Myocardial ischemia and reperfusion injury remain significant causes of morbidity and mortality whereby alterations in the balance between matrix metalloproteinase and tissue inhibitor of metalloproteinase have been identified as contributory biological mechanisms. This novel translational study advances the concept of targeted delivery of recombinant proteins to modify adverse myocardial remodeling in ischemia-reperfusion injury.
Keywords: myocardial infarction, tissue inhibitors of metalloproteinase, recombinant proteins, myocardial remodeling
Abstract
Ischemia-reperfusion (IR) and myocardial infarction (MI) cause adverse left ventricular (LV) remodeling and heart failure and are facilitated by an imbalance in matrix metalloproteinase (MMP) activation and the endogenous tissue inhibitors of metalloproteinase (TIMPs). We have identified that myocardial injections of recombinant TIMP-3 (rTIMP-3; human full length) can interrupt post-MI remodeling. However, whether and to what degree intracoronary delivery of rTIMP-3 post-IR is feasible and effective remained to be established. Pigs (25 kg) underwent coronary catheterization and balloon occlusion of the left anterior descending coronary artery (LAD) for 90 min whereby at the final 4 min, rTIMP-3 (30 mg, n = 9) or saline was infused in the distal LAD. LV echocardiography was performed at 3–28 days post-IR, and LV ejection fraction (EF) and LV end-diastolic volume were measured. LV EF fell and LV end-diastolic volume increased from baseline (pre-IR) values (66 ± 1% and 40 ± 1 ml, respectively, means ± standard deviation) in both groups; however, the extent of LV dilation was reduced in the rTIMP-3 group by 40% at 28 days post-IR (P < 0.05) and the fall in LV EF was attenuated. Despite equivalent plasma troponin levels (14 ± 3 ng/ml), computed MI size at 28 days was reduced by over 45% in the rTIMP-3 group (P < 0.05), indicating that rTIMP-3 treatment abrogated MI expansion post-IR. Plasma NH2-terminal pro-brain natriuretic peptide levels, an index of heart failure progression, were reduced by 25% in the rTIMP-3 group compared with MI saline values (P < 0.05). Although the imbalance between MMPs and TIMPs has been recognized as a contributory factor for post-MI remodeling, therapeutic strategies targeting this imbalance have not been forthcoming. This study is the first to demonstrate that a relevant delivery approach (intracoronary) using rTIMP can alter the course of post-MI remodeling.
NEW & NOTEWORTHY Myocardial ischemia and reperfusion injury remain significant causes of morbidity and mortality whereby alterations in the balance between matrix metalloproteinase and tissue inhibitor of metalloproteinase have been identified as contributory biological mechanisms. This novel translational study advances the concept of targeted delivery of recombinant proteins to modify adverse myocardial remodeling in ischemia-reperfusion injury.
a critical structural event in the progression of heart failure after a myocardial infarction (MI) is left ventricular (LV) remodeling, which can be defined as changes in LV geometry and structure (5, 13, 21, 25). Although LV remodeling is a multifactorial process, post-MI remodeling is characterized by continuous turnover of the extracellular matrix (ECM) within the MI region, causing mural wall thinning, LV chamber dilation, and, eventually, pump dysfunction (15–17, 19, 21, 24). A family of proteolytic enzymes that contribute to ECM remodeling, particularly in terms of post-MI remodeling, are the matrix metalloproteinases (MMPs) (17, 19, 21). For example, induction and release of MMPs have been identified in patients post-MI and associated with the rate and extent of LV remodeling (24). Basic studies have provided mechanistic evidence that modulating MMP activity would favorably alter the course of post-MI remodeling (12, 15–17, 19, 21). However, clinical translation of these studies into therapies that alter MMP induction/activation have encountered problematic issues, such as systemic delivery of pharmacological MMP inhibitors (6, 11, 20). An alternative approach would be a more localized delivery approach, thus potentially overcoming an issue that has impeded progress in terms of therapeutic strategies targeting the MMP system and post-MI remodeling. One biological pathway that can result in MMP inhibition is through the synthesis and release of tissue inhibitors of metalloproteinase (TIMPs) (3, 4, 17, 21). However, the effects of individual TIMPs are not uniform in terms of MMP inhibitory profiles, processing of biological signaling cascades, and effects on fibroblast growth/viability (7, 9, 14, 18, 26). For example, one specific TIMP, TIMP-3, influences multiple biological processes, which include binding to the ECM (14, 26), cytokine processing (4, 18), and fibroblast phenotype (9, 14, 18, 26). The potential importance of TIMP-3 has been buttressed by transgenic studies whereby deletion of TIMP-3 causes adverse remodeling and acceleration to heart failure (12). These findings would suggest that localized augmentation of TIMP-3 within the MI region would affect ECM remodeling and hence adverse LV remodeling. In an initial feasibility study, this laboratory demonstrated that direct myocardial injection of full-length recombinant TIMP-3 (rTIMP-3) into the MI region favorably altered the natural history of post-MI remodeling (7). This initial study, however, did not address a number of issues that hold translational relevance. First, past animal studies of TIMP-3 have primarily used a coronary ligation model of MI (7, 15, 17, 19, 21), whereas in the clinical context, MI arises most often after an ischemia-reperfusion (IR) event. Second, our past study of direct myocardial injection of TIMP-3 at the time of MI, using a cardiac surgical approach, does not hold translational relevance. Specifically, the clinical scenario that would hold the most translational relevance is to deliver TIMP-3 at the time of reperfusion using a catheter-based technique (1, 22). Accordingly, the central hypothesis of the present study was that intracoronary delivery of rTIMP-3 at the time of reperfusion, recapitulating the clinical context of a percutaneous coronary intervention, would significantly alter adverse LV remodeling in the post-IR period.
METHODS
Overview.
This study used a large-animal (pig) model of IR achieved through the use of conventional clinical coronary catheterization approaches, whereby intracoronary infusion of rTIMP-3 occurred just before reperfusion. The period of ischemia was to simulate what is considered a relevant “door-to-balloon” time (90 min) (1, 22), and the main response variable was the time course of LV remodeling, as defined as the rate and extent of LV dilation post-IR.
rTIMP-3 and MMP inhibition.
Full-length human TIMP-3 (rTIMP-3) was expressed in a Chinese hamster ovary cell line and purified using previously described methods (7). Using a global MMP fluorescent peptide assay, 60 μM of MMP substrate (Omni MMP Substrate, BML-P126, Enzo) and 10 nM of recombinant, active MMP construct (SE-237/SE-244, BioMol) were incubated in the presence and absence of increasing concentrations of rTIMP-3. This MMP substrate contains a consensus domain for the catalytic site of all MMP types. All reactions and measurements were performed at 37°C and in triplicate. Fluorescence of the cleaved substrate was measured (FLUOstar, BMG Laboratories) at an emission/excitation wavelength of 280/360 nm. An exponential decay in MMP activity occurred with increasing concentrations of rTIMP-3, as shown in Fig. 1. The exponential fit of this curve yielded a computed effective 50% inhibitory concentration (IC50) of ~7 µg/ml of rTIMP-3. This MMP inhibitory profile and IC50 are consistent with active MMP inhibition for TIMPs (20, 21).
Fig. 1.
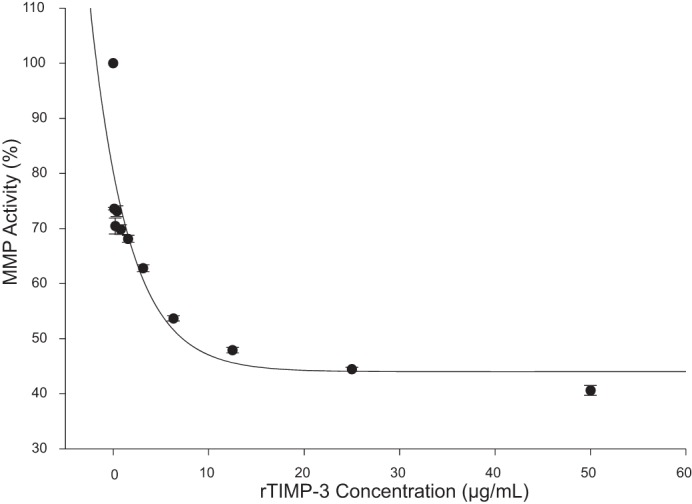
Recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) used in this study inhibited matrix metalloproteinase (MMP) activity, on the basis of a fluorescent substrate activity assay, in a negative exponential fashion, with a computed IC50 of ~7 µg/ml. All assays were performed in triplicate.
Intracoronary rTIMP-3 delivery, retention, and dosing rationale.
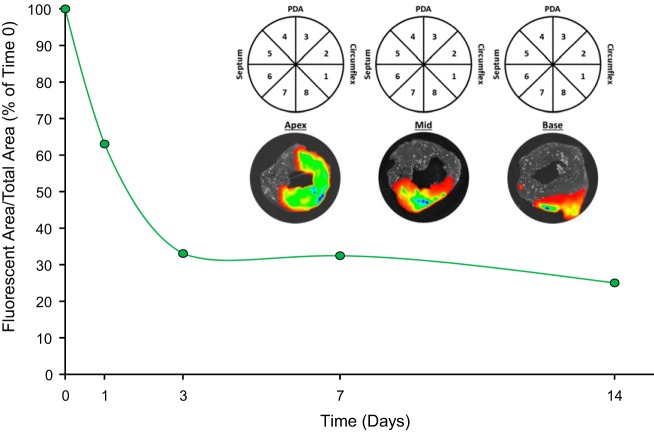
The first set of experiments was performed to confirm the spatial distribution and retention of rTIMP-3 in the targeted IR region, whereby rTIMP-3 was fluorescently labeled. Specifically, rTIMP-3 was labeled with IRDye 800 by diluting to 2.5 mg/ml rTIMP-3 in 50 mM sodium borate (pH 8.0), 50 mM NaCl, 2 mM EDTA, and 5% trehalose and adding ~4× molar excess of DyLight 800 (20 mM stock in DMSO, ~2% final, DyLight 800 NHS ester; Thermo Scientific). The reaction was incubated for 1.5 h at 37°C for 200 mM. l-Arginine was then added to prevent aggregation and subjected to column separation (Sephadex G25 column with PBS, pH 7.4). The final labeled rTIMP-3 was prepared by dialysis (overnight in PBS) and in a final concentration of 5 mg/ml, whereby confirmation of labeling was confirmed by analytical size exclusion chromatography (at 280 vs. 777 nm). These aliquots were maintained at −70°C. To compute the distribution and relative content of the labeled rTIMP-3, pigs (25 kg, n = 5) underwent intracoronary infusion (1 ml, 5 mg/ml) at the final 4 min of the ischemia protocol, as described in greater detail below. At specific post-IR time points (0, 1, 3, 7, and 14 days), circumferential LV sections were subjected to epi-illumination imaging (Xenogen IVIS, Perkin Elmer, Waltham, MA). The settings for the imaging system were predicated on the IRDye 800 spectra (745/800-nm excitation/emission) and the signal was collected over a 0.5-s exposure window. The digitized images (Living Image Software, Perkin Elmer) were then subjected to planimetry (ImageJ software, National Institutes of Health, Bethesda, MD) to determine the total LV circumferential area for that region, and the final results were expressed as the area occupied by rTIMP-3. A representative set of LV images from each region, with injection of rTIMP-3 immediately before reperfusion, is shown in Fig. 2. Using an exponential fit model, the computed rTIMP-3 retention half-life was ~3 days (2.97 days). Next, a theoretical model for computing myocardial volume within the MI region was then applied to develop a targeted intracoronary dose (7). Briefly, if the centralized MI region was considered to have target area of 2 × 2 cm2 and assumed to have a maximal thickness of 1 cm (as quantified by echocardiography), then the volume of the MI targeted region was calculated as 4 cm3. On the basis of the IC50 from the MMP inhibition curve and the computed rTIMP-3 retention times and using this volume of distribution computation, it was determined that an intracoronary dose of 30 mg would conservatively provide a relevant rTIMP-3 concentration (at least double the IC50) within the MI region for 7–10 days post-IR.
Fig. 2.
Recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) used in this study was fluorescently labeled with IRDye 800 before intracoronary injection. Intracoronary injection of fluorescently labeled rTIMP-3 was performed over the last 4 min of the ischemia-reperfusion (IR) protocol in pigs (n = 5, 1 pig/time point), whereby the left ventricle (LV) was harvested immediately after injection and reperfusion or at 0, 1, 3, 7, and 14 days postinjection and ischemia-reperfusion. Inset: representative circumferential LV slices taken at the midpoint of the IR region immediately after labeled rTIMP-3 injection using ex vivo epi-illumination imaging and superimposing the infrared fluorescent signal. The LV planimetry maps used for this analysis are shown. A robust signal, reflecting retention of rTIMP-3 in the myocardial segments, was observed in the apical and midregion with a lower-intensity signal in the basal region, all consistent with the lower left anterior descending coronary artery distribution. PDA, posterior descending artery region. These planimetry maps were used to quantify the fluorescent signal, which was then normalized to the immediate post-IR injection time point (time 0) to establish the rTIMP-3 retention curve. Using a negative exponential curve fit, the estimated retention half-life of labeled rTIMP-3 was ~3 days.
Catheterization, IR induction, and protocol.
Mature pigs (n = 17, Yorkshire, male, 20–25 kg) were administered amiodarone (200 mg po) and aspirin (81 mg po) for 3 days preoperatively and a broad-spectrum antibiotic [Draxxin (2.5 mg/kg im)] at least once preoperatively. On the day of surgery, the pigs were sedated [ketamine-acepromazine-atropine (22/1.1/0.04 mg/kg im)], intubated, and then maintained on 1.5–2% isoflurane delivered in an oxygen/nitrous mixture (3:1 l/min). Buprenorphine (0.05 mg/kg im) was administered as presurgery analgesia. An intravenous infusion (via ear vein cannula) of Benadryl (25 mg) in conjunction with a continuous lidocaine infusion (4 mg·kg−1·h−1) and lactated Ringer solution (10 ml·kg−1·h−1) was initiated. The region encompassing the right femoral artery was prepared in a sterile fashion, the main branch of the femoral artery was surgically exposed, and a catheter introducer (6F Input Introducer Sheath, Medtronic) was positioned and stabilized in the artery. A bolus of heparin was administered (4,000 units iv) before placement to the guide catheter followed by an additional bolus every hour (1,000 units iv). Under fluoroscopic guidance (GE OEC 9600, Salt Lake City, UT), a coronary angiography catheter/launcher (5F Launcher guiding catheter 0.058-in. HIS, Medtronic) was placed in the left coronary ostia, and an angioplasty balloon catheter containing an injection lumen (3 × 10-mm Sprinter OTW balloon catheter, Medtronic) was positioned in the lower portion of the left anterior descending coronary artery (LAD; as defined as 1 cm below the first diagonal). The LAD was occluded by balloon inflation (12-atm balloon inflation pressure, Everest 30 disposable inflation device, Medtronic) and maintained for 90 min. At the final 4 min of the ischemic period, rTIMP-3 or saline was infused, contained within a 3-ml volume, over a 4-min interval through the lumen of the balloon occlusion catheter.
Before the initiation of the procedure, pigs were randomly assigned in a blinded fashion to the following groups: saline (IR-saline; n = 8) or rTIMP-3 (IR-rTIMP-3; n = 9) infusion. The balloon was then deflated, and the catheter system was disengaged and removed. The femoral artery was ligated and the incision was closed. A transdermal fentanyl patch was placed for 3 days for postoperative analgesia. All experimental protocols were approved by the Institutional Animal Care and Use Committees of the University of South Carolina and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
LV function and geometry.
The day before randomization and IR induction, the animals were sedated [diazepam (200 mg po), Barr Laboratories, Pomona, NY] and echocardiography was performed (GE Vivid E9 Dimension Ultrasound System: M5S 1.5–4.6 MHz active matrix array sector transducer probe) to measure LV volumes and area, posterior LV free wall thickness, fractional area shortening, and ejection fraction as previously described (7, 19). The pigs were returned to the laboratory under identical sedation/study conditions at 3, 7, 14, and 28 days post-IR. To improve resolution and thereby compute LV end-systolic volume and hence LV ejection fraction, contrast echocardiography was performed at the terminal post-IR time point. Specifically, 2 ml of contrast media (Optison, GE HealthCare) were injected rapidly in a peripheral ear vein followed by a rapid (1 min) infusion of 10 ml saline while simultaneously visualizing the LV from a transthoracic apical view. At the completion of these measurements, pigs were again anesthetized (5% isoflurane) and the LV region containing the MI region and that of the remote region (LV posterior wall encompassing the posterior descending artery) were harvested. LV sections were then subjected to histochemical staining for MI size and formalin fixation for histomorphometry and subsequent RNA isolation (RNALater, Qiagen). An additional group of pigs (n = 6) of identical age and weight was included in this terminal study protocol to obtain referent normal LV myocardium for comparative analysis.
Plasma troponin, NH2-terminal pro-brain natriuretic peptide, and MI size.
Decanted plasma was used to measure pig specific troponin I and NH2-terminal pro-brain natriuretic peptide (NT-proBNP) using a standard ELISA format and internal standards (Kamiya Biomedical, Seattle, WA). Circumferential LV slices (5 mm) from the base to apex, incorporating the entire MI region, were subjected to histochemical staining and direct infarct size quantitation. Briefly, LV sections were incubated in triphenyltetrazolium chloride (TTC) solution (Sigma, room temperature, 20 min), and digital images of these stained sections were then subjected to densitometry. Infarct size was determined by convention, area negative for TTC staining (infarct) normalized to the total area at risk (circumflex artery distribution area), and expressed as a percentage (7).
Histomorphometry and mRNA profiling.
The formalin-fixed full-thickness LV samples were embedded, sectioned (7 µm), and stained with picro-Sirius red for fibrillar collagen, and the percent area of collagen within the remote and MI regions was computed using computer-assisted morphometry (7, 19). For this analysis, a minimum of five fields were digitized within each region. LV sections from the remote region were also subjected to histochemical staining using silver impregnation to accentuate cardiac myocyte profiles (whereby the nucleus could be identified and centrally located), which were then digitized, and myocyte cross-sectional area was computed. A minimum of 50 profiles from each section were digitized to develop a representative frequency distribution for myocyte cross-sectional area. All images were obtained at an original ×20 magnification (Nikon E600 with Q imaging software/Image-Pro Plus version 4.5).
RNA was extracted from the LV samples (RNeasy Fibrous Tissue Mini Kit, Qiagen), and the quality and quantity of the extracted RNA were determined (Experion Automated Electrophoresis System, Bio-Rad Laboratories, Hercules, CA). The RNA was reverse transcribed (iScript cDNA Synthesis Kit, Bio-Rad), and pig-specific primer/probe arrays for apoptosis and inflammation were assembled (RT2 Profiler PCR Custom Array, PASS-0122 and CAPS11182, respectively, Qiagen). The reaction was performed (RT2 SYBR Green qPCR Mastermix, Qiagen) and quantified by real-time PCR (CFX96 real-time PCR detection system, Bio-Rad). The real-time PCR fluorescence signal was converted to threshold cycle (Ct) normalized to GAPDH (ΔCt method). All PCR assays were performed in duplicate.
Data analysis.
The comparative analysis (STATA, College Station, TX) was performed using multiway ANOVA followed by pairwise adjusted comparisons using the least-significant-difference test. In terms of the change from baseline, a t-test was performed, whereby the transformed data were computed using a null hypothesis of a zero mean value. The fibrillar collagen percent area was subjected to ANOVA followed by the least-significant-difference test. The myocyte cross-sectional area measurements were placed into categorical quartiles, and the distribution was examined by χ2-analysis. Statistical analyses were performed (STATA). Data are presented as means ± SD.
RESULTS
All of the pigs survived the IR protocol, and early changes in post-IR plasma troponin I levels, a reflection of myocardial injury/ischemia, are shown in Fig. 3. Plasma troponin I levels were at nominal detection levels (<0.05 ng/ml) at baseline (pre-IR) but, as expected, increased significantly by 2 h post-IR. The pattern of plasma troponin I release was identical between those pigs randomized to the IR-saline and IR-rTIMP-3 groups. At 28 days post-IR, plasma troponin I had returned to within baseline levels.
Fig. 3.
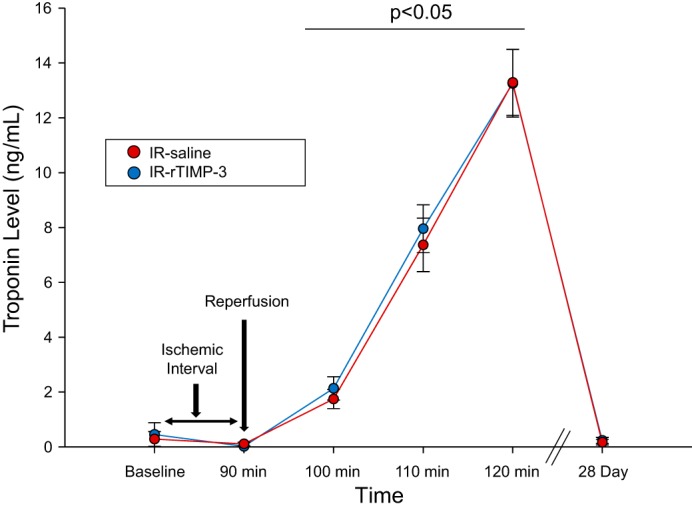
Plasma troponin levels were measured before the induction of ischemia-reperfusion (IR; baseline), after 90 min of coronary occlusion, up to 2 h post-IR, and at terminal study at 28 days post-IR. As expected, plasma troponin I levels were nominally detectable using this high-sensitivity assay at baseline and after 90 min of coronary balloon occlusion (no reperfusion) but surged with release of the intracoronary balloon and institution of reperfusion. Plasma troponin I levels were equivalent to baseline values at 28 days post-IR. Whereas plasma troponin I levels were increased at 100–120 min compared with baseline values (bar: P < 0.05), there were no differences in these values between groups. IR-saline, intracoronary infusion of 3 ml of saline during the final 4 min of balloon occlusion; IR-rTIMP-3, intracoronary infusion of 30 mg recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) contained within 3 ml of saline during the final 4 min of balloon occlusion (IR-saline, n = 8; IR-rTIMP-3, n = 9).
The primary response variables for this study were the rate and extent of LV dilation post-IR, and the results are shown in Fig. 4 in absolute terms and as a function of 3-day post-IR values. In both IR groups, LV dilation as defined as increased LV end-diastolic volume increased from baseline values in a time-dependent manner. However, the magnitude of the LV dilation was reduced in the IR-rTIMP-3 group at 14 and 28 days post-IR. When computed as a function of day 3 post-IR values, LV dilation was reduced by ~40% in the IR-rTIMP-3 group compared with the IR-saline group at the later post-IR time points.
Fig. 4.
The main response variable for this study was left ventricular (LV) dilation, as defined as changes in LV end-diastolic volume over time, using transthoracic echocardiography. Whereas LV dilation occurred in both ischemia-reperfusion (IR) groups as a function of time (LV volumes greater than baseline at all post-IR time points, irrespective of treatment group, P < 0.05), the degree of LV dilation was reduced in the IR- recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) group at 14 and 28 days post-IR when determined as absolute volumetric values or as a change from relative baseline values (inset). +P < 0.05 vs. respective IR-saline values (IR-saline, n = 8; IR-rTIMP-3, n = 9).
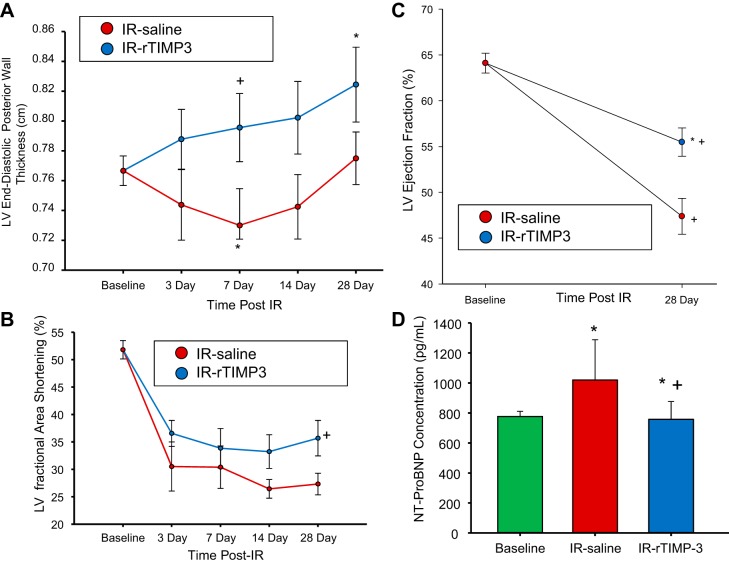
Secondary response variables for this study included LV posterior wall thickness measured at the midventricular region, LV fractional area shortening computed as a function of the entire LV area at systole and diastole, LV ejection fraction determined at baseline and at 28 days post-IR, and plasma NT-proBNP levels at 28 days post-IR (Fig. 5). LV posterior wall thickness was measured at the midventricular, circumferential orientation and, as such, assessed relative wall thickness within the border region of the MI. LV posterior wall thickness fell in the IR-saline group at 7 days post-IR and returned to within baseline values at later post-IR time points. In contrast, this MI/border region remained within baseline values in the IR-rTIMP-3 group at early post-IR time points and actually increased by 28 days post-IR. LV fractional area shortening fell in both groups at all post-IR time points but was higher in the IR-rTIMP-3 group at 28 days post-IR. LV ejection fraction fell in both groups as a function of baseline values, but this was significantly attenuated in the IR-rTIMP-3 group. Plasma NT-proBNP increased in the IR-saline group at 28 days post-IR and was unchanged from baseline values in the IR-rTIMP-3 group.
Fig. 5.
Secondary response variables for this study included left ventricular (LV) posterior wall thickness at end-diastole, fractional area shortening, ejection fraction, and plasma NH2-terminal pro-brain natriuretic peptide (NT-proBNP) levels. A: LV posterior wall thickness fell in the ischemia-reperfusion (IR)-saline group at 7 days post-IR but remained similar to baseline values in the IR-recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) group at this same time point. LV posterior wall thickness actually increased from baseline values in the IR-rTIMP-3 group at 28 days post-IR. B: LV fractional area shortening fell in both groups across all time points (P < 0.05) but was higher at 28 days post-IR in the IR-rTIMP-3 group. C: before IR (baseline), LV end-systolic volumes as well as end-diastolic volumes were measured, and LV ejection fraction was determined. To provide enhanced endocardial border recognition, contrast echocardiography was performed at 28 days post-IR to again determine LV end-systolic volume and hence LV ejection fraction. LV ejection fraction fell in both groups as a function of baseline values, but this was significantly attenuated in the IR-rTIMP-3 group. D: plasma NT-proBNP levels increased in the IR-saline group at 28 days post-IR and were similar to baseline values in the IR-rTIMP-3 group. *P < 0.05 vs. baseline values; +P < 0.05 vs. respective IR-saline values (IR-saline, n = 8; IR-rTIMP-3, n = 9).
LV circumferential sections taken through the midregion were used to assess MI size (Fig. 6). In absolute terms, MI size relative to total LV area appeared lower in the IR-rTIMP-3 group but did not reach statistical significance. However, when MI size was computed for the rTIMP-3 group as a function of IR-saline values, a significant reduction in relative MI size was observed. Specifically, rTIMP-3 reduced relative MI size by ~45% when computed as a function of untreated IR values.
Fig. 6.
Relative myocardial infarction (MI) size, computed as a function of total left ventricular (LV) area, was computed using triphenyltetrazolium chloride staining and planimetry. A: representative LV circumferential sections cut across the central portion of the MI are shown for both groups identifying the relative increase in MI thickness and reduced overall area in the ischemia-reperfusion (IR)-recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) group. B: although absolute MI size was lower in the IR-rTIMP-3 group, this did not reach statistical significance (P = 0.09). C: however, when MI size was computed for the rTIMP-3 group as a function of IR-saline values, a significant reduction in relative MI size was observed. Specifically, rTIMP-3 reduced relative MI size by ~45% when computed as a function of untreated IR values. D: collagen content as assessed by histochemical methods increased in both the MI and remote regions at 28 days post-IR compared with referent control values. Within the MI region, total fibrillar collagen content was lower in the IR-rTIMP-3 group. There was no difference in fibrillar collagen content within the remote region between IR groups. E: myocyte cross-sectional area was assessed within the remote, viable myocardial region as well as within the identical region of referent controls. The cross-sectional area values were then placed into a categorical, quartile distribution and assessed by χ2-analysis. At 28 days post-IR, there was a shift in the distribution of myocytes from those <180 µm2 to 181–265 µm2 irrespective of treatment. However, there was a significant shift in the distribution of myocyte cross-sectional area values of >266 µm2 in the IR-rTIMP-3 group with a greater frequency of myocytes of <350 µm2 and a reduced frequency of myocytes of >350 µm2. PDA, posterior descending artery region. *P < 0.05 vs. baseline values or referent control values; +P < 0.05 vs. respective IR-saline values (IR-saline, n = 8; IR-rTIMP-3, n = 9; referent control, n = 6).
Histochemical staining of LV sections from the MI and remote region was performed to determine fibrillar collagen content and compared with respect to referent control values (Fig. 6). Fibrillar collagen content increased from referent control values in both post-IR groups and in both regions. As expected, a substantial increase in collagen content was observed within the MI region but was reduced in the IR-rTIMP-3 group. Relative collagen content increased to a similar degree within the remote region in both IR groups. Myocyte cross-sectional area was measured within the remote region and compared with referent control values, whereby these values were examined by a categorical analysis and frequency distribution (Fig. 6). There was an overall shift to the right in myocyte cross-sectional area from values of ≤180 µm2 to the category of 181–265 µm2, indicative of myocyte hypertrophy. At higher myocyte cross-sectional values, a shift in the distribution occurred within the IR-rTIMP-3 group whereby there was a higher distribution in the 266- to 350-µm2 category and less in the >350-µm2 category.
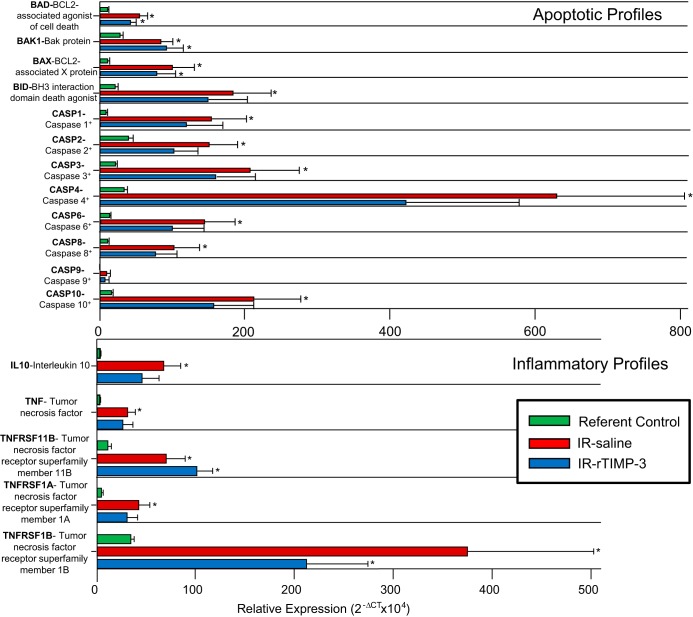
Indexes of proapoptosis and inflammation were determined by RT-PCR and porcine-specific arrays. Representative mRNA profiles with respect to referent control values are shown in Fig. 7. Overall, markers of proapoptosis and inflammatory pathways increased at 28 days post-IR, which included an induction of cell death domains (BAD/BAX), the family of caspases, IL-10, TNF, and TNF receptor subtypes. Although many of these factors were also increased in the IR-rTIMP-3 group, several were reduced and as a result did not reach statistical significance from referent control values. This included the family of caspases, IL-10, TNF, and TNF receptor subtype IA.
Fig. 7.
Left ventricular (LV) myocardial samples were subjected to RT-PCR for the relative expression of proapoptotic signals (top) and for indexes of inflammation (bottom). Overall, there was a significant increase in the relative expression of proapoptotic factors at 28 days postischemia-reperfusion (post-IR) compared with referent control values. Although increased, the relative magnitude was lower in the IR-recombinant tissue inhibitor of metalloproteinase-3 (rTIMP-3) group, notably for the family of caspases. In terms of inflammation, a rise in IL-10, TNF, and TNF receptor expression increased at 28 days post-IR, and this relative increase was reduced for IL-10, TNF, and TNF receptor type IA in the IR-rTIMP-3 group. *P < 0.05 vs. referent control values (IR-saline, n = 6; IR-rTIMP-3, n = 6; referent control, n = 6).
DISCUSSION
Although significant progress has been made in terms of reducing mortality associated with acute myocardial ischemia (acute coronary syndromes), IR injury and resultant MI remain significant contributory factors for the development and progression of heart failure (2). Although the development of heart failure secondary to IR is due to a number of factors, a structural event is LV remodeling, most commonly defined as LV dilation, and changes in overall LV geometry. Thus, developing therapeutic strategies that interrupt this process would, in turn, likely prevent the progression to heart failure. One biological system identified to play a contributory role in LV dilation in the post-MI period consists of MMPs and TIMPs. Specifically, it has been postulated that an imbalance between MMP induction/activation and TIMPs occurs within the myocardium post-MI, which, in turn, promulgates adverse LV remodeling (16, 17, 21). Clinical observational studies and mechanistic animal studies have clearly supported the concept that modifying the MMP-TIMP balance post-MI will influence LV remodeling (12, 15, 16, 19, 24). For example, modifying myocardial levels of a specific TIMP, TIMP-3, either through transgenic means or by direct myocardial injection of rTIMP-3, has been shown to alter the natural history of post-MI remodeling (7, 12). However, translation of these past studies to a relevant model of IR and a clinically applicable method for rTIMP-3 delivery is necessary if this therapeutic target is to move forward. The present study used a large-animal model of IR and conventional coronary catheterization approaches to examine the effects of intracoronary delivery of rTIMP-3 on post-IR LV remodeling. The new and important findings from this study were twofold. First, intracoronary delivery of rTIMP-3 at the time of reperfusion attenuated LV dilation for a sustained period of time post-IR (28 days). Second, intracoronary delivery of rTIMP-3, reduced LV wall thinning, and increased LV pump function at 28 days post-IR were accompanied by a reduction in a biomarker for heart failure progression (NT-proBNP). This attenuation of the natural history of post-MI remodeling with rTIMP-3 was associated with reduced indexes of apoptosis and inflammation. This unique translational study provides the first direct evidence that intracoronary delivery of a rTIMP at the time of reperfusion after a prolonged period of ischemia attenuates a critical determinant of heart failure progression, LV dilation.
Although pharmacological targeting of the MMP-TIMP system remains an area of research interest in terms of a therapeutic target, systemic delivery of pharmacological MMP inhibitors has encountered problematic issues regarding dosing, efficacy, and adverse effects (6, 11, 20). An alternative approach is to locally alter the balance between MMP and TIMPs through genetic or recombinant protein methods (7, 9, 12, 15, 23). For example, direct myocardial injection of a gel that released rTIMP-3 into the MI region has been shown to have favorable effects on LV structure and function (7, 23). Although minimally invasive/robotic surgical approaches to access the LV epicardial surface are rapidly evolving (8, 10) and thus provide a platform for direct myocardial injections of gels containing recombinant proteins, a more readily translatable approach for delivery of rTIMP-3 was pursued in the present study. Specifically, a simulation of an acute coronary syndrome followed by reperfusion was used in pigs, whereby just before angioplasty balloon deflation, intracoronary infusion of rTIMP-3 was infused into the downstream coronary vasculature. The results from this study demonstrated that this single bolus infusion of rTIMP-3 significantly reduced the extent and rate of LV dilation. The relative attenuation in LV dilation observed in the present study is similar to that achieved by myocardial injection of a gel releasing rTIMP-3 (7). Thus, although additional studies are warranted, this is the first to demonstrate that intracoronary delivery of rTIMP-3 in the context of IR injury can be translated into a persistent effect on LV remodeling.
The mechanistic basis for the observation that intracoronary rTIMP-3 infusion reduced the extent of LV dilation in the post-IR period is likely to be multifactorial. First, the degree of MI expansion was reduced with rTIMP-3 delivery as evidenced by a preservation of LV wall thickness and a relative reduction in overall MI area. Although not directly measured in the present study, the preservation of LV wall thickness was likely due to a local effect on ECM turnover and stability, which, in turn, would reduce LV wall thinning. However, although a reduction in MI expansion was likely a key factor in the reduction in LV dilation, it cannot be discounted that rTIMP-3 infusion affected a number of biological pathways. For example, TIMP-3 influences cytokine production, such as TNF-α (3, 4), and thus a reduction in proinflammatory mediators in the post-IR period may also play a role in favorable effects on LV remodeling. To more carefully examine this possibility, the present study examined the relative expression of TNF and TNF receptor subtypes. At 28 days post-IR, TNF and TNF receptor steady-state mRNA levels were increased and were modestly attenuated with rTIMP-3 delivery. In addition, IL-10, which is canonically considered an anti-inflammatory cytokine and is associated with the generalized inflammatory process in the post-MI period, was also attenuated, again albeit to a modest degree, with rTIMP-3 delivery. These results would suggest that one contributory factor for the favorable effects on post-MI remodeling with intracoronary rTIMP-3 delivery was attenuation in local inflammatory processes. However, it must be recognized that these measurements were performed at one point in time and are measurements of steady-state mRNA levels. TIMP-3 can affect TNF processing at the posttranslational level (3, 4), and thus the mRNA measurements would not be reflective of this aspect of TIMP-3 biology.
LV systolic function was assessed in the present study as LV fractional area shortening and by LV ejection fraction as reflections of overall LV pump function. The relative change in LV area from diastole to systole is a reasonable surrogate for assessment of LV pump performance. Because of the heterogeneity in LV end systole, which can occur as a result of dyskinesia in the MI region, contrast echocardiography was performed to quantify LV end-systolic volume and hence global LV ejection fraction at 28 days post-IR. Both measurements identified that LV pump function was higher with intracoronary infusion of rTIMP-3 in the post-IR period. The basis for this improvement was likely due to a favorable effect on LV afterload. Specifically, the reduction in LV end-diastolic volume and the increased posterior wall thickness in the post-IR period with intracoronary delivery of rTIMP-3 would result in a concordant reduction in LV wall stress, a fundamental determinant of LV afterload. To examine potential cellular/extracellular mechanisms for this effect, morphometric measurements of myocyte cross-sectional area within the remote viable myocardium as well as fibrillar collagen content within the MI and remote regions were determined. A shift in myocyte cross-sectional area occurred with rTIMP-3 delivery, suggestive of a change in myocyte hypertrophy/growth. A fundamental stimulus for myocyte growth is LV wall stress, and thus the shift in the pattern of myocyte cross-sectional area to lower values with rTIMP-3 delivery would be consistent with a reduction in this biomechanical stimulus. Within the MI region, a relative reduction in fibrillary collagen content was observed at 28 days after IR and rTIMP-3 delivery. Although this observation may be counterintuitive, rTIMP-3 delivery may have stabilized ECM turnover and as a result reduced the stimulus for collagen synthesis. This also identifies a limitation of this histochemical approach in that fibrillar collagen content reflects only one constituent of the ECM and also does not reflect the geometry and structure of the fibrillar collagen network. Nevertheless, the observation that rTIMP-3 delivery reduced relative fibrillar collagen content within the MI region and was associated with no change within the remote region compared with untreated IR values suggests that this therapeutic approach was not associated with a profibrotic response.
The present study also identified that despite an equivalent degree of initial IR injury, as assessed by plasma troponin I levels, intracoronary delivery of rTIMP-3 reduced relative MI size at 28 days post-IR. This relative reduction in MI area at 28 days post-MI was likely due, in large part, to a reduction in MI expansion but may have also been due to reduced myocyte loss in the post-IR period. Specifically, TIMP-3 has been shown to reduce markers of myocyte apoptosis in a rodent MI model (23). Studies in rodent models of MI and pressure overload have identified that TIMP-3 can influence several growth/viability pathways (9, 12). To begin to address this issue, the present study examined steady-state levels for proapoptotic factors within the MI region at 28 days post-IR. Proapoptotic factors were robustly increased post-IR, with several factors modestly reduced with rTIMP-3 delivery. Since TNF is a known upstream cytokine that can induce proapoptotic signaling, such as caspase induction, the relative reduction in proapoptotic factors with rTMP-3 delivery may have been secondary to the reduction in steady-state mRNA levels for TNF and TNF receptor profiles. A past in vitro study has identified that TIMP-3 can mitigate fibroblast apoptosis directly, suggesting a possible independent pathway for TIMP-3 to influence cell viability (18). Thus, the relative reduction in proapoptotic factors, notably members of the caspase cascade, may have been due to alterations in upstream inflammatory mediators as well as direct cellular effects. At 28 days post-IR, the MI region is most predominantly populated by fibroblasts/myofibroblasts; however, inflammatory cells and endothelial/smooth muscle cells associated with neovascularization are also present (15). Thus, whether and to what degree rTIMP-3 delivery modified apoptotic programs in a specific cell type within the MI region remain to be established. Moreover, since this measurement was at one point in time post-IR, whether rTIMP-3 delivery induced temporal changes in post-IR apoptotic expression profiles remains unknown. Nevertheless, this is the first study to identify that a large number of proapoptotic factors appeared to be modified through intracoronary delivery of a rTIMP at the time of reperfusion. On the basis of the findings of the present study, future studies focused on biological pathways by which rTIMP-3 affected post-IR remodeling over and above that of direct MMP inhibition are warranted.
The focus of the present study was to translate past mechanistic studies regarding TIMP-3 into a clinically relevant context of post-IR remodeling. However, there are several limitations that must be recognized. First, the intracoronary dose of rTIMP-3 selected in this study was based on estimates/approximations of myocardial volumes, distribution, and retention of rTIMP-3. This study was intended to be a translational, proof-of-principle study, and therefore additional studies that examine dose-dependent effects would be warranted. Second, MMP activity within the myocardium was not measured in the present study as the terminal studies were 28 days after the intracoronary delivery of rTIMP-3. However, our past study (7) has demonstrated that direct myocardial delivery of rTIMP-3 in the early postischemic period reduced interstitial MMP activity. Third, the present study examined LV remodeling over an interval of time whereby dynamic changes in LV geometry and function after an ischemic event have been documented in humans and animals (13, 17, 19, 21, 24, 25). Whether and to what degree the effects of rTIMP-3 on post-IR remodeling persist with longer followup periods remain to be established. However, there is evidence from the present study to support the concept that intracoronary delivery of rTIMP-3 modified the natural history and progression of LV failure post-IR. Specifically, NT-proBNP, a biomarker for the development and progression to heart failure, did not increase in the post-IR period with intracoronary rTIMP-3 delivery. Fourth, the present study used rTIMP-3, but it must be recognized that there are four known TIMPs with diverse biological functions (3, 4, 7, 9, 14, 18, 26). Thus, examining the potential differential/interactive effects of TIMPs to that of TIMP-3 would be an appropriate research direction. Although future studies are warranted, the present study provides continued support for the concept that localized modulation of the MMP-TIMP balance within the vulnerable postischemic myocardium is a relevant therapeutic target.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-111090 and HL-113352, a merit award from the Veterans Health Administration, and a basic research grant from Amgen Incorporated.
DISCLOSURES
S. Smith, A. Y. Khakoo, and T. Lee are employees of Amgen Incorporated, which manufactures drugs for a wide range of diseases, including cardiovascular disease. The other authors declare that they have no competing interests. The rTIMP-3 formulations were furnished as a material transfer agreement from Amgen to F. G. Spinale.
AUTHOR CONTRIBUTIONS
S.S., A.Y.K., T.L., and F.G.S. conceived and designed research; S.C.B., H.D., J.J., L.A.F., P.E.P., K.N.Z., K.M., C.F.V., and F.G.S. performed experiments; S.C.B., H.D., J.J., L.A.F., P.E.P., K.N.Z., K.M., C.F.V., and F.G.S. analyzed data; S.C.B., H.D., J.J., L.A.F., P.E.P., K.N.Z., K.M., C.F.V., S.S., A.Y.K., T.L., and F.G.S. interpreted results of experiments; F.G.S. prepared figures; F.G.S. drafted manuscript; F.G.S. edited and revised manuscript; F.G.S. approved final version of manuscript.
REFERENCES
- 1.Bagai A, Dangas GD, Stone GW, Granger CB. Reperfusion strategies in acute coronary syndromes. Circ Res 114: 1918–1928, 2014. doi: 10.1161/CIRCRESAHA.114.302744. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [Corrigendum. Circulation 135: March 2017, p. e646. doi:.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20: 161–168, 2010. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colucci WS, Braunwald E. Pathophysiology of heart failure. In: Braunwald's Heart Disease: a Textbook of Cardiovascular Medicine (7th ed.). St. Louis, MO: Saunders Elsevier, 2005, chapt. 16, p. 509–538. [Google Scholar]
- 6.Dormán G, Cseh S, Hajdú I, Barna L, Kónya D, Kupai K, Kovács L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs 70: 949–964, 2010. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, O’Neill JW, Shuman JA, Novack CP, Zellars KN, Pettaway S, Black RA, Khakoo A, Lee T, Mukherjee R, Gorman JH, Gorman RC, Burdick JA, Spinale FG. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med 6: 223ra21, 2014. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejiofor JI, Leacche M, Byrne JG. Robotic CABG and hybrid approaches: the current landscape. Prog Cardiovasc Dis 58: 356–364, 2015. doi: 10.1016/j.pcad.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103: 268–280, 2014. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Rivas D, Yang Y, Ng C. Advances in uniportal video-assisted thoracoscopic surgery: pushing the envelope. Thorac Surg Clin 26: 187–201, 2016. doi: 10.1016/j.thorsurg.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol 48: 15–20, 2006. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 4: 98–108, 2011. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 269: 9352–9360, 1994. [PubMed] [Google Scholar]
- 15.Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation 105: 753–758, 2002. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 16.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol 91: 134–140, 2016. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 30: 31–41, 2012. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol 288: H461–H468, 2005. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003. doi: 10.1161/01.CIR.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev 9: 63–79, 2004. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 21.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sutton NR, Gurm HS. Door to balloon time: is there a point that is too short? Prog Cardiovasc Dis 58: 230–240, 2015. doi: 10.1016/j.pcad.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Uchinaka A, Kawaguchi N, Mori S, Hamada Y, Miyagawa S, Saito A, Sawa Y, Matsuura N. Tissue inhibitor of metalloproteinase-1 and -3 improves cardiac function in an ischemic cardiomyopathy model rat. Tissue Eng Part A 20: 3073–3084, 2014. doi: 10.1089/ten.tea.2013.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 114: 1020–1027, 2006. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 25.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol 97, 10A: 13F–25F, 2006. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Yu WH, Yu S, Meng Q, Brew K, Woessner JF Jr. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem 275: 31226–31232, 2000. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]