Abstract
Hypertension is a prevalent pathology that increases risk for numerous cardiovascular diseases. Because the etiology of hypertension varies across patients, specific and effective therapeutic approaches are needed. The role of renal sympathetic nerves is established in numerous forms of hypertension, but their contribution to salt sensitivity and interaction with factors such as endothelin-1 are poorly understood. Rats deficient of functional ETB receptors (ETB-def) on all tissues except sympathetic nerves are hypertensive and exhibit salt-sensitive increases in blood pressure. We hypothesized that renal sympathetic nerves contribute to hypertension and salt sensitivity in ETB-def rats. The hypothesis was tested through bilateral renal sympathetic nerve denervation and measuring blood pressure during normal salt (0.49% NaCl) and high-salt (4.0% NaCl) diets. Denervation reduced mean arterial pressure in ETB-def rats compared with sham-operated controls by 12 ± 3 (SE) mmHg; however, denervation did not affect the increase in blood pressure after 2 wk of high-salt diet (+19 ± 3 vs. +16 ± 3 mmHg relative to normal salt diet; denervated vs. sham, respectively). Denervation reduced cardiac sympathetic-to-parasympathetic tone [low frequency-high frequency (LF/HF)] during normal salt diet and vasomotor LF/HF tone during high-salt diet in ETB-def rats. We conclude that the renal sympathetic nerves contribute to the hypertension but not to salt sensitivity of ETB-def rats.
Keywords: sympathetic nerves, autonomics, circadian, endothelin B
hypertension is the most prominent cardiovascular disorder and a well-established risk factor for the development of further cardiovascular complications (43a). Many manifestations of hypertension are associated with increased sympathetic nervous system tone (18, 27, 53, 63) and dietary salt sensitivity (28). Although the associations between salt intake, sympathetic tone, and hypertension are well established (10, 34, 53, 59, 62), the precise mechanisms contributing to these correlations remain poorly understood, resulting in less effective therapeutic approaches.
One such therapy, renal sympathetic denervation, has had a tumultuous road of application. Early clinical trials using radio frequency ablation of the renal sympathetic nerves provided encouraging results for the utility of denervation in resistant hypertensive patients (16, 17, 17a, 32). More recently, however, a randomized, double-blinded, sham-controlled study failed to demonstrate the efficacy of the technique relative to sham procedure (4, 9). Subsequent discussion has examined potential explanations for the failure of this particular trial along with the applicability of the procedure in treating various forms of hypertension (3, 15, 26, 40, 52). Although the fate of renal sympathetic denervation in clinical application is currently uncertain, a large number of preclinical studies demonstrate an important role for renal sympathetic nerves in mediating hypertension in many experimental models. For instance, renal denervation attenuates established Dahl salt-sensitive hypertension (19) but does not affect the development of Dahl salt-sensitive hypertension (45). Selective afferent renal sympathetic denervation attenuates mineralocorticoid-induced hypertension (5, 20). Interestingly, renal denervation has also been suggested to decrease renal inflammation as a mediating factor or consequence of hypertension (5, 65) but not in all models (2). It is therefore unclear what role the renal sympathetic nerves play in mediating various forms of hypertension and which specific etiologies would respond effectively to renal sympathetic denervation. This presents an ideal situation for applying personalized medicine strategies for the use of renal denervation.
Another major contributor to blood pressure control and sodium homeostasis is the endothelin-1 (ET-1) system. ET-1 contributes to the development of both systemic (47, 55, 56) and pulmonary hypertension (33). ET-1 exerts its physiological actions in mammals through the endothelin A (ETA) and B (ETB) receptors. Vascular smooth muscle ETA receptor activation mediates the potent vasoconstrictive properties of ET-1, and ETB receptor activation on endothelial cells mediates a nitric oxide-dependent vasorelaxation (37). In addition to the vascular effects of ETB receptors, their presence in the collecting duct of the kidney contributes to long-term regulation of blood pressure and sodium homeostasis by promoting natriuresis (30, 35, 37, 51, 56), and infusion of exogenous ET-1 induces salt-sensitive hypertension (42). Interestingly, genetic removal of ETB receptors from vascular smooth muscle cells increases blood pressure but does not alter salt sensitivity (41). Although the actions of ETB receptors in the kidney and endothelium promote a lowering of blood pressure, several studies have shown that ETB receptors on postganglionic sympathetic neurons are likely excitatory (14, 38, 39). We have previously demonstrated that acute ETB receptor activation results in an α1-adrenergic-mediated pressor response in a rat model of ETB receptor deficiency (ETB-def; 6), in which functional ETB receptors are only expressed on sympathetic neurons (21–23). This study also showed that ETB-def rats have elevated sympathetic tone compared with controls (6). The ETB-def rats are a low-renin, salt-sensitive model of hypertension (22); however, it is unknown whether the renal sympathetic nerves play any role in mediating the hypertension and salt sensitivity in this model.
We hypothesized that renal sympathetic nerves contribute to hypertension and salt sensitivity in ETB-def rats. We tested this hypothesis by applying bilateral renal sympathetic denervation (DNx) to ETB-def and transgenic control (TG) littermates and evaluating circadian blood pressure and autonomic tone during normal salt (0.49% NaCl) and high-salt (4.0% NaCl) diets by telemetry. Furthermore, previous telemetry studies from our group and others show that ETB-def rats on a high-salt diet have an exaggerated diurnal blood pressure rhythm. Therefore we also examined whether denervation affected autonomic tone in a circadian manner with ETB-def rats on normal salt and high-salt diets. As secondary aims we investigated whether denervation altered renal production of ET-1 and markers of renal injury in ETB-def rats following high-salt diet. Because alterations to the diurnal rhythm of blood pressure are known risk factors for the development of cardiovascular disease (24, 29, 61) and little is known about the effect of salt on diurnal autonomic tone, we also sought to evaluate the effects of salt and denervation on diurnal control of autonomic tone.
MATERIALS AND METHODS
Animal use.
A total of 40 male ETB-def and littermate TG control rats, age 12–14 wk old, with starting weights of 250–350 g from our own colony were used in the present study. These rats are from the spotting lethal (sl) strain, which harbors a naturally occurring mutation in the ETB gene resulting in expression of nonfunctional ETB receptors. Rats homozygous for the mutation die from megacolon because of the absence of enteric nerve development. The strain is rescued from this phenotype by insertion of a transgene containing human ETB gene under dopamine β-hydroxylase promotor control (21–23). ETB-def rats refer to those homozygous for the sl mutation (sl+/+) but possessing the transgene. TG controls are sl−/− and also express the transgene. Genotype was assessed by PCR on tail snips performed at weaning. One rat died following telemetry and denervation surgery, and two TG DNx rats were excluded from final analysis because of a high renal cortical norepinephrine content measured at the end of study. The final numbers of rats used were 23 for the high-salt protocol and 14 for the normal salt protocol. Rats were housed in a temperature-controlled room with lights on at 7 AM, hereinafter referred to as zeitgeber time (ZT) 0, and lights off at 7 PM (ZT 12). At the end of the experiment, rats were anesthetized with isoflurane, a midline incision was performed, and blood was drawn from the abdominal aorta. The whole blood was then centrifuged for collection of plasma. Both kidneys were visibly dissected into cortex, outer medulla, and inner medulla and flash frozen in liquid nitrogen. All rats were euthanized at ZT 0 ± 1 (SE) h. All tissues were stored at −80°C. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and conformed to the National Institutes of Health ethical use of animal subjects as detailed in the Guide for the Care and Use of Laboratory Animals.
High-salt protocol.
In brief, rats were anesthetized by inhaled isoflurane, and a midline incision was made, intestines were retracted, and the abdominal aorta and renal arteries were visualized. Renal sympathetic nerves were dissected from the adventitia of the renal arteries and mechanically stripped. After mechanical denervation, a 10% phenol solution was painted on the renal arteries. The contralateral kidney was similarly denervated. Sham surgery was performed similarly except that nerves were not mechanically stripped and the artery was painted with saline. After denervation, telemetry catheters (S-10; Data Sciences International, Minneapolis, MN) were inserted into the abdominal aorta distal to the renal arteries and fixed with tissue glue. The muscle layer was closed with 6-0 prolene suture, and the skin layer was closed with surgical staples.
Rats were allowed to recover from renal denervation and telemetry implantation for at least 7 days. Blood pressure recordings were taken via telemetry at a sampling rate of 1 kHz (8) in 10-min segments every 20 min. All data collection was completed using Ponemah software version 6.0, and data analysis was done off-line in Ponemah version 6.2 (Data Sciences International). Blood pressure, heart rate, pulse pressure, and locomotor activity were binned in 9-h segments of active (ZT 14–22) and inactive (ZT 2–10) periods. Heart rate frequency domain analysis was completed using 10-s bins of low frequency (LF; 0.2–0.6 Hz) and high frequency (HF; 1.0–3.0 Hz) using the maximum derivative of the blood pressure signal for interbeat interval triggering. Blood pressure frequency domain analysis used the same frequency cutoffs triggered by systolic (maximum) blood pressure from the pulse wave analyzed in 10-min bins.
Mean arterial pressure (MAP) and frequency domain parameters were subjected to cosinor analysis for evaluation of diurnal rhythm variation in sympathetic tone (Prism 7.0; GraphPad Software, La Jolla, CA). Data were binned into 1-h segments, and these hour bins were averaged across 3 consecutive normal salt days and 3 consecutive high-salt days (days 8–10). Cosinor analysis was done on each individual subject using a custom nonlinear regression equation defined as:
A period of 24 h was assumed as this matched the light cycle of the room. Mean estimated statistic of rhythm (MESOR), amplitude, and acrophase were reported from the regression for each individual animal.
Spontaneous baroreflex sensitivity was calculated from inactive (ZT 4–8) and active (ZT 16–20) segments by exporting the raw pulse wave data from Ponemah and importing it into HemoLab (Harald Stauss, Iowa City, IA). Baroreflex gain was determined using the sequence method (7, 57).
Rats were maintained on a normal salt diet of 0.49% NaCl (TD.96208; Envigo, Indianapolis, IN). Rats were moved to metabolic cages 3 days before diet change. Measurements and urine collections were taken twice per day, once corresponding to the active period (ZT 12–24) and once corresponding to the inactive period (ZT 0–12) following an acclimation period of 24 h. Diet was switched to high salt (4.0% NaCl, TD.92034) at ZT 12, and rats remained in metabolic cages. Rats were then returned to home cages at ZT 12 of day 2 of high-salt diet and remained there until ZT 12 of day 11 of high-salt diet when they were once more housed in metabolic cages for 2 days of collection. Rats were returned to home cages for at least 2 days before euthanasia.
Normal salt controls.
For collection of normal salt tissues a second group of rats was used. Rats were denervated through a flank incision exposing the renal artery, all visible nerves were mechanically stripped with fine forceps, and the artery was painted with a 10% phenol solution. Sham surgery was performed similarly except that nerves were not mechanically stripped and the renal artery was painted with saline.
Animals were treated similarly to the high-salt protocol in regard to handling and time frame with the exception that rats continued to receive normal salt diet throughout the protocol and the second metabolic cage collection period was omitted.
Tissue and urine analyses.
Renal inner medulla sections were analyzed for ET-1 content as previously described (50). In brief, inner medulla sections were pulverized and homogenized in a solution of 1 M acetic acid (Sigma-Aldrich, St. Louis, MO) with 10 µg/ml pepstatin A (Sigma-Aldrich). Total protein content was determined by a Bradford assay (Bio-Rad, Hercules, CA). Endothelin-1 content was determined by an ELISA (QET00B; R&D Systems, Minneapolis, MN) according to manufacturer’s instructions.
Renal cortex samples were sectioned and weighed. Samples were homogenized in 5-µl 1 mM EDTA with 0.01 N HCl per 1 mg of tissue wet weight. Homogenates were centrifuged at 4°C at 23,000 rpm for 30 min, the supernatant was recovered, and the pellet was resuspended in 1 ml of homogenization solution. Total protein content of both the supernatant and pellet was determined by Bradford assay (Bio-Rad), and total norepinephrine content was determined via ELISA (BA E-6200; Rocky Mountain Diagnostics, Colorado Springs, CO) according to the manufacturers’ instructions.
Urine and plasma electrolyte concentrations were determined using EasyLyte (Medica, Bedford, MA) according to the manufacturer’s instructions. Urine and plasma ET-1 concentrations were measured via ELISA (QET00B; R&D Systems). Urine albumin was measured via ELISA (GWB-1B2B4B; GenWay Biotech, San Diego, CA) as per manufacturer’s instructions.
Statistical analysis.
Comparisons between groups were evaluated using either one-way or two-way ANOVA with Tukey’s posttest for multiple comparisons for testing between groups (genotype and denervation effects) and Sidak’s posttest for multiple comparisons for evaluating the effect of diet. Statistical significance was designated for two-tailed P values <0.05.
RESULTS
Characteristics of experimental animals.
Renal denervation was confirmed by measuring norepinephrine (NE) content from renal cortex tissues. Data are described in Table 1 along with final body and organ weights. There were no differences in weight gain, heart weight, or spleen weight across groups regardless of genotype, DNx, or diet (Table 1). Similarly, we did not observe any differences in food or water intake across genotype or treatment groups, nor did we observe any changes in active/inactive period urine volume or urinary Na+, K+, or Cl− excretion between genotype or treatment groups (Table 2). After a high-salt diet, water intake, urine volume, and electrolyte excretion increased relative to normal salt diet as expected, but there was no significant difference between groups on high salt (Table 2).
Table 1.
Renal cortex norepinephrine content and final body and organ weights
| Normal Salt |
High Salt |
|||||||
|---|---|---|---|---|---|---|---|---|
| TG Sham | TG DNx | ETB-def Sham | ETB-def DNx | TG Sham | TG DNx | ETB-def Sham | ETB-def DNx | |
| n | 5 | 3 | 3 | 3 | 6 | 4 | 7 | 6 |
| Cortex NE, ng/mg | 1.15 ± 0.54 | 0.08 ± 0.01* | 0.82 ± 0.35 | 0.04 ± 0.03† | 2.8 ± 0.53 | 0.24 ± 0.06* | 2.71 ± 0.49 | 0.24 ± 0.14† |
| Starting BW, g | 328 ± 18 | 305 ± 14 | 322 ± 21 | 324 ± 17 | 306 ± 10 | 318 ± 8 | 304 ± 8 | 283 ± 8 |
| Final BW, g | 372 ± 7 | 345 ± 5 | 368 ± 14 | 363 ± 8 | 354 ± 8 | 358 ± 7 | 348 ± 6 | 332 ± 7 |
| ΔBW, g | 44 ± 3 | 40 ± 4 | 47 ± 6 | 39 ± 3 | 48 ± 6 | 43 ± 6 | 44 ± 3 | 49 ± 4 |
| HW/BW, mg/g | 3.28 ± 0.06 | 3.20 ± 0.09 | 3.19 ± 0.03 | 3.18 ± 0.07 | 3.29 ± 0.06 | 3.46 ± 0.10 | 3.43 ± 0.05 | 3.28 ± 0.05 |
| SW/BW, mg/g | 1.89 ± 0.05 | 1.79 ± 0.04 | 1.76 ± 0.07 | 1.90 ± 0.05 | 1.80 ± 0.03 | 1.80 ± 0.10 | 2.01 ± 0.17 | 2.37 ± 0.22 |
Values are means ± SE. DNx, denervated; NE, norepinephrine; BW, body weight; HW, heart weight; SW, spleen weight; TG, transgenic; DNx, denervated; ETB, endothelin B. Two-way ANOVA with Tukey’s test for multiple comparisons:
P < 0.05 vs. TG sham;
P < 0.05 vs. ETB-def sham.
Table 2.
Metabolic cage parameters for normal salt and high-salt diets
| Normal Salt |
High Salt |
|||||||
|---|---|---|---|---|---|---|---|---|
| TG Sham | TG DNx | ETB-def Sham | ETB-def DNx | TG Sham | TG DNx | ETB-def Sham | ETB-def DNx | |
| n | 6 | 5 | 7 | 6 | 6 | 5 | 7 | 6 |
| AP water, ml | 18.0 ± 1.0 | 20.6 ± 0.9 | 18.9 ± 0.7 | 20.4 ± 0.5 | 39.8 ± 2.5† | 46.1 ± 1.8† | 44.1 ± 2.5† | 47.4 ± 3.4† |
| IP water, ml | 5.3 ± 0.4 | 6.3 ± 0.9 | 6.2 ± 0.6 | 4.4 ± 0.4 | 8.6 ± 0.4† | 8.9 ± 0.9 | 8.7 ± 1.4 | 6.8 ± 1.1 |
| AP food, g | 14.6 ± 0.8 | 15.5 ± 0.4 | 15.2 ± 0.4 | 16.6 ± 0.5 | 15.3 ± 0.9 | 17.1 ± 0.6 | 17.1 ± 1.0 | 17.7 ± 0.5 |
| IP food, g | 4.6 ± 0.5 | 4.7 ± 0.4 | 4.8 ± 0.4 | 4.4 ± 0.7 | 4.0 ± 0.3 | 4.1 ± 0.4 | 4.6 ± 0.7 | 3.0 ± 0.4 |
| AP urine, ml | 5.6 ± 0.6 | 6.1 ± 0.7 | 6.3 ± 0.4 | 6.7 ± 0.5 | 21.6 ± 1.8† | 29.4 ± 2.5*† | 25.7 ± 1.8† | 26.9 ± 1.5† |
| IP urine, ml | 4.1 ± 0.3 | 4.8 ± 0.7 | 4.9 ± 0.5 | 4.4 ± 0.3 | 9.2 ± 0.8† | 11.1 ± 0.6† | 12.0 ± 0.9† | 12.1 ± 0.8† |
| AP Na+, µeq/12 h | 877 ± 55 | 717 ± 67 | 797 ± 51 | 796 ± 46 | 8,132 ± 578† | 10,199 ± 1,307† | 8,579 ± 655† | 8,258 ± 250† |
| IP Na+, µeq/12 h | 666 ± 14 | 783 ± 42 | 748 ± 59 | 710 ± 80 | 3,592 ± 164† | 4,607 ± 297† | 4,006 ± 310† | 4,123 ± 263† |
| AP K+, µeq/12 h | 1,423 ± 98 | 1,437 ± 100 | 1,534 ± 79 | 1,556 ± 71 | 2,415 ± 185† | 2,731 ± 256† | 2,524 ± 170† | 2,504 ± 156† |
| IP K+, µeq/12 h | 666 ± 24 | 707 ± 49 | 662 ± 60 | 592 ± 49 | 843 ± 36 | 957 ± 71† | 938 ± 79† | 793 ± 55 |
| AP Cl−, µeq/12 h | 1,002 ± 68 | 850 ± 58 | 1,031 ± 53 | 1,015 ± 66 | 7,862 ± 570† | 9,471 ± 1,103† | 8,237 ± 660† | 8,384 ± 591† |
| IP Cl−, µeq/12 h | 813 ± 25 | 871 ± 25 | 873 ± 63 | 824 ± 95 | 3,629 ± 141† | 4,437 ± 264† | 3,837 ± 278† | 3,929 ± 223† |
Values are means ± SE. TG, transgenic; DNx, denervated; ETB, endothelin B; AP, active period (zeitgeber time 12–24); IP, inactive period (zeitgeber time 0–12). Two-way ANOVA with Tukey’s and Sidak’s test for multiple comparisons:
P < 0.05 vs. TG sham;
P < 0.05 vs. normal salt.
Hemodynamic measurements.
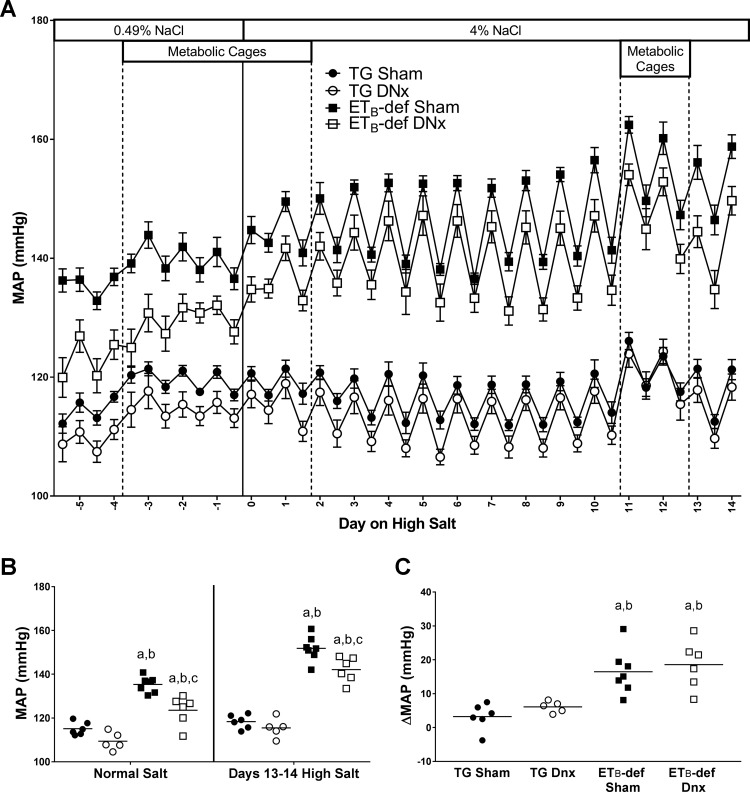
Similar to previously described results, ETB-def rats had elevated blood pressure relative to TG control animals during normal salt diet (44). Bilateral renal denervation reduced blood pressure by ~10 mmHg in ETB-def rats (Fig. 1, A and B). After 13–14 days of high-salt diet, ETB-def animals with intact renal nerves had an increase in mean arterial blood pressure of 16 ± 3 mmHg. A similar increase in blood pressure of 19 ± 3 mmHg was also observed in ETB-def DNx rats following high salt. As such, the denervated group continued to have an ~10 mmHg lower blood pressure than intact ETB-def rats (Fig. 1, A and B). Neither denervation nor diet had a significant effect on blood pressure in TG sham rats. Heart rate was not significantly different across groups apart from ETB-def DNx being higher than TG sham during normal salt diet (361 ± 4 vs. 341 ± 3 beats/min, respectively). High-salt diet significantly decreased heart rate in all groups except TG sham, and there were no significant differences in heart rate between groups following high-salt diet (TG sham, 335 ± 4; TG DNx, 339 ± 4; ETB-def sham, 333 ± 5; ETB-def DNx, 341 ± 4 beats/min). We failed to detect a difference in locomotor activity across genotype, DNx, or salt diet (data not shown).
Fig. 1.
Mean arterial pressure (MAP) throughout the experimental protocol. A: active/inactive MAP during normal salt and high-salt diet. B: 24-h MAP during normal salt diet and days 13–14 of high-salt diet. C: change in MAP from normal salt to days 12–14 of high-salt diet. DNx, denervated; TG, transgenic; ETB, endothelin B. aP < 0.05 vs. TG sham; bP < 0.05 vs. TG DNx; cP < 0.05 vs. ETB-def sham; two-way ANOVA with Sidak’s post hoc test for multiple comparisons (B); one-way ANOVA with Tukey’s post hoc test for multiple comparisons (C). DNx, denervated; TG, transgenic; ETB, endothelin B.TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
We evaluated the chronic pressure-natriuresis relationship using the 12-h sodium excretion and blood pressure values. ETB-def sham rats had a lower slope of the chronic pressure-natriuresis relationship compared with TG sham controls (334 ± 18 vs. 647 ± 110 µeq/MAP, respectively). Denervation did not significantly affect the slope of either ETB-def (324 ± 28 µeq/MAP) or TG (914 ± 185 µeq/MAP) compared with the respective sham groups.
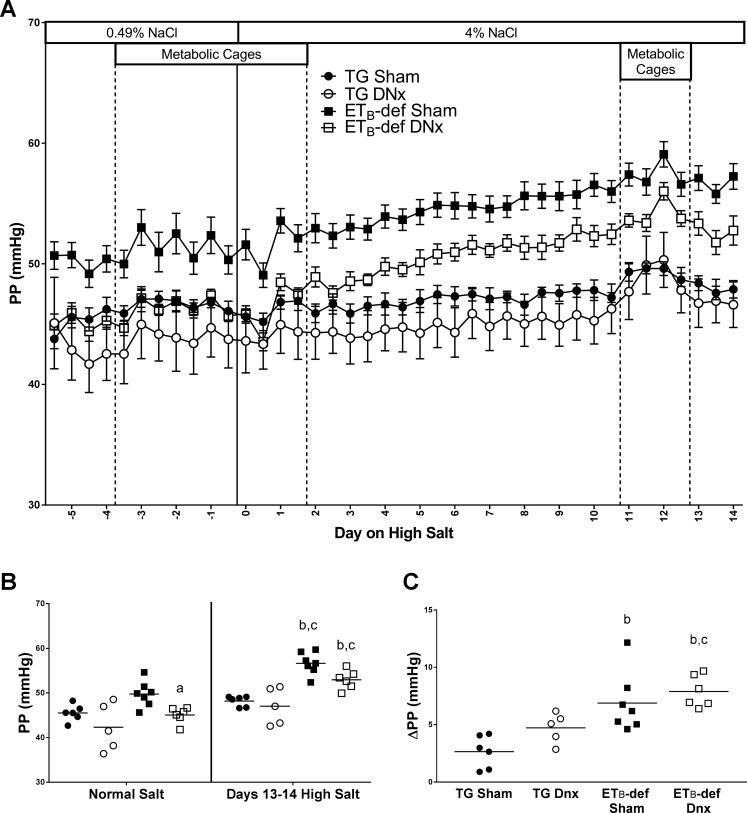
Along with the increased MAP, ETB-def sham rats tended to have a higher pulse pressure during normal salt diet. This was attenuated to TG levels by denervation (Fig. 2, A and B). After a high-salt diet, pulse pressure increased in both ETB-def sham and DNx groups to a similar extent (Fig. 2, A and C). Denervation and salt diet had no significant effect on the pulse pressure of TG rats.
Fig. 2.
Pulse pressure (PP) throughout the experimental protocol. A: active/inactive PP during normal salt and high-salt diet. B: 24-h PP during normal salt diet and days 13–14 of high-salt diet. C: change in PP from normal salt to days 12–14 of high-salt diet. DNx, denervated; TG, transgenic; ETB, endothelin B. aP < 0.05 vs. ETB-def sham; bP < 0.05 vs. TG sham; cP < 0.05 vs. TG DNx; two-way ANOVA with Sidak’s post hoc test for multiple comparisons (B); one-way ANOVA with Tukey’s post hoc test for multiple comparisons (C). TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
Circadian analysis of hemodynamic and autonomic parameters.
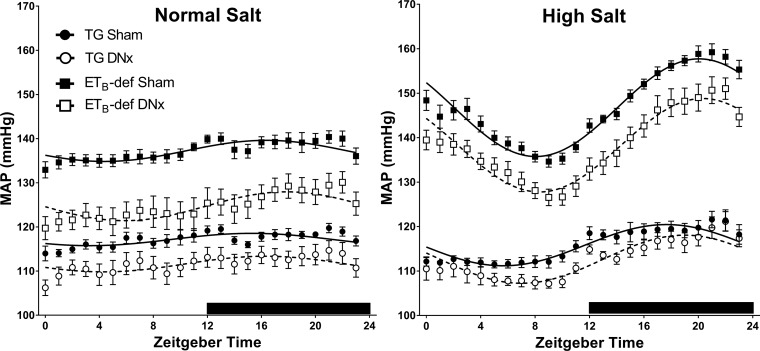
There were no significant differences between genotypes or DNx versus sham in relation to the diurnal rhythm of blood pressure during normal salt diet. Although the ETB-def sham rats had an elevated MESOR value, which represents the level around which the diurnal rhythm oscillates, there were no differences between groups in amplitude or acrophase (Fig. 3 and Table 3). High-salt diet caused an increase in MAP amplitude in all groups relative to normal salt diet, and this amplitude was further exaggerated in ETB-def rats relative to TG regardless of DNx or sham surgery. There was also a significant phase shift toward later ZT in ETB-def rats relative to TG rats that was not influenced by DNx.
Fig. 3.
Cosinor analysis of MAP. Average hourly data ± SE and cosinor curves from bins of 3 consecutive days during normal salt or high-salt diet. DNx, denervated; TG, transgenic; ETB, endothelin B. Quantified data for cosinor parameters are described in Table 3. TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
Table 3.
Cosinor analysis of MAP, heart rate LF/HF, and blood pressure LF/HF
| Normal Salt |
High Salt |
|||||||
|---|---|---|---|---|---|---|---|---|
| TG Sham | TG DNx | ETB-def Sham | ETB-def DNx |
TG Sham | TG DNx | ETB-def Sham | ETB-def DNx |
|
| n | 6 | 5 | 7 | 6 | 6 | 5 | 7 | 6 |
| MAP | ||||||||
| MESOR | 117 ± 0.9 | 112 ± 2.0 | 137 ± 1.4* | 125 ± 2.8† | 116 ± 1.2 | 113 ± 1.6 | 147 ± 1.0‡ | 133 ± 4.8†‡ |
| Amplitude | 1.9 ± 0.3 | 1.9 ± 0.5 | 2.6 ± 0.3 | 3.5 ± 0.4 | 4.7 ± 0.7‡ | 5.6 ± 0.6‡ | 11.3 ± 1.1*‡ | 9.5 ± 1.0*‡ |
| Acrophase (ZT) | 16.1 ± 0.2 | 16.5 ± 0.9 | 16.5 ± 0.6 | 17.9 ± 0.7 | 17.6 ± 0.2 | 18.9 ± 0.5‡ | 20.0 ± 0.3*‡ | 20.4 ± 0.5*‡ |
| HRV LF/HF | ||||||||
| MESOR | 0.93 ± 0.06 | 0.89 ± 0.03 | 1.32 ± 0.16* | 0.95 ± 0.04† | 0.80 ± 0.07 | 0.72 ± 0.04 | 1.02 ± 0.04‡ | 0.89 ± 0.07 |
| Amplitude | 0.16 ± 0.05 | 0.12 ± 0.01 | 0.34 ± 0.11 | 0.16 ± 0.05 | 0.25 ± 0.04 | 0.14 ± 0.03 | 0.26 ± 0.02 | 0.18 ± 0.03 |
| Acrophase (ZT) | 16.55 ± 0.14 | 14.88 ± 0.19* | 17.93 ± 0.24* | 15.51 ± 0.13*† | 15.42 ± 0.10‡ | 15.45 ± 0.40 | 13.50 ± 0.16*‡ | 13.64 ± 0.37*‡ |
| BPV LF/HF | ||||||||
| MESOR | 1.48 ± 0.09 | 1.18 ± 0.05 | 1.50 ± 0.06 | 1.25 ± 0.10 | 1.68 ± 0.11 | 1.34 ± 0.08 | 2.13 ± 0.13*‡ | 1.72 ± 0.11*†‡ |
| Amplitude | 0.29 ± 0.06 | 0.44 ± 0.05 | 0.34 ± 0.06 | 0.43 ± 0.07 | 0.56 ± 0.05‡ | 0.51 ± 0.08 | 0.87 ± 0.06*‡ | 0.68 ± 0.08‡ |
| Acrophase (ZT) | 17.41 ± 0.41 | 17.44 ± 0.48 | 17.55 ± 0.69 | 17.84 ± 0.33 | 17.48 ± 0.49 | 17.91 ± 0.31 | 17.79 ± 0.55 | 18.00 ± 0.42 |
Values are means ± SE. TG, transgenic; DNx, denervated; ETB, endothelin B; MAP, mean arterial pressure; HRV, heart rate variability; BPV, blood pressure variability; LF/HF, low frequency-high frequency; MESOR, mean estimated statistic of rhythm; ZT, zeitgeber time. Two-way ANOVA with Tukey’s and Sidak’s test for multiple comparisons:
P < 0.05 vs. TG sham;
P < 0.05 vs. ETB-def sham;
P < 0.05 vs. normal salt.
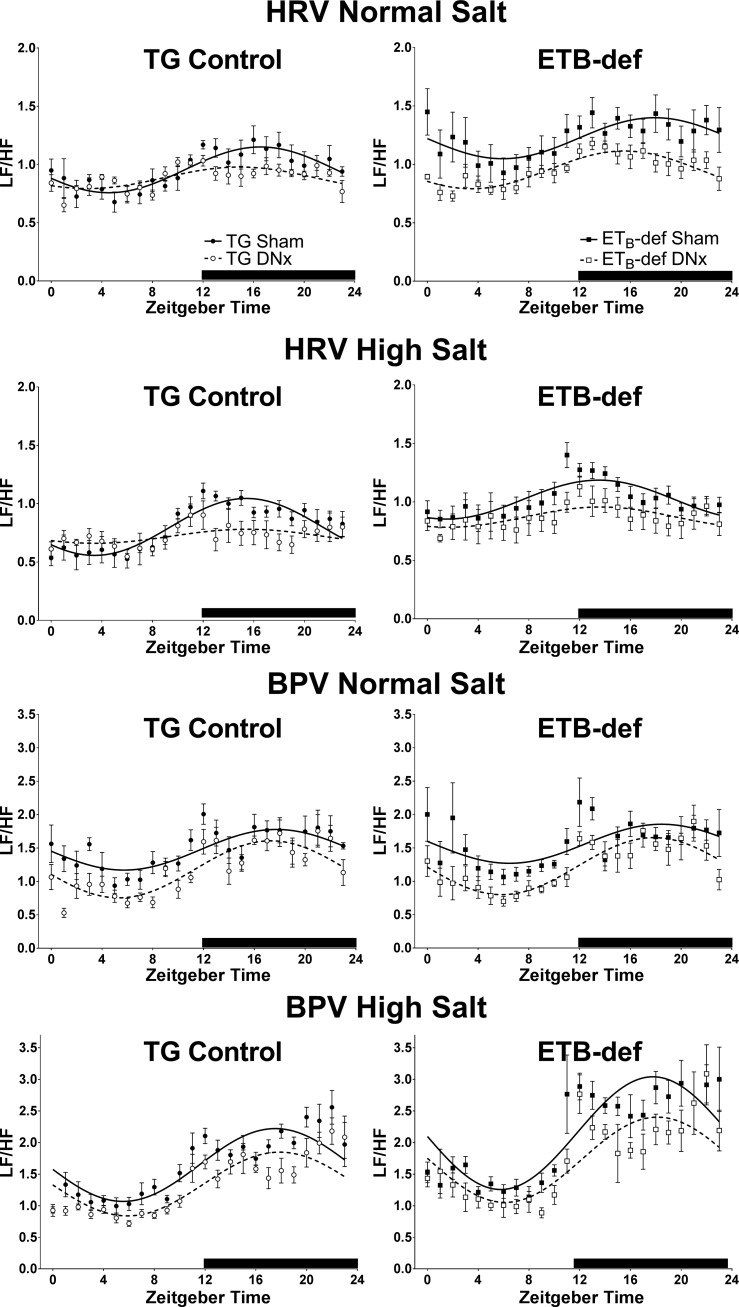
We assessed autonomic tone using frequency analysis of heart rate variability and blood pressure variability as the ratio of LF to HF power density. We evaluated the diurnal rhythm of LF/HF to investigate any changes to autonomic tone that may occur throughout the active and inactive periods as well as to evaluate whether or not denervation had an effect on the diurnal rhythm of autonomic tone. Composite cosinor curves are displayed in Fig. 4, and the analysis of individual animals is summarized in Table 3.
Fig. 4.
Cosinor analysis of LF/HF tone of heart rate and blood pressure. Average hourly data ± SE and cosinor curves from bins of 3 consecutive days during normal salt or high-salt diet. HRV, heart rate variability; BPV, blood pressure variability. DNx, denervated; TG, transgenic; ETB, endothelin B. Quantified data for cosinor parameters are described in Table 3. TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
We found that with respect to heart rate LF/HF, ETB-def sham animals had an elevated LF/HF MESOR during normal salt diet indicating that ETB-def sham animals have overall higher sympathetic-to-parasympathetic cardiac tone relative to TG sham rats (Fig. 4 and Table 3). Denervation significantly attenuated the heart rate LF/HF MESOR in ETB-def animals during normal salt. High-salt diet reduced LF/HF MESOR in ETB-def sham rats but did not statistically change LF/HF in other groups. There were no significant differences observed in the amplitude of the heart rate LF/HF diurnal rhythm; however, multiple phase shifts were seen between groups and salt diet. In both TG and ETB-def groups on normal salt diet, denervation resulted in an earlier acrophase (time of peak) compared with sham. The effect of denervation was absent during high salt although a significant genotype effect was uncovered; specifically, both ETB-def sham and ETB-def DNx rats had an earlier peak LF/HF time of ~2 h compared with TG rats.
Blood pressure LF/HF MESOR, amplitude, and acrophase were not statistically different between genotypes or denervation/sham during normal salt. However, high-salt diet resulted in a significant increase in LF/HF MESOR in ETB-def relative to normal salt diet in both sham and DNx groups; this increase following a high-salt diet was not seen in TG rats (Fig. 4 and Table 3). During high-salt diet, ETB-def DNx rats had lower LF/HF MESOR compared with ETB-def sham. High-salt diet also resulted in an increased LF/HF amplitude in both ETB-def sham and ETB-def DNx rats compared with normal salt diet. We failed to detect a significant phase shifts in blood pressure LF/HF following high-salt diet.
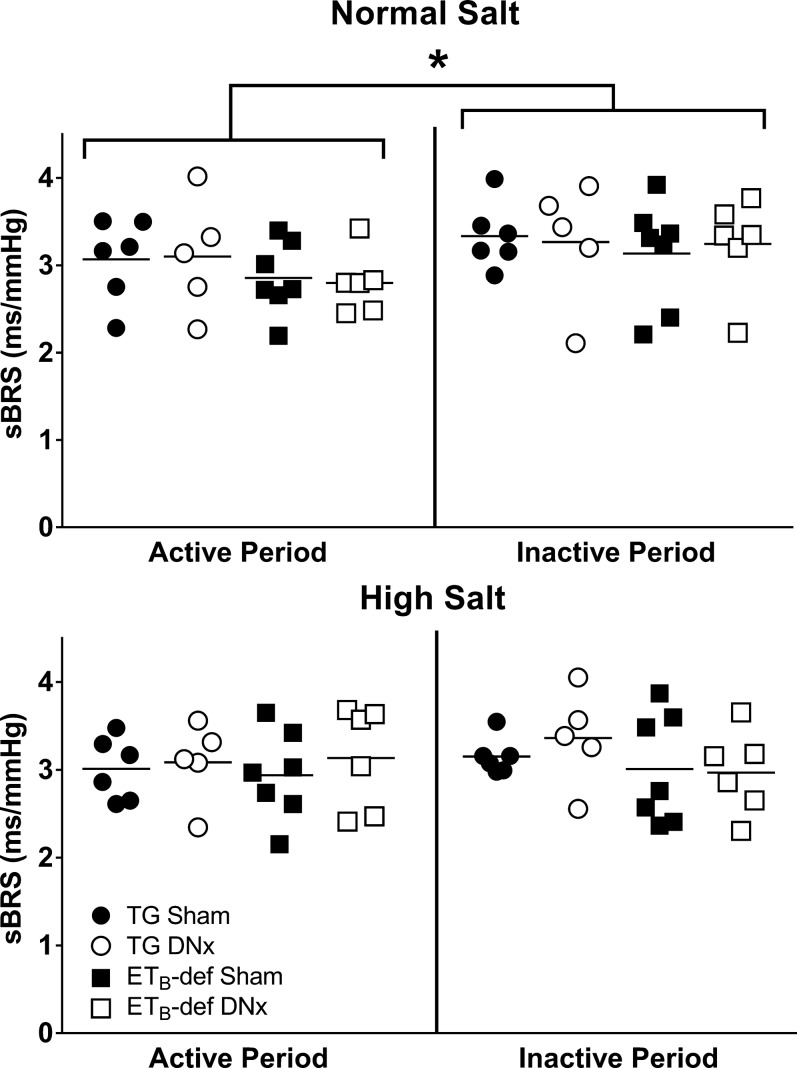
We measured spontaneous baroreflex sensitivity (sBRS) during peak inactive and active periods. During normal salt diet there was a significant interaction of variance between active and inactive sBRS by two-way ANOVA (P = 0.01); however, no significant differences were observed between genotype or treatment under post hoc analysis (Fig. 5, top). After 13–14 days of a high-salt diet, there was no significant interaction between active and inactive sBRS by two-way ANOVA (P = 0.35). Similar to normal salt periods, no significant differences between groups were observed (Fig. 5, bottom). There was no significant difference within subjects between normal salt and high-salt diets during either active (P = 0.30) or inactive (P = 0.19) periods. The sBRS results remained similar when the up and down sequences were individually analyzed (data not shown).
Fig. 5.
Spontaneous baroreflex sensitivity (sBRS) during active and inactive periods during normal salt (top) or high-salt (bottom) diet. DNx, denervated; TG, transgenic; ETB, endothelin B. *P < 0.05 active vs. inactive period by two-way ANOVA. TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
Endothelin-1.
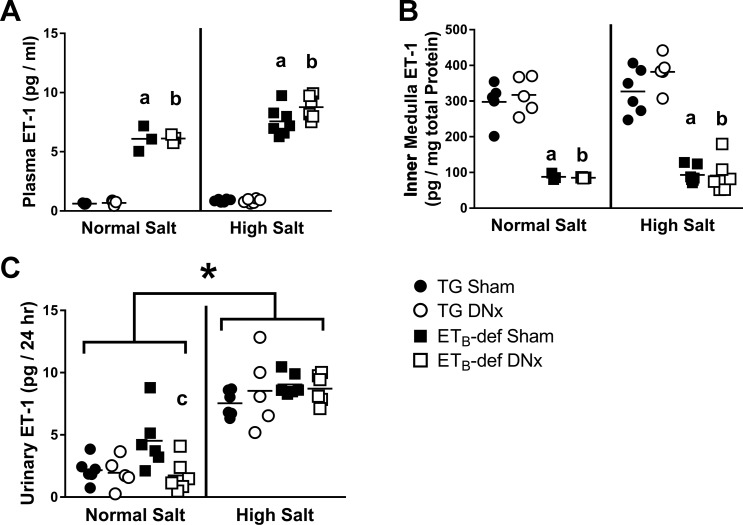
To investigate potential contributing factors toward the ability of denervation to reduce blood pressure in this model, we evaluated plasma, kidney tissue content, and renal production of ET-1. Plasma levels of ET-1 have previously been reported to be elevated in the ETB-def model (44) because of lack of clearance by ETB receptors in the lungs. We also observed this dramatic elevation of plasma ET-1 concentration in ETB-def animals in our study, which was not significantly affected by denervation (Fig. 6A). Kidney cortex and outer medulla express relatively little ET-1; however, inner medullary ET-1 levels are normally robust (50). We observed significantly lower inner medulla ET-1 content in ETB-def rats compared with TG during both normal and high salt, which was not significantly affected by renal denervation (Fig. 6B). We failed to observe any differences between groups in cortical (TG sham, 3.7 ± 0.7; TG DNx, 4.0 ± 0.6; ETB-def sham, 3.7 ± 0.3; ETB-def DNx, 3.9 ± 1.1; pg ET-1/mg total protein; n = 3–4 per group; one-way ANOVA, P = 0.99) or outer medullary (TG sham, 9.9 ± 0.7; TG DNx, 8.4 ± 0.5; ETB-def sham, 11.4 ± 1.1; ETB-def DNx, 12.4 ± 3.1; pg ET-1/mg total protein; n = 3–4 per group; one-way ANOVA, P = 0.39) ET-1 content. Urinary ET-1 excretion is an established marker for renal production of ET-1. Here we found that urinary excretion of ET-1 was not significantly different between ETB-def sham and TG sham (P = 0.07) on normal salt diet, although ETB-def DNx had lower urinary ET-1 excretion relative to ETB-def sham (Fig. 6C). All groups increased urinary ET-1 excretion on high-salt diet, although denervation had no significant effect on this parameter (Fig. 6C).
Fig. 6.
ET-1 levels during normal salt and high-salt diets. A: plasma endothelin-1 (ET-1) content. B: renal inner medullary ET-1 content. C: renal production of ET-1 measured by 24-h urinary excretion of ET-1. DNx, denervated; TG, transgenic; ETB, endothelin B. aP < 0.05 vs. TG sham; bP < 0.05 vs. TG DNx; cP < 0.05 vs. ETB-def sham; one-way ANOVA with Tukey’s post hoc test for multiple comparisons (A–C). *P < 0.05 active vs. inactive period by two-way ANOVA; two-way ANOVA with Sidak’s post hoc test for multiple comparisons (C). TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
Albuminuria.
As an initial investigation of the potential effect of high-salt diet and hypertension on renal injury, we evaluated whether 2 wk of high-salt diet produced any changes in urinary albumin excretion. We observed an increase in urinary albumin excretion in ETB-def rats fed a high-salt diet (Fig. 7), but this effect was not significantly attenuated by denervation (P = 0.06 vs. ETB-def sham).
Fig. 7.
Urinary albumin excretion during normal salt and high-salt diet. DNx, denervated; TG, transgenic; ETB, endothelin B. aP < 0.05 vs. TG sham; one-way ANOVA with Tukey’s post hoc test for multiple comparisons within each diet. TG sham, n = 6; TG DNx, n = 5; ETB-def sham, n = 7; ETB-def DNx, n = 6.
DISCUSSION
The major findings of the present study are that the hypertension in rats lacking functional ETB receptors on all tissues except sympathetic nerves has a neurogenic component, which can be attenuated by renal sympathetic denervation. However, denervation does not affect the salt-sensitive increase in blood pressure in ETB-def rats. Utilizing a novel approach to assess autonomic tone in a circadian manner, we also observed disrupted diurnal autonomic tone in ETB-def rats following high-salt diet that is partially attenuated by renal sympathetic denervation. These findings support our hypothesis that renal sympathetic nerves contribute to hypertension in ETB-def rats. However, renal sympathetic nerves do not appear to contribute to salt sensitivity in rats lacking ETB receptors in nonneuronal tissue.
In an acute setting, we have previously shown that anesthetized ETB-def rats have elevated sympathetic tone relative to TG controls (6). Here we provide further evidence toward the higher sympathetic tone of ETB-def rats compared with genetic controls with our observation that the MESOR LF-to-HF ratio of heart rate frequency variability is elevated in ETB-def rats (Figs. 5 and 6). This suggests that one potential mechanism for the higher blood pressure during normal salt diet in the ETB-def rats could be due to increased sympathetic tone. Furthermore, this hypertension is attenuated in ETB-def rats following bilateral renal sympathetic denervation and is not dependent on changes in sodium handling (Table 2). This effect cannot be due to the transgene that expresses the functional ETB receptor since DNx had no effect in TG littermate controls. Interestingly, denervation did not completely attenuate the hypertension in the ETB-def animals as blood pressure remained elevated compared with TG controls implicating nonrenal sympathetic mechanisms contributing to the hypertension in this model on normal salt diet.
Denervation had no effect on the increase in blood pressure following high-salt diet (Fig. 1), nor did it affect the slope of the chronic pressure-natriuresis curve in ETB-def rats. This indicates that the salt sensitivity of this model is due to mechanisms not dependent on renal sympathetic nerves. Previous studies have specified a role for ETB receptors in the distal nephron contributing to natriuresis (12, 13, 30). Because this model lacks functional ETB receptors except in sympathetic tissues, the salt sensitivity is likely due to an impaired natriuresis that requires a shift in the chronic pressure-natriuresis relationship to maintain sodium homeostasis. Because denervation in this model would not be expected to change ETB receptor expression or function in the distal nephron, our intervention did not affect the response of this genotype to high-salt diet. Another possible explanation for the lack of an effect of denervation on salt sensitivity could be altered central responses to high-salt diet. High-salt diet is known to increase the activation of presympathetic centers of the brain (10, 11) increasing sympathetic tone (1, 53, 59). It is possible that in this model the increased blood pressure following high salt is a result of central mechanisms increasing sympathetic tone and thus blood pressure, which would be independent of contributions from renal nerves. As such, both sham-operated controls and denervated ETB-def rats had an increased vasomotor sympathetic/parasympathetic tone following high-salt diet (Table 3 and Fig. 4).
A previous study has shown an attenuation of blood pressure following DNx and an attenuation of the increase in blood pressure following administration of DOCA-salt (31), a finding which appears to conflict with our own. Interestingly, in this study by Jacob et al., renal denervation also reduced salt appetite. When the sham rats were restricted to consuming amounts of sodium similar to those of the DNx group, the attenuation of the increase in blood pressure following DOCA-salt was abolished. Our findings demonstrate no significant differences between sham and DNx animals in sodium or water handling during either normal salt or high-salt diet. Therefore, there are likely model differences in the contribution of the renal nerves toward salt appetite between our study and that of Jacob et al. In another model of salt sensitivity, the Dahl salt-sensitive rat, renal DNx has been shown to not affect the development of hypertension (45) although it does reduce blood pressure in established hypertension (19).
Another observation of our study comprised the differential effects of high-salt diet on different autonomic beds in ETB-def rats. High-salt diet decreased cardiac sympathetic/parasympathetic tone while increasing vasomotor sympathetic/parasympathetic tone (Table 3). This was not evident in TG control animals, and denervation had no effect on these changes in either genotype, once again suggesting mechanisms unrelated to renal nerve activity. These findings point to differential effects of high-salt diet on different autonomic beds. Consistent with this notion of differential sympathetic tone in response to high salt are data from Stocker et al. demonstrating greater increases in lumbar sympathetic tone than in renal sympathetic tone following water deprivation (58). We have previously reported evidence that ET may act differentially between cardiac and vasomotor sympathetic beds (6), and others have also suggested differential regulation of ETB receptor activation between sympathetic beds such as the splanchnic/mesenteric (39). More work is needed to elucidate potential sites and mechanisms contributing to differential control of various autonomic sites, particularly in the context of contributions by ET-1 and dietary salt.
Numerous recent studies have focused on diurnal rhythms in blood pressure as alterations to this rhythm are strongly correlated with many poor clinical outcomes (24, 29, 61). Less understood is whether alterations in diurnal autonomic tone can contribute to the progression of cardiovascular diseases. In human cohorts, cosinor analysis has shown that an earlier acrophase (time at peak) and higher amplitude of LF/HF are correlated with obesity in adolescents (48), and feeding time has been linked with circadian alterations of autonomic function (66, 67). Of particular interest with respect to circadian control of blood pressure in the present study is the increase in blood pressure LF/HF amplitude in ETB-def rats following high-salt diet (Fig. 4 and Table 3). This increased amplitude is also reflected in the diurnal rhythm of blood pressure (Fig. 1 and Fig. 3) as there is an increased difference in day/night MAP in ETB-def rats during high-salt diet. It is likely that the increase in diurnal amplitude of sympathetic tone to vascular beds is mediating, at least in part, this increased diurnal difference in blood pressure during high-salt diet. A previous report by Simmonds et al. demonstrated that high-salt diet increased blood pressure variability only during the night (active) period (53). Furthermore, this study observed increases in evoked sympathetic reflexes in anesthetized animals after 2 wk of high-salt diet that were prevented by ablation of the anteroventral third ventricular region. These ablations implicate osmosensitive centers of the brain in mediating the increased sympathetic response to high dietary salt. The influence of the ET system in mediating central responses to dietary salt is poorly understood and may provide important avenues for understanding the sensitivity of cardiovascular complications to high-salt diet.
Prior studies suggest an interaction of ET with baroreflex control (43, 46, 49). However, our results fail to demonstrate any differences in baroreflex sensitivity in conscious rats during either normal salt diet or high-salt diet regardless of genotype and renal nerve denervation. It is important to note that the spontaneous baroreflex sensitivity measurement only provides information related to the baroreflex gain and does not give insight into the upper or lower plateaus of the baroreflex curve. Therefore, it remains possible that ETB receptor deficiency, high-salt diet, and denervation may affect aspects of baroreflex other than the gain, but the limitations of the conscious, sBRS measurement preclude our ability to comment directly on components of the baroreflex curve beyond the gain. Nonetheless, it is still interesting that there was no effect of salt or ETB receptor deficiency on sBRS control of heart rate. Because we evaluated these parameters during periods of stable blood pressure (normal salt diet and established hypertension following high-salt diet), it is likely that baroreflex resetting occurred.
The spontaneously hypertensive rat has much lower levels of papillary ET-1 content relative to Wistar-Kyoto (WKY) controls, and denervation reduces the level of papillary ET-1 in WKY but not spontaneously hypertensive rats (25). Similar to these previous findings, our present study demonstrates much lower inner medullary ET-1 content in the hypertensive ETB-def rat compared with the normotensive TG controls (Fig. 6). However, unlike WKY rats (25), renal denervation did not affect inner medullary ET-1 content in either genotype. Reduced medullary ET-1 has been demonstrated in other salt-sensitive models such as the Dahl salt-sensitive rat (54), and these reduced ET-1 levels likely contribute to salt sensitivity by reducing ET-1/ETB receptor-mediated natriuresis (30, 35, 51). The Dahl salt-sensitive rat also has reduced levels of medullary ETB receptor (54); however, it is unclear what contributes to these altered levels of ET-1 and ETB receptor. Because ET-1 receptors bind ligand irreversibly, the levels of medullary ET-1 measured experimentally may simply be a reflection of the ET-1 bound to the high concentration of ETB receptor present in this tissue. This would be consistent with the Dahl salt-sensitive and salt-resistant rats in which neither medullary ET-1 content nor ETB receptor expression changed between low and high salt although renal production of ET-1 increased following high salt (54). This same phenomenon was observed in our present study in that rats deficient of ETB receptors had dramatically lower medullary ET-1 content that did not change following high-salt diet even though renal production of ET-1 increased in all groups following high salt (Fig. 6).
We observed increased urine albumin excretion in ETB-def rats on high-salt diet, and denervation prevented this increase (Fig. 7). Several recent studies demonstrated a protective effect of denervation (5, 65) thought to be conferred by removal of efferent sympathetic nerve activity on the kidney. This phenomenon is relatively less well studied in humans, although one report has demonstrated a very profound reduction in renal damage following denervation in patients with chronic kidney disease (36). We cannot directly comment on the protective mechanisms from our present study, and it is possible that the reduction in blood pressure in denervated rats is the driving force behind this protection. However, our results add supportive evidence to the existing literature on the potential protective effects of renal denervation on renal function during hypertension.
One potential limitation of the present study is the method of denervation. Here we applied total denervation by mechanical stripping of the renal nerves and application of a phenol solution. This prevents us from specifically evaluating the contribution of efferent versus afferent renal sympathetic nerves. As renal denervation had not yet been evaluated in this model, we sought first to determine the overall contribution of the renal nerves before exploring specific applications. Because we failed to observe an effect related to blood pressure, renal ET-1 content/production, and pressure-natriuresis following high-salt diet, future work can specifically address the mechanisms involved in mediating the effects observed under normal salt diet by exploring the influence of the afferent renal sympathetic system.
Perspectives and Significance
Our findings support the hypothesis that the ETB receptor system in sympathetic nerves participates in maintaining blood pressure given our observation that bilateral renal sympathetic denervation attenuated hypertension in a model of reduced ETB function. This role, however, appears related to nonneuronal ET-1 activity given that the ETB-def rats maintain ETB receptors in nervous tissue, which is likely due to the impaired natriuresis caused by reduced renal medullary ET-1/ETB receptor function. Strengthening this conclusion is the lack of observable changes in medullary ET-1 content following denervation.
We also demonstrate ETB involvement in circadian control of cardiac and vasomotor autonomic tone. Specifically, during normal salt diet, denervation reduced cardiac LF/HF. Vasomotor LF/HF tone increased following high-salt diet, but this increase was attenuated by renal denervation suggesting a potential afferent mechanism of sympathetic denervation toward altering global autonomic tone in this model of hypertension. These findings demonstrate the need for future work in clarifying the contributions of the ET-1 system in afferent versus efferent mechanisms.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL69999 and HL95499 and American Heart Association (AHA) Grant 15SFRN2390002 (to J. S. Pollock and D. M. Pollock) and NHLBI Grant 127178 (to J. S. Speed). B. K. Becker was supported by a National Institutes of Health institutional training grant (NHLBI Grant HL7457). D. Chen was supported as an AHA Strategically Focused Research Network Hypertension postdoctoral fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.K.B. and D.M.P. conceived and designed research; B.K.B., A.C.F., D.C., M.K., and C.J. performed experiments; B.K.B., A.C.F., D.C., M.K., C.J., and J.S.S. analyzed data; B.K.B., M.K., J.S.S., J.S.P., and D.M.P. interpreted results of experiments; B.K.B. prepared figures; B.K.B. drafted manuscript; B.K.B., D.C., M.K., J.S.S., J.S.P., and D.M.P. edited and revised manuscript; B.K.B., A.C.F., D.C., M.K., C.J., J.S.S., J.S.P., and D.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of J. Miller Allan, Jackson Colson, and Xiaofen Liu.
REFERENCES
- 1.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension 54: 308–314, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asirvatham-Jeyaraj N, Fiege JK, Han R, Foss J, Banek CT, Burbach BJ, Razzoli M, Bartolomucci A, Shimizu Y, Panoskaltsis-Mortari A, Osborn JW. Renal denervation normalizes arterial pressure with no effect on glucose metabolism or renal inflammation in obese hypertensive mice. Hypertension 68: 929–936, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakris GL, Bhatt DL. Reply: a mechanistic explanation for the minimal impact of renal denervation on 24-h ambulatory blood pressure in SIMPLICITY HTN-3. J Am Coll Cardiol 65: 959–960, 2015. doi: 10.1016/j.jacc.2014.11.058. [DOI] [PubMed] [Google Scholar]
- 4.Bakris GL, Townsend RR, Liu M, Cohen SA, D’Agostino R, Flack JM, Kandzari DE, Katzen BT, Leon MB, Mauri L, Negoita M, O’Neill WW, Oparil S, Rocha-Singh K, Bhatt DL; SYMPLICITY HTN-3 Investigators . Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol 64: 1071–1078, 2014. doi: 10.1016/j.jacc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker BK, Speed JS, Powell M, Pollock DM. Activation of neuronal endothelin B receptors mediates pressor response through alpha-1 adrenergic receptors. Physiol Rep 5: e13077, 2017. doi: 10.14814/phy2.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985. [PubMed] [Google Scholar]
- 8.Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010. doi: 10.1088/0967-3334/31/9/009. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370: 1393–1401, 2014. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 10.Budzikowski AS, Vahid-Ansari F, Leenen FH. Chronic activation of brain areas by high-sodium diet in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 274: H2046–H2052, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Budzikowski AS, Vahid-Ansari F, Robertson GS, Leenen FH. Patterns of neuronal activation during development of sodium sensitive hypertension in SHR. Hypertension 30: 1572–1577, 1997. doi: 10.1161/01.HYP.30.6.1572. [DOI] [PubMed] [Google Scholar]
- 12.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL. Increased O2·− production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension 43: 1048–1054, 2004. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- 15.Esler M, Guo L. The future of renal denervation. Auton Neurosci 204: 131–138, 2017. doi: 10.1016/j.autneu.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, Mahfoud F, Schlaich MP. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J 35: 1752–1759, 2014. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA; Symplicity HTN-2 Investigators . Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126: 2976–2982, 2012. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 17a.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903–1909, 2010. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 18.Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26: 463–475, 2012. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- 19.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 310: R262–R267, 2016. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci USA 93: 867–872, 1996. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 102: 1092–1101, 1998. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giles TD. Circadian rhythm of blood pressure and the relation to cardiovascular events. J Hypertens Suppl 24, Suppl 2: S11–S16, 2006. doi: 10.1097/01.hjh.0000220098.12154.88. [DOI] [PubMed] [Google Scholar]
- 25.Girchev R, Bäcker A, Markova P, Kramer HJ. Impaired response of the denervated kidney to endothelin receptor blockade in normotensive and spontaneously hypertensive rats. Kidney Int 65: 982–989, 2004. doi: 10.1111/j.1523-1755.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 116: 976–990, 2015. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 28.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2: 2393–2442, 2012. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 29.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 57: 3–10, 2011. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 30.Hyndman KA, Bugaj V, Mironova E, Stockand JD, Pollock JS. NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. Am J Physiol Renal Physiol 308: F244–F251, 2015. doi: 10.1152/ajprenal.00596.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol 289: H1519–H1529, 2005. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 35: 528–535, 2012. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 41: 851–876, 2001. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 34.Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension 69: 163–170, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kittikulsuth W, Pollock JS, Pollock DM. Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. Am J Physiol Renal Physiol 303: F659–F666, 2012. doi: 10.1152/ajprenal.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiuchi MG, Graciano ML, Carreira MA, Kiuchi T, Chen S, Lugon JR. Long-term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens (Greenwich) 18: 190–196, 2016. doi: 10.1111/jch.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau YE, Galligan JJ, Kreulen DL, Fink GD. Activation of ETB receptors increases superoxide levels in sympathetic ganglia in vivo. Am J Physiol Regul Integr Comp Physiol 290: R90–R95, 2006. doi: 10.1152/ajpregu.00505.2005. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Dai X, Watts S, Kreulen D, Fink G. Increased superoxide levels in ganglia and sympathoexcitation are involved in sarafotoxin 6c-induced hypertension. Am J Physiol Regul Integr Comp Physiol 295: R1546–R1554, 2008. doi: 10.1152/ajpregu.00783.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Böhm M. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J 38: 93–100, 2017. doi: 10.1093/eurheartj/ehw325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller E, Czopek A, Duthie KM, Kirkby NS, van de Putte EE, Christen S, Kimmitt RA, Moorhouse R, Castellan RF, Kotelevtsev YV, Kuc RE, Davenport AP, Dhaun N, Webb DJ, Hadoke PW. Smooth muscle endothelin B receptors regulate blood pressure but not vascular function or neointimal remodeling. Hypertension 69: 275–285, 2017. doi: 10.1161/HYPERTENSIONAHA.115.07031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortensen LH, Fink GD. Salt-dependency of endothelin-induced, chronic hypertension in conscious rats. Hypertension 19: 549–554, 1992. doi: 10.1161/01.HYP.19.6.549. [DOI] [PubMed] [Google Scholar]
- 43.Mosqueda-Garcia R, Appalsamy M, Fernandez-Violante R, Hamakubo T. Modulatory effects of endothelin on baroreflex activation in the nucleus of the solitary tract. Eur J Pharmacol 351: 203–207, 1998. doi: 10.1016/S0014-2999(98)00365-3. [DOI] [PubMed] [Google Scholar]
- 43a.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 44.Ohkita M, Wang Y, Nguyen ND, Tsai YH, Williams SC, Wiseman RC, Killen PD, Li S, Yanagisawa M, Gariepy CE. Extrarenal ETB plays a significant role in controlling cardiovascular responses to high dietary sodium in rats. Hypertension 45: 940–946, 2005. doi: 10.1161/01.HYP.0000161878.81141.62. [DOI] [PubMed] [Google Scholar]
- 45.Osborn JL, Roman RJ, Ewens JD. Renal nerves and the development of Dahl salt-sensitive hypertension. Hypertension 11: 523–528, 1988. doi: 10.1161/01.HYP.11.6.523. [DOI] [PubMed] [Google Scholar]
- 46.Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol (1985) 112: 187–196, 2012. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollock DM. Renal endothelin in hypertension. Curr Opin Nephrol Hypertens 9: 157–164, 2000. doi: 10.1097/00041552-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Colón S, He F, Bixler EO, Fernandez-Mendoza J, Vgontzas AN, Berg A, Kawasawa YI, Liao D. The circadian pattern of cardiac autonomic modulation and obesity in adolescents. Clin Auton Res 24: 265–273, 2014. doi: 10.1007/s10286-014-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi NF, Maliszewska-Scislo M, Chen H. Central endothelin: effects on vasopressin and the arterial baroreflex in doxorubicin heart failure rats. Can J Physiol Pharmacol 86: 343–352, 2008. doi: 10.1139/Y08-027. [DOI] [PubMed] [Google Scholar]
- 50.Sasser JM, Pollock JS, Pollock DM. Renal endothelin in chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol 283: R243–R248, 2002. doi: 10.1152/ajpregu.00086.2002. [DOI] [PubMed] [Google Scholar]
- 51.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw JA, Warren JL. Resistant hypertension and renal denervation where to now? Cardiovasc Ther 33: 9–14, 2015. doi: 10.1111/1755-5922.12103. [DOI] [PubMed] [Google Scholar]
- 53.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speed JS, LaMarca B, Berry H, Cockrell K, George EM, Granger JP. Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 301: R519–R523, 2011. doi: 10.1152/ajpregu.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension 61: 1142–1145, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speed JS, Pollock DM. New clues towards solving the mystery of endothelin and blood pressure regulation. Hypertension 66: 275–277, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 58.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 15: 538–546, 2013. doi: 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Ye Z, Li Y, Zhang J, Zhang Q, Ma X, Peng H, Lou T. Prognostic value of reverse dipper blood pressure pattern in chronic kidney disease patients not undergoing dialysis: prospective cohort study. Sci Rep 6: 34932, 2016. doi: 10.1038/srep34932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang JM, Veerasingham SJ, Tan J, Leenen FH. Effects of high salt intake on brain AT1 receptor densities in Dahl rats. Am J Physiol Heart Circ Physiol 285: H1949–H1955, 2003. doi: 10.1152/ajpheart.00744.2002. [DOI] [PubMed] [Google Scholar]
- 63.Wehrwein EA, Yoshimoto M, Guzman P, Shah A, Kreulen DL, Osborn JW. Role of cardiac sympathetic nerves in blood pressure regulation. Auton Neurosci 183: 30–35, 2014. doi: 10.1016/j.autneu.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J II, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Togo F, Kawano Y. Influence of dietary behavior on the circadian rhythm of the autonomic nervous system as assessed by heart rate variability. Physiol Behav 118: 122–128, 2013. doi: 10.1016/j.physbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, Nakai A, Togo F, Kawano Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur J Appl Physiol 113: 2603–2611, 2013. doi: 10.1007/s00421-013-2702-z. [DOI] [PubMed] [Google Scholar]