Abstract
Atherosclerotic peripheral artery disease primarily manifests in the medium- to large-sized conduit arteries of the lower extremities. However, the factors underlying this increased vulnerability of leg macrovasculature to disease are largely unidentified. On the basis of recent studies, we propose that excessive time spent in the sitting position and the ensuing reduction in leg blood flow-induced shear stress cause endothelial cell dysfunction, a key predisposing factor to peripheral artery disease. In particular, this review summarizes the findings from laboratory-based sitting studies revealing acute leg vascular dysfunction with prolonged sitting in young healthy subjects, discusses the primary physiological mechanisms and the potential long-term implications of such leg vasculopathy with repeated exposure to prolonged sitting, as well as identifies strategies that may be effective at evading it.
Keywords: blood flow, endothelial function, physical inactivity, sedentarism, shear stress
peripheral artery disease (PAD), which affects 155 million people worldwide (21a), is characterized by the formation of atherosclerotic lesions largely affecting the medium- to large-sized conduit arteries of the lower limbs (1, 31, 44, 60, 63). Despite its high prevalence, the factors contributing to this increased vulnerability of leg macrovasculature to disease surprisingly remain largely unknown. The observation that atherosclerotic lesions are distributed nonuniformly and develop primarily in the lower extremities (1, 31, 44, 60, 63) support the notion that localized factors may be involved in the pathophysiology of leg vascular disease. In this regard, a critical instigator of leg vascular disease may be low shear stress, a proatherogenic hemodynamic environment (9, 14, 26, 28, 48, 65) to which the arteries of the lower extremities are subjected during sitting (42, 46, 47, 55, 56, 69). Given that for many the majority of awake hours are spent in the sitting position (24, 25, 37), this review supports the premise that excessive sitting and the consequent repeated exposure to reduced leg vascular shear stress perturbs the endothelium and renders the vasculature of the lower limbs vulnerable to disease, which manifests when additional risk factors (e.g., type 2 diabetes, smoking, and aging) are superimposed (Fig. 1). Consistent with this idea, epidemiological data are available showing that sedentary time is associated with low levels of the ankle-brachial index, predictive of leg PAD, in an asymptomatic middle-aged to older population (32).
Fig. 1.
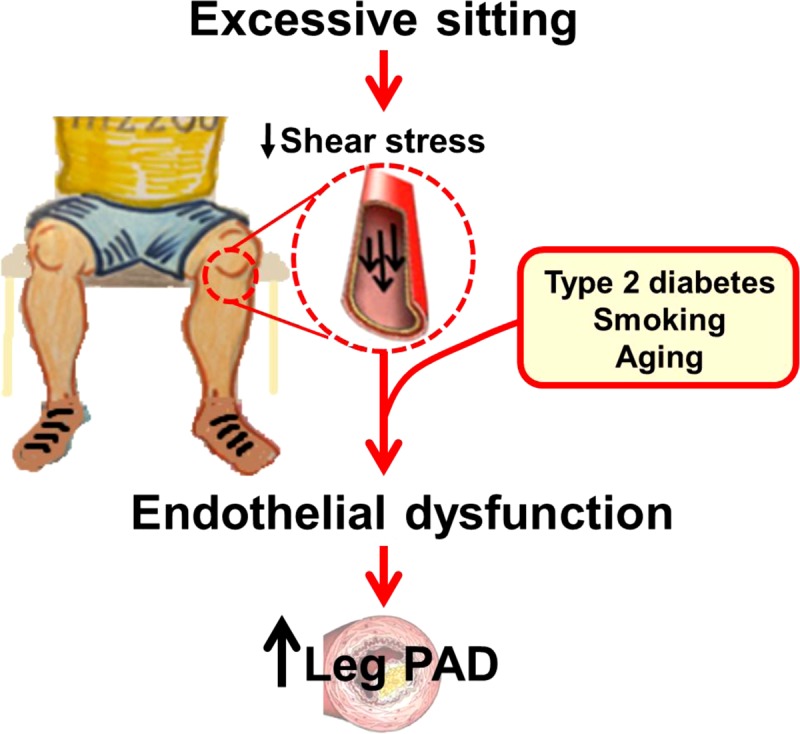
Schematic illustrating the potential link between excessive sitting time and increased risk for leg peripheral artery disease (PAD). Such detrimental effects of prolonged and repeated sitting would be exacerbated when additional risk factors such as type 2 diabetes, smoking, or aging are superimposed.
Accordingly, the purpose of the present review is to summarize the findings from laboratory-based sitting studies documenting leg vascular dysfunction with prolonged sitting, discuss the underlying physiological mechanisms and the potential clinical implications of a leg vasculopathy with repeated exposure to prolonged sitting, as well as recognize strategies that may be effective at circumventing it.
Uninterrupted Sitting Impairs Leg Endothelial Function
Accumulating evidence demonstrates that prolonged exposure to the sitting position (e.g., from 1 to 6 h) impairs endothelial function in the leg vasculature, including the popliteal and femoral arteries (42, 46, 47, 55, 56, 69). Importantly, endothelial dysfunction is implicated as a principal feature of the initiation and progression of atherosclerotic lesions (41, 76). Endothelial dysfunction with prolonged sitting is specific to the leg vasculature [e.g., it does not manifest in upper extremities (brachial artery)] (55, 70) and it is transient. That is, resuming walking activity after uninterrupted sitting restores normal leg vascular function (55). However, the fact that endothelial dysfunction with persistent sitting is acute should not be regarded as inconsequential, as the average individual spends a major portion of their day in the seated position (24, 25, 37). This signifies that the endothelium of lower extremity conduit arteries may display a dysfunctional phenotype during a large fraction of the day (granting time-course studies throughout a typical sitting day are needed). The transient and repeated nature of leg endothelial dysfunction caused by sitting resembles the also temporary impairment of endothelial function during the postprandial state (75). In 1979, Zilversmit (79) published a landmark paper in Circulation entitled “Atherogenesis: a postprandial phenomenon.” Indeed, the repetitive postprandial oxidative stress and endothelial dysfunction has emerged as one of the key initiators in the etiology of cardiovascular disease, more so than traditional risk factors, and the postprandial period is presently considered in the treatment of diabetes to avert vascular complications (75). Similarly, here, we submit the idea that sitting vasculopathy, the recurring and extended episodes of leg conduit artery endothelial dysfunction associated with excessive sitting, may contribute to the genesis of leg PAD.
An interesting observation is that the detrimental impact of prolonged sitting on leg endothelial function appears to be less profound in young adult women compared with men (74). The mechanisms for this sexual dimorphism in leg vascular dysfunction with sitting remain unclear. However, because prepubertal girls are not immune to leg vascular dysfunction with sitting (42), it is possible that cyclic elevations in sex hormones are required for vascular protection against sitting in women. Interestingly, and of clinical significance, the finding that young adult women are generally more protected from sitting-induced impairments in leg conduit artery endothelial function compared with men (74) parallels the lower rates of atherosclerotic disease (54) and leg PAD (20) in women.
An important aspect that should be discussed is that studies documenting leg endothelial dysfunction with sitting have been performed in a laboratory setting. The laboratory-based model of prolonged sitting that has been commonly used (42, 46, 47, 55, 56, 69) involves sitting without any leg movement. Additional home- and work-based sitting studies are needed to determine the leg vascular effects of sitting in a more real-life scenario in which possibly longer and more frequent periods of inactive sitting are occasionally interrupted (e.g., a typical workday). Another important consideration is the extent to which the leg vascular responses to sitting observed in healthy young subjects can be extrapolated to older populations with existing vascular dysfunction and at risk for cardiovascular disease. It is possible that these individuals exhibit less of an impairment in endothelial function with sitting because the endothelium is already compromised (i.e., “flooring effect”). Nevertheless, future studies are warranted to examine the effects of sitting in older at risk populations.
Role of Reduced Shear Stress in Mediating Leg Endothelial Dysfunction With Sitting
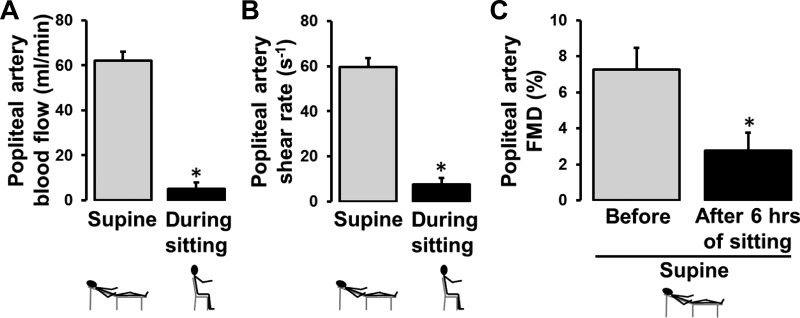
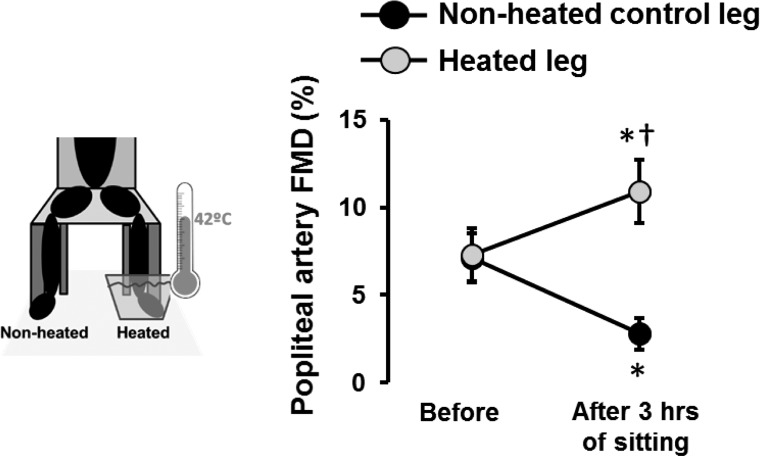
An important and consistent finding is that leg blood flow is markedly reduced during sitting (Fig. 2). Given that shear stress is an important physiological signal for maintaining endothelial health (11–13, 16, 23, 27, 33, 34, 38, 59, 61, 71, 78), we tested whether the sustained reduction of shear stress during sitting mediates leg endothelial dysfunction. Specifically, we examined whether preventing the reduction in leg blood flow and shear stress during sitting would abolish the detrimental effects of sitting on popliteal artery endothelial function (56). We performed bilateral measurements of popliteal artery flow-mediated dilation (FMD) before and after 3 h of sitting during which one foot was submerged in 42°C water to increase blood flow and thus shear stress, whereas the contralateral leg remained dry and served as an internal control. We found that preventing the reduction of shear stress during prolonged sitting with local heating abolished the impairment in popliteal artery FMD (Fig. 3), supporting the view that sitting-induced leg endothelial dysfunction is mediated by a reduction in shear stress (56).
Fig. 2.
Prolonged sitting is associated with a marked reduction in leg blood flow (A) and shear rate (B), which leads to impaired popliteal artery flow-mediated dilation (FMD; C). FMD measures were performed in the supine position before and after 6 h of uninterrupted sitting. n = 11. *P < 0.05. [Modified from Restaino et al. (55).]
Fig. 3.
Preventing sitting-induced reductions in lower limb blood flow with foot heating preserves leg endothelial function. Popliteal artery flow-mediated dilation (FMD) measures were performed in the supine position in both legs before and after sitting for 3 h during which time one foot was constantly submerged in 42°C water. The water reached up to the ankle (i.e., malleolus). n = 11. *P < 0.05 vs. before; †P < 0.05 between legs. [Modified from Restaino et al. (56).]
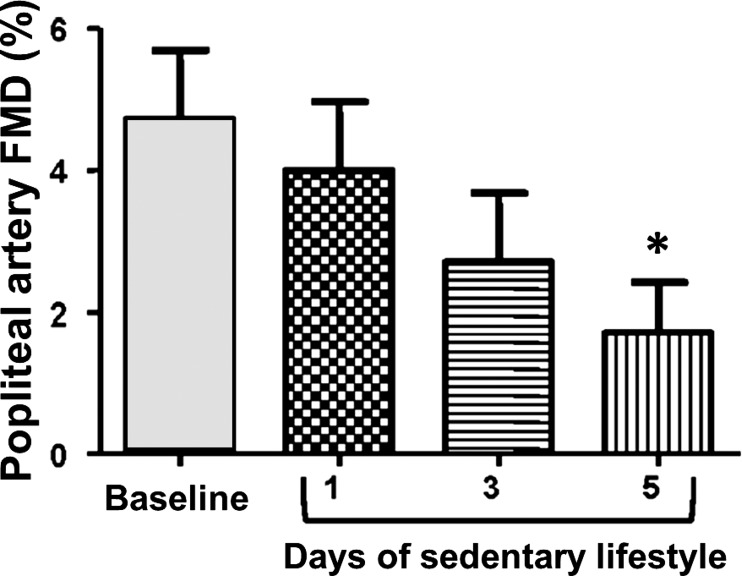
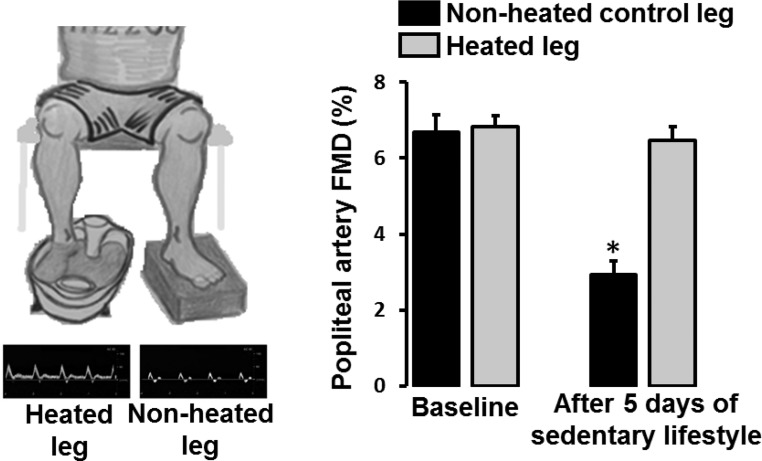
In concert with this hypothesis, we recently reported that when active individuals adopt a sedentary behavior, characterized by extensive sitting and reduced number of steps (from >10,000 to <5,000) for 5 days, endothelial dysfunction manifests in the popliteal but not brachial arteries (Fig. 4) (8). The finding that endothelial dysfunction is primarily displayed in the popliteal artery could be related to the fact that legs, upon reduction of locomotion, are subjected to a greater decrease in blood flow and thus conduit artery shear stress, relative to the upper extremities which likely retain similar levels of activity. To test the hypothesis that “knockdown” of shear stress in the leg vasculature is indeed the underlying factor mediating leg endothelial dysfunction following the adoption of a sedentary lifestyle, we followed a similar approach to that of our previous study (8). That is, we recruited active subjects and performed bilateral measurements of popliteal artery FMD before and after 5 days of increased sitting time and reduced number of steps (66). During the inactive period, one foot was submerged in 42°C water for 30 min, 3 times/day, to increase blood flow and thus shear stress, while the opposite leg operated as an internal control. We found that subjecting one leg to foot heating, and consequent increases in leg blood flow, throughout the 5-day inactivity period prevented the decline in popliteal artery FMD in that leg (Fig. 5) (66). Thus, these findings further support the idea that leg endothelial dysfunction caused by sedentary behavior can be obliterated by “replenishing” the conduit artery shear stress stimulus that is “lost” during sitting and/or reduced activity.
Fig. 4.
Exposure to a sedentary lifestyle for 5 days impairs popliteal artery flow-mediated dilation (FMD ) in young healthy men. Popliteal artery FMD measures were performed in the supine position at baseline and at days 1, 3, and 5 of adoption of a sedentary lifestyle. n = 11. *P < 0.05 vs. baseline. [Modified from Boyle et al. (8).]
Fig. 5.
Impaired popliteal artery flow-mediated dilation (FMD) after 5 days of adoption of a sedentary lifestyle is prevented by daily application of local (foot) heating (42°C, 3 times/day, 30 min/session) to increase leg blood flow. FMD measures were performed in the supine position in both legs at baseline and after 5 days of adoption of a sedentary lifestyle during which period one foot was intermittently submerged in 42°C water. The water reached up to the ankle (i.e., malleolus). Original screen shots from Doppler ultrasound images of the popliteal artery blood velocity during unilateral foot heating are also included. n = 13. *P < 0.05 vs. baseline. [Modified from Teixeira et al. (66).]
Potential Molecular Transducers of Sitting-Induced Endothelial Dysfunction
Although reduced leg vascular shear stress is likely the primary instigator of prolonged sitting-induced endothelial dysfunction in the conduit arteries of the lower extremities, the underlying molecular transducers of sitting leg vasculopathy have not been interrogated. On the basis of previous work, we propose that endothelin-1 (ET-1), which is primarily expressed in endothelial cells, may be implicated in the pathophysiology of leg endothelial dysfunction with excessive sitting. This proposal is largely founded on data demonstrating that ET-1 increases formation of vascular reactive oxygen species, reduces nitric oxide bioavailability, impairs endothelium-dependent dilation, and accelerates atherosclerosis (5, 18, 19, 35, 52, 73). Importantly, ET-1 production is stimulated by lowering of shear stress (45). In this regard, we reported that cessation of wheel running in rats for 7 days upregulates expression of ET-1 in arteries feeding the contracting hindlimbs (e.g., the iliac artery) but not in arteries that perfuse noncontracting tissues such as the kidneys (e.g., the renal artery) (49), supporting the notion that induction of ET-1 with inactivity is specific to the vasculature experiencing the removal of vascular shear stress. Of importance, shear stress-induced downregulation of ET-1 production in cultured endothelial cells is deterred when cells are treated with N-nitro-l-arginine methyl ester (l-NAME), indicating suppression of ET-1 by shear is, at least in part, nitric oxide dependent (45). The link between nitric oxide and ET-1 (3) is also supported by our data, demonstrating that chronic treatment with l-NAME, a nitric oxide synthase inhibitor, increases vascular expression of ET-1 in rats (50). Furthermore, data are also available indicating that the inactive lower extremities of spinal cord injury patients, chronically subjected to low vascular shear stress, display increased ET-1-mediated basal vascular tone (67). Moreover, previous work has demonstrated that low-flow-induced constriction is largely ET-1 mediated (62). Together, the literature supports the hypothesis that ET-1 may be a primary molecular transducer of leg endothelial dysfunction caused by sitting and low shear stress. However, it should be emphasized that no studies are available documenting an increase in circulating levels of ET-1 or an upregulation of ET-1 in the vasculature of the lower limbs after prolonged exposure to sitting. Of note, conditions with an increased risk for development of PAD, such as aging, type 2 diabetes, hypertension, and smoking, are all associated with an upregulation of ET-1 (7, 10, 22, 36, 57, 64). Thus, it is conceivable that the combination of such risk factors with reduced leg vascular exposure to shear stress, secondary to an overabundance of sitting, leads to excessive ET-1 production, which mediates a proatherogenic endothelial cell phenotype in the leg vasculature. Stated differently, it is possible that undue sitting-associated low shear stress in the leg vasculature accelerates leg vascular disease via an ET-1-dependent mechanism. Future studies are needed to test this hypothesis as well as to examine the contribution of other molecular mechanisms underlying endothelial dysfunction in the leg vasculature with sitting. It can be anticipated that these efforts will require significant experimental creativity and probably the incorporation of animal models and in vitro approaches. Ultimately, an understanding of the molecular mechanisms by which sitting causes leg endothelial dysfunction may be crucial for the identification of new targets and strategies to combat PAD, the prevalence of which remains high likely owing to the rising sedentary society.
Factors Contributing to Reduced Leg Blood Flow During Sitting
Given that sitting-induced leg vascular dysfunction appears to be attributable to the reduction in leg blood flow-induced shear stress, a discussion of potential factors and mechanisms contributing to leg vascular resistance during sitting is warranted. It is plausible that during sitting increased hydrostatic pressure within the leg vasculature causes blood pooling within the venous circulation, and in support of this we have consistently found an increase in calf circumference during sitting relative to supine (55, 74). This effect may be aggravated by reduced skeletal muscle activity during sitting, which eliminates any influence of the muscle pump in facilitating venous return (17). In addition, it is possible that during sitting venous return from the lower limbs is further limited by the increased pressure on the back of the thighs. Thus, venous distension-induced arterial constriction and increased hydrostatic pressure-induced myogenic constriction (30) are possible contributors to increased leg vascular resistance during sitting. In addition, because muscle sympathetic nerve activity is increased in the upright position (53), adrenergic vasoconstriction may also contribute to the elevated leg vascular resistance. In support of a possible involvement of increased sympathetic nerve activity, although modest, we observe that during sitting there is an increase in blood pressure relative to the supine position (46, 47, 74). Also, as noted above, it is possible that elevations in ET-1 during the prolonged sitting period could exacerbate increases in limb vascular resistance and result in reductions in upstream conduit artery blood flow-induced shear stress.
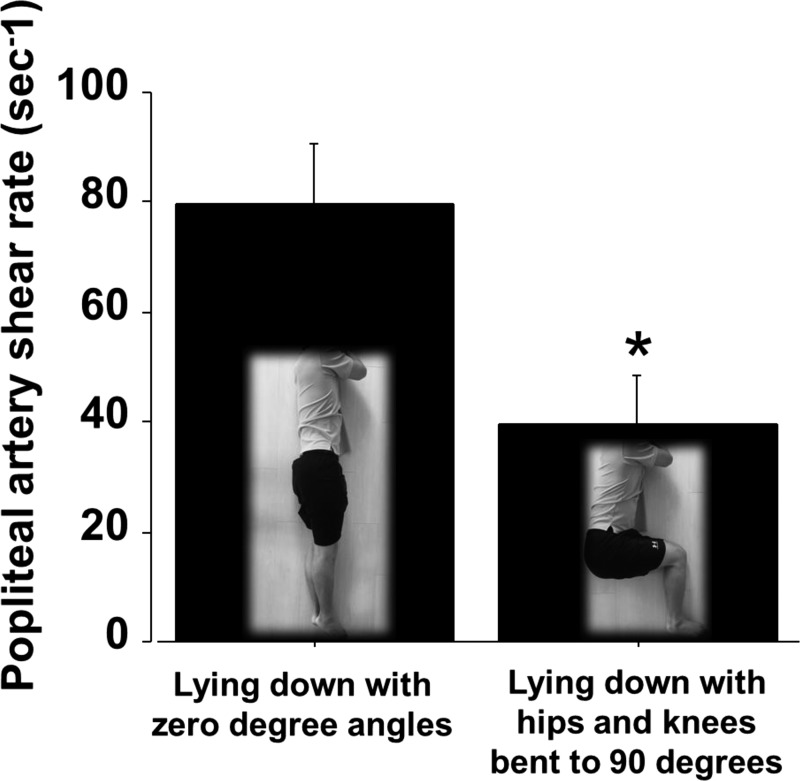
Furthermore, because leg blood flow is immediately reduced upon sitting (74), it is possible that other factors, likely biomechanical, may also be contributing to the decrease in leg blood flow during sitting. Along these lines, we recently reasoned that flexion of the hips and knees with sitting, and associated “arterial bending,” may hinder limb blood flow as a result of increased resistance (47). Accordingly, we performed measurements of popliteal artery blood flow with the subjects lying down on their side and the body positioned straight (0° angles) versus lying down on their side and hips and knees bent to 90° (i.e., mimicking the sitting position). In agreement with our line of thought, we found that reproducing the sitting position (hip and knee joints bent to 90°) markedly reduced popliteal artery blood flow and shear rate by 45% compared with lying down with the body positioned straight (Fig. 6) (47). This recent finding suggests that reduced leg blood flow during sitting is partially attributed to arterial angulations. In support of this idea, previous work has demonstrated that femoral artery blood flow can be greatly altered in response to passive knee extension and flexion (39). Specifically, leg blood flow is reduced during knee flexion and increased during knee extension (39).
Fig. 6.
“Arterial bending” reduces popliteal artery shear rate. Popliteal artery shear rate measurements were performed while the subject was lying down in two different positions, each adopted for 5 min. Popliteal artery blood flow was also reduced by ~45% (data not shown). n = 8 *P < 0.05. [Modified from Morishima et al. (47).]
Strategies to Circumvent Sitting-Induced Leg Vascular Dysfunction
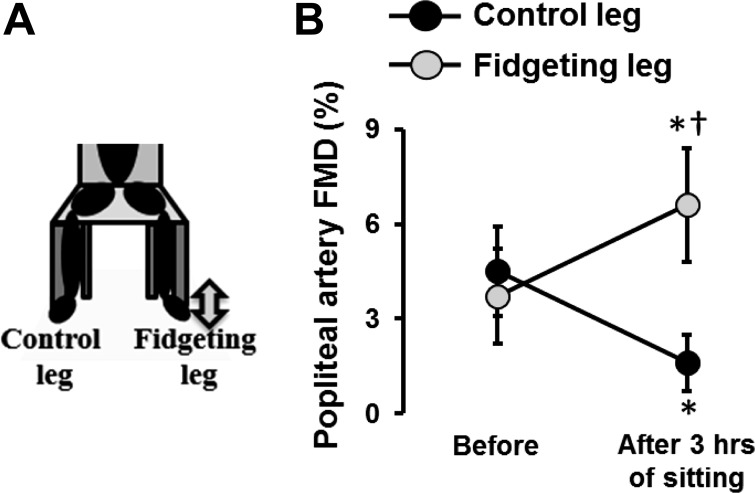
On the basis of the abovementioned discussion regarding the role of reduced shear stress in mediating leg vascular dysfunction with sitting, it follows that strategies that are effective at increasing or preserving leg blood flow during sitting are also vascular protective against sitting. In this context, we tested the premise that active sitting (or “fidgeting”) and concomitant increases in leg blood flow and conduit artery shear stress would prevent leg endothelial dysfunction caused by sitting (46). As such, we performed bilateral assessments of popliteal artery FMD before and after a 3-h sitting period during which one leg was subjected to intermittent fidgeting (1 min on/4 min off) while the contralateral leg continued still throughout and functioned as an internal control. We found that popliteal artery FMD was blunted after 3 h of sitting in the control leg but improved in the fidgeting leg (46) (Fig. 7), providing evidence that extended sitting-induced leg endothelial dysfunction is preventable with small and consistent amounts of leg movement while sitting, probably through the repeated intermittent increases in vascular shear stress. Furthermore, evidence is also available indicating that leg endothelial dysfunction can be prevented when excessive sitting is broken up by short (i.e., 5 min) bouts of walking every hour (69).
Fig. 7.
Intermittent fidgeting and increases in lower limb blood flow prevent prolonged sitting-induced leg endothelial dysfunction. Popliteal artery flow-mediated dilation (FMD) measures were performed in the supine position in both legs before and after sitting for 3 h during which time one leg was subjected to intermittent fidgeting (1 min on/4 min off). n = 11. *P < 0.05 vs. before; †P < 0.05 between legs. [Modified from Morishima et al. (46).]
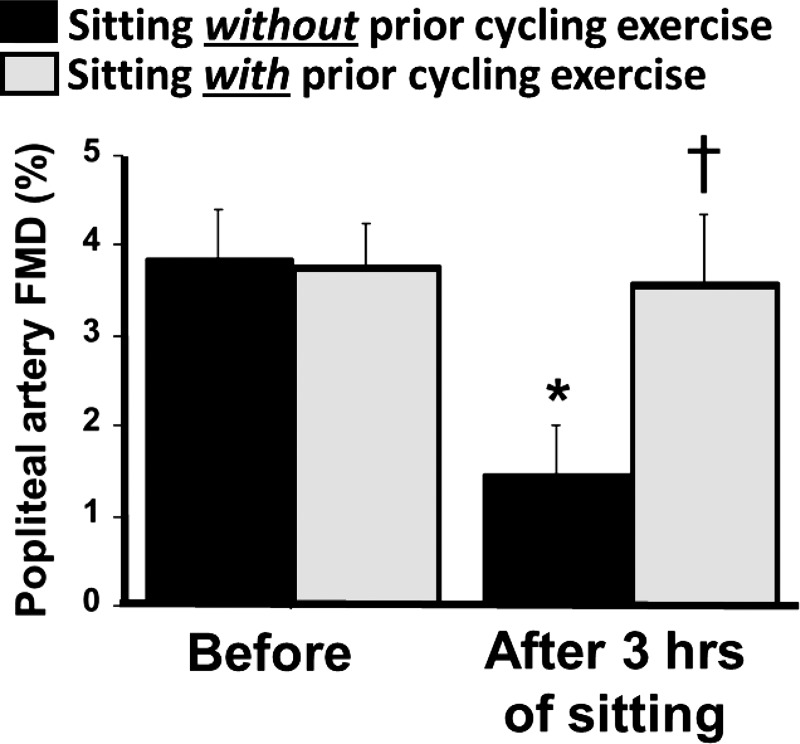
Most recently, we also reported that leg endothelial dysfunction caused by 3 h of sitting was prevented when sitting was preceded by a 45-min bout of cycling exercise (Fig. 8) (47). This finding suggests that prior exercise affords a window of vascular protection against subsequent sitting; however, the mechanisms by which this occurs and the duration of this protection remain to be determined. In addition, we demonstrated that standing for 3 h does not affect leg endothelial dysfunction (47), suggesting that in the absence of exercise, standing represents an effective substitute to sitting for preserving leg conduit artery endothelial function. The nondetrimental vascular effects of standing, relative to sitting, are likely attributable to the fact that the reduction in leg blood flow during standing is significantly less to that during sitting (47). The greater preservation of leg blood flow during standing may be explained by the fact that sustaining the standing position requires greater skeletal muscle activity compared with sitting. In addition, it is possible that elimination of the arterial angulations with standing may contribute to the greater popliteal artery blood flow and shear rate compared with sitting. Along these lines, it is reasonable to propose that sitting with legs unbent (i.e., elevated) would not cause the same magnitude of leg blood flow reduction as that occurring with regular sitting. Hence, one could hypothesize that “sitting with the legs up” would be a more vascular-favorable form of sitting; however, this interesting hypothesis remains to be tested.
Fig. 8.
Prior exercise prevents prolonged sitting-induced leg endothelial dysfunction. Popliteal artery flow-mediated dilation (FMD) measures were performed in the supine position before and after 3 h of sitting on two different visits: with vs. without prior exercise. The exercise bout consisted of 45 min of cycling exercise at a moderate intensity (average workload = 86 ± 5 W, average relative exercise intensity = 71 ± 3% age-predicted maximal heart rate). n = 15. *P < 0.05 vs. before; †P < 0.05 between conditions. [Modified from Morishima et al. (47).]
Perspectives
In the United States, PAD affects ~8 to 12 million men and women, and it has been projected that by the year 2050 this devastating disease will affect ~19 million Americans over the age of 65 yr. The hallmark symptom of PAD is intermittent claudication, which manifests as ischemic limb pain on exertion, and contributes to the patient’s limited mobility and diminished quality of life. In addition, patients with PAD have a significantly higher risk of mortality from cardiovascular disease (40). Atherosclerotic PAD is a major complication of type 2 diabetes and is also worsened with aging and by smoking (4, 21, 43, 58). Surprisingly, however, the reason PAD primarily manifests in the vasculature of the lower extremities, while upper limbs are atheroresistant, remains largely unknown. In this review, we summarized evidence supporting the contention that the ubiquitous adherence to the sitting position produces a pathologically primed endothelial cell phenotype in the conduit arteries feeding the lower limbs. Indeed, we assert that overabundance of sitting and the consequent reduced exposure to shear stress is likely a key culprit that puts the femoral and popliteal arteries at increased risk for atherogenesis, which reveals when added risk factors are overlaid (Fig. 1). In support of this, epidemiological data indicate that sedentary time is associated with increased indices of PAD (32) as well as increased cardiovascular disease and mortality, independent of other risk factors (6, 15, 29, 51, 68, 72, 77). We predict that too much sitting will soon be considered an insidious behavioral health hazard in the same way as we now recognize other environmental risk factors including second-hand smoking. Accordingly, we advocate that cultural, policy, and environmental changes that limit sitting time are needed to foster leg vascular health. When prolonged and repeated periods of sitting are unescapable, strategies that prevent the marked reduction in leg blood flow and conduit artery shear stress during sitting should be implemented to evade sitting vasculopathy.
GRANTS
J. Padilla was supported by National Institutes of Health Grants K01 HL-125503 and R21 DK-105368; P. Fadel was supported by National Institutes of Health Grant R01 HL-127071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.P. and P.J.F. conceived and designed research; J.P. and P.J.F. interpreted results of experiments; J.P. prepared figures; J.P. drafted manuscript; J.P. and P.J.F. edited and revised manuscript; J.P. and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Xavier Padilla for the drawings in Figs. 1 and 5.
REFERENCES
- 1.Aboyans V, McClelland RL, Allison MA, McDermott MM, Blumenthal RS, Macura K, Criqui MH; The Multi-Ethnic Study of Atherosclerosis . Lower extremity peripheral artery disease in the absence of traditional risk factors. Atherosclerosis 214: 169–173, 2011. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Fail Rev 8: 107–115, 2003. doi: 10.1023/A:1022155206928. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 26: 3333–3341, 2003. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 5.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation 110: 2233–2240, 2004. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 6.Borodulin K, Kärki A, Laatikainen T, Peltonen M, Luoto R. Daily sedentary time and risk of cardiovascular disease: The National FINRISK 2002 Study. J Phys Act Health 12: 904–908, 2015. doi: 10.1123/jpah.2013-0364. [DOI] [PubMed] [Google Scholar]
- 7.Bossard M, Pumpol K, van der Lely S, Aeschbacher S, Schoen T, Krisai P, Lam T, Todd J, Estis J, Risch M, Risch L, Conen D. Plasma endothelin-1 and cardiovascular risk among young and healthy adults. Atherosclerosis 239: 186–191, 2015. doi: 10.1016/j.atherosclerosis.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 8.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol 115: 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan MT, Duckles H, Feng S, Hsiao ST, Kim HR, Serbanovic-Canic J, Evans PC. Mechanoresponsive networks controlling vascular inflammation. Arterioscler Thromb Vasc Biol 34: 2199–2205, 2014. doi: 10.1161/ATVBAHA.114.303424. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002. doi: 10.1161/01.CIR.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 11.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379–2393, 2007. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, de Crom R, van Haperen R, Helderman F, Mousavi Gourabi B, van Damme LC, Kirschbaum SW, Slager CJ, van der Steen AF, Krams R. The role of shear stress in atherosclerosis: action through gene expression and inflammation? Cell Biochem Biophys 41: 279–294, 2004. doi: 10.1385/CBB:41:2:279. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113: 2744–2753, 2006. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 14.Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf) 219: 382–408, 2017. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 15.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol 61: 2346–2354, 2013. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol 145: 323–333, 2005. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun 269: 713–717, 2000. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- 20.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 20: 384–392, 1991. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 21.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382: 1329–1340, 2013. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 21a.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1545–1602, 2016. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 58: 2238–2245, 2009. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 25.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 31: 369–371, 2008. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 26.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol 18: 527–533, 2007. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: implications for cardiovascular disease. Curr Cardiovasc Risk Rep 6: 331–346, 2012. doi: 10.1007/s12170-012-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 61: 615–621, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998–1005, 2009. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 30.Kitano A, Shoemaker JK, Ichinose M, Wada H, Nishiyasu T. Comparison of cardiovascular responses between lower body negative pressure and head-up tilt. J Appl Physiol 98: 2081–2086, 2005. doi: 10.1152/japplphysiol.00563.2004. [DOI] [PubMed] [Google Scholar]
- 31.Kröger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 32.Kulinski JP, Sanghavi M, Ayers CR, Das SR, Banerjee S, Berry JD, Addo T, De Lemos JA, Kumbhani DJ. Association between low ankle-brachial index and accelerometer-derived sedentary and exercise time in the asymptomatic general population. Vasc Med 20: 332–338, 2015. doi: 10.1177/1358863X15573837. [DOI] [PubMed] [Google Scholar]
- 33.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 35.Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 33: 2306–2315, 2013. doi: 10.1161/ATVBAHA.113.302028. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoud AM, Szczurek MR, Blackburn BK, Mey JT, Chen Z, Robinson AT, Bian JT, Unterman TG, Minshall RD, Brown MD, Kirwan JP, Phillips SA, Haus JM. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol Rep 4: e12895, 2016. doi: 10.14814/phy2.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 167: 875–881, 2008. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005. doi: 10.1152/japplphysiol.01263.2003. [DOI] [PubMed] [Google Scholar]
- 39.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol 112: 560–565, 2012. doi: 10.1152/japplphysiol.01223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med 73, Suppl 4: S2–S7, 2006. doi: 10.3949/ccjm.73.Suppl_4.S2. [DOI] [PubMed] [Google Scholar]
- 41.McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation 84: 1273–1278, 1991. doi: 10.1161/01.CIR.84.3.1273. [DOI] [PubMed] [Google Scholar]
- 42.McManus AM, Ainslie PN, Green DJ, Simair RG, Smith K, Lewis N. Impact of prolonged sitting on vascular function in young girls. Exp Physiol 100: 1379–1387, 2015. doi: 10.1113/EP085355. [DOI] [PubMed] [Google Scholar]
- 43.Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B, Harrap S, Marre M, Chalmers J; ADVANCE Collaborative Group . Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care 39: 1796–1803, 2016. doi: 10.2337/dc16-0588. [DOI] [PubMed] [Google Scholar]
- 44.Moore WS. Vascular Surgery: a Comprehensive Review. Philadelphia: W. B. Saunders Company, 2002. [Google Scholar]
- 45.Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525: 761–770, 2000. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Padilla J. Prior exercise and standing as strategies to circumvent sitting-induced leg endothelial dysfunction. Clin Sci (Lond) 131: 1045–1053, 2017. doi: 10.1042/CS20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padilla J, Jenkins NT, Laughlin MH, Fadel PJ. Blood pressure regulation VIII: resistance vessel tone and implications for a pro-atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol 114: 531–544, 2014. doi: 10.1007/s00421-013-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilla J, Jenkins NT, Roberts MD, Arce-Esquivel AA, Martin JS, Laughlin MH, Booth FW. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp Physiol 98: 337–347, 2013. doi: 10.1113/expphysiol.2012.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padilla J, Jenkins NT, Thorne PK, Lansford KA, Fleming NJ, Bayless DS, Sheldon RD, Rector RS, Laughlin MH. Differential regulation of adipose tissue and vascular inflammatory gene expression by chronic systemic inhibition of NOS in lean and obese rats. Physiol Rep 2: e00225, 2014. doi: 10.1002/phy2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, Colditz GA, Thun MJ. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol 172: 419–429, 2010. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 91: 507–516, 2012. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 53.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol 264: H1–H7, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 55.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee SY, Kim YS. Peripheral arterial disease in patients with type 2 diabetes mellitus. Diabetes Metab J 39: 283–290, 2015. doi: 10.4093/dmj.2015.39.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ridger V, Krams R, Carpi A, Evans PC. Hemodynamic parameters regulating vascular inflammation and atherosclerosis: a brief update. Biomed Pharmacother 62: 536–540, 2008. doi: 10.1016/j.biopha.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 60.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 61.Siasos G, Tousoulis D, Siasou Z, Stefanadis C, Papavassiliou AG. Shear stress, protein kinases and atherosclerosis. Curr Med Chem 14: 1567–1572, 2007. doi: 10.2174/092986707780831087. [DOI] [PubMed] [Google Scholar]
- 62.Spieker LE, Lüscher TF, Noll G. ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol 42: 315–318, 2003. doi: 10.1097/00005344-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374, 1995. doi: 10.1161/01.CIR.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 64.Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med 37: 1558–1566, 2016. doi: 10.3892/ijmm.2016.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res 88: 877–887, 2001. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- 66.Teixeira AL, Padilla J, Vianna LC. Impaired popliteal artery flow-mediated dilation caused by reduced daily physical activity is prevented by increased shear stress. J Appl Physiol 123: 49–54, 2017. doi: 10.1152/japplphysiol.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thijssen DHJ, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MTE, Smits P. A causal role for endothelin-1 in the vascular adaptation to skeletal muscle deconditioning in spinal cord injury. Arterioscler Thromb Vasc Biol 27: 325–331, 2007. doi: 10.1161/01.ATV.0000253502.83167.31. [DOI] [PubMed] [Google Scholar]
- 68.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med 41: 207–215, 2011. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 70.Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound 12: 50, 2014. doi: 10.1186/1476-7120-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 72.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med 172: 494–500, 2012. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 73.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol (Oxf) 219: 22–96, 2017. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 74.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 75.Wallace JP, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: a review. Int J Clin Pract 64: 389–403, 2010. doi: 10.1111/j.1742-1241.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 76.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 77.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 55: 2895–2905, 2012. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 78.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol 98: 940–946, 2005. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- 79.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation 60: 473–485, 1979. doi: 10.1161/01.CIR.60.3.473. [DOI] [PubMed] [Google Scholar]