H2S is a gasotransmitter capable of producing a decrease in mean arterial pressure and heart rate. The hypotensive and bradycardic effects of H2S can be dissociated, as shown with cardiac pacing experiments. Responses were not blocked by diltiazem, ivabradine, methylene blue, or glybenclamide.
Keywords: H2S donors, ATP-sensitive potassium channels, hypotension, bradycardia, L-type calcium channels
Abstract
The actions of hydrogen sulfide (H2S) on the heart and vasculature have been extensively reported. However, the mechanisms underlying the effects of H2S are unclear in the anesthetized rat. The objective of the present study was to investigate the effect of H2S on the electrocardiogram and examine the relationship between H2S-induced changes in heart rate (HR), mean arterial pressure (MAP), and respiratory function. Intravenous administration of the H2S donor Na2S in the anesthetized Sprague-Dawley rat decreased MAP and HR and produced changes in respiratory function. The administration of Na2S significantly increased the RR interval at some doses but had no effect on PR or corrected QT(n)-B intervals. In experiments where respiration was maintained with a mechanical ventilator, we observed that Na2S-induced decreases in MAP and HR were independent of respiration. In experiments where respiration was maintained by mechanical ventilation and HR was maintained by cardiac pacing, Na2S-induced changes in MAP were not significantly altered, whereas changes in HR were abolished. Coadministration of glybenclamide significantly increased MAP and HR responses at some doses, but methylene blue, diltiazem, and ivabradine had no significant effect compared with control. The decreases in MAP and HR in response to Na2S could be dissociated and were independent of changes in respiratory function, ATP-sensitive K+ channels, methylene blue-sensitive mechanism involving L-type voltage-sensitive Ca2+ channels, or hyperpolarization-activated cyclic nucleotide-gated channels. Cardiovascular responses observed in spontaneously hypertensive rats were more robust than those in Sprague-Dawley rats.
NEW & NOTEWORTHY H2S is a gasotransmitter capable of producing a decrease in mean arterial pressure and heart rate. The hypotensive and bradycardic effects of H2S can be dissociated, as shown with cardiac pacing experiments. Responses were not blocked by diltiazem, ivabradine, methylene blue, or glybenclamide.
hydrogen sulfide (H2S), nitric oxide (NO), and carbon monoxide (CO) are gaseous agents that have diverse concentration-dependent actions on the cardiovascular system (14, 22, 23, 42). Exposure to all three gaseous agents in high concentration can produce death, whereas administration of these agents in lower doses causes vasodilation and has a beneficial effect in a number of disease models (20, 34, 36). In low concentrations, H2S can act as an endothelium-derived vasodilator factor that can help maintain systemic arterial pressure at physiological levels (11, 43). In contrast to a beneficial physiological role in blood pressure regulation at low concentration, a high concentration of H2S may act as a chemical poison, causing hypotension, cardiac failure, respiratory failure, and death (18, 19, 27, 55). Acute, potentially fatal H2S exposures in humans occur intentionally as a method of suicide and unintentionally in the petroleum, pulp and paper, agriculture, aquaculture, and other industries (28, 65).
Sulfur pools in vivo are typically broken down into free H2S (which exists in equilibrium with HS− and S2−), acid-labile sulfur, and sulfane sulfur, the chemistry of which has been previously reviewed (50, 62). Endogenous enzymatic production of H2S is mediated by cystathionine γ-lyase (CSE), cystathionine β-synthase, and 3-mercaptopyruvate sulfurtransferase (48, 51, 57). H2S production in the vascular system is largely mediated by CSE, and CSE knockout mice develop hypertension similar to that observed in endothelial NO synthase knockout mice (66, 70). Modulation of endogenous CSE levels affects disease progression in myocardial ischemia-reperfusion and pressure overload models, whereas CSE overexpression demonstrated protective effects and CSE deletion demonstrated deleterious effects (6, 12, 29, 35). In rodent models of myocardial ischemia, the CSE-selective inhibitor propargylglycine worsened outcomes, whereas treatment with the H2S donor sodium hydrosulfide (NaHS) improved outcomes (72). H2S donors have demonstrated therapeutic benefit in a number of cardiovascular disease models (5, 7, 17, 26, 46).
H2S has been shown to be endogenously produced by the heart and NaHS in ex vivo Langendorff preparations reduced heart rate (HR), coronary perfusive flow, and left ventricular pressure (17, 47). Administration of NaHS reduced action potential duration and decelerated sinus rhythm in the isolated rat atria and inhibited the second half of slow diastolic depolarization in the murine sinus node (1, 2). In isolated rat ventricular cardiomyocytes, Na2S has been shown to block L-type Ca2+ channels, and in human pluripotent stem cell-derived cardiomyocytes, NaHS has been shown to inhibit slow and rapid delayed rectifier K+ channels and hyperpolarization-activated inward current (If) (59, 63). Inhalation of H2S in mice decreased respiratory rate, induced sinus bradycardia, and decreased cardiac output while maintaining stroke volume and mean arterial pressure (MAP) for several hours (61).
In addition to inducing a nonvagally mediated decrease in HR, H2S can induce changes in respiration that include stimulatory effects at low doses and apnea at higher doses (18, 19, 24, 31). It has been demonstrated that an afferent signal sent from the lungs through the vagus is primarily responsible for modulating the apneic response to H2S in the rat (3). Inhalation of NaHS has been shown to sensitize rat capsaicin-sensitive lung vagal neurons to chemical and mechanical stimuli by increasing Ca2+ transients (21).
It has been reported that ATP-sensitive K+ (KATP) channel activation is a major mechanism by which H2S and H2S donors relax vascular smooth muscle and that other pathways may be involved (9, 13, 26, 68–70). In addition to relaxing vascular smooth muscle and decreasing systemic arterial pressure, it has been reported that H2S donors can decrease HR and that a methylene blue-sensitive effect on L-type Ca2+ channels is a major target for H2S toxicity (19, 27). Typically, a decrease in systemic arterial pressure will normally induce a baroreceptor-mediated increase in HR. H2S donor-induced hypotension has been shown to be accompanied by a decrease in HR that was insensitive to parasympathetic blockade by atropine (67).
The present study was undertaken to investigate the effects of the H2S donor Na2S on the cardiac electrophysiology of the rat and to determine the relationship between changes in MAP and HR in a cardiac pacing model. In addition, the role of changes in respiratory function in mediating the cardiovascular response to the H2S donor were investigated in animals with normal spontaneous respiration and in mechanically ventilated animals. The roles of KATP channels, a methylene blue-sensitive effect on L-type Ca2+ channels, the direct effect of L-type Ca2+, and the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel’s effect on If in mediating cardiovascular responses to the H2S donor were investigated. Chronic treatment with NaHS for 5 wk has been shown to significantly reduce MAP in spontaneously hypertensive rats (SHRs), and to a greater degree than in Wistar-Kyoto rats (71). In the last set of experiments, the effect of acute administration of Na2S in the SHR was investigated. The results of the present study provide electrocardiographic evidence that the major effect of Na2S is on the sinus node and that decreases in MAP and HR can be dissociated. The present data indicate that changes in respiratory function do not have a major effect in mediating cardiovascular responses to the H2S donor at the doses used in the present study. Cardiovascular responses to Na2S are not mediated by KATP channel activation, a methylene blue-sensitive effect on L-type voltage-sensitive channels, or the HCN channel. Acute responses to Na2S appear to be more robust in the SHR than the Sprague-Dawley (S-D) rat.
MATERIALS AND METHODS
All experiments were approved by the Institutional Animal Care and Use Committee of the Tulane University School of Medicine, and all procedures were conducted in accordance with institutional guidelines. Animals were maintained on a 12:12-h light-dark cycle and were provided food and water ad libitum. In these experiments, 64 male S-D rats (275–493 g) and 8 SHRs (291–349 g) were anesthetized with an intraperitoneal injection of thiobutabarbital (Inactin, Sigma-Aldrich, St. Louis, MO) in a dose of 100 mg/kg. Supplemental doses of Inactin were administered intraperitoneally to maintain a uniform level of anesthesia. Animals were placed in the supine position, and body temperature was maintained with the use of Deltaphase Isothermal Pads (Braintree Scientific, Braintree, MA). The trachea was cannulated with a short segment of polyethylene (PE)-240 tubing to maintain a patent airway. In experiments measuring respiratory frequency and airflow amplitude, the tracheal tube was connected to a pneumotachometer (RX237B, Biopac, Santa-Barbara, CA), and the animals spontaneously breathed room air. In experiments where the animal was artificially ventilated, the tracheal tube was connected to a ventilator (model 683 Small Animal Ventilator, Harvard Apparatus, Holliston, MA) and ventilated at a tidal volume of 10 ml/kg at a rate of 70 breaths/min with 100% O2; decamethonium (Sigma-Aldrich) was administered at a dose of 0.8 mg/kg iv with supplemental doses of 0.4 mg/kg administered as needed to induce neuromuscular blockade. In all other experiments, the animals spontaneously breathed room air enriched with 100% O2.
A lead II electrocardiogram was measured with 12-mm needle electrodes (EL452, Biopac) placed subcutaneously with the negative electrode placed near the right shoulder, the positive electrode placed to the left of the xyphoid space, and the ground electrode placed near the left shoulder. The left jugular vein was catheterized with PE-50 tubing for the systemic injection of drugs and fluids. The left carotid artery was cannulated with PE-50 tubing, and systemic arterial pressure was measured with a pressure transducer (Namic Perceptor DT, Navilyst Medical, Malborough, MA). For experiments with cardiac pacing, the right jugular was cannulated and a bipolar pacing catheter (REF 401769, St. Jude Medical, St. Paul, MN) was advanced to the right atrium, and the position was confirmed with fluoroscopy (OEC 6600, GE, Boston, MA). The heart was paced with a Grass stimulator (model S88, Grass Medical Instruments, Quincy, MA) and isolation unit at a voltage which was 10–15% above threshold with 5-ms pulses at rates of 347−488 beats/min. Systemic arterial pressure and MAP were obtained by electronic averaging of the pressure signal, and the HR signal was measured from the pressure signal. Hemodynamic, electrocardiographic, and respiratory data were continuously recorded and displayed with a data-acquisition system (MP 100A-CE, Biopac) and stored on a personal computer. Bazett’s formula was used for Q-T correction as follows: QTc(n)-B = QT/(RR/f)(1/2), where QTc is the corrected QT interval and f = 150 ms (32). At the conclusion of the experiments, anesthetized animals were euthanized with an intravenous injection of saturated KCl solution, and a bilateral thoracotomy was performed to ensure euthanasia.
Drugs.
Na2S, diltiazem, decamethonium, and methylene blue (Sigma-Aldrich) were dissolved in 0.9% saline. Glybenclamide (Sigma-Aldrich) was dissolved in 4% glucose and 0.1 N NaOH solution. Ivabradine (Corlanor, Amgen, Thousand Oaks, CA) was suspended in sterile PBS and administered by oral gavage. The dosages used in this study were determined from dosages used in previous studies and pilot experiments in our laboratory (10, 27, 39, 41, 53, 58, 67). New measurement techniques for sulfide pools have been developed, and considerable debate in the literature has focused on ascertaining the true physiological levels of H2S (15, 34, 44, 64). The rapid clearance of free H2S from the blood has led to concerns regarding concentrations used in some recent studies (18, 31, 54, 64). Administration of sulfide donors in dosages less than or equal to those in the present study have shown therapeutic benefit in a number of in vivo experimental models (4, 25, 33, 38, 45, 52).
Statistics.
Data are expressed as means ± SE and were analyzed using Student’s t-test for paired and unpaired data; n is the number of animals. A P value of <0.05 was used as the criterion for statistical significance.
RESULTS
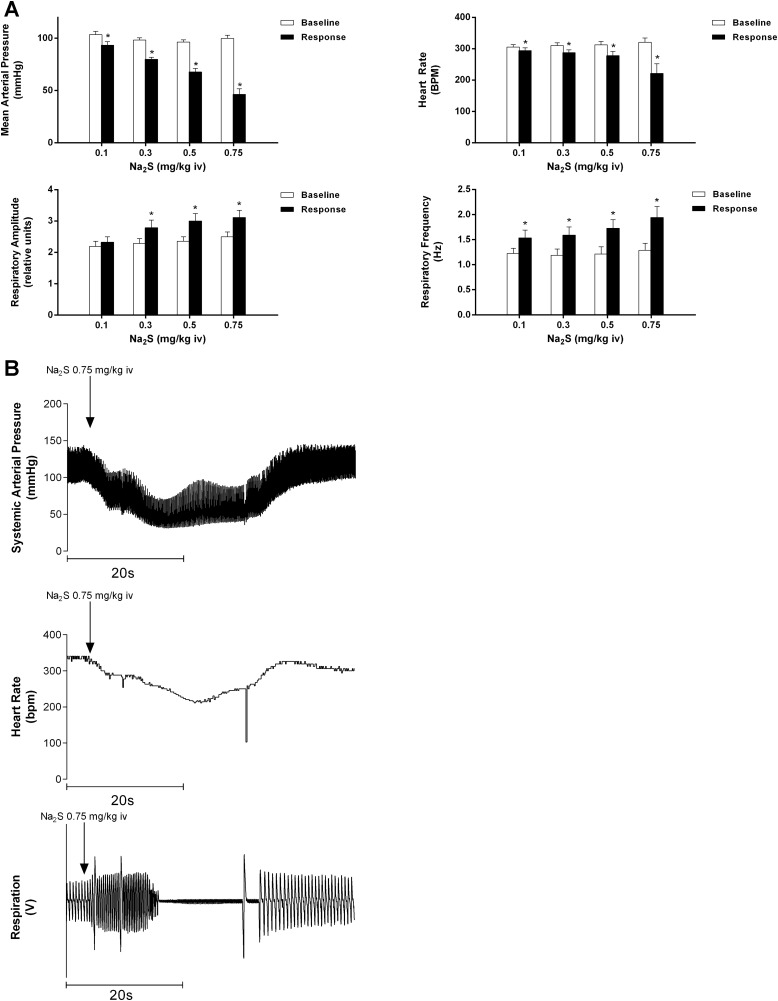
The effect of Na2S in doses of 0.1–0.75 mg/kg iv on MAP, HR, respiratory amplitude, and respiratory frequency are shown in Fig. 1A. Significant decreases in MAP and HR were observed at all doses of Na2S compared with baseline. Injection of Na2S in doses of 0.3–0.75 mg/kg iv significantly increased respiratory amplitude, whereas respiratory frequency was increased at all doses compared with baseline. The injections of Na2S in a dose of 0.75 mg/kg iv were notable in that after periods of increased respiratory amplitude and frequency, transient apneic periods occurred in six of the eight rats (mean duration: 30.73 ± 9.107 s). Records from an experiment illustrating the observed apnea are shown in Fig. 1B.
Fig. 1.
A: bar graphs showing the effect of intravenous injections of Na2S at doses of 0.1–0.75 mg/kg on mean arterial pressure, heart rate, respiratory frequency, and respiratory amplitude. *P < 0.05 compared with baseline, paired comparison; n = 8. B: records from an experiment showing the effect of intravenous injection of Na2S at 0.75 mg/kg iv on systemic arterial pressure, heart rate, and respiratory airflow. Transient apnea was observed in six of eight animals (mean duration: 30.73 ± 9.107 s) at the 0.75 mg/kg dose.
Effect of respiration.
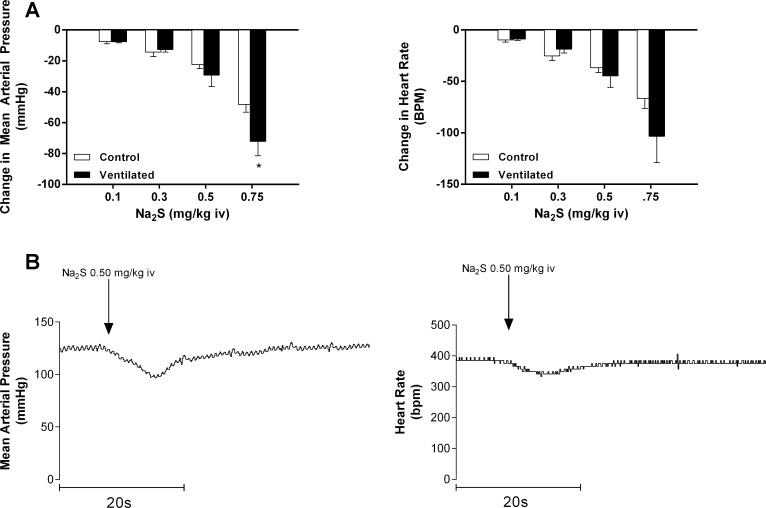
To investigate the relationships between changes in respiratory function and cardiovascular responses to the H2S donor, the decreases in MAP and HR were compared during normal spontaneous respiration and when the rats were ventilated at constant rate and volume with a positive pressure respirator (Fig. 2A). The decreases in HR in response to Na2S were not significantly different in doses of 0.1–0.75 mg/kg iv, but, interestingly, the decreases in MAP in the response to intravenous injections of Na2S were significantly increased at the 0.75 mg/kg dose and not significantly different at doses of 0.1–0.5 mg/kg. Records from an experiment illustrating reductions in MAP and HR are shown in Fig. 2B.
Fig. 2.
A: bar graphs comparing the effect of intravenous injections of Na2S at doses of 0.1–0.75 mg/kg on mean arterial pressure and heart rate during spontaneous breathing and mechanical ventilation. *P < 0.05, paired comparison; n = 6. B: records from an experiment showing the effect of intravenous injection of Na2S at 0.50 mg/kg on mean arterial pressure and heart rate.
Effect on the electrocardiogram.
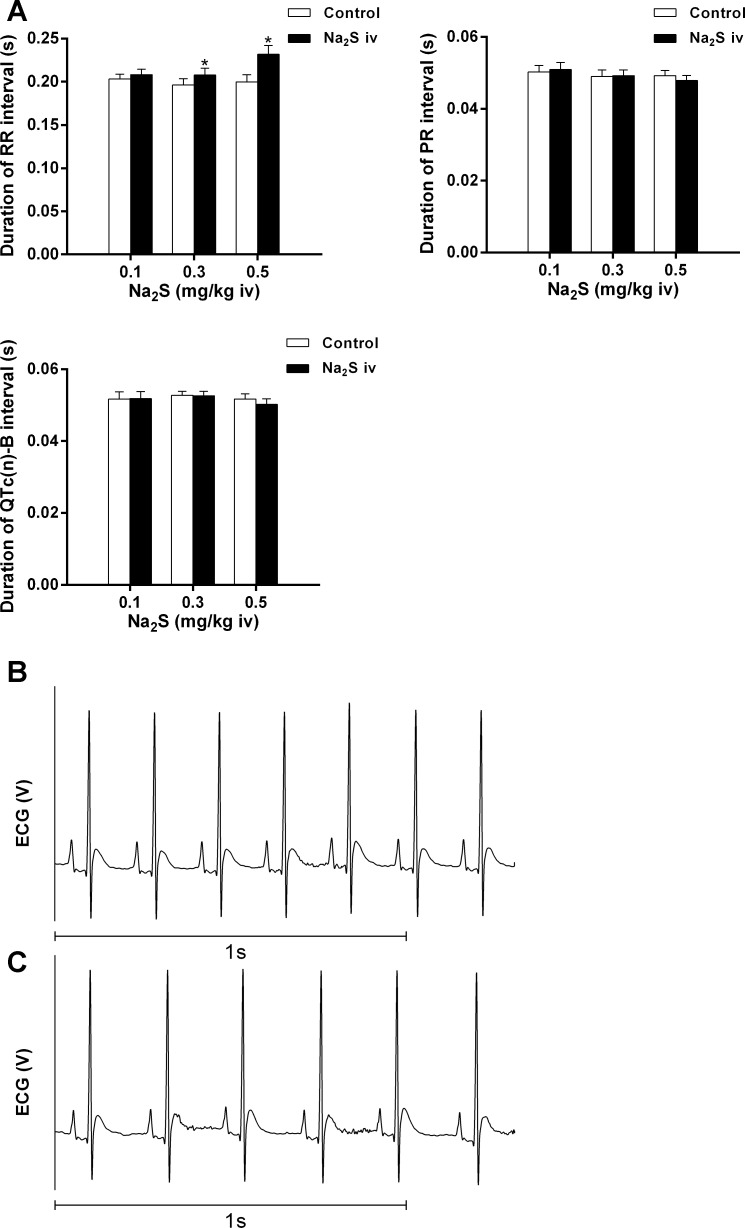
The effect of Na2S on the electrocardiogram in doses of 0.1–0.5 mg/kg iv was investigated and significantly increased RR intervals at the 0.3 and 0.5 mg/kg iv doses but had no significant effect on the PR interval or the QTc(n)-B interval (Fig. 3A). Records from an experiment showing the baseline electrocardiogram are shown in Fig. 3B; records during the Na2S-induced bradycardia are shown in Fig. 3C.
Fig. 3.
A: bar graphs showing the effect of intravenous injections of Na2S on RR, PR, and corrected QT(n)-B [QTc(n)-B] intervals on the electrocardiogram. *P < 0.05, paired comparison; n = 8. B and C: records from an experiment showing the baseline electrocardiogram (B) and the electrocardiogram during 0.5 mg/kg iv Na2S-induced bradycardia (C).
Effect of cardiac pacing.
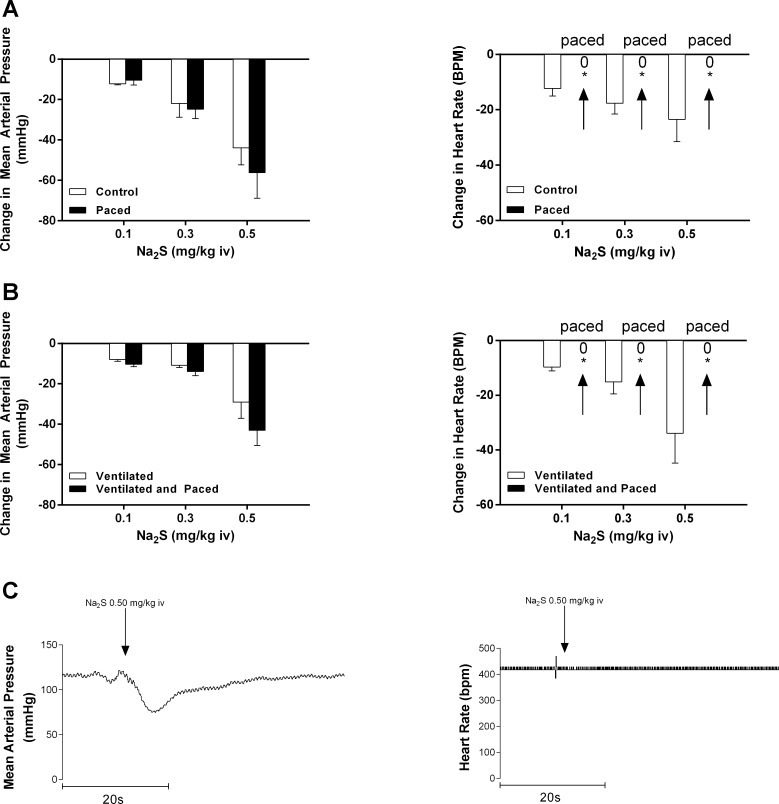
The relationship between the changes in MAP and HR were investigated in experiments in which the HR was paced in both spontaneously breathing (Fig. 4A) and mechanically ventilated rats (Fig. 4B). Intravenous Na2S administration in doses of 0.1–0.5 mg/kg in both paced spontaneously breathing and paced mechanically ventilated rats produced decreases in MAP that were not different from decreases in MAP in respective unpaced controls, whereas changes in HR were abolished with pacing.
Fig. 4.
Bar graphs showing the effect of cardiac pacing on the decrease in mean arterial pressure and heart rate in response to intravenous injections of Na2S at doses of 0.1–0.5 mg/kg in spontaneously breathing animals [*P < 0.05, paired comparison; n = 5 (A)] and mechanically ventilated animals [*P < 0.05, paired comparison; n = 5 (B)]. The decreases in mean arterial pressure in response to Na2S were not different than control responses in the unpaced heart at doses of 0.1–0.5 mg/kg iv. The decreases in mean arterial pressure at doses of 0.1–0.5 mg/kg iv were associated with no change in heart rate in response to the intravenous injections of Na2S. C: records from an experiment illustrating a decrease in mean arterial pressure in response to a 0.5 mg/kg iv injection of Na2S in a mechanically ventilated and paced animal.
Effect of glybenclamide.
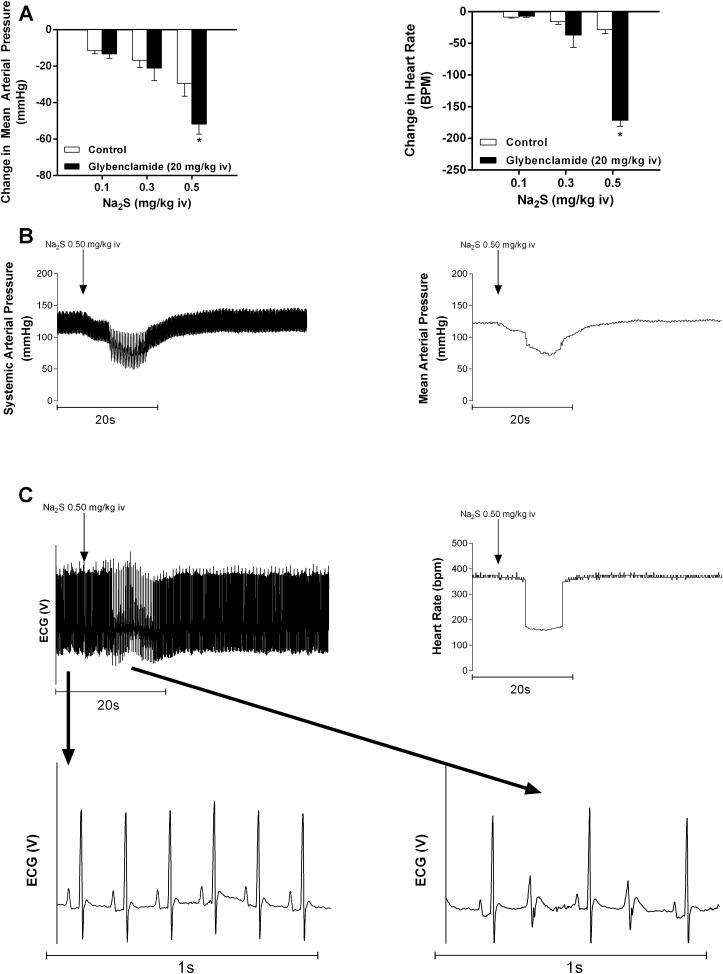
It has been previously reported that vasorelaxant responses to the H2S donor are blocked by glybenclamide, suggesting that KATP channel activation is a major mechanism by which H2S relaxes vascular smooth muscle (68). However, glybenclamide did not attenuate responses to the H2S donors in the intact anesthetized rat (67). In a previous study, glybenclamide was solubilized using organic solvents such as DMSO, which could potentially influence the response (16). In the present study, glybenclamide was solubilized using a glucose-sodium hydroxide vehicle, which had no significant effect on MAP or HR. Rats treated with glybenclamide at a high dose of 20 mg/kg iv demonstrated no significant difference in response to intravenous injections of Na2S at lower doses compared with control, but the 0.5 mg/kg dose significantly increased MAP and HR responses compared with control (Fig. 5A). Interestingly, this potentiated response after glybenclamide administration was associated with the development of a bigeminal rhythm in seven of seven of the rats (mean duration: 10.3 ± 1.37 s) treated with 0.5 mg/kg iv Na2S (Fig. 5, B and C).
Fig. 5.
A: bar graphs showing the effect of a high dose of glybenclamide on the decrease in mean arterial pressure and heart rate in response to intravenous injections of Na2S in doses of 0.1–0.5 mg/kg. *P < 0.05, paired comparison; n = 7. B: records from an experiment illustrating the changes in systemic arterial pressure, mean arterial pressure, heart rate, and electrocardiogram in response to an injection of 0.50 mg/kg iv Na2S in a glybenclamide-treated animal. C: records from an experiment showing high-resolution electrocardiographic traces showing the baseline sinus rhythm and the bigeminal rhythm when Na2S was injected at a dose of 0.5 mg/kg iv. A bigeminal rhythm was noted in seven of seven rats (mean duration: 10.3 ± 1.37 s) treated with a dose of 0.5 mg/kg.
Effect of methylene blue, diltiazem, and ivabradine.
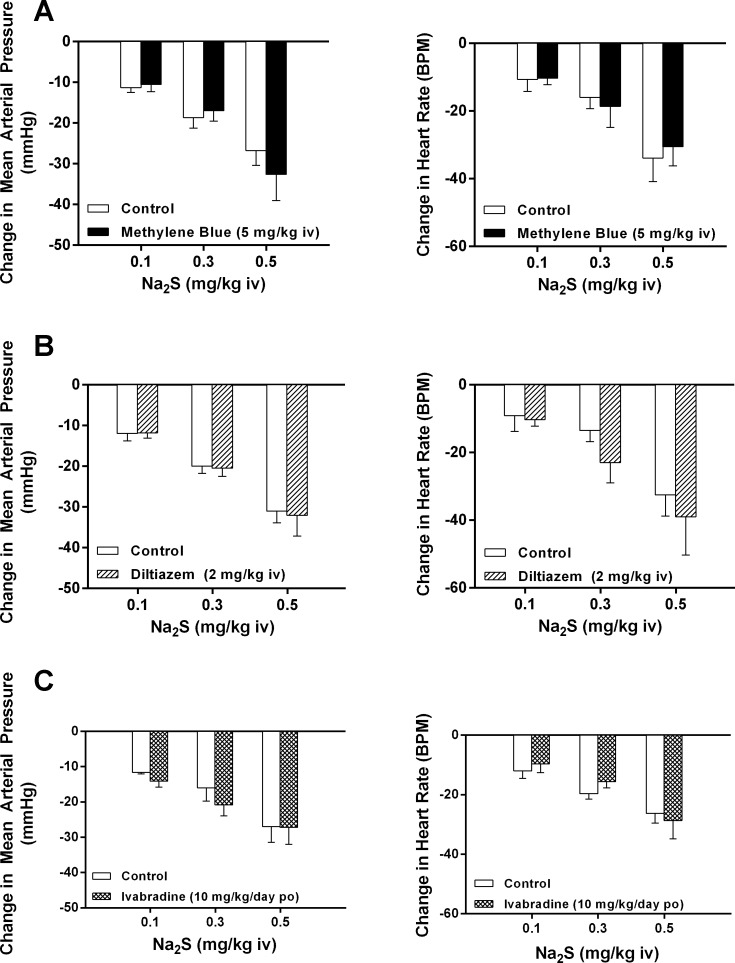
It has been previously reported that methylene blue can be used as a rescue treatment for the fatal cardiotoxic effects of NaHS infusion on the heart by an effect on voltage-dependent Ca2+ channels in anesthetized rats (27). The effect of methylene blue pretreatment on cardiovascular responses to Na2S was investigated, and these data are shown in Fig. 6A. Intravenous injection of methylene blue at a single dose of 5 mg/kg or multiple doses of 5 mg/kg did not change the decreases in MAP or HR in response to intravenous injections of Na2S at doses of 0.1–0.5 mg/kg. Intravenous injection of the L-type voltage-dependent Ca2+ channel antagonist diltiazem at a dose of 2 mg/kg did not alter the decreases in MAP or HR in response to intravenous injections of Na2S (Fig. 6B).
Fig. 6.
A: bar graphs showing the effect of methylene blue at a dose of 5 mg/kg iv on the decreases in mean arterial pressure and heart rate in response to intravenous injections of Na2S at a dose of 0.1–0.5 mg/kg (paired comparison; n = 7). B: effect of the L-type voltage-dependent Ca2+ entry blocking agent diltiazem on the decreases in mean arterial pressure and heart rate in response to intravenous injections of Na2S at doses of 0.1–0.5 mg/kg (paired comparison, n = 7). C: effect of the hyperpolarization-activated inward current (If) channel inhibitor ivabradine on the decrease in mean arterial pressure and heart rate in response of intravenous injections of Na2S (unpaired comparison; n = 5 control and n = 6 ivabradine).
It has been suggested in the literature that H2S donors may modulate If (63). Treatment with the HCN channel antagonist ivabradine, which modulates If at a dose of 10 mg/kg by gavage for 3 days, did not attenuate the decrease in MAP or HR in response to intravenous injections of Na2S in the anesthetized rat (Fig. 6C).
Effect of Na2S on SHRs.
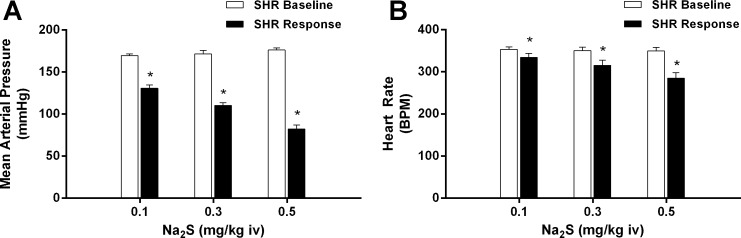
The effect of Na2S at doses of 0.1–0.5 mg/kg iv on MAP and HR in SHRs are shown in Fig. 7. Intravenous injections of the H2S donor produced dose-dependent significant decreases in MAP and HR in SHRs that were more robust than those observed in S-D rats.
Fig. 7.
Bar graphs showing the effect of intravenous injections of Na2S at doses of 0.1–0.5 mg/kg on mean arterial pressure (A) and heart rate (B) in spontaneously hypertensive rats (SHRs). *P < 0.05 compared with baseline, paired comparison; n = 8.
DISCUSSION
The present study found that the H2S donor Na2S decreases systemic arterial pressure and HR in anesthetized rats and is consistent with previous studies (20, 56, 66, 67). The major findings in the present study indicate that the effects on arterial pressure and HR can be separated by cardiac pacing. In experiments where atrial pacing was used, the Na2S-induced decrease in MAP was preserved, whereas the decrease in HR was abolished, which suggests that the bradycardic response of Na2S in the anesthetized rat may be primarily mediated by the sinoatrial node. It has been previously reported that the decrease in HR in response to Na2S is not blocked by atropine, suggesting that it is not mediated by an increase in vagal tone (67). In the present study, the decrease in HR was associated with no change in the PR or QTc(n)-B intervals on the electrocardiogram. The results of the electrocardiogram experiments are consistent with the results of experiments with atropine and are also consistent with the hypothesis that the major effects of the H2S donor Na2S on HR are mediated by an effect on the sinus node.
It has been previously reported that H2S can produce respiratory stimulation and, at higher doses, apnea (19). In the present study, intravenous injections of Na2S at most doses significantly increased respiratory amplitude and significantly increased respiratory rate at all dosages. The 0.75 mg/kg dose was noted to produce transient apnea in some experiments. The Na2S-induced bradycardia was not different between spontaneously breathing and mechanically ventilated animals, and the decreases in MAP were similar at all doses but the 0.75 mg/kg dose, in which the mechanically ventilated animals exhibited a greater decrease in MAP. These data suggest that the respiratory changes do not play a substantial role in mediating the hypotension and bradycardia observed in the present study.
It has been previously reported that H2S donors decrease total peripheral resistance in the anesthetized rat, and the mechanism of the vasodilator response to H2S has been investigated in many studies (9). Although a previous study indicated that the decrease in systemic arterial pressure and HR in response to H2S donors were not attenuated by the KATP channel antagonist glybenclamide, it has been reported that KATP channel activation is a major mechanism by which H2S relaxes vascular smooth muscle (9, 13, 26, 68–70). H2S donors may also have central effects, and microinjections of NaHS into the nucleus tractus solitarius produced hypotension and bradycadia that was attenuated with prior intranucleus tractus solitarius administration of glybenclamide and the nonselective glutamate antagonist kynurenic acid (49). In an attempt to clarify the role of KATP channel activation in mediating responses to H2S, a different method that does not require the use of organic solvents such as DMSO to prepare glybenclamide solutions was used in the present study. It has been previously reported that systemic administration of glybenclamide may fail to achieve therapeutic levels in the central nervous system, and concerns have been raised about neuronal toxicity in centrally administered H2S donors (37, 54). The potentiated responses observed at the 0.5 mg/kg dose may be a result of the occurrence of runs of a bigeminal rhythm in the anesthetized rat suggesting a potential interaction of Na2S and KATP channels, although the lack of blockade at the lower dosages in the absence of an arrhythmia suggests that peripheral KATP channel activation does not mediate the hypotensive or bradycardic response to the H2S donor in the anesthetized rat (8, 30). Genetic disruption of the KATP channel has been shown to increase susceptibility to catecholamine induced after depolarizations, and it is possible that adrenergic reflexes in response to Na2S-induced hypotension may have contributed to the observed dysrhythmia noted at the 0.5 mg/kg iv dose (40).
In the development of effective countermeasures against acute H2S intoxication, studies in the literature have indicated that methylene blue counteracts fatal H2S infusion-induced cardiac depression by restoring L-type Ca2+ channel activity (19, 27, 55). The decreases in arterial pressure and HR in response to intravenous injections of Na2S were not attenuated by methylene blue at a dose of 5 mg/kg iv, which was used in the previous study, or by repeated injections of methylene blue at this dose (27). These results suggest that the decreases in arterial pressure and HR in response to the H2S donor Na2S in the range of doses used in the present study were not mediated by a methylene blue-sensitive mechanism.
It has also been previously reported that changes in L-type Ca2+ channel activity may be involved in mediating H2S toxicity-induced cardiac depression (27). However, in the present study, the L-type Ca2+ channel antagonist diltiazem did not attenuate the decreases in systemic arterial pressure or HR in response to Na2S, suggesting that H2S-induced hypotension and bradycardia may not involve an effect on L-type Ca2+ channels.
Ivabradine is a blocker of the HCN channel that contributes to If in the sinus node and decreases HR in patients with heart failure (60). NaHS has been observed to modulate If in in vitro studies (63). Treatment with ivabradine at a dose of 10 mg/kg by gavage for 3 days did not alter responses to Na2S, suggesting that the decrease in HR in response to the H2S donor is not mediated by activation of the If channel in the sinus node of the rat.
In conclusion, the results of the present study show that decreases in systemic arterial pressure and HR in response to intravenous injections of the H2S donor Na2S can be dissociated using cardiac pacing to prevent bradycardia. The present data show that changes in respiration do not contribute to the decreases in arterial pressure and HR in that similar decreases were observed during normal spontaneous ventilation and in mechanically ventilated rats. The decreases in systemic arterial pressure and HR could not be blocked by different formulations and high doses of glybenclamide, suggesting that KATP channel activation is not a major mechanism mediating hypotensive or bradycardic responses to the H2S donor in the intact rat model. The cardiovascular responses to Na2S were not attenuated by methylene blue, ivabradine, or diltiazem, suggesting that a methylene blue-H2S chemical reaction or that an effect on the HCN channel in the sinus node or L-type Ca2+ channels are not involved in mediating responses to the H2S donor. The hemodynamic responses observed in SHRs were more robust than those in S-D rats. Although this is consistent with chronic studies between Wistar-Kyoto and SHRs, some of the observed difference in the present study may be due to differences between the Wistar-Kyoto and S-D background. Because of the relatively narrow therapeutic window of exogenously administered sulfide donors, future development of slow-release direct or indirect sulfide donors with suitable pharmacokinetics may hold promise in the treatment of many pathological processes.
GRANTS
This work was supported by American Heart Association National Center Scientist Development Grant 14SDG20490359 (to P.V.G.K) and National Institutes of Health Grants NS-094834 (to P.V.G.K) and HL-62000 (to P.J.K).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.W.S., B.M.S., and P.K. conceived and designed research; K.W.S. and B.M.S. performed experiments; K.W.S., B.M.S., A.L.C., T.J.C., R.A.C., B.T.G., and P.K. analyzed data; K.W.S., B.M.S., P.V.G.K., E.K.K., T.D.G., and P.K. interpreted results of experiments; K.W.S., B.M.S., A.L.C., T.J.C., and P.K. prepared figures; K.W.S., B.M.S., and P.K. drafted manuscript; K.W.S., B.M.S., A.L.C., T.J.C., R.A.C., B.T.G., P.V.G.K., E.K.K., T.D.G., and P.K. edited and revised manuscript; K.W.S., B.M.S., A.L.C., T.J.C., R.A.C., B.T.G., P.V.G.K., E.K.K., T.D.G., and P.K. approved final version of manuscript.
REFERENCES
- 1.Abramochkin DV. Modulation of sinoatrial node pacemaker activity by carbon monoxide and hydrogen sulfide. Dokl Biol Sci 453: 338–341, 2013. doi: 10.1134/S0012496613060094. [DOI] [PubMed] [Google Scholar]
- 2.Abramochkin DV, Moiseenko LS, Kuzmin VS. The effect of hydrogen sulfide on electrical activity of rat atrial myocardium. Bull Exp Biol Med 147: 683–686, 2009. doi: 10.1007/s10517-009-0607-y. [DOI] [PubMed] [Google Scholar]
- 3.Almeida AF, Guidotti TL. Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicol Sci 50: 287–293, 1999. doi: 10.1093/toxsci/50.2.287. [DOI] [PubMed] [Google Scholar]
- 4.Aslami H, Beurskens CJ, de Beer FM, Kuipers MT, Roelofs JJ, Hegeman MA, Van der Sluijs KF, Schultz MJ, Juffermans NP. A short course of infusion of a hydrogen sulfide-donor attenuates endotoxemia induced organ injury via stimulation of anti-inflammatory pathways, with no additional protection from prolonged infusion. Cytokine 61: 614–621, 2013. doi: 10.1016/j.cyto.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 6.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 122: 11–19, 2010. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-Aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol 53: 94–98, 2010. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 10.Downing SJ, Edwards D, Hollingsworth M. Diltiazem pharmacokinetics in the rat and relationship between its serum concentration and uterine and cardiovascular effects. Br J Pharmacol 91: 735–745, 1987. doi: 10.1111/j.1476-5381.1987.tb11271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards G, Feletou M, Weston AH. Hydrogen sulfide as an endothelium-derived hyperpolarizing factor in rodent mesenteric arteries. Circ Res 110: e13–e16, 2012. doi: 10.1161/CIRCRESAHA.111.259309. [DOI] [PubMed] [Google Scholar]
- 12.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42: 539–548, 2005. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels 28: 52–61, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 16.Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28: 1317–1330, 2014. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 17.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 18.Haouzi P. Is exogenous hydrogen sulfide a relevant tool to address physiological questions on hydrogen sulfide? Respir Physiol Neurobiol 229: 5–10, 2016. doi: 10.1016/j.resp.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haouzi P, Sonobe T, Judenherc-Haouzi A. Developing effective countermeasures against acute hydrogen sulfide intoxication: challenges and limitations. Ann N Y Acad Sci 1374: 29–40, 2016. doi: 10.1111/nyas.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CC, Lin RL, Lee LY, Lin YS. Hydrogen sulfide induces hypersensitivity of rat capsaicin-sensitive lung vagal neurons: role of TRPA1 receptors. Am J Physiol Regul Integr Comp Physiol 305: R769–R779, 2013. doi: 10.1152/ajpregu.00202.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 65: 1–21, 1989. doi: 10.1161/01.RES.65.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br J Pharmacol 157: 944–951, 2009. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa K, Kimmoun A, Collin S, Ganster F, Fremont-Orlowski S, Asfar P, Mertes PM, Levy B. Compared effects of inhibition and exogenous administration of hydrogen sulphide in ischaemia-reperfusion injury. Crit Care 17: R129, 2013. doi: 10.1186/cc12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury−evidence for a role of KATP channels. Basic Res Cardiol 101: 53–60, 2006. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 27.Judenherc-Haouzi A, Zhang XQ, Sonobe T, Song J, Rannals MD, Wang J, Tubbs N, Cheung JY, Haouzi P. Methylene blue counteracts H2S toxicity-induced cardiac depression by restoring L-type Ca channel activity. Am J Physiol Regul Integr Comp Physiol 310: R1030–R1044, 2016. doi: 10.1152/ajpregu.00527.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamijo Y, Takai M, Fujita Y, Hirose Y, Iwasaki Y, Ishihara S. A multicenter retrospective survey on a suicide trend using hydrogen sulfide in Japan. Clin Toxicol (Phila) 51: 425–428, 2013. doi: 10.3109/15563650.2013.799676. [DOI] [PubMed] [Google Scholar]
- 29.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 111: 3182–3187, 2014. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiss L, Deitch EA, Szabó C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008. doi: 10.1016/j.lfs.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol 305: R630–R638, 2013. doi: 10.1152/ajpregu.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol 641: 187–192, 2010. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Knapp J, Heinzmann A, Schneider A, Padosch SA, Böttiger BW, Teschendorf P, Popp E. Hypothermia and neuroprotection by sulfide after cardiac arrest and cardiopulmonary resuscitation. Resuscitation 82: 1076–1080, 2011. doi: 10.1016/j.resuscitation.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35: 5–20, 2013. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubo S, Doe I, Kurokawa Y, Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J Pharmacol Sci 104: 392–396, 2007. doi: 10.1254/jphs.SC0070199. [DOI] [PubMed] [Google Scholar]
- 37.Lahmann C, Kramer HB, Ashcroft FM. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One 10: e0134476, 2015. doi: 10.1371/journal.pone.0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langston JW, Toombs CF. Defining the minimally effective dose and schedule for parenteral hydrogen sulfide: long-term benefits in a rat model of hindlimb ischemia. Med Gas Res 5: 5, 2015. doi: 10.1186/s13618-015-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecuona E, Saldías F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema and downregulates alveolar epithelial cell Na,K-adenosine triphosphatase function. Chest 116, Suppl: 29S–30S, 1999. doi: 10.1378/chest.116.suppl_1.29S. [DOI] [PubMed] [Google Scholar]
- 40.Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O’Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes 53, Suppl 3: S165–S168, 2004. doi: 10.2337/diabetes.53.suppl_3.S165. [DOI] [PubMed] [Google Scholar]
- 41.Misawa M, Takata T. Effects of platelet-activating factor on rat airways. Jpn J Pharmacol 48: 7–13, 1988. doi: 10.1254/jjp.48.7. [DOI] [PubMed] [Google Scholar]
- 42.Murad F, Forstermann U, Nakane M, Schmidt H, Pollock J, Sheng H, Matsumoto T, Warner T, Mitchell J, Tracey R. The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol 58, Suppl 2: 150P–157P, 1992. [PubMed] [Google Scholar]
- 43.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Pan H, Chen D, Liu B, Xie X, Zhang J, Yang G. Effects of sodium hydrosulfide on intestinal mucosal injury in a rat model of cardiac arrest and cardiopulmonary resuscitation. Life Sci 93: 24–29, 2013. doi: 10.1016/j.lfs.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porokhya MV, Abramochkin DV, Abramov AA, Kuzmin VS, Sukhova GS. Inotropic effects of gaseous transmitters in isolated rat heart preparation. Bull Exp Biol Med 153: 855–857, 2012. doi: 10.1007/s10517-012-1843-0. [DOI] [PubMed] [Google Scholar]
- 48.Porter PN, Grishaver MS, Jones OW. Characterization of human cystathionine β-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine β-synthase. Biochim Biophys Acta 364: 128–139, 1974. doi: 10.1016/0005-2744(74)90140-5. [DOI] [PubMed] [Google Scholar]
- 49.Qiao W, Yang L, Li XY, Cao N, Wang WZ, Chai C, Lu Y. The cardiovascular inhibition functions of hydrogen sulfide within the nucleus tractus solitarii are mediated by the activation of KATP channels and glutamate receptors mechanisms. Pharmazie 66: 287–292, 2011. [PubMed] [Google Scholar]
- 50.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 52.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 26: 154–161, 2006. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 53.Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Paramonova N, Isajeva D, Rostoka E, Sjakste T, Kalvinsh I, Sjakste N. Comparison of the effects of glibenclamide on metabolic parameters, GLUT1 expression, and liver injury in rats with severe and mild streptozotocin-induced diabetes mellitus. Medicina (Kaunas) 48: 532–543, 2012. [PubMed] [Google Scholar]
- 54.Sonobe T, Haouzi P. H2S concentrations in the heart after acute H2S administration: methodological and physiological considerations. Am J Physiol Heart Circ Physiol 311: H1445–H1458, 2016. doi: 10.1152/ajpheart.00464.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila) 53: 525–539, 2015. doi: 10.3109/15563650.2015.1043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srilatha B, Adaikan PG, Li L, Moore PK. Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med 4: 1304–1311, 2007. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 57.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277, 1982. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suffredini S, Stillitano F, Comini L, Bouly M, Brogioni S, Ceconi C, Ferrari R, Mugelli A, Cerbai E. Long-term treatment with ivabradine in post-myocardial infarcted rats counteracts f-channel overexpression. Br J Pharmacol 165: 1457–1466, 2012. doi: 10.1111/j.1476-5381.2011.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 60.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376: 875–885, 2010. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 61.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, Zapol WM. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 108: 659–668, 2008. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 63.Wei H, Zhang G, Qiu S, Lu J, Sheng J, Manasi, Tan G, Wong P, Gan SU, Shim W. Hydrogen sulfide suppresses outward rectifier potassium currents in human pluripotent stem cell-derived cardiomyocytes. PLoS One 7: e50641, 2012. doi: 10.1371/journal.pone.0050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930–R1937, 2008. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 65.Woodall GM Jr, Smith RL, Granville GC. Proceedings of the Hydrogen Sulfide Health Research and Risk Assessment Symposium October 31-November 2, 2000. Inhal Toxicol 17: 593–639, 2005. doi: 10.1080/08958370591000618. [DOI] [PubMed] [Google Scholar]
- 66.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo D, Jupiter RC, Pankey EA, Reddy VG, Edward JA, Swan KW, Peak TC, Mostany R, Kadowitz PJ. Analysis of cardiovascular responses to the H2S donors Na2S and NaHS in the rat. Am J Physiol Heart Circ Physiol 309: H605–H614, 2015. doi: 10.1152/ajpheart.00171.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening KATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 69.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–H480, 2002. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 70.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res 31: 1619–1630, 2008. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 72.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985) 102: 261–268, 2007. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]