Abstract
Placental hypoxia is associated with maternal hypertension, placental insufficiency, and fetal growth restriction. In the pregnant guinea pig, prenatal hypoxia during early gestation inhibits cytotrophoblast invasion of spiral arteries, increases maternal blood pressure, and induces fetal growth restriction. In this study the impact of chronic maternal hypoxia on fetal heart structure was evaluated using four-dimensional echocardiography with spatiotemporal image correlation and tomographic ultrasound, and uterine and umbilical artery resistance/pulsatility indexes and fetal heart function were evaluated using pulsed-wave Doppler ultrasound. Pregnant guinea pigs were exposed to normoxia (n = 7) or hypoxia (10.5% O2, n = 9) at 28–30 days gestation, which was maintained until full term (65 days). At full term, fetal heart structure and outflow tracts were evaluated in the four-chamber view. Fetal heart diastolic function was assessed by E wave-to-A wave diastolic filling ratios (E/A ratios) of both ventricles and systolic function by the myocardial performance index (or Tie) of left ventricles of normoxic (n = 21) and hypoxic (n = 17) fetuses. There were no structural abnormalities in fetal hearts. However, hypoxia induced asymmetric fetal growth restriction and increased the placental/fetal weight compared with normoxic controls. Hypoxia increased Doppler resistance and pulsatility indexes in the uterine, but not umbilical, arteries, had no effect on the Tie index, and increased the E/A ratio in left, but not right, ventricles. Thus, prolonged hypoxia, starting at midgestation, increases uterine artery resistance and generates fetal growth restriction at full term. Furthermore, the enhanced cardiac diastolic filling with no changes in systolic function or umbilical artery resistance suggests that the fetal guinea pig systemic circulation undergoes a compensated, adaptive response to prolonged hypoxia exposure.
Keywords: pregnancy hypoxia, placental insufficiency, maternal hypertension, Doppler ultrasound, fetal echocardiography, guinea pig
gestational hypoxia is associated with increased uterine artery resistance, reduced spiral artery remodeling, and placental insufficiency, leading to preeclamptic-like symptoms of maternal hypertension, renal dysfunction, and fetal growth restriction (8, 18, 22, 37, 48). Conditions of hypoxia during pregnancy can occur as a result of cardiorespiratory disease (26), anemia (11), smoking (28), and living at high altitude (4, 15, 33–35, 55), generating reduced placental oxygenation and fetal hypoxemia, one of the most significant clinical challenges of pregnancy (18, 19).
Several animal models have been used to investigate the underlying mechanisms contributing to hypoxia-induced pregnancy complications and the role of impaired oxygenation in placental and fetal responses. For example, the hemodynamic responses of the fetal circulation to hypoxia have been well characterized using chronically instrumented fetal sheep; these studies have contributed significantly to our understanding of reflex and neuroendocrine responses to acute and chronic hypoxia (2, 13, 18, 19, 44, 46, 47). The fetal response is associated with sustained redistribution of blood flow toward the brain, heart, and adrenal glands and away from peripheral organs as an adaptive response to the limited O2 availability (18, 19, 47), evidenced as reduced body weights of fetuses in hypoxic animals (16) and pregnant women living at high altitude (6). In addition to hemodynamic responses, chronic hypoxia has been shown to have a pathophysiological effect on the fetal heart. For example, hypoxia reduces cardiomyocyte proliferation and increases apoptosis (3, 29) and induces ventricular wall thinning (45) and cardiac cell hypertrophy (30). In addition, hypoxia stimulates fetal cardiac dysfunction in animal models (16, 17, 36) via molecular mechanisms associated with altered gene expression related to contractile dysfunction (5, 25, 39), ventricular wall remodeling (41, 45), oxidative and nitrosative stress (1, 14, 16, 41, 59), and epigenetic programming (17, 42, 43). These studies demonstrate the impact of hypoxia on fetal hemodynamic and cardiac mechanisms, although the mechanisms that mediate the progressive adaptations in the fetus and placenta to prolonged hypoxia remain unknown. This is expected, given the dependency of fetal responses on gestational age at the time of exposure, differences in placentation and O2 sensitivity, and differences in relative fetal maturity among species.
The pregnant guinea pig has been described as an excellent animal model for studying placental development, because its placenta is hemochorial, with trophoblast cell types similar to the human, and has deep trophoblast invasion into the maternal decidua and a longer gestational period than other rodent species (10, 27, 32). We recently generated a pregnant guinea pig model of midgestation hypoxia that exhibits failure of cytotrophoblasts to invade spiral arteries of the placenta, elevated maternal blood pressure, and reduced fetal body weight at full term (60). Histological changes in the hypoxic placenta were associated with an increased junctional zone, enhanced decidualization of the stroma, and increased proliferation of cytotrophoblasts in the subplacenta (a placental source of cytotrophoblasts) and labyrinth (60). We have also demonstrated that hypoxia may induce placental insufficiency in the guinea pig, indicated by a decrease in fetal body weight, despite a maintained placental weight. In other studies, 6–21 days of hypoxia during gestation in the pregnant rat increased placental weight but did not change fetal body weight (48), while 15–21 days of hypoxia decreased both placental and fetal body weight measured at full term (51). Thus, differences in the compensatory response of the placenta/fetus to chronic hypoxia may be related to differences in O2 sensitivity, as well as the relative stages of placental development at the time of exposure.
The purpose of this study is to characterize the maternal and fetal hemodynamic responses at full term to prolonged hypoxia starting in midgestation. Based on our previous studies of hypoxia exposure during late gestation (i.e., the fetal growth phase) (1, 14, 57, 58), we hypothesize that prolonged hypoxia will inhibit placental perfusion, increase uterine artery and umbilical artery resistance, and inhibit fetal heart function. This is the first study to apply Doppler ultrasound technology and fetal echocardiography to evaluate the in vivo consequences of prolonged hypoxia in the pregnant guinea pig.
METHODS
Animal Model
All animal procedures using guinea pigs were approved by the University of Maryland Institutional Animal Care and Use Committee in accordance with Association for Assessment and Accreditation of Laboratory Animal Care-accredited procedures (Animal Welfare Assurance no. A3200-01).
Generation of Hypoxia During Midgestation Pregnancy
Timed-mated pregnancies were generated by mating male breeders with female guinea pigs (~500 g, Hartley-Duncan guinea pigs; Elm Hill Laboratories, Chelmsford, MA) following opening of the vaginal membrane. After 3 days, female guinea pigs were separated from the males. Pregnancy was confirmed by palpation, with the presence of a solid 1-cm-diameter lump corresponding to 20–23 days of gestation (27). Pregnant guinea pigs were housed in separate animal boxes in room air (normoxia) or 10.5% O2 (hypoxia; altitude equivalent ~18,200 ft) in a Plexiglas chamber at 28–30 days gestation and maintained in their respective environments for the duration (35 days) of pregnancy (full term = 65 days). The O2 content of the gas mixture within the chamber was monitored with an O2 probe (Oxysensor, Remington Bioinstruments, Redfield, NY) and regulated with a servocontroller that mixes 100% N2 and room air to obtain 10.5% O2. The gas mixture of the chamber exited through canisters containing soda lime to remove CO2 and silica gel to remove excess water and ammonia. All animals were housed in the same room under identical 12:12-h light-dark cycles and room temperature.
Pregnant guinea pigs were exposed to a hypoxia level (10.5% O2) that reduces maternal guinea pig arterial O2 saturation from 97.7% (in normoxia) to 66.1% (in hypoxia) (1). This level was chosen, since it has been shown to generate hypoxia in the placenta (60), fetal heart ventricle (i.e., increased hypoxia-inducible factor-1α protein expression) (58), and liver tissue (hypoxyprobe staining) (38). The gestational age chosen for hypoxia exposure (28–30 days) is based on the timing of trophoblast invasion into uteroplacental arteries of the placenta (~21 days gestation) (27), which ensures successful implantation but disrupts the progressive nature of invasion of spiral artery remodeling (60).
Food (g·day−1·100 g body wt−1) and water (ml·day−1·100 g body wt−1) intake was routinely monitored over 4-day intervals throughout pregnancy starting at 20 days gestation. For hypoxic animals, exposure to room air was minimized to <1 min during cage cleaning, measurement of food and water consumption, and body weight determinations.
Doppler ultrasound exams were performed in room air on normoxic (n = 7) and hypoxic (n = 9) pregnant guinea pigs and their respective fetuses (n = 21 normoxic and n = 17 hypoxic) at near term (63–65 days gestation) under light anesthesia (ketamine, 40 mg/kg body wt ip) to minimize animal movement. Ketamine at 75 mg/kg ip has previously been shown to reduce guinea pig heart rate by 16% (54). Despite the use of anesthesia, we did not detect significant differences in maternal or fetal heart rates between groups. Immediately after the animals were examined, supplemental anesthesia [ketamine (80 mg/kg ip)-xylazine (6 mg/kg sc)] was administered, and lidocaine (0.3%) was injected subcutaneously to attain an anesthetic state for tissue collection following abdominal incision and hysterotomy. Near-term fetuses and placentas were excised and weighed, and fetuses were sexed.
Doppler Ultrasound Hemodynamics Assessment
A Voluson E8 ultrasound unit (GE Healthcare Ultrasound, Milwaukee, WI) with a 4– to 8-MHz transabdominal probe was used to perform sonograms on full-term normoxic and hypoxic pregnant guinea pigs. Fetal positions were identified within the uterine horns by ultrasound and confirmed at the time of dissection. Maternal right and left uterine arteries of each pregnant sow were identified at the crossover of the external iliac artery branch and identified by pulsed-wave color Doppler and waveform profiles. Doppler waveforms of randomized single umbilical arteries were obtained from each fetus in the litter, identified by color flow around the adjacent border of the fetal bladder. Maternal uterine artery and fetal umbilical artery Doppler resistance index [RI = (systolic velocity – diastolic velocity)/systolic velocity] and pulsatility index [PI = (systolic velocity – diastolic velocity)/mean velocity] were calculated/averaged from at least three consecutive waveforms and recorded. Flow waveform velocities were measured with a <30° angle of insonation. Maternal and fetal heart rates were obtained from uterine and umbilical artery velocity waveforms, respectively.
Fetal Heart Structure Assessment
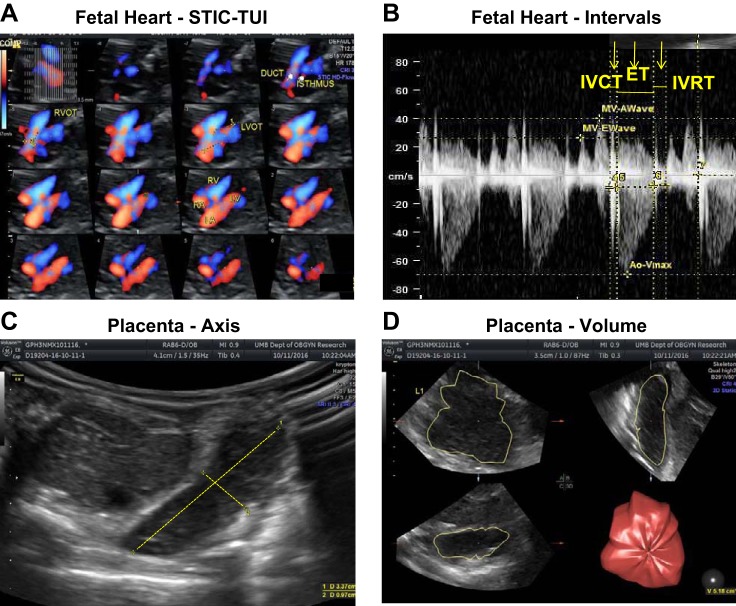
Fetal guinea pigs were evaluated for heart structure using four-dimensional echocardiography with spatiotemporal image correlation (STIC) and tomographic ultrasound imaging (TUI) along with color (Fig. 1A). A four-chamber view of the fetal heart was identified by color Doppler. Low-frequency color Doppler was used with the following settings: color flow map 1, low flow resolution, artifact off, line filter 2, line density 7, and balance 200. The STIC volume was acquired at the level of the four-chamber view. Acquisition time was 10 s at a sweep angle set at 20° (63).
Fig. 1.
Spatiotemporal image correlation (STIC) and tomographic ultrasound imaging (TUI) of fetal heart (A), Doppler images of cardiac intervals (B), and 2-dimensional (C) and 3-dimensional (D) images of placenta. Four-dimensional echocardiography with STIC-TUI, along with color Doppler, was used to evaluate structural features of the fetal heart. Doppler velocity waveforms of right and left outflow tracts were obtained in the 4-chamber view to measure time intervals during the cardiac cycle. 2-Dimensional placental images were obtained using Doppler ultrasound for short and long axes of the placenta. 3-Dimensional placental images were obtained using virtual organ computer-aided analysis (VOCAL) software to determine placental volume. ET, ejection time; IVCT, intraventricular contraction time; IVRT, intraventricular relaxation time.
TUI is applied to acquire multiple cross-sectional images of adjusted slice thickness at specific distances from the four-chamber view to evaluate cardiac structure (Fig. 1A). These steps standardized the number of images to be evaluated and included all the images from the four-chamber view up to the top of the aortic arch. Anatomic landmarks were obtained and evaluated from this postprocessing software. Fetal heart structures, such as the descending aorta, two equal-sized atria and ventricles, two atrioventricular valves, left and right outflow tracts, and aortic and pulmonary duct arches of similar size in transverse view, as well as forward blood flow in both arches, were evaluated for abnormalities (63).
Fetal Heart Function Assessment
Fetal heart systolic and diastolic function was assessed once a satisfactory four-chamber view had been obtained. The Doppler gate was placed inferior to each of the four atrioventricular valves to measure inflow and outflow tract velocities throughout the cardiac cycle. Consecutive waveforms were obtained, and time intervals of diastolic filling and systolic ejection were recorded (Fig. 1B).
Diastolic function was assessed by the velocity waveforms of the mitral and tricuspid valves during ventricular filling. The first [early (E)] wave is generated by the flow velocity during passive ventricular filling from the atria, and the second [atrial (A)] wave is generated by filling during atrial contraction. The E/A ratio is obtained from the maximal flow velocities of each wave and reflects the relative contribution of passive vs. active diastolic filling of the ventricle as indexes of diastolic function (52). For calculation of volumetric flow of outflow tracts, right and left outflow tract diameters were measured at the time of valve closure (end systole).
Systolic function was evaluated by measurement of velocity waveforms of the aortic valve to determine the myocardial performance (Tie) index [(IVCT + IVRT)/ET]. Isovolumetric contraction time (IVCT) was calculated from the time interval between mitral closure and aortic valve opening, relaxation time (IVRT) from aortic valve closure to mitral valve opening, and ejection (ET) time from aortic valve opening to aortic valve closure (Fig. 1B).
Placental Weight/Volume Analysis
Placental dimensions (2 dimensions; short and long axes (Fig. 1C)] and volume [3 dimensions (Fig. 1D)] were evaluated in utero, and placental weight was measured ex utero at the time of dissection. A Voluson E8 ultrasound machine (GE Healthcare Ultrasound) with a 4- to 8-MHz transducer was used for volume analysis. Initially, the volume box was adjusted to scan the entire placenta. After the entire volume was scanned, the three orthogonal ultrasonographic sections were analyzed. The longest view of the placenta on the A plane of the three orthogonal ultrasonographic sections was chosen as the reference image. The volume was then measured by the rotational technique with virtual organ computer-aided analysis (VOCAL) software (3-dimensional Sonoview, GE Healthcare on the Voluson E8). This method consists of outlining the contour of the placenta six times at each 15° rotation of the image. After the complete rotation was finished, the placental volume was automatically calculated by the VOCAL software. The placenta images illustrate how short and long axes are measured to derive placental thickness and length, respectively, for determination of placental volume.
Statistical Analysis
Systat software (San Jose, CA) was used for statistical analyses. Data are expressed as means ± SE. Maternal body weight and food and water intake rates of normoxic and hypoxic groups were compared using a two-way repeated-measures analysis of variance (ANOVA) with days and treatment as independent variables. If differences in the mean values following the ANOVA were significant (P < 0.05), a Holm-Sidak test was applied as a post hoc comparison between groups. Between-groups (sex and treatment) comparisons of fetal body weights, organ weights, and Doppler indexes of placental volume were made using a two-way ANOVA followed by a Holm-Sidak multiple-comparison post hoc test. Doppler indexes of uterine and umbilical artery hemodynamics and fetal heart function of normoxic and hypoxic groups were compared using a Student’s t-test with a significance of P < 0.05. The fetuses and placentas from all the litters were combined under each single treatment group for determination of fetal body and organ weights; n = 1 indicates a single fetus under each treatment or a single pregnant sow.
RESULTS
Effects of Hypoxia on Maternal Body Weight and Food and Water Intake
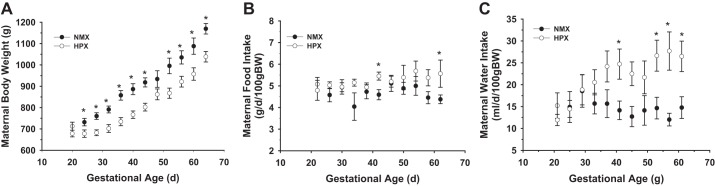
The body weight profile of pregnant sows was significantly different between normoxic and hypoxic groups (Fig. 2A). Hypoxia caused a transient decrease in maternal body weight measured at the time of exposure and a decrease in body weight gain up to 30 days gestation, at which time there was a parallel increase in body weight until full term. However, there was no significant difference in maternal food intake between groups (Fig. 2B) throughout the course of gestation, although there was a consistent and significant increase in maternal water intake rate after 50 days gestation in hypoxic animals compared with normoxic controls (Fig. 2C).
Fig. 2.
Effect of chronic hypoxia on maternal body weight (A) and maternal food (B) and water (C) intake. Maternal body weight was measured at 4-day intervals, along with food and water consumption of normoxic (n = 7) and hypoxic (10.5% O2; n = 9) animals. Differences between groups were identified by 2-way repeated-measures ANOVA followed by a post hoc (Holm-Sidak) multiple-comparison test. *P < 0.05 vs. normoxic control.
Effects of Hypoxia on Fetal Body and Organ Weights
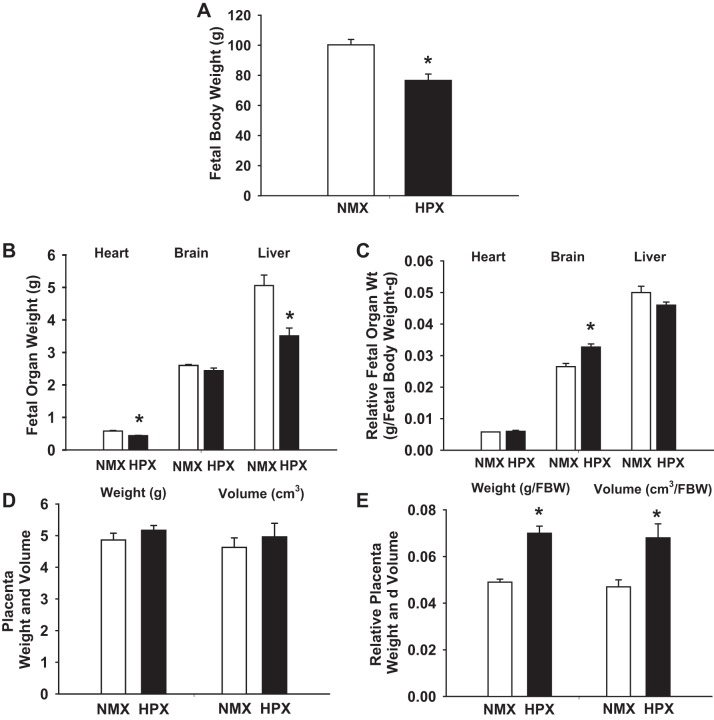
Near-term fetal body weight was decreased (P < 0.001) by 23% in chronically hypoxic animals compared with normoxic controls (Fig. 3A). The average litter size was 3.0 ± 0.5 fetuses for normoxic and 2.1 ± 0.4 fetuses for hypoxic guinea pigs. Chronic hypoxia decreased (P < 0.001) fetal heart and liver weights but had no significant effect on fetal brain weight. Relative organ weight was calculated from fetal organ weight-to-body weight ratio. Chronic hypoxia increased (P < 0.001) relative fetal brain weight by 23% but had no significant effect on relative fetal liver or heart weight (Fig. 3, B and C). Table 1 illustrates the effects of chronic hypoxia on fetal parameters based on sex differences derived from the combined fetal group described above. Hypoxia-induced changes in fetal body and organ weights were similar to those of each combined fetal group, with no sex-related differences.
Fig. 3.
Effect of chronic hypoxia on full-term fetal body and organ weights and volume. Fetal body weight (A) and organ weights of normoxic (n = 21, NMX) and hypoxic (n = 17, HPX) fetuses were obtained at full term and measured as absolute (B) and relative (C) organ weight (normalized to fetal body weight). Absolute placental weight and volume (D) were normalized to fetal body weight (FBW) as relative placental weight and volume (E). Values are means ± SE. *P < 0.05 vs. normoxic control.
Table 1.
Effects of hypoxia on fetal guinea pig organ and placental weights at full term (65 days)
| Male |
Female |
|||
|---|---|---|---|---|
| Normoxia (n = 11) | Hypoxia (n = 7) | Normoxia (n = 10) | Hypoxia (n = 10) | |
| Fetus | ||||
| Body wt, g | 94.7 ± 4.4 | 75.9 ± 4.0* | 106.4 ± 5.3 | 77.3 ± 6.8** |
| Heart wt, g | 0.56 ± 0.03 | 0.45 ± 0.02* | 0.60 ± 0.03 | 0.44 ± 0.02*** |
| Brain wt, g | 2.61 ± 0.04 | 2.51 ± 0.04 | 2.60 ± 0.05 | 2.39 ± 0.14 |
| Liver wt, g | 4.85 ± 0.46 | 3.24 ± 0.20* | 5.30 ± 0.46 | 3.70 ± 0.39* |
| Heart wt/body wt | 0.0059 ± 0.0002 | 0.0060 ± 0.0002 | 0.0057 ± 0.0002 | 0.0060 ± 0.0004 |
| Brain wt/body wt | 0.028 ± 0.001 | 0.034 ± 0.002* | 0.025 ± 0.001 | 0.032 ± 0.002** |
| Liver wt/body wt | 0.050 ± 0.003 | 0.043 ± 0.001 | 0.049 ± 0.003 | 0.048 ± 0.002 |
| Placenta | ||||
| Weight, g | 4.73 ± 0.36 | 5.13 ± 0.08 | 5.01 ± 0.23 | 5.20 ± 0.25 |
| Volume, cm3 | 4.85 ± 0.56 (5) | 5.58 ± 0.95 (5) | 4.44 ± 0.32 (6) | 4.99 ± 0.53 (10) |
| Placental wt/fetal body wt | 0.050 ± 0.002 | 0.070 ± 0.003*** | 0.047 ± 0.001 | 0.068 ± 0.005*** |
| Placental volume/fetal body wt | 0.051 ± 0.005 (5) | 0.081 ± 0.013*** (5) | 0.043 ± 0.004 (6) | 0.067 ± 0.007*** (10) |
| Length, cm | 3.03 ± 0.22 (5) | 2.86 ± 0.15 (5) | 3.18 ± 0.16 (6) | 2.88 ± 0.12 (10) |
| Thickness, cm | 1.01 ± 0.05 (5) | 0.86 ± 0.12 (5) | 1.05 ± 0.11 (6) | 1.37 ± 0.22 (10) |
Values are means ± SE; n values for placental volumes and dimensions are shown in parentheses. All groups were compared using 2-way ANOVA, with sex and treatment as variables, followed by Holm-Sidak multiple-comparison post hoc test. There were no sex-related differences regardless of treatment.
P < 0.05,
P < 0.01,
P < 0.001 vs. normoxia for each sex.
Effects of Hypoxia on Doppler Indexes of Maternal and Fetal Arteries
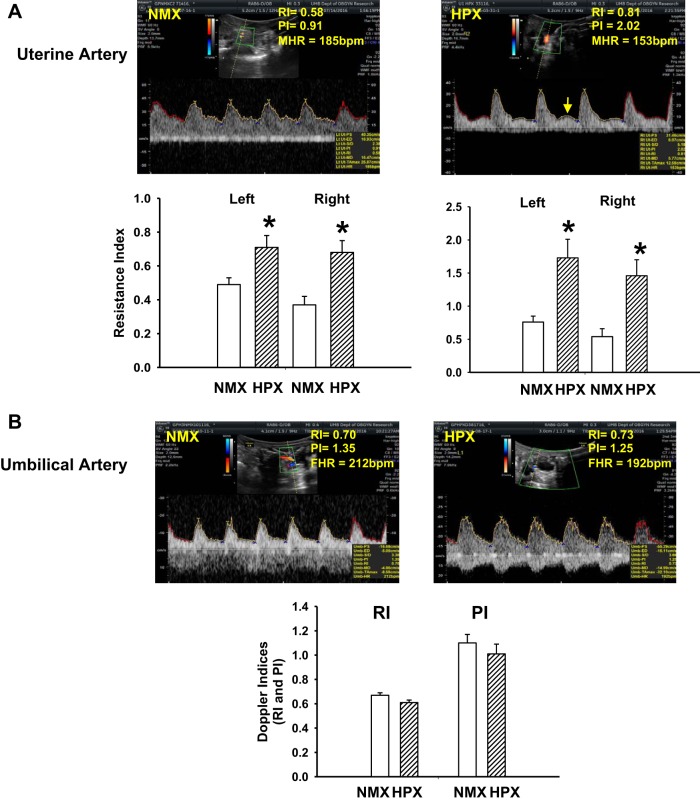
Doppler ultrasound waveform analysis was used to determine the effects of hypoxia on flow velocity in maternal uterine and umbilical arteries. Chronic hypoxia significantly increased RI and PI in uterine arteries of left and right uterine horns (Fig. 4A). In half of the traces (4 of 8 hypoxic vs. 0 of 7 normoxic guinea pigs), there was evidence of diastolic notching in hypoxic animals. Doppler indexes of umbilical arteries were obtained from three consecutive waveforms. There was no effect of chronic hypoxia on RI or PI values compared with normoxic controls (Fig. 4B).
Fig. 4.
Doppler ultrasound indexes of resistance (RI) and pulsatility (PI) of uterine artery of normoxic (n = 7) and hypoxic (n = 9) pregnant guinea pigs (A) and umbilical artery of normoxic (n = 21) and hypoxic (n = 17) fetal guinea pigs (B). Doppler velocity waveforms are illustrated for maternal uterine artery (A; arrow indicates diastolic notching) and umbilical artery (B). MHR and FHR, maternal and fetal heart rate. RI and PI values (means ± SE) of normoxic and hypoxic animals are shown below images. *P < 0.05 vs. normoxic control.
Effects of Hypoxia on Fetal Heart Structure and Function
Chronic hypoxia had no effect on generation of gross structural abnormalities within the fetal heart based on images obtained from STIC-TUI (Fig. 1A). Four-dimensional echocardiography and STIC-TUI identified the specific heart structures for evaluation of heart defects. Despite the lack of hypoxia-induced defects in the current study, this is a useful application for visualization of potential fetal heart abnormalities in utero.
Diastolic heart function of the right and left ventricles of normoxic and hypoxic fetuses was measured in utero from the E and A waveforms of mitral and tricuspid heart valves’ peak velocities (Table 2). Chronic hypoxia significantly increased the E/A ratio of the left (mitral valve), but not the right (tricuspid valve), ventricle. The increase in left ventricle E/A ratio is due to a significant increase in the E wave, whereas E and A waves were similarly increased in the right ventricle.
Table 2.
Effects of hypoxia on in vivo heart function of full-term fetal guinea pigs
| Normoxia (n = 21) | Hypoxia (n = 17) | |
|---|---|---|
| Maternal HR, beats/min | 180.6 ± 8.6 | 161.1 ± 6.2 |
| Fetal HR, beats/min | 196.1 ± 6.2 | 209.8 ± 6.7 |
| Systolic function | ||
| IVCT, ms | 0.026 ± 0.001 | 0.030 ± 0.003 |
| ET, ms | 0.126 ± 0.005 | 0.132 ± 0.005 |
| IVRT, ms | 0.046 ± 0.002 | 0.050 ± 0.003 |
| Tie index | 0.577 ± 0.019 | 0.622 ± 0.027 |
| Diastolic function | ||
| Mitral valve | ||
| E wave velocity | 20.4 ± 1.3 | 24.4 ± 2.3* |
| E/A ratio | 0.60 ± 0.03 | 0.72 ± 0.04* |
| Tricuspid valve | ||
| E wave velocity | 22.4 ± 1.5 | 30.5 ± 4.1 |
| A wave velocity | 33.0 ± 2.0 | 45.1 ± 6.0 |
| E/A ratio | 0.711 ± 0.035 | 0.66 ± 0.02 |
| Outflow-left | ||
| Aorta | ||
| Diameter, cm | 0.194 ± 0.006 | 0.203 ± 0.008 |
| CSA, cm3 | 0.030 ± 0.002 | 0.033 ± 0.003 |
| Vmax, cm/s | 43.45 ± 3.04 | 58.82 ± 14.81 |
| Flow, cm3/ms | 1.209 ± 0.149 | 1.654 ± 0.378 |
| Outflow-right | ||
| Pulmonary artery | ||
| Diameter, cm | 0.205 ± 0.007 | 0.190 ± 0.010 |
| CSA, cm3 | 0.034 ± 0.002 | 0.030 ± 0.003 |
| Vmax, cm/s | 48.05 ± 3.88 | 47.74 ± 10.05 |
| Flow, cm3/s | 1.66 ± 0.21 | 1.46 ± 0.31 |
Values are means ± SE. HR, heart rate; IVCT, intraventricular contraction time; IVRT, intraventricular relaxation time; ET, ejection time; Tie index, IVCT + IVRT/ET; CSA, cross-sectional area; Vmax, maximal flow velocity. All groups were compared using Student’s t-test.
P < 0.05 vs. normoxia.
Myocardial performance was measured as the Tie index to determine the effect of chronic hypoxia on systolic function. Chronic hypoxia had no significant effect on maternal or fetal heart rates measured at the time of the Doppler exam under normoxic conditions (Table 2). Table 2 also lists the cardiovascular parameters measured to determine the effect of hypoxia on fetal heart function. There were no differences in the time intervals of IVCT, ET, and IVRT between the treatment groups. As a result, chronic hypoxia had no significant effect on systolic heart function as measured by the Tie index.
Doppler ultrasound was used to obtain diameters of right (main pulmonary artery) and left (aorta) outflow tracts of the fetal heart (Table 2). Chronic hypoxia had no effect on right or left outflow tract diameters or their corresponding cross-sectional areas. Using peak maximal velocities of the Doppler waveforms from the aortic and main pulmonary arteries and their respective cross-sectional areas, maximal blood flow (in cm3/s) of the aorta and main pulmonary arteries was calculated as outflow tract area (in cm2) × maximal flow velocity (in cm/s). Chronic hypoxia increased maximal aortic blood flow by 36.4% [1.21 ± 0.15 cm3/s (normoxia) vs. 1.65 ± 0.38 cm3/s (hypoxia), P = 0.17] and decreased main pulmonary blood flow by 12.0% [1.66 ± 0.21 cm3/s (normoxia) vs. 1.46 ± 0.31 cm3/s (hypoxia), P = 0.08].
Effects of Hypoxia on Placental Weight and Volume
Chronic hypoxia had no significant effect on absolute placental weight or volume. However, hypoxia significantly increased (P < 0.001) relative placental weight and volume compared with normoxic controls (Fig. 3, D and E). Hypoxia had no effect on absolute placental weight, volume, length, or thickness but increased (P < 0.001) relative weight and volume in male and female fetuses (Table 1). There were no sex-related differences in any of the placental parameters measured.
DISCUSSION
This study evaluated the effect of hypoxia from midgestation to late gestation on hemodynamic responses of the maternal, placental, and fetal compartments in the pregnant guinea pig. Results from Doppler ultrasound demonstrate that chronic hypoxia increases maternal uterine artery resistance, maintains placental size, and may redistribute fetal cardiac output toward the brain by an enhanced diastolic filling of the left ventricle of the fetal heart. For example, fetal left ventricle diastolic filling was enhanced, rather than decreased, and systolic function remained unchanged, indicating that contractile function is unaffected or compensates during the course of hypoxic pregnancy. This animal model identifies several compensatory and decompensatory cardiovascular responses to chronic hypoxia that likely reflect placental and fetal consequences to prolonged exposure during pregnancy.
Maternal Response
Chronic hypoxia decreases body weight of the pregnant sows within days of exposure during midgestation. Given that maternal food consumption was not altered by hypoxia, reduced fetal growth in utero may be mediated by altered nutrient transport, rather than availability, since hypoxia may inhibit nutrient transport mechanisms in the placenta (12). We speculate that the increase in water consumption in hypoxic animals may compensate for an increase in respiratory water loss that may be stimulated by an increase in ventilatory rate (not measured) (23).
Uterine artery response.
Chronic hypoxia increased uterine vascular resistance, as indicated by the increase in RI/PI and suggests altered remodeling of the uterine circulation. This may contribute to the maternal hypertension measured previously in pregnant guinea pigs under the same hypoxic conditions (60). Normal uterine artery remodeling is necessary to increase uterine blood flow during pregnancy. In the normal pregnant guinea pig, Verkeste et al. (64) showed that trophoblast invasion in the uterine circulation advances to mesometrial and myometrial artery branches upstream of the spiral arteries but stops at the main arcade artery branches. In response to high-altitude hypoxia, vascular remodeling of uterine arteries of pregnant guinea pigs was altered by mechanisms associated with decreased vascular smooth muscle proliferation and DNA synthesis (49, 50), diminished flow-stimulated vasodilation, and reduced nitric oxide-dependent relaxation (31, 65). Hypoxia exposure and cytotrophoblast invasion of spiral arteries (27) occurred at the same gestational age as the hypoxia-induced decrease in DNA synthesis of uterine vascular smooth muscle (50) and inhibited cytotrophoblast invasion of spiral arteries (60). Thus, hypoxia may decrease the remodeling and diameter at upstream (uterine artery) sites and disrupt normal spiral artery remodeling at downstream sites. In the human placenta with inhibited trophoblast invasion, reduced spiral artery remodeling is not considered to contribute significantly to the increase in uterine artery resistance/pulsatility (7), arguing for a greater contribution of altered upstream uterine artery remodeling. In the guinea pig placenta, upstream and downstream vessels are altered by hypoxia, which may reduce uterine blood flow and placental perfusion. However, the effect of hypoxia on spiral artery remodeling may differ in the guinea pig, since maternal blood enters labyrinth arteries, rather than an intervillous space, as in the human placenta. How hypoxia affects in vivo vessel growth (i.e., angiogenesis) remains unclear, since indirect mechanisms of flow shear stress, synthesis of vasoactive factors, and/or factors regulating cell growth also contribute to uterine vessel remodeling (40). It should be noted that, in the current study, Doppler ultrasound measures flow velocity, and measurements of volumetric flow could not be calculated from vessel diameters because of the limitation of imaging the small-sized arteries. Therefore, in studies where diameter measurements cannot be obtained, inferences to blood flow changes should be evaluated cautiously.
Fetal Response
Umbilical artery response.
Chronic hypoxia generated asymmetric growth restriction of guinea pig fetuses, with a redistribution of blood flow during chronic hypoxia toward vital organs such as the brain, heart, and adrenal glands and away from peripheral circulations as an adaptive response to the reduced oxygenation (20, 47). In the current study the growth changes in the fetal organs, such as increased relative brain weight and reduced relative liver weight, support the expected flow redistribution during chronic hypoxia (i.e., brain sparing and reduced hepatic blood flow) (47).
To evaluate the effect of hypoxia on the fetal side of the placenta, Doppler indexes of the umbilical artery were evaluated. An increase in umbilical artery resistance/pulsatility would indicate an increase in vascular resistance on the fetal side of the placenta and generate an increased afterload to the fetal heart. Because of the decrease in fetal growth and increased relative placental size, we expected hypoxia to increase umbilical artery resistance/pulsatility as evidence of increased placental vascular resistance. In a previous study using fetal guinea pigs, Doppler ultrasound characterized a progressive reduction in umbilical artery resistance and pulsatility with advancing gestation in normal pregnancy (61). Doppler indexes of normoxic fetal guinea pigs in the current study were similar to those of umbilical artery RI (0.5–0.6) and PI (0.9) values previously reported (21, 61, 62). Yet hypoxia had no significant effect on either umbilical artery index. It is possible that placental perfusion may be affected differently on different sides of the placenta, resulting in enhanced perfusion on the fetal side concomitant with reduced perfusion on the maternal side. Our previous study (60) shows reduced spiral artery remodeling and morphological changes in enlarged blood spaces and sites of coagulation that could account for altered perfusion patterns within the hypoxic placenta.
Fetal heart function.
The fetal heart responses to chronic hypoxia take into consideration aspects related to preload, afterload, and contractile function. Chronic hypoxia had no effect on umbilical artery Doppler indexes (afterload) or fetal left heart systolic function (contractile function), but it increased the E/A ratio of the left ventricle (preload; index of diastolic filling) (20). The hypoxia-induced increase in the E/A ratio suggests an increased preload mediated by enhanced blood flow through the foramen ovale. In human fetal hearts, the E/A ratios are <1.0 and increase with advancing gestation due to progressive increases in the E waveform velocity, while the A waveform velocity remains the same (20). The increase in the E/A ratio of the left ventricle supports enhanced passive diastolic filling in the hypoxic fetal heart. The increased filling would be expected to increase carotid artery blood flow and accommodate brain sparing during hypoxia (20), which is supported by the trend toward an increased aortic blood flow and reduced main pulmonary blood flow calculated from the outflow tract flow velocities.
The enhanced filling of the fetal heart during hypoxia has been shown to be affected by an increased venous return mediated by increased distribution of umbilical venous blood through the ductus venosus in fetal sheep (12, 44, 46, 56). For example, fetal hypoxemia increased umbilical vein resistance and decreased ductus venosus resistance in fetal sheep (44). In addition, fetal hypoxemia increased the percentage of umbilical vein blood bypassing the liver through the ductus venous (46), thereby contributing to enhanced venous return to the heart. In contrast, Edelstone et al. (13) showed no effect of ovine fetal hypoxemia on ductus venosus or liver blood flow. The vascular response of diverting umbilical blood flow through the ductus venosus and bypassing the liver in response to fetal hypoxemia varies among studies and may be due to differences in perfusion pressures within the fetal circulation and the level of hypoxemia (12). These studies suggest that regulation of the ductus venosus resistance by hypoxemia is an adaptive mechanism for enhancing venous return and cardiac filling. In the guinea pig, vascular mechanisms that enhance venous return during hypoxemia differ from those in the fetal sheep because of the absence of a functional ductus venosus and umbilical vein blood flow that is distributed directly to the liver (10). Thus an enhanced venous return during fetal hypoxemia may occur instead by diverting hepatic blood flow directly to the vena cava, since unequal perfusion patterns have been demonstrated within the right lobe (9), allowing for umbilical blood flow to bypass the hepatic sinusoids (54).
Few studies have reported the effects of hypoxia on systolic function of the fetal heart in vivo in small animal models. However, it is well supported that chronic hypoxia negatively impacts fetal heart function in several studies, including our own, via disruption of normal gene expression (1, 14, 43), generation of oxidative and nitrosative stress (18, 41, 59), and disruption of normal cardiac remodeling (18, 29). In addition, fetal hypoxia induces lasting consequences that are manifest as increased risk of ischemia-reperfusion injury measured in isolated heart preparations of offspring rats (66). Based on results from hypoxia studies using ex vivo heart preparations (14, 16, 41, 51, 57, 58), we expected to measure reduced systolic function in vivo. Yet we measured no significant change in the Tie index or individual cardiac time intervals in hypoxic hearts, indicating no change in left ventricular systolic function. We conclude that the lack of change in umbilical artery resistance with maintained placental growth may reduce afterload and, therefore, workload of the hypoxic fetal heart. We attribute this fetal response to prolonged exposure to hypoxia, which provides sufficient time for the cardiovascular system to adapt. This may indicate systemic plasticity of the fetal circulation in response to prolonged hypoxia that differs from conditions of hypoxia of shorter durations (i.e., late gestation), which limits the time interval for compensation. An additional compensatory mechanism, such as increased coronary artery perfusion (24), may also be supportive of heart function in the chronically hypoxic fetus.
Placental Response
Placental weight and volume were maintained under conditions of hypoxia, resulting in increased placental-to-fetal weight ratios. Morphological changes (60) that accompany this response to hypoxia may be associated with altered blood perfusion, O2 and nutrient transport, and/or uteroplacental artery development. However, although placental growth was maintained during hypoxia, fetal growth was restricted, suggesting a less efficient nutrient transfer to the fetus (8). The inefficient nature of the hypoxic guinea pig placenta is further supported by the morphological changes in enlarged blood spaces, altered trophoblast proliferation, and reduced spiral artery remodeling previously identified (60). The interactions between altered placental morphology, fetal growth restriction, and placental perfusion are integrally related to the hemodynamics of the fetal circulation, and these relationships remain poorly understood with respect to chronic hypoxia.
Perspectives and Significance
In response to early exposure to hypoxia, the pregnant guinea pig exhibits increased uterine artery resistance, reduced uteroplacental vessel remodeling, and failure of trophoblasts to invade spiral arteries (60). On the fetal side, the hypoxic guinea pig placenta exhibits sustained placental weight and volume, likely due to enhanced trophoblast proliferation in the subplacenta and labyrinth and increased blood spaces in the labyrinth (60). There appears to be no deficit in fetal heart function but, rather, increased preload of the left ventricle, increased aortic blood flow, and, presumably, redistribution of blood flow to the brain via the aortic arch branches and carotid artery. The unexpected finding is that fetal heart function measured in vivo in an intact circulation of the guinea pig is not disrupted by hypoxia, despite fetal growth restriction. Regardless, this study demonstrates the functional capacity of the guinea pig sow and fetus to adapt to prolonged hypoxic stress. Future studies will investigate the response of the compensatory mechanisms to shorter durations of hypoxia with regard to uteroplacental vessel remodeling, placental perfusion, and fetal heart and circulatory function.
GRANTS
This project is supported in part by National Heart, Lung, and Blood Institute Grant HL-126859 (L. P. Thompson).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T. and L.P.T. conceived and designed research; S.T. and G.W.A. performed experiments; S.T., G.W.A., and L.P.T. interpreted results of experiments; S.T. and L.P.T. drafted manuscript; S.T., G.W.A., and L.P.T. edited and revised manuscript; S.T., G.W.A., and L.P.T. approved final version of manuscript; L.P.T. analyzed data; L.P.T. prepared figures.
ACKNOWLEDGMENTS
The authors thank Gerard Pinkas for technical assistance in generating and maintaining the animal colony.
REFERENCES
- 1.Al-Hasan YM, Evans LC, Pinkas GA, Dabkowski ER, Stanley WC, Thompson LP. Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal guinea pig organs. Reprod Sci 20: 299–307, 2013. doi: 10.1177/1933719112453509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, Botting KJ, Cross CM, Itani N, Skeffington KL, Beck C, Giussani DA. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 594: 1247–1264, 2016. doi: 10.1113/JP271091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567, 1989. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne VA, Stiffel VM, Pearce WJ, Longo LD, Gilbert RD. Activator calcium and myocardial contractility in fetal sheep exposed to long-term high-altitude hypoxia. Am J Physiol Heart Circ Physiol 272: H1196–H1204, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Browne VA, Julian CG, Toledo-Jaldin L, Cioffi-Ragan D, Vargas E, Moore LG. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 370: 20140068, 2015. doi: 10.1098/rstb.2014.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482, 2009. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 96: 1509–1565, 2016. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter AM. The blood supply to the abdominal organs of the fetal guinea-pig. J Dev Physiol 6: 407–416, 1984. [PubMed] [Google Scholar]
- 10.Carter AM. Animal models of human placentation—a review. Placenta 28 Suppl A: S41–S477, 2007. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Davis L, Thornburg KL, Giraud GD. The effects of anaemia as a programming agent in the fetal heart. J Physiol 565: 35–41, 2005. doi: 10.1113/jphysiol.2004.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimasuay KG, Boeuf P, Powell TL, Jansson T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol 7: 12, 2016. doi: 10.3389/fphys.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstone DI, Rudolph AM, Hemann MA. Effects of hypoxemia and decreasing umbilical flow on liver and ductus venosus blood flows in fetal lambs. Am J Physiol Heart Circ Physiol 238: H656–H663, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Evans LC, Liu H, Pinkas GA, Thompson LP. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr Res 71: 25–31, 2012. doi: 10.1038/pr.2011.10. [DOI] [PubMed] [Google Scholar]
- 15.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7: e31017, 2012. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giussani DA, Davidge ST. Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4: 328–337, 2013. doi: 10.1017/S204017441300010X. [DOI] [PubMed] [Google Scholar]
- 18.Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington KL, Itani N, Wooding FB, Cross CM, Allison BJ. Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol 814: 77–87, 2014. doi: 10.1007/978-1-4939-1031-1_7. [DOI] [PubMed] [Google Scholar]
- 19.Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594: 1215–1230, 2016. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey ME, Messing B, Cohen SM, Valsky DV, Yagel S. Functional assessment of the fetal heart: a review. Ultrasound Obstet Gynecol 39: 131–144, 2012. doi: 10.1002/uog.9064. [DOI] [PubMed] [Google Scholar]
- 21.Herrera EA, Alegría R, Farias M, Díaz-López F, Hernández C, Uauy R, Regnault TR, Casanello P, Krause BJ. Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs. J Physiol 594: 1553–1561, 2016. doi: 10.1113/JP271467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol 45: 189–200, 2006. doi: 10.1016/S1028-4559(09)60224-2. [DOI] [PubMed] [Google Scholar]
- 23.Ivy CM, Scott GR. Ventilatory acclimatization to hypoxia in mice: methodological considerations. Respir Physiol Neurobiol 235: 95–103, 2017. doi: 10.1016/j.resp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Jonker SS, Davis L, Soman D, Belcik JT, Davidson BP, Atkinson TM, Wilburn A, Louey S, Giraud GD, Lindner JR. Functional adaptations of the coronary microcirculation to anaemia in fetal sheep. J Physiol 594: 6165–6174, 2016. doi: 10.1113/JP272696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamitomo M, Onishi J, Gutierrez I, Stiffel VM, Gilbert RD. Effects of long-term hypoxia and development on cardiac contractile proteins in fetal and adult sheep. J Soc Gynecol Investig 9: 335–341, 2002. doi: 10.1177/107155760200900603. [DOI] [PubMed] [Google Scholar]
- 26.Katz O, Sheiner E. Asthma and pregnancy: a review of two decades. Expert Rev Respir Med 2: 97–107, 2008. doi: 10.1586/17476348.2.1.97. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann P, Davidoff M. The guinea-pig placenta. Advances in anatomy, embryology, and cell biology. Adv Anat Embryol Cell Biol 53: 5–91, 1977. [DOI] [PubMed] [Google Scholar]
- 28.Longo LD. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol 129: 69–103, 1977. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- 29.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 580: 639–648, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, Hohimer AR, Semenza GL. Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol 178: 527–534, 1998. doi: 10.1016/S0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 31.Mateev S, Sillau AH, Mouser R, McCullough RE, White MM, Young DA, Moore LG. Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am J Physiol Heart Circ Physiol 284: H820–H829, 2003. doi: 10.1152/ajpheart.00701.2002. [DOI] [PubMed] [Google Scholar]
- 32.Mess A. The guinea pig placenta: model of placental growth dynamics. Placenta 28: 812–815, 2007. doi: 10.1016/j.placenta.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in Tibetan women during pregnancy at 3,658 m. Am J Phys Anthropol 114: 42–53, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4: 141–156, 2003. doi: 10.1089/152702903322022767. [DOI] [PubMed] [Google Scholar]
- 35.Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol 178: 181–190, 2011. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35: 730–743, 2008. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 37.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost 7: 375–384, 2009. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 38.Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol 199: 78e1–78e6, 2008. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Onishi J, Kamitomo M, Stiffel VM, Gilbert RD. Effects of long-term high-altitude hypoxia on myocardial protein kinase A activity and troponin I isoforms in fetal and nonpregnant sheep. J Soc Gynecol Investig 10: 189–193, 2003. doi: 10.1016/S1071-5576(03)00042-X. [DOI] [PubMed] [Google Scholar]
- 40.Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation 21: 38–47, 2014. doi: 10.1111/micc.12080. [DOI] [PubMed] [Google Scholar]
- 41.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med 10: 653–666, 2010. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res 107: 365–373, 2010. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCε gene in foetal rat hearts. Cardiovasc Res 93: 302–310, 2012. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulick RP, Meyers RL, Rudolph CD, Rudolph AM. Venous responses to hypoxemia in the fetal lamb. J Dev Physiol 14: 81–88, 1990. [PubMed] [Google Scholar]
- 45.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuss ML, Rudolph AM. Distribution and recirculation of umbilical and systemic venous blood flow in fetal lambs during hypoxia. J Dev Physiol 2: 71–84, 1980. [PubMed] [Google Scholar]
- 47.Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 119: 717–723, 1998. doi: 10.1016/S1095-6433(98)01010-1. [DOI] [PubMed] [Google Scholar]
- 48.Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova-Davies T, Spasic-Boskovic O, Dunster C, Mudway IS, Kelly FJ, Burton GJ, Poston L, Giussani DA. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590: 1377–1387, 2012. doi: 10.1113/jphysiol.2011.226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockwell LC, Keyes LE, Moore LG. Chronic hypoxia diminishes pregnancy-associated DNA synthesis in guinea pig uteroplacental arteries. Placenta 21: 313–319, 2000. doi: 10.1053/plac.1999.0487. [DOI] [PubMed] [Google Scholar]
- 50.Rockwell LC, Dempsey EC, Moore LG. Chronic hypoxia diminishes the proliferative response of guinea pig uterine artery vascular smooth muscle cells in vitro. High Alt Med Biol 7: 237–244, 2006. doi: 10.1089/ham.2006.7.237. [DOI] [PubMed] [Google Scholar]
- 51.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res 90: 285–294, 2011. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 52.Russell NE, McAuliffe FM. First-trimester fetal cardiac function. J Ultrasound Med 27: 379–383, 2008. doi: 10.7863/jum.2008.27.3.379. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz S, Tacke S, Guth B, Henke J. Comparison of physiological parameters and anaesthesia specific observations during isoflurane, ketamine-xylazine or medetomidine-midazolam-fentanyl anaesthesia in male guinea pigs. PLoS One 22: e0161258, 2016. doi: 10.1371/journal.pone.0161258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silver M, Barnes RJ, Comline RS, Burton GJ. Placental blood flow: some fetal and maternal cardiovascular adjustments during gestation. J Reprod Fertil Suppl 31: 139–160, 1982. [PubMed] [Google Scholar]
- 55.Soria R, Julian CG, Vargas E, Moore LG, Giussani DA. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res 74: 633–638, 2013. doi: 10.1038/pr.2013.150. [DOI] [PubMed] [Google Scholar]
- 56.Tchirikov M, Schröder HJ, Hecher K. Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol 27: 452–461, 2006. doi: 10.1002/uog.2747. [DOI] [PubMed] [Google Scholar]
- 57.Thompson LP, Dong Y. Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts. J Soc Gynecol Investig 12: 388–395, 2005. doi: 10.1016/j.jsgi.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Thompson L, Dong Y, Evans L. Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatr Res 65: 188–192, 2009. doi: 10.1203/PDR.0b013e31818d6ad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy 2012: 582748, 2012. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod 128: 1–10, 2016. doi: 10.1095/biolreprod.116.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner AJ, Trudinger BJ. Ultrasound measurement of biparietal diameter and umbilical artery blood flow in the normal fetal guinea pig. Comp Med 50: 379–384, 2000. [PubMed] [Google Scholar]
- 62.Turner AJ, Trudinger BJ. A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 30: 236–240, 2009. doi: 10.1016/j.placenta.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Turan S, Turan OM, Ty-Torredes K, Harman CR, Baschat AA. Standardization of the first-trimester fetal cardiac examination using spatiotemporal image correlation with tomographic ultrasound and color Doppler imaging. Ultrasound Obstet Gynecol 33: 652–656, 2009. doi: 10.1002/uog.6372. [DOI] [PubMed] [Google Scholar]
- 64.Verkeste CM, Slangen BFM, Daemen M, van Straaten H, Kohnen G, Kaufmann P, Peeters LLH. The extent of trophoblast invasion in the preplacental vasculature of the guinea-pig. Placenta 19: 49–54, 1998. doi: 10.1016/S0143-4004(98)90098-4. [DOI] [PubMed] [Google Scholar]
- 65.White MM, McCullough RE, Dyckes R, Robertson AD, Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am J Physiol Heart Circ Physiol 278: H2069–H2075, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase Cε. J Pharmacol Exp Ther 330: 624–632, 2009. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]