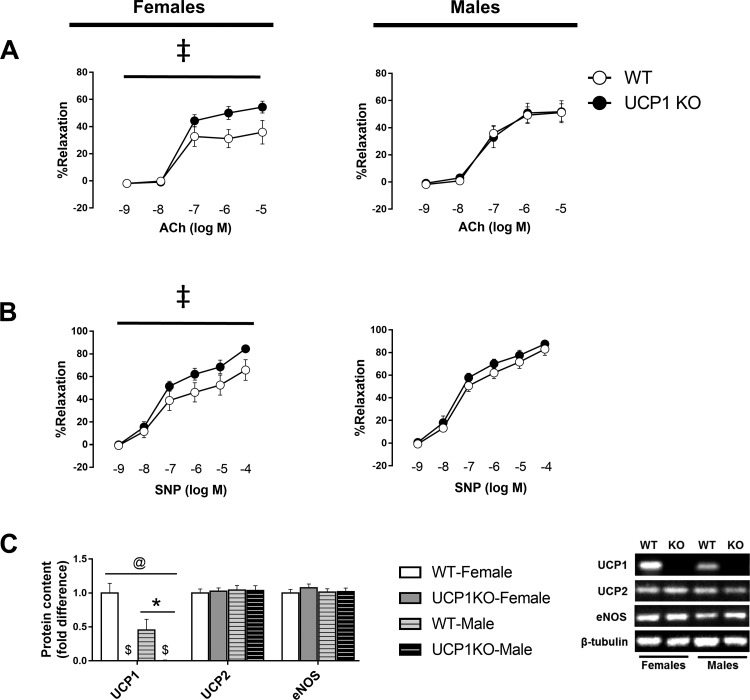
Confirming our model of whole body UCP1 deletion, aortic UCP1 protein levels were completely absent in UCP1 KO mice (Fig. 3C). Notably, we found significantly greater aortic UCP1 protein abundance in WT females compared with males (Fig. 3C). In addition, both endothelium-dependent and -independent vasorelaxation were enhanced in UCP1 KO female mice, whereas no effect of UCP1 ablation was noted in male mice (Fig. 3, A and B). These changes in vasomotor function were not associated with differences in eNOS or UCP2 protein content (Fig. 3C).
Fig. 3.
Effects of sex and UCP1 ablation on vascular function, arterial stiffness, and thoracic aorta protein content. Ex vivo vasorelaxation in response to increasing doses of acetylcholine (A) and sodium nitroprusside (B). C: protein levels of UCP1 and UCP2 in thoracic aorta; 4 μg of protein was loaded per lane. Data are expressed as means ± SE. WT, wild-type; KO, knockout; ACh, acetylcholine; SNP, sodium nitroprusside. *P < 0.05, main effect of sex; $P < 0.05, main effect of genotype. Using a dissecting scope, care was taken to completely strip periadventitial BAT from thoracic aortae. @P < 0.05, sex × genotype interaction. ‡P < 0.05, dose × genotype interaction. n = 9–13/group.
Lack of UCP1 does not increase markers of oxidative stress in BAT, and free radical scavenger, tempol, does not prevent UCP1 deficiency-induced metabolic dysfunction.
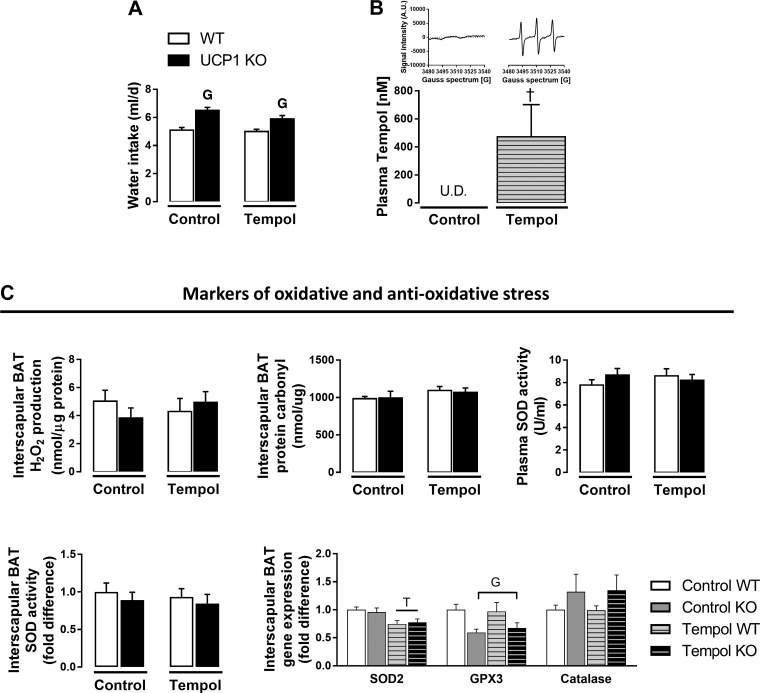
Tempol was significantly elevated in plasma indicated by an increase in EPR spectra (Fig. 4, A and B). Water intake was higher in UCP1 KO mice but did not differ by treatment (Fig. 4A). UCP1 KO animals exhibited increased body weight, lean mass, subcutaneous AT, and liver weight regardless of tempol treatment, whereas a tendency for a genotype × treatment interaction was demonstrated for interscapular BAT (P = 0.056), such that tempol attenuated a UCP1 KO-induced increase in interscapular BAT mass (Table 3). However, neither tempol nor UCP1 ablation affected markers of oxidative stress in interscapular BAT (Fig. 4C). Data on antioxidant markers are also summarized in Fig. 4C. As shown, mRNA expression of mitochondrial superoxide dismutase (SOD2) in interscapular BAT was decreased in tempol-treated animals. However, this tempol effect was not associated with a reduction in SOD activity assessed in interscapular BAT or plasma (Fig. 4C). UCP1 deletion attenuated GPX3 mRNA expression in interscapular BAT, with no effect of tempol. Furthermore, we found no significant genotype or treatment effects on catalase mRNA expression in interscapular BAT (Fig. 4C). Similarly, no differences in fat mass, percent body fat, visceral fat pad mass, or thoracic periaortic BAT mass were evident by genotype or treatment (Table 3). Despite the increase in circulating tempol levels, tempol did not prevent UCP1 deficiency-induced hyperinsulinemia, hyperglycemia, increased markers of IR (HOMA-IR and AT-IR), or liver steatosis (main effect of genotype, Fig. 5, A–E). Likewise, UCP1 KO-induced increase in total cholesterol and HDLc were not attenuated by tempol; however, LDLc was lower in tempol-treated animals (Fig. 5F).
Fig. 4.
Effects of tempol administration for 8 wk and UCP1 ablation on oxidative and antioxidative stress. A: water intake. B: plasma tempol levels and representative EPR spectra (inset). C: interscapular BAT H2O2 generation, protein carbonyls, plasma and interscapular BAT SOD activity, and antioxidant mRNA markers in interscapular BAT. Data are expressed as means ± SE. BAT, brown adipose tissue; WT, wild-type; KO, knockout; H2O2, hydrogen peroxide. GP < 0.05 main effect of genotype. †P < 0.05 main effect of treatment. N = 9–13/group for water intake and oxidative stress markers. A subset of animals was used for analysis of circulating levels of tempol (controls, n = 8; tempol, n = 8).
Table 3.
Effects of tempol and UCP1 ablation on body weight, body composition, and tissue weights
| Control |
Tempol |
|||
|---|---|---|---|---|
| Variable | WT | UCP1 KO | WT | UCP1 KO |
| Body wt, g | 28.8 ± 0.7 | 32.4 ± 0.6† | 28.6 ± 0.8 | 31.5 ± 0.6† |
| Fat mass, g | 3.5 ± 0.4 | 4.3 ± 0.3 | 3.9 ± 0.4 | 4.2 ± 0.3 |
| Lean mass, g | 23.8 ± 0.5 | 26.2 ± 0.5† | 23.3 ± 0.4 | 25.8 ± 0.5† |
| Body fat, % | 12.0 ± 0.7 | 13.2 ± 1.1 | 13.5 ± 1.2 | 13.4 ± 1.0 |
| Subcutaneous AT, g | 0.29 ± 0.01 | 0.38 ± 0.02† | 0.30 ± 0.02 | 0.31 ± 0.02† |
| Visceral white AT, g | 0.54 ± 0.04 | 0.59 ± 0.05 | 0.58 ± 0.07 | 0.59 ± 0.04 |
| Interscapular BAT, g | 0.08 ± 0.01 | 0.13 ± 0.01† | 0.10 ± 0.01 | 0.11 ± 0.01† |
| Thoracic periaortic BAT, g | 0.02 ± 0.001 | 0.03 ± 0.001 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| Liver wt, g | 1.35 ± 0.03 | 1.50 ± 0.04† | 1.3 ± 0.05 | 1.5 ± 0.03† |
Values are means ± SE.
P < 0.05, main effect of genotype.
Fig. 5.
Effects of tempol administration for 8 wk and UCP1 ablation on insulin resistance, fatty liver, and blood lipids in WT and UCP1 KO mice. A: fasting glucose. B: fasting insulin. C: HOMA-IR. D: AT-IR. E: liver TG. F: blood lipids. Data are expressed as means ± SE. AT, adipose tissue; WT, wild-type; KO, knockout; HOMA-IR, homeostasis model assessment of insulin resistance; AT-IR, adipose tissue insulin resistance; TG, triglycerides. *P < 0.05, main effect of treatment; $P < 0.05, main effect of genotype; @P < 0.05, treatment × genotype interaction. n = 9–13/group.
DISCUSSION
In the present study, we found that in normal chow-fed mice housed at 25°C, constitutive expression of UCP1 protein in BAT depots is similar between females and males, despite females having greater UCP1 gene expression in those depots, and that sex differences in insulin sensitivity are not UCP1 dependent. That is, ablation of UCP1 does not fully normalize differences in insulin sensitivity between females and males. In contrast, we show that thoracic aortic UCP1 protein content is greater in females than males and that, unexpectedly, deletion of UCP1 actually enhances aortic vasomotor function in females only. Last, contrary to our hypothesis, lack of UCP1 does not increase markers of oxidative stress in BAT in regular chow-fed mice, and the free radical scavenger tempol does not prevent UCP1 deficiency-induced metabolic dysfunction, thus indicating that oxidative stress may not contribute to the metabolic derangements instigated by UCP1 deficiency under normal dietary conditions. Importantly, future research should establish whether, in the setting of obesity, metabolic dysfunction exacerbated by loss of UCP1 is attributed to increased oxidative stress.
Prior studies indicate that females are more insulin sensitive than males (1, 37, 64). We tested the hypothesis that one factor contributing to the increased insulin sensitivity phenotype in females could be their greater expression of UCP1 and BAT content, as previously reported by others (12, 16, 29, 47, 49, 51). Indeed, BAT from female rodents has larger mitochondria and cristae density than males, and thus female rodents may exhibit greater BAT mitochondrial oxidative capacity (49). In accordance with others (1, 37, 64), we found that insulin sensitivity was greater in females than males; however, we did not find sex differences in BAT UCP1 protein content. Moreover, loss of UCP1 increased hyperinsulinemia, AT-IR, and liver steatosis similarly between females and males, suggesting that sex differences in insulin sensitivity are not fully dependent on UCP1. Hence, UCP1 appears to be required for maintenance of insulin sensitivity independent of sex in mice housed at 25°C. Of note, previous studies reporting greater BAT expression of UCP1 in females, housed animals at 20–22°C or colder temperatures (47, 49, 51, 60), exposing animals to greater levels of thermostress compared with the current investigation. Along these lines, pharmacological induction of UCP1, via the β-3 agonist (CL316,243), is greater in female mice compared with males (30), supporting the premise that females, when stimulated, are in fact more responsive than males to the recruitment of brown adipocytes and activation of BAT. Another stimulus known to increase UCP1 expression is an obesogenic diet, which may be related to increased sympathetic nerve activity (4, 51, 54). In this context, compared with males, female rodents fed a high-fat diet gain less weight and exhibit higher expression of BAT proteins involved in thermogenesis, and oxidative metabolism, while proteins involved in lipid accumulation are reduced (10). It is reasonable to posit that a stressor (metabolic, environmental, or pharmacological) is required to reveal UCP1’s contribution to sex differences in insulin sensitivity. Indeed, additional studies are warranted to examine the hypothesis that in the setting of diet-induced obesity or cold stimulation, greater induction of UCP1 in females is an underlying mechanism by which females are more insulin sensitive than their male counterparts.
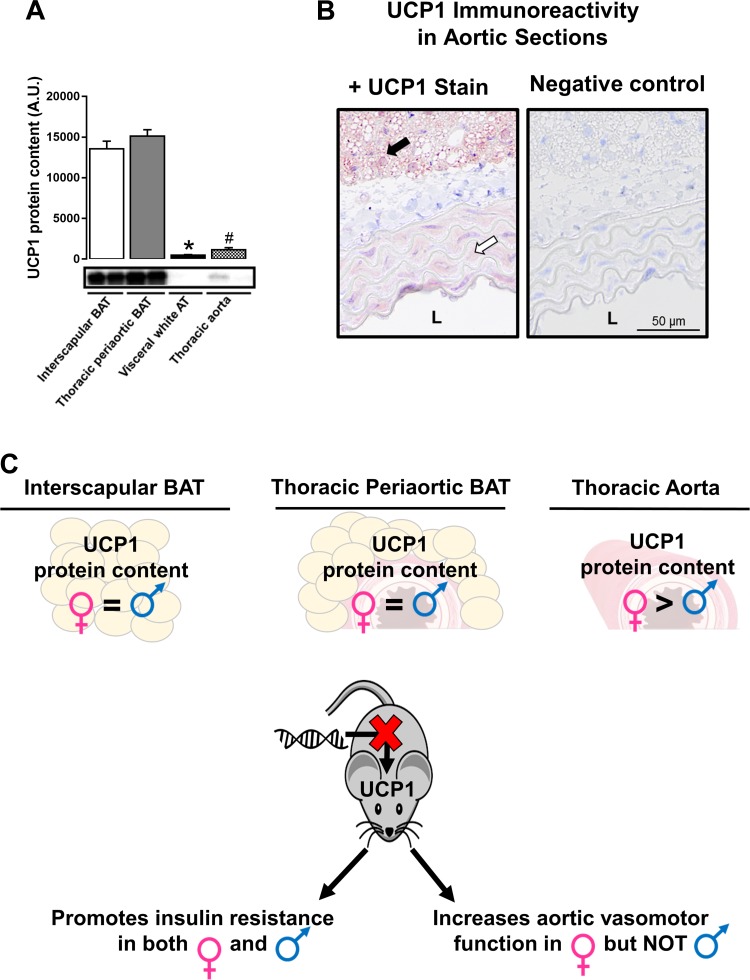
Although the role of UCP1 in regulating energy homeostasis is largely appreciated, its contribution to modulating vascular function is less understood. In the current investigation, we provide evidence that UCP1 protein is expressed in vascular tissues (i.e., aorta) although, as expected, the expression levels are distinctly lower than that in thoracic periaortic and interscapular BAT (Fig. 6, A and B). To our knowledge, very few reports are available demonstrating expression of UCP1 in vascular cells (15). Notably, we report for the first time that aortic UCP1 protein levels are twofold higher in females than males (Fig. 3C), and this elevation in aortic UCP1 expression was accompanied by reduced aortic vasomotor function. Of significance, compared with males, deletion of UCP1 enhanced both endothelium- and smooth muscle-dependent aortic vasorelaxation in female mice such that sex differences were normalized, indicating a UCP1-dependent sexual dimorphism on aortic function. This finding that loss of UCP1 augmented aortic vasorelaxation is especially notable given that UCP1-deficient mice exhibited whitening of periaortic BAT and evidence of metabolic dysfunction. Since previous work indicates that UCP2 is vascular-protective (26, 32, 59), we postulated that enhanced aortic vasomotor function with loss of UCP1 could be attributed to a compensatory upregulation of UCP2 particularly in females. However, contrary to this idea, we found that aortic UCP2 protein content was neither affected by UCP1 ablation nor different between males and females. Likewise, no differences in aortic expression of eNOS was noted between groups. Thus the mechanisms by which deletion of UCP1 enhances vasomotor function in females remain to be determined.
Fig. 6.
Schematic summary of major findings. A: tissue-specific expression of UCP1. B: histology images (20× objective) of aortic sections and adjacent periaortic BAT stained for UCP1 identified as the maroon color or negative control. C: the dichotomous role of UCP1 deletion on metabolic and vascular function in regular chow-fed diet mice housed at 25°C; 4 μg of protein was loaded per lane. In A, using a dissecting scope, care was taken to completely strip peri-adventitial BAT from thoracic aortas. In B, black arrow indicates strong positive immunoreactivity of UCP1 in periaortic BAT connected to the aortic wall. White arrow indicates modest positive immunoreactivity of UCP1 in the media of the aortic wall. L, lumen. *P < 0.05 vs. all groups; #P < 0.05 vs. interscapular and thoracic periaortic BAT.
Differences in sex hormones are thought to contribute to the dimorphic responses often observed in BAT function and UCP1 expression between males and females (reviewed in 46). In this context, it is possible that the sex-dependent link between UCP1 and vasomotor function reported herein is related to differences in sex hormones. To begin to address this hypothesis, we assessed ex vivo aortic vasorelaxation in ovariectomized WT (n = 9) and UCP1 KO (n = 10) mice from a paralleled study (i.e., age-matched female littermates). Ovariectomy was performed 14 wk before euthanasia. UCP1 KO ovariectomized mice also exhibited improved aortic vasorelaxation in response to ACh or SNP compared with WT ovariectomized mice (data not shown), and the magnitude of improvement with loss of UCP1 was the same as that we report in ovary-intact females (Fig. 3). Accordingly, we conclude that ovarian hormones may not fully account for the sex-dependent link between UCP1 and vasomotor function.
Currently, the ontology of UCP1 expression and function in vascular cells is unknown. Despite widely reported metabolic improvements caused by cold exposure, a recent report demonstrated that daily exposure to 5°C in mice increases their susceptibility to atherosclerotic lesions, an effect that was driven by UCP1-induced lipolysis (18). This cold-induced response increased cholesterol synthesis and hypercholesterolemia, which may have contributed to vascular disease (18). In this regard, we found that deletion of UCP1 improved aortic vasomotor function and reduced circulating cholesterol levels in females but not males. Furthermore, our data are also consistent with work by Bernal-Mizrachi et al. (2), who demonstrated that transgenic overexpression of UCP1 in aortic smooth muscle cells increased blood pressure and atherosclerotic lesions, and decreased nitric oxide bioavailability in mice. Similarly, respiratory uncoupling is increased in aortas from atherosclerosis-prone pigeons compared with healthy controls (53). Moreover, administration of the mitochondrial uncoupler, dinitrophenol, increased smooth muscle contraction in rabbits (45), which is implicated in the pathogenesis of hypertension. Together, these data suggest that mitochondrial inefficiency may be detrimental to vascular health. Our findings provide novel evidence that UCP1 is expressed in vascular tissues and differentially regulates vasomotor function in a sex-dependent fashion. Further investigation is warranted to determine the mechanisms by which UCP1 modulates vascular function.
The activation of uncoupling proteins has been postulated to attenuate superoxide generation, thus serving as endogenous antioxidants (9, 38, 69). Accordingly, we hypothesized that metabolic dysfunction induced by loss of UCP1 would in part be attributable to increased oxidative stress in UCP1-expressing tissues. In contrast to our hypothesis, deletion of UCP1 did not increase markers of oxidative stress in BAT and, furthermore, treatment with tempol, an established ROS scavenger (19, 39, 48), did not prevent UCP1 ablation-induced hyperinsulinemia, liver steatosis, or increased whitening of BAT depots despite a notable increase in circulating tempol levels. This lack of induction of oxidative stress in UCP1-deficient mice, combined with the lack of restorative effects of the ROS scavenger tempol, suggests that metabolic derangements associated with loss of UCP1 alone are not attributable to increased oxidative stress. Further work is needed to examine if oxidative stress is implicated in mediating metabolic dysfunction in UCP1-null mice exposed to an obesogenic environment (e.g., Western diet). In addition, more research is needed to uncover the mechanisms by which UCP1 regulates metabolic function independent of changes in adiposity (67).
In conclusion, we report a dichotomous role of UCP1 in metabolic and vascular tissues (Fig. 6C), suggesting that caution should be exercised when considering UCP1 as a potential therapeutic target. Moreover, results from the present study suggest that the metabolic disruptions caused by UCP1 ablation may not be contingent upon increased oxidative stress.
GRANTS
This study was supported in part by grants from the Cardiometabolic Disease Research Foundation (J. Padilla), Sears Trust Research Foundation (J. Padilla), National Institutes of Health (NIH) (Grants K01-HL-125503 and R21-DK-105368 to J. Padilla), University of Missouri (MU) Research Council (V. J. Vieira-Potter), MU Richard Wallace Faculty Incentive Grant (V. J. Vieira-Potter), NIH Initiative for Maximizing Student Diversity (IMSD) EXPRESS Fellows Program R25GM056901 (J. R. Ball and T. L. Gaines), and American Egg Board Fellowship no. 00050021 (N. C. Winn). The content is solely the responsibility of the authors and does not represent views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.C.W., Z.I.G., M.L.G., M.L.W., R.J.W., S.L.C., J.R.B., T.L.G., and J.P. performed experiments; N.C.W., Z.I.G., M.L.G., and J.P. analyzed data; N.C.W., Z.I.G., M.L.G., H.S.S., V.J.V.-P., and J.P. interpreted results of experiments; N.C.W. prepared figures; N.C.W. drafted manuscript; N.C.W., Z.I.G., M.L.G., M.L.W., S.L.C., J.A.K., H.S.S., V.J.V.-P., and J.P. edited and revised manuscript; N.C.W., Z.I.G., M.L.G., M.L.W., R.J.W., S.L.C., J.R.B., T.L.G., N.G.K., J.A.K., H.S.S., V.J.V.-P., and J.P. approved final version of manuscript; M.L.G., N.G.K., H.S.S., V.J.V.-P., and J.P. conceived and designed research.
ACKNOWLEDGMENTS
We greatly acknowledge D. Szy for technical assistance. The ESR Facility at The University of Iowa provided invaluable support and was supported in part by the Holden Comprehensive Cancer Center (P30CA086862).
REFERENCES
- 1.Amengual-Cladera E, Lladó I, Gianotti M, Proenza AM. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism 61: 1108–1117, 2012. doi: 10.1016/j.metabol.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435: 502–506, 2005. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 3.Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte EE, Haman F, Richard D, Carpentier AC. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64: 2388–2397, 2015. doi: 10.2337/db14-1651. [DOI] [PubMed] [Google Scholar]
- 4.Brooks SL, Rothwell NJ, Stock MJ, Goodbody AE, Trayhurn P. Increased proton conductance pathway in brown adipose tissue mitochondria of rats exhibiting diet-induced t hermogenesis. Nature 286: 274–276, 1980. doi: 10.1038/286274a0. [DOI] [PubMed] [Google Scholar]
- 5.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 34: 1621–1630, 2014. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buettner GR. Ascorbate oxidation: UV absorbance of ascorbate and ESR spectroscopy of the ascorbyl radical as assays for iron. Free Radic Res Commun 10: 5–9, 1990. doi: 10.3109/10715769009145927. [DOI] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes 34, Suppl 1: S7–S16, 2010. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 9.Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, Casteilla L. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63: 3253–3265, 2014. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 10.Choi DK, Oh TS, Choi JW, Mukherjee R, Wang X, Liu H, Yun JW. Gender difference in proteome of brown adipose tissues between male and female rats exposed to a high fat diet. Cell Physiol Biochem 28: 933–948, 2011. doi: 10.1159/000335807. [DOI] [PubMed] [Google Scholar]
- 11.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 44: 1267–1270, 2003. [PubMed] [Google Scholar]
- 13.Contreras GA, Thelen K, Ayala-Lopez N, Watts SW. The distribution and adipogenic potential of perivascular adipose tissue adipocyte progenitors is dependent on sexual dimorphism and vessel location. Physiol Rep 4: e12993, 2016. doi: 10.14814/phy2.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couet WR, Brasch RC, Sosnovsky G, Tozer TN. Factors affecting nitroxide reduction in ascorbate solution and tissue homogenates. Magn Reson Imaging 3: 83–88, 1985. doi: 10.1016/0730-725X(85)90012-8. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi Ren, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 83: 807–816, 2006. doi: 10.1016/j.exer.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding W, Wang B, Zhang M, Gu Y. Tempol, a Superoxide dismutase-mimetic drug, ameliorates progression of renal disease in CKD mice. Cell Physiol Biochem 36: 2170–2182, 2015. doi: 10.1159/000430183. [DOI] [PubMed] [Google Scholar]
- 18.Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, Zhang C, Seki T, Hosaka K, Wahlberg E, Yang J, Zhang L, Länne T, Sun B, Li X, Liu Y, Zhang Y, Cao Y. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 18: 118–129, 2013. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebenezer PJ, Mariappan N, Elks CM, Haque M, Francis J. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity (Silver Spring) 17: 1994–2002, 2009. doi: 10.1038/oby.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437, 2011. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gálvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64, 2008. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 24.Gaskill BN, Garner JP. Letter-to-the-editor on “Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans”. Mol Metab 3: 335–336, 2013. doi: 10.1016/j.molmet.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6, Suppl 1: 60–75, 2009. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Hernández A, Perdomo L, de las Heras N, Beneit N, Escribano O, Otero YF, Guillén C, Díaz-Castroverde S, Gozalbo-López B, Cachofeiro V, Lahera V, Benito M. Antagonistic effect of TNF-alpha and insulin on uncoupling protein 2 (UCP-2) expression and vascular damage. Cardiovasc Diabetol 13: 108, 2014. doi: 10.1186/s12933-014-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn SM, Sullivan FJ, DeLuca AM, Krishna CM, Wersto N, Venzon D, Russo A, Mitchell JB. Evaluation of tempol radioprotection in a murine tumor model. Free Radic Biol Med 22: 1211–1216, 1997. doi: 10.1016/S0891-5849(96)00556-4. [DOI] [PubMed] [Google Scholar]
- 28.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 51: 993–999, 2011. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Krynyckyi BR, Machac J, Kim CK. Temporal relation between temperature change and FDG uptake in brown adipose tissue. Eur J Nucl Med Mol Imaging 35: 984–989, 2008. doi: 10.1007/s00259-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 30.Kim SN, Jung YS, Kwon HJ, Seong JK, Granneman JG, Lee YH. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol Sex Differ 7: 67, 2016. doi: 10.1186/s13293-016-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopecký J, Rossmeisl M, Hodný Z, Syrový I, Horáková M, Kolárová P. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol Endocrinol Metab 270: E776–E786, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, Huang Y. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal 21: 1571–1581, 2014. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 34.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition 26: 686–693, 2010. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 34: 949–959, 2010. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6: 14, 2015. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oelkrug R, Goetze N, Meyer CW, Jastroch M. Antioxidant properties of UCP1 are evolutionarily conserved in mammals and buffer mitochondrial reactive oxygen species. Free Radic Biol Med 77: 210–216, 2014. doi: 10.1016/j.freeradbiomed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Onuma S, Nakanishi K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism 53: 1305–1308, 2004. doi: 10.1016/j.metabol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Padilla J, Jenkins NT, Lee S, Zhang H, Cui J, Zuidema MY, Zhang C, Hill MA, Perfield JW II, Ibdah JA, Booth FW, Davis JW, Laughlin MH, Rector RS. Vascular transcriptional alterations produced by juvenile obesity in Ossabaw swine. Physiol Genomics 45: 434–446, 2013. doi: 10.1152/physiolgenomics.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padilla J, Jenkins NT, Roberts MD, Arce-Esquivel AA, Martin JS, Laughlin MH, Booth FW. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp Physiol 98: 337–347, 2013. doi: 10.1113/expphysiol.2012.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 65: 1238–1246, 2016. doi: 10.1016/j.metabol.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paletta JT, Pink M, Foley B, Rajca S, Rajca A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org Lett 14: 5322–5325, 2012. doi: 10.1021/ol302506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettersson G. Effect of dinitrophenol and anoxia on isometric tension in rabbit colon smooth muscle. Acta Pharmacol Toxicol (Copenh) 57: 184–189, 1985. doi: 10.1111/bcpt.1985.57.3.184. [DOI] [PubMed] [Google Scholar]
- 46.Quarta C, Mazza R, Pasquali R, Pagotto U. Role of sex hormones in modulation of brown adipose tissue activity. J Mol Endocrinol 49: R1–R7, 2012. doi: 10.1530/JME-12-0043. [DOI] [PubMed] [Google Scholar]
- 47.Quevedo S, Roca P, Picó C, Palou A. Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflugers Arch 436: 689–695, 1998. doi: 10.1007/s004240050690. [DOI] [PubMed] [Google Scholar]
- 48.Rafikova O, Salah EM, Tofovic SP. Renal and metabolic effects of tempol in obese ZSF1 rats–distinct role for superoxide and hydrogen peroxide in diabetic renal injury. Metabolism 57: 1434–1444, 2008. doi: 10.1016/j.metabol.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem 277: 42958–42963, 2002. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez AM, Quevedo-Coli S, Roca P, Palou A. Sex-dependent dietary obesity, induction of UCPs, and leptin expression in rat adipose tissues. Obes Res 9: 579–588, 2001. doi: 10.1038/oby.2001.75. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez E, Monjo M, Rodríguez-Cuenca S, Pujol E, Amengual B, Roca P, Palou A. Sexual dimorphism in the adrenergic control of rat brown adipose tissue response to overfeeding. Pflugers Arch 442: 396–403, 2001. doi: 10.1007/s004240100556. [DOI] [PubMed] [Google Scholar]
- 52.Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol 298: H1991–H2000, 2010. doi: 10.1152/ajpheart.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santerre RF, Nicolosi RJ, Smith SC. Respiratory control in preatherosclerotic susceptible and resistant pigeon aortas. Exp Mol Pathol 20: 397–406, 1974. doi: 10.1016/0014-4800(74)90069-0. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Invest 72: 361–370, 1983. doi: 10.1172/JCI110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell 163: 560–569, 2015. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2: 5–9, 2012. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speakman JR, Keijer J. Not so nuanced: Reply to the comments of Gaskill and Garner on ‘Not so hot: Optimal housing temperatures for mice to mimic the environment of humans’. Mol Metab 3: 337, 2013. doi: 10.1016/j.molmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, Cheang WS, Wang Y, Yao X, Huang Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res 110: 1211–1216, 2012. doi: 10.1161/CIRCRESAHA.111.262170. [DOI] [PubMed] [Google Scholar]
- 60.Valle A, Santandreu FM, García-Palmer FJ, Roca P, Oliver J. The serum levels of 17beta-estradiol, progesterone and triiodothyronine correlate with brown adipose tissue thermogenic parameters during aging. Cell Physiol Biochem 22: 337–346, 2008. doi: 10.1159/000149812. [DOI] [PubMed] [Google Scholar]
- 61.Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5: 241, 2015. doi: 10.3389/fendo.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venkataraman S, Martin SM, Schafer FQ, Buettner GR. Detailed methods for the quantification of nitric oxide in aqueous solutions using either an oxygen monitor or EPR. Free Radic Biol Med 29: 580–585, 2000. doi: 10.1016/S0891-5849(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 63.Vieira-Potter VJ, Lee S, Bayless DS, Scroggins RJ, Welly RJ, Fleming NJ, Smith TN, Meers GM, Hill MA, Rector RS, Padilla J. Disconnect between adipose tissue inflammation and cardiometabolic dysfunction in Ossabaw pigs. Obesity (Silver Spring) 23: 2421–2429, 2015. doi: 10.1002/oby.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vital P, Larrieta E, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol 190: 425–432, 2006. doi: 10.1677/joe.1.06596. [DOI] [PubMed] [Google Scholar]
- 65.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, Li B, Wang W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One 10: e0123795, 2015. doi: 10.1371/journal.pone.0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 312: R74–R84, 2017. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao C, Goldgof M, Gavrilova O, Reitman ML. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring) 23: 1450–1459, 2015. doi: 10.1002/oby.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou SS, Cao LL, Xu WD, Cao J, Zhao ZJ. Effect of temperature on oxidative stress, antioxidant levels and uncoupling protein expression in striped hamsters. Comp Biochem Physiol A Mol Integr Physiol 189: 84–90, 2015. doi: 10.1016/j.cbpa.2015.07.017. [DOI] [PubMed] [Google Scholar]