Activation of TNF-α receptor 1 in the subfornical organ (SFO) contributes to sympathetic excitation in heart failure rats by increasing inflammation and renin-angiotensin system activity in the SFO and downstream in the hypothalamic paraventricular nucleus. Cytokine receptors in the SFO may be a target for central intervention in cardiovascular conditions characterized by peripheral inflammation.
Keywords: hypothalamic paraventricular nucleus, proinflammatory cytokines, renin-angiotensin system
Abstract
In systolic heart failure (HF), circulating proinflammatory cytokines upregulate inflammation and renin-angiotensin system (RAS) activity in cardiovascular regions of the brain, contributing to sympathetic excitation and cardiac dysfunction. Important among these is the subfornical organ (SFO), a forebrain circumventricular organ that lacks an effective blood-brain barrier and senses circulating humors. We hypothesized that the tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) in the SFO contributes to sympathetic excitation and cardiac dysfunction in HF rats. Rats received SFO microinjections of a TNFR1 shRNA or a scrambled shRNA lentiviral vector carrying green fluorescent protein, or vehicle. One week later, some rats were euthanized to confirm the accuracy of the SFO microinjections and the transfection potential of the lentiviral vector. Other rats underwent coronary artery ligation (CL) to induce HF or a sham operation. Four weeks after CL, vehicle- and scrambled shRNA-treated HF rats had significant increases in TNFR1 mRNA and protein, NF-κB activity, and mRNA for inflammatory mediators, RAS components and c-Fos protein in the SFO and downstream in the hypothalamic paraventricular nucleus, along with increased plasma norepinephrine levels and impaired cardiac function, compared with vehicle-treated sham-operated rats. In HF rats treated with TNFR1 shRNA, TNFR1 was reduced in the SFO but not paraventricular nucleus, and the central and peripheral manifestations of HF were ameliorated. In sham-operated rats treated with TNFR1 shRNA, TNFR1 expression was also reduced in the SFO but there were no other effects. These results suggest a key role for TNFR1 in the SFO in the pathophysiology of systolic HF.
NEW & NOTEWORTHY Activation of TNF-α receptor 1 in the subfornical organ (SFO) contributes to sympathetic excitation in heart failure rats by increasing inflammation and renin-angiotensin system activity in the SFO and downstream in the hypothalamic paraventricular nucleus. Cytokine receptors in the SFO may be a target for central intervention in cardiovascular conditions characterized by peripheral inflammation.
in patients with systolic heart failure (HF), increased circulating proinflammatory cytokines (PICs) are associated with an adverse prognosis (5, 6). Rats with HF after myocardial infarction, simulating a common cause of systolic HF in humans, have increased PICs in the circulation and also in cardiovascular regions of the brain (12, 13, 28, 29). Interventions that reduce circulating PICs or the activity of PICs in the brain can reduce sympathetic nerve activity and ameliorate the peripheral manifestations of HF in these rats (23, 24, 27, 29).
We recently demonstrated in normal rats that peripheral administration of PICs activates the sympathetic nervous system (SNS) and that this response is largely prevented by lesioning the subfornical organ (SFO) (59), a forebrain circumventricular organ that senses circulating humoral factors (45). Moreover, we found that microinjection of PICs directly into the SFO activates the SNS while upregulating inflammation and renin-angiotensin system (RAS) activity both in the SFO and downstream in the hypothalamic paraventricular nucleus (PVN) (58), a forebrain source of presympathetic neurons that has been implicated in the pathophysiology of HF and hypertension (3, 35, 66). These findings from acute studies in normal rats suggest that the SFO is an important interface between peripheral inflammation, as manifested by circulating PICs, and the central nervous system mechanisms that drive SNS activity. However, the significance of a PIC influence on SFO neurons to the pathophysiology of HF, a condition in which circulating PICs are chronically elevated and many other factors that may contribute to activation of the SNS are also present, has not been determined.
The present study addressed the influence of the prototypical PIC tumor necrosis factor-α (TNF-α), acting upon the TNF-α p55 receptor in the SFO, on central nervous system mechanisms contributing to sympathetic nerve activity in rats with HF induced by myocardial infarction. The TNF-α p55 receptor, also known as TNF-α receptor 1 (TNFR1), is the TNF-α receptor most abundantly expressed in the brain, with particular intensity in blood-brain barrier-related structures, including the circumventricular organs (47), and is thought to mediate most of the adverse central nervous system effects of TNF-α (44). Furthermore, the expression of TNFR1 in these brain regions is upregulated by circulating TNF-α (47), which is known to be increased in the rat model of ischemia-induced HF (12, 28, 29, 63). Neurons, astrocytes, and microglia are all known to express TNFR1 (51, 55), and we previously reported that TNFR1 is expressed in neurons, astrocytes, and unlabeled cellular elements in the SFO of normal rats (59). The present study examined the effect of shRNA knockdown of TNFR1 in SFO on RAS activity and inflammatory mechanisms known to drive sympathetic activity in the SFO and PVN of HF rats. Additional experiments were performed in normal rats to test the hypothesis that TNFR1 in the SFO mediate the sympathetic and hemodynamic responses to circulating TNF-α.
METHODS
Animals
Adult male Sprague-Dawley rats weighing 250–300 g were obtained from Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in temperature (23 ± 2°C)- and light-controlled animal quarters, and standard rat chow and water were given ad libitum. Experiments were performed in accordance with the National Institutes of Healthʼs Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Protocol I. TNFR1 Knockdown in SFO: HF Rats
One hundred eleven rats underwent SFO microinjection of a TNFR1 shRNA (n = 37) or a scrambled control (Con) shRNA (n = 35) lentiviral vector encoding green fluorescent protein (GFP), or vehicle (Veh; artificial cerebrospinal fluid, n = 39).
One week later, some rats that received SFO microinjection of TNFR1 shRNA (n = 3) or Veh (n = 3) were euthanized for immunohistochemistry to verify the accuracy of microinjection and efficacy of transfection of the SFO.
The remaining 105 rats underwent either coronary artery ligation (CL) to induce HF or sham operation (Sham), resulting in six study groups: HF + TNFR1 shRNA (n = 21), HF + Con shRNA (n = 22), HF + Veh (n = 23), Sham + TNFR1 shRNA (n = 13), Sham + Con shRNA (n = 13), and Sham + Veh (n = 13).
Fifteen rats died within 24 h of CL (HF + TNFR1 shRNA: n = 4, HF + Con shRNA: n = 6, and HF + Veh: n = 5), and an additional six rats died between 24 h and the end of the protocol (HF + TNFR1 shRNA: n = 1, HF + Con shRNA: n = 1, and HF + Veh: n = 4). Six rats with small myocardial infarction (ischemic zone ≤ 30%) were excluded from the study. All surviving rats with large myocardial infarction (n = 39) were used for data analysis. There were no deaths among Sham rats.
An echocardiogram was performed within 24 h of CL or sham operation to evaluate left ventricular (LV) function. A second echocardiogram was performed 4 wk later to determine treatment effects. At the conclusion of the 5-wk protocol, six rats from each treatment group were euthanized to collect brain tissue for real-time PCR and blood, heart, and lung tissues to assess peripheral indicators of HF. Seven rats from each treatment group were anesthetized for invasive study of cardiac hemodynamics and then euthanized to collect brain tissues for Western blot analysis.
SFO microinjections.
Rats were anesthetized [ketamine (100 mg/kg) and xylazine (10 mg/kg ip)] and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skull was exposed by a longitudinal incision, a small hole was made 0.8 mm caudal and 1.7 mm lateral to the bregma (to avoid the sagittal sinus), and a 30-gauge guide cannula was advanced at a 25° angle toward the midline to a position 5 mm ventral to the cortical surface. A 35-gauge injection cannula connected to a 1-μl Hamilton microsyringe was inserted into the guide cannula and extended 0.5 mm beyond the tip of the guide cannula. TNFR1 shRNA (0.3 µl of 7.6 × 108 TU/ml) or the same dose of a Con shRNA lentiviral vector, both carrying GFP (OriGene Technologies, Rockville, MD), or Veh, was then injected into the SFO over 15–30 s. The injection cannula was left at the injection site for an additional 5 min before it was removed from the brain. The scalp incision was closed with sutures.
Induction of HF.
CL to induce HF or sham surgery was performed as previously described (67, 69).
Indicators of severity of HF.
echocardiography.
Echocardiography was performed under ketamine sedation (60 mg/kg ip). The ischemic zone as a percentage of LV circumference (%IZ), LV ejection fraction (EF), LV end-diastolic volume (LVEDV), and the LV volume-to-mass ratio were assessed as previously described (67, 69).
cardiac hemodynamics.
Hemodynamics were assessed in urethane (1.5 g/kg ip)-anesthetized rats immediately before euthanasia. A Millar catheter was inserted via the right carotid artery into the aorta to measure systolic blood pressure (SBP) and diastolic blood pressure (DBP), and it was then advanced across the aortic valve into the LV to measure LV end-diastolic pressure (LVEDP), LV peak systolic pressure (LVPSP), and maximum rate of rise of LV pressure (dP/dtmax). Heart rate (HR) was derived from the arterial pressure waveform, as previously described (64, 69).
plasma norepinephrine.
Trunk blood was collected at the time of euthanasia in tubes containing EDTA (1 mM) and sodium metabisulfite (4 mM) to prevent norepinephrine (NE) degradation. The plasma level of NE was measured with a commercial ELISA kit (Labor Diagnostika Nord, Nordhorn, Germany) according to the manufacturerʼs instructions.
anatomic measures.
Wet lung weight, LV weight and right ventricular (RV) weight, with respect to body weight, were measured as indexes of pulmonary congestion and cardiac remodeling.
Immunofluorescent studies.
Deeply anesthetized rats were transcardially perfused with 4% paraformaldehyde in 1 × PBS. Brains were removed and postfixed in 4% paraformaldehyde in 1 × PBS for 24 h at 4°C followed by cryoprotection by soaking in 30% sucrose for 48 h at 4°C. Brains were frozen in OCT compound on dry ice, and coronal sections (20 μm) of target tissues were cut using a cryostat and stored at −80°C for subsequent staining. For detection of TNFR1 expression, sections were incubated with rabbit polyclonal primary antibody to TNFR1 (catalog no. ab19139, 1: 200, Abcam, Cambridge, MA) followed by secondary antibody Alexa fluor 568 goat anti-rabbit IgG (catalog no. ab175471, 1:200, Abcam). GFP and immunostaining for TNFR1 were visualized with a confocal laser scanning microscope (Zeiss LSM 710, Carl Zeiss Oberkochen, Germany).
Molecular studies.
quantification of mrna expression.
The SFO and PVN regions, including small amounts of surrounding tissues, were punched using a 15-gauge needle stub (inner diameter: 1.5 mm), and total RNA was extracted with TRI Reagent (Molecular Research Center, Cincinnati, OH). mRNA expression of TNFR1, the inflammatory mediators TNF-α, interleukin-1β (IL-1β), cyclooxygenase (COX)-1 and COX-2, and the RAS components angiotensin-converting enzyme (ACE) and ANG II type 1 receptor (AT1R) were analyzed with SYBR green real-time PCR after reverse transcription of total RNA, as previously described (66). The sequences for the primers are shown in Table 1. Real-time PCR was performed using the ABI Prism 7000 sequence detection system (Applied Biosystems, Carlsbad, CA). Values were corrected by GAPDH and calculated using the following formula x = (where Ct is threshold cycle) (37). All mRNA data were expressed as fold changes relative to the Sham + Veh group.
Table 1.
Sequences for primers
| Gene | Sequences |
|---|---|
| TNFR1 | |
| Forward primer | 5′-GGTTCCTTTGTGGCACTTGGT-3′ |
| Reverse primer | 5′-CTCTTGGTGACCGGGAGAAG-3′ |
| ACE | |
| Forward primer | 5′-GTGTTGTGGAACGAATACGC-3′ |
| Reverse primer | 5′-CCTTCTTTATGATCCGCTTGA-3′ |
| AT1R | |
| Forward primer | 5′-GGATGGTTCTCAGAGAGAGTACAT-3′ |
| Reverse primer | 5′-CCTGCCCTCTTGTACCTGTTG-3′ |
| TNF-α | |
| Forward primer | 5′-CCTTATCTACTCCCAGGTTCTC-3′ |
| Reverse primer | 5′-TTTCTCCTGGTATGAATGGC-3′ |
| IL-1β | |
| Forward primer | 5′-CGACAGAATCTAGTTGTCC-3′ |
| Reverse primer | 5′-TCATAAACACTCTCATCCACAC-3′ |
| COX-1 | |
| Forward primer | 5′-AGAGATCACCAATGCCAGCT-3′ |
| Reverse primer | 5′-ACTGGATGGTACGCTTGGTC-3′ |
| COX-2 | |
| Forward primer | 5′-AAGGGAGTCTGGAACATTGTGAAC-3′ |
| Reverse primer | 5′-CAAATGTGATCTGGACGTCAACA-3′ |
| GAPDH | |
| Forward primer | 5′-AAGGTCATCCCAGAGCTGAA-3′ |
| Reverse primer | 5′-ATGTAGGCCATGAGGTCCAC-3′ |
TNFR1, tumor necrosis factor-α receptor 1; IL-1β, interleukin-1β; ACE, angiotensin-converting enzyme; AT1R, angiotensin II type 1 receptor; COX, cyclooxygenase.
western blot analysis.
Protein from the SFO and PVN punches was extracted using cell lysis buffer (Cell Signaling Technology, Danvers, MA). Protein levels for TNFR1, phosphorylated (p)-NF-κB p65, NF-κB inhibitory protein IκB-α, and c-Fos (a marker of neuronal activation) were measured by Western blot analysis, as previously described (69), using primary antibodies to TNFR1 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), p-NF-κB p65, IκB-α, and β-actin (1:500, 1:500, and 1:1,000, respectively, Cell Signaling Technology), and c-Fos (1:200, Santa Cruz Biotechnology). The density of the bands was quantified using Image Laboratory analysis software (Bio-Rad, Hercules, CA). All data were corrected by β-actin.
Protocol II. TNFR1 Knockdown in SFO: Normal Rats
Additional experiments were performed to determine whether the sympathoexcitatory response to blood-borne TNF-α is mediated by its actions upon TNFR1 in the SFO. Twenty-four rats underwent SFO microinjection of TNFR1 shRNA, Con shRNA, or Veh (n = 8 for each group). Two weeks later, four rats from each group were euthanized to collect brain tissue to measure TNFR1 expression by real-time PCR. The remaining four rats in each group were anesthetized for terminal electrophysiological and hemodynamic recording studies to measure renal sympathetic nerve activity (RSNA), mean blood pressure (MBP), and HR at baseline and continuously for 4 or 5 h after intracarotid artery (ICA) injections of TNF-α (0.5 μg/kg). The ICA route was chosen to preferentially target the forebrain region (19) and to avoid potential peripheral effects of TNF-α. The dose and methods used for ICA TNF-α injection were the same as those used in previous studies (68, 70).
Preparation for electrophysiological and hemodynamic recordings.
Rats were prepared for electrophysiological and hemodynamic recordings, as previously described (59, 68, 70). Briefly, rats were anesthetized with urethane (1.5 g/kg ip, supplemented as needed with 0.1 g/kg), and the left femoral artery was cannulated with PE-50 tubing filled with heparinized saline (20 U/ml) to measure blood pressure. HR was derived from the arterial pressure waveform. A left flank incision was made to expose the renal nerve, which was dissected free from surrounding tissue and placed on bipolar silver wire recording electrodes to record RSNA. Before the incisions were closed, Kwik-Cast silicon sealant (World Precision Instruments, Sarasota, FL) was applied to stabilize the nerve and electrodes. During recording, body temperature was maintained at 37 ± 1°C using a heating pad and heat lamp.
Data acquisition.
Data were acquired with a Cambridge Electronics Design (CED) laboratory interface (model 1401, CED, Cambridge, UK) using previously described methods (59). Hemodynamic data and sympathetic nerve activity were analyzed with CED Spike2 software. MBP (in mmHg), HR (in beats/min), and integrated RSNA (in mV) were averaged over 5-min intervals after ICA injection of TNF-α and compared with baseline values averaged over a 5-min interval immediately before each intervention. Changes in integrated RSNA were reported as percent changes from baseline control.
Statistical Analysis
All data are expressed as means ± SE. TNFR1 mRNA data from normal rats in protocol 2 were analyzed using one-way ANOVA followed by a multiple-comparison test. The rest of the data was analyzed using two-way ANOVA followed by multiple-comparison tests. P < 0.05 was considered statistically significant.
RESULTS
Protocol I. TNFR1 Knockdown in SFO: HF Rats
Effectiveness of SFO TNFR1 knockdown.
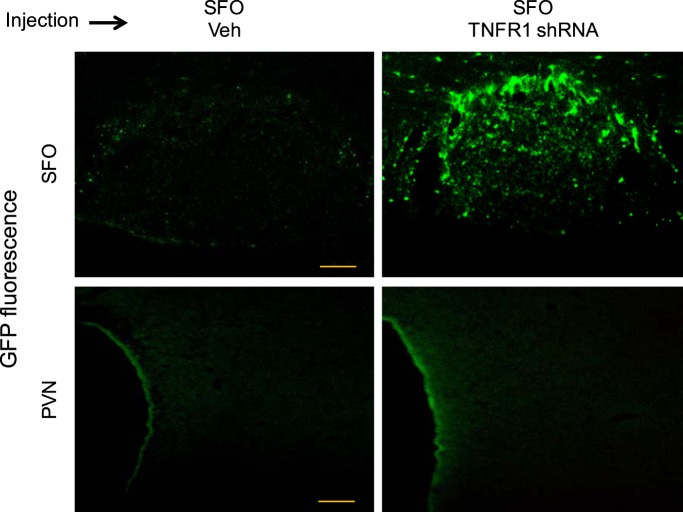
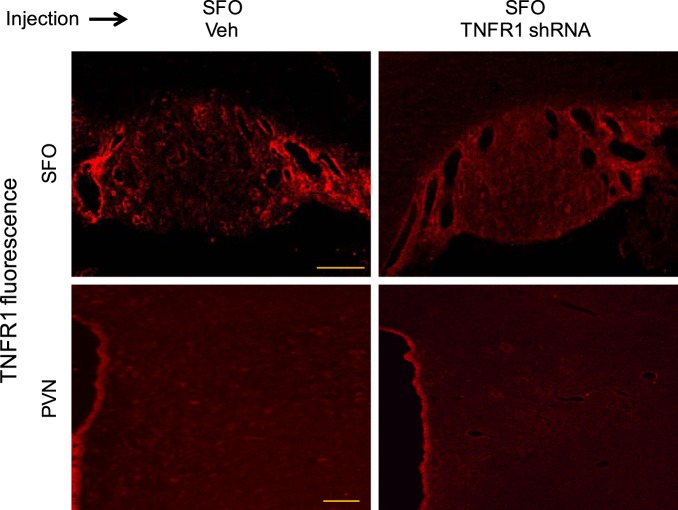
In the rats studied 1 wk after SFO microinjections of the TNFR1 shRNA lentiviral vector, GFP immunoreactivity was discretely localized in the SFO (Fig. 1). No GFP was observed in immediately surrounding tissues or in the PVN. TNFR1 immunoreactivity was reduced in the SFO, but TNFR1 immunoreactivity in the PVN, which was minimal in these otherwise normal rats, was unaffected (Fig. 2).
Fig. 1.
Green fluorescent protein (GFP) expression in the subfornical organ (SFO) and surrounding area (top) and in the hypothalamic paraventricular nucleus (PVN; bottom) 1 wk after SFO microinjection of vehicle (Veh; left) or tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) shRNA carrying GFP (right). In rats that received SFO microinjection of TNFR1 shRNA, GFP expression was observed in the SFO, with little expression in the surrounding tissue, but not in the PVN. Scale bar = 100 µm.
Fig. 2.
Tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) immunoreactivity (red) in the subfornical organ (SFO) and surrounding area (top) and in the hypothalamic paraventricular nucleus (PVN; bottom) 1 wk after SFO microinjection of vehicle (Veh; left) or TNFR1 shRNA (right). SFO microinjection of TNFR1 shRNA reduced TNFR1 immunoreactivity in the SFO but did not affect TNFR1 immunoreactivity in the PVN, which was minimal. Scale bar = 100 µm.
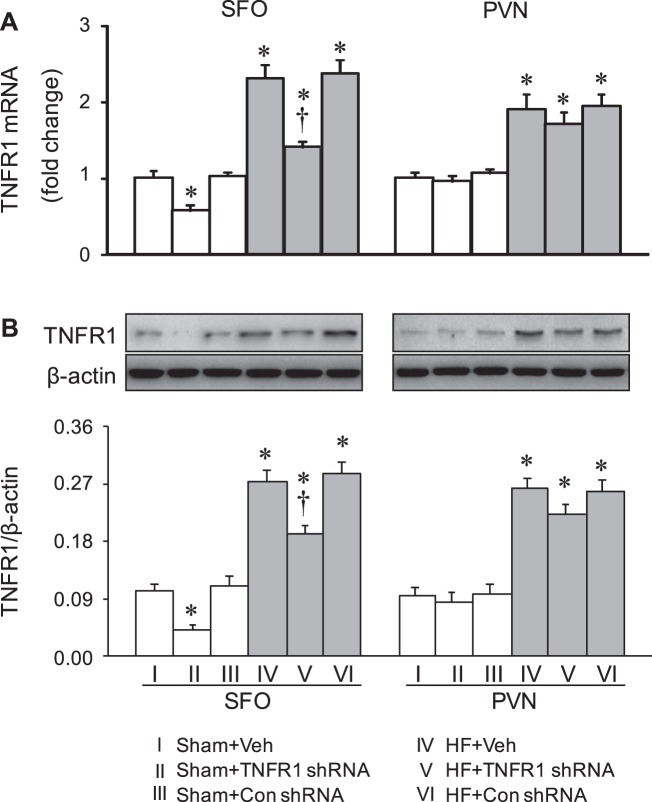
Four weeks after CL, HF + Veh rats had markedly higher mRNA (Fig. 3A) and protein (Fig. 3B) expression of TNFR1 in the SFO and in the PVN compared with Sham + Veh rats. Compared with HF + Veh rats, HF + TNFR1 shRNA rats had significantly lower mRNA and protein expression of TNFR1 in the SFO but not in the PVN. However, mRNA and protein levels of TNFR1 in the SFO were still higher than those in Sham + Veh rats. mRNA and protein expression of TNFR1 in HF + Con shRNA rats were similar to that in HF + Veh rats. Sham + TNFR1 shRNA rats also exhibited significantly lower mRNA and protein expression of TNFR1 in the SFO but not in the PVN, compared with Sham + Veh rats and Sham + Con shRNA rats.
Fig. 3.
mRNA expression (A) and protein levels (B) of tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) in the subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) in rats 4 wk after coronary artery ligation to induce heart failure (HF) or a sham operation (Sham) and 5 wk after SFO microinjection of TNFR1 shRNA, scrambled (Con) shRNA, or vehicle (Veh). Values are expressed as means ± SE; n = 6 or 7 for each group. Values for mRNA are expressed as fold changes compared with the Sham + Veh group; values for protein levels were corrected by β-actin. Data were analyzed with two-way ANOVA followed by multiple-comparison tests. [For mRNA expression: SFO, HF effect: F(1, 30) = 176, P < 0.0001; treatment effect: F(2, 30) = 30, P < 0.0001; PVN, HF effect: F(1, 30) = 75, P < 0.0001; treatment effect: F(2, 30) = 1.13, P = 0.34. For protein levels: SFO, HF effect: F(1, 36) = 207, P < 0.0001; treatment effect: F(2, 36) = 21, P < 0.0001. PVN, HF effect: F(1, 36) = 145, P < 0.0001; treatment effect: F(2, 36) = 1.50, P = 0.24]. *P < 0.05 vs. Sham + Veh; †P < 0.05, HF + TNFR1 shRNA vs. HF + Veh.
These observations confirmed the accuracy of the microinjection technique and the efficacy of TNFR1 knockdown in the SFO.
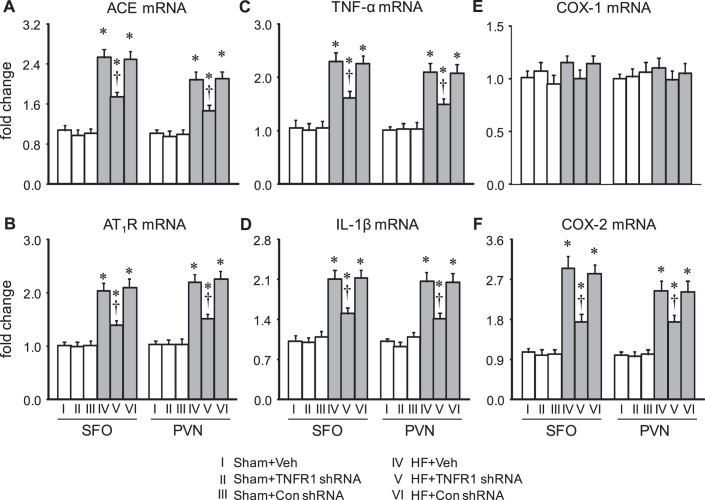
Effect on expression of excitatory mediators.
Four weeks after CL, HF + Veh rats had markedly higher mRNA expression of the RAS components ACE and AT1R (Fig. 4, A and B) and the inflammatory mediators TNF-α, IL-1β (Fig. 4, C and D), and COX-2 (Fig. 4F) in the SFO and in the PVN compared with Sham + Veh rats. mRNA expression of ACE, AT1R, TNF-α, IL-1β, and COX-2 in the SFO and PVN in HF + Con shRNA rats was similar to that in HF + Veh rats. In HF + TNFR1 shRNA rats, mRNA expression of these excitatory mediators was significantly lower in both the SFO and PVN. There was no change in mRNA expression of inflammatory or RAS mediators in the SFO or PVN in Sham + TNFR1 shRNA rats, even though the TNFR1 shRNA treatment reduced TNFR1 expression in these rats.
Fig. 4.
mRNA expression of renin-angiotensin system components angiotensin-converting enzyme (ACE; A) and angiotensin II (ANG II) type 1 receptor (AT1R; B) and inflammatory mediators tumor necrosis factor-α (TNF-α; C), interleukin-1β (IL-1β; D), cyclooxygenase (COX)-1 (E), and COX-2 (F) in the subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) in rats 4 wk after coronary artery ligation to induce heart failure (HF) or a sham operation (Sham) and 5 wk after SFO microinjection of TNF-α receptor 1 (TNFR1) shRNA, scrambled (Con) shRNA, or vehicle (Veh). Values are expressed as means ± SE and expressed as fold changes compared with the Sham + Veh group; n = 6 for each group. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [A, SFO, HF effect: F(1, 30) = 59, P < 0.0001; treatment effect: F(2, 30) = 3.53, P = 0.04. PVN, HF effect: F(1, 30) = 48, P < 0.0001; treatment effect: F(2, 30) = 3.86, P = 0.03. B, SFO, HF effect: F(1, 30) = 72, P < 0.0001; treatment effect: F(2, 30) = 6.83, P = 0.004. PVN, HF effect: F(1, 30) = 55, P < 0.0001; treatment effect: F(2, 30) = 3.43, P = 0.05. C, SFO, HF effect: F(1, 30) = 66, P < 0.0001; treatment effect: F(2, 30) = 3.68, P = 0.04. PVN, HF effect: F(1, 30) = 57, P < 0.0001; treatment effect: F(2, 30) = 3.55, P = 0.04. D, SFO, HF effect: F(1, 30) = 68, P < 0.0001; treatment effect: F(2, 30) = 4.56, P = 0.02. PVN, HF effect: F(1, 30) = 52, P < 0.0001; treatment effect: F(2, 30) = 4.68, P = 0.02. E, SFO, HF effect: F(1, 30) = 1.99, P = 0.17; treatment effect: F(2, 30) = 0.17, P = 0.84. PVN, HF effect: F(1, 30) = 0.06, P = 0.81; treatment effect: F(2, 30) = 0.21, P = 0.81. F, SFO, HF effect: F(1, 30) = 70, P < 0.0001; treatment effect: F(2, 30) = 5.84, P = 0.007. PVN, HF effect: F(1, 30) = 83, P < 0.0001; treatment effect: F(2, 30) = 3.76, P = 0.04]. *P < 0.05 vs. Sham + Veh; †P < 0.05, HF + TNFR1 shRNA vs. HF + Veh.
There was no difference across the six experimental groups in COX-1 mRNA in the SFO or PVN (Fig. 4E).
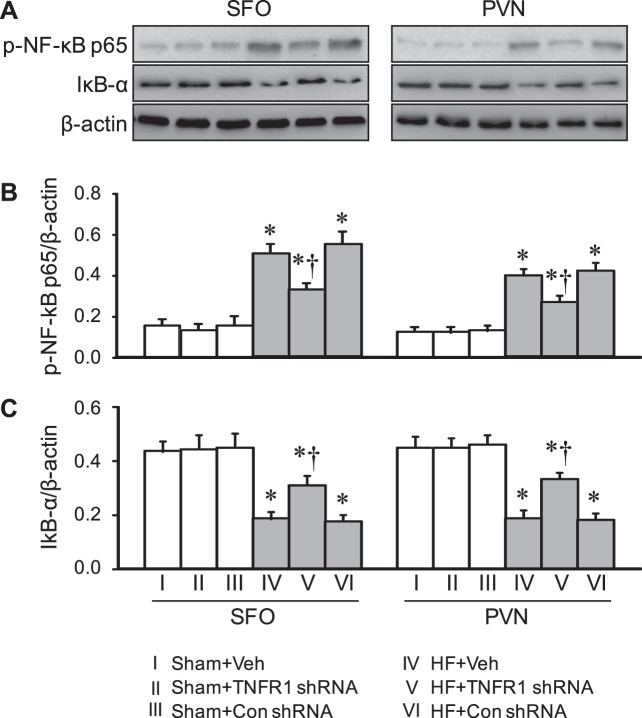
Effect on NF-κB activity.
Compared with Sham + Veh rats, HF + Veh rats had higher protein levels of p-NF-κB p65 (Fig. 5, A and B) and lower protein levels of IκB-α (Fig. 5, A and C) in both the SFO and PVN, consistent with activation of NF-κB. Compared with HF + Veh rats, HF + TNFR1 shRNA rats but not HF + Con shRNA rats had significantly lower protein levels of p-NF-κB p65 and higher protein levels of IκB-α in both the SFO and PVN. Protein levels of p-NF-κB p65 and IκB-α in the SFO and PVN were similar across all three Sham groups.
Fig. 5.
NF-kB activity in the subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) in rats 4 wk after coronary artery ligation to induce heart failure (HF) or sham operation (Sham) and 5 wk after SFO microinjection of tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) shRNA, scrambled (Con) shRNA, or vehicle (Veh). A: representative Western blots. B: phosphorylatled (p-)NF-κB p65 protein. C: IκB-α protein. Values are expressed as means ± SE and were corrected by β-actin; n = 7 for each group. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [B, SFO, HF effect: F(1, 36) = 149, P < 0.0001; treatment effect: F(2, 36) = 7.02, P = 0.003. PVN, HF effect: F(1, 36) = 231, P < 0.0001; treatment effect: F(2, 36) = 12, P = 0.0001. C, SFO, HF effect: F(1, 36) = 86, P < 0.0001; treatment effect: F(2, 36) = 4.19, P = 0.02. PVN, HF effect: F(1, 36) = 112, P < 0.0001; treatment effect: F(2, 36) = 4.06, P = 0.03]. *P < 0.05 vs. Sham + Veh; †P < 0.05, HF + TNFR1 shRNA vs. HF + Veh.
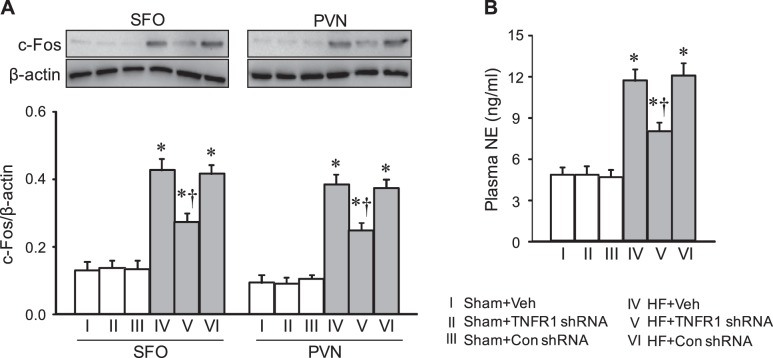
Effect on c-Fos expression.
Compared with Sham + Veh rats, HF + Veh rats exhibited higher c-Fos protein levels in both the SFO and PVN (Fig. 6A). c-Fos levels in the SFO and PVN were significantly lower in HF + TNFR1 shRNA rats but not HF + Con shRNA rats. There were no differences in c-Fos protein levels in the SFO or PVN among the three Sham groups.
Fig. 6.
Protein levels of c-Fos (A), a marker of neuronal excitation, in the subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) and plasma levels of norepinephrine (NE; B), a marker of sympathetic excitation, in rats 4 wk after coronary artery ligation to induce heart failure (HF) or sham operation (Sham) and 5 wk after SFO microinjection of tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) shRNA, scrambled (Con) shRNA, or vehicle (Veh). Values are expressed as means ± SE; n = 6 or 7 for each group. Values for protein levels were corrected by β-actin. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [A, SFO, HF effect: F(1, 36) = 163, P < 0.0001; treatment effect: F(2, 36) = 6.89, P = 0.003. PVN, HF effect: F(1, 36) = 256, P < 0.0001; treatment effect: F(2, 36) = 10, P = 0.0003. B, HF effect: F(1, 30) = 59, P < 0.0001; treatment effect: F(2, 30) = 3.46, P = 0.04]. *P < 0.05 vs. Sham + Veh; †P < 0.05, HF + TNFR1 shRNA vs. HF + Veh.
Effect on plasma NE.
HF + Veh rats had higher levels of plasma NE, a general indicator of sympathetic activity, than Sham + Veh rats (Fig. 6B). HF + TNFR1 shRNA rats had significantly lower plasma NE levels than HF + Veh and HF + Con shRNA rats, but plasma NE levels in HF + TNFR1 shRNA rats were still higher than those in Sham + Veh rats. There were no differences in plasma NE levels among the three Sham groups.
Effects on peripheral manifestations of HF.
Echocardiography performed within 24 h of CL revealed that HF + Veh rats, HF + TNFR1 shRNA rats, and HF + Con shRNA rats had similar infarct sizes and similar degrees of cardiac dysfunction, as indicated by LVEF and LVEDV (Table 2). Four weeks after CL, LV function was further compromised in HF + Veh rats, with increased LVEDV. Treatment with TNFR1 shRNA or Con shRNA had no effect on these echocardiographic indexes of LV function in HF rats. Echocardiographic indexes of cardiac function were similar among the three Sham groups.
Table 2.
Echocardiographic measurements
| Sham + Veh | Sham + TNFR1 shRNA | Sham + Con shRNA | HF + Veh | HF + TNFR1 shRNA | HF + Con shRNA | |
|---|---|---|---|---|---|---|
| Variables at baseline | ||||||
| LVEDV, ml | 0.46 ± 0.03 | 0.47 ± 0.02 | 0.46 ± 0.03 | 0.75 ± 0.04* | 0.79 ± 0.04* | 0.79 ± 0.03* |
| LV volume/mass, µl/mg | 0.54 ± 0.04 | 0.57 ± 0.03 | 0.57 ± 0.02 | 1.22 ± 0.08* | 1.26 ± 0.09* | 1.24 ± 0.08* |
| LVEF | 0.73 ± 0.03 | 0.71 ± 0.04 | 0.70 ± 0.03 | 0.36 ± 0.04* | 0.33 ± 0.03* | 0.31 ± 0.03* |
| %IZ | 42 ± 5* | 43 ± 2* | 43 ± 2* | |||
| Variables at week 4 | ||||||
| LVEDV, ml | 0.51 ± 0.03 | 0.49 ± 0.01 | 0.51 ± 0.02 | 1.13 ± 0.11*† | 1.00 ± 0.10* | 1.12 ± 0.10*† |
| LV volume/mass, µl/mg | 0.64 ± 0.04 | 0.61 ± 0.03 | 0.60 ± 0.03 | 1.47 ± 0.9* | 1.42 ± 0.12* | 1.48 ± 0.06* |
| LVEF | 0.74 ± 0.04 | 0.74 ± 0.03 | 0.75 ± 0.03 | 0.28 ± 0.03* | 0.32 ± 0.03* | 0.29 ± 0.02* |
| %IZ | 44 ± 2* | 42 ± 2* | 44 ± 2* |
Values are expressed as means ± SE; n = 13 animals/group. Sham, sham operation; Veh, vehicle; Con, control; HF, heart failure; LVEDV, left ventricular (LV) end-diastolic volume; LVEF, LV ejection fraction; %IZ, ischemic zone as a percentage of LV circumference. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [LVEDV: time effect, F(1, 144) = 30, P < 0.0001, and group effect, F(5, 144) = 39, P < 0.0001; LV volume/mass: time effect, F(1, 144) = 12, P = 0.0008, and group effect, F(5, 144) = 76, P < 0.0001; LVEF: time effect, F(1, 144) = 0.13, P = 0.72, and group effect, F(5, 144) = 115, P < 0.0001; %IZ: time effect, F(1, 144) = 0.004, P = 0.95, and group effect, F(5, 144) = 532, P < 0.0001].
P < 0.05 vs. the Sham + Veh group at the same week;
P < 0.05 vs. HF + Veh or HF + Con shRNA groups at 4 wk vs. 24 h.
Four weeks after CL, RV-to-body weight and wet lung-to-body weight ratios were substantially higher in HF + Veh rats compared with Sham + Veh rats (Table 3). Treatment with TNFR1 shRNA improved the wet lung-to-body weight and RV-to-body weight ratios, indicators of pulmonary congestion and RV remodeling, respectively. Treatment with TNFR1 shRNA had no effect on RV-to-body weight and wet lung-to-body weight ratios in Sham rats.
Table 3.
Anatomic and hemodynamic measurements at week 4
| Sham + Veh | Sham + TNFR1 shRNA | Sham + Con shRNA | HF + Veh | HF + TNFR1 shRNA | HF + Con shRNA | |
|---|---|---|---|---|---|---|
| Body weight, g | 355 ± 2 | 351 ± 3 | 352 ± 4 | 348 ± 9 | 350 ± 5 | 352 ± 6 |
| LV/body weight, mg/g | 2.44 ± 0.15 | 2.39 ± 0.11 | 2.40 ± 0.09 | 2.35 ± 0.11 | 2.46 ± 0.08 | 2.40 ± 0.07 |
| Right ventricle/body weight, mg/g | 0.52 ± 0.03 | 0.51 ± 0.03 | 0.51 ± 0.02 | 0.90 ± 0.06* | 0.69 ± 0.05* † | 0.91 ± 0.07* |
| Lung/body weight, mg/g | 4.26 ± 0.37 | 4.23 ± 0.15 | 4.22 ± 0.19 | 9.07 ± 0.95* | 6.67 ± 0.45* † | 8.98 ± 0.47* |
| Heart rate, beats/min | 362 ± 11 | 366 ± 7 | 360 ± 6 | 368 ± 7 | 370 ± 15 | 369 ± 10 |
| Systolic blood pressure, mmHg | 126 ± 2 | 122 ± 3 | 128 ± 2 | 108 ± 3* | 110 ± 3* | 106 ± 4 |
| Diastolic blood pressure, mmHg | 90 ± 2 | 89 ± 4 | 92 ± 4 | 87 ± 3 | 86 ± 3 | 84 ± 4 |
| LV peak systolic pressure, mmHg | 119 ± 2 | 118 ± 3 | 120 ± 3 | 99 ± 2* | 104 ± 3* | 99 ± 3* |
| LV end-diastolic pressure, mmHg | 3.38 ± 0.39 | 3.44 ± 0.41 | 3.44 ± 0.34 | 16.07 ± 0.89* | 12.74 ± 0.88* † | 16.51 ± 1.15* |
| LV dP/dtmax, mmHg/s | 9080 ± 350 | 9015 ± 285 | 9100 ± 306 | 6022 ± 215* | 7362 ± 229* † | 6081 ± 231* |
Values are expressed as means ± SE; n = 6 animals/group for anatomic measurements and n = 7 animals/group for hemodynamic measurements. Sham, sham operation; Veh, vehicle; Con, control; HF, heart failure; LV, left ventricular. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [body weight: HF effect, F(1, 30) = 0.37, P = 0.55, and treatment effect, F(2, 30) = 0.04, P = 0.96; LV/body weight: HF effect, F(1, 30) = 0.006, P = 0.94, and treatment effect, F(2, 30) = 0.04, P = 0.95; right ventricle/body weight: HF effect, F(1, 30) = 70, P < 0.0001, and treatment effect, F(2, 30) = 3.67, P = 0.04; lung/body weight: HF effect, F(1, 30) = 95, P < 0.0001, and treatment effect, F(2, 30) = 3.69, P = 0.04; heart rate: HF effect, F(1, 36) = 0.57, P = 0.46, and treatment effect, F(2, 36) = 0.07, P = 0.94; systolic blood pressure: HF effect, F(1, 36) = 35, P < 0.0001, and treatment effect, F(2, 36) = 0.04, P = 0.96; diastolic blood pressure: HF effect, F(1, 36) = 3.18, P = 0.08, and treatment effect, F(2, 36) = 0.05, P = 0.95; LV peak systolic pressure: HF effect, F(1, 36) = 92, P < 0.0001, and treatment effect, F(2, 36) = 0.77, P = 0.47; LV end-diastolic pressure: HF effect, F(1, 36) = 367, P < 0.0001, and treatment effect, F(2, 36) = 3.76, P = 0.03; LV dP/dtmax: HF effect, F(1, 36) = 133, P < 0.0001, and treatment effect, F(2, 36) = 3.41, P = 0.04].
P < 0.05, vs. the Sham + Veh group.
P < 0.05, HF + TNFR1 shRNA group vs. HF + Veh group.
Hemodynamic recordings (Table 3) showed that SBP, LVPSP, and LV dP/dtmax were lower and LVEDP was higher in HF + Veh rats than Sham + Veh rats. HF rats treated with TNFR1 shRNA but not Con shRNA had lower LVEDP and higher LV dP/dtmax than HF + Veh rats. Of note, these values were still significantly different from those in Sham + Veh rats. SBP and LVPSP in HF rats were not affected by treatment with TNFR1 shRNA or Con shRNA. The %IZ among the HF rats (n = 7 for each group) that underwent hemodynamic measurements was similar at baseline (HF + Veh: 42 ± 2, HF + TNFR1 shRNA: 42 ± 4, and HF + Con shRNA: 44 ± 3) and at 4 wk after CL (HF + Veh: 44 ± 3, HF + TNFR1 shRNA: 43 ± 3, and HF + Con shRNA: 40 ± 3). TNFR1 shRNA or Con shRNA did not affect the hemodynamic parameters in Sham rats.
There were no significant differences in body weight, LV-to-body weight ratio, HR, and DBP across any of the experimental groups.
Protocol II. TNFR1 Knockdown in SFO: Normal Rats
Effects on sympathetic and hemodynamic responses to blood-borne TNF-α.
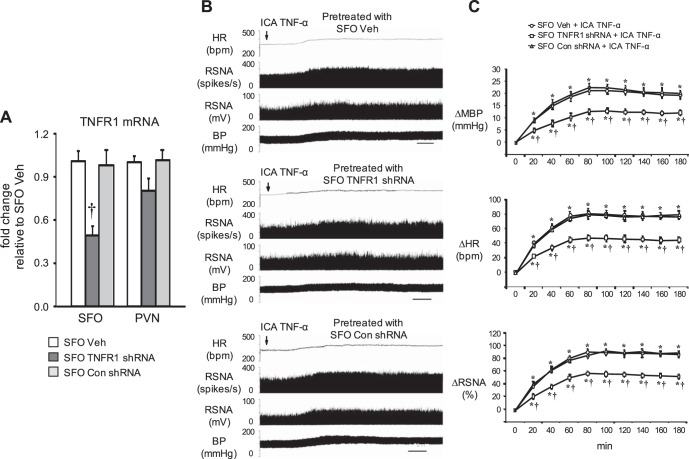
Two weeks after SFO microinjection of TNFR1 shRNA, Con shRNA, or Veh, TNFR1 mRNA expression was significantly reduced in the SFO but not in the PVN in TNFR1 shRNA-treated rats compared with Con shRNA-treated or Veh-treated rats (Fig. 7A).
Fig. 7.
Effects of tumor necrosis factor-α (TNF-α) receptor 1 (TNFR1) knockdown in the subfornical organ (SFO) on sympathoexcitatory responses to intracarotid artery (ICA) injection of TNF-α. Data were acquired 2 wk after SFO microinjection of TNFR1 shRNA, scrambled (Con) shRNA, or vehicle (Veh). A: expression of TNFR1 mRNA in the SFO and the hypothalamic paraventricular nucleus (PVN). Data were analyzed with one-way ANOVA followed by multiple-comparison tests [SFO, F(2, 9) = 13, P = 0.003; PVN, F(2, 9) = 1.8, P = 0.22]. B: representative original tracings showing the effects of SFO TNFR1 knockdown on responses of heart rate (HR), renal sympathetic nerve activity (RSNA, shown as integrated nerve activity and as windowed spike activity), and blood pressure (BP) to ICA injection of TNF-α. Scale bar = 20 min. C: grouped data showing that ICA TNF-α-induced increases in HR, RSNA, and mean BP (MBP) were diminished by SFO pretreatment with TNFR1 shRNA but not Con shRNA. Data were analyzed with two-way ANOVA followed by multiple-comparison tests [MBP time effect: F(9, 90) = 84, P < 0.0001; group effect: F(2, 90) = 121, P < 0.0001. HR, time effect: F(9, 90) = 100, P < 0.0001; group effect: F (2, 90) = 179, P < 0.0001. RSNA, time effect: F(9, 90) = 121, P < 0.0001; group effect: F(2, 90) = 139, P < 0.0001]. Values are expressed as means ± SE; n = 4 for each group. *P < 0.05, vs. baseline; †P < 0.05, vs. SFO Veh or SFO Con shRNA.
ICA injection of TNF-α in Veh-treated rats induced substantial (P < 0.05) increases in MBP, HR, and RSNA, beginning within 20–30 min after the injection. The peak responses in MBP (from 96.8 ± 3.3 to 118.9.8 ± 4.1 mmHg), HR (from 335.7 ± 11.4 to 413.5 ± 15.2 beats/min), and integrated RSNA (93.5 ± 4.2% increase from baseline) occurred 1.5–2.5 h after the ICA injections and lasted for 4–5 h and sometimes even longer (Fig. 7, B and C). There were no significant differences between Con shRNA- and Veh-treated rats in the increases in MBP, HR, and RSNA elicited by ICA injection of TNF-α. Compared with Con shRNA- and Veh-treated rats, TNFR1 shRNA-treated rats had significantly smaller increases in RSNA, MBP, and HR in response to ICA TNF-α (Fig. 7, B and C).
DISCUSSION
The SFO, which lacks an effective blood-brain barrier and can sense circulating humoral substances (54), contributes significantly to the increased sympathetic nerve activity characteristic of HF (39). Neurons in the SFO project directly to the PVN (36, 38, 46), a forebrain center that has been implicated as a source of the excessive sympathetic excitation in HF (23, 50, 60, 62). PVN presympathetic neurons, in turn, project directly, or indirectly via a synapse in the rostral ventrolateral medulla, to the intermediolateral cell column of the spinal cord (1), where they activate preganglionic neurons that regulate sympathetic drive to the heart, kidney, and vascular tree. In normal rats, stimulation of either the SFO or PVN evokes a sympathetic and hemodynamic response (10, 30, 38, 43), and the sympathoexcitatory response to stimulation of SFO can be blocked at the PVN level (10, 38). Neuroactive substances that increase in the circulation in HF may perpetuate sympathetic overactivity by activating this descending excitatory neural pathway. Sympathetic overactivity in HF contributes to volume accumulation and peripheral vasoconstriction, increasing stress on the failing heart, and increases the risk of lethal arrhythmias, a common cause of death in HF (49).
Our previous study (59) revealed that the SFO plays a major role in mediating the sympathetic responses to systemically administered PICs in normal rats. The present study tested the hypothesis that the prototypical PIC TNF-α, acting upon TNFR1 in the SFO, contributes to sympathetic excitation and the progression of HF in rats after CL, an experimental model in which circulating PICs are increased (12, 28, 29, 63). The novel findings of this study are 1) in HF rats, expression of TNFR1 was increased in the SFO and downstream in the PVN; 2) knockdown of TNFR1 selectively in the SFO with TNFR1 shRNA reduced HF-induced increases in mRNA expression of the RAS components ACE and AT1R, the inflammatory mediators TNF-α, IL-1β, and COX-2, and NF-κB activity, all of which are associated with increased sympathetic nerve activity, in the SFO and PVN; 3) knockdown of TNFR1 selectively in the SFO attenuated HF-induced c-Fos in SFO and PVN, suggesting reduced central neuronal excitation, and HF-induced increases in plasma NE, suggesting a general reduction in sympathetic activity; 4) knockdown of TNFR1 selectively in the SFO of HF rats was associated with improved cardiac hemodynamics and with less pulmonary congestion and RV remodeling; and 5) in normal rats, knockdown of TNFR1 selectively in the SFO significantly reduced the sympathetically mediated pressor response to ICA administration of TNF-α, confirming that TNFR1 in the SFO respond to circulating TNF-α. These findings are the first to demonstrate in an experimental model of HF that TNF-α, acting upon its receptors in the SFO, upregulates brain RAS activity and inflammation in the SFO and PVN. Thus, while other brain tissues associated with the blood-brain barrier also respond to circulating PICs (47), the SFO plays a major role as an interface between blood-borne products of inflammation and central nervous system mechanisms driving sympathetic activity in HF.
We previously reported that blockade of circulating cytokines reduces sympathetic activity in HF rats, but the mechanism for that effect was not determined (28). Several mechanisms have been proposed by which circulating PICs, which are lipophobic and too large to penetrate the blood-brain barrier, might initiate central inflammation and activate sympathetic drive (52). TNF-α and IL-1β may act upon their receptors on endothelial and perivascular cells in the cerebral vasculature to induce COX-2 activity and the production of PGE2 (9, 34), which readily crosses the blood-brain barrier and can increase sympathetic excitation (20). Consistent with that mechanism, we found that eliminating perivascular macrophages in the cerebral circulation without altering circulating PICs reduced plasma NE levels in rats with systolic HF (68). It has also been suggested that active transport of cytokines across the blood-brain barrier might increase central inflammation (2). Arguing against that mechanism, we found that central administration of the cytokine synthesis inhibitor pentoxifylline normalized TNF-α and IL-1β in the PVN of HF rats (and reduced sympathetic activity) without altering circulating IL-1β levels (29), consistent with the view that the PICs inside the blood-brain barrier are locally produced.
The present data in HF rats, along with our recently published studies concerning the role of the SFO in normal rats (58, 59), support a third possibility: that circulating cytokines exert their central effects by activating cells in circumventricular organs that lack a blood-brain barrier (47). We found that the sympathoexcitatory responses to TNF-α and IL-1β, administered either intravenously or via the ICA route, were significantly attenuated in rats with SFO lesions (59). Moreover, direct microinjections of TNF-α and IL-1β into the SFO upregulated mRNA expression for key components of the RAS and mediators of central inflammation in both the SFO and PVN. The associated increases in blood pressure, HR, and RSNA were attenuated by pretreating the SFO with microinjections of agents that counter RAS and COX-2 activity (58).
Those studies examined the acute effects of exogenous PICs, administered to normal rats in doses designed to elicit a sympathoexcitatory response. In contrast, the present study addressed the effects mediated by endogenous PICs acting upon on a specific cytokine receptor, TNFR1, in a specific forebrain circumventricular organ (the SFO) and in a pathophysiological model of HF. In rats with established systolic HF, in which circulating PICs are chronically elevated (12, 28, 29, 63), downregulating TNFR1 in the SFO and thereby reducing the effects of TNF-α at the SFO level alone, significantly reduced sympathetic activity and improved cardiac function. Although we cannot exclude the possibility that TNF-α produced locally in the SFO may have contributed to the activation of TNFR1 in the SFO of these HF rats, the demonstration that knockdown of TNFR1 in the SFO also reduced the sympathoexcitatory response to ICA administration of TNF-α in normal rats confirms that TNFR1 in the SFO respond to blood-borne TNF-α. Together, these findings suggest that circulating PICs act upon the SFO to induce and/or promote the central neuroinflammation and augmented RAS activity in the SFO and PVN that drives sympathetic excitation in HF. These observations demonstrate that blood-borne PICs have effects on the central nervous system that promote adverse outcomes in HF, in addition to their direct effects on the heart (33, 40–42). They strengthen the argument for an aggressive pursuit of ways to counter the effects of inflammation, which is not specifically targeted by current HF therapy.
In normal rats, TNFR1 is expressed abundantly in the SFO but only sparsely in the PVN (58, 59). This finding was confirmed in Veh-treated Sham rats in the present study. However, Veh-treated HF rats had significant increases in TNFR1 mRNA expression, along with TNF-α mRNA, in both the SFO and PVN. This result is consistent with previous studies in normal animals showing an increase in brain TNFR1 mRNA expression in response to peripheral lipopolysaccharide or TNF-α administration (47, 58), and suggests that proinflammatory cytokines upregulate their receptors in the brain under inflammatory conditions.
In the present study, knockdown of TNFR1 selectively in the SFO was associated with less RAS activity and inflammation and less neuronal excitation in the PVN, along with lower plasma NE levels. This effect on excitatory mediators in the PVN, downstream in the central sympathoexcitatory pathway, is consistent with our previous study (58), showing that microinjection of TNF-α or IL-1β into SFO elicits increases in excitatory mediators in the PVN, and suggests that the SFO orchestrates the central sympathoexcitatory response to peripheral inflammation. The persistent increase in TNFR1 in the PVN after TNFR1 knockdown in the SFO of HF rats is unexplained by the findings of this study but may reflect other influences on the PVN in HF. For example, the organum vasculosum of the lamina terminalis, another circumventricular organ that expresses TNFR1 (47) and responds to circulating TNF-α (48), also projects to PVN and may have similar effects to stimulate PVN inflammation. In addition, ANG II, which is upregulated in the PVN of HF rats, may stimulate TNF-α production in PVN (26). In the SFO, at least, TNF-α appears to upregulate the expression of TNFR1 (58).
Previous studies of the role of central inflammation in HF have focused primarily on the effects of PICs in the PVN, justifiably so because of its role as a source of sympathetic excitation in HF. Those studies have examined the effects of TNF-α within the blood-brain barrier, using intracerebroventricular administration (17, 24) or direct PVN microinjections (27) of agents that reduce or block the effects of TNF-α. They have demonstrated “cross talk” between PICs and the RAS in the PVN, but they have not directly addressed factors leading to upregulation of these two excitatory systems in the PVN. To our knowledge, the present study is the first to examine in a HF model the effects of TNF-α on a circumventricular organ innervating the PVN. The results strongly suggest that circulating PICs, acting upon their receptors in the SFO, foster the development of the inflammatory/excitatory milieu in the PVN that drives sympathetic excitation in HF. Considering that the SFO is but one of many neural and humoral sources of afferent input to the PVN in HF, the magnitude of the effects of TNF-α activity in the SFO on PVN neurochemistry and sympathetic drive in HF is somewhat surprising and suggests that this discrete nucleus, strategically positioned outside the blood-brain barrier, might be an ideal target for interventions designed to counter the effects of central inflammation. We know that TNFR1 is expressed by both neuronal and glial elements of the SFO (58, 59). Further studies to determine which particular cell types in the SFO contribute to this PIC-induced upregulation of excitatory mediators in the PVN may assist in the development of a targeted therapy.
A particularly interesting aspect of this study is the demonstration that TNF-α acting upon the TNFR1 in the SFO increases RAS activity in the SFO and PVN. We have previously reported that systemic infusion of ANG II increased mRNA expression for proinflammatory cytokines in the SFO and PVN in rats (65), and it was recently reported that deletion of neuronal AT1R prevents upregulation of the TNF-α gene in DOCA-salt hypertensive mice (61). These findings imply a significant degree of redundancy at the SFO level in the effects of circulating ANG II and PICs, two critical indicators of peripheral stress. In HF rats, a reciprocal facilitatory interaction between the RAS and inflammation is clearly present in the PVN, where blockade of proinflammatory cytokines reduces RAS activity (27) and blockade of RAS activity reduces proinflammatory cytokine activity (25). In the SFO, as in the PVN, both systems contribute to sympathetic excitation in HF, and interrupting either may have beneficial effects.
The reduction in TNFR1 expression in the SFO, and the resulting decrease in plasma NE levels, had no effect on echocardiographic indexes of LV function in HF rats. This result is consistent with previous studies from our laboratory (14, 15, 29, 56, 57) and other laboratories (18, 25, 32) in which interventions that reduce central sympathetic drive in HF after a large myocardial infarction do not improve echocardiographic indexes of LV function. Some of these studies (18, 32) even used systemic administration of agents that might be expected to have direct beneficial effects on cardiac function in addition to their central effects on sympathetic drive to the heart. Some of the same investigators, using the same method to induce HF, have shown dramatic improvements in echocardiographic indexes of LV function after central interventions in sympathetic drive (24, 27). Studies in which lesser degrees of LV dysfunction were induced by smaller myocardial infarctions have also shown improvement in echocardiographic indexes of LV function after central interventions (21, 22). The reasons for these variable effects of central interventions on echocardiographic indicators of LV function are not obvious, but they may include differences in the residual circulating levels of NE, which remained above control levels in the present study, and of other humoral factors that have direct effects on cardiac function and cardiac remodeling, e.g., ANG II (16, 53) and the circulating PICs themselves (7, 33, 40).
Despite the lack of improvement in echocardiographic indexes of cardiac function, we did observe improvements in cardiac hemodynamics, including an increase in LV dP/dtmax and a reduction in LVEDP, a decrease in the wet lung-to-body weight ratio as an index of pulmonary congestion, and a reduction in the RV-to-body weight ratio as an index of RV remodeling. Other investigators intervening centrally to reduce sympathetic excitation in this rat model of HF have observed similar improvements in cardiac hemodynamics without associated improvements in echocardiographic indexes of LV function (18, 25, 32). These hemodynamic improvements likely reflect the vascular and renal effects of reduced sympathetic nerve activity, with reductions in preload and afterload, manifest as a decrease in LVEDP and an increase in dP/dtmax, improving the functional capacity of the still damaged heart.
A novel aspect of the present study is that it evaluated the effect of selectively blocking only one of the two receptors for TNF-α, rather than blocking all effects of TNF-α, as previous studies have done using an antibody complex, such as etanercept, or a synthesis inhibitor, such as pentoxifylline (27, 29). The p75 TNF-α receptor, also known as TNFR2, is sparsely expressed in the brain under normal conditions (47), but there is evidence that it may have protective effects (4, 8, 11, 33, 44). There is a possibility that TNF-α activation of TNFR2 may contribute to the beneficial effects of knocking down TNFR1 in the SFO. The potential for a protective role of TNFR2 in central cardiovascular regulation has yet to be investigated.
Limitations of the Study
Microinjection of TNFR1 shRNA into the SFO of HF rats resulted in only a partial (~50%) reduction in TNFR1 mRNA and protein. A greater reduction in TNFR1 may have been more effective in the reducing the excitatory neurochemical milieu of the PVN and ameliorating the development of HF. While complete knockdown of TNFR1 expression is not expected with this technique, a larger TNFR1 shRNA dose might have been more effective. We cannot exclude the possibility that off-target microinjections might have minimized our findings, since localization of microinjection sites was not performed on the tissue used for molecular measurement, but the significant reductions in TNFR1 mRNA and protein in the SFO argue against that as a significant limitation.
Another potential technical limitation of this study is that blood for plasma NE measurement was collected from anesthetized rats at the time of euthanasia. Despite the presence of an anesthetic effect, there were significant increases in plasma NE in HF + Veh rats compared with Sham + Veh rats, and treatment of the HF rats with TNFR1 shRNA resulted in plasma NE levels that were significantly lower than those of HF + Veh or HF + Con shRNA rats, although still higher than those of Sham + Veh rats. Thus, while anesthesia clearly affected the NE measurements, it did not mask a treatment effect.
SFO neurons innervate both parvocellular and magnocellular divisions of the PVN (31), raising the possibility that TNF-α actions at the SFO level influence neuroendocrine as well as presympathetic neurons. The present study was narrowly focused on the effects of TNFR1 in the SFO on the sympathoexcitatory milieu of the PVN, without consideration of other functions of SFO neurons projecting directly or indirectly to the PVN or of their neurochemical phenotypes. Further studies will be needed to address these issues and to determine the effect of TNF-α actions in the SFO on PVN neurons regulating the release of arginine vasopressin and adrenocorticotropic hormone, which may also contribute to the progression of HF.
Perspectives
Much attention has been directed to the effects of circulating ANG II and aldosterone on SFO-mediated mechanisms (thirst, Na+ appetite, and sympathetic drive) that are highly relevant to the progression of the HF syndrome. The present study suggests a similar major influence of circulating PICs on the SFO, at least with regard to activation of the SNS, including the possibility that PICs may upregulate RAS components in the SFO in HF and thereby prime SFO neurons to respond to circulating ANG II. Although PIC receptors are known to be expressed in circumventricular organs, their significance with regard to sympathetic activation by circulating PICs in HF has not previously been addressed. The present study strongly supports the hypothesis that HF-induced increases in circulating TNF-α act upon TNFR1 in the SFO to upregulate RAS activity and inflammatory mediators in the SFO and downstream in the PVN, thereby contributing to sympathetic excitation and cardiac dysfunction in HF. PIC-driven SFO-mediated increases in sympathetic activity may act upon the kidneys to stimulate renin release and increase Na+ retention and volume accumulation (i.e., preload), upon the vasculature to increase vasoconstriction (i.e., afterload), and upon the heart itself to promote the progression of HF and precipitate lethal ventricular arrhythmias. TNFR1 in the SFO may be a novel and potentially readily accessible target for therapeutic intervention to prevent these adverse central effects of circulating PICs. Whether PICs affect other SFO functions (e.g., salt appetite and vasopressin release) relevant to the HF syndrome remains to be determined.
GRANTS
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, and by National Institutes of Health Grants R01-HL-073986 (to R. B. Felder) and S10-OD-019941 (to R. M. Weiss).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Y., S.-G.W., and R.B.F. conceived and designed research; Y.Y. and S.-G.W. performed experiments; Y.Y., S.-G.W., and R.M.W. analyzed data; Y.Y., S.-G.W., and R.B.F. interpreted results of experiments; Y.Y. and S.-G.W. prepared figures; Y.Y. and R.B.F. drafted manuscript; Y.Y., S.-G.W., R.M.W., and R.B.F. edited and revised manuscript; Y.Y., S.-G.W., R.M.W., and R.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Kathy Zimmerman for her diligent and expert assistance in the performance of the echocardiograms.
REFERENCES
- 1.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1α, murine IL-1 alpha and murine IL-1β are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 259: 988–996, 1991. [PubMed] [Google Scholar]
- 3.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59: 113–121, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immun 30: 45–53, 2013. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059, 2001. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 6.Dibbs Z, Kurrelmeyer K, Kalra D, Seta Y, Wang F, Bozkurt B, Baumgarten G, Sivasubramanian N, Mann DL. Cytokines in heart failure: pathogenetic mechanisms and potential treatment. Proc Assoc Am Physicians 111: 423–428, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, Wilson EM, Spinale FG, Mann DL. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation 109: 262–268, 2004. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 8.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 75: 104–112, 1997. doi: 10.1016/S0165-5728(97)00009-X. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci 17: 7166–7179, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson AV, Renaud LP. Hypothalamic paraventricular nucleus lesions decrease pressor responses to subfornical organ stimulation. Brain Res 305: 361–364, 1984. doi: 10.1016/0006-8993(84)90443-8. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 22: RC216, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 13.Francis J, Wei SG, Weiss RM, Beltz T, Johnson AK, Felder RB. Forebrain-mediated adaptations to myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 282: H1898–H1906, 2002. doi: 10.1152/ajpheart.00488.2001. [DOI] [PubMed] [Google Scholar]
- 14.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol 287: H2138–H2146, 2004. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 15.Francis J, Weiss RM, Johnson AK, Felder RB. Central mineralocorticoid receptor blockade decreases plasma TNF-α after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol 284: R328–R335, 2003. doi: 10.1152/ajpregu.00376.2002. [DOI] [PubMed] [Google Scholar]
- 16.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev 16: 13–21, 2011. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 17.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol 106: 273–286, 2011. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail 10: 625–634, 2008. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haywood JR, Fink GD, Buggy J, Phillips MI, Brody MJ. The area postrema plays no role in the pressor action of angiotensin in the rat. Am J Physiol Heart Circ Physiol 239: H108–H113, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman WE, Schmid PG. Cardiovascular and antidiuretic effects of central prostaglandin E2. J Physiol 288: 159–169, 1979. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang BS, Chen A, Ahmad M, Wang HW, Leenen FH. Mineralocorticoid and AT1 receptors in the paraventricular nucleus contribute to sympathetic hyperactivity and cardiac dysfunction in rats post myocardial infarct. J Physiol 592: 3273–3286, 2014. doi: 10.1113/jphysiol.2014.276584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang BS, White RA, Ahmad M, Tan J, Jeng AY, Leenen FH. Central infusion of aldosterone synthase inhibitor attenuates left ventricular dysfunction and remodelling in rats after myocardial infarction. Cardiovasc Res 81: 574–581, 2009. doi: 10.1093/cvr/cvn222. [DOI] [PubMed] [Google Scholar]
- 23.Kang YM, Gao F, Li HH, Cardinale JP, Elks C, Zang WJ, Yu XJ, Xu YY, Qi J, Yang Q, Francis J. NF-κB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res Cardiol 106: 1087–1097, 2011. doi: 10.1007/s00395-011-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-α modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83: 737–746, 2009. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res 79: 671–678, 2008. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-κB activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82: 503–512, 2009. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, Zhao XF, Zhang J, Zhang LH, Yang ZM, Francis J. TNF-α in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med 222: 251–263, 2010. doi: 10.1620/tjem.222.251. [DOI] [PubMed] [Google Scholar]
- 28.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–766, 2006. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 29.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Kawano H, Masuko S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience 169: 1227–1234, 2010. doi: 10.1016/j.neuroscience.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 32.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298: H1546–H1555, 2010. doi: 10.1152/ajpheart.01006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinbongard P, Schulz R, Heusch G. TNF-α in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev 16: 49–69, 2011. doi: 10.1007/s10741-010-9180-8. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem 70: 452–466, 1998. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- 35.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 36.Lind RW, Swanson LW, Sawchenko PE. Anatomical evidence that neural circuits related to the subfornical organ contain angiotensin II. Brain Res Bull 15: 79–82, 1985. doi: 10.1016/0361-9230(85)90064-4. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ CT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012. doi: 10.1152/ajpregu.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llewellyn TL, Sharma NM, Zheng H, Patel KP. Effects of exercise training on SFO-mediated sympathoexcitation during chronic heart failure. Am J Physiol Heart Circ Physiol 306: H121–H131, 2014. doi: 10.1152/ajpheart.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116: 1254–1268, 2015. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariappan N, Elks CM, Haque M, Francis J. Interaction of TNF with angiotensin II contributes to mitochondrial oxidative stress and cardiac damage in rats. PLoS One 7: e46568, 2012. doi: 10.1371/journal.pone.0046568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-α-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol 293: H2726–H2737, 2007. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 43.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res 577: 261–267, 1992. doi: 10.1016/0006-8993(92)90282-E. [DOI] [PubMed] [Google Scholar]
- 44.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5: 45, 2008. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: III–XII, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Miselis RR. The subfornical organ’s neural connections and their role in water balance. Peptides 3: 501–502, 1982. doi: 10.1016/0196-9781(82)90115-2. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience 93: 1449–1464, 1999. doi: 10.1016/S0306-4522(99)00225-0. [DOI] [PubMed] [Google Scholar]
- 48.Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis 31, Suppl 5: S178–S184, 2000. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 49.Ng GA. Neuro-cardiac interaction in malignant ventricular arrhythmia and sudden cardiac death. Auton Neurosci 199: 66–79, 2016. doi: 10.1016/j.autneu.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev 5: 73–86, 2000. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 51.Probert L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 302: 2–22, 2015. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Rivest S, Lacroix S, Vallières L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223: 22–38, 2000. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 53.Sayer G, Bhat G. The renin-angiotensin-aldosterone system and heart failure. Cardiol Clin 32: 21–32, vii, 2014. doi: 10.1016/j.ccl.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state--central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol 299: R405–R415, 2010. doi: 10.1152/ajpregu.00103.2010. [DOI] [PubMed] [Google Scholar]
- 55.Twohig JP, Cuff SM, Yong AA, Wang EC. The role of tumor necrosis factor receptor superfamily members in mammalian brain development, function and homeostasis. Rev Neurosci 22: 509–533, 2011. doi: 10.1515/RNS.2011.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei SG, Yu Y, Weiss RM, Felder RB. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am J Physiol Heart Circ Physiol 311: H871–H880, 2016. doi: 10.1152/ajpheart.00362.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei SG, Yu Y, Weiss RM, Felder RB. Inhibition of brain mitogen-activated protein kinase signaling reduces central endoplasmic reticulum stress and inflammation and sympathetic nerve activity in heart failure rats. Hypertension 67: 229–236, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 65: 1126–1133, 2015. doi: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62: 118–125, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu B, Zheng H, Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 302: H1700–H1711, 2012. doi: 10.1152/ajpheart.00722.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res 121: 43–55, 2017. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, Yi QY, Zhang J, Zhu GQ, Zhu Z, Kang YM. Interaction between AT1 receptor and NF-κB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol 13: 381–390, 2013. doi: 10.1007/s12012-013-9219-x. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor-κB. Hypertension 49: 511–518, 2007. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y, Xue BJ, Wei SG, Zhang ZH, Beltz TG, Guo F, Johnson AK, Felder RB. Activation of central PPAR-γ attenuates angiotensin II-induced hypertension. Hypertension 66: 403–411, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, Johnson AK, Felder RB. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension 61: 842–849, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 101: 304–312, 2007. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- 68.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 55: 652–659, 2010. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y, Zhang ZH, Wei SG, Weiss RM, Felder RB. Peroxisome proliferator-activated receptor-γ regulates inflammation and renin-angiotensin system activity in the hypothalamic paraventricular nucleus and ameliorates peripheral manifestations of heart failure. Hypertension 59: 477–484, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang ZH, Felder RB. Hypothalamic corticotrophin-releasing factor and norepinephrine mediate sympathetic and cardiovascular responses to acute intracarotid injection of tumour necrosis factor-α in the rat. J Neuroendocrinol 20: 978–987, 2008. doi: 10.1111/j.1365-2826.2008.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]