Relatively little is known about the mechanisms that govern contractions of lymphatic vessels. σ1-Receptor activation has been shown to reduce the fractional pump flow of isolated rat mesenteric collecting lymphatics. The σ1-receptor was localized mainly in the endothelium, and blockade of nitric oxide synthase inhibited the effects of afobazole.
Keywords: collecting lymphatic, afobazole, σ1-receptor, lymphatic endothelium, nitric oxide
Abstract
Recently, it has been reported that a σ-receptor antagonist could reduce inflammation-induced edema. Lymphatic vessels play an essential role in removing excess interstitial fluid. We tested the hypothesis that activation of σ-receptors would reduce or weaken collecting lymphatic contractions. We used isolated, cannulated rat mesenteric collecting lymphatic vessels to study contractions in response to the σ-receptor agonist afobazole in the absence and presence of different σ-receptor antagonists. We used RT-PCR and Western blot analysis to investigate whether these vessels express the σ1-receptor and immunofluorescence confocal microscopy to examine localization of the σ1-receptor in the collecting lymphatic wall. Using N-nitro-l-arginine methyl ester (l-NAME) pretreatment before afobazole in isolated lymphatics, we tested the role of nitric oxide (NO) signaling. Finally, we used 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate fluorescence as an indicator to test whether afobazole increases NO release in cultured lymphatic endothelial cells. Our results show that afobazole (50–150 µM) elevated end-systolic diameter and generally reduced pump efficiency and that this response could be partially blocked by the σ1-receptor antagonists BD 1047 and BD 1063 but not by the σ2-receptor antagonist SM-21. σ1-Receptor mRNA and protein were detected in lysates from isolated rat mesenteric collecting lymphatics. Confocal images with anti-σ1-receptor antibody labeling suggested localization in the lymphatic endothelium. Blockade of NO synthases with l-NAME inhibited the effects of afobazole. Finally, afobazole elicited increases in NO production from cultured lymphatic endothelial cells. Our findings suggest that the σ1-receptor limits collecting lymphatic pumping through a NO-dependent mechanism.
NEW & NOTEWORTHY Relatively little is known about the mechanisms that govern contractions of lymphatic vessels. σ1-Receptor activation has been shown to reduce the fractional pump flow of isolated rat mesenteric collecting lymphatics. The σ1-receptor was localized mainly in the endothelium, and blockade of nitric oxide synthase inhibited the effects of afobazole.
afobazole is an anxiolytic drug that acts on σ-receptors, a family of proteins that modulate a variety of signals within cells (11, 12, 40). The σ-receptor family consists of two known subtypes: σ1 and σ2. While σ1- and σ2-receptors are highly expressed in the central nervous system, they have also been found in the liver, kidney, heart, and eye as well as endocrine organs (9). The σ1- and σ2-receptors have also been shown to differentially regulate acid-sensing ion channels and voltage-gated Ca2+ channel activity (23, 55). Within cells, σ-receptors localize to the plasma membrane or intracellular membranes and have the ability to translocate within different subcellular compartments when treated with agonistic compounds (9). In the mitochondria-associated membrane of the endoplasmic reticulum, σ1-receptors appear to act as chaperones stabilizing the inositol trisphosphate receptor and are involved in Ca2+ transfer from the endoplasmic reticulum to mitochondria (22). Many drugs used to treat Alzheimer’s disease or drug/alcohol dependence have moderate to high affinity for σ1-receptors (9). Endogenous sphingolipids, N,N-dimethyltryptamine, and neuroactive steroids such as dehydroepiandosterone can bind to σ1-receptors, but their potential roles as physiological ligands remain to be determined (15, 28, 33, 42).
Drugs that affect intracellular free Ca2+ concentration ([Ca2+]i) have been shown to have mild to profound effects on the pump function of collecting lymphatics (5, 7, 14, 26, 38, 46). Unlike blood vessels, which have the heart as a central pump to drive flow, collecting lymphatics are organized into a series of segments that contract phasically and drive lymph flow (6). These segments, called lymphangions, are separated by one-way luminal valves to prevent backflow of lymph and have a smooth muscle layer that expresses a unique combination of contractile proteins found in both cardiac and smooth muscle (13, 35). Some researchers have adopted the term “lymphatic muscle” to describe this smooth muscle layer because of these unique properties (35). Like other smooth muscle cell types, contraction of lymphatic smooth muscle can be driven by increases in [Ca2+]i (4, 37, 49). The phasic contractions are driven by oscillating action potentials that elicit transient increases in [Ca2+]i within lymphatic smooth muscle cells (24). In addition, these vessels display some degree of tone between phasic contractions, which is also a Ca2+-dependent process (3, 26, 46). L- and T-type voltage-gated Ca2+ channels appear to have an important role in the mechanism (27), although there is some controversy about this (51). Release and uptake of Ca2+ from internal stores also play an important role (46, 53). In addition, other signaling pathways, such as those driven by PKC and Rho/Rho kinase, also affect the contractile mechanisms in lymphatic vessels, probably by affecting the sensitivity to Ca2+ (26, 35, 47). Finally, the lymphatic endothelium can also affect lymphatic pumping. Endothelial cells may respond to agonists or physical forces such as shear stress, which can cause activation of endothelial nitric oxide (NO) and release of NO, which can then elicit relaxation of lymphatic smooth muscle through a cGMP-dependent mechanism (16, 18–20, 25). Collectively, many important details about the lymphatic contractile mechanism are known; however, much remains unknown, and the potential role of σ-receptors in the control of lymphatic pumping has not been explored.
In the present study, we investigated how afobazole affects the contractions of isolated rat mesenteric collecting lymphatic vessels. We tested the hypothesis that the σ-receptor agonist afobazole would depress lymphatic contractions and investigated the expression and localization of the σ1-receptor in rat mesenteric collecting lymphatics. The experimental model consisted of isolated, perfused collecting lymphatics from the rat mesentery. This model was chosen because it allowed us to study the direct effect of afobazole on lymphatic contractions without confounding influences from interstitial fluid flow and with tight control of the inflow and outflow pressures (6).
MATERIALS AND METHODS
Materials.
Afobazole was generously provided by IBC Genarium (Moscow, Russia). BD 1047 dihydrobromide (catalog no. 0956), BD 1063 dihydrochloride (catalog no. 0883), SM-21 maleate (catalog no. 0751), and N-nitro-l-arginine methyl ester (l-NAME) hydrochloride (catalog no. 0665) were obtained from Tocris Bioscience/R&D Systems (Minneapolis, MN). 4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM; catalog no. D23844) was from Life Technologies (Carlsbad, CA). Acetylcholine chloride (catalog no. A9101-10VL) and bradykinin (catalog no. B3259) were from Sigma-Aldrich (St. Louis, MO). Crystallized bovine albumin (purified BSA; catalog no. 10856), used for preparing physiological salt solutions, was from Affymetrix (Santa Clara, CA). Donkey serum (catalog no. D9663), used for blocking solutions, was from Sigma-Aldrich. Rabbit anti-σ1-receptor (catalog no. 42-3300) was from Invitrogen (Camarillo, CA). Mouse anti-α/γ-smooth muscle actin (catalog no. Mab1522) was from Millipore (Billerica, MA). Goat anti-vascular-endothelial (VE)-cadherin (catalog no. sc-6458) was from Santa Cruz Biotechnology (Santa Cruz, CA). Donkey anti-rabbit horseradish peroxidase-conjugated IgG (catalog no. ab97064) was from Abcam (Cambridge, MA). Alexa 488 donkey anti-rabbit IgG (catalog no. A21206), Alexa 488 donkey anti-mouse IgG (catalog no. A21202), Alexa 555 donkey anti-goat IgG (catalog no. A21432), Alexa 647 donkey anti-mouse IgG (catalog no. A31571), and Alexa fluor 633 hydrazide were from Life Technologies (Grand Island, NY). SIGMAR1 (catalog no. Rn00578590_m1) and GAPDH (catalog no. Rn01775763_g1) primers (TaqMan gene expression assays), used for PCR, were from Applied Biosystems/ThermoFisher Scientific (Waltham, MA). All other chemicals and reagents, unless otherwise specified, were purchased from Sigma-Aldrich.
Animal care and use.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of South Florida under protocol number IS00002179. The experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., 2011) and are reported here in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines. A total of 42 male Sprague-Dawley rats (5–9 wk of age) were purchased from Charles River (Wilmington, MA) and housed in a temperature (22°C)- and illumination (12:12-h light-dark cycle)-controlled environment. After arrival, rats were allowed to acclimate for 1 wk; standard rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan, Indianapolis, IN) and water were provided ad libitum. When possible, multiple lymphatics or tissue samples were obtained from a single rat for different experiments to minimize the total number of rats used. All possible measures were taken to minimize pain or suffering, including administration of general anesthesia before the experiments (see below). All rats were euthanized by extension of the laparotomy into the chest cavity and injection of Euthasol/SomnaSol (0.1 ml/450 g body wt) directly into the cardiac ventricle, in accordance with American Veterinary Medical Association guidelines for the euthanasia of animals.
Mesenteric collecting lymphatic isolation.
Collecting lymphatics were isolated as previously described (25). Briefly, rats were anesthetized (ketamine and xylazine at 90 and 9 mg/kg ip, respectively), and the depth of anesthesia was checked using interdigital pinch and palpebral blink reflex. A midline laparotomy was performed, and the small intestine and mesentery were exteriorized, excised, and placed in ice-cold albumin-physiological salt solution (APSS; 120 mM NaCl, 4.7 mM KCl, 2 mM CaCl2·2H2O, 1.2 mM MgSO4·7H2O, 1.2 mM NaH2PO4, 2 mM Na pyruvate, 5 mM glucose, 0.02 mM EDTA, 3 mM MOPS, and 1% BSA). Rats were then immediately euthanized (see Animal care and use). In each experiment, a section of mesentery that included the terminal ileum was pinned in a dissection chamber containing ice-cold APSS, and, with the aid of a stereomicroscope, a collecting lymphatic vessel (60- to 150-µm internal diameter and 0.5 cm long) was carefully dissected from surrounding adipose and connective tissue. The isolated lymphatic was transferred to an isolated vessel chamber (Living Systems Instrumentation, Burlington, VT) and mounted onto two resistance-matched glass micropipettes and secured with nylon thread. The chamber was transferred to an Accu-Scope 3032 inverted microscope equipped with a halogen lamp, ×10 objective, and charge-coupled device camera for video image acquisition (Living Systems). Intraluminal pressure was imposed using APSS-filled gravity manometers that fed to each micropipette. All experiments were performed with the vessel bathed in 37°C APSS with the imposed pressure from both micropipettes set at 2 cmH2O and a 30- to 45-min stabilization period to allow the vessel to equilibrate for baseline measurements. Only lymphatic vessels that displayed phasic contractions that reduced internal diameter during systole by ≥25% of the diastolic diameter were considered sufficiently viable for use in these studies.
Experimental protocols.
After cannulation, the vessels were allowed to equilibrate for 30–45 min to establish baseline contractions. When steady baseline pumping was established, the impact of the σ-receptor agonist afobazole on lymphatic contractions was evaluated by the addition of afobazole to achieve final concentrations of 50, 100, and 150 µM in the bath. These concentrations were chosen on the basis of previous work in which 50–150 µM afobazole was effective for inhibiting ATP-induced migration of glial cells and also mitigating acidosis-induced Ca2+ overload in neurons (11, 12). To investigate the roles of σ1- versus σ2-receptor activation by afobazole, lymphatics were treated for 20 min with the selective σ1-receptor antagonist BD 1047 (200 nM), the selective σ1-receptor antagonist BD 1063 (200 nM), or the selective σ2-receptor antagonist SM-21 (2 µM) before the addition of afobazole (50, 100, and 150 µM). These concentrations were also chosen on the basis of previous work (11, 12). The role of NO signaling was investigated using the NO synthase (NOS) inhibitor l-NAME (200 µM), which we previously used to block NOS (8, 25). Vessel diameters were tracked throughout each experiment, and the bath solution was changed to Ca2+-free APSS at the end of each experiment to determine the maximal passive diameter (MaxD) at the same luminal pressure, 2 cmH2O. Parameters used to characterize lymphatic pump function were calculated from the data (see Data analyses).
Total RNA isolation and PCR.
Mesenteric collecting lymphatic vessels (10 vessels/rat) were freshly isolated, placed on ice, sonicated, snap frozen in 300 µl of QIAzol lysis reagent (Qiagen, Valencia, CA), and stored at −80°C. Total RNA was extracted using chloroform and isopropanol, and samples were treated with DNase to remove genomic DNA. The RNA was then purified using the RNeasy MinElute Cleanup Kit (Qiagen) according to the manufacturer’s specifications, and the RNA pellet was eluted in 14 µl RNase-free water. RNA concentration and purity were determined using a spectrophotometer (NanoVue Plus, GE Healthcare, Piscataway, NJ). Total RNA (50 ng) was reverse transcribed into cDNA using the ProtoScript first-strand cDNA synthesis kit (New England Biolabs, Ipswich, MA). The cDNA was combined with TaqMan Fast Universal PCR Master Mix (Applied Biosystems/ThermoFisher Scientific) and primers specific for rat SIGMAR1 or rat GAPDH. PCRs were performed as follows: a 10-min hold at 95°C, 95°C for 15 s, and 60°C for 1 min (40 cycles) using a CFX Connect real-time PCR detection system (Bio-Rad, Hercules, CA). Primer sequences (National Center for Biotechnology Information reference sequence) were NM_030996.1 (SIGMAR1) and NM_017008.4 (GAPDH). PCR products were run on a 2% agarose gel, and bands were made detectable with SYBR green I nucleic acid gel stain (Life Technologies/ThermoFisher Scientific) and visualized under ultraviolet light using the Bio-Rad Chemi Doc XRS+ system with Quantity 1-D analysis software (Bio-Rad).
Immunoblot protocol.
Approximately 10 collecting lymphatic vessel segments/rat, freshly isolated from the mesentery, were placed in 200 µl of 1× RIPA buffer (4°C) containing Halt Protease and Phosphatase Inhibitor Cocktail (Pierce, Rockford, IL). The mixture was sonicated twice at 4°C for 5 s using a sonic dismembrator (model FB-120, Fisher Scientific, Asheville, NC) and frozen at −80°C. Protein concentrations were determined using the BCA protein assay (Pierce/Thermo Scientific, Waltham, MA) to equilibrate samples. Lysate was mixed with 4× NuPage LDS sample buffer containing reducing agent (Invitrogen, Grand Island, NY). Samples containing 30 µg protein were loaded into 4–20% Novex Bis-Tris gels (Invitrogen) for SDS-PAGE to separate proteins. The NexusPointer prestained protein ladder (BioNexus, Oakland, CA) was used to determine molecular weight versus mobility. Proteins were transferred from the gels to Immobilon-P PVDF membranes (Millipore) by wet transfer. Membranes were blocked with 5% BSA in Tris-buffered saline with Tween 20. The primary antibody was 1:1,000-diluted rabbit anti-σ1-receptor (catalog no. 42-3300, Invitrogen). The secondary antibody was 1:10,000-diluted donkey anti-rabbit IgG-HRP (catalog no. ab97064). Bands were visualized using West Pico SuperSignal reagent (Pierce/Thermo Scientific) and a Chemi Doc XRS+ system with Quantity 1-D analysis software (5-min exposure, Bio-Rad).
Immunofluorescence labeling and confocal microscopy.
Rat mesenteric lymphatics were isolated and excised, and for each isolated vessel, one end was mounted onto a glass micropipette filled with APSS in a custom chamber for fixation and labeling, as previously described (25). The other end of the lymphatic was left free to float in an APSS bath to allow for APSS to be pulled into or pushed out of the vessel lumen by gentle application of negative or positive pressure, respectively, on the micropipette via an attached 1-ml syringe. Each vessel was fixed with 4% paraformaldehyde for 10–15 min at room temperature with positive pressure in the vessel lumen and then washed twice for 5 min each with 100 mM glycine buffer and once for 5 min with Ca2+/Mg2+-free Dulbecco’s PBS (CMF-DPBS). Ice-cold (−10 to −20°C) acetone was applied for 5 min to permeabilize the cell membranes, which were then washed three times for 5 min each with CMF-DPBS. A blocking solution consisting of 5% normal donkey serum in CMF-DPBS was applied for 30 min at room temperature, and vessels were incubated overnight with combinations of primary antibodies in antibody dilution buffer (151 mM NaCl, 17 mM trisodium citrate, 2% donkey serum, 1% BSA, 0.05% Triton X-100, and 0.02% NaN3) at 4°C: 1:3,000-diltued mouse anti-α/γ-smooth muscle actin (catalog no. Mab1522), 1:50-diluted goat anti-VE-cadherin (catalog no. sc-6458), and 1:60-diluted rabbit anti-σ1-receptor. Labeling controls received antibody dilution buffer containing no primary antibody. After an overnight incubation, vessels were rinsed (3 times for 10 min) with antibody wash solution (151 mM NaCl, 17 mM trisodium citrate, and 0.05% Triton X-100). Vessels were then incubated for 30–60 min at room temperature with antibody dilution buffer containing secondary antibodies: 1:400-diluted Alexa 488 donkey anti-rabbit IgG (catalog no. A21206), 1:400-diluted Alexa 488 donkey anti-mouse IgG (catalog no. A21202), 1:400-diluted Alexa 555 donkey anti-goat IgG (catalog no. A21432), and 1:400-diluted Alexa 647 donkey anti-mouse IgG (A31571). Alexa Fluor 633 hydrazide (1.0 μM) was also added during this time period to label extracellular matrix elastin fibers (10, 41) in some of the whole mounts. Three 10-min rinses with antibody wash solution were performed, and each vessel was removed from its cannula, placed on a glass slide with a SecureSeal imaging spacer in 20 µl of Prolong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (Life Technologies) or VECTASHIELD with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA), and covered with a no. 1 glass coverslip. Care was taken to keep the lymphatic vessel lumen patent for better view of vessel structure. Confocal z-stack images (step size = 0.5–0.75 μm) of the vessels were obtained with an Olympus FV1200 spectral inverted laser scanning confocal microscope and a ×60 (PLAPON60×Oil, 1.42 or 1.30 numerical aperture) objective with ×1.3–1.5 zoom at the Lisa Muma Weitz Advanced Microscopy and Cell Imaging Core at the University of South Florida. Imaris software (Bitplane, Concord, MA) was used to produce three-dimensional models of z stacks for interpretation. FIJI/ImageJ open-source imaging software (http://fiji.sc) was also used to view and process confocal image stacks into the figures.
Cell culture.
Primary human dermal lymphatic endothelial cells (HDLEC-juvenile, PromoCell, Heidelberg, Germany) were seeded onto gelatin (0.75%)-coated 100-mm culture dishes. Cells were routinely maintained in endothelial cell growth medium (EGM2-MV, Lonza, Walkersville, MD), with medium changed every 48 h. For all experiments, passage 2–3 endothelial cells were used. For NO imaging experiments, confluent endothelial monolayers were washed with 1× DPBS, incubated in trypsin-EDTA (0.25%), neutralized with EGM2-MV, and pelleted at 2,000 rpm/min. Resuspended endothelial cells were seeded on 18-mm round microscope coverglasses coated with gelatin (0.75%) at a density of 0.1 × 106 cells and grown to 70–80% confluence.
NO imaging measurements.
Changes in intracellular NO concentrations were measured in cultured primary endothelial cells using fluorescence imaging techniques and the NO-sensitive dye DAF-FM. Cells were loaded using membrane-permeable DAF-FM. Cells seeded on coverslips (see Cell culture) were incubated for 1 h at 37°C in HBSS (135 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4) with 8 μM DAF-FM and 0.8% dMSO. Coverslips were washed in DAF-FM-free HBSS before experiments were performed. Cells were illuminated with 495-nm light for 400 ms at 0.3 Hz (Lambda DG-4, Sutter Instruments, Novato CA), and fluorescence emissions at 535 nm were collected using a digital charge-coupled device camera (Sensicam, Cooke, Auburn Hills, MI). Imaged cells were perfused with control solution and solutions containing 150 μM afobazole, 10 μM acetylcholine, or 1 μM bradykinin using a perfusion system consisting of 250-μm-diameter glass tubes positioned 500 μm away with flow rates of 300 μl/min.
Data analyses.
For the lymphatic pumping experiments, data sets of lymphatic diameter over time, before and after various interventions such as pressure steps or addition of drugs, were used for analysis. Parameters used to characterize lymphatic pump function were determined from the lymphatic intraluminal diameter measurements in the manner previously described (16, 26). These parameters include contraction frequency (CF), end-diastolic diameter (EDD), end-systolic diameter (ESD), amplitude of contraction (AMP; AMP = EDD − ESD), ejection fraction [EF; EF = (EDD2 − ESD2)/(EDD2)], and fractional pump flow (FPF; FPF = CF × EF). EDD, ESD, and AMP were normalized to MaxD to account for variability in the resting diameter of different lymphatics. Baseline data for all drug experiments were the averages for the 2-min period before drug administration. For each concentration of afobazole, the 2-min increment starting 4 min after the addition was used to calculate the mean data (i.e., means represent 4–6 min after the addition of each concentration, and each concentration was held for 10 min). These time points after afobazole administration represented the peak responses. When σ-receptor antagonists or l-NAME was added, we used the last 2-min period just before addition of afobazole to calculate the mean parameters due to any effects of the antagonist alone.
Summarized data are means ± SE. For all experiments, we tested the hypothesis that the various experimental interventions would produce a difference in the baseline pumping parameters just before the addition of afobazole, which served as controls. Prism 6 (GraphPad Software, La Jolla, CA) was used for statistical analyses, with the threshold for significance set at P < 0.05. The responses of lymphatic vessels over time to various treatments were evaluated by repeated-measures ANOVA without assumption of sphericity and with application of the Geisser-Greenhouse correction to minimize the chance of a type I error. When a significant trend was identified by the ANOVA, we used Dunnett’s test (with individual variances, rather than the pooled variance, because sphericity was not assumed) to compare individual time points with a single control. For experiments on the impact of afobazole alone on lymphatic contractions, the baseline period was used as the control in Dunnett’s test. For experiments with a σ-receptor antagonist added before afobazole, the period during exposure to the antagonist alone served as the control for Dunnett’s test, because we wanted to compare this particular treatment with both baseline and the afobazole treatment groups. When two different groups (e.g., l-NAME vs. vehicle control) receiving treatments over time were compared, a repeated-measures two-way ANOVA design was used, with Dunnett’s test used for post hoc analysis when appropriate. P values are shown for all multiple comparisons that were significant and, in some cases, those that “looked significant” but were not. P values represent the multiplicity-adjusted P value for the post hoc test or the overall ANOVA P value, whichever was greater.
For the NO imaging experiments using cultured cells, data files were collected with SlideBook 4.02 (Intelligent Imaging Innovations). Emission intensities of individual fluorescent cells were measured as functions of time and exported to SigmaPlot (version 11, Systat Software, San Jose, CA). Intensities were normalized to initial magnitudes and slopes (bleaching rates) using linear fits of 5-min control measurements for each cell. Changes in NO production were determined as changes in the slope of the emission intensity versus time traces for each cell, before and after normalization. Summarized data are presented as means ± SE. Repeated-measures one-way ANOVA followed by Dunnett’s test was used, and significance was accepted at P < 0.05.
RESULTS
Afobazole reduces rat mesenteric lymphatic pumping.
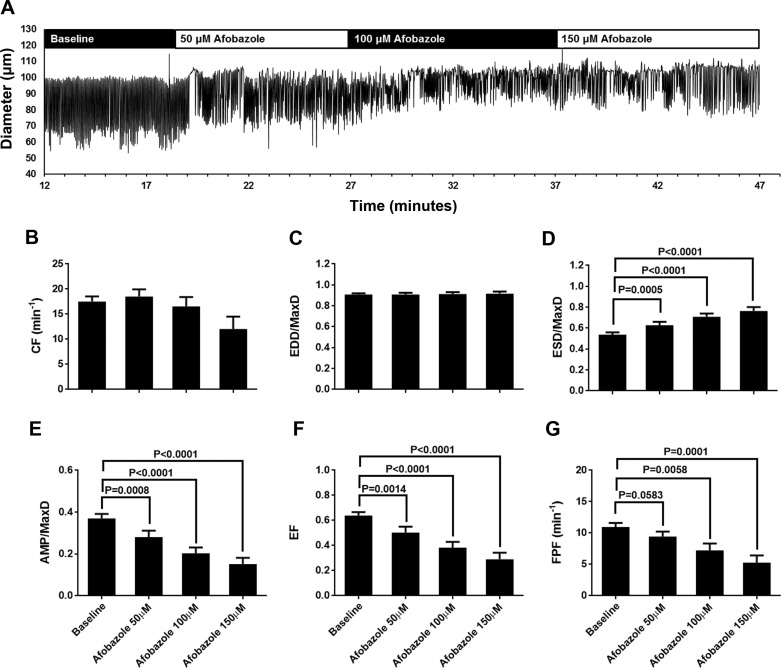
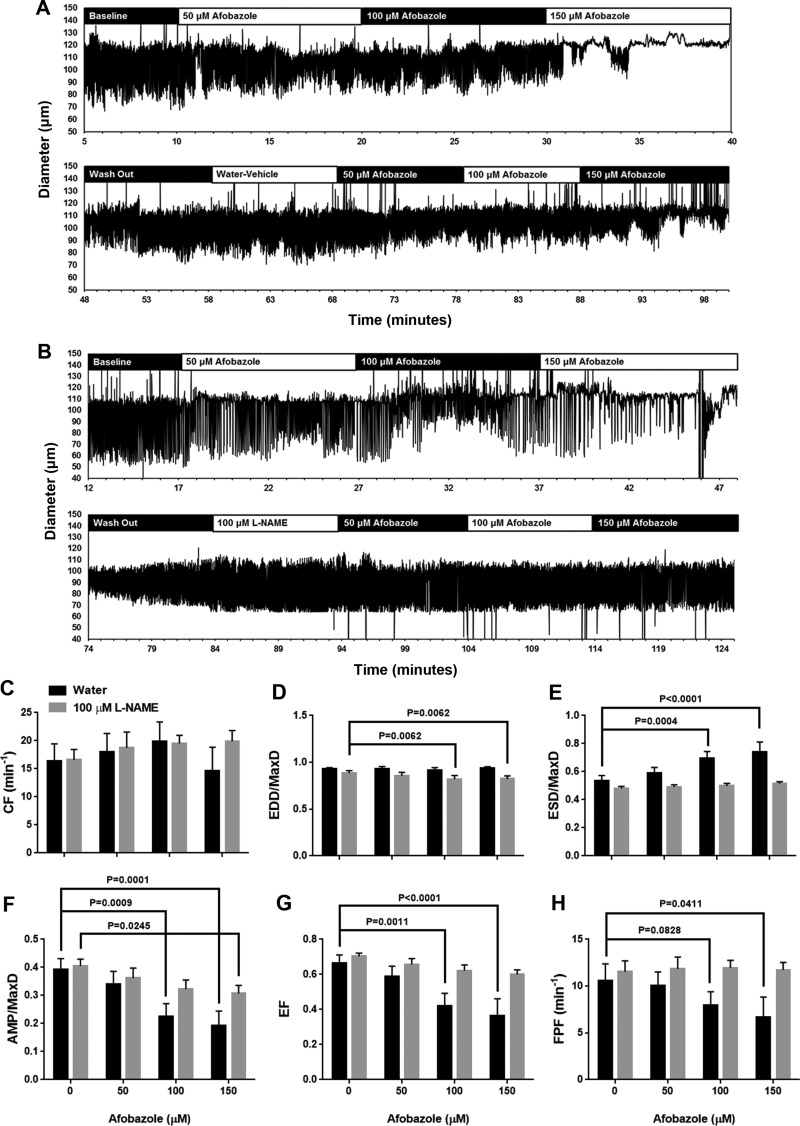
We investigated the impact of the σ-receptor agonist afobazole on the intrinsic pumping of isolated rat mesenteric lymphatics (Fig. 1). As shown in the representative trace in Fig. 1A, with afobazole treatment (50, 100, and 150 µM) there was an apparent elevation in ESD, resulting in a smaller AMP. Summarized data from 19 different afobazole-treated lymphatics (each isolated from a different rat) are shown in Fig. 1, B–G. Afobazole did not cause a significant change in CF (Fig. 1B) or EDD/MaxD (Fig. 1C). However, afobazole significantly increased ESD/MaxD compared with baseline, starting with the lowest concentration (50 µM) and in a concentration-related fashion (Fig. 1D). Afobazole also significantly decreased AMP/MaxD and EF at all concentrations tested in a concentration-related manner (Fig. 1, E and F). Finally, 100 and 150 µM afobazole significantly decreased FPF, the product of CF and EF, which is a measure of overall pump flow, compared with baseline (Fig. 1G). To our knowledge, these data provide the first demonstration that afobazole can modulate contractions of collecting lymphatics.
Fig. 1.
Afobazole modulates rat mesenteric lymphatic contractions. A: representative trace of mesenteric lymphatic pumping in the presence of increasing concentrations of the σ1-receptor agonist afobazole (50–150 µM). B–G: summarized data (means ± SE) from experiments with 19 cannulated lymphatic vessels, each from a different rat. Data represent average values of each parameter obtained during the last 2 min of the baseline period and a 2-min period during each afobazole concentration starting 4 min after administration of afobazole. B and C: 50–150 µM afobazole caused no significant trend in contraction frequency (CF; P = 0.0502) or end-diastolic diameter (EDD) normalized to maximal passive diameter (MaxD; P = 0.5815) as determined by repeated-measures ANOVA. D: 50–150 µM afobazole significantly increased EDD/MaxD. E and F: 50–150 µM afobazole significantly decreased amplitude of contraction (AMP) normalized to MaxD (AMP/MaxD) and ejection fraction (EF). G: 100 or 150 µM afobazole significantly decreased fractional pump flow (FPF) compared with baseline. Data were analyzed by repeated-measures ANOVA with the Geisser-Greenhouse correction; when this test identified a significant trend, multiple comparisons were performed using Dunnett’s test with baseline as the control.
The σ1-receptor mediates afobazole-induced changes in lymphatic pumping.
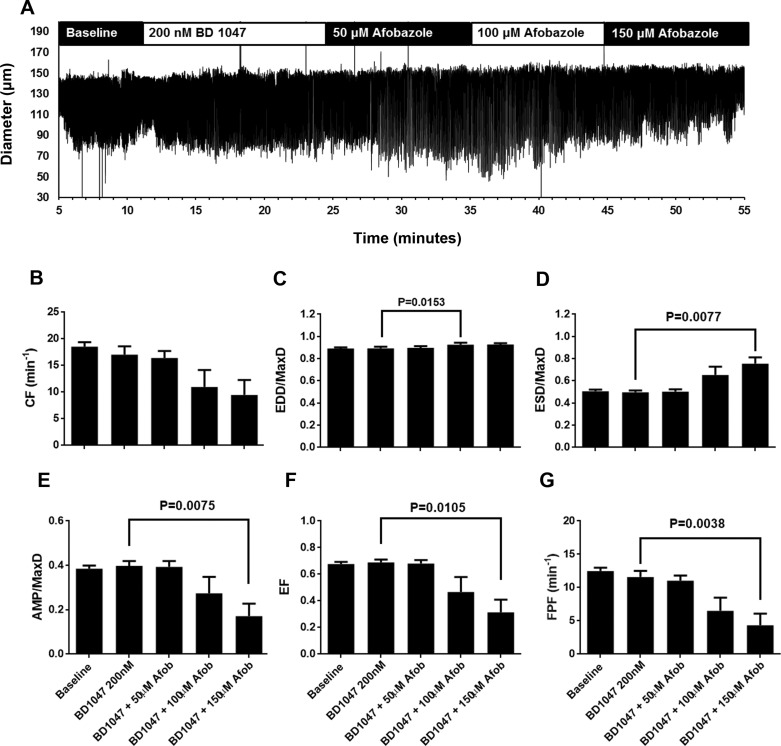
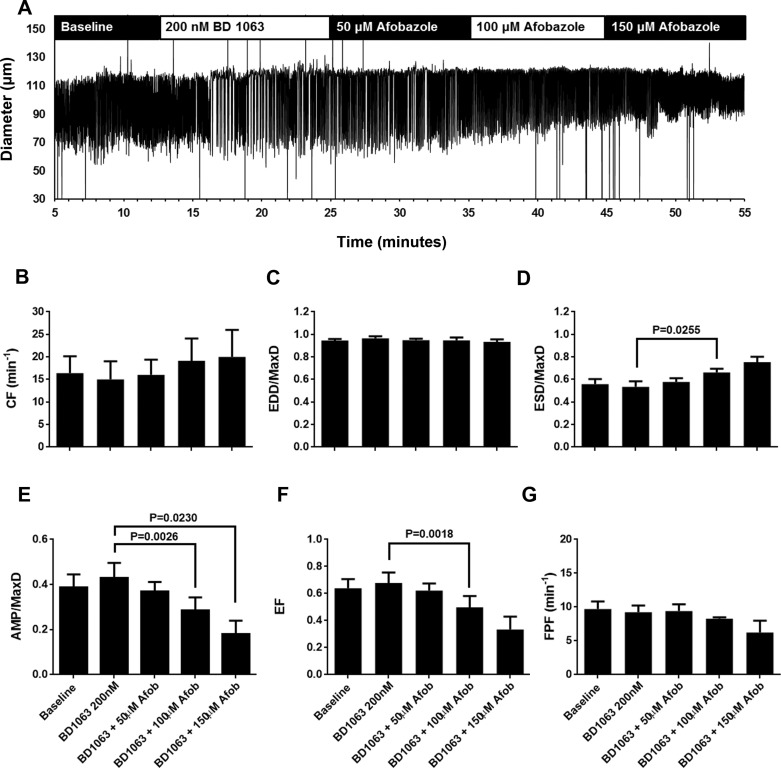
Because afobazole is known to be an agonist for σ1-receptors, we next tested the ability of σ1-receptor antagonists to block the effects of afobazole on isolated rat mesenteric collecting lymphatics. Lymphatics were pretreated with the selective σ1-receptor antagonist BD 1047 before the addition of afobazole (Fig. 2). As shown in the representative trace in Fig. 2A, no remarkable changes in the contractile patterns were apparent after addition of BD 1047, and after the subsequent addition of afobazole the effects on contraction appeared diminished compared with the trace shown in Fig. 1A. In the summarized data, in the presence of BD 1047, 50 µM afobazole did not elicit significant changes in the lymphatic pumping parameters (Fig. 2, B–G), in contrast to the changes in ESD, AMP, EF, and FPF observed with 50 µM afobazole alone (Fig. 1). While 100 µM afobazole caused an increase in EDD/MaxD in the presence of BD 1047 (Fig. 2C), significant changes in ESD, AMP, EF, and FPF were not detected until 150 µM afobazole was applied (Fig. 2, D–G). We also tested a second selective σ1-receptor antagonist, BD 1063 (Fig. 3). When we examined the diameter traces shown in Fig. 3A, the response to afobazole after treatment with BD 1063 appeared to be diminished compared with that shown in Fig. 1A. In addition, no significant changes in the pumping parameters were detected with the addition of 50 µM afobazole in the presence of BD 1063 (Fig. 3, B–G). Only after the addition of 100 µM afobazole were the significant changes in ESD, AMP, and EF observed. While the effect of BD 1063 alone on AMP was significantly different from the effect of BD 1063 + 150 µM afobazole (Fig. 3E), other parameters, such as ESD and EF, despite having means that appear very different from BD 1063 alone, had large enough variances that their P values were >0.05 and, thus, were not accepted as significantly different. In addition, with BD 1063 pretreatment, afobazole caused no significant changes in FPF at any of the concentrations tested (Fig. 3G), unlike the results we observed with afobazole alone (Fig. 1G). Combined with BD 1047 or BD 1063, a higher concentration of afobazole was required to elicit significant changes in ESD, AMP, EF, or FPF, suggesting that afobazole is acting, at least in part, through σ1-receptor activation.
Fig. 2.
BD 1047, a specific antagonist of the σ1-receptor, reduced effects of afobazole (Afob) on lymphatic contractions. A: representative trace of vessel diameter as a function of time during baseline, in the presence of BD 1047 (200 nM), and after subsequent addition of 50, 100, and 150 μM afobazole. B–G: summarized data (means ± SE) from 7 isolated lymphatic vessels, each from a different rat. Data represent average values of each parameter obtained during the last 2 min of the baseline and BD 1047 periods and a 2-min period during each afobazole concentration starting 4 min after administration of afobazole. B: pretreatment with BD 1047 followed by afobazole caused an apparent trend in contraction frequency (CF) that was significant (P = 0.0266) when tested by repeated-measures ANOVA. However, multiple comparisons with Dunnett’s test revealed no significant differences between BD 1047 treatment alone and the time points after which afobazole was administered. C: in the presence of BD 1047, treatment with 100 µM (but not 50 or 150 µM) afobazole caused a significant increase in end-diastolic diameter (EDD) normalized to maximal passive diameter (MaxD; 0.891 ± 0.010 in the presence of BD 1047 alone vs. 0.9267 ± 0.018 in the presence of BD 1047 + 100 µM afobazole, mean ± SE, P = 0.0183). D: only 150 µM afobazole caused a significant increase in end-systolic diameter (ESD) normalized to MaxD (ESD/MaxD) in the presence of BD 1047. E–G: only 150 µM afobazole caused a significant decrease in amplitude of contraction (AMP) normalized to MaxD (AMP/MaxD), ejection fraction (EF), and fractional pump flow (FPF) in the presence of BD 1047. Data were analyzed by repeated-measures ANOVA with the Geisser-Greenhouse correction; when this test identified a significant trend, multiple comparisons were performed using Dunnett’s test with the inhibitor alone as the control.
Fig. 3.
BD 1063, a specific σ1-receptor antagonist, reduced effects of afobazole (Afob) on lymphatic contraction. A: representative trace of pumping of an isolated, cannulated rat mesenteric lymphatic vessel during baseline, in the presence of BD 1063 (200 nM), and after subsequent addition of 50, 100, and 150 μM afobazole. B–G: summarized data (means ± SE) from 4 isolated lymphatic vessels, each from a different rat. Data represent average values of each parameter obtained during the last 2 min of the baseline and BD 1063 periods and a 2-min period during each afobazole concentration starting 4 min after administration of afobazole. B and C: in the presence of BD 1063, afobazole (50–150 µM) caused no significant trend in contraction frequency (CF; P = 0.2752) or end-diastolic diameter (EDD) normalized to maximal passive diameter (MaxD; P = 0.3963) as determined by repeated-measures ANOVA. D: only 100 µM afobazole caused a significant increase in end-systolic diameter (ESD) normalized to MaxD (ESD/MaxD) in the presence of BD 1063 compared with BD 1063 alone. Treatment with 150 µM afobazole, despite achieving a higher mean than 100 µM afobazole treatment, showed more variability, and compared with BD 1063 alone, produced a P value that was not considered significant. E: only 100 and 150 µM afobazole in the presence of BD 1063 significantly reduced amplitude of contraction (AMP) normalized to MaxD (AMP/MaxD) compared with BD 1063 alone. F: only 100 µM afobazole in the presence of BD 1063 significantly reduced ejection fraction (EF) compared with BD 1063 alone. Despite a lower mean EF, 150 µM afobazole in this case had a greater variance and, compared with BD 1063 alone, produced a P value that was not considered significant. G: no significant changes in fractional pump flow (FPF) were apparent when afobazole was applied at 50–150 µM in the presence of BD 1063 (P = 0.2492, repeated-measures ANOVA). Data were analyzed by repeated-measures ANOVA with the Geisser-Greenhouse correction; when this test identified a significant trend, multiple comparisons were performed using Dunnett’s test with the inhibitor alone as the control.
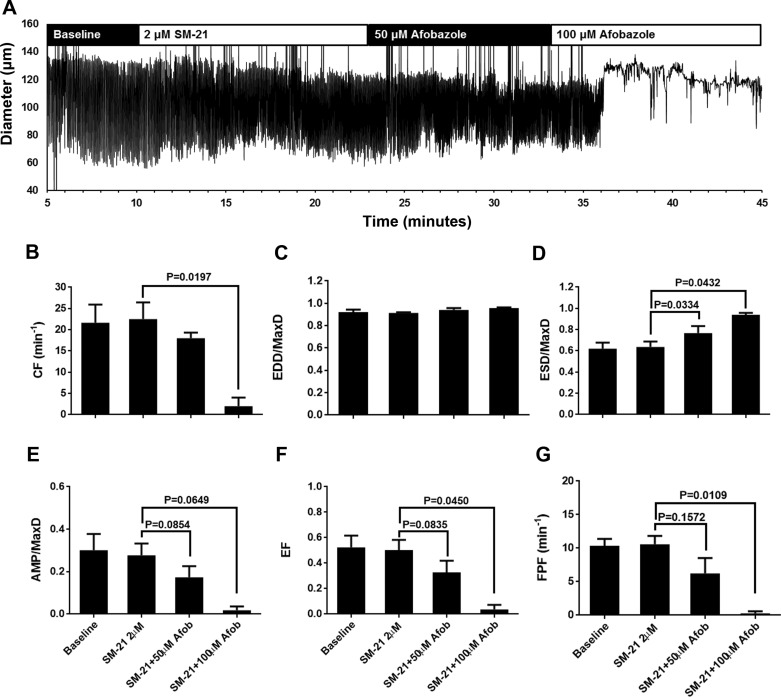
We also tested the ability of a specific antagonist of the σ2-receptor, SM-21, to inhibit afobazole-induced changes in lymphatic contractions. SM-21, applied at a final bath concentration of 2 µM, did not change the pumping pattern of isolated rat mesenteric lymphatics from baseline and also did not appear to inhibit the effects of afobazole (Fig. 4A). In three of the four lymphatic vessels tested, pumping ceased after the addition of 100 µM afobazole when SM-21 was present, resulting in a significant decrease in CF compared with SM-21 alone (Fig. 4B). EDD/MaxD did not change with SM-21 or with subsequent afobazole (Fig. 4C). In addition, in the presence of SM-21, 50 µM afobazole elicited a significant elevation in ESD/MaxD (Fig. 4D), similar to that observed with afobazole alone (Fig. 1D). While significant decreases in AMP/MaxD, EF, and FPF were not achieved with 50 µM afobazole, when 100 µM afobazole was added, these parameters decreased markedly (Fig. 4, E and F). These data suggest that the σ2-receptor does not mediate the afobazole-induced increase in ESD/MaxD in rat mesenteric collecting lymphatic contractions. Interestingly, blockade of this receptor appears to facilitate a reduction in CF by afobazole.
Fig. 4.
SM-21, a specific antagonist of the σ2-receptor, did not block the effects of afobazole (Afob). A: representative trace of an isolated rat mesenteric lymphatic pumping in the presence of SM-21 (2 μM) and afobazole (50 and 100 μM). Lymphatics frequently stopped pumping with 100 µM afobazole in the presence of SM-21, so only 50 and 100 µM afobazole were used for analysis. B–G: summarized data (means ± SE) from 4 isolated lymphatic vessels, each from a different rat. Data represent average values of each parameter obtained during the last 2 min of baseline and SM-21 periods and a 2-min period during each afobazole concentration starting 4 min after administration of afobazole. Mean values shown for the 100 µM afobazole groups include lymphatics that stopped pumping. B and C: application of 100 µM afobazole in the presence of SM-21 caused a significant decrease in contraction frequency (CF) and no change in end-diastolic diameter (EDD) normalized to maximal passive diameter (MaxD). D: 50 and 100 μM afobazole significantly elevated end-systolic diameter (ESD) normalized to MaxD (ESD/MaxD) in the presence of SM-21 compared with SM-21 alone. For lymphatics that stopped pumping, diameter/MaxD was used for the ESD/MaxD calculations. E–G: in the presence of SM-21 and compared with SM-21 alone, 100 µM afobazole significantly decreased amplitude of contraction normalized to MaxD (AMP/MaxD), ejection fraction (EF), and fractional pump flow (FPF). For lymphatics that stopped pumping, the value for these parameters was zero. Data were analyzed by repeated-measures ANOVA with the Geisser-Greenhouse correction; when this test identified a significant trend, multiple comparisons were performed using Dunnett’s test with the inhibitor alone as the control.
The σ1-receptor is expressed in rat mesenteric lymphatics.
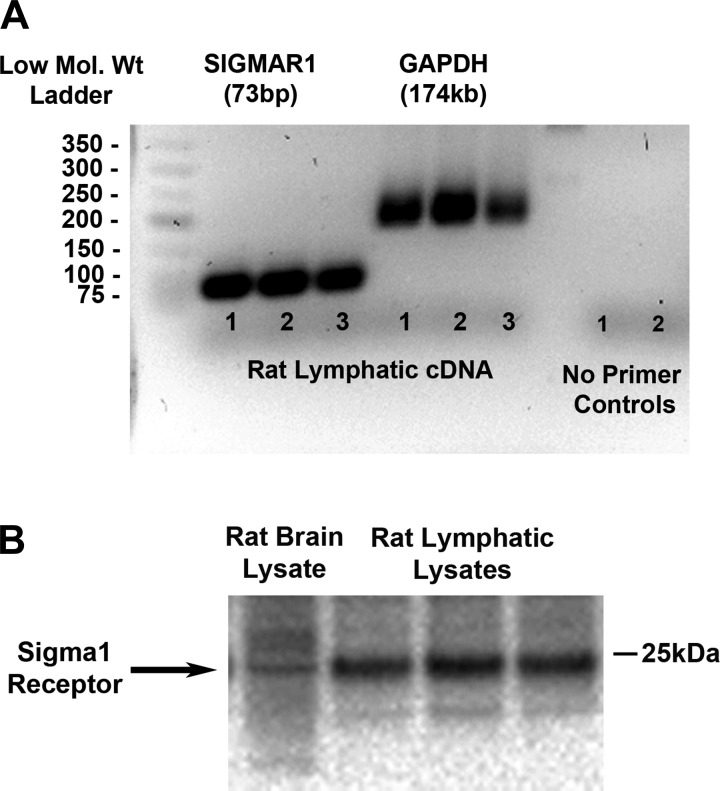
Before this study, only the σ1-receptor had been cloned (21). Thus, primers and antibodies were available for the σ1-receptor and not for the σ2-receptor, which was revealed to be TMEM97 after our study was completed (1). Based on our data above using the pharmacological agents, we expected to find σ1-receptor mRNA and protein expression in rat collecting mesenteric lymphatics. Data obtained from PCR experiments showed that primers specific for the rat σ1-receptor (SIGMAR1) amplified a 74-bp cDNA product (3 samples from 3 rats), consistent with the amplicon size for the σ1-receptor described by the manufacturer (Applied Biosystems) (Fig. 5A). Primers specific for rat GAPDH were also used and amplified a 174-bp cDNA product. Two rat lymphatic cDNA samples were also run with no primers as controls. Western blot analysis revealed that the σ1-receptor (∼25 kDa) is present in protein lysate (30 µg) obtained from isolated rat mesenteric lymphatic vessels (Fig. 5B). Bands represent samples from three different rats. Rat brain lysate (10 µg) was used as a positive control. Together, these data show positive identification of the σ1-receptor in isolated rat mesenteric lymphatic vessels.
Fig. 5.
σ1-Receptor is expressed in rat mesenteric lymphatics. A: rat mesenteric lymphatics were harvested (10 lymphatic vessels per rat), and total RNA (50 ng) from mesenteric lymphatic vessels (3 samples from 3 different rats) was reverse transcribed into cDNA and RT-PCR was performed with specific primer/probe (FAM) sets for rat SIGMAR1 (NM_030996.1) and rat GAPDH (NM_017008.4). Two cDNA samples were also run with no primers as controls. B: protein lysates (6 samples from 6 rats, only 3 are shown) were prepared for SDS-PAGE and Western blot analysis for the detection of σ1-receptor expression. With use of a rabbit anti-σ1-receptor antibody (Invitrogen) at 0.25 µg/ml (specific for the mouse and rat but not human) and donkey anti-rabbit horseradish peroxidase-conjugated secondary antibody at 1:10,000, a single band was detected at ∼25 kDa in rat lymphatic (30 µg) and rat brain (10 µg) lysates.
The σ1-receptor is mainly localized in the intimal layer of rat mesenteric lymphatic collecting vessels.
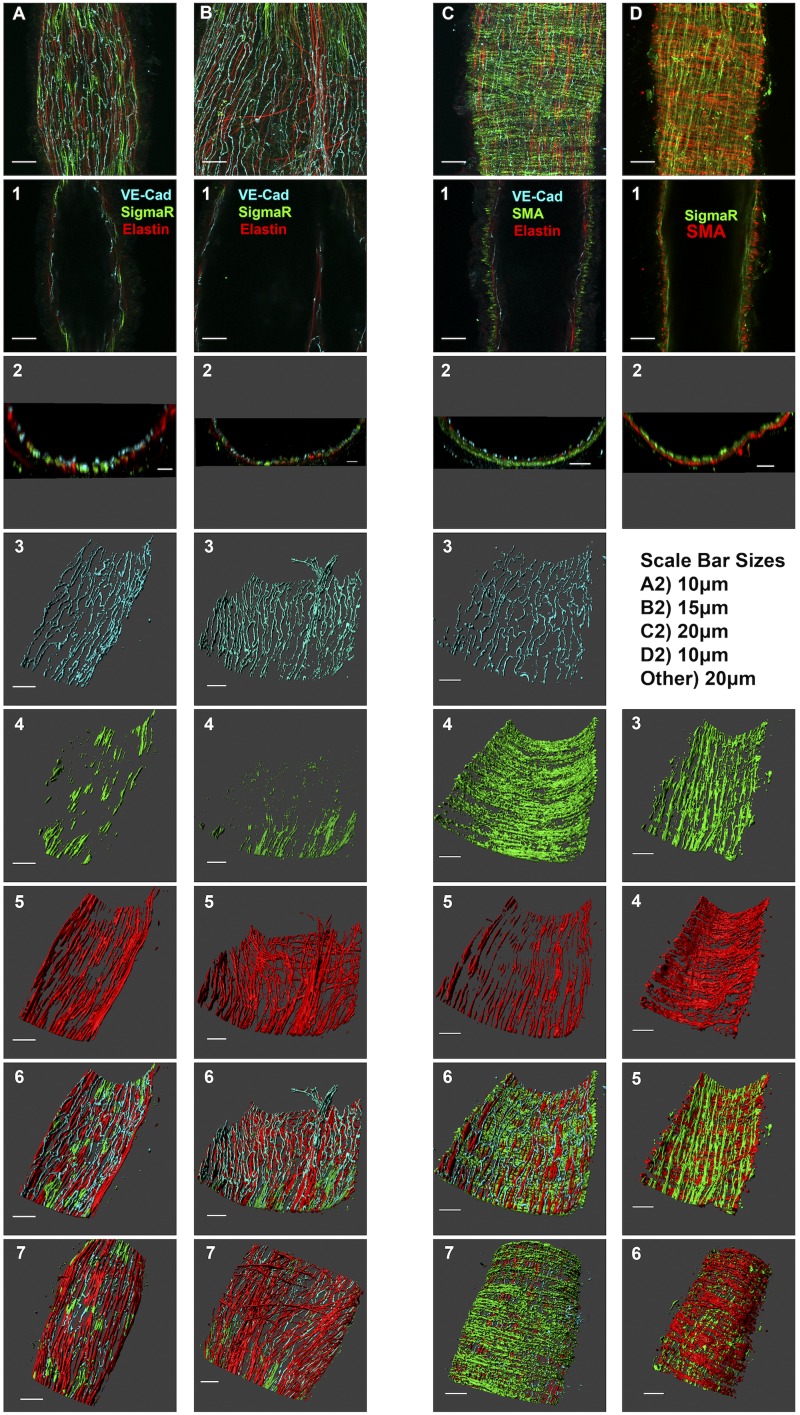
The σ1-receptor was also visualized using whole mount immunofluorescence labeling and laser scanning confocal microscopy of isolated rat mesenteric lymphatic vessels (Fig. 6). In preliminary experiments, the labeling pattern of the σ1-receptor appeared fibrous and near the inner layer of the vessel (data not shown), so we counterlabeled the vessels to view the endothelial and elastin layers. A maximum-intensity projection of a confocal z-stack of a rat isolated mesenteric lymphatic vessel, with labeling of the σ1-receptor, VE-cadherin (an endothelial cell junction marker), and elastin is shown in Fig. 6A. In addition, views of a single confocal slice (Fig. 6A,1), an orthogonal slice (cross section; Fig. 6A), and three-dimensional renderings of each channel (Fig. 6A,3−5) as well as composite views of the luminal (Fig. 6A,6) and abluminal (Fig. 6A,7) sides of the vessel wall are shown. This vessel can also be viewed in Supplemental Movie S1 (see Supplemental Material for this article, available at the American Journal of Physiology-Heart and Circulatory Physiology website). Both punctate and fibrous immunoreactive sites were observed with the σ1-receptor antibody (Fig. 6A,4). In the three-dimensional reconstructions (Fig. 6A,6 and 7), the punctate sites appearred to be within endothelial cells, whereas the fibrous sites appearred to be located at the endothelial-elastin interface. In a different vessel labeled in the same fashion, but also showing an intraluminal valve leaflet, a somewhat similar pattern of σ1-receptor, VE-cadherin, and elastin labeling was generally observed, but also with little σ1-receptor within the valve leaflet (Fig. 6B; also see Supplemental Movie S2). To confirm that the σ1-receptor labeling was largely located in the endothelial and inner elastin layer, and not in the smooth muscle, we also labeled for smooth muscle actin. Figure 6C and Supplemental Movie S3 show the distinction between the endothelial, elastin, and smooth muscle layers with VE-cadherin, elastin, and smooth muscle actin labeling, respectively. Labeling of the σ1-receptor and counterlabeling with smooth muscle actin are shown in Fig. 6D and Supplemental Movie S4. σ1-Receptor labeling was mostly found on the inner (luminal) side of the smooth muscle layer. Collectively, these confocal imaging experiments suggest that the σ1-receptor is mostly located in the inner, endothelial layer of rat collecting lymphatic vessels and in close contact with parts of elastin fibers that are adjacent to the endothelium.
Fig. 6.
Localization of the σ1-receptor in isolated rat collecting lymphatic vessels. A and B: images of two different collecting lymphatic vessels that were labeled for vascular-endothelial (VE)-cadherin, the σ1-receptor, and elastin. C: labeling for VE-cadherin, elastin, and smooth muscle actin. D: σ1-receptor and smooth muscle actin labeling. Top images show a maximum-intensity z-projection obtained with a ×60 objective. A−D,1: representative confocal slices about halfway through the z-stack, with the target proteins denoted by the colors in the label. A−D,2: orthogonal projections (5 µm thickness) representing a cross-sectional view of the vessel. Images 3–5 in A–C and images 3 and 4 in D show three-dimensional representations of individual channels, with the luminal side of the vessel facing upward. Image 6 in A–C and image 5 in D show three-dimensional composites of all channels, with the luminal side of the vessel facing upward. Image 7 in A–C and image 6 in D show three-dimensional composites of all channels but with the abluminal side of the vessel facing upward. Each labeling scheme is representative of 3 vessels from 3 rats each.
Inhibition of NOS attenuates the afobazole-induced reduction of lymphatic pumping.
Because the σ1-receptor appeared to be located mainly in association with the endothelium, we explored the possibility that the increases in ESD/MaxD and reductions in AMP/MaxD, EF, and FPF may be due to an endothelium-dependent mechanism. Production of NO in endothelial cells seemed a logical starting point because of the well-known role of NO in relaxation of blood vessels and lymphatic vessels. To test the role of a NO-dependent mechanism, the NOS inhibitor l-NAME or a vehicle control (water) was applied before afobazole (Fig. 7). In these experiments, we made sure to test the response of each lymphatic vessel to afobazole first in the absence of l-NAME or vehicle and then after a washout, in the presence of l-NAME or vehicle. The trace in Fig. 7A shows the results from an isolated lymphatic vessel that was treated sequentially with 50, 100, and 150 µM afobazole; after a washout period, the vessel was treated with vehicle (water) and then again with 50, 100, and 150 µM afobazole. In this trace of diameter over time, it is evident that, during both rounds of afobazole treatment, the lymphatic vessel responded in a fashion similar to that shown in Fig. 1, although the response to the second application of afobazole might be somewhat diminished (Fig. 7A). The trace in Fig. 7B shows the response to application of l-NAME after the washout period. In this case, after l-NAME treatment, when afobazole was applied a second time, no apparent response was detected (Fig. 7B). Summarized data showing the responses to 50, 100, and 150 µM afobazole in the presence of vehicle (water) or l-NAME are shown in Fig. 7, C–H. No differences in CF were detected in either group (Fig. 7C). In the vehicle-treated group, afobazole caused no changes in EDD/MaxD. However, in the l-NAME-treated group, a significant decrease in EDD/MaxD was detected after the addition of 100 and 150 µM afobazole compared with l-NAME treatment alone (Fig. 7D). In the vehicle-treated group, 100 and 150 µM afobazole elicited a significant increase in ESD/MaxD. However, in the presence of l-NAME, this was abolished (Fig. 7E). In the vehicle-treated group, afobazole at 100 and 150 µM also elicited significant decreases in AMP/MaxD but, in the presence of l-NAME, elicited a significant decrease only when applied at 150 µM (Fig. 7F). Significant reductions in EF and FPF elicited by 100 and 150 µM afobazole in the vehicle group were absent in the l-NAME-treated group (Fig. 7, G and H). Collectively, these data suggest that pretreatment with l-NAME inhibits afobazole-induced depression of lymphatic pumping.
Fig. 7.
The nitric oxide synthase inhibitor N-nitro-l-arginine methyl ester (l-NAME) blocks afobazole-induced changes in lymphatic pumping. A: representative trace of a lymphatic vessel first treated for 10 min each with 50, 100, and 150 µM afobazole followed by a washout period for reestablishment of contractions similar to baseline and then treated with vehicle (water) and 50, 100, and 150 µM afobazole. B: representative trace of a lymphatic vessel first treated for 10 min each with 50, 100, and 150 µM afobazole followed by a washout period for reestablishment of contractions similar to baseline and then treated with l-NAME and 50, 100, and 150 µM afobazole. C–H: summarized data (means ± SE) from 5 isolated lymphatic vessels in the water-treated group and 7 isolated lymphatic vessels in the l-NAME-treated group. Each vessel was from a different rat. Data represent average values of each parameter obtained during the last 2 min of baseline and water or l-NAME periods and a 2-min period during each afobazole concentration staring 4 min after the administration of afobazole. C: contraction frequency (CF) did not significantly change in either group (two-way ANOVA, P = 0.1470). D: end-diastolic diameter (EDD) normalized to maximal passive diameter (MaxD) was significantly elevated in l-NAME-treated lymphatics after treatment with 100 and 150 µM afobazole. E: end-systolic diameter (ESD) normalized to MaxD (ESD/MaxD) was significantly elevated in the water- but not l-NAME-treated group after 100 and 150 µM afobazole. F: amplitude of contraction (AMP) normalized to MaxD (AMP/MaxD) was significantly reduced in the water-treated group after 100 and 150 µM afobazole but only after 150 µM afobazole in the l-NAME-treated group. G: ejection fraction (EF) was significantly reduced in the water-treated group after treatment with 100 and 150 µM afobazole but not in the l-NAME-treated group. H: fractional pump flow (FPF) was significantly reduced in the water- but not l-NAME-treated group after 150 µM afobazole. Data were analyzed by repeated-measures two-way ANOVA with the Geisser-Greenhouse correction; when this test identified a significant trend, multiple comparisons were performed using Dunnett’s test.
Afobazole elicits elevated NO production in cultured lymphatic endothelial cells.
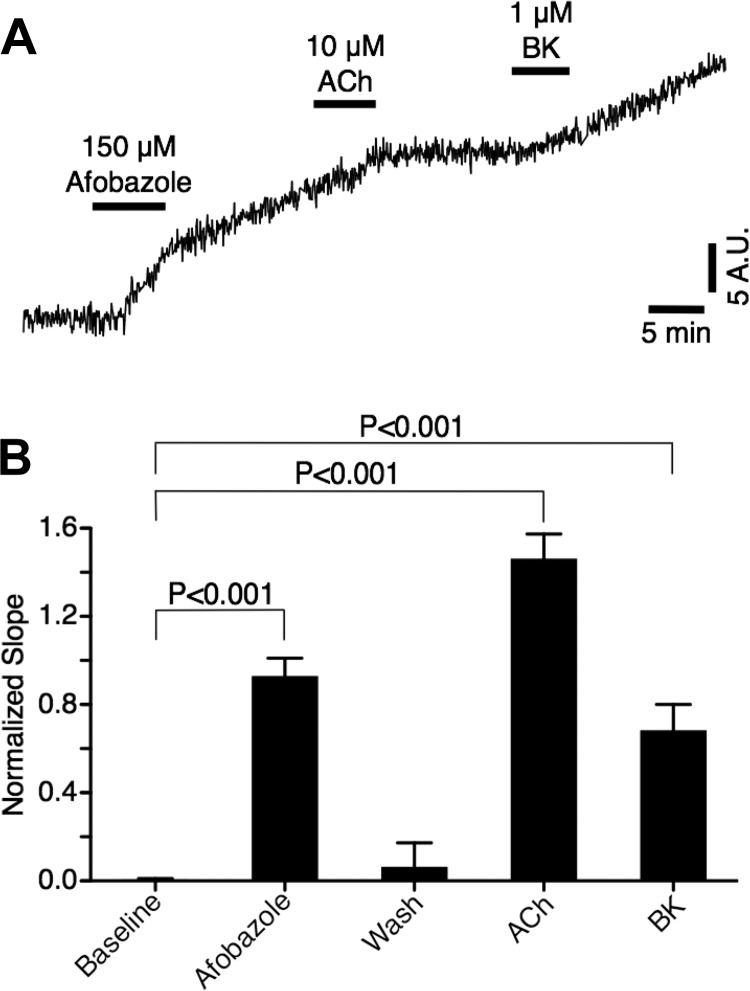
With our finding that l-NAME could attenuate the afobazole-induced elevations in ESD/MaxD and related decreases in AMP/MaxD, EF, and FPF, we next investigated whether afobazole elicits an increase in NO release from lymphatic endothelial cells. We chose a cultured lymphatic endothelial cell model to avoid movement artifacts that would be expected to occur in an isolated collecting lymphatic vessel. We loaded the cells with the indicator DAF-FM, which becomes fluorescent when it comes into contact with NO. In 90 of the 144 cells studied, afobazole (150 µM) elicited an apparent increase in DAF-FM fluorescence. A trace of the emitted fluorescence (normalized to baseline slope) from a single cell that responded with an increase in DAF-FM fluorescence in response to afobazole is shown in Fig. 8A. We also treated the cells with acetylcholine or bradykinin, both positive controls for endothelial NO release, and in this particular trace, these two treatments caused modest increases in fluorescence. The data summarized from the 90 cells in which afobazole elicited an increase in DAF-FM fluorescence are shown in Fig. 8B. In these cells, afobazole caused a significant elevation in DAF-FM fluorescence, as did the positive controls acetylcholine and bradykinin (Fig. 8B), suggesting that afobazole can elicit increases in NO production in lymphatic endothelial cells.
Fig. 8.
Afobazole elevates lymphatic endothelial cell nitric oxide (NO) production. A: trace of change in fluorescence from baseline (F/F0) of a single cultured dermal lymphatic endothelial cell (passage 3) loaded with 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) to detect NO production. DAF-FM is essentially nonfluorescent until it interacts with NO to produce a fluorescent benzotriazole. The time course shows the accumulation of this fluorescent benzotriazole and changes in slope associated with treatments and washouts. Apparent elevations in the slope were visible shortly after afobazole treatment or after treatment with positive controls for endothelium-derived NO production, acetylcholine (ACh) and bradykinin (BK). AU, arbitrary units. B: summarized data from 90 lymphatic endothelial cells that responded to afobazole. Data were analyzed by one-way ANOVA followed by Dunnett’s test, with all groups compared with baseline as control.
DISCUSSION
The novel finding of the present study is that the σ1-receptor is expressed in rat mesenteric collecting lymphatic vessels and that its activation modulates lymphatic contractions through a mechanism that involves NO production in endothelial cells. Specifically, in lymphatic vessels, ESD was increased in response to the σ1-receptor agonist afobazole, whereas AMP, EF, and FPF were significantly decreased. This is the first demonstration that contractile activity of lymphatic vessels can be modulated through activation of the σ1-receptor. The σ1-receptor is localized mainly in the endothelial layer of collecting lymphatics, in some areas in close contact with the elastin fibers that surround the endothelium of mesenteric lymphatic vessels. The finding that σ1-receptor activation can attenuate mesenteric contractions is important, because it identifies a new potential therapeutic target that may be useful for lymphatic disorders.
To our knowledge, this is the first study to assess the impact of the anxiolytic afobazole, an agonist of the σ1-receptor, on lymphatic pumping. Our goals were 1) to assess the extent to which σ-receptor activation would have an impact on lymphatic pumping and 2) to determine the expression and localization of the σ1-receptor in mesenteric lymphatics. We decided to investigate σ-receptors, because their agonists were reported to modulate Ca2+ channel activities (11, 12, 32) and also because a recent report led us to suspect that the σ1-receptor may increase susceptibility to edema formation (40). Parenti et al. (40) reported that the σ1-receptor antagonistic compound (−)-MRV3 can inhibit paw edema in male Sprague-Dawley rats after carrageenan treatment to induce inflammatory pain. In the present study, the agonist afobazole significantly increased ESD/MaxD, leading to reductions in AMP/MaxD, EF, and FPF (Fig. 1) in isolated rat mesenteric lymphatics. Data from our σ-receptor antagonist (BD 1047, BD 1063, and SM-21) experiments suggest primarily involvement of the σ1-receptor and limited involvement of the σ2-receptor in the afobazole-induced changes in lymphatic pumping. While it is not clear whether the reduction in edema observed by Parenti et al. is attributable in part to changes in lymphatic clearance, this is certainly a possibility (34). Future studies designed to measure peripheral limb edema formation in animals treated with σ1-receptor agonists or antagonists would provide insight into this important question. One potential limitation with our use of afobazole is its affinity for the melatonin MT1 receptor (43). However, because both σ1-receptor antagonists attenuated the response to afobazole and also because melatonin has been shown to cause vasoconstriction in blood vessels, probably due to inhibition of NO signaling (44, 45, 50, 52, 54), we believe it is unlikely that the MT1 receptor is contributing to the response observed in our study. Also, during σ2-receptor blockade, we observed that 100 µM afobazole caused a significant decrease in CF (Fig. 4B) that was not seen in the absence of this inhibitor or with any other inhibitor. As of this writing, cloning of the σ2-receptor has not been achieved (21), limiting our ability to comprehensively investigate its role. Therefore, we can only speculate that it appears that the σ2-receptor may have a role in preserving phasic contractions during activation of the σ1-receptor with a drug like afobazole.
With the pharmacological evidence of the involvement of the σ1-receptor, we determined in the present study whether it is expressed in rat mesenteric collecting lymphatic vessels. As expected, σ1-receptor protein and mRNA were detected in isolated rat mesenteric lymphatic collecting vessel lysates (Fig. 5). Zhang et al. (56) detected the protein in primary human lung-lymphatic endothelial cell and dermal human microvascular endothelial cell lysates, while a vascular study (48) has shown expression in rat aortic lysates. The results of our immunofluorescence confocal microscopy indicate that the σ1-receptor localizes primarily in the endothelium, with a certain degree of the σ1-receptor in close contact with elastin fibers that surround the intimal layer (Fig. 6). We were initially surprised that the σ1-receptor was not localized in the smooth muscle of the lymphatic vessels (Fig. 7), because Tagashira and colleagues (48) found the σ1-receptor in vascular smooth muscle cells and endothelial cells of the aorta. The reason for this difference in expression remains to be determined; however, it may be due to the markedly different functions of the aorta vs. collecting lymphatics.
Our data also show that the σ1-receptor is closely localized with what appear to be elastin fibers between the endothelial and smooth muscle layers. Our observation of these elastin fibers reflects a previous study by Arkill et al. (2), who showed anatomic evidence of an extensive extracellular matrix near the luminal side of the bovine mesenteric lymphatic vessel wall. They observed that endothelial cells were attached to an elastin-rich matrix that has fibers running predominantly parallel to the vessel axis (2). Interestingly, the σ1-receptor has also been shown to be associated with cell-matrix interactions (β1-integrin) in breast cancer cell lines (39). Together, these findings indicate that the σ1-receptor is localized at the endothelial interface with the elastin network in mesenteric collecting lymphatic vessels. At this stage, the function of this interaction is unclear, but the close interaction with the elastin network hints at a role in mechanotransduction.
The endothelial localization of the σ1-receptor, combined with elevated ESD/MaxD in response to afobazole, which could be interpreted as weaker phasic contractions, suggests that an endothelial-derived relaxation factor such as NO (25, 29, 30, 36) may be involved in the mechanism. We found that pretreatment of lymphatic vessels with l-NAME could completely block the afobazole-induced elevation in ESD/MaxD and also significantly attenuate the decreases in AMP/MaxD, EF, and FPF. In addition, we observed that afobazole could elicit elevations in NO in cultured lymphatic endothelial cells. Together, these data support the idea that endothelial production of NO mediates the afobazole-induced changes in lymphatic pump function. It is worth noting that NO donors or stimuli that activate NO production, such as elevated flow or administration of acetylcholine, have been reported to cause increases in lymphatic diastolic diameter, in some cases with cessation of pumping (16, 25, 30). Afobazole did not cause an increase in EDD/MaxD in the present study, but we also observed that, in the presence of l-NAME, afobazole treatment led to a decrease in EDD/MaxD (Fig. 7). This finding suggests that other, unidentified NO-independent signals are also activated by afobazole and may be counteracting the ability of NO to cause relaxation during the diastolic phase of lymphatic pumping. One limitation of our study was that we used lymphatic endothelial cells derived from human skin, which would be derived from multiple lymphatic vessel types (lymphatic capillary, precollectors, and collecting lymphatics) (6) and might be one reason why we observed elevated NO production in response to afobazole in only a subset of all cells studied.
In summary, our data provide the first characterization of the σ1-receptor in collecting lymphatic vessels. Activation of the σ1-receptor with afobazole elicits an elevation in ESD/MaxD, resulting in decreased AMP/MaxD, EF, and FPF. The mechanism involves activation of NOS in lymphatic endothelial cells. These new findings uncover a novel pharmacological target on lymphatic vessels that could potentially be useful for individuals who have lymphatic disorders or others who are at risk for edema formation.
GRANTS
The research reported in this publication was supported by the University of South Florida and National Heart, Lung, and Blood Institute Grants L40-HL-097863 and R01-HL-098215.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N.T., J.C., and J.W.B. conceived and designed research; A.N.T. and B.C. performed experiments; A.N.T., B.C., and J.W.B. analyzed data; A.N.T., C.K., J.C., B.C., T.E.T.-C., and J.W.B. interpreted results of experiments; A.N.T. prepared figures; A.N.T. drafted manuscript; A.N.T., C.K., J.C., B.C., T.E.T.-C., and J.W.B. edited and revised manuscript; A.N.T., C.K., J.C., B.C., T.E.T.-C., and J.W.B. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Kruse AC. Identification of the gene that codes for the σ2-receptor. Proc Natl Acad Sci USA 114: 7160–7165, 2017. doi: 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkill KP, Moger J, Winlove CP. The structure and mechanical properties of collecting lymphatic vessels: an investigation using multimodal nonlinear microscopy. J Anat 216: 547–555, 2010. doi: 10.1111/j.1469-7580.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atchison DJ, Johnston MG. Role of extra- and intracellular Ca2+ in the lymphatic myogenic response. Am J Physiol Regul Integr Comp Physiol 272: R326–R333, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Azuma T, Ohhashi T, Roddie IC. Bradykinin-induced contractions of bovine mesenteric lymphatics. J Physiol 342: 217–227, 1983. doi: 10.1113/jphysiol.1983.sp014847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res 96: 46–54, 2014. doi: 10.1016/j.mvr.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 9: 3–11, 2011. doi: 10.1089/lrb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 284: H92–H100, 2003. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- 9.Brune S, Pricl S, Wünsch B. Structure of the σ1-receptor and its ligand binding site. J Med Chem 56: 9809–9819, 2013. doi: 10.1021/jm400660u. [DOI] [PubMed] [Google Scholar]
- 10.Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, Dora KA, Yang Y, Davis MJ, Pohl U, Meininger GA, Hill MA. Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arterioscler Thromb Vasc Biol 31: 2889–2896, 2011. doi: 10.1161/ATVBAHA.111.236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas J, Behensky A, Deng W, Katnik C. Afobazole modulates neuronal response to ischemia and acidosis via activation of σ1-receptors. J Pharmacol Exp Ther 339: 152–160, 2011. doi: 10.1124/jpet.111.182774. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas J, Rodriguez A, Behensky A, Katnik C. Afobazole modulates microglial function via activation of both σ1- and σ2-receptors. J Pharmacol Exp Ther 339: 161–172, 2011. doi: 10.1124/jpet.111.182816. [DOI] [PubMed] [Google Scholar]
- 13.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295: H587–H597, 2008. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous σ1-receptor regulator. Science 323: 934–937, 2009. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591: 4549–4565, 2013. doi: 10.1113/jphysiol.2013.258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation 14: 827–839, 2007. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- 20.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Zhen X. σ2-Receptor ligands: neurobiological effects. Curr Med Chem 22: 989–1003, 2015. doi: 10.2174/0929867322666150114163607. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Su T-PP. σ1-Receptors (σ1-binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306: 718–725, 2003. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- 23.Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. σ1-Receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther 327: 491–502, 2008. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- 24.Jamalian S, Davis MJ, Zawieja DC, Moore JE Jr. Network scale modeling of lymph transport and its effective pumping parameters. PLoS One 11: e0148384, 2016. doi: 10.1371/journal.pone.0148384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation 21: 593–605, 2014. doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz KH, Souza-Smith FM, Moor AN, Breslin JW. Rho kinase enhances contractions of rat mesenteric collecting lymphatics. PLoS One 9: e94082, 2014. doi: 10.1371/journal.pone.0094082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Roizes S, von der Weid P-YY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592: 5409–5427, 2014. doi: 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the σ1-receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev 37: 116–132, 2001. doi: 10.1016/S0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno R, Dörnyei G, Koller A, Kaley G. Myogenic responses of isolated lymphatics: modulation by endothelium. Microcirculation 4: 413–420, 1997. doi: 10.3109/10739689709146805. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Monnet FP. σ1-Receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell 97: 873–883, 2005. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- 33.Moriguchi S, Shinoda Y, Yamamoto Y, Sasaki Y, Miyajima K, Tagashira H, Fukunaga K. Stimulation of the σ1-receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One 8: e60863, 2013. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 124: 915–921, 2014. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21: 640–648, 2014. doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohhashi T, Azuma T. Electrical activity and ultrastructure of bovine mesenteric lymphatics. Lymphology 12: 4–6, 1979. [PubMed] [Google Scholar]
- 38.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980. [DOI] [PubMed] [Google Scholar]
- 39.Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. σ1-Receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res 67: 11166–11175, 2007. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- 40.Parenti C, Marrazzo A, Aricò G, Cantarella G, Prezzavento O, Ronsisvalle S, Scoto GM, Ronsisvalle G. Effects of a selective σ1-antagonist compound on inflammatory pain. Inflammation 37: 261–266, 2014. doi: 10.1007/s10753-013-9736-6. [DOI] [PubMed] [Google Scholar]
- 41.Rahbar E, Weimer J, Gibbs H, Yeh AT, Bertram CD, Davis MJ, Hill MA, Zawieja DC, Moore JE Jr. Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat Res Biol 10: 152–163, 2012. doi: 10.1089/lrb.2011.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The σ1-receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol 609: 19–26, 2009. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seredenin SB, Voronin MV. [Neuroreceptor mechanisms of the afobazole effect]. Eksp Klin Farmakol 72: 3–11, 2009. [PubMed] [Google Scholar]
- 44.Shukla P, Sun C, O’Rourke ST. Melatonin inhibits nitric oxide signaling by increasing PDE5 phosphorylation in coronary arteries. Am J Physiol Heart Circ Physiol 303: H1418–H1425, 2012. doi: 10.1152/ajpheart.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva CL, Tamura EK, Macedo SM, Cecon E, Bueno-Alves L, Farsky SH, Ferreira ZS, Markus RP. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br J Pharmacol 151: 195–205, 2007. doi: 10.1038/sj.bjp.0707225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 2011: 3438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza-Smith FM, Molina PE, Breslin JW. Reduced RhoA activity mediates acute alcohol intoxication-induced inhibition of lymphatic myogenic constriction despite increased cytosolic [Ca2+]. Microcirculation 20: 377–384, 2013. doi: 10.1111/micc.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tagashira H, Matsumoto T, Taguchi K, Zhang C, Han F, Ishida K, Nemoto S, Kobayashi T, Fukunaga K. Vascular endothelial σ1-receptor stimulation with SA4503 rescues aortic relaxation via Akt/eNOS signaling in ovariectomized rats with aortic banding. Circ J 77: 2831–2840, 2013. doi: 10.1253/circj.CJ-13-0256. [DOI] [PubMed] [Google Scholar]
- 49.Takeshita T, Kawahara M, Fujii K, Kinoshita H, Morio M. The effects of vasoactive drugs on halothane inhibition of contractions of rat mesenteric lymphatics. Lymphology 22: 194–198, 1989. [PubMed] [Google Scholar]
- 50.Tamura EK, Cecon E, Monteiro AW, Silva CL, Markus RP. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J Pineal Res 46: 268–274, 2009. doi: 10.1111/j.1600-079X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 51.Telinius N, Mohanakumar S, Majgaard J, Kim S, Pilegaard H, Pahle E, Nielsen J, de Leval M, Aalkjaer C, Hjortdal V, Boedtkjer DB. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 592: 4697–4714, 2014. doi: 10.1113/jphysiol.2014.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tunstall RR, Shukla P, Grazul-Bilska A, Sun C, O’Rourke ST. MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J Pharmacol Exp Ther 336: 127–133, 2011. doi: 10.1124/jpet.110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von der Weid P-YY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295: H1989–H2000, 2008. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 54.Yang Q, Scalbert E, Delagrange P, Vanhoutte PM, O’Rourke ST. Melatonin potentiates contractile responses to serotonin in isolated porcine coronary arteries. Am J Physiol Heart Circ Physiol 280: H76–H82, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Cuevas J. σ1-Receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol 87: 2867–2879, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Jiang S, Yu J, Kuzontkoski PM, Groopman JE. Cocaine enhances HIV-1 gp120-induced lymphatic endothelial dysfunction in the lung. Physiol Rep 3: e12482, 2015. doi: 10.14814/phy2.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.