In a three-dimensional microtissue model, which lowers baseline activation of cardiac fibroblasts but enables cell-cell, paracrine, and cell-extracellular matrix interactions, we demonstrate that selective cardiac fibroblast activation by enhanced Gq signaling, a pathophysiological trigger in the diseased heart, modulates cardiomyocyte electrical activity, leading to proarrhythmogenic automaticity.
Keywords: three-dimensional models, activated cardiac fibroblasts, cardiac myocytes, automaticity, arrhythmias
Abstract
Cardiac fibroblasts (CFs) are known to regulate cardiomyocyte (CM) function in vivo and in two-dimensional in vitro cultures. This study examined the effect of CF activation on the regulation of CM electrical activity in a three-dimensional (3-D) microtissue environment. Using a scaffold-free 3-D platform with interspersed neonatal rat ventricular CMs and CFs, Gq-mediated signaling was selectively enhanced in CFs by Gαq adenoviral infection before coseeding with CMs in nonadhesive hydrogels. After 3 days, the microtissues were analyzed by signaling assay, histological staining, quantitative PCR, Western blots, optical mapping with voltage- or Ca2+-sensitive dyes, and microelectrode recordings of CF resting membrane potential (RMPCF). Enhanced Gq signaling in CFs increased microtissue size and profibrotic and prohypertrophic markers. Expression of constitutively active Gαq in CFs prolonged CM action potential duration (by 33%) and rise time (by 31%), prolonged Ca2+ transient duration (by 98%) and rise time (by 65%), and caused abnormal electrical activity based on depolarization-induced automaticity. Constitutive Gq activation in CFs also depolarized RMPCF from –33 to −20 mV and increased connexin 43 and connexin 45 expression. Computational modeling confers that elevated RMPCF and increased cell-cell coupling between CMs and CFs in a 3-D environment could lead to automaticity. In conclusion, our data demonstrate that CF activation alone is capable of altering action potential and Ca2+ transient characteristics of CMs, leading to proarrhythmic electrical activity. Our results also emphasize the importance of a 3-D environment where cell-cell interactions are prevalent, underscoring that CF activation in 3-D tissue plays a significant role in modulating CM electrophysiology and arrhythmias.
NEW & NOTEWORTHY In a three-dimensional microtissue model, which lowers baseline activation of cardiac fibroblasts but enables cell-cell, paracrine, and cell-extracellular matrix interactions, we demonstrate that selective cardiac fibroblast activation by enhanced Gq signaling, a pathophysiological trigger in the diseased heart, modulates cardiomyocyte electrical activity, leading to proarrhythmogenic automaticity.
cardiac fibroblasts (CFs) play a major role in normal cardiac physiology and in the response of the heart to pathological stress and injury. They form a three-dimensional (3-D) cellular scaffold around the cardiac myocytes (CMs) and maintain and remodel the extracellular matrix (ECM), thereby contributing to the integrity and connectivity of the myocardial architecture (19). In response to hemodynamic stress or injury, CFs become activated and assume a myofibroblast (MFb) phenotype with heightened proliferation, contractile properties, and collagen deposition (for reviews, see Refs. 15 and 30). Although CFs are considered nonexcitable cells, they have been shown to modulate active and passive electrical properties in CMs (for reviews, see Refs. 55 and 64).
Two-dimensional (2-D) cell culture models have long been used to investigate the regulation of CMs by CFs in vitro. Immunohistochemistry of heterotypic cell pairs and cocultured monolayers have shown that CFs can directly couple with CMs via gap junctions and adherens junctions (e.g., Refs. 58, 66, and 84). Strips of intervening CFs were also shown to couple otherwise uncoupled CMs and thereby enable action potential (AP) propagation (23), but the conduction velocity was extremely slow, which can greatly facilitate the occurrence of triggered activity and reentry (70, 72). In standard 2-D cell culture, CFs rapidly transition into MFbs, which has been exploited to study MFb effects on CMs by some groups (5, 60), but concerns exist that CF activation induced by substrate stiffness does not fully replicate the activation process in vivo (for reviews, see Refs. 64 and 85). Another approach to studying MFb effects has used activated CFs isolated from infarcted hearts (87). Together, these studies have shown that MFbs slow conduction, change AP duration (APD), and induce ectopic activity in CMs by partial depolarization of CMs (5, 60, 61, 87). Despite longstanding investigations, establishing an active role for CFs in regulating CM electrical function in vivo has been a formidable challenge and matter of considerable debate (for a review, see Ref. 47). Novel technologies enabled recent demonstrations of nonexcitable cardiac cells that follow CM APs in the intact heart which represent strong evidence in support of functional CM-CF coupling in vivo (54, 71).
In contrast to 2-D culture, in which cell-cell interactions are limited to a modest number of planar interactions, cells can interact with each other in multiple directions in a three-dimensional (3-D) tissue environment. Both 3-D scaffold-supported and self-assembled in vitro cultures are important intermediaries between 2-D cultures and in vivo tissues (65). 3-D platforms heighten cell-cell and cell-ECM contact and establish molecular concentration gradients (6). In scaffold-based systems, interactions between cells and external cues dominate. The advantages of self-assembly are organotypic density, architecture and stiffness, maximal cell-to-cell communication and movement among different cell types, self-produced ECM, and cell-driven biomechanical forces (1). Electrical signal in the heart is propagated through gap junctions; thus, interconnectivity is essential to determine the potential for arrhythmogenesis. To determine the full roll of CFs in CM regulation, CM-CF interactions must be evaluated in these controllable but more biomimetic environments.
In this study, we used a scaffold-free 3-D model we previously developed, in which cell-cell interactions between neonatal rat ventricular CMs and CFs dominate but the cells can also interact via paracrine and ECM molecules. We have previously shown self-assembly into viable microtissues with intermingled CMs and CFs, reminiscent of the myocardium, with characteristic CM resting membrane potential (RMP) and AP shape (17). Our original study pointed to the importance of CF inclusion, as AP was prolonged when CFs were interspersed with CMs and showed feasibility for cell-type specific gene manipulation. In the present study, we selectively enhanced Gq signaling in CFs to examine the effect of activated CFs on the structural microenvironment and CM electrical function. We chose the Gq signaling pathway because it is commonly activated in the diseased heart, when the release of angiotensin II (ANG II), endothelin-1 (ET-1), and other factors in response to hemodynamic stress and injury leads to the activation of Gq protein-coupled receptors (GqPCRs) on both CMs and CFs (49). To examine the effect of enhanced Gq signaling when it is restricted to CFs on CM function in a 3-D microtissue environment, we overexpressed the Gq protein α-subunit or constitutively active Gαq (GαqQ209L) via adenoviral gene transfer in CFs before engineering of the microtissues. Here, we show that enhanced Gq signaling in CFs increased the size of microtissues also containing CMs as well as early profibrotic and prohypertrophic marker gene expression. Optical mapping showed prolongations in CM AP and intracellular Ca2+ transient durations and rise times as well as a markedly increased occurrence of abnormal electrical activity based on depolarization-induced automaticity. Increases in the CF resting membrane potential (RMPCF) and connexin (Cx) expression in CFs with enhanced Gq signaling were computationally modeled for their functional significance, as was the importance of the 3-D environment on the observed arrhythmogenic phenotype. Our findings suggest that elevated CM RMP via enhanced coupling to more depolarized CFGαqQL, observed in a microtissue context to our knowledge for the first time in this study, may contribute under pathophysiological conditions to electrophysiological anomalies and arrhythmogenicity in the intact heart together with potential paracrine effects.
MATERIALS AND METHODS
Reagents.
The following reagents were purchased from Sigma-Aldrich (St. Louis, MO): trypsin from the porcine pancreas, FBS, 100 U/ml penicillin and 100 mg/ml streptomycin, bromodeoxyuridine (BrdU), glucose, lithium chloride, sucrose, hematoxylin, eosin, BSA, NaCl, KCl, MgCl2, CaCl2, NaOH, borax, ammonium formate, sodium formate, and carbenoxolone. The following reagents were purchased from ThermoFisher Scientific (Waltham, MA): DMEM/F-12, HEPES, trypsin-EDTA, formic acid, human embryonic kidney (HEK)293A cells (RRID:CVCL_6910), Superfrost Plus Microscope Slides, TRIzol, agarose, Live/Dead Viability/Cytotoxicity kit, CellTracker Orange CMRA, ProLong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole, TaqMan Reverse Transcription Reagents, TaqManGene Expression Assays, TaqMan Gene Expression master mix, SuperSignal West Pico or Femto Substrate, di-4-ANEPPS, and Rhod2-AM. The sources for all other reagents are specified below.
Isolation and 2-D culture of neonatal rat ventricular CMs and CFs.
The cardiac tissue harvesting procedures conformed with the Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training, and the protocol was approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital. CMs and CFs were isolated from postnatal day 2 Sprague-Dawley rats as previously described (17). Serial enzymatic digestion of isolated ventricles with trypsin (1 mg/ml) and collagenase type 2 (0.6 mg/ml, Worthington Biochemical, Lakewood, NJ) followed by discontinuous Percoll (GE, Fairfield, CT) gradient centrifugation of the cardiac cell suspension yielded CM- and CF-enriched cell fractions. To allow the cells to recover from isolation, CMs and CFs were cultured in 2-D (3 ×106 cells/100-mm dish) in DMEM/F-12 supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin (referred to as “medium”). The medium for CMs was supplemented with 50 mM BrdU to inhibit the proliferation of the few CFs present in the CM fraction (see below). After 3 days, cells were detached with trypsin-EDTA (3–4 min at 37°C) and replated into nonadhesive hydrogels to generate 3-D microtissues (see below).
Average cell yields per pup were 2.4 ± 0.8 × 106 CMs/pup and 3.4 ± 1.1 × 106 CFs/pup. The purity of CM and CF fractions was 94.9 ± 2.3% and 97.3 ± 1.6%, respectively, as determined by immunostaining of fixed cells after 3 days in 2-D culture with antibodies recognizing cell type-specific markers [i.e., vimentin for CFs and α-sarcomeric actinin (αSA) for CMs] as previously described (17). After trypsinization, 61.2 ± 17.6% of CMs and 50.0 ± 15.3% of CFs were recovered compared with the original cell numbers seeded.
Adenoviral infection of CFs.
Adenoviruses encoding wild-type or constitutively active GαqQ209L (Ad-Gαq and Ad-GαqQL, respectively) were kindly provided by Dr. J. Heller Brown (University of California, San Diego, CA). Empty adenovirus (Ad-Ctr) and uninfected cells served as negative controls, and Ad-GFP was used to visualize the effectiveness and uniformity of multiplicity of infection (MOI)-dependent infection. All adenoviruses were amplified in HEK293A cells and titered using an Adeno-X qPCR Titration Kit (Takara Bio USA, Mountain View, CA).
Ad-Gαq and Ad-GαqQL were originally generated by Adams et al. (2) and used to activate Gαq signaling in neonatal rat ventricular CMs in that study. In the present study, adenoviral infections of neonatal rat ventricular CFs in 2-D culture were achieved at the intended MOIs by cell counting at the day of infection, as previously described for adult rat ventricular CFs (93). To generate cardiac microtissues with adenovirus-infected CFs, before being cell seeded in 3-D culture, CFs were infected in suspension after trypsinization and cell counting in serum-free DMEM/F-12 for 2 h. Cells were then collected by centrifugation and resuspended in medium, which was repeated twice before seeding in 3-D (or 2-D) culture as described below.
Fabrication of hydrogels and 3-D culture.
Scaffold-free 3-D microtissues were generated using nonadhesive agarose gels with cylindrical microwells with hemispherical bottoms to guide self-assembly. Sterilized 2% (wt/vol) agarose was pipetted into molds designed for 6-, 12-, or 24-well plates with 400- or 800-μm-diameter rounded pegs (Microtissues, Providence, RI). After being cooled to room temperature (<5 min), the agarose gels were separated from the molds and transferred to single wells of 6-, 12-, or 24-well plates. For equilibration, medium was added to each well (3 ml for 6-well plates, 2 ml for 12-well plates, or 1 ml for 24-well plates), and air bubbles were removed with a vacuum chamber (400 μm diameter only, Lindberg/Blue M, ThermoFisher Scientific). Hydrogels were equilibrated overnight at 37°C in a humidified incubator with 5% CO2.
Depending on the specific applications, CFs in suspension were added alone or in combination with CMs (at 1:1 or 1:2 ratios) to the center of the hydrogel seeding chamber with the indicated number of recesses (100 μl for 24-well format with 35 recesses, 225 μl for 12-well format with 81 recesses, and 500 μl for 6-well format with 822 recesses) and allowed to settle into the recesses for 30 min. Medium was then added to each well (3 ml for 6-well molds, 2 ml for 12-well molds, and 1 ml for 24-well molds), and cells were cultured for 2−4 days as indicated.
Phospholipase C-β activity assay.
Total inositol phosphate (IP) formation, an indirect measure of phospholipase C (PLC)-β activity, was assayed in 2-D culture as previously described for adult CFs (93). To determine total IP formation in 3-D cultures, microtissues were collected from 6 hydrogels in the 12-well format (3 × 105 cells each) per condition 48 h after adenoviral infection (see above). They were harvested by inverting hydrogels in PBS and centrifuging the culture plates at 63 g for 2 min. Once removal from the hydrogel was confirmed by light microscopy, the microtissues in PBS from each hydrogel were collected into a microcentifuge tube and centrifuged (19 g for 1 min at room temperature). The supernatant was gently aspirated and the microtissues were pooled. Microtissues were then labeled with myo-[3H]inositol (4 µCi/ml; Perkin Elmer, Waltham, MA) in inositol-free DMEM (MP Biomedical, Solon, OH) for 4 h at 37°C, with inversion every 15–30 min for mixing. They were then briefly centrifuged, 10 mM LiCl was added to each tube, tubes were inverted to mix, and samples were incubated for 1 h at 37°C. After gentle centrifugation (82 g, 1 min, 4°C), media were removed, and the microtissues were washed with PBS once. IPs were extracted with 1 ml of 20 mM formic acid on a nutator at 4°C for 1 h. Extracts were neutralized to pH 7.5 with a solution containing 150 mM KOH and 7.5 mM HEPES and centrifuged at 4°C for 2 min at 16,000 g. Clear supernatants were loaded onto 0.5-ml Dowex AG1-X8 (Bio-Rad, Hercules, CA) anion exchange columns, equilibrated with 2 ml of 1 M NaOH followed by 2 ml of 1 M formic acid and 5 × 5 ml of H2O. After sample application, columns were washed with 5 ml of H2O and 5 ml of 5 mM borax-60 mM sodium formate before total IPs were eluted with 3 ml of 0.9 mM ammonium formate-0.1 M formic acid directly into vials containing 15 ml of Ultima Gold XR scintillation cocktail (Perkin Elmer). Each sample was assayed in triplicate.
Live/Dead and CellTracker staining.
Cell viability of microtissues was examined using the Live/Dead Viability/Cytotoxicity kit. Cells were stained with 2 μM calcein-AM and 4 μM ethidium homodimer-1 (in 1 ml PBS) at 37°C for 30 min followed by image acquisition. Before seeding with Ad-GFP-infected CFs, CMs were labeled with CellTracker Orange CMRA (10 μM) as previously described (17).
Image acquisition and processing.
Phase-contrast and epifluorescent images of cells and microtissues were captured with a Nikon TE2000-U and a black and white/color digital camera (MicroVideo Instruments, Avon, MA) and acquired and analyzed with NIS Elements software. Confocal images were acquired with a Nikon C1si confocal (Nikon) using diode lasers at 488 and 561 nm as previously described (17).
Microtissue size analysis.
Stitched 4× phase-contrast images of whole 24-well microtissue hydrogels (0.6 × 106 cells each) were acquired and analyzed. Image thresholding and particle size analysis was used in NIS Elements to determine the top view cross-sectional area of individual microtissues across each mold.
3-D tissue sections.
Microtissues in 24-well hydrogels (0.6 × 106 cells) were fixed with 4% (vol/vol) paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and 8% (wt/vol) sucrose in PBS overnight at room temperature. Molds were then rinsed twice with PBS and fully equilibrated (as indicated by their sinking, usually over 12 h) with 15% and then 30% (wt/vol) sucrose in PBS. Whole agarose gels containing microtissues were removed from sucrose, blotted dry, and embedded in Tissue-Tek CRYO-OCT Compound (Ted Pella, Redding, CA). The microtissues are free floating in the cylindrical recesses and can be located at different heights within the mold during the embedding process. Blocks were stored at −80°C, sectioned on a Leica CM3050 cryostat microtome (Leica Biosystems, Buffalo Grove, IL) into 10-μm-thick sections, and placed on Superfrost Plus slides. After being air dried for 15 min, sections were postfixed in 4% paraformaldehyde in PBS.
Histological analysis.
Cryosections were stained with hematoxylin and eosin and Sirius Red, after they were thawed for 10 min at 37°C, placed in 95% ethanol for 1 min, and rinsed with distilled H2O for 30 s. For hematoxylin and eosin, a 4-min incubation in hematoxylin and a rinse with running tap water for 2 min were followed by five dips into 1% HCl in 70% alcohol and washes in running tap water and 95% ethanol (1 min each). Slides were then stained in eosin for 25 s and rinsed with 95% ethanol for 15–20 s (with agitation). For Sirius Red, slides were placed in Sirius Red stain (Electron Microscopy Sciences) for at least 1 h and washed twice for 30 s in 0.5% acetic acid. All slides were then rinsed three times in 100% ethanol for 30 s, cleared in xylene for 5 min, and mounted with SHUR/Mount (Electron Microscopy Sciences).
Western blot analysis.
Microtissues were harvested from two to three hydrogels in the 6-well format (1 × 106 cells each) after 2−4 days in 3-D culture as described above, lysed in 0.2 ml Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) containing complete mini protease inhibitor cocktail (Roche, Pleasanton, CA), and sonicated for 3 × 10 s on ice. Protein concentrations were measured with a Bradford microassay (Bio-Rad, Hercules, CA) with BSA as the standard. Equal amounts of protein (10 μg/lane) were separated on 4–20% TGX Gels (Bio-Rad), transferred to nitrocellulose membranes, and probed with the following primary antibodies at indicated dilutions: α-smooth muscle actin (αSMA; 1:400, RRID:AB_262054, Sigma), Cx43 (1:5,000, RRID:AB_2294590, Cell Signaling), Cx45 (1:1,000, RRID:AB_91014, Millipore), fibronectin (FN; 1:1,200, RRID:AB_476976, Sigma), GAPDH (1:1,000, RRID:AB_561053, Cell Signaling), Gαq/11/14 (1:100, RRID:AB_10842057, Santa Cruz Biotechnology), and vimentin (1:500, RRID:AB_477627, Sigma). After incubation with the appropriate peroxidase-coupled secondary antibodies, proteins of interest were visualized by chemiluminescence (SuperSignal West Pico or Femto substrate).
mRNA analysis.
Microtissues were pooled from two to three hydrogels in the 12-well format (1.4 × 106 cells each) for each condition after 3 days in 3-D culture as described above. They were sonicated for 3 × 10 s on ice in 1.0 ml TRIzol before extraction of total RNA. Total mRNA from CFs in 2-D culture (0.875 × 106 cells/6-well plate) was isolated using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) and pooled from two to three wells. All RNA samples were reverse transcribed using TaqMan Reverse Transcription Reagents and subjected to quantitative PCR (qPCR) using FAM-labeled TaqMan MGB probes for the indicated genes plus 18S and TaqMan Gene Expression Master Mix according to the manufacturer’s instructions. Absence of template during qPCR was used as negative control. qPCR cycling was performed at 95°C for 10 min and then 95°C for 15 s and 60°C for 1 min for a total of 45 cycles. Each sample was assayed in duplicate, and the threshold cycle (CT) values corresponding to the qPCR cycle number at which fluorescence emission in real time reaches a threshold above the baseline emission were determined using QuantStudio Real-Time PCR software. Data were analyzed by the comparative CT method (78) and are presented as fold changes in mRNA expression normalized to 18S and relative to microtissues containing Ad-Ctr-infected CFs (CFCtr or CM:CFCtr).
Optical mapping.
An Olympus MVX10 microscope was used to image 1.2 × 1.2-mm2 regions. Microtissues were loaded with voltage-sensitive di-4-ANEPPS (5 μM for 10 min at 35°C) for measurements of membrane potential (Vm) or Rhod2-AM (4 μM) for cytosolic [Ca2+]i. Fluorescence images were acquired at 979 frames/s using a Photometrics Evolve +128 EMCCD camera (2 × 2 binning to 64 × 64 pixels, 18.7 × 18.7-µm2 resolution, 1.2 × 1.2-mm2 field of view) and an Olympus MXV10 macroview optical system. Fluorescence images were filtered using nonlinear bilateral filter (spatial filter: 5 × 5 window, temporal filter: 13 point window) to preserve AP upstrokes from blurring. Typically, four microtissues were recorded simultaneously per scan at this magnification. A single spheroid is typically covered by ~60 pixels at this magnification. All 35 microtissues in 24-well hydrogels (0.6 × 106 cells) were recorded. To evaluate automaticity, all tissues were initially recorded for 5 s without stimulation. Although longer time recording (20 s) may detect spontaneous activity from CM:CFCTR, a recording time of 5 s was chosen to avoid potential phototoxic damages caused by extended excitation light exposure that can promote spontaneous activity. Tissues were then field stimulated at a basic pacing cycle length of 1 Hz, with a biphasic stimulation pulse strength of 10 V/cm and duration of 4 ms. Carbenoxolone (CBX; 100 μM) was then added for 10 min to partially inhibit gap junctions, and the same pacing protocol was repeated. For washout experiments, automaticity was recorded without pacing, after perfusion of CBX for 30 min, and after washout for 30 min.
Resting membrane potential recordings.
Standard microelectrode techniques were used for membrane potential recordings with a 15-MΩ microelectrode with pipette filled with 3 mol/l KCl. Because CFs and CMs cannot be distinguished in 3-D cultures, spherical microtissues containing only infected CFs were formed in hydrogels (24-well format, 0.6 × 106 cells) and transferred to coverslips after 2 days of 3-D culture. Tissues were bathed in Tyrode solution containing (in mmol/l) 140 NaCl, 5.4 KCl, 1 MgCl2, 10 HEPES, 1.8 CaCl2, and 5.5 glucose (pH 7.4). Recordings were low-pass filtered at 100 kHz and digitized at 200 kHz. Vm was also recorded in infected CFs in standard 2-D cell cultures for comparison using an Axopatch-200B amplifier (Molecular Devices, Foster City, CA) and the perforated current-clamp technique. For Vm recordings, pipettes were filled with (in mmol/l) 120 potassium gluconate, 20 KCl, 5 NaCl, 5 HEPES, and 5 MgATP (pH 7.2). Cells were bathed in Tyrode solution, and β-escin (50 μM) was added to the pipette solution. Pipette resistances were 5–8 MΩ. Recordings were low-pass filtered at 10 kHz and digitized at 20 kHz. All experiments were carried out at room temperature.
Computational modeling of CM-CF coupling.
Effects of RMPCF and gap junction coupling on CM APs were studied using a computational CM AP model that is electrotonically connected with several CFs. The Vm of a CM or CF in a coupled CM-CF pair is governed by the following equation:
where CCM = 125 pF is the membrane capacitance of a CM, VCM is the Vm of a CM, t is time, Iion is the corresponding membrane current, n is the number of coupled CFs to a single CM (CM connected to n CFs, and a CF has only one connection with the CM), and ggap,CM-CF is the gap junction conductance between the CM and the kth connected CF whose Vm is VCF,k. We used the Luo and Rudy AP model (52) with a modified maximum conductance of inward rectifier K+ current (IK1; GK1,max = 0.5) to match with our previous experimental results (17). The passive model CF was modeled as follows (90):
where CCF = 25 pF, CF current (ICF) = gm,CF (VCF − RMPCF), and gm,CF is the membrane conductance of a CF. In this study, RMPCF was varied from −50 to 0 mV, and the coupling conductance between CM-CF (ggap,CM-CF) from 0 to 10 nS to investigate the effects of RMPCF and ggap,CM-CF on automaticity. gm,CF was set to 4 nS based on previous experimental and computational studies (13, 39, 44, 48, 62, 74, 79, 80, 89, 90). Numerical calculation of the differential equations was performed using the Euler method with a time step of Δt = 0.01 ms.
To investigate the effect of CM-CF interactions in a 3-D environment, a cube of 25 × 25 × 25 cells was modeled, which had a sufficient number of cells to reflect a single microtissue. In the 3-D cube, each cell was connected to six neighboring cells through gap junctions, and the CMs and CFs were randomly distributed in the cube with CM:CF = 1:1 or 1:2 ratio. ggap,CM-CM was set to 10 nS, and ggap,CM-CF and ggap,CF-CF were set to 5 nS. This simulation was carried out on a multicore graphic processing unit (Geforce GTX Titan, NVidia) with double precision.
Statistical analyses.
Technical replicates were averaged to a single value. Values are expressed as means with SD. n is reported as the number of microtissues/cells, number of replicates, and number of repeated experiments where appropriate and as indicated. Statistical analyses were performed using one-way ANOVA with Bonferroni post hoc tests, Student’s two-tailed paired and unpaired t-test, or Fisher’s exact test as appropriate.
RESULTS
Microtissue model development with CMs interspersed with Gq-activated CFs.
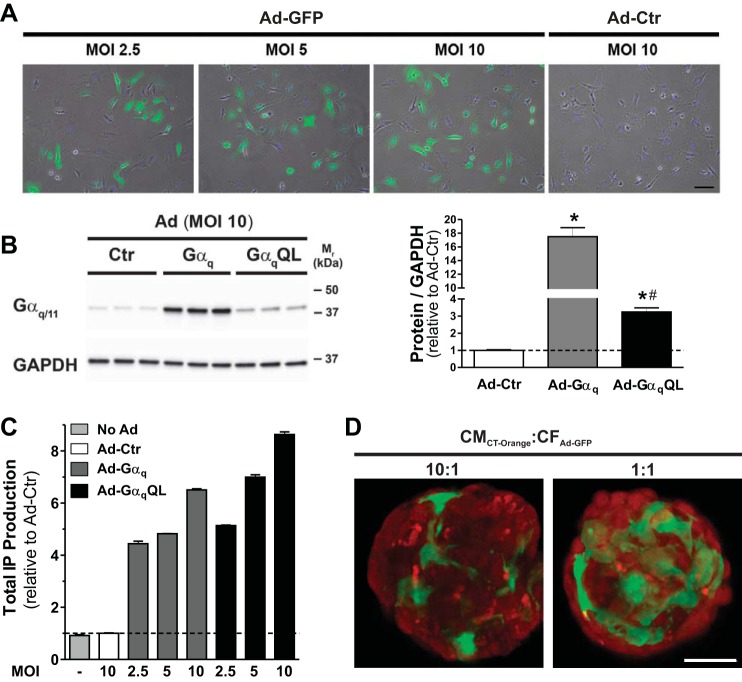
To study the modulation of CM electrical activity by Gαq-activated cardiac CFs, we leveraged a scaffold-free cardiac microtissue model we previously developed in which neonatal rat ventricular CMs and CFs self-assemble in nonadhesive hydrogels to form spheroidal microtissues with high interspersion of the two cell types reminiscent of the myocardium (17). Gq activation was achieved in CFs with adenoviruses encoding the α-subunit of heterotrimeric Gq protein (Gαq) or a constitutively active mutant (Q209L) of Gαq [GαqQL (16)]. First, we confirmed the effectiveness of adenovirus-mediated gene transfer in neonatal rat CFs. Figure 1A shows the uniformity and efficiency of adenoviral infections in CFs in 2-D cultures: although there was variability in fluorescent intensity between individual cells 3 days after infection with Ad-GFP at three different MOIs, the number of infected CFs increased in a MOI-dependent manner. The number of GFP-positive CFs (as a percentage of total cells identified by nuclear Hoechst staining) was 44.1 ± 2.8% at MOI 2.5 (n = 120), 65.2 ± 8.0 at MOI 5 (n = 136), and 73.7 ± 8.7% at MOI 10 (n = 154). We next assessed the effect of adenovirus-mediated gene transfer of wild-type or constitutively active Gαq in CFs on both Gαq protein expression (using an antibody recognizing Gαq and the closely related Gα11 isoform; Fig. 1B) and Gq signaling (using total IP formation as an indirect measure for the activity of PLC-β, the canonical direct downstream target of Gαq; Fig. 1C): despite a more modest increase in Gαq protein expression in CFs infected with Ad-GαqQL compared with Ad-Gαq at MOI 10 (3.3-fold vs. 17-fold of Ad-Ctr), Ad-GαqQL induced a more pronounced increase in PLC-β activity than Ad-Gαq (8.6-fold vs. 6.5-fold of Ad-Ctr). This was expected based on prior studies in other cells (see discussion) and is due to the Q209L point mutation that renders the protein constitutively active.
Fig. 1.
Adenoviral infection of neonatal rat ventricular cardiac fibroblasts (CFs). A: representative merged epifluorescent and phase contrast images of neonatal rat ventricular CFs in two-dimensional (2-D) cell culture, 3 days after infection with Ad-GFP (or Ad-Ctr) at indicated multiplicities of infection (MOIs). Cells were stained with Hoechst for 20 min before mounting. Scale bar = 100 μm. B: representative Western blot of Gαq/11 expression in cell lysates (10 µg protein/lane) collected from CFs in 2-D cultures, 3 days after infection with indicated adenoviruses at MOI 10 (left). GAPDH was used as the loading control. Group data are means ± SD (right). *P < 0.05 vs. Ad-Ctr; #P < 0.05 vs. Ad-Gαq (ANOVA). C: basal inositol phosphate (IP) production in CFs 72 h after infection with the indicated adenoviruses at MOI 2.5, 5, and 10. Three wells from a 6-well plate were pooled for each sample (n = 3 for each condition). Similar results were obtained in two other experiments testing a total of two and four batches of Ad-Gαq and Ad-GαqQL, respectively. D: representative confocal images from sections of cardiomyocyte (CM):CF microtissues composed of a 10:1 or 1:1 ratio of CellTracker Orange-labeled CMs (CMCT-Orange) and Ad-GFP-infected CFs (CFAd-GFP) 2 days after coseeding in hydrogels. Scale bar = 50 μm.
To generate cardiac microtissues with CF-restricted activation of Gαq signaling, adenoviral infections of CFs were performed before coseeding with CMs into the hydrogels and self-assembly. To confirm CF-restricted adenoviral infection, Ad-GFP-infected CFs (CFAd-GFP) were coseeded with CellTracker Orange CMRA-labeled CMs (CMCT-Orange). Minimal fluorescent overlap on confocal images in CMCT-Orange:CFAd-GFP microtissues at 10:1 and 1:1 ratios 2 days after coseeding supported Ad-mediated gene transfer predominantly in CFs (Fig. 1D).
We next assessed the effect of CF-restricted Ad-Gαq and Ad-GαqQL infections on total IP formation in 3-D microtissues composed of either infected CFs alone (CFAd) and in combination with equal numbers of uninfected CMs (CM:CFAd; Fig. 2A). Since microtissues containing uninfected or Ad-Ctr-infected CFs showed similar results, only Ad-Ctr data are shown. In microtissues composed of CFs only, Ad-Gαq and Ad-GαqQL increased PLC-β activity 6.1-fold and 7.3-fold compared with Ad-Ctr, respectively, 3 days after infection (Fig. 2A). CM:CFAd microtissues also showed markedly elevated PLC-β activity, but the effect of Ad-GαqQL was less pronounced than Ad-GαqWT (4.9 ± 1.0-fold and 8.5 ± 1.2-fold, respectively; Fig. 2A). Similar results were observed in 2-D cocultures of CMs and adenovirus-infected CFs (5.0 ± 0.5-fold for Ad-GαqQL and 7.9 ± 0.1-fold for Ad-Gαq; data not shown).
Fig. 2.
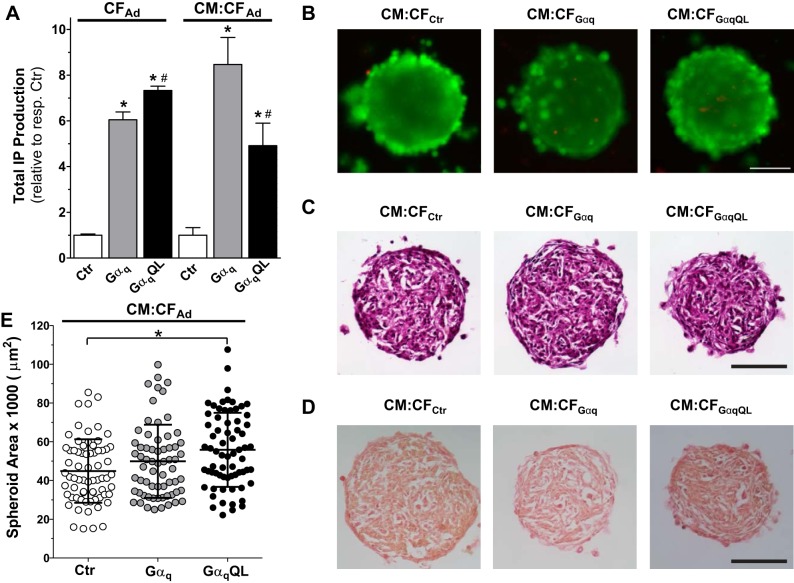
Cardiac fibroblast (CF)-restricted Gαq activation in spherical cardiac microtissues. A: basal inositol phosphate (IP) production in microtissues after adenoviral infection of CFs with the indicated adenoviruses for 2 h before cell seeding in nonadhesive hydrogels alone (CFAd) or with cardiomyocytes (CMs) (CM:CFAd). Microtissues from 6 hydrogels in the 12-well format were pooled for each sample (n = 3 for each condition). *P < 0.05 vs. control (Ctr); #P < 0.05 vs. Gαq (ANOVA). B: fluorescent images of representative Live/Dead-stained whole CM:CFAd microtissues containing the indicated infected CFs 3 days after infection and cell seeding. Scale bar = 100 µm. C and D: bright-field images of representative hematoxylin and eosin (C)- and Sirius Red (D)-stained cryosections (10 µm thick) of CM:CFAd microtissues fixed after 3 days in three-dimensional (3-D) culture. Scale bars = 100 µm. E: area of CM:CFAd microtissues after 3 days in 3-D culture. Values are means ± SD; n = 65–70 microtissues/condition. *P < 0.05 (ANOVA).
Microtissues containing Ad-Gαq-infected CFs were viable, as illustrated by representative Live/Dead-stained CM:CF microtissues (Fig. 2B). Consistent with our prior study (17), immunostaining with antibodies against αSA and vimentin confirmed that CMs and CFs self-assembled to be highly interspersed (data not shown). Hematoxylin and eosin staining of microtissue cryosections did not reveal any obvious differences among CM:CFCtr, CM:CFGαq, and CM:CFGαqQL at various depths into the microtissue (Fig. 2C), and representative images of Sirius Red-stained sections suggested no gross differences between CM:CF microtissues containing Gαq-activated CFs compared with control CFs (Fig. 2D). Although the images shown are likely from different planes because the location of the microtissues within the recesses cannot be controlled during the embedding process (see materials and methods), they are representative of the morphology seen across different planes and spheroids.
GαqQL expression in CFs increased the microtissue area of CM:CFGαqQL microtissues over CM-CFCtr (Fig. 2E). CM:CFCtr microtissues were 44,900 ± 16,400 μm2 (n = 69 microtissues) in cross-sectional area, which equates to a radius of 120 ± 22 μm and a volume of 7.15 × 106 ± 3.91 × 106 μm3, whereas CM:CFGαqQL microtissues were 55,800 ± 19,100 μm2 (n = 70 microtissues) in area (r = 133 ± 23 μm, volume = 9.85 × 106 ± 5.11 × 106 μm3, P < 0.05). The mean values for CM:CFGαq microtissues (n = 65 microtissues) were higher than CM:CFCtr and lower than CM:CFGαqQL (49,900 ± 18,900 μm2, r = 126 ± 24 μm, volume = 8.38 × 106 ± 4.79 × 106 μm3) but did not reach statistical significance (Fig. 2E).
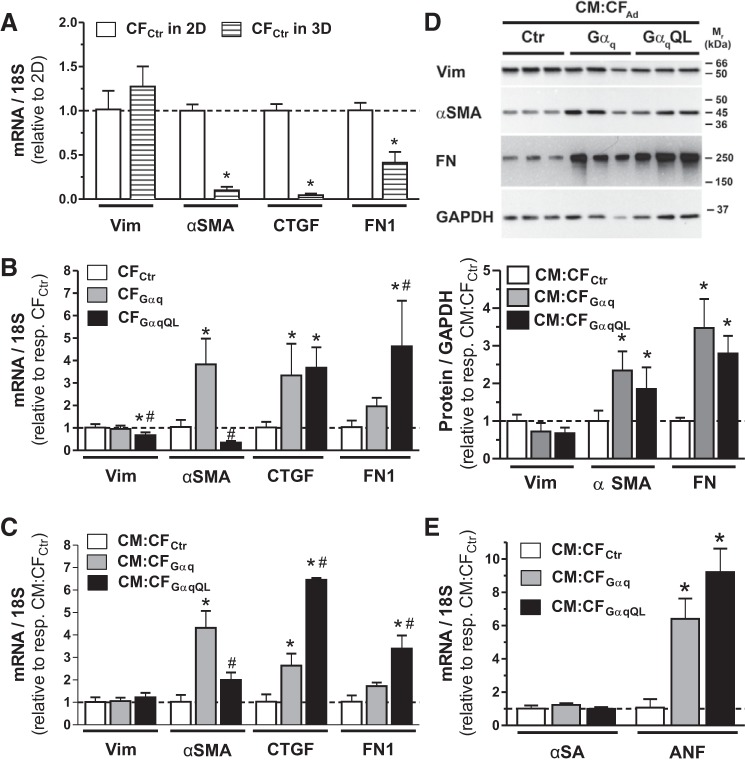
We next examined the expression of select marker genes for MFb (αSMA) and fibrogenesis (CTGF and FN1) using qPCR analysis. Vimentin was used for comparison because it is expressed in both unactivated and activated CFs in vitro and in vivo (3). First, we compared their expression in 3-D CF microtissues composed of control CFs to cells cultured as a monolayer in conventional 2-D culture for the same duration (3 days): mRNA levels of αSMA, CTGF, and FN1 were markedly reduced in 3-D culture (by 90 ± 4%, 96 ± 2%, and 59 ± 12%, respectively), whereas vimentin expression was comparable to 2-D culture (Fig. 3A). Next, we assessed the effect of CF activation by Gαq on the expression of these markers in microtissues containing CFs either alone (Fig. 3B) or together with CMs (Fig. 3C). In contrast to vimentin, αSMA mRNA was increased (3.8 ± 1.1-fold) in microtissues composed of Gαq-expressing CFs but not those composed of GαqQL-expressing CFs, whereas CTGF was increased to a comparable level in both (3.3 ± 1.4-fold in CFGαq and 3.7 ± 0.9-fold in CFGαqQL) and FN1 was increased more in CFGαqQL (4.6 ± 2.0-fold) than CFGαq (2.0 ± 0.4-fold; Fig. 3B). We observed similar trends in CM:CF microtissues containing Gαq-activated CFs (Fig. 3C). The only major difference was a more pronounced increase for CTGF in CM:CFGαqQL (6.5 ± 0.1-fold) than CM:CFGαq (2.6 ± 0.5-fold), which points to a contribution from the CMs. The representative Western blots shown in Fig. 3D demonstrate that vimentin, αSMA, and FN1 protein expression correlated overall with the mRNA levels in CM:CF microtissues. Furthermore, CM:CF microtissues containing Gαq-activated CFs had comparable αSA mRNA levels to CM:CFCtr but increased atrial natriuretic factor (ANF) mRNA (6.4 ± 1.2-fold in CM:CFGαq and 9.2 ± 1.4-fold in CM:CFGαqQL; Fig. 3E).
Fig. 3.
Cardiac fibroblast (CF)-restricted Gαq overexpression leads to CF activation and atrial natriuretic factor (ANF) expression. CFs were infected with the indicated adenoviruses for 2 h before being seeded. CFs were seeded alone in standard two-dimensional (2-D) culture (A) or were seeded in hydrogels in three-dimensional (3-D) culture alone (CFAd; B) or together with CMs (1:1, CM:CFAd) (C–E). After the indicated days, cells or microtissues were harvested for mRNA extraction (pooled from 2−3 wells for each condition, 6-well and 12-well plates for 2-D and 3-D, respectively) or protein extraction (pooled from 3 hydrogels in 6-well format for each condition). A: relative mRNA expression for the indicated proteins assessed by quantitative PCR (qPCR) analysis of cells cultured in 2-D monolayers or in 3-D as described. Values are means ± SD; n = 5–6 per condition. *P < 0.05 vs. 2-D (Student’s t-test). B and C: relative mRNA expression for the indicated proteins in CFAd (B) or CM:CFAd microtissues (C) assessed by qPCR analysis after 3 days in 3-D culture. Values are means ± SD for n = 6 (B) or n = 3 (C) samples/condition. *P < 0.05 vs. control (Ctr); #P < 0.05 vs. Gαq (ANOVA). D, top: representative Western blots of microtissue lysates after 2 days in 3-D culture (10 µg protein/lane) probed with the indicated antibodies. GAPDH was used as the loading control. Similar changes were observed after 3 and 4 days in culture (not shown). Bottom: pooled group data. Values are means ± SD; n = 4–7 samples/condition. *P < 0.05 vs. Ctr (ANOVA). E: relative mRNA expression of the indicated proteins in CM:CFAd microtissues assessed by qPCR analysis after 3 days in 3-D culture. Values are means ± SD; n = 3 samples/condition. *P < 0.05 vs. Ctr (ANOVA). αSA, α sarcomeric actinin; αSMA, α-smooth muscle actin; CTGF, connective tissue growth factor; FN, fibronectin; Vim, vimentin.
APs and Ca2+ transients in CM:CF microtissues containing Gq-activated CFs.
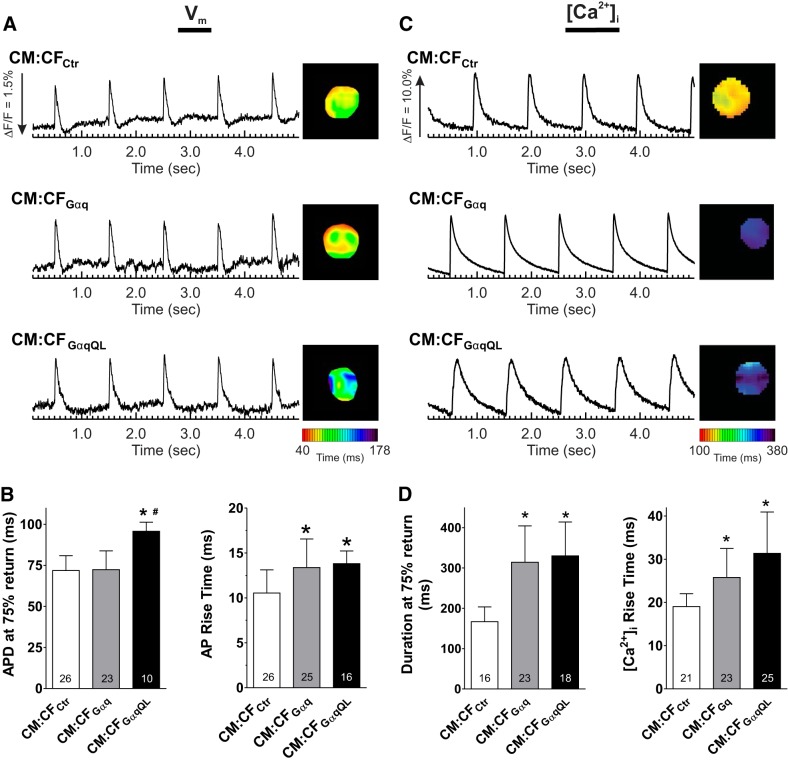
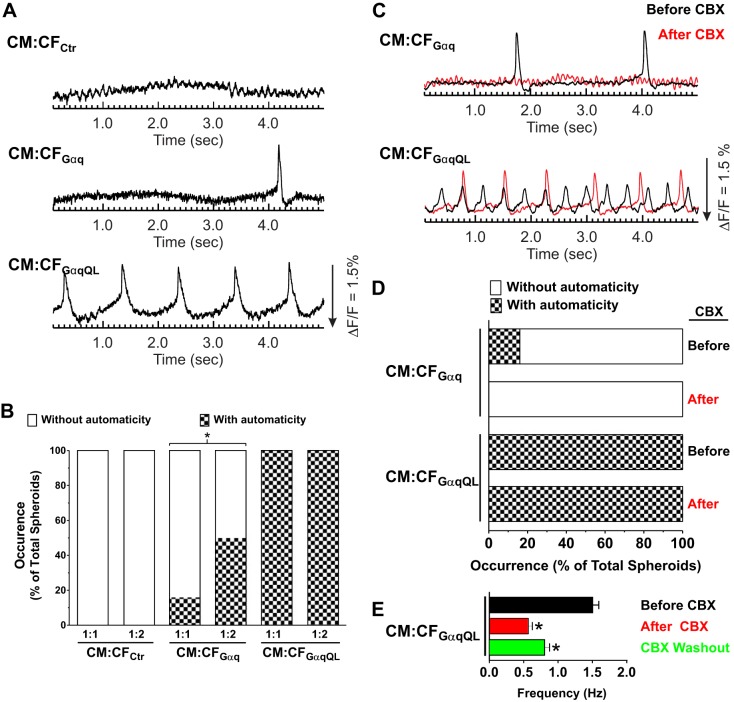
To investigate the modulation of CM electrical activity by CFs, optical mapping was used to record APs and [Ca2+]i from paced (1 Hz) CM:CF microtissues using voltage- and Ca2+-sensitive dyes, respectively. Voltage and Ca2+ could not be recorded in the same microtissues, but the data presented were all recorded from the same batches of isolated cells. Representative AP traces and APD maps from CM:CF microtissues are shown in Fig. 4A. APD at 75% repolarization was comparable for CM:CFCtr and CM:CFGαq but prolonged in CM:CFGαqQL by more than 20 ms (95.7 ± 5.6 vs. 71.8 ± 9.1 ms in CM:CFCtr). The number of CM:CFGαqQL microtissues analyzed was lower compared with the other conditions because of automaticity that persisted despite field stimulation (see below). Gq activation in CM:CF microtissues also altered AP rise time, which was prolonged in both CM:CFGαq (13.4 ± 3.2 ms) and CM:CFGαqQL (13.8 ± 1.4 ms) compared with CM:CFCtr (10.5 ± 2.6 ms; Fig. 4B). Representative [Ca2+]i traces and duration maps from optical mapping of Rhod2-AM-loaded CM:CF microtissues are shown in Fig. 4C. The Ca2+ transient duration nearly doubled in both CM:CFGαq (314.1 ± 90.1 ms) and CM:CFGαqQL (330.1 ± 83.7 ms) over CM:CFCtr (166.8 ± 36.6 ms), and the Ca2+ transient rise time increased by more than 6 ms in CM:CFGαq (25.8 ± 6.7 ms) and by more than 12 ms in CM:CFGαqQL (31.4 ± 9.6 ms) compared with CM:CFCtr (19.0 ± 3.0 ms; Fig. 4D).
Fig. 4.
Gαq activation in cardiac fibroblasts (CFs) prolongs action potential (AP) and Ca2+ transient duration and rise time in cardiomyocyte (CM):CF microtissues. CMs and CFs infected with the indicated adenoviruses were coseeded at a 1:1 ratio in hydrogels. After 3 days in three-dimensional (3-D) culture, microtissues were loaded with di-4-ANEPPS or Rhod2-AM for optical mapping of membrane potentials (Vm; left) or Ca2+ transients ([Ca2+]i; right) under paced conditions (1 Hz). A and C: representative traces and duration maps of paced Vm and [Ca2+]i recordings obtained from the indicated microtissues paced at 1 Hz. B and D: quantification of the duration at 75% return (left) and rise time (right) of APs and [Ca2+]i in CM:CFAd (1:1) microtissues. Values were averaged from all the pixels of single spheroids (~60 pixels/spheroid). Values are means ± SD for the indicated numbers. *P < 0.05 vs. control (Ctr); #P < 0.05 vs. Gαq (ANOVA). Data shown are from a representative experiment in which both Vm and [Ca2+]i were recorded in the same batch of microtissues. Comparable recordings and differences between the experimental groups were obtained in two and four additional independent experiments for Vm and [Ca2+]i, respectively. APD, AP duration.
Increased automaticity in CM:CF microtissues containing Gq-activated CFs.
Although neonatal rat CMs spontaneously contract in culture, depolarization-induced automaticity is not typical. Since we observed persistent automaticity in CM:CFGαqQL when the microtissues were subjected to electrical field pacing (see above), we next examined the occurrence and frequency of spontaneous membrane potential activity in CM:CF microtissues without pacing. As shown in Fig. 5, A and B, none of the interspersed CM:CF microtissues with control CFs showed any spontaneous Vm activity in the 5-s intervals acquired, regardless of the ratio of CM:CF (1:1 or 1:2). In CM:CFGαq with a 1:1 ratio, a few microtissues showed depolarization-induced spontaneous Vm activity (16%), and the occurrence of this automaticity increased to 50% with a 1:2 CM:CFGαq ratio. In contrast, automaticity was observed in all CM:CFGαqQL microtissues, even at a 1:1 ratio.
Fig. 5.
Gαq activation in cardiac fibroblasts (CFs) causes automaticity in unpaced cardiomyocyte (CM):CF microtissues that is mitigated by partial gap junction inhibition. CMs and CFs infected with the indicated adenovirus were coseeded in 1:1 or 1:2 ratios in hydrogels. After 3 days in three-dimensional (3-D) culture, microtissues were loaded with di-4-ANNEPS and optically mapped in the absence of electrical stimulation. A: representative membrane potential (Vm) traces of 1:1 CM:CFAd microtissues illustrating automacity in unpaced microtissues. B: group data for the occurrence of automacity within 5-s recordings, expressed as a percentage of total microtissues recorded for each condition per hydrogel. *P < 0.05 (Fisher’s exact test). C: representative Vm traces of unpaced CM:CFGαq and CM:CFGαqQL microtissues (1:1) from A that were optically mapped in the absence of electrical stimulation both before (black) and after (red) administration of carbenoxolone (CBX; 100 μM, 10 min), illustrating that automacity was mitigated by CBX. The traces recorded before and after CBX are from the same microtissue. D: group data for the occurrence of automacity within 5-s recordings before and after 100 μM CBX administration for 10 min (expressed as percentages of total microtissues recorded for each condition). E: unpaced CM:CFGαqQL microtissues were perfused with 100 μM CBX for 30 min followed by a washout period of 30 min. Averaged frequency of the automaticity observed before CBX (n = 34), after CBX (n = 31), and after CBX washout (n = 27) is shown. Values are means ± SD. *P < 0.05 (Student’s t-test).
To elucidate the potential role of gap junctions in automaticity in CMs regulated by Gαq-activated CFs, we treated CM:CF (1:1) microtissues with the gap junction inhibitor CBX at a concentration expected to block half of gap junctions [100 μM (68, 81)] for 10 min. CBX treatment abolished automaticity in CM:CFGαq microtissues (Fig. 5, C and D). Although the occurrence was not completely suppressed in CM:CFGαqQL microtissues, their frequency was lowered significantly (from 1.6 ± 0.7 to 1.0 ± 0.3 Hz, n = 20, P < 0.05 by Student’s t-test). The importance of gap junctions in automaticity was further indicated with CBX perfusion and washout (30 min each; Fig. 5E). The frequency of automaticity was partially recovered after the washout (1.56 ± 0.42, 0.57 ± 0.29, and 0.81 ± 0.31 Hz for before, after CBX, and after washout, respectively, P < 0.01). Complete washout of CBX is implausible with our experimental timeline and 3-D tissue environment (21, 34).
Gap junction protein expression in microtissues containing Gq-activated CFs.
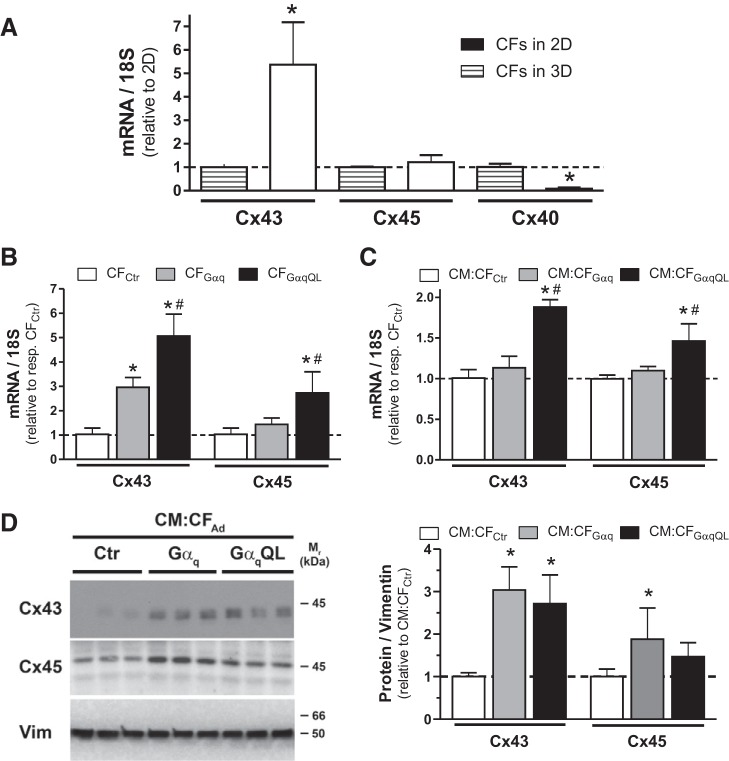
We then examined the expression of gap junction proteins at both mRNA and protein levels. To determine Cx expression changes in CFs on enhanced Gq signaling, we first examined CF microtissues without interspersed CMs. Analysis of Cx expression in CFs cultured in 2-D monolayer versus 3-D culture for 3 days revealed marked isoform-specific differences: Cx43 mRNA expression was 5.4 ± 1.8 times higher in 3-D than 2-D, whereas Cx45 expression was not significantly changed, and Cx40 expression was markedly decreased (by 92%) in 3-D (Fig. 6A). We then compared Cx43 and Cx45 mRNA expression changes in 3-D CFs with enhanced Gq signaling compared with CFCtr microtissues and observed upregulation of both Cx43 and Cx45 mRNAs, which was more pronounced in CF microtissues that expressed GαqQL (Fig. 6B): Cx43 mRNA was increased 2.9 ± 0.4 times with Gαq overexpression and 5.1 ± 0.9 times with GαqQL expression compared with control. Cx45 mRNA expression in CFGαq was only 1.4 ± 0.3 times higher than in CFCtr but significantly increased (2.7 ± 0.9 times) in CFGαqQL. Similar trends in mRNA changes were observed in microtissues composed of interspersed CMs and CFs (Fig. 6C), although it should be noted that baseline Cx expression was much higher in CM:CF microtissues than in CF microtissues (data not shown). Although overall less pronounced than in CF-only microtissues (see Fig. 6B), the relative increase in Cx43 and Cx45 mRNA on Gαq activation in CFs interspersed with CMs was significant in CM:CFGαqQL (Fig. 6C): Cx43 and Cx45 mRNA levels were increased 1.9 ± 0.1 and 1.6 ± 0.3 times compared with CM:CFCtr. Increased Cx43 expression was also observed at the protein level in CM:CFGαq and CM:CFGαqQL compared with CM:CFCtr, whereas upregulation of Cx45 protein reached statistical significance in CM:CFGαq only (Fig. 6D). Taken together, these results point toward a potential role of enhanced CM-CF coupling with enhanced Gq signaling in CF.
Fig. 6.
Gαq activation in cardiac fibroblasts (CFs) increases gap junction protein expression in CFAd and CM:CFAd microtissues. CFs were infected with indicated adenoviruses for 2 h before being seeded in hydrogels alone (CFAd) or together with CMs (CM:CFAd, 1:1). After 3 days in three-dimensional (3-D) culture, microtissues were harvested for mRNA extraction (pooled from 3 hydrogels in 12-well format per condition) and protein extraction (pooled from 3 hydrogels in 6-well format per condition). A: relative mRNA expression of indicated connexin (Cx) isoforms assessed by quantitative PCR (qPCR) analysis of CFs cultured in two-dimensional (2-D) monolayers for 3 days (pooled from 2−3 wells from a 6-well plate) or in 3-D as described. Values are means ± SD; n = 5–6 samples/condition). *P < 0.05 vs. 2-D (Student’s t-test). B and C: relative mRNA expression of Cx43 and Cx45 in CFAd and CM:CFAd assessed qPCR analysis after 3 days in 3-D culture. Values are means ± SD; n = 6 samples/condition. *P < 0.05 vs. control (Ctr); #P < 0.05 vs. Gαq (ANOVA). D: representative Western blots (left) of microtissue lysates after 3 days in 3-D culture (10 µg protein/lane) probed with the indicated antibodies. Vimentin was used as the loading control. Similar results were obtained after 2 days (not shown). Group data (right) are means ± SD; n = 5 samples/condition. *P < 0.05 vs. Ctr (ANOVA).
Resting membrane potential in Gq-activated CFs.
Since the RMP of CFs is an important factor to determine the magnitude of current flow between CMs and CFs, we next measured RMP in Gαq-activated CFs. Because CFs and CMs cannot be distinguished in 3-D CM:CF microtissues, RMPCF recordings were acquired from CF microtissues through insertion of a microelectrode. In CFCtr and CFGαq microtissues, RMP amounted to −33.0 ± 8.4 and −31.9 ± 13.0 mV, respectively, whereas in CFGαqQL microtissues it was depolarized by more than 10 mV to −20.0 ± 7.2 mV (Fig. 7, right). Additional perforated patch-clamp recordings were acquired in CFs in traditional 2-D culture to validate these findings, and we observed comparable results (Fig. 7, left): the RMP was overall slightly lower in CFs cultured in 2-D, but as in 3-D CFGαqQL microtissues the RMP in 2-D CFGαqQL was significantly elevated (−25.8 ± 9.5 mV) compared with 2-D CFCtr (−39.8 ± 6.3 mV). In 2-D CFGαq, RMP was slightly elevated (−29.7 ± 10.0 mV), but this did not reach statistical significance compared with 2-D CFCtr.
Fig. 7.
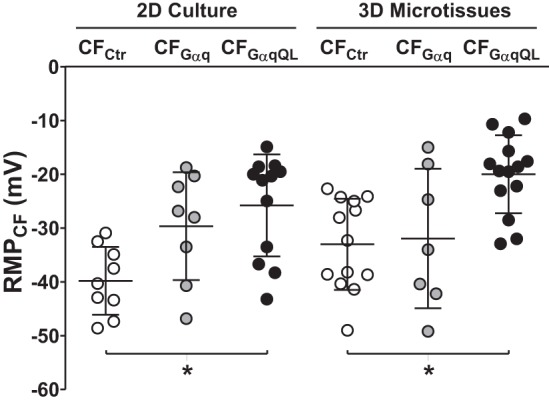
Elevated resting membrane potential (RMP) in GαqQL-activated cardiac fibroblasts (CFs). CFs were infected with the indicated adenoviruses for 2 h before being seeded alone in two-dimensional (2-D) or three-dimensional (3-D) cultures. For electrophysiology experiments, cells were digested after 3 days in 2-D culture and allowed to reattach for 6–12 h before being patch clamped, and microtissues were harvested after 2 days in 3-D culture and allowed to attach to coverslips for 24 h before being probed with a microelectrode. Depicted are resting membrane potentials from individual recordings and group data (means ± SD; n = 8–14 each). *P < 0.05 vs. control (Ctr; ANOVA).
Modeling the effects of gap junctional coupling and RMP on automaticity.
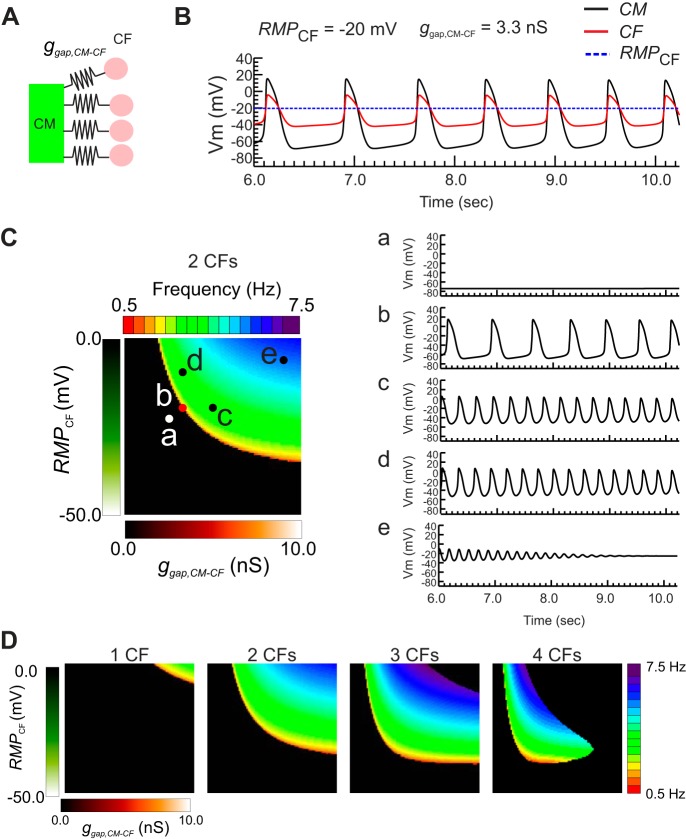
Our experimental results show that Cx43 and Cx45 expression was higher in CFGαqQL and RMPCF of CFGαqQL was more depolarized. We hypothesized that the automaticity in 3-D CM:CFGαqQL microtissues was associated with RMPCF and electrotonic coupling conductance (ggap,CM-CF) between CM and CF through gap junctions. To investigate the conditions of CM-CF coupling promoting automaticity, we used a computational model of CM AP electrically coupled with multiple passive CFs, as shown in Fig. 8A (see materials and methods for detailed model parameters). This computational model replicated the automaticity observed experimentally (Fig. 5). Figure 8B shows Vm traces of a CM (black) and a CF (red) when the CM and CFs were electrically coupled (ggap,CM-CF = 3.3 nS). A map of automaticity frequency when two CFs were connected to the CM is shown in Fig. 8C. Representative Vm traces of the CM from five parameter sets from the frequency map are shown on the right. When CM-CF coupling strength and RMPCF were low, RMPCM remained at −80 mV, and no spontaneous activity occurred (Fig. 8C,a). When RMPCF and CM-CF coupling strength were increased, AP oscillations appeared (Fig. 8C,b). The oscillations accelerated as ggap,CM-CF (Fig. 8C,c) and RMPCF (Fig. 8C,d) increased. Further increase of ggap,CM-CF or RMPCF caused RMPCM to stabilize at close to RMPCF after few oscillations (Fig. 8C,e). Figure 8D shows frequency maps of automaticity when the CM was connected to one to four CFs, which shows that increasing the number of coupled CFs causes automaticity to occur at lower RMPCF and ggap,CM-CF. This agrees with the observation that more frequent automaticity occurred in CM:CFGαq microtissues when the CM:CF ratio increased from 1:1 to 1:2 (Fig. 5B). Thus, our computational results suggest that RMPCF, ggap,CM-CF, and the number of electrically connected CFs are important parameters for the genesis of automaticity in the CM:CF experimental model, but RMPCF is the most sensitive one.
Fig. 8.
Computer modeling study predicts that elevated resting membrane potential of cardiac fibroblasts (RMPCF) and increased cardiac myocyte (CM)-cardiac fibroblast (CF) coupling facilitate automaticity. A: schematics of CM-CF coupling in the computational modeling study. The number of CFs connected to a single CM was increased from n = 1 to n = 4. B: representative membrane potential (Vm) traces from CM (black), CF (red), and reference trace of unconnected CF RMP (dotted blue). Vm of CF also oscillated in parallel with CM through electrotonic current flow between CM and CF through gap junctions. C: map of automaticity frequencies in the parameter space of gap coupling conductance between CM-CF (ggap,CM-CF; x-axis) and RMPCF (y-axis) when n = 2 CFs were connected (left). RMPCF was varied from −50 to 0 mV, and ggap,CM-CF was varied from 0 to 10 nS. Right, representative Vm traces of CM from the locations marked on the frequency map. The characteristics of Vm traces can be categorized into five groups: without automaticity at low RMPCF and/or ggapCM-CF (a), automaticity associated with a slow rise of Vm during the resting period (b), acceleration of automaticity when ggap,CM-CF increases (c), acceleration of automaticity caused by elevation of RMPCF (d), and Vm reaches close to RMPCF with dampening Vm oscillations (e). D: maps of automaticity frequencies in the same parameter space of ggap,CM-CF (x-axis) and RMPCF (y-axis) with one to four CFs coupled to CM. The map for two CFs is the same as in C. Increase of connected CFs promoted automaticity at lower RMPCF and ggap,CM-CF.
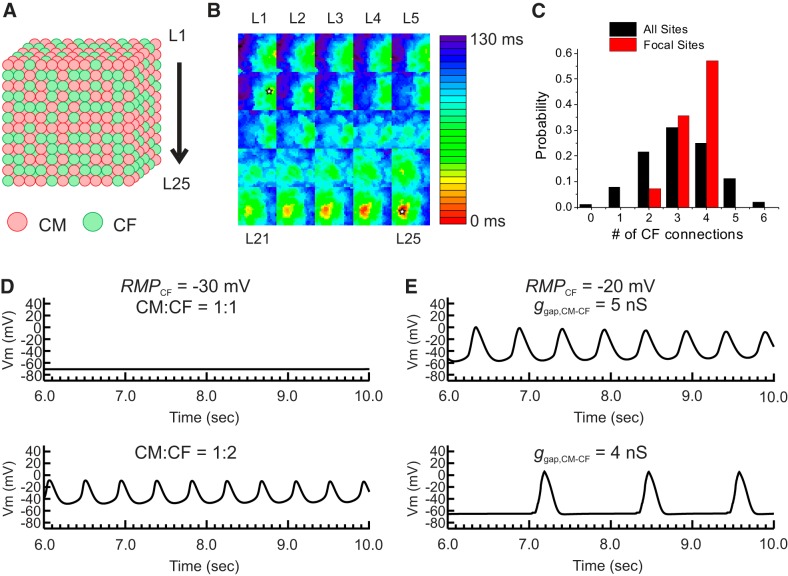
Since cell-cell contact is increased in a 3-D environment through increase of contact surfaces in 3-D, it is possible that automaticity may occur even at lower RMPCF and ggap,CM-CF. We further investigated the 3-D effect on automaticity using a 3-D cube (Fig. 9A). In this configuration, cells were considered as a cube of which each surface connects to a neighboring cell (RMPCF = −20 mV, ggap,CM-CF = 5 nS). Figure 9B shows an example of activation map of automaticity (see Supplemental Movie S2A in the Supplemental Material; Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website). The 3-D cube model demonstrated automaticity originating from the location where a higher number of CFs were present (Fig. 9C). When RMPCF was set at −30 mV and the CM:CF ratio increased to 1:2, automaticity increased (Fig. 9D), which replicates the experimental results we obtained for CM:CFGαq (Fig. 5B). When RMPCF was increased to −20 mV based on our recordings from CFGαqQL microtissues (Fig. 7), automaticity also increased (Fig. 9E, top trace). Reduction of ggap,CM-CF from 5 to 4 nS (Fig. 9E, bottom trace) resulted in a slower oscillation, replicating the lowered frequency (Hz) of automaticity in our experiments of CM:CFGαqQL microtissues after partial gap junction inhibition with CBX (Fig. 5, D and E). These simulation results underscore the importance of the 3-D environment on arrhythmogenic effects of activated CFs by increasing CM:CF connections.
Fig. 9.
Computational modeling study of the three-dimensional (3-D) effect of cardiomyocyte (CM)-cardiac fibroblast (CF) interactions on automaticity. A: schematics of 3-D cube modeling. CMs and CFs were randomly distributed to have 1:1 or 1:2 ratios in a cube of 25 × 2 5 × 25 cells. B: representative activation map of automaticity from the 3-D cube. The activation maps of each layer labeled as L1−L25 in A are shown in 5 × 5 grids, starting the top layer in the top left corner. In this set of CM:CF distribution, automaticity was originated from two sites (open star). C: the number of CFs connected to a single CM in 3-D cube. The parameter set was chosen based on the single CM-CF simulation shown in Fig. 8. The resting membrane potential of a CF (RMPCF) was set to −20 mV and gap junction coupling conductance between CM-CF (ggap,CM-CF) was set to 5 nS. With these parameter settings, automaticity was not observed when n = 1 CF was connected (Fig. 8D). A total of 12 randomly generated CM:CF = 1:1 distributions were tested. The black histogram bars indicate the number of CFs connected to CM from all the CMs in the 3-D cube, which shows that the majority of CMs have connections with three CFs on average. The red histogram bars represent the number of CFs connected only to CMs that initiate automaticity. The initiation sites of automaticity showed a larger number of CFs connected to CMs. D: effect of increased CFs on automaticity. RMPCF was set to −30 mV and showed no automaticity. When the CM:CF ratio was increased from 1:1 to 1:2, automaticity appeared, suggesting that a higher density of CF promotes automaticity as seen experimentally for CM:CFGαq (see Fig. 5B). E: effect of ggap,CM-CF on automaticity. A reduction of ggap,CM-CF from 5 to 4 nS decreased the frequency of automaticity, which replicates the effect of carbenoxolone we observed experimentally in CM:CFGαqQL (see Fig. 5E). Vm, membrane potential.
DISCUSSION
The goal of this study was to investigate the influence of activated CFs on CM electrophysiology in a 3-D microtissue environment. CF activation via GqPCR stimulation commonly occurs in the diseased heart, but the increased abundance of GqPCR agonists (such as ANG II and ET-1) has concomitant effects on CMs. To dissect the effect of enhanced Gq signaling in CFs on CM function in a 3-D microtissue environment, we adenovirally overexpressed the α-subunit of Gq protein (wild-type or a constitutively active Q209L mutant) in CFs before microtissue self-assembly with CMs. We showed that CF-restricted overexpression of GαqQL leads to the expected increase in Gq signaling in microtissues that are viable, slightly enlarged, and show increased expression of profibrotic and prohypertrophic marker genes. Optical mapping demonstrated prolongations of both the durations and rise times of APs and Ca2+ transients during pacing at 1 Hz and increased automaticity in unstimulated microtissues that was mitigated by partial gap junction inhibition. Here, we report an increase in Cx43 and Cx45 expression in GαqQL-activated CFs when seeded alone or together with CMs in 3-D cultures and significant RMP elevation in GαqQL-activated CFs. Computer modeling revealed that RMP elevation in CM:CF microtissues containing GαqQL-activated CFs is a key factor, promoting depolarization-induced automaticity by increasing the current flow from CFs. Computer modeling also revealed that gap junctional conductance between CMs and CFs and the number of electrically connected CFs are important factors for the genesis of automaticity, and these influences underscore the importance of the 3-D environment on arrhythmogenic effects by increasing the connections between CMs and of CFs.
Scaffold-free 3-D microtissues as an experimental model to investigate CM regulation by Gq-activated CFs.
The 3-D tissue-engineered approach used in this study provides several advantages. First, CMs and CFs are highly interspersed in this scaffold-free cardiac microtissue model akin to the native myocardium, as previously reported (17), and the cells can interact with each other through cell-cell, cell-ECM, and cell-paracrine interactions. We used a self-assembly approach rather than a scaffold-based approach because it facilitates this organotypic interspersion (17), with maximized cell-to-cell communication and ECM self-produced by the cells (for a review, see Ref. 1). Second, the CM:CF ratios can be experimentally controlled to recapitulate native ratios. The relative proportion of CMs and CFs varies considerably, depending on the species, developmental and disease states, and methodologies used to identify and count cell numbers that have evolved over time (for reviews, see Refs. 45 and 95). On the basis of an initial report (7) estimating that CFs constitute ~60% of the total cell number in ventricles from adult healthy rats, we primarily focused on 1:1 ratios of CMs and CFs in this study and also used 2:1 ratios when examining automaticity. Third, a large number of individual microtissues can be generated in versatile formats, and they are suitable for molecular and functional investigations. Fourth, cells can be cultured in the absence of unnaturally stiff or adherent substrate. Substrate mechanics are known to influence the morphology and function of cardiac and other cells (18, 82). This is especially true of CFs, which are known to assume an activated MFb phenotype when plated on unnaturally stiff cell culture substrates (for a review, see Ref. 85). Young’s modulus of fibroblast spheroids was shown to be 1.8–3.3 kPa for NIH3T3 cells (37), which much more closely resembles that of native heart tissue [4–500 kPa (for a review, see Ref. 82)] than that of conventional polystyrene, glass, or polydimethylsiloxane [MPa-GPa (56)]. Mechanics along with cell adhesions and diffusible factors constitute the differences between 2-D and 3-D cell culture and native tissue environments (6). In this study, we show that compared with conventional 2-D culture, CFs cultured in self-assembled 3-D microtissues have markedly reduced mRNA levels of αSMA [a contractile intermediate filament-associated protein commonly used as a marker to identify activated MFbs (30)], CTGF [a matricellular protein and well-characterized mediator of fibrotic response (49)], and FN1 (a glycoprotein of the ECM synthetized by fibroblasts), whereas the expression of vimentin was comparable (Fig. 3A). These data indicate that the CFs present in microtissues have lower baseline activation than those on stiff 2-D substrates, which have been mostly used to investigate the effect of CFs on CM electrical properties to date. Marked differences in gene and protein expression between 2-D monolayer and 3-D spheroid cultures have been reported for tumor and nontumor cells (22, 77), but to our knowledge this has not yet been systemically examined for fibroblasts of cardiac or other origin.
Since CFs express several different GqPCRs including ANG II type 1 (88) and ET-1 (28, 40) receptors, we overexpressed Gαq protein as the common mediator of their effects to mimic the resulting increased in Gq-mediated signal transduction. Consistent with previous reports in cell lines [COS-1 cells (69)], neonatal rat CMs (2), and transgenic mice with CM-restricted Gαq overexpression (14, 59), GαqQL overexpression in CFs induced a more pronounced increase in PLC-β activity compared with wild-type Gαq overexpression (Fig. 1C), despite much lower amounts of protein expression at comparable infection conditions (Fig. 1B), because the Q209L point mutation renders the protein constitutively active. Importantly, we provide evidence for the efficacy of adenovirus-mediated gene transfer in CFs (Fig. 1A) and CF-restricted adenoviral infection in our mixed CM:CF microtissues (Fig. 1D), consistent with previous reports (93, 17).
As expected, overexpression of Gαq or the more active mutant GαqQL increased the activity of the direct canonical downstream target PLC-β in microtissues composed of adenovirus-infected CFs only (CFAd; Fig. 2A). In the presence of CMs (CM:CFAd), PLC-β was markedly increased compared with controls as well, but the effect was less pronounced when CFs were infected with Ad-GαqQL than Ad-Gαq, reflecting a differential contribution from the CMs. Enhanced Gq signaling did not alter microtissue viability (Fig. 2B), gross morphology (Fig. 2C), or Sirius Red-based collagen staining (Fig. 2D), but we observed a modest increase in CM:CF microtissue area on CF Gq activation that was significant for GαqQL (Fig. 2E). This could be due to changes in cell sizes and/or ECM deposition but needs to be interpreted with caution, because both packing density and the size of CFs or CMs are difficult to assess in self-assembled interspersed microtissues.
We substantiated that enhanced Gq signaling in CFs leads to CF activation by examining the expression of well-known MFb and profibrotic marker genes in both CFAd (Fig. 3B) or CM:CFAd (Fig. 3, C and D) microtissues. In contrast to vimentin, αSMA, CTGF, and FN1 were markedly increased when CFs were infected with Ad-Gαq or Ad-GαqQL. However, the regulation of their expression in cardiac microtissues appears to be complex. First, Gαq-induced upregulation of CTGF mRNA was more pronounced for GαqQL versus Gαq in the presence of CMs (CM:CFAd; Fig. 3C) but comparable in their absence (CFAd, Fig. 3B), suggesting CTGF expression in CMs in response to CF-restricted Gαq activation. CTGF is an early profibrotic marker whose upregulation precedes collagen deposition (75), and its expression in response to TGF-β and other stimuli has been reported not only in CFs but also CMs (11, 43). Second, a comparable increase in FN protein levels in CM:CFAd microtissues containing Gαq- or GαqQL-activated CFs (Fig. 3D), despite a graded increase in FN1 mRNA expression (Fig. 3C), points toward posttranscriptional regulation. Finally, similarly, αSMA protein expression was upregulated in CM:CF microtissues containing Gαq- and GαqQL-activated CFs (Fig. 3D), but αSMA mRNA was only increased when the CFs were activated by Gαq (but not GαqQL), in both the presence (CM:CF microtissues, Fig. 3C) or absence of CMs (CF microtissues, Fig. 3B). Although αSMA-positive CFs are generally referred to as MFbs (57), a recent single cell expression study (41) showed a lower correlation of αSMA expression to the activated phenotype than previously assumed.
Taken together, CF-restricted enhancement of Gq signaling before their assembly in microtissues alone or in combination with CMs resulted in viable microtissues with increased early profibrotic marker gene expression after 3 days. The additional increase in ANF mRNA expression (Fig. 3E) is consistent with well-established paracrine effects of activated CFs on CM hypertrophy (e.g., Refs. 27, 51, and 67).
Gq-activated CFs prolong CM APs and Ca2+ transients.
We previously showed that the APD in our self-assembled cardiac microtissues was overall comparable to other models and that the inclusion of CFs increases APD compared with microtissues composed of only CMs (17). In this study, we show that GαqQL-activated CFs further prolong APD compared with control CFs (Fig. 4B). This finding is consistent with APD prolongation reported in neonatal rat ventricular CM monolayers cocultured in a 1:1 ratio with CFs that had been precultured to assume a MFb phenotype (5). However, a reduction in APD can also occur depending on RMPCF, CF density, and degree of coupling to CMs. For example, this was shown for neonatal rat CM monolayer cocultures with activated CFs obtained from infarcted compared with healthy hearts (87). The effect of CF coupling on APD can be bimodal, potentially due to the impact on activation of L-type Ca2+ current as well as repolarization. Computer modeling (90) suggested that increase of CFs coupled with CMs maintains the plateau Vm negative, which, in turn, increases driving force of L-type Ca2+ channels and increases APD slightly. However, increase of coupling with CF can suppress the AP plateau and accelerate AP repolarization, shortening APD (53). Computer modeling also predicts that APD is prolonged when RMPCF is less negative, provided that a sufficient number of CFs are present and the coupling is strong enough (32). This seems to be the case in CM:CFGαqQL microtissues, as RMPCF was indeed less negative (Fig. 7), and increased coupling was suggested by increased Cx43 expression (Fig. 6) and the effectiveness of partial gap junction inhibition (Fig. 5). Paracrine effects may contribute to the APD prolongation observed in CM:CFGαqQL microtissues, as previously shown for CMs in 2-D culture when they were exposed to conditioned medium from CFs in the absence of direct cell-cell contact (42, 67).
AP rise time also increased in microtissues with Gαq- or GαqQL-activated CFs (Fig. 4B). The slow rise of AP (slow AP upstroke velocity) associated with slow conduction has been observed in 2-D culture (5, 31, 60) as well as in computer modeling studies (8, 32, 33, 76) and was attributed to the dissipation of depolarizing currents to the neighboring CFs via a source-sink relationship. The slow AP upstroke could reduce conduction velocity and promote conduction blocks as seen in 2-D culture. Since the size of CM:CF microtissues are relatively small within 150 μm diameter, it was difficult to measure the impact of CF activation on conduction.
The activation of CFs promoted significant changes in CM Ca2+ transients (Fig. 4, C and D), as both rise time and duration were prolonged along with APD prolongation. Although we did not quantify the shape of Ca2+ transients, the increase of rise time was associated with an altered peak: CM:CFGαqQL microtissues tended to show a two-step Ca2+ rise with a rapid rise phase during the initial rise of Ca2+ transients and a slow secondary rise during the peak (Fig. 4C), which contributes to the increase of Ca2+ transient rise time. The mechanisms underlying the changes in [Ca2+]i kinetics are not clear. Although slowing of the Ca2+ transient upstroke can be explained by slow AP upstroke through electrotonic CM-CF coupling, Ca2+ transient prolongation is difficult to explain solely by CM-CF coupling since [Ca2+]i recovery is much slower than AP repolarization (Fig. 4, B and D). Further studies are needed to examine potential paracrine effects from CFs on Ca2+ transients in CMs.
Gq-activated CFs promote automaticity in CMs.
Abnormal electrical activity including spontaneous ectopic activity and automaticity has been frequently observed and associated with induction of atrial and ventricular arrhythmias in various cardiac diseases, such as ischemia-reperfusion, myocardial infarction, and heart failure (4, 20, 35, 63). The cause of ectopic activity has been conventionally linked to the excessive inward current during the diastole, which causes slow depolarization leading to the triggering of APs. These extra APs increase risks for ventricular tachyarrhythmias, if they propagate and form reentry. Increasing evidence supports potential roles of activated CFs in generating abnormal automaticity. For example, using micropatterned strands of neonatal rat ventricular CMs coated with CFs that were activated by preculture on a stiff substrate and referred to as MFbs based on αSMA expression, Miragoli et al. showed MFb-induced and density-dependent synchronized spontaneous activity based on depolarization-induced abnormal automaticity (ectopic activity) (61). Similarly, another group showed a marked increase in ectopic activity in conventional 2-D monolayers of neonatal rat ventricular CMs when they were cocultured with similarly preactivated MFbs (5). Involvement of gap junctional coupling was suggested in this study by a reduction in ectopic activity when Cx43 was downregulated in MFbs via RNA inhibition.
In the present study, we examined the role of activated CFs on automaticity in a 3-D environment, both experimentally and via computer simulation, and used a more physiological mode of CF activation. We showed that CF activation by enhanced Gαq signaling markedly induces automaticity in CM:CF microtissues and that this effect was more pronounced when the number of CFs was increased and when Gαq was constitutively activated (Fig. 5, A and B). Partial gap junction blockade had an inhibitory effect on the occurrence and frequency of automaticity (Fig. 5, C–E), which together with the increased Cx expression (Fig. 6; discussed below) points toward the possibility of enhanced gap junctional coupling between Gαq-activated CFs and CMs. Our computer simulations indeed supported the notion that gap junctional coupling (Figs. 8C and 9E) and the number of electrically connected CFs (Fig. 8D and Fig. 9, C and D) are important factors for the generation of automaticity in the CM:CF microtissue model. Other mechanisms of hetercellular interactions, such as paracrine effects (67) and nanotubes (29, 71), may contribute as well.
Gq-activated CFs and the 3-D environment increase Cx expression.
Cell-cell connectivity can be affected by the presence, expression pattern, and functionality of the Cx family of proteins, the building units of functional gap junctions (36). For CFs that were cultured in microtissue or 2-D culture for the same duration, we showed pronounced and isoform-specific differences in mRNA expression for Cx43 [>5-fold upregulated, consistent with Ref. 9, Cx45 (unchanged), and Cx40 (downregulated by 92%); Fig. 6A]. This observation is another example of the marked expression differences between 2-D monolayer and 3-D microtissue cultures that were discussed above. Further investigation of Cx expression in CF microtissues showed up to 5-fold upregulation of Cx43 mRNA in activated CFs and a 2.5-fold increase in Cx45 mRNA in CFGαqQL (Fig. 6B). Cx upregulation also occurs in CFs activated in vivo postmyocardial infarct, as shown in situ for both Cx43 and Cx45 in a sheep coronary occlusion model (10) and in CFs isolated from the infarct scar of mice (94). In microtissues containing both CMs and CFs activated by Gαq and/or GαqQL, Cx43 and Cx45 upregulation was also observed, with some differences between mRNA and protein expression levels (Fig. 6, C and D). Importantly, Cx expression per se is not an indication for functional gap junction channels, because the oligomerization of Cxs into hemichannels (connexons), the delivery of connexons to the plasma membrane, and the formation of gap junction and their stability and function are complex processes that are subject to various posttranslational modifications and other regulatory processes (83).
In 2-D culture, it has been well established that Cx proteins exist at the sites of heterocellular contact between CMs and CFs (12, 23, 24, 60, 74), and some studies have shown functional coupling (73, 87). However, whether or not gap junctional coupling occurs in the 3-D tissue context and how this may be altered under pathological stimulation and in disease has long been a subject of investigation and debate (for reviews, see Refs. 47, 55, and 64). Strong evidence in support of functional CM-CF coupling in vivo was only recently reported when it was demonstrated that nonexcitable cells in the heart can closely follow CM APs. To that end, expression of a voltage-sensitive fluorescent protein in non-CMs was used to record AP-like signals in cryoinjured scar tissue (71), and current injected into the healthy myocardium could be recorded in adjacent scar tissue, but no signals were recorded when Cx43 was knocked down in non-CMs (54).
Enhanced coupling between CMs and CFs can influence AP characteristics of CMs in several ways depending on RMPCF: 1) by providing inward currents during the diastolic period to facilitate depolarization of the transmembrane potential of CMs, 2) by providing outward currents during the upstroke and plateau phases of the AP, and 3) by delaying AP repolarization during the late phase of repolarization (for a review, see Ref. 92). Given the challenges in relating Cx expression to connexon formation and gap junction functionality, we used computer modeling to assess the potential impact of functional gap junctional coupling between CM and Gq-activated CFs in our microtissues and were able to replicate key features of the propensity for automaticity in CM:CFGαqQL microtissues (see above). Our simulations suggests that with coupling above a threshold, automaticity is mostly dependent on the electrophysiological properties of CFs, such as overall ionic conductance, capacitance, and RMPCF (see below). They also emphasize the importance of a 3-D environment where cell-cell contact increases in all directions. In 3-D, the probability of a number of CFs connecting to CMs is higher because of potential contact over their entire cell surface areas compared with limited side-to-side contacts in 2-D. Despite similar trends in RMPCF between 2-D and 3-D cultures, the efficacy of triggering automaticity is increased in 3-D simulation, underscoring the influence of CM-CF coupling in a 3-D environment.
Although computer modeling in this study mainly focused on CM-CF coupling, CM-CM or CF-CF coupling may also modulate automaticity. A previous computer modeling study (91) showed that enhancing CM-CM coupling suppressed triggered activity through the source-sink effect, i.e., an enhanced CM-CM coupling strength potentiates the sink effect, making spontaneous depolarization more difficult. Therefore, the increase of CM-CM coupling in 3-D microtissues should have reduced automaticity, but our experimental results showed increased automaticity in GαqQL-activated microtissues. However, it is also possible that CM-CM coupling can increase automaticity via synchronization of automaticity of CMs, as seen in sinoatrial pacemaker. Further studies are needed to delineate homo/heterocellular coupling of CMs and CFs in modulating automaticity.
Elevated RMP in Gq-activated CFs.
RMPCF is an important factor because the difference between Vm,CM and Vm,CF determines the driving force of electrotonic current flow between CMs and CFs, and the importance of RMPCF elevation on triggered activity has been also shown in computer simulations of CM-CF interactions (96). Since fluorescence recordings by optical mapping do not provide the absolute Vm, we used high-resistance microelectrodes to determine the RMPCF in 2-D culture or 3-D microtissues. Consistent with previous reports in neonatal (26, 60, 74) and adult ventricular CFs (13), the RMP of control CFs (Fig. 7) was much less negative compared with CMs. Importantly, RMP in CFs infected with Ad-GαqQL was markedly elevated (by 13–14 mV) in both 2-D and 3-D culture conditions (Fig. 7). RMP recorded in CFGαqQL microtissues (−20.0 ± 7.2 mV) was comparable to that reported for neonatal rat ventricular CFs that were activated by 8- to 9-day culture on rigid 2-D substrates [−20.4 + 0.4 mV (60) and −17 + 6 mV (5)]. Therefore, our present study showed that a biological trigger common to the diseased heart (i.e., enhanced Gq signaling) is sufficient to elevate RMPCF and can do so in a soft microtissue context.
CFs express multiple ion channels (50), with heterogeneity reported depending on the cardiac region (chamber), activation (disease) state, and recording conditions (acutely isolated or cultured) (for reviews, see Refs. 55 and 86). The ionic mechanisms determining RMPCF are still poorly understood. Indepth electrophysiological studies will be needed to delineate the determinants of the RMPCF under baseline conditions and the mechanism underlying RMP depolarization in activated CFs, which may depend on the nature of the stimulus. Mechanical strain also influences RMPCF (26, 38, 48), which needs to be considered when interpreting RMPCF values obtained in freshly isolated, cultured CFs and those in microtissue context.
Potential relevance and limitations.
Establishing an active role for CFs in regulating CM electrical function in the intact heart has been a formidable challenge because of the intricate interspersion of the two cell types [virtually every CM borders one or more CFs (46)] and exposure to the same environment, so that the contribution of individual cell types to integrated tissue function cannot be easily determined. Previous investigations of the effect of activated CFs on CM electrical activity in vitro were mainly performed on rigid 2-D substrates that can mechanically activate CFs. The present study extended this question to a 3-D microtissue environment that is associated with a lower baseline activation state of the CFs and enables 3-D cell-cell, paracrine, and cell-ECM interactions as present in the cardiac tissue. Using this model, we present experimental and modeling evidence suggesting that Gq-activated CFs can contribute to arrhythmogenesis by direct electrotonic modulation of CMs and highlighting the importance of the 3-D environment on arrythmogenic effects.
A limitation of our experimental model is that Gαq activation in CFs is initiated shortly after self-assembly of the bioengineered microtissues and not within already established tissue as is the case in the intact diseased heart. The present study also used a simplified CM AP model and passive CF computational model. Although IK1 conductance was adjusted to the previous voltage-clamp data in the same 3-D microtissue construct, this model does not incorporate potential electrical remodeling caused by paracrine effects from Gq-activated activated CFs. The CF model in this study is also limited without incorporation of ionic currents such as Clcn-3 and Kir2.1. Further studies are needed to understand how potential CM remodeling and homo-/heterocellular couplings contribute to depolarization-induced abnormal automaticity. Transgenic mice with CF-restricted Gαq expression will be required to extrapolate our findings from the in vitro microtissue environment to the in vivo setting, along with characterizations of activated CFs in diseased hearts with regard to their size, their relative density and localization, their changes in Gq signaling, their cellular electrophysiology and ability to form homocellular and heterocellular gap junctions, and the resulting effect on the integrated function of the ventricular myocardium. There is growing interest to modulate CF activation to mitigate fibrosis (25). Our results demonstrate that CF activation in response to enhanced Gq signaling, a common pathophysiological trigger in the diseased heart, can greatly influence AP and Ca2+ handling of CMs, leading to arrhythmogenic ectopic activity, suggesting potential antiarrhythmic benefits of targeting CFs in cardiac disease.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute (NHLBI) Postdoctoral Fellowships T32-HL-094300 and 1-F32-HL-126311 and American Heart Association Grant 15POST22740017 (to C.M. Kofron) and by NHLBI Grants R21-HL-1139181 and R01-HL-114784 (to U. Mende).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M.K., T.Y.K., B.-R.C., and U.M. conceived and designed research; C.M.K., T.Y.K., M.E.K., A.X., F.F., and E.P. performed experiments; C.M.K., T.Y.K., M.E.K., A.X., B.-R.C., and U.M. analyzed data; C.M.K., T.Y.K., M.E.K., A.X., Z.Q., B.-R.C., and U.M. interpreted results of experiments; C.M.K., T.Y.K., M.E.K., B.-R.C., and U.M. prepared figures; C.M.K., T.Y.K., B.-R.C., and U.M. drafted manuscript; C.M.K., T.Y.K., M.E.K., A.X., Z.Q., B.-R.C., and U.M. edited and revised manuscript; C.M.K., T.Y.K., M.E.K., A.X., Z.Q., B.-R.C., and U.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the use of Applied Biosystems real-time PCR systems in the Genomics Core Facility of Brown University (funded by the National Institutes of Health, National Science Foundation, Lifespan Rhode Island Hospital, and Division of Biology and Medicine, Brown University). We thank Bethany Desroches for contributions to the experiment with CellTracker-labeled cardiomyocytes and the Core Research Laboratories at Lifespan for confocal image acquisition.
REFERENCES
- 1.Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 12: 1347–1360, 2012. doi: 10.1517/14712598.2012.707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW 2nd. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 95: 10140–10145, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Müller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635, 2014. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 4.Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin 3: 23–45, 2011. doi: 10.1016/j.ccep.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askar SF, Bingen BO, Swildens J, Ypey DL, van der Laarse A, Atsma DE, Zeppenfeld K, Schalij MJ, de Vries AA, Pijnappels DA. Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: role of maximal diastolic potential. Cardiovasc Res 93: 434–444, 2012. doi: 10.1093/cvr/cvr351. [DOI] [PubMed] [Google Scholar]
- 6.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125: 3015–3024, 2012. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293: H1883–H1891, 2007. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 8.Brown TR, Krogh-Madsen T, Christini DJ. Illuminating myocyte-fibroblast homotypic and heterotypic gap junction dynamics using dynamic clamp. Biophys J 111: 785–797, 2016. doi: 10.1016/j.bpj.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng 9: 1243–1253, 2003. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 10.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-β in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32: 1805–1819, 2000. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 12.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol 583: 225–236, 2007. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol 288: H2931–H2939, 2005. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW 2nd. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA 94: 8121–8126, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70: 9–18, 2014. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vivo M, Chen J, Codina J, Iyengar R. Enhanced phospholipase C stimulation and transformation in NIH-3T3 cells expressing Q209LGq-alpha-subunits. J Biol Chem 267: 18263–18266, 1992. [PubMed] [Google Scholar]
- 17.Desroches BR, Zhang P, Choi BR, King ME, Maldonado AE, Li W, Rago A, Liu G, Nath N, Hartmann KM, Yang B, Koren G, Morgan JR, Mende U. Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells. Am J Physiol Heart Circ Physiol 302: H2031–H2042, 2012. doi: 10.1152/ajpheart.00743.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 19.Dostal D, Glaser S, Baudino TA. Cardiac fibroblast physiology and pathology. Compr Physiol 5: 887–909, 2015. doi: 10.1002/cphy.c140053. [DOI] [PubMed] [Google Scholar]
- 20.Ducceschi V, Di Micco G, Sarubbi B, Russo B, Santangelo L, Iacono A. Ionic mechanisms of ischemia-related ventricular arrhythmias. Clin Cardiol 19: 325–331, 1996. doi: 10.1002/clc.4960190409. [DOI] [PubMed] [Google Scholar]
- 21.Elsen FP, Shields EJ, Roe MT, Vandam RJ, Kelty JD. Carbenoxolone induced depression of rhythmogenesis in the pre-Bötzinger Complex. BMC Neurosci 9: 46, 2008. doi: 10.1186/1471-2202-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaedtke L, Thoenes L, Culmsee C, Mayer B, Wagner E. Proteomic analysis reveals differences in protein expression in spheroid versus monolayer cultures of low-passage colon carcinoma cells. J Proteome Res 6: 4111–4118, 2007. doi: 10.1021/pr0700596. [DOI] [PubMed] [Google Scholar]
- 23.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 93: 421–428, 2003. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 24.Goshima K. Formation of nexuses and electrotonic transmission between myocardial and FL cells in monolayer culture. Exp Cell Res 63: 124–130, 1970. doi: 10.1016/0014-4827(70)90339-3. [DOI] [PubMed] [Google Scholar]
- 25.Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 15: 620–638, 2016. doi: 10.1038/nrd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grand T, Salvarani N, Jousset F, Rohr S. Aggravation of cardiac myofibroblast arrhythmogeneicity by mechanical stress. Cardiovasc Res 104: 489–500, 2014. doi: 10.1093/cvr/cvu227. [DOI] [PubMed] [Google Scholar]
- 27.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-β1 and endothelin-1 from fibroblasts. Cardiovasc Res 40: 352–363, 1998. doi: 10.1016/S0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 28.Hafizi S, Wharton J, Chester AH, Yacoub MH. Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem 14: 285–292, 2004. doi: 10.1159/000080338. [DOI] [PubMed] [Google Scholar]
- 29.He K, Shi X, Zhang X, Dang S, Ma X, Liu F, Xu M, Lv Z, Han D, Fang X, Zhang Y. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res 92: 39–47, 2011. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 30.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou L, Hu B, Jalife J. Genetically engineered excitable cardiac myofibroblasts coupled to cardiomyocytes rescue normal propagation and reduce arrhythmia complexity in heterocellular monolayers. PLoS One 8: e55400, 2013. doi: 10.1371/journal.pone.0055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008. doi: 10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacquemet V, Henriquez CS. Modelling cardiac fibroblasts: interactions with myocytes and their impact on impulse propagation. Europace 9, Suppl 6: vi29–vi37, 2007. doi: 10.1093/europace/eum207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahromi SS, Wentlandt K, Piran S, Carlen PL. Anticonvulsant actions of gap junctional blockers in an in vitro seizure model. J Neurophysiol 88: 1893–1902, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Janse MJ. Electrophysiology of arrhythmias. Arch Mal Coeur Vaiss 92: 9–16, 1999. [PubMed] [Google Scholar]
- 36.Jansen JA, van Veen TA, de Bakker JM, van Rijen HV. Cardiac connexins and impulse propagation. J Mol Cell Cardiol 48: 76–82, 2010. doi: 10.1016/j.yjmcc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, Tschumperlin DJ. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol 312: C277–C285, 2017. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res 57: 793–803, 2003. doi: 10.1016/S0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 39.Kamkin A, Kiseleva I, Wagner KD, Pylaev A, Leiterer KP, Theres H, Scholz H, Günther J, Isenberg G. A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol Heart Circ Physiol 282: H842–H849, 2002. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- 40.Katwa LC, Guarda E, Weber KT. Endothelin receptors in cultured adult rat cardiac fibroblasts. Cardiovasc Res 27: 2125–2129, 1993. doi: 10.1093/cvr/27.12.2125. [DOI] [PubMed] [Google Scholar]
- 41.Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, Poetsch A, Hoelper S, Conway SJ, Möllmann H, Looso M, Troidl C, Offermanns S, Wettschureck N. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res 118: 1906–1917, 2016. doi: 10.1161/CIRCRESAHA.116.308643. [DOI] [PubMed] [Google Scholar]
- 42.Kaur K, Zarzoso M, Ponce-Balbuena D, Guerrero-Serna G, Hou L, Musa H, Jalife J. TGF-β1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS One 8: e55391, 2013. doi: 10.1371/journal.pone.0055391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol 37: 603–606, 2004. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, Wagner KD, Persson PB, Günther J. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J Mol Cell Cardiol 30: 1083–1093, 1998. doi: 10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 45.Kofron CM, Mende U. In vitro models of the cardiac microenvironment to study myocyte and non-myocyte crosstalk: bioinspired approaches beyond the polystyrene dish. J Physiol 595: 3891–3905, 2017. doi: 10.1113/JP273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol 38, Suppl: 45–50, 2005. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 47.Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol 70: 37–46, 2014. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79: 943–956, 1994. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 49.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 116: 1269–1276, 2015. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 50.Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One 4: e7307, 2009. doi: 10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long CS, Henrich CJ, Simpson PC. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul 2: 1081–1095, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res 68: 1501–1526, 1991. doi: 10.1161/01.RES.68.6.1501. [DOI] [PubMed] [Google Scholar]
- 53.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 92: 4121–4132, 2007. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahoney VM, Mezzano V, Mirams GR, Maass K, Li Z, Cerrone M, Vasquez C, Bapat A, Delmar M, Morley GE. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci Rep 6: 26744, 2016. doi: 10.1038/srep26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahoney VM, Mezzano V, Morley GE. A review of the literature on cardiac electrical activity between fibroblasts and myocytes. Prog Biophys Mol Biol 120: 128–133, 2016. doi: 10.1016/j.pbiomolbio.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices 7: 281–293, 2005. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 57.Matthijs Blankesteijn W. Has the search for a marker of activated fibroblasts finally come to an end? J Mol Cell Cardiol 88: 120–123, 2015. doi: 10.1016/j.yjmcc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 58.McSpadden LC, Nguyen H, Bursac N. Size and ionic currents of unexcitable cells coupled to cardiomyocytes distinctly modulate cardiac action potential shape and pacemaking activity in micropatterned cell pairs. Circ Arrhythm Electrophysiol 5: 821–830, 2012. doi: 10.1161/CIRCEP.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95: 13893–13898, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 61.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 101: 755–758, 2007. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 62.Nayak AR, Shajahan TK, Panfilov AV, Pandit R. Spiral-wave dynamics in a mathematical model of human ventricular tissue with myocytes and fibroblasts. PLoS One 8: e72950, 2013. doi: 10.1371/journal.pone.0072950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nuss HB, Kääb S, Kass DA, Tomaselli GF, Marbán E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol 277: H80–H91, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Ongstad E, Kohl P. Fibroblast-myocyte coupling in the heart: potential relevance for therapeutic interventions. J Mol Cell Cardiol 91: 238–246, 2016. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8: 839–845, 2007. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 66.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol 295: H390–H400, 2008. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res 83: 688–697, 2009. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pijnappels DA, Schalij MJ, van Tuyn J, Ypey DL, de Vries AA, van der Wall EE, van der Laarse A, Atsma DE. Progressive increase in conduction velocity across human mesenchymal stem cells is mediated by enhanced electrical coupling. Cardiovasc Res 72: 282–291, 2006. doi: 10.1016/j.cardiores.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Qian NX, Winitz S, Johnson GL. Epitope-tagged Gq alpha subunits: expression of GTPase-deficient alpha subunits persistently stimulates phosphatidylinositol-specific phospholipase C but not mitogen-activated protein kinase activity regulated by the M1 muscarinic acetylcholine receptor. Proc Natl Acad Sci USA 90: 4077–4081, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu Z, Nivala M, Weiss JN. Calcium alternans in cardiac myocytes: order from disorder. J Mol Cell Cardiol 58: 100–109, 2013. doi: 10.1016/j.yjmcc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O’Toole ET, Knöpfel T, Kohl P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc Natl Acad Sci USA 113: 14852–14857, 2016. doi: 10.1073/pnas.1611184114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohr S. Arrhythmogenic implications of fibroblast-myocyte interactions. Circ Arrhythm Electrophysiol 5: 442–452, 2012. doi: 10.1161/CIRCEP.110.957647. [DOI] [PubMed] [Google Scholar]
- 73.Rook MB, Jongsma HJ, de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch 414: 95–98, 1989. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- 74.Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol 263: C959–C977, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Rosin NL, Falkenham A, Sopel MJ, Lee TD, Légaré JF. Regulation and role of connective tissue growth factor in AngII-induced myocardial fibrosis. Am J Pathol 182: 714–726, 2013. doi: 10.1016/j.ajpath.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Sachse FB, Moreno AP, Seemann G, Abildskov JA. A model of electrical conduction in cardiac tissue including fibroblasts. Ann Biomed Eng 37: 874–889, 2009. doi: 10.1007/s10439-009-9667-4. [DOI] [PubMed] [Google Scholar]
- 77.Sakai Y, Yamagami S, Nakazawa K. Comparative analysis of gene expression in rat liver tissue and monolayer- and spheroid-cultured hepatocytes. Cells Tissues Organs 191: 281–288, 2010. doi: 10.1159/000272316. [DOI] [PubMed] [Google Scholar]
- 78.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 79.Shibukawa Y, Chilton EL, Maccannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J 88: 3924–3935, 2005. doi: 10.1529/biophysj.104.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sridhar S, Vandersickel N, Panfilov AV. Effect of myocyte-fibroblast coupling on the onset of pathological dynamics in a model of ventricular tissue. Sci Rep 7: 40985, 2017. doi: 10.1038/srep40985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strom M, Wan X, Poelzing S, Ficker E, Rosenbaum DS. Gap junction heterogeneity as mechanism for electrophysiologically distinct properties across the ventricular wall. Am J Physiol Heart Circ Physiol 298: H787–H794, 2010. doi: 10.1152/ajpheart.00887.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tallawi M, Rai R, Boccaccini AR, Aifantis KE. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng Part B Rev 21: 157–165, 2015. doi: 10.1089/ten.teb.2014.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thévenin AF, Kowal TJ, Fong JT, Kells RM, Fisher CG, Falk MM. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology (Bethesda) 28: 93–116, 2013. doi: 10.1152/physiol.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation 123: 2083–2093, 2011. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol 93: 133–142, 2016. doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 86.Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol 57: 380–388, 2011. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res 107: 1011–1020, 2010. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation 88: 2849–2861, 1993. doi: 10.1161/01.CIR.88.6.2849. [DOI] [PubMed] [Google Scholar]
- 89.Xie Y, Garfinkel A, Camelliti P, Kohl P, Weiss JN, Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: A computational study. Heart Rhythm 6: 1641–1649, 2009. doi: 10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Y, Garfinkel A, Weiss JN, Qu Z. Cardiac alternans induced by fibroblast-myocyte coupling: mechanistic insights from computational models. Am J Physiol Heart Circ Physiol 297: H775–H784, 2009. doi: 10.1152/ajpheart.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J 99: 1408–1415, 2010. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 89: 744–753, 2011. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol 301: H147–H156, 2011. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol 19: e233–e240, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou P, Pu WT. Recounting cardiac cellular composition. Circ Res 118: 368–370, 2016. doi: 10.1161/CIRCRESAHA.116.308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zimik S, Nayak AR, Pandit R. A computational study of the factors influencing the PVC-triggering ability of a cluster of early afterdepolarization-capable myocytes. PLoS One 10: e0144979, 2015. doi: 10.1371/journal.pone.0144979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.