Abstract
Although the rate of fatty acid release from adipose tissue into the systemic circulation is very high in most obese adults, some obese adults maintain relatively low rates of fatty acid release, which helps protect them against the development of systemic insulin resistance. The primary aim of this study was to identify factors in adipose tissue that may underlie low vs. high rates of fatty acid mobilization in a relatively homogeneous cohort of obese adults. We measured systemic fatty acid rate of appearance (FA Ra) via 13C-palmitate isotope dilution, and we obtained subcutaneous abdominal adipose tissue samples from 30 obese adults (BMI: 38 ± 1 kg/m2, age: 30 ± 2 yr) after an overnight fast. We then measured insulin sensitivity using a hyperinsulinemic-euglycemic clamp. Confirming our previous work, insulin sensitivity was inversely proportional to FA Ra (R2 = 0.50; P < 0.001). Immunoblot analysis of subcutaneous adipose tissue samples revealed that, compared with obese adults with high FA Ra, those with low FA Ra had lower markers of lipase activation and higher abundance of glycerol-3-phosphate acyltransferase, which is a primary enzyme for fatty acid esterification. Microarray and pathway analysis provided evidence of lower fibrosis and lower SAPK/JNK pathway activation in obese adults with low FA Ra compared with those with high FA Ra. Our findings suggest that alterations in factors regulating triglyceride storage in adipose tissue, along with lower fibrosis and inflammatory pathway activation, may underlie maintenance of a relatively low FA Ra in obesity, which may help protect against the development of insulin resistance.
Keywords: adipose tissue, fatty acid metabolism, healthy obesity, insulin resistance
obesity is associated with insulin resistance, which is central to the development of many cardiometabolic diseases. However, up to one-third of obese adults remain metabolically healthy (i.e., not insulin resistant) (52); these “insulin-sensitive” obese adults have fewer metabolic health complications, and their mortality rates are similar to lean, healthy individuals (6, 19). Although it remains unclear why some obese adults remain insulin sensitive, mounting evidence suggests adipose tissue plays an important role.
Much of the insulin resistance observed in obesity is a consequence of excessive release of fatty acids into systemic circulation (fatty acid mobilization, FA Ra) and the resultant ectopic lipid deposition that disrupts insulin signaling in peripheral tissues. In fact, several studies report profound insulin resistance in healthy, lean subjects after short-term lipid and heparin infusion to mimic the high FA Ra found in obesity (24, 25). Conversely, drugs that lower systemic fatty acid availability in obese adults can reverse insulin resistance (3, 41). Our laboratory and others have demonstrated that the degree of FA Ra dictates the degree of insulin resistance in obesity and that obese adults who maintain a relatively low basal FA Ra remain insulin sensitive (28, 50). However, although it is clear that the rate of FA Ra from adipose tissue can greatly impact insulin resistance, little is known about factors that may help sequester excess fatty acids in adipose tissue, and, thereby, limit ectopic lipid deposition.
The accumulation of visceral adipose tissue is often linked with the severity of cardiometabolic disease risk (40); however, the excess accumulation of visceral fat most likely results from the subcutaneous adipose tissue’s inability to effectively store excess nutrients. Nielsen et al. (33) demonstrated that ~90% of circulating fatty acids are derived from subcutaneous adipose tissue, with nearly 70% of these fatty acids coming from abdominal subcutaneous adipose tissue. Moreover, despite the anatomical proximity of visceral adipose tissue to the liver, the vast majority of fatty acids in the hepatic circulation are also derived from abdominal subcutaneous adipose tissue (33). Human studies using deuterated water to track long-term turnover and storage of triacylglycerol (TG) found that TG synthesis and storage are impaired in the subcutaneous adipose tissue of insulin-resistant obese individuals compared with obese adults who maintain normal insulin sensitivity (1). These data demonstrate the importance of subcutaneous adipose tissue in the control of FA Ra and, subsequently, the control of ectopic lipid deposition and insulin sensitivity.
The factors responsible for differences in FA Ra in obesity are not clear, and although the enzymes regulating TG hydrolysis and esterification are well described, the regulation of fatty acid release from adipose tissue is far more complex. Various adipose tissue factors have been postulated to be important mediators of adipose tissue function, including cell size (30), lipid storage capacity (49), adipogenesis (39), angiogenesis (8), extracellular matrix (ECM) dynamics (10), and inflammation (29). Advancing our understanding about mechanisms that allow some obese adults to maintain low FA Ra could lead to targeted interventions that could markedly improve metabolic health. The primary purpose of this study was to determine factors that protect some obese adults from developing high FA Ra from adipose tissue.
MATERIALS AND METHODS
Subjects
Thirty sedentary, premenopausal women (n = 25) and obese men (n = 5) (BMI: 30–45 kg/m2) ages 18–40 yr were recruited for this study. Participants with coronary heart disease, Type 2 diabetes, hypertension, or clinically significant hypertriacylglycerolemia (plasma TG >150 mg/dl) were excluded. Participants were not taking regular medications known to affect metabolic processes, and some women were taking contraceptive medication. All subjects were nonsmokers, weight stable (±2 kg) for 6 mo, and had not participated in regular exercise for at least 6 mo. Body composition was assessed using dual-energy X-ray absorptiometry (Lunar DPX DEXA Scanner). Written, informed consent was obtained from all subjects before initiating participation, and all study procedures were approved by the University of Michigan’s Institutional Review Board.
Experimental Procedures
Subjects were admitted to the Michigan Clinical Research Unit at 0700 after an overnight fast. We obtained a baseline blood sample and an ~100–200-mg subcutaneous adipose tissue sample from the abdominal region ~6 cm lateral to the umbilicus. The sample was cleaned with saline, blotted dry, and quickly frozen in liquid nitrogen. At ~0900, we began a constant-rate infusion of [1-13C]-palmitate (0.04 µmol·kg−1·min−1); after 45 min of infusion, three arterialized blood samples were obtained from a heated hand vein in 5-min intervals for determination of FA Ra. At ~1000, we began a hyperinsulinemic-euglycemic clamp to assess peripheral insulin sensitivity using a primed 2-h insulin infusion at a rate of 100 mU·m−2·min−1 (9). This insulin infusion rate was selected to inhibit hepatic glucose production, even in insulin-resistant subjects (4), allowing us to assess insulin sensitivity, largely independent of the liver. Plasma glucose concentration was monitored every 5 min during the clamp study with a glucose autoanalyzer (Yellow Springs Instruments, Yellow Springs, OH), and glucose was infused at a variable rate to maintain plasma glucose concentration at each participant’s baseline fasting glucose concentration. Stability of glucose concentration was achieved during the last 20 min of insulin infusion. On average, plasma glucose concentration during the final 20 min of the clamp was 98 ± 5% of baseline fasting glucose concentration values, across all 30 subjects (means ± SD). Insulin sensitivity was defined as the glucose infusion rate (GIR; mg/min) during the last 20 min of the hyperinsulinemic-euglycemic clamp (steady-state) divided by fat-free mass (FFM; kg).
Analytical Procedures
Plasma fatty acid kinetics.
The tracer-to-tracee ratio for plasma palmitate was determined by gas chromatography-mass spectrometry (MSD 5973; Agilent Technologies, Wilmington, DE), as previously described (32). Palmitate rate of appearance (Ra) into plasma was calculated using the Steele equation for steady-state conditions (47). FA Ra was calculated by dividing palmitate Ra by the ratio of plasma palmitate to total plasma fatty acid concentration.
Subject stratification.
Subjects were divided into tertiles based on the magnitude of their FA Ra. For our main comparisons, we examined differences between subjects that maintained a relatively low FA Ra despite being obese (LOW-FA; n = 10) to those with a high FA Ra (HIGH-FA; n = 10).
Plasma measurements.
Plasma glucose (Thermo Fisher Scientific, Waltham, MA), fatty acids (Wako Chemicals, Richmond, VA), TG (Sigma Aldrich, St. Louis, MO), and total cholesterol (Wako Chemicals) concentrations were measured using commercially available colorimetric assay kits. Plasma insulin concentration was measured by radioimmunoassay (EMD Millipore, St. Charles, MO).
mRNA expression.
RNA was isolated from subcutaneous adipose tissue (~50 mg) using a commercially available kit (Aurum total RNA fatty and fibrous tissue kit; Bio-Rad, Hercules, CA), quantified spectrophotometrically, and reverse transcribed (high-capacity cDNA reverse transcription kit; Thermo Fisher Scientific). Predesigned PrimeTime quantitative PCR assays (Integrated DNA Technologies, San Diego, CA) were used for mRNA analyses. Real-time quantitative PCR data were normalized to peptidylprolyl isomerase A (PPIA) and β2-microglobulin (B2M) expression using the −∆Ct method (44).
Microarray Analysis
Microarray analysis of adipose tissue gene expression was performed by the University of Michigan DNA Sequencing Core following the manufacturerʼs recommendations. RNA from the five subjects with highest and lowest FA RA was hybridized to Human Gene ST 2.1 strips (Affymetrix, Santa Clara, CA). The fold difference in gene expression between LOW-FA and HIGH-FA was determined with ArrayStar version 12.1 (DNASTAR, Rockville, MD). The upstream regulator module of Ingenuity Pathway Analysis (IPA) software (Qiagen, Redwood City, CA) was used to identify upstream transcriptional regulators, potentially explaining differences in microarray gene expression. We performed gene set enrichment analysis with IPA using logistic regression (LRPath: http://lrpath.ncibi.org/) (42) to test for predefined biologically relevant gene sets containing more significant genes than expected by chance between LOW-FA and HIGH-FA. The data discussed in this publication have been deposited in NCBIʼs Gene Expression Omnibus and are accessible through GEO Series accession number GSE95777.
Western Blot Analysis
Adipose tissue was homogenized in tissue lysis buffer (Cellytic MT cell lysis reagent; Sigma-Aldrich), with commercially available proteinase and phosphatase inhibitors (P8340, P5726, and P0044; Sigma-Aldrich). Protein concentration was determined using the bicinchoninic acid method (Thermo Fisher Scientific). Fifteen to twenty micrograms of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were Ponceau stained to confirm equal loading. Membranes were blocked, incubated in primary antibodies overnight at 4°C, washed, and incubated with appropriate secondary antibodies for 1 h. Blots were developed using enhanced chemiluminescence (Clarity Western ECL substrate, Bio-Rad), imaged, and quantified via densitometry (Image Laboratory Software, Bio-Rad). The primary antibodies used were phosphorylated extracellular signal-regulated kinase (p-ERK1/2Thr202/Tyr204; no. 9101; Cell Signaling, Danvers, MA), extracellular signal-regulated kinase (p-ERK1/2Thr202/Tyr204 no. 9102; Cell Signaling), phosphorylated hormone-sensitive lipase (p-HSLser660) (no. 4126; Cell Signaling), hormone-sensitive lipase (HSL) (no. 4107; Cell Signaling), p-HSLser563 (no. 4139; Cell Signaling), p-HSLser565 (no. 4137; Cell Signaling), adipose triglyceride lipase (ATGL) (no. 2138; Cell Signaling), glycerol-3-phosphate acyltransferase 1 (GPAT1; PRS4613; Sigma-Aldrich), diacylglycerol acyltransferase 1 (DGAT1) (NB110-41487; Novus Biologicals, Littleton, CO), phospho-signal transducer and activator of transcription 3 (p-Stat3Tyr705) (no. 9131; Cell Signaling), STAT3 (no. 4904; Cell Signaling), phospho-p38 MAPKThr180/Tyr182 (no. 9211; Cell Signaling), p38 MAPK (no. 9212; Cell Signaling), monocyte chemotactant protein 1 (MCP1) (no. 2720; Cell Signaling), galectin-3 (MAC2) (ab2785; Abcam, Cambridge, MA), and fatty acid translocase (FAT/CD36) (EPR6573; Abcam).
Statistical Analysis
Simple linear regression was used to examine the relationship between FA Ra and insulin sensitivity. Unpaired Student’s t-tests were used to test for between-group differences (LOW-FA vs. HIGH-FA) in all measured outcome variables. The linear regression and Student’s t-tests were processed using SigmaPlot 13.0. Statistical significance was defined as P < 0.05. Gene expression differences in the microarray data were determined in ArrayStar version 12.1 using a moderated t-test (45). The fold difference values and P values generated from ArrayStar were then used for directional tests in LRpath gene ontology analysis. For all immunoblot and gene expression analyses, we used Grubb’s outlier test (18) to detect outliers in the data sets using GraphPad statistical software (https://graphpad.com/quickcalcs/). Using this test, we found one outlier value in each of the immunoblot analyses of pHSL/HSL, HSL, and CD36 and removed these data points. We also found and removed one outlier from each of the mRNA expression analyses for SORL1 and SPP1.
RESULTS
FA Ra and Cohort Stratification
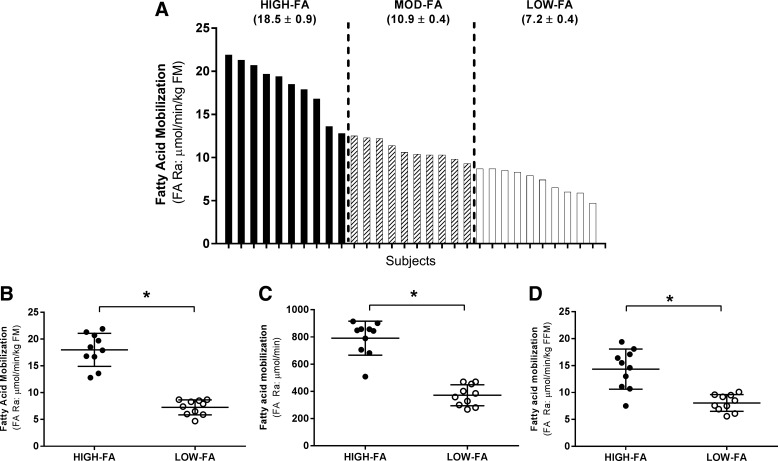
As expected, FA Ra varied widely among our subjects (Fig. 1A). Subjects were stratified into FA Ra tertiles (Fig. 1A), allowing for direct comparisons between subjects with the highest FA Ra values (“HIGH-FA”; ≥12.8 μmol·min−1·kg−1 FM; n = 10) vs. subjects with the lowest FA Ra values (“LOW-FA”; ≤8.7 μmol·min−1·kg FM−1; n = 10). Subjects with intermediate/moderate FA Ra values (“MOD-FA”; 8.8–12.7 μmol·min−1kg·FM−1; n = 10) were excluded from our primary analyses (i.e., HIGH-FA vs. LOW-FA; Fig. 1B), but they were included in our correlational analyses across the entire cohort. Expressing FA Ra relative to fat mass (FM) allowed us to evaluate differences in FA Ra independently of adipose tissue mass. Importantly, however, FA Ra remained significantly different (P < 0.05) between HIGH-FA and LOW-FA, even when not normalized (Fig. 1C) or when expressed relative to FFM (Fig. 1D).
Fig. 1.
Fatty acid rate of appearance (FA Ra) variability across all subjects. FA Ra across the entire cohort (n = 30; A) normalized to fat mass (FM; B) in HIGH-FA (•) vs. LOW-FA (∘) normalized to FM (C) in HIGH-FA vs. LOW-FA expressed as total FA Ra (D) in HIGH-FA vs. LOW-FA normalized to fat-free mass (FFM). Data are expressed as means ± SD. *P < 0.05 vs. HIGH-FA.
Subject Characteristics
Age, body mass, waist circumference, hip circumference, and BMI were not different between groups (Table 1). However, LOW-FA had a significantly higher percentage of body fat (P < 0.01) and lower FFM (P < 0.01) compared with HIGH-FA (Table 1). Although the HIGH-FA group contained three males and seven females, while the LOW-FA contained only females (n = 10), the between-group differences in body composition were still evident even when comparing only the female subjects in HIGH-FA vs. LOW-FA [body fat (%): 47 ± 6 vs 53 ± 3; FFM (kg): 54 ± 9 vs 46 ± 4, respectively; both P < 0.05], while all other other physical characteristics remained similar (data not shown). There were no significant differences in fasting plasma concentrations of insulin, glucose, fatty acids, TG, or total cholesterol (Table 1).
Table 1.
Subject characteristics
| HIGH-FA | LOW-FA | |
|---|---|---|
| Sex (M/F) | 3/7 | 0/10 |
| Age, yr | 28 ± 2 | 34 ± 3 |
| BMI, kg/m2 | 36 ± 1 | 37 ± 1 |
| Mass, kg | 106 ± 6 | 99 ± 3 |
| Fat mass, kg | 45 ± 5 | 52 ± 2 |
| Fat free mass, kg | 61 ± 4 | 47 ± 1* |
| Body fat, % | 44 ± 2 | 53 ± 1* |
| Waist circumference, cm | 107 ± 3 | 111 ± 5 |
| Hip circumference, cm | 123 ± 3 | 124 ± 4 |
| Glucose, mM | 4.8 ± 0.2 | 4.7 ± 0.1 |
| Insulin, µU/ml | 21.3 ± 0.4 | 19.5 ± 2.3 |
| NEFA, µM | 615 ± 66 | 515 ± 53 |
| Triglycerides, mg/dl | 68 ± 13 | 50 ± 9 |
| Total cholesterol, mg/dl | 161 ± 7 | 143 ± 11 |
Values are expressed as means ± SD. Plasma concentrations measured after an overnight fast. NEFA, nonesterified fatty acids.
Significant difference compared with HIGH-FA, P < 0.05.
FA Ra and Insulin Sensitivity
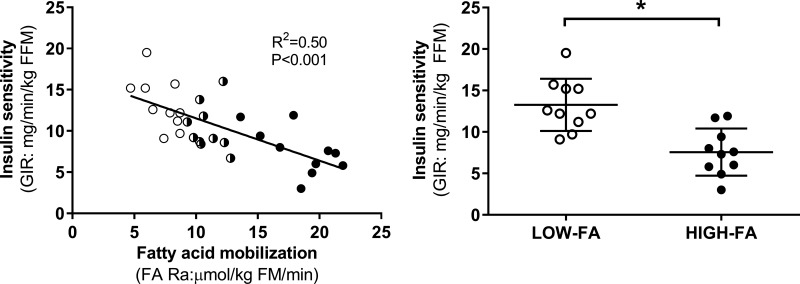
Subjects exhibited a wide range of insulin-mediated glucose uptake (i.e., insulin sensitivity) during the hyperinsulinemic-euglycemic clamp. In agreement with our previous findings (50), we found a highly significant negative correlation between insulin sensitivity and FA Ra across the entire cohort (P < 0.001; Fig. 2A), and 50% of the variability in insulin sensitivity among subjects was explained by the magnitude of FA Ra (R2 = 0.50). In addition, mean insulin sensitivity was nearly 85% greater in LOW-FA vs. HIGH-FA (P = 0.003; Fig. 2B). While the insulin sensitivity data presented in Fig. 2 were normalized per FFM, it is important to note that insulin sensitivity was also significantly higher in LOW-FA compared with HIGH-FA when not normalized to FFM [GIR (mg/min): 610 ± 128 vs. 446 ± 182; P < 0.05]. Furthermore, because the steady-state insulin concentration in plasma (SSI) during the clamp was not different between LOW-FA and HIGH-FA [SSI (µU/ml): 287 ± 64 vs. 324 ± 78; P = 0.28], insulin sensitivity also remained significantly different between the groups when normalized to SSI (GIR/SSI; 2.2 ± 0.5 vs. 1.4 ± 0.5; P = 0.005). To assess whether differences in the sex distribution in our cohorts (HIGH-FA: seven women and three men; LOW-FA: 10 women and 0 men) influenced our findings, we also compared insulin sensitivity between only the female subjects in our groups and found that the differences in insulin sensitivity remained significant when the three men were removed from the HIGH-FA group (GIR (mg·min−1·kg FFM−1): 13.3 ± 3.0 vs. 7.8 ± 2.2, for LOW-FA and HIGH-FA, respectively; P = 0.002); Therefore, it appears that the inclusion of men in the HIGH-FA group was not responsible for the observed differences in insulin sensitivity between the HIGH-FA and LOW-FA groups.
Fig. 2.
FA Ra and insulin sensitivity. A: correlation between insulin-mediated glucose uptake during the clamp (insulin sensitivity) and FA Ra [LOW-FA (n = 10; ∘), MOD-FA (n = 10; half black, half white circles), HIGH-FA (n = 10; •)]. B: insulin sensitivity in LOW-FA vs. HIGH-FA. Data are expressed as means ± SD *P < 0.05 vs. HIGH-FA.
Adipose Tissue
Lipolysis and esterification markers.
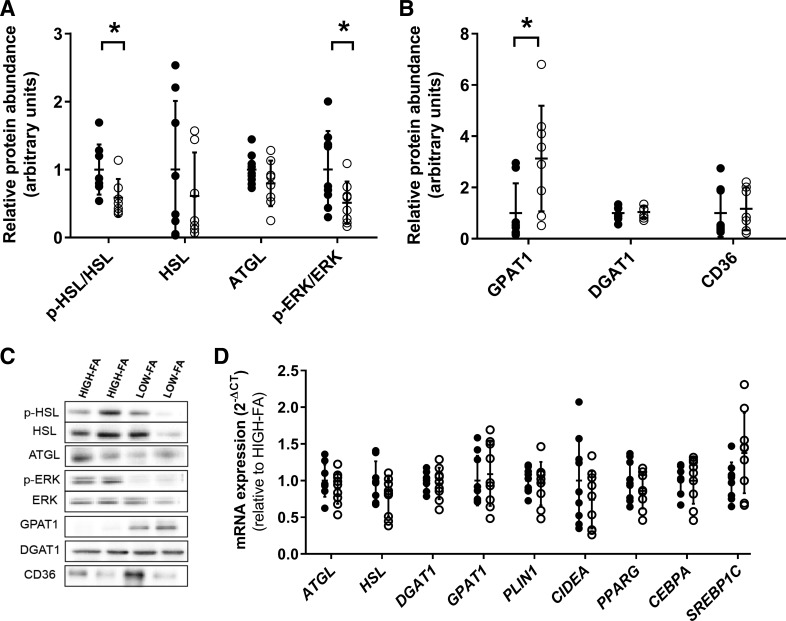
We found a lower abundance (P = 0.02) of phosphorylated HSL at serine-660 (HSLser660), a marker of increased HSL activity, in LOW-FA compared with HIGH-FA (Fig. 3, A and C). There were no differences in phosphorylated HSL at serine-563 (p-HSLser563) (P = 0.27), another marker of increased HSL activity, or phosphorylation of serine-565 (p-HSLser565) (P = 0.47), a marker of inhibition of HSL activity (data not shown). Interestingly, abundance of p-HSLser660/HSL was significantly correlated to FA Ra across the entire cohort (R2 = 0.31, P = 0.003). There was no difference in HSL mRNA expression between groups (Fig. 3D). In contrast to HSL, neither protein abundance nor mRNA expression of ATGL was different between groups (Fig. 3, A, C, and D). ERK pathway activation, which increases lipolysis, was also significantly lower in LOW-FA vs. HIGH-FA (p-ERKThr202/Tyr204/ERK; P = 0.05; Fig. 3, A and C). We also found three-fold greater protein abundance of GPAT1 in LOW-FA compared with HIGH-FA (P = 0.02; Fig. 3, B and C), despite no difference in GPAT1 mRNA expression (Fig. 3D). There were no differences in protein abundance or mRNA expression of DGAT1 (Fig. 3, A–D).
Fig. 3.
Markers of lipolysis, esterification, and fatty acid uptake. A: relative protein abundance of proteins related to lipolysis normalized to HIGH-FA. B: relative protein abundance of proteins related to esterification and fatty acid uptake expressed relative to HIGH-FA. C: representative images for Western blot analysis of proteins related to lipolysis, esterification, and fatty acid uptake. D: mRNA expression of factors related to lipolysis, esterification, lipogenesis, and fatty acid storage in LOW-FA (n = 9; ∘) compared with HIGH-FA (n = 9; •). Expression values were normalized to the mean of the housekeeping genes PPIA and B2M and then expressed relative to HIGH-FA. Data are expressed as means ± SD. *P < 0.05 vs. HIGH-FA.
Markers of lipogenesis, adipogenesis, lipid storage, and transport.
mRNA expression of factors involved in lipid droplet storage (PLIN1 and CIDEA) was not different between LOW-FA and HIGH-FA (Fig. 3D). mRNA expression of factors involved in lipogenic and adipogenic processes (i.e., PPARG, CEBPA, and SREBP1C) was also similar between LOW-FA and HIGH-FA (Fig. 3D). Lastly, we found no differences between LOW-FA and HIGH-FA in protein abundance of CD36, a primary fatty acid transporter (Fig. 3, B and C).
LOW-FA vs. HIGH-FA Gene Expression Profile
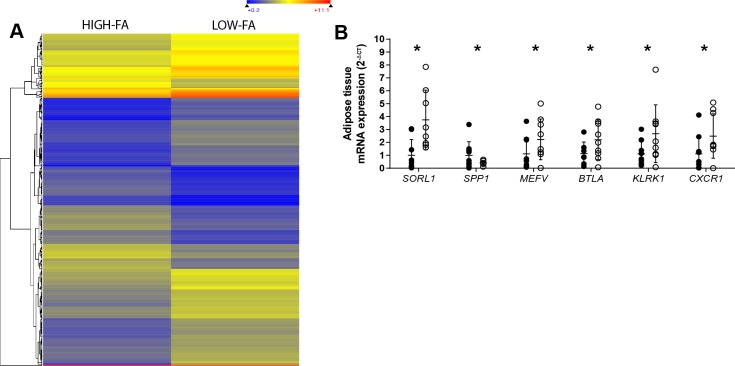
We performed gene ontology (GO) analyses on the microarray data from LOW-FA and HIGH-FA to identify novel alterations in biological pathways that may underlie the observed differences in FA Ra between LOW-FA and HIGH-FA. Table 2 summarizes the three most highly upregulated and downregulated GO terms in LOW-FA compared with HIGH-FA that were statistically significant and biologically relevant. LOW-FA had lower enrichment of pathways related to ECM structure, organization, and disassembly. Surprisingly, the most highly upregulated GO processes in LOW-FA were antigen receptor-mediated signaling, defense response, and lymphocyte differentiation, suggesting greater immune activity in LOW-FA adipose tissue. Of the 413 statistically significant differentially (>2-fold) expressed genes in LOW-FA compared with HIGH-FA (Fig. 4A), 210 genes were upregulated, and 203 were downregulated in LOW-FA vs. HIGH-FA. We examined the most biologically relevant genes to identify those with novel involvement in adipose tissue metabolism. Out of the 47 most relevant genes (Table 3), 40 were related to the adipose tissue immune response, while other candidate genes were related to fatty acid metabolism, ECM, and mitochondria. Follow-up quantitative PCR (qPCR) validation analyses performed for sortilin-related receptor 1 (SORL1), secreted phosphoprotein 1 (SPP1), pyrin innate immunity regulator (MEFV), B and T Lymphocyte Associated (BTLA), killer cell lectin-like receptor K1 (KLRK1), and C-X-C motif chemokine receptor 1 (CXCR1) confirmed significant differences in mRNA expression between HIGH-FA and LOW-FA for these six genes (P < 0.05; Fig. 4B). The observed differences in SORL1 between groups were of high interest to us, considering its role in enhancing cellular insulin signaling (43), which, in turn, could impact lipolytic rate.
Table 2.
Gene ontology analysis of adipose tissue genes in LOW-FA compared with HIGH-FA
| GO Term | GO Description | Q Value | Genes | Direction |
|---|---|---|---|---|
| GO:0022617 | extracellular matrix disassembly | 6.04E-06 | FN1, TIMP1, COL1A2, COL6A1, LAMB1, COL5A2, COL16A1 | down |
| GO:0030198 | extracellular matrix organization | 6.04E-06 | APP1, FN1, TIMP1, COL1A2, ITGA3, CD151, SERPINH1, COL6A1, LAMB1, MUSK, COL5A1, APLP1, COL16A1, CCDC80 | down |
| GO:0043062 | extracellular structure organization | 6.04E-06 | APP1, FN1, TIMP1, COL1A2, ITGA3, CD151, SERPINH1, COL6A1, LAMB1, MUSK, COL5A1, APLP1, COL16A1, CCDC80 | down |
| GO:0050851 | antigen receptor-mediated signaling pathway | 4.57E-04 | LCK, CD38, UBA52 | up |
| GO:0098542 | defense response to other organism | 0.001437458 | LCK, DDX58, ISG15, DMBT1, BCL3, IFITM1, HERC5, BNIP3L, HIST1H2BJ | up |
| GO:0030098 | lymphocyte differentiation | 0.00292656 | LCK, ATP7A, POU1F1, CD27, BCL3, SLC46A2 | up |
Fig. 4.
Microarray and gene expression analysis of adipose tissue. A: heat map representing genes twofold differentially expressed in LOW-FA compared with HIGH-FA. B: qPCR validation of mRNA expression of six genes found to be greater than twofold different in LOW-FA (n = 9, ∘) compared with HIGH-FA (n = 9, •). Expression values were normalized to the mean of the housekeeping genes PPIA and B2M and then expressed relative to HIGH-FA. Data are expressed as means ± SD. *P < 0.05 vs. HIGH-FA.
Table 3.
Most biologically relevant genes over twofold different in LOW-FA compared with HIGH-FA
| Gene Symbol | Gene | Fold Change |
|---|---|---|
| Fatty acid metabolism | ||
| AADAC | Arylacetamide deacetylase | 0.24 |
| FFAR3 | Free fatty acid receptor 3 | 0.40 |
| SORL1 | Sortilin-related receptor | 2.71 |
| Extracellular matrix | ||
| MMP9 | Matrix metalloproteinase 9 | 0.41 |
| MMP7 | Matrix metalloproteinase 7 | 0.45 |
| MXRA5 | Matrix-remodeling associated 5 | 0.45 |
| Immune response | ||
| HLA-DRB1 | Major histocompatibility complex, class II, DR β1 | 0.35 |
| Antigen presentation | ||
| HLA-DQB1 | Major histocompatibility complex, class II, DQ β1 | 2.88 |
| LILRA | Leukocyte immunoglobulin-like receptor A1 | 2.40 |
| T cell | ||
| CCL19 | Chemokine (C-C motif) ligand 19 | 0.48 |
| TRBV11 | T-cell receptor β variable 11–1 | 0.46 |
| TRGJP2 | T-cell receptor γ joining P2 | 3.80 |
| TRGJ2 | T-cell receptor γ joining 2 | 3.03 |
| TAGAP | T-cell activation RhoGTPase activating protein | 2.79 |
| TRAJ17 | T-cell receptor alpha joining 17 | 2.39 |
| TRAT1 | T-cell receptor associated transmembrane adaptor 1 | 2.35 |
| TRAJ49 | T-cell receptor | 2.35 |
| TRAJ14 | T-cell receptor | 2.19 |
| TRAJ42 | T-cell receptor | 2.19 |
| TRAJ47 | T-cell receptor | 2.18 |
| T-cell activation | ||
| NKG7 | Natural killer cell granule protein 7 | 2.77 |
| BTLA | B and T lymphocyte associated | 2.73 |
| TXK | Nonreceptor tyrosine kinase | 2.31 |
| KLRK1 | killer cell lectin-like receptor K1 | 2.20 |
| GZMA | Granzyme A | 3.15 |
| CD3D | CD3 | 2.16 |
| Antiinflammatory | ||
| MEFV | Mediterranean fever | 2.53 |
| SPP1 | Secreted phosphoprotein 1 | 0.35 |
| LBP | Lipopolysaccharide binding protein | 0.43 |
| FPR3 | Formyl peptide receptor 3 | 0.54 |
| CSF3R | Colony stimulating factor 3 | 2.36 |
| Neutrophils | ||
| CXCR1 | Chemokine (C-X-C motif) ligand 1 | 4.20 |
| VNN2 | Vanin 2 | 3.60 |
| Lymphocyte related | ||
| SELL | Selectin L | 3.40 |
| FPR1 | Formyl peptide receptor 1 | 2.73 |
| S1PR4 | Shpingosine-1-phosphate receptor 4 | 2.44 |
| MS4A1 | Membrane spanning protein 4 A1 | 2.55 |
| Cytokines−/− other | ||
| IL23A | Interleukin 23 subunit alpha | 3.15 |
| IL18 | Interleukin 18 receptor accessory protein | 2.33 |
| CLEC1B | C-type lectin domain family 1 member B | 3.01 |
| RNASE2 | Ribonuclease A family member 2 | 2.75 |
| GBP5 | Guanylate binding protein 5 | 2.75 |
| IFIT1B | Interferon-induced protein | 2.47 |
| DEFA | Defensin | 2.42 |
| EMR3 | EGF-like module-containing mucin-like hormone receptor-like 3 | 2.38 |
| MNDA | Myeloid cell nuclear differentiation antigen | 2.36 |
| Mitochondria | ||
| YME1L1 | YME1-like 1 ATPase | 3.15 |
Inflammation and Fibrosis
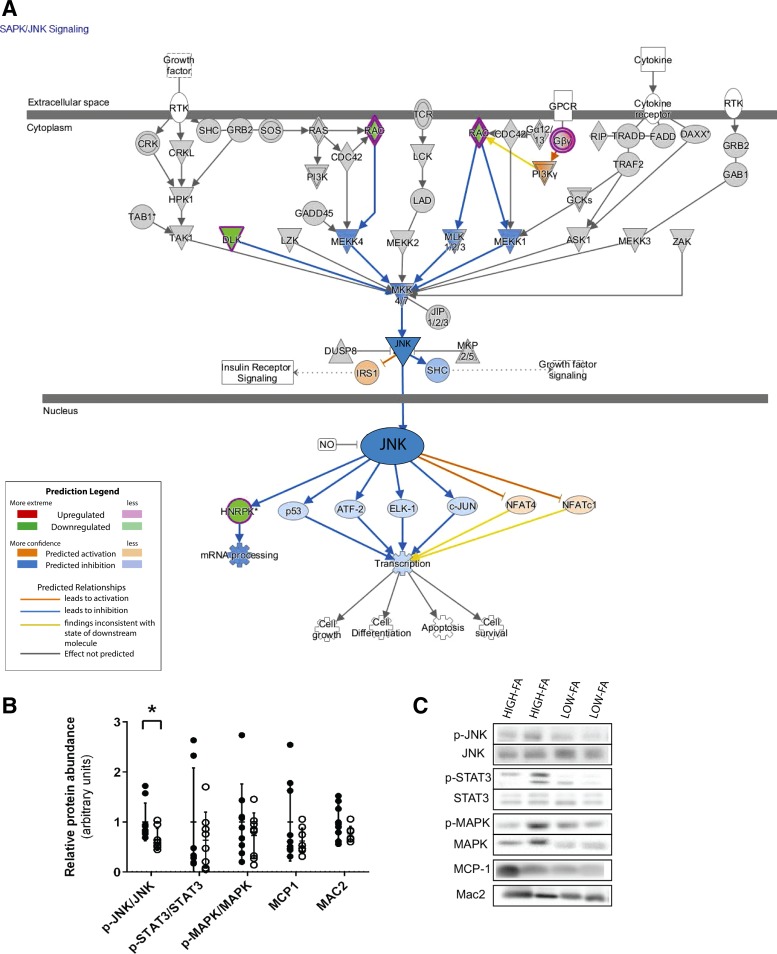
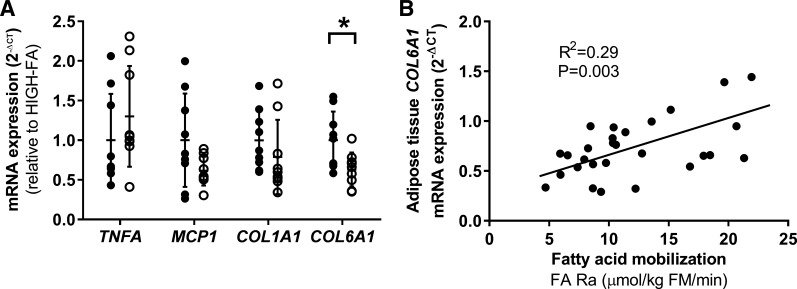
IPA analysis predicted significant (P = 0.04) downregulation of the stress/inflammatory SAPK/JNK pathway in LOW-FA vs. HIGH-FA (Fig. 5A). Follow-up Western blot analysis confirmed significantly lower JNK pathway activation (p-JNKThr183/Tyr185/JNK) in LOW-FA compared with HIGH-FA (P = 0.04; Fig. 5, B and C). We found no differences between groups for other stress-related signaling pathways (i.e., p38 MAPK or STAT3; Fig. 5, B and C). There were also no differences in protein expression of MCP-1 or MAC2, a crude marker of macrophage abundance (Fig. 5, B and C). qPCR analysis revealed no differences in mRNA expression of some canonical markers of inflammation (TNFA and MCP1) or COL1A1 between LOW-FA and HIGH-FA (Fig. 6A). However, in agreement with the GO analysis, COL6A1 mRNA expression was lower in LOW-FA vs. HIGH-FA (P = 0.009; Fig. 6A), and COL6A1 expression was positively correlated with FA Ra (R2 = 0.29; P = 0.003; Fig. 6B).
Fig. 5.
SAPK/JNK and stress/inflammatory pathway activation. A: Ingenuity Pathway Analysis identified the SAPK/JNK pathway to be significantly downregulated in LOW-FA compared with HIGH-FA (P = 0.04). B: relative protein abundance of proteins related to stress pathway activation and inflammation expressed relative to HIGH-FA (LOW-FA; n = 8, ∘; HIGH-FA; n = 9, •). Data are expressed as means ± SD *P < 0.05 vs. HIGH-FA.
Fig. 6.
Markers of inflammation and fibrosis. A: mRNA expression of factors related to inflammation and fibrosis in LOW-FA (n = 7–9; ∘) compared with HIGH-FA (n = 7–9; •). Expression values were normalized to the mean of the housekeeping genes PPIA and B2M and then expressed relative to HIGH-FA. Data are expressed as means ± SD *P < 0.05 vs. HIGH-FA. B: correlation between COL6A1 mRNA expression and FA Ra across 28 subjects.
DISCUSSION
Our observation that FA Ra from subcutaneous adipose tissue is an important mediator of insulin resistance in obesity is consistent with previous work from our laboratory (50) and others (28). Our findings suggest lower lipase activation, greater fatty acid esterification capacity, and alterations in ECM organization and fibrosis in subcutaneous adipose tissue may all contribute to the attenuated fatty acid release in our LOW-FA cohort, perhaps through an enhanced ability to sequester and store fatty acids as TG within adipocytes. In contrast to our hypothesis, our microarray data revealed upregulated markers of immune activity in subcutaneous adipose tissue from LOW-FA vs. HIGH-FA. Because insulin sensitivity was relatively high in our LOW-FA subjects, these findings from our microarray conflict with the overly simplistic notion that upregulated adipose tissue immune activity directly underlies the development of insulin resistance.
The inability of subcutaneous adipose tissue to adequately sequester excess fatty acids, which may lead to excessive FA Ra, is causally linked with a host of cardiometabolic complications, including insulin resistance (2). The importance of storing fatty acids in adipose tissue and preventing excessive exposure of fatty acids to peripheral tissues is demonstrated clinically in patients with lipodystrophy, a condition characterized by an extraordinarily low capacity to store fatty acids in subcutaneous adipose tissue, leading to extreme insulin resistance (14). Lipodystrophic mice develop similar metabolic complications; however, these complications (e.g., insulin resistance and ectopic lipid deposition) can be reversed with transplantation of well-functioning subcutaneous adipose tissue (15). Although it may seem counterintuitive, the ability to expand subcutaneous adipose tissue to accommodate nutrient oversupply may actually help prevent insulin resistance by enhancing the capacity to sequester fatty acids—thereby, preventing excess fatty acid release and ectopic lipid deposition in insulin-sensitive tissues (17). Along these lines, this is one of the main mechanisms of action for thiazolidinedione treatment for Type 2 diabetes (5). Our data suggest that LOW-FA subjects may have a naturally enhanced ability to expand and store excess nutrients as TG in subcutaneous adipose tissue. While our data capture only a snapshot of the fat storage capacity in our subjects, other human studies that tracked long-term TG turnover and storage using deuterated water reported impaired TG synthesis and storage in subcutaneous adipose tissue of insulin-resistant obese adults (1). Our data further support the importance of subcutaneous adipose tissue in the control of FA Ra and, subsequently, peripheral tissue insulin sensitivity.
Differences in the expression, abundance, and activation of enzymes controlling lipolysis and esterification among our subjects may contribute to the variability in their FA Ra and may impact whole body insulin resistance. Partial inhibition of lipolysis in obese mice through pharmacological or genetic manipulation of HSL resulted in lower fatty acid availability and improved insulin sensitivity without affecting fat mass (16). Lower markers of lipolysis are also correlated with reduced indices of insulin resistance in obese humans, independently of fat mass (16). In agreement, our LOW-FA group had lower markers of HSL activation (p-HSLser660/HSL) and ERK (p-ERKThr202/Tyr204/ERK) signaling, which are indicative of lower lipolytic activation (7, 27). Systemic fatty acid release from subcutaneous adipose tissue can also be attenuated by upregulated esterification, which can synthesize TGs from locally released fatty acids before they enter the circulation. Our finding that GPAT protein abundance [i.e., the enzyme catalyzing the first committed step of the esterification pathway (23)] was threefold greater in LOW-FA than the HIGH-FA Group suggests greater esterification capacity may have also contributed to a lower systemic FA release. Together, our findings suggest that obese adults who maintain relatively low FA Ra (LOW-FA) may have reduced activation of basal lipolysis and an increased capacity for fatty acid esterification compared with obese adults with high FA Ra.
Our finding that markers of ECM organization and assembly were lower in LOW-FA vs. HIGH-FA is very intriguing and among the most novel findings of our study. Furthermore, to our knowledge, this is the first study demonstrating a strong direct correlation between FA Ra and collagen VI expression in human adipose tissue. Therefore, ECM-mediated alterations in adipose tissue fatty acid metabolism may be an important contributor to the well-described relationship between excess accumulation of certain adipose tissue ECM components (including collagen VI) and insulin resistance in obesity (11). Subcutaneous adipose tissue collagen VI expression has been found to be positively correlated with BMI, fat mass, and insulin resistance, and is upregulated during short-term overfeeding (37). In agreement, mice lacking collagen VI were protected from high-fat diet-induced insulin resistance (26). The maintenance of insulin sensitivity in these mice was accompanied by larger, more hypertrophied adipocytes despite similar fat mass, indicating the lack of collagen VI may have allowed the adipocytes to expand with less constraint, providing improved capacity for fatty acid storage. Having less subcutaneous adipose tissue fibrosis in our human subjects may also have allowed for an increased adipocyte expansion, with potential for enhanced adipocyte lipid storage capacity, lower mobilization of fatty acids, and a resultant lower ectopic lipid deposition. This would likely be advantageous for maintaining insulin sensitivity in obese humans as well.
Fibrotic adipose tissue is also often accompanied by an elevated inflammatory profile (29, 46), which is a key contributor to metabolic dysfunction in obesity (48). However, despite evidence for higher adipose tissue fibrosis and lower insulin sensitivity in HIGH-FA vs. LOW-FA, our finding that adipose tissue immune activity was lower in HIGH-FA compared with LOW-FA conflicts with this notion. The adipose tissue immune response is very complex, and inflammatory status clearly cannot be classified simply as “proinflammatory” and “anti-inflammatory.” The regulation of macrophages, neutrophils, lymphocytes, and T-cell activity has many redundant, overlapping mechanisms controlling whether these cells confer pathogenic or protective functions. While macrophages are commonly linked to the development and maintenance of fibrosis, they are also involved in the inhibition and reversal of fibrosis (12, 31), which corroborates our data. Although overall immune activity may be elevated, there could be a compensatory rise in mechanisms that mitigate the detrimental effects of increased immune activity on adipose tissue function, such as MEFV and BTLA. MEFV plays a role in the degradation of several inflammasome components (36), while BTLA is involved in inhibiting the Th1 “proinflammatory” T-cell response (51). Furthermore, our LOW-FA group had higher mRNA expression of C-X-C motif chemokine receptor 1 (CXCR1), a powerful chemoattractant factor that activates neutrophils (22) and lower expression of secreted phosphoprotein 1 (SPP1), which is related to induction of the inflammatory response in obesity (34). Unfortunately, without specific sorting and analysis of the adipose tissue immune cells, we can only speculate on the “protective” vs. “pathogenic” immune cell abundance and activity in the LOW-FA group. Nevertheless, these data suggest an uncoupling of the immune inflammatory response and the development of insulin resistance in obese, yet otherwise healthy, individuals.
Despite evidence of higher overall adipose tissue immune activity, SAP/JNK pathway activation was lower in adipose tissue from LOW-FA compared with HIGH-FA. The SAPK/JNK pathway is activated in multiple tissues in obesity, including adipose tissue, and is implicated in orchestrating the relationship between inflammation and poor metabolic outcomes (35). While the SAPK/JNK pathway can interfere with insulin signaling and promote insulin resistance (21), SAPK/JNK pathway activation in macrophages may also be important for controlling basal fatty acid release from adipose tissue. For example, reducing JNK pathway activity in macrophages alters macrophage polarization and lowers the expression and release of proinflammatory cytokines that may cause excess FA Ra (20, 38). Therefore, the lower SAPK/JNK pathway activation in LOW-FA may explain why this group can maintain a lower FA Ra despite elevated overall immune activity. Our findings demonstrate that preserving low JNK pathway activation in adipose tissue in obesity may be important for maintaining low FA Ra and preserving whole body insulin sensitivity in humans.
Another novel finding was the greater mRNA expression of sortilin-related receptor (SORL1) in adipose tissue from LOW-FA vs. HIGH-FA. SORL1 is best known for its role in the neurodegenerative processes involved in Alzheimer’s disease (13). However, Schmidt et al. (43) demonstrated an important role for SORL1 in modulating adipose tissue metabolic processes. For example, SORL1 overexpression in mice reduced TG hydrolysis, while inactivation of SORL1 increased TG breakdown and fatty acid release from the adipose tissue (43). The proposed mechanism for SORL1 action on adipose tissue lipolysis is to enhance cellular insulin signaling (43) and, thereby, increase the antilipolytic response to insulin. This enhanced effect on insulin signaling may be particularly relevant at basal (i.e., relatively low) insulin concentrations, which is when FA Ra was measured in our study. Perhaps the elevated SORL1 expression we found in subcutaneous adipose tissue from our LOW-FA subjects may be beneficial for sequestering fatty acids, reducing their release into the systemic circulation.
While our data provide important insight into possible mechanisms protecting some obese adults from developing insulin resistance, there are some limitations to this study. First, although our findings identify several factors that may underlie differences in FA Ra among our cohort of obese subjects, our study does not directly address causation, which is always very challenging in human studies. Second, because our measurements of FA Ra, gene expression, and protein abundance were performed on samples collected during the postabsorptive state, our conclusions do not address alterations in adipose tissue in the postprandial state (i.e., insulin-stimulated). Relevant to this, we acknowledge that varying degrees of adipose tissue resistance to the antilipolytic effects of insulin could also contribute to differences in FA Ra among our subjects. However, because our measurements were made in the postabsorptive state (i.e., when plasma insulin concentration was at its lowest point), the contribution of differences in adipose tissue insulin resistance on FA Ra was likely rather small in our study. More importantly, we contend that ectopic lipid deposition (and its negative impact on whole body insulin resistance) is primarily affected by the much higher FA Ra that occurs in the postabsorptive state, which is why we focused on assessing differences in FA Ra among our subjects after an overnight fast. Unfortunately, because we could not measure plasma catecholamines due to inadequate sample availability, we do not know whether differences in circulating catecholamines may have also contributed to the differences in FA Ra between our groups. Finally, we did not have adequate adipose tissue samples to perform histological measurements, which could provide more information about adipose tissue fibrosis and inflammation. These measures will be incorporated in follow-up studies.
In conclusion, our findings support the importance of FA Ra from adipose tissue in modulating the degree of insulin resistance in obese adults. Obese adults who can maintain low FA Ra may do so via reduced activation of lipolytic pathways (ERK and HSL) and enhanced fatty acid esterification via increased protein content of GPAT. The reduced activation of lipolytic pathways may also be mediated, in part, by increased SORL1 expression and/or reduced SAPK/JNK pathway activation. Furthermore, obese adults who maintain low FA Ra have lower markers of fibrosis and ECM deposition, potentially enhancing their ability to store fatty acids as TG within adipocytes and protecting them from ectopic lipid deposition. Expanding our understanding about what dictates systemic fatty acid release from adipose tissue may lead to the development of treatments or therapeutic interventions that can reduce and/or prevent insulin resistance in obesity.
GRANTS
This work was primarily supported by National Institutes of Health (NIH) Grant no. R01 DK-077966, and the American Diabetes Association (ADA) Grant no. 1-16-ICTS-048, both awarded to J. F. Horowitz. Additional support for this project was provided by the Michigan Clinical Research Unit (NIH Grant 2UL1TR000433-06) and the Human Phenotyping Core of the University of Michigan Nutrition and Obesity Research Center (NIH Grant P30-DK089503).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.W.V.P. and J.F.H. conceived and designed research; D.W.V.P., L.M.G., J.F.H., and A.Y.W. performed experiments; D.W.V.P., L.M.G., A.Y.W., and J.F.H. analyzed data; D.W.V.P., L.M.G., A.Y.W., and J.F.H. interpreted results of experiments; D.W.V.P. prepared figures; D.W.V.P. and J.F.H. drafted manuscript; D.W.V.P., L.M.G., and J.F.H. edited and revised manuscript; D.W.V.P., L.M.G., A.Y.W., and J.F.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are thankful to Dr. Alexander Hinko for his assistance with the fatty acid tracer analysis. We are thankful for the assistance of the nursing staff of the Michigan Clinical Research Unit for their support throughout this study. We are also especially grateful to Dr. Christopher Mendias and his laboratory at the University of Michigan for their critical assistance and guidance with the microarray analysis and Western blot analysis. Finally, we are particularly grateful to all of the study subjects for their participation on this project.
REFERENCES
- 1.Allister CA, Liu LF, Lamendola CA, Craig CM, Cushman SW, Hellerstein MK, McLaughlin TL. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res 56: 435–439, 2015. doi: 10.1194/jlr.M052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner P, Rydén M. Fatty acids, obesity and insulin resistance. Obes Facts 8: 147–155, 2015. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54: 3148–3153, 2005. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna RC, Leif G, Kraemer N, Ferrannini E, Del Prato S, DeFronzo RA. Obesity and insulin resistance in humans: a dose-response study [doi]. Metabolism 39: 452–459, 1990. doi: 10.1016/0026-0495(90)90002-T. [DOI] [PubMed] [Google Scholar]
- 5.Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 57: 2272–2279, 2008. doi: 10.2337/db08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, Perseghin G. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34: 210–215, 2011. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol 18: 2123–2131, 2004. doi: 10.1210/me.2004-0193. [DOI] [PubMed] [Google Scholar]
- 8.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 1842: 463–472, 2014. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Divoux A, Clement K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev 12: e494–e503, 2011. doi: 10.1111/j.1467-789X.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 11.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59: 2817–2825, 2010. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005. doi: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsky D, Szeszko P, Yu L, Honer WG, De Jager PL, Schneider JA, Malhotra AK, Lencz T, Ikuta T, Pipitone J, Chakravarty MM, Lobaugh NJ, Mulsant BH, Pollock BG, Kennedy JL, Bennett DA, Voineskos AN. The SORL1 gene and convergent neural risk for Alzheimer’s disease across the human lifespan. Mol Psychiatry 19: 1125–1132, 2014. doi: 10.1038/mp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am 33: 305–331, 2004. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105: 271–278, 2000. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL, Houssier M, Roussel B, Besse-Patin A, Combes M, Mir L, Monbrun L, Bézaire V, Prunet-Marcassus B, Waget A, Vila I, Caspar-Bauguil S, Louche K, Marques MA, Mairal A, Renoud ML, Galitzky J, Holm C, Mouisel E, Thalamas C, Viguerie N, Sulpice T, Burcelin R, Arner P, Langin D. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11: e1001485, 2013. doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev 65: S7–S12, 2007. doi: 10.1301/nr.2007.jun.S7-S12. [DOI] [PubMed] [Google Scholar]
- 18.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics 11: 1–21, 1969. doi: 10.1080/00401706.1969.10490657. [DOI] [Google Scholar]
- 19.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 97: 2482–2488, 2012. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339: 218–222, 2013. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 22.Hu N, Westra J, Rutgers A, Doornbos-Van der Meer B, Huitema MG, Stegeman CA, Abdulahad WH, Satchell SC, Mathieson PW, Heeringa P, Kallenberg CGM. Decreased CXCR1 and CXCR2 expression on neutrophils in anti-neutrophil cytoplasmic autoantibody-associated vasculitides potentially increases neutrophil adhesion and impairs migration. Arthritis Res Ther 13: R201, 2011. doi: 10.1186/ar3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igal RA, Wang S, Gonzalez-Baró M, Coleman RA. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J Biol Chem 276: 42205–42212, 2001. doi: 10.1074/jbc.M103386200. [DOI] [PubMed] [Google Scholar]
- 24.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 25.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92: 91–98, 1993. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. J Clin Endocrinol Metab 97: E1219–E1223, 2012. doi: 10.1210/jc.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Santibañez G, Lumeng CN-K. Macrophages and the regulation of adipose tissue remodeling. Annu Rev Nutr 34: 57–76, 2014. doi: 10.1146/annurev-nutr-071812-161113. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW. Enhanced proportion of small adipose cells in insulin-resistant vs. insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50: 1707–1715, 2007. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 31.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newsom SA, Schenk S, Thomas KM, Harber MP, Knuth ND, Goldenberg N, Horowitz JF. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity. J Appl Physiol (1985) 108: 554–560, 2010. doi: 10.1152/japplphysiol.01106.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117: 2877–2888, 2007. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal M, Febbraio MA, Lancaster GI. The roles of c-Jun NH-terminal kinases (JNKs) in obesity and insulin resistance. J Physiol 594: 267–279, 2016. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer HD, Grütter C, Grütter M, Tschopp J. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1β processing. Cell Death Differ 14: 1457–1466, 2007. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 37.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 94: 5155–5162, 2009. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160: 745–758, 2015. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896, 2006. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 40.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 282: E657–E663, 2002. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 41.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48: 1836–1841, 1999. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 42.Sartor MA, Leikauf GD, Medvedovic M. LRpath: a logistic regression approach for identifying enriched biological groups in gene expression data. Bioinformatics 25: 211–217, 2009. doi: 10.1093/bioinformatics/btn592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt V, Schulz N, Yan X, Schürmann A, Kempa S, Kern M, Blüher M, Poy MN, Olivecrona G, Willnow TE. SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J Clin Invest 126: 2706–2720, 2016. doi: 10.1172/JCI84708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 45.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: e3, 2004. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 46.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 299: E1016–E1027, 2010. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 82: 420–430, 1959. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 48.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J 59: 849–857, 2012. doi: 10.1507/endocrj.EJ12-0271. [DOI] [PubMed] [Google Scholar]
- 49.Tuvdendorj D, Chandalia M, Batbayar T, Saraf M, Beysen C, Murphy EJ, Abate N. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. Am J Physiol Endocrinol Metab 305: E999–E1006, 2013. doi: 10.1152/ajpendo.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Pelt DW, Newsom SA, Schenk S, Horowitz JF. Relatively low endogenous fatty acid mobilization and uptake helps preserve insulin sensitivity in obese women. Int J Obes 39: 149–155, 2015. doi: 10.1038/ijo.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4: 670–679, 2003. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 52.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 168: 1617–1624, 2008. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]