Abstract
Taste stimuli have a temperature that can stimulate thermosensitive neural machinery in the mouth during gustatory experience. Although taste and oral temperature are sometimes discussed as different oral sensory modalities, there is a body of literature that demonstrates temperature is an important component and modulator of the intensity of gustatory neural and perceptual responses. Available data indicate that the influence of temperature on taste, herein referred to as “thermogustation,” can vary across taste qualities, can also vary among stimuli presumed to share a common taste quality, and is conditioned on taste stimulus concentration, with neuronal and psychophysical data revealing larger modulatory effects of temperature on gustatory responding to weakened taste solutions compared with concentrated. What is more, thermogustation is evidenced to involve interplay between mouth and stimulus temperature. Given these and other dependencies, identifying principles by which thermal input affects gustatory information flow in the nervous system may be important for ultimately unravelling the organization of neural circuits for taste and defining their involvement with multisensory processing related to flavor. Yet thermal effects are relatively understudied in gustatory neuroscience. Major gaps in our understanding of the mechanisms and consequences of thermogustation include delineating supporting receptors, the potential involvement of oral thermal and somatosensory trigeminal neurons in thermogustatory interactions, and the broader operational roles of temperature in gustatory processing. This review will discuss these and other issues in the context of the literature relevant to understanding thermogustation.
Keywords: flavor, multisensory, somatosensation, taste, temperature
gustatory neurobiology is partly concerned with elucidating how information about taste stimuli is represented by neural activity. Two variables commonly considered to parameterize this process are taste stimulus concentration and quality. Taste qualities are human-defined categories that intend to capture similarities and differences among taste stimuli based on descriptive sensory features. Taste qualities reflect familiar taste sensations, such as the “sweet” quality perceived when tasting sugars or the “salty” quality of table salt (sodium chloride). The concept of taste qualities appears to at least partially translate to animal models used in flavor neurobiology research, as rodents performing in orosensory behavioral tasks can discriminate, for example, sweet from umami taste stimuli (33, 64) and bitter tastants from salts (127) when stimulus intensity cues are minimized. Concentration, on the other hand, is a continuous physical property of taste stimuli that reflects the amount of taste chemical solute present in the stimulus medium. The intensity of evoked neural firing and the magnitude of perceptual and behavioral responses to taste stimuli are proportional to stimulus concentration within a broad range (e.g., 31, 53, 83).
Although important for indexing gustation, taste quality and stimulus concentration are only two of multiple variables that influence sensory processing in neural circuits for taste. Psychological factors, including the attentive state of the animal (42) and anticipations about the tastes of sapid stimuli (129), can affect firing patterns of cortical gustatory neurons. Gustatory neural activity is also influenced by cross-modal interactions with other flavor senses, such as olfaction (e.g., 37, 43, 114, 121). For example, recent neurophysiological data show that spike firing rates to taste stimuli can change with olfactory context in a subpopulation of brain stem gustatory neurons (37). Furthermore, there is a body of literature that describes how neural and perceptual responses to taste stimuli show sensitivity to the somatosensory features of sapid stimuli and the oral environment, with the bulk of this work focused on the modulatory effect of temperature.
It is intuitive that temperature plays an important role in our daily experience and enjoyment of food flavor. We associate and expect particular temperatures with certain flavors and tastes, with variation in serving temperature changing the oral sensory attributes of particular ingesta (e.g., coffee; 130) and also their appeal (19). Taste stimuli always possess a temperature that may stimulate thermosensory machinery on oral skin to induce thermal sensations that accompany taste. Furthermore, oral thermal signals are evidenced to influence gustatory information processing. Neurophysiological and psychophysical data show that stimulus and oral mucosa temperature can, under particular conditions, modulate the intensity of sensory responses to and detection of select taste stimuli (e.g., 8, 16, 53, 86). These observations suggest temperature operates as an important variable of gustatory information flow in the nervous system. However, unless specifically focused on temperature effects, functional studies on taste have infrequently considered temperature as a modulator of gustatory neural firing compared with, for example, stimulus concentration, which has been, probably without exception, always considered. Ironically, a handful of neural and perceptual studies on thermal effects on taste have identified that, for some taste stimuli and qualities, concentration interacts with temperature to influence gustatory activity, as described below.
This review will discuss the role of temperature in gustatory information processing. There are several lines of data on this topic, including results demonstrating that temperature can modulate the actions of select molecular mechanisms for gustatory transduction, neurophysiological taste responses in peripheral and central neurons, and perceptual and behavioral reactions to taste stimuli. This review will generally progress by providing a brief description of the anatomy and neural substrates largely affiliated with studies of temperature and taste, followed by discussion of thermal data relevant to different taste qualities. Some open questions will also be discussed. The text will not exhaustively cover all papers on temperature modulation of taste or thermogustation, but will attend to some key historical and recent findings.
As discussed elsewhere, the investigation of how temperature affects taste perception has an impressive, centuries-long history (1). Data on this topic are sometimes challenging to interpret across studies because of wide differences in methods and results (1). Functional data concerning thermal effects on neuronal taste processing also show some discrepancies, possibly attributable in part to differences in methodologies. Nonetheless, such vagaries speak to the complex nature of the effect of temperature on taste that emerges from the literature. In example, temperature effects are not readily generalized across all taste qualities and, in some cases, depend on cofactors.
A Primer to Gustatory and Oral Thermosensory Neuroanatomy
The general layout of the mammalian gustatory system has been detailed in earlier reviews (e.g., 39, 65, 119, 122, 128) and will be only partially discussed here in the context of the neuroanatomy largely associated with functional studies on thermogustation. Taste stimuli are transduced through interaction with ion channels and G protein-coupled receptors (GPCRs) expressed by taste bud cells (TBCs) in various papillae on oral epithelia. Some TBCs are innervated by fibers of cranial nerves, which link the output of gustatory reception mechanisms to the brain for perceptual and behavioral processing. The chorda tympani (CT) branch of the facial (VII) nerve supplies sensation to TBCs of the fungiform papillae on the rostral tongue and of the rostral foliate papillae on the caudal tongue. The glossopharyngeal (IX) nerve innervates TBCs in the more caudal foliate and circumvallate lingual papillae (see Ref. 28). The greater superficial petrosal branch of nerve VII innervates taste buds on the palate (59, 95). It is noteworthy that nerves VII and IX also contain orosensory fibers implicated for somatosensory processing (e.g., 14, 36, 44, 152) and fibers dually responsive to taste and oral somatosensory input including temperature (e.g., 41, 91, 103, 152). Although the bulk of the data supporting these observations were derived from rodent models, bimodal sensitivity to temperature and taste is also evident in the human CT nerve (35, 99). Commonly referred to in press and spoken word as “gustatory fibers,” taste-active peripheral neurons can display cross-modal sensitivity to touch and thermal stimuli applied to oral mucosa. This observation suggests that neural interactions between taste and somatosensation, including temperature, can begin at a peripheral level.
Temperature influence on peripheral taste-sensitive neurons is partly due to, or associated with, the reliance of some gustatory transduction processes on temperature-sensitive molecular effectors. These include the warmth-activated transient receptor potential (TRP) melastatin 5 (TRPM5) cation channel involved with GPCR-mediated transduction cascades for sweet, umami, and bitter taste stimuli (30, 109, 155). Additionally, epithelial sodium channels (ENaCs), involved with sodium taste transduction and sensory-discriminative function (61, 118, 126), display Na+ currents and conductances sensitive to temperature change (5, 6, 25, 26). Temperature also appears capable of activating gustatory-sensitive nerves via mechanisms that do not involve taste receptors, as evidenced by electrophysiological recordings of oral thermal responses from the CT nerves of taste-deficient mice (40).
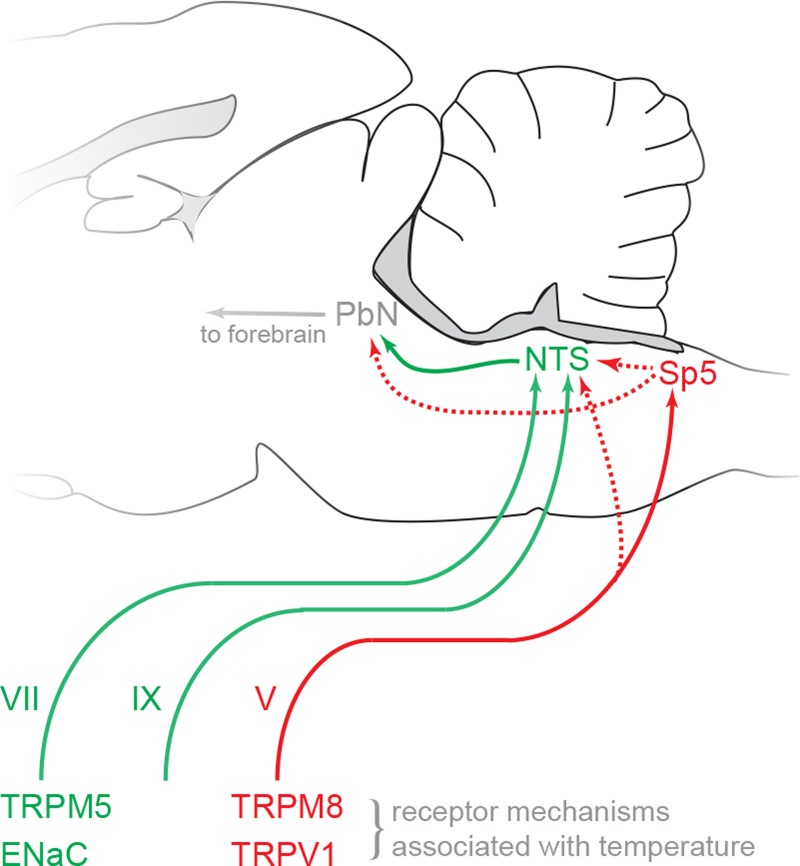
In mammals, taste-active fibers of cranial nerves VII and IX bilaterally terminate in the rostral nucleus tractus solitarii (NTS) in the medulla (Fig. 1). The NTS contains neurons that respond to oral thermal stimulation and also to temperature and taste (83, 102, 132, 136, 144). The pattern of connectivity between the NTS and other brain regions suggests it plays a pivotal role in gustatory processing related to oral sensation and ingestive decisions. Gustatory-sensitive regions of the NTS maintain efferent connections with medullary circuits associated with oral motor behaviors (57, 135). Ascending taste-active NTS projection neurons reach thalamic (primate; 10) or parabrachial (rodent; 97) targets that ultimately interface with circuitry involved with sensory-discriminative and affective processing. In rodents, the parabrachial nucleus contains gustatory-responsive neurons sensitive to change in oral temperature and to general oral somatosensory stimulation (60, 98, 100, 101, 137), implicating this structure, along with the NTS, with important roles in taste-somatosensory integration. Thalamic and cortical structures housing taste-sensitive neurons also possess units with dual responsiveness to oral temperature and gustatory input (e.g., 67, 139, 140).
Fig. 1.
Schematic of peripheral and early central nervous system (CNS) pathways in the rodent associated with thermogustatory and oral thermal processing. This schematic does not intend to show anatomically precise scale or projection routes and highlights only forward connections between peripheral and central pathways related to the literature discussed in this review. Cranial nerves known to mediate thermogustatory and oral thermal input to CNS structures are marked by Roman numerals. Pathways and receptor mechanisms implicated for taste and thermal processing are marked green. Trigeminal-specific pathways and mechanisms are marked red. Dotted red arrows denote known trigeminal projections [references: V → NTS (28, 38); Sp5 → NTS (154); Sp5 → PbN (23)] of unknown function in thermotaste processing. ENaC, epithelial sodium channel; TRP, transient receptor potential; TRPM5, TRP melastatin 5 ion channel; TRPM8, TRP melastatin 8 ion channel; TRPV1, TRP vanilloid 1 ion channel; NTS, nucleus tractus solitarius; Sp5, spinal trigeminal nucleus; PbN, parabrachial nucleus.
Although neural sensitivity to oral temperature is distributed along the gustatory neuraxis, most functional studies on thermogustation have focused on the peripheral nervous system. Thus there exists only a paucity of data on how systematic change in temperature, of taste solutions or the mouth, can affect central gustatory processing. Paradoxically, the initial single-unit electrophysiology studies of taste-active neurons in the rat NTS, carried out in Carl Pfaffmann’s laboratory in the late 1950s and early 1960s, tested for and found responses to oral thermal stimulation in these cells (83, 110). Although this early observation gave rise to a potential role for NTS neurons in oral thermal and gustatory interaction, the first neurophysiological studies that specifically detailed the influence of temperature on taste responding and coding in the rodent NTS appeared more than 50 years later, beginning in 2013. These works party identified that NTS neurons can display interactive and unique responses to particular combinations of thermal and gustatory input, such as warmth paired with sweet stimuli such as sucrose (144, 145), discussed below.
Taste neurons can fire to oral temperature, albeit comparably dominant signals concerning detection and discrimination among oral thermal qualities are carried by trigeminal neurons (72), which mediate cephalic and oral somatosensation. Fibers of the trigeminal (V) nerve express TRP channels implicated for temperature sensation, including TRP melastatin 8 (TRPM8), activated by cool temperatures and the minty-cooling agent menthol (2, 9, 34, 87, 108), and the nocisensor TRP vanilloid 1 (TRPV1), which is sensitive to capsaicin (chili pepper burn) and also heat under select conditions (e.g., 21, 22). TRPV1 was also identified as a developmental marker of the cell lineage that mediates thermosensation in somatosensory pathways (88). Lingual nerve fibers involved in part with tongue thermosensation (81, 111) emanate from the mandibular trunk of the trigeminal nerve. Lingual fibers are evidenced to partly innervate tissue of the taste bud-containing fungiform papillae (89) but to not penetrate resident taste buds (2, 34), which are innervated by the chorda tympani branch of nerve VII. This arrangement presumably supports detection of spatially correlated taste and trigemino-somatic features of oral stimuli.
Although trigeminal sensory fibers heavily project to the central trigeminal nuclear complex (84, 85), several anatomical and physiological studies have shown that a subset of mandibular trigeminal fibers reach the NTS (12, 13, 15, 27, 28, 38, 58, 76, 85, 143). Lingual nerve fibers that arrive at the NTS can display apparent terminal swellings, possibly synapses, with neurons demonstrated to receive synaptic input from the CT nerve involved with taste transmission (38). Accordingly, electrical stimulation of mandibular trigeminal processes can cause spike firing in taste-active NTS units in rats, with some evidence that activation of trigeminal afferents can affect taste activity in NTS cells (13, 15). What is more, anatomical data have identified that neurons in the spinal trigeminal nucleus pars caudalis, involved in part with the coding of oral temperature (e.g., 20, 72, 153), project to areas of the parabrachial nucleus associated with gustatory processing (23). Altogether, these data open the possibility that oral thermal activity in taste-sensitive brain neurons is partly contributed by trigeminal convergence (Fig. 1). Yet direct evidence supporting this postulate is currently lacking.
Early Functional Observations
Descriptions of temperature sensitivity by taste-active nerves appear in classic studies of sensory physiology (36, 156). Temperature began to receive attention as a variable of gustatory neural responses in peripheral nerve electrophysiological studies carried out in the 1950s and 1960s. Thermal control equipment used in these early functional works was arguably crude compared with modern standards and may have included fish aquaria laden with ice cubes to cool bottles of taste solutions, or equipped with “knife heaters” for warming taste stimuli (1). Nevertheless, several of the early reports described patterns of effects of temperature on taste that have since been replicated or rediscovered by more recent studies on neuronal thermo-taste processing. One common trend that comes across from the early functional investigations was that when temperature was found to influence taste, it appeared to operate as a quantitative variable of the intensity of the gustatory response. This trend is apparent in some manner in likely all studies of thermogustation that have identified effects of temperature on perceptual, behavioral, or neural responses to taste input.
What appears to be one of the first detailed electrophysiological investigation of temperature influence on gustatory neural activity was completed in 1953 as a doctoral dissertation carried out in Pfaffmann’s laboratory. This manuscript was never published as a primary scientific paper but was cited by and influenced several subsequent studies on temperature-taste interactions. In this dissertation, Abbott (1) made integrated recordings from the rat CT nerve to, in part, study the effect of change in oral and stimulus temperature on gustatory activity to solutions of acidic (sour) stimuli and salts, including KCl and the sodium halides NaBr and NaCl. Gustatory scientists generally accept the latter stimulus, sodium chloride, as a prototypical salty taste stimulus. Abbott’s work found change in oral and stimulus temperature caused no effect on nerve responses to solutions of the sour tastant HCl, but induced appreciable modulation of nerve activity to lingual delivery of salts. For instance, responses to 0.1, 0.3, and 1 M solutions of KCl and NaBr showed near or absolute maximal firing at 37°C. For multiple concentrations, both of these salts tended to give their second largest responses when warmed to 45°C but induced suppressed firing when cooled to 22°C or 15°C.
Abbott noted temperature could also modulate nerve activity to NaCl, albeit the manner of the effect differed from the other salts. Unlike KCl and NaBr, oral presence of 0.03, 0.1, 0.3, and 1.0 M NaCl induced responses that were maximal at 22°C, with a small reduction in evoked firing present at 37°C. Further suppression in nerve firing was observed when solutions of 0.03, 0.1, and 0.3 M NaCl were cooled to 15°C, and when 0.03 M NaCl was heated to 45°C. However, cooling and warming to 15° and 45°C, respectively, produced only nominal suppression of CT nerve activity to 1.0 M NaCl solutions compared with 22° or 37°C (1). Altogether, these data began to suggest that the effect of temperature on neuronal taste processing was, in part, conditioned on the taste stimulus under study, a trend recapitulated in subsequent (e.g., 149) and recent (e.g., 78, 104, 145) functional studies on thermogustation. Furthermore, Abbott’s observation that temperature effects on nerve firing to NaCl lessened at elevated concentrations suggested thermal influence on taste processing is conditioned on stimulus concentration, an effect borne out and detailed in later single-unit neurophysiological studies of sodium, and also sweet, thermogustatory processing (145, 148).
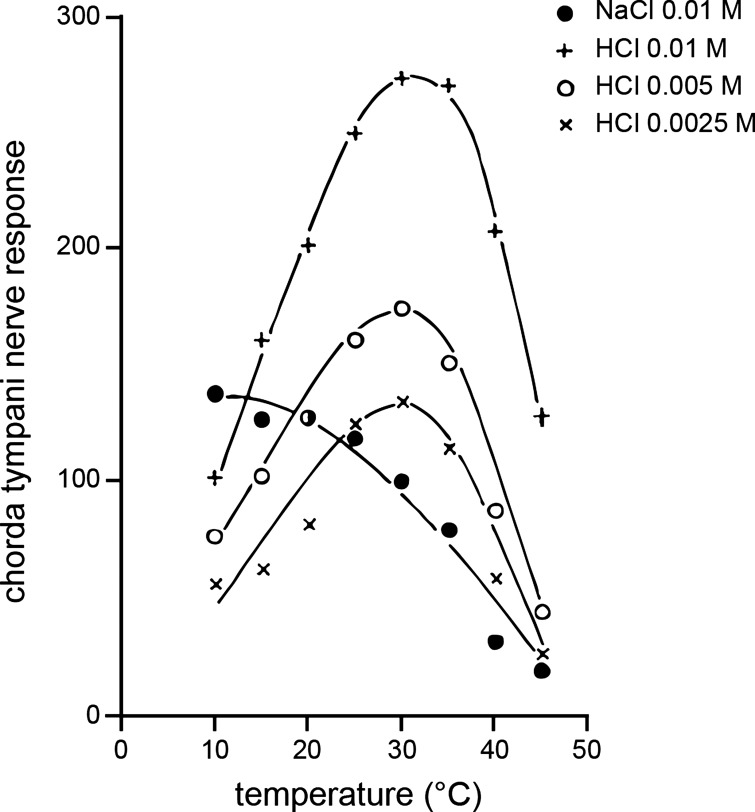
It is noteworthy that thermogustatory electrophysiological studies that emerged shortly after Abbott’s work did show some differences in results. These differences partly resided in, for example, the “optimal” temperatures for sodium solutions to induce maximal gustatory firing in CT afferents, which other data identified to fall between ~25° and 40°C for 0.1 M NaCl (94, 149). Moreover, a subset of follower studies identified that temperature can, unlike in Abbott’s report, affect integrated and unit gustatory responses to HCl solutions in the rat CT nerve (148, 149, 150; see Fig. 2). Yet several of these works did continue to reveal that temperature could induce unique effects on firing to different taste stimuli, including those representative of different taste qualities (Fig. 2), and that thermal effects on gustatory responding could depend on stimulus concentration.
Fig. 2.
Effects of temperature can differ across taste qualities. Example electrophysiological data depicting the mean standardized maximum response of the rat chorda tympani nerve (ordinate) to reduced concentrations of sodium chloride (NaCl; “salty”) and hydrochloric acid (HCl; “sour”) plotted against temperature (abscissa). This work aimed to determine whether lower concentrations of HCl would elicit a decrease in gustatory nerve firing with warming, as shown for a low concentration (0.01 M) of NaCl. Yet across concentrations, nerve responses to HCl peaked with warming to around 30°C. Adapted from Yamashita and Sato (149) with permission.
Effects of Temperature on Sodium Taste
Neural data.
Concentration would prove to be a critical variable for investigation and interpretation of effects of temperature on sodium taste transmission, as exemplified by classic data. Sodium salts continued to be focal stimuli in early electrophysiological studies on thermotaste processing in the CT nerve possibly because, in part, they induce strong and readily indexed taste responses in this afferent. The inclusion of temperature in some of these investigations was partly viewed as a way to shed light on the reception mechanisms of gustatory transduction, which at the time were unknown. Potential transduction mechanisms were discussed to include either a physical interaction between the taste chemical and a taste sensor element or an enzymatic reaction that led to gustatory neural signaling, with the latter presumably evidenced by elevated thermal sensitivity of gustatory nerve responses (see Ref. 115). Along this line, Beidler (11) published data in 1954 from rats showing that chemosensory CT nerve responses to oral presence of sodium stimuli were insensitive to change in temperature between 20°C and 30°C. From this he concluded the initial steps of sodium gustatory transduction involved a physical interaction between taste chemical and receptor “substance” rather than an enzymatic process. Beidler’s conclusion, of course, agrees with more recent research that has shown sodium salts at least partly interact with ENaCs on taste bud cells to induce gustatory transduction (e.g., 24, 118). Yet his noted absence of a thermal effect may have been due to stimulus conditions. Beidler’s data were obtained using temperatures that might be considered to represent only moderate shifts in cooling applied to a relatively high, 0.5 M concentration of NaCl. In comparison, the near simultaneous, unpublished work of Abbott suggested that CT nerve responses to elevated concentrations of NaCl (1 M in Abbott’s case) showed good resistance against change with temperature, whereas responses to lower NaCl concentrations displayed notable change when tested over a broad range of cooling and warming steps (1).
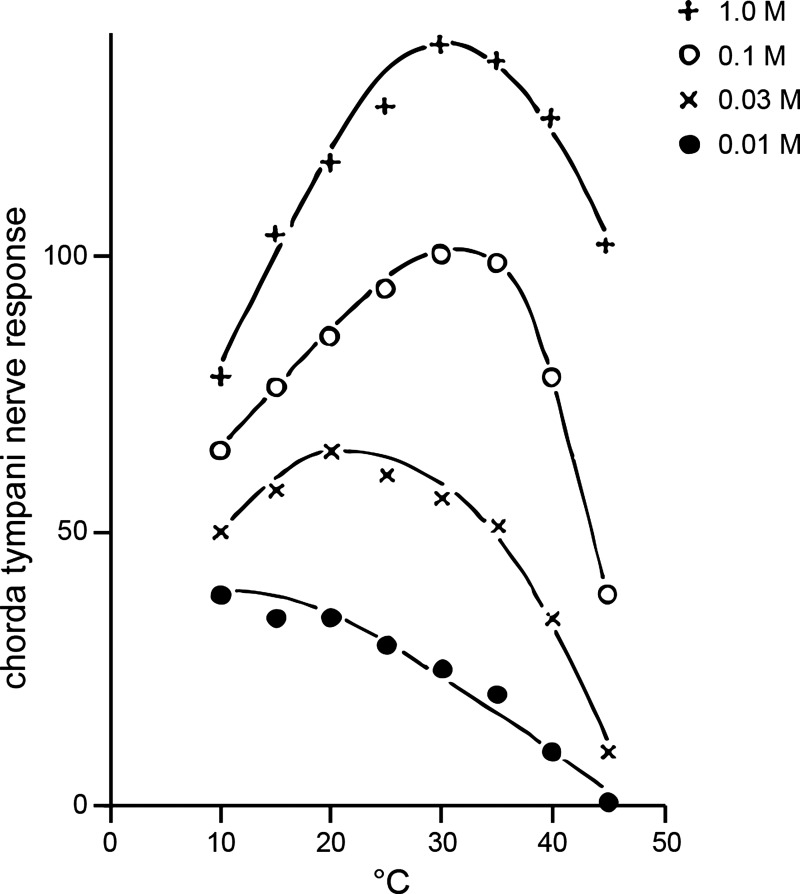
Work that followed on the heels of Abbott’s and Beidler’s pioneering investigations began to delineate the role of stimulus concentration in neuronal thermogustatory responses to sodium salts. Using integrated recordings from the rat CT nerve, Yamashita and Sato (149) demonstrated in 1965 that the temperature that optimized CT nerve activity to oral presence of NaCl was positively associated with stimulus concentration within a select range. As shown in Fig. 3, they found that a 0.1 M solution of NaCl gave maximal activity at around 30°C, whereas progressively weaker NaCl concentrations induced their largest responses when stimulation temperature was stepped to cooler values: 0.03 and 0.01 M caused maximal firing at, respectively, 20° and 10°C. Somewhat similar to Beidler’s (11) report, Yamashita and Sato (149) noted that nerve responses to a given concentration of NaCl appeared to show only relatively small change with temperature shifts between 20°C and 30°C, albeit more extreme cold (10°C) and hot (45°C) temperatures were found to strongly modulate activity to individual concentrations compared with moderate temperatures (Fig. 3).
Fig. 3.
Effect of temperature on gustatory responding can rely on stimulus concentration. Example electrophysiological data depicting the mean standardized initial response of the rat chorda tympani nerve (ordinate) to various concentrations of sodium chloride (NaCl) plotted against temperature (abscissa). The temperature that optimizes nerve firing to 0.01, 0.03, and 0.1 M NaCl is seen to systematically increase with concentration. Adapted from Yamashita and Sato (149) with permission.
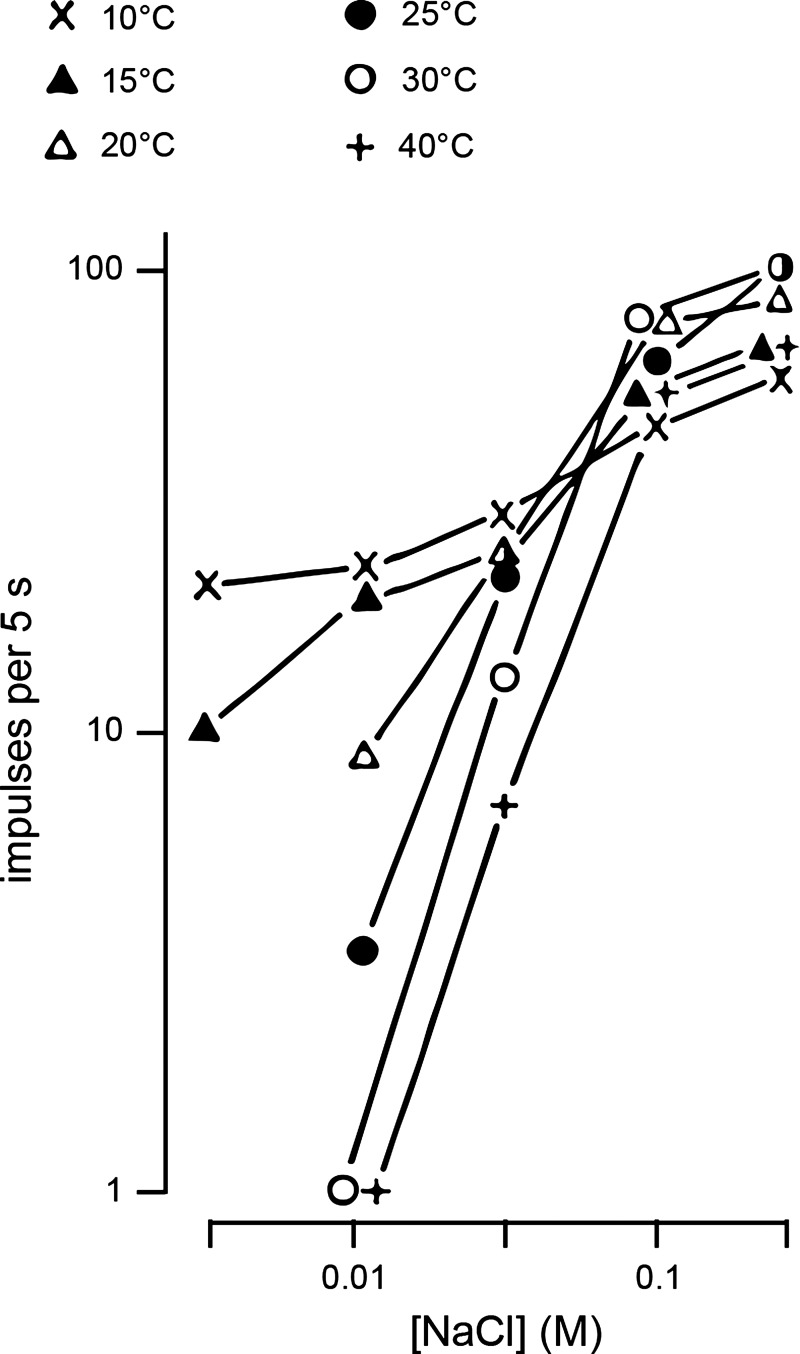
Yamashita et al. (148) went on in a publication from 1970 to detail the combined effect of temperature and concentration on sodium taste responses in individual rat CT fibers. This work tested a sample of units with weak to high concentrations (0.003 to 0.3 M) of NaCl delivered to the tongue at multiple cool and warm temperatures (between 10°C and 40°C). Thermal effects noted in the sodium-sensitive CT neurons subjected to this protocol were evidenced to partly associate with neural breadth of responsiveness to taste stimuli. For instance, CT fibers activated by NaCl, sucrose, and HCl showed facilitated firing across low and high NaCl concentrations with cooling. On the other hand, units with more selective tuning displayed facilitated activity to mainly lower concentrations (e.g., ≤0.03) of NaCl with cooling, with increased firing to elevated NaCl concentrations noted for select warming conditions (Fig. 4). But more globally, inspection of data and plots in this paper reveals that temperature could cause changes in firing to NaCl that were not constant across concentrations, suggesting there was an interaction between temperature and concentration on sodium activity. For instance, warming of the tongue and stimulus solutions from 15°C, to 20°C, to 25°C, and then to 30°C increased the mean slope of the neuronal concentration-response function for NaCl across the analyzed fibers (148). For some cell types including units oriented toward NaCl, increments in the slope of this function with warming were also accompanied by decrements in its intercept, causing wide separation of functions on the left as opposed to the right (Fig. 4). This reflected an ability of temperature to produce greater change in firing by this cell class to weakly concentrated NaCl solutions (e.g., 0.01 M) as opposed to high (e.g., 0.1 M), with, in general, a progressive lessening of the magnitude of temperature effects when NaCl concentration was raised (Fig. 4). It is noteworthy that only a small sample of individual fibers was reported in Yamashita et al. (148), which cautions some interpretations. Nonetheless, their description that temperature could induce larger modulatory effects on responses to weakened taste solutions as opposed to concentrated was later identified to also apply to single-neuron responses to temperature-varied sweet stimuli (145), as discussed below. All of the above data indicate that temperature and concentration can operate in a nonindependent manner to shape the magnitude of gustatory firing.
Fig. 4.
Temperature and concentration interact to shape gustatory neural firing. Example electrophysiological data obtained from sodium-sensitive rat chorda tympani neurons showing their mean spike firing (ordinate) to a series of low to high concentrations of NaCl (abscissa) applied to the tongue at multiple cool and warm temperatures. Data are plotted in double logarithmic coordinates. Cooling markedly enhanced neural firing to low concentrations of NaCl (e.g., 0.01 M), whereas select warmer temperatures could enhance firing to high concentrations (e.g., 0.3 M), albeit the latter effect was relatively smaller. The ability of temperature to modulate unit firing to NaCl is generally decreased at high concentrations. These data represent an early description of how temperature and concentration can interactively control gustatory response magnitude in single neurons. Adapted from Yamashita, Ogawa, Kiyohara, and Sato (148) with permission.
Receptor mechanisms of sodium thermogustation: a potential role for ENaC.
The most parsimonious mechanistic explanation for the effect of temperature on the magnitude of sodium firing is that the operation of gustatory receptor mechanisms for Na+ is somehow sensitive to temperature. Although a definitive explanation is presently lacking, there are several lines of evidence that raise the possibility that temperature effects on sodium taste may be at least partly contributed by the direct actions of temperature on Na+ currents mediated by ENaCs expressed on taste cells. In early electrophysiological work relevant to this topic, Nakamura and Kurihara (94) identified that integrated gustatory responses to NaCl in the rat CT nerve are composed of two temperature-responsive components sensitive to blockade by lingual delivery of amiloride, an antagonist of ENaC-mediated sodium taste transmission (e.g., 61). They discovered that amiloride-blockable activity to a reduced, 0.01 M concentration of NaCl peaked when solutions were cooled to around 10°C. On the other hand, amiloride-sensitive nerve activity to an elevated 0.3 M concentration of NaCl was maximal at around 30°C. An intermediate 0.1 M concentration of NaCl induced amiloride-blockable nerve firing that was facilitated at two temperatures, each hovering near the optimal temperatures for 0.01 and 0.3 M. Nakamura and Kurihara (94) interpreted this result as evidence for two amiloride-sensitive taste receptor “sites” for NaCl, with one site showing its strongest activation to lower concentrations of NaCl when cooled and the other activated maximally by higher concentrations delivered warmed. Although these data leave the definition of receptor site open to interpretation, they do suggest that ENaC is involved with sodium thermogustation and could mediate a dual effect of temperature on sodium taste.
Nakamura and Kurihara’s results agreed in part with some of the early rodent whole nerve data that showed there is a positive association between stimulus concentration and temperature that optimizes integrated gustatory firing to NaCl in the CT nerve (149), as discussed above. This agreement reflects the marked contribution of amiloride-sensitive, ENaC-dependent fibers to NaCl activity in this afferent (e.g., 24, 61, 96). Relatedly, Yamashita et al. (148) provided evidence that sodium-oriented CT fibers, which typically show amiloride-blockable gustatory activity to NaCl (e.g., 62, 63, 80), could demonstrate increases in firing to low and elevated NaCl concentrations with, respectively, cooling and warming (Fig. 4). Moreover, a recent study on amiloride-sensitive, sodium-oriented mouse NTS neurons identified that gustatory activity to a low intensity/perithreshold 0.004 M solution of NaCl was augmented by cooling, whereas warming could increase firing to an elevated, 0.3 M NaCl concentration (77). All of these data also point to an involvement of ENaC with cooling and warming effects on sodium taste transmission.
Biophysical studies have revealed that amiloride-blockable Na+ currents carried by ENaC are indeed sensitive to temperature change. Electrophysiological experiments on ENaC conducted in an artificial expression system revealed decreases in temperature from 35°C to 10°C induced systematic and sizable increases in whole cell Na+ current flow that were completely inhibited by the addition of amiloride (5). Over a range of temperatures from 44°C to 6°C, maximum ENaC-mediated amiloride-sensitive Na+ current was achieved at 6°C, with half-maximal current noted at ~25°C (5). Other biophysical work has identified that cooling can increase the steady-state (tonic) component of the amiloride-sensitive Na+ current passed by ENaC (25, 26). What is more, Chraïbi and Horisberger (25) used patch-clamp recordings of amiloride-sensitive currents passed by human ENaCs in an expression system to show that whereas cooling from ~30°C to 15°C increased the number of open ENaC channels, raising temperature from 15°C to around 30°C increased ENaC single channel conductance. Although these biophysical results can be only cautiously generalized to in vivo gustatory neural data, this twofold effect of temperature on ENaC operation does seem to agree with the ability of both cooling and warming to modulate amiloride-sensitive taste activity to NaCl in rodent CT (94) and NTS (77) neurons.
It is important to acknowledge that some electrophysiological data suggest that ENaC may not support thermal effects on sodium taste transmission. Single-unit recording studies of rat CT neurons have reported that shifts in oral and stimulus temperature among cold, moderate, and warm values (e.g., 10°C, 25°C, and 40°C) cause no reliable change in firing to 0.1 M NaCl in sodium-specialist fibers, which display responses to lingual presence of sodium stimuli that can be inhibited by amiloride (16, 80). Although one of these papers did seem to discover a trend for this ENaC-dependent fiber class to show maximal firing to NaCl at 25°C opposed to other temperatures, this trend did not reach statistical significance (16). Methodological differences may have contributed to discrepancies between these and the above data. Nevertheless, future direct studies on temperature influence on ENaC as it functions in taste cells in situ will be important to ultimately define the role of this channel in sodium thermogustation.
Thermo-TRP channels and ENaC-independent sodium taste.
Multiple studies have proposed that temperature-sensitive TRP channels mediate the ENaC-independent component of gustatory neural activity to sodium. This response component is generally indexed as residual firing to sodium stimuli that remains after blockade of oral ENaCs by a competitive antagonist, such as amiloride or benzamil (e.g., 112). Intuitively, an involvement of thermo-TRP channels with sodium transduction could importantly contribute to sodium thermogustation. However, inspection of the literature reveals, in some cases, wide disagreement among studies and a muddled picture of how thermo-TRP channels generally function in sodium taste transmission in mammals.
For instance, some reports have documented that ENaC-independent gustatory activity to NaCl is contributed in part by the warmth-sensitive TRPM5 ion channel, which, beyond sodium taste, has defined roles in GPCR gustatory transduction processes (30, 109, 155). Using whole nerve recordings, Ren et al. (112) reported that mice genetically deficient for TRPM5 display significantly smaller CT nerve responses to oral presence of 0.1 M NaCl compared with controls and that this effect is due to an absence of ENaC-independent sodium activity in the mutant line. In principally similar fashion, Damak et al. (30) revealed TRPM5 knockout mice showed reduced CT nerve activity to oral delivery of 0.1 M, and also 0.03 M, NaCl compared with wild-type mice. However, these reductions in nerve firing were small and of undetermined significance, as, in their paper, TRPM5 null and wild-type mice subjected to orosensory intake tests showed no difference in licking behavior to multiple concentrations of NaCl up to 0.1 M (30).
Other studies have implicated the heat-sensitive TRPV1 ion channel in salt taste transduction. Lyall and colleagues (82) first proposed that a variant of TRPV1 mediates the ENaC-independent gustatory response component to NaCl. Using CT nerve recordings, they showed in rats that lingual presence of pharmacological agonists of TRPV1 increased heat-potentiated responses to NaCl that were insensitive to benzamil, and that this effect was lost in mice genetically deficient for TRPV1 (82). In partial accord with this finding, other data have shown that ENaC-independent gustatory activity to NaCl in mouse CT fibers is suppressed, but not abolish, by oral delivery of a potent antagonist of TRPV1 (4). Although such data support a role for TRPV1, or some variant product of its gene, in sodium taste transduction, they contrast with molecular genetic labeling studies that have revealed no evidence of TRPV1 expression in fungiform taste buds or receptor cells innervated by CT fibers (88). Furthermore, some electrophysiological studies conducted in TRPV1 null mice found that CT nerve responses to oral delivery of a concentration series of NaCl did not differ between these mutants and wild-type controls (124). Finally, psychophysical and conditioned generalization behavioral studies that used mice genetically deficient for TRPV1 revealed no evidence that this ion channel is needed for the gustatory detection of sodium salts (124, 138). A more detailed discussion of the evidence and pitfalls concerning the involvement of TRPV1 with taste is presented by Roper (113). Overall, there are still questions regarding how TRPV1 and other thermo-TRP channels might be involved with sodium taste transduction and thermogustation.
Temperature effects on sodium taste: psychophysical data.
Interpreting the significance of effects noted in functional studies is aided by comparison with relevant psychophysical and behavioral findings. Yet studies on sodium thermogustatory behaviors in rodents are scarce. There are data that pertain to temperature effects on rodent saline preference (46), albeit gustation was not a focus of the methods used in this work. Although several psychophysical studies on human sodium thermogustation have been completed, comparison of these data with physiological results is complicated by several issues, including a lack of accord among the human data. For example, Moskowitz (90) reported warming stimulus solutions from 25°C to 35°C notably enhanced taste intensity ratings for NaCl in humans. This was evidenced by a warmth-induced increase in the intercept of the psychophysical function for NaCl (90), which relates sodium concentration to perceived intensity. On the other hand, Green and Frankmann (54) demonstrated in humans that warming from 20°C to 36°C had no effect on the perceived gustatory intensity of NaCl, with similar psychophysical functions for NaCl obtained across temperatures. Differences between these studies may partly relate to methods of temperature control. Moskowitz’s study tested taste solutions at temperatures that may have, in some cases, differed from that of the tongue. In contrast, Green and Frankmann controlled the temperature of the tongue using a prerinse method and measurement of tongue surface temperature, with NaCl solutions tested at cool and warm temperatures intended to be isothermal with the adapted lingual value. Although there are other methodological differences between these studies to consider (54), it is curious if these discrepant results obtained under different methods of oral thermal control partially hint to a role for mouth temperature, and its balance with stimulus temperature, in sodium thermogustation. Along this vein, change in oral adaptation temperature can influence electrophysiological taste activity to NaCl in NTS oral sensory neurons in mice (77).
Studies on the effects of temperature on gustatory sensitivity to NaCl have sometimes shown better agreement. Pangborn et al. (107) reported humans working in a task to discriminate sips of solutions that contained either water or near-threshold concentrations of NaCl showed greater performance when stimulus temperatures were 22°C or 37°C as opposed to 0°C or 55°C. Using a discrimination task with different stimulus presentation methodology, McBurney et al. (86) similarly identified that humans showed the lowest threshold to report NaCl taste when solution temperature was between 22°C and 32°C as opposed to temperatures above (42°C) or markedly below (17°C) physiological warm. These data suggest the detection of near-threshold or weak sodium solutions is facilitated at moderate or cool temperatures. In at least partial agreement, mouse neurophysiological data have demonstrated innocuous cooling, but not warming, can enhance brain gustatory responses to oral presence of a perithreshold concentration of NaCl (77).
Additional human psychophysical data suggest temperature may influence sodium gustatory perception along multiple dimensions. The length of time humans perceive the taste of NaCl in the mouth increases with cooling (17), suggesting gustatory adaptation to sodium is affected by temperature. Warming to relatively extreme temperatures encountered in cooked foods can, in untrained subjects, decrease the saltiness of soup-base products, including chicken broth and miso soup, but not salt water of comparable sodium concentration (71), suggesting thermal effects could also vary in some cases with sodium-food context.
Effects of Temperature on Sweet Taste
Neural data.
Sweet is a gustatory quality that has received considerable attention in recent psychophysical, neurophysiological, and molecular studies of thermogustation. This emphasis has been probably driven in part by discoveries of notably strong effects of temperature on gustatory neural activity to sweet stimuli in functional studies, which have generally shown additivity between sweet inputs and warming. Yamashita and Sato (149) reported in 1965 that oral presence of solutions of 0.5 M sucrose induced maximal firing in the rat CT nerve when stimuli were warmed to 30°C, with cooling below or further warming above this value causing systematic inhibition of nerve firing. More recent recording studies from gustatory nerves and ganglia have generally reproduced this result in rats, mice, and other species, but in some cases suggested peak nerve activity to sucrose arose over a slightly broader range of innocuous warm, near physiological temperatures between ~30°C and 40°C (16, 78, 79, 93, 104, 133). Similar effects of warming and cooling have been reported for CT nerve activity to other sweet stimuli, including monosaccharides (e.g., glucose) and artificial sweeteners (e.g., saccharin) (78, 79, 93, 104, 133). Moreover, work that analyzed the effect of temperature on rodent CT nerve activity to stimuli from multiple taste qualities demonstrated increasing temperature from 10°C to 30°C caused a substantial steepening of the rate of warmth-induced increase in nerve firing to 0.5 M sucrose that was not apparent among representative bitter, salty, or sour stimuli (104).
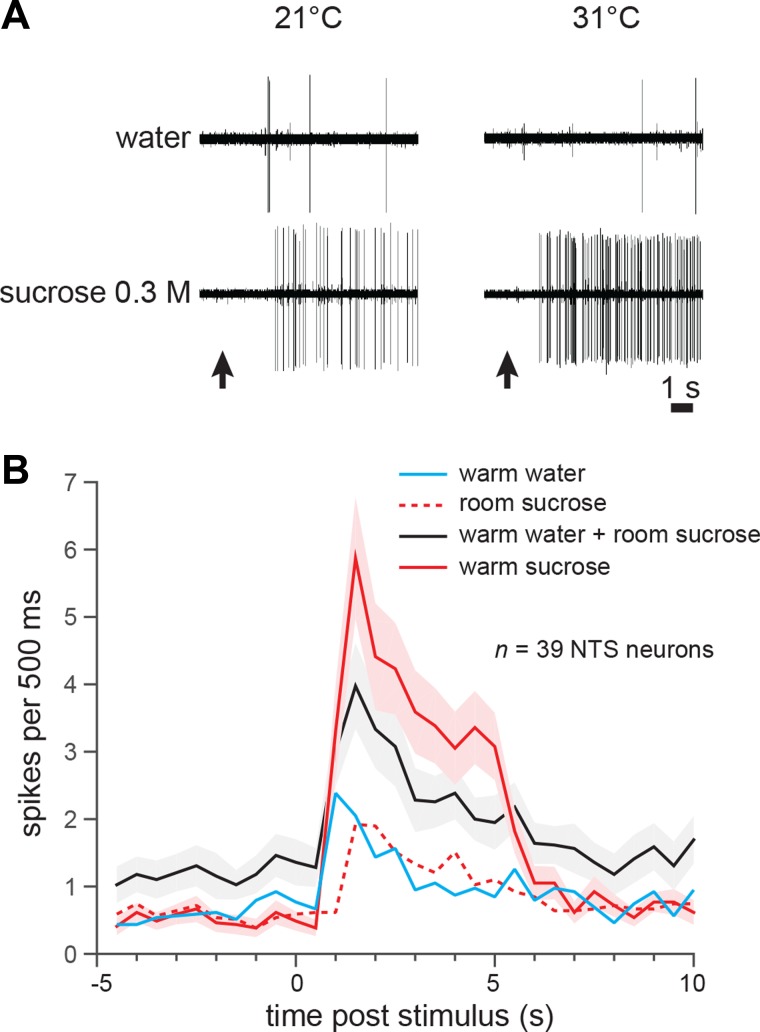
A steep rate of increase in neural firing to sucrose with oral warming was also reported in the initial study on the effects of temperature on gustatory processing in NTS neurons. This work, conducted in mice, identified that warming taste solutions from ~22°C to 30°C caused a supralinear increase in neural firing to oral presence of a moderate concentration of sucrose (144). The effect was considered supralinear, or superadditive, because the enhanced firing rate to 30°C sucrose was significantly higher than the increase predicted by simple addition of responses to oral presence of this stimulus at 22°C and to water warmed to 30°C (see Fig. 5, A and B). Thus warming appeared to positively interact with some element of the ascending neural signal for sucrose rather than simply “adding on” spikes to an independent neural message for sucrose taste (e.g., Fig. 5A). With consideration for the average response of all cells sampled in this work, the superadditive effect of warming observed for firing to sucrose was absent for gustatory responses to bitter, salty, or sour stimuli, but was apparent for an umami mixture (144), as discussed below.
Fig. 5.
Superadditive effects of temperature on gustatory neural responding. A: example raw electrophysiological sweeps depicting spiking activity by an individual taste-active neuron in the mouse nucleus tractus solitarius (NTS) to oral delivery of water (top) and 0.3 M sucrose (bottom) at 21°C (left) and 31°C (right). Temperature values reflect oral stimulus temperature within ±1°C. Upward arrow denotes stimulus onset. As shown, this neuron was mostly insensitive to thermal stimulation in the absence of taste, giving 0.6 and 0.3 spikes/s to water at 21°C and 31°C, respectively. However, the cell was responsive to sucrose and increased its firing to this input from 4.1 to 14.4 spikes/s with warming from 21°C to 31°C. This increase reflects a +251% change in firing that is considered superadditive; the increase far exceeds the response level that would be achieved if sucrose and warmth inputs to this neuron combined through simple addition (i.e., the sum of activity to 21°C sucrose and 31°C water). Unpublished cell; methods for data acquisition as described in Wilson and Lemon (144). B: peristimulus time histograms (PSTHs; 500 ms bins) showing the mean spike firing (ordinate) of 39 mouse NTS neurons during 5-s oral deliveries of warm (~30°C) water and 0.1 M sucrose tested at room (~22°C) and warm temperatures. The black-lined PSTH is the addition of the PSTHs for room temperature sucrose and warm water; this additive-predicted trace represents the response level expected if enhanced firing to warm sucrose was caused by simple addition of activity to warmth and room temperature sucrose. Yet during the stimulus period (0 to 5 s), the additive-predicted PSTH is lower than the PSTH for warm sucrose (shaded regions represent ± SE). Furthermore, the mean number of spikes present during the stimulus period was greater for warm sucrose compared with the additive-predicted response (paired t-test on normally distributed data: t38 = 3.3, P = 0.002). Thus the increase in firing to sucrose with warming is superadditive. Data are from neurons described in Wilson and Lemon (144).
Peripheral (79, 133) and central (145) neurophysiological work that detailed the facilitative effect of warming on gustatory neuronal activity to sucrose identified this phenomenon was conditioned on stimulus concentration. Wilson and Lemon (145) tested taste-active, sucrose “best” NTS neurons in mice with a sucrose thermoconcentration series spanning perithreshold (0.05 M) to overly strong (0.56 M) intensities at multiple cool and warm temperatures. This study revealed that increasing stimulus temperature caused unprecedented enhancement of spiking activity to oral sucrose at primarily lower stimulus concentrations. For example, warming from 18°C to 30°C increased average unit firing to 0.1 M sucrose by 1,049%, whereas this same warming step caused only a 152% increase in gustatory activity to 0.56 M sucrose. What is more, the slope of the NTS neuronal concentration-response function for sucrose was found to significantly and systematically decrease while concomitantly displaying increasing intercept when stimulus temperatures were raised from 18°C, to 22°C, to 30°C. As observed for sodium thermogustatory processing (148; Fig. 4), neuronal concentration-response functions for sucrose obtained at these temperatures generally showed progressively greater separation on the left (i.e., low concentrations) as opposed to the right (i.e., high concentrations), with temperature-varied functions displaying near or complete convergence at an elevated sucrose concentration of around 0.5 M. This relationship indicated the degree to which temperature could modify gustatory neural activity to sucrose was inversely proportional to stimulus concentration, with activity to increasing sucrose concentrations generally becoming more resistant to change with thermal influence (145). The magnitude dimension of gustatory firing to sucrose appears to be interactively controlled by temperature and concentration, with temperature exerting larger effects on sucrose taste processing at lower stimulus concentrations.
Temperature and sweet taste: psychophysical data.
Psychophysical studies have indicated that particular combinations of sweet tastes and oral warming can induce interactive perceptual effects. One suggestion of such interaction appeared in a paper from von Békésy (141) published in 1964. Using an apparatus that allowed for simultaneous delivery of taste stimuli and temperature-controlled water to separate halves of the human tongue, von Békésy set out to measure whether particular hemicombinations of these inputs affected spatial localization of the oral stimulus. It was reported that delivery of warmth to one side of the tongue and bitter or sweet (cane sugar) stimuli to the other formed a sensation localized to the middle of the tongue. Such interaction with warmth did not arise with hemilingual delivery of salty or sour solutions, which were conversely reported to interact with cooling of the opposite lingual surface (141). Although von Békésy’s observations suggested the percepts of, in part, sweet stimuli and oral warmth could combine in a complementary manner, his paper contains an idiosyncratic lack of data presentation and analysis, leaving the reader to partially intuit certain aspects of the reported effects.
A handful of subsequent human psychophysical studies that adopted a quantitative approach revealed interactions between sweet taste and temperature involve facilitation by warming of the perceived gustatory intensity of sucrose. Specifically, these investigations used in part a “sip-and-spit” technique, where taste solutions are sampled orally then expectorated, to show that innocuous oral and stimulus warming could increase the intercept of the psychophysical function for sucrose (8, 18, 54). Notably, these studies also identified that raising temperature can, under some conditions, decrease this function’s slope. The combination of these effects caused temperature-varied perceptual response functions for sucrose to separate more at lower stimulus concentrations compared with high, suggesting growth in perceived sweetness with warming was inversely related to sucrose concentration. In example, Bartoshuk and colleagues (8) showed facilitation of the perceived sweetness of sucrose with warming from 12°C to 44°C weakened as sucrose concertation was raised in quarter-log increments from 0.03 M, with facilitation by warming largely lost at 0.5 M. The intersection point of temperature-varied psychophysical functions for sucrose was estimated from data to be 0.52 M (8). Similarly, other psychophysical studies identified the facilitatory effect of warming on the perceived sweetness of sucrose was markedly attenuated or absent when concentration was increased to near half-molar values (18, 54). Beyond demonstrating the concentration dependence of human sucrose thermogustation, these data closely associate with mouse neurophysiological results that showed temperature can cause greater modulation of cellular gustatory activity to low sucrose concentrations compared with high, with thermal effects on unit firing showing nearly complete or absolute inhibition at around 0.5 M (145). This correlation between human perceptual and mouse functional data reinforces the notion that neural systems for sweet taste in these species share, at least in part, common operational principles (73).
It is important to point out that not all psychophysical data on sweet thermogustation agree, with some reports conversely identifying, in example, no significant effect of warming or temperature change on the perceived gustatory intensity of sucrose and other sugars (117, 131). This discrepancy has been discussed to possibly have roots in methodology, including differences across studies in rating tasks and use of methods for stimulus delivery that may facilitate inconsistent intraoral stimulation patterns (56). From this, a recent study from Green and Nachtigal (56) investigated thermal effects on sweet taste in humans using an approach that both temporally controlled and spatially restricted thermotaste stimulation to the anterior tongue protruding from the mouth. Under these methods, Green and Nachtigal identified that cooling taste solutions could indeed inhibit initial sweetness intensity ratings, but, notably, did so in a stimulus-specific manner. For example, cooling to ≤10°C suppressed the initial sweetness of sucrose and select artificial sweeteners but had not effect on the sweetness of fructose. Furthermore, cooling to 21°C could advance gustatory adaptation to sucrose, fructose, and glucose, whereas the rate of sweet taste adaptation for saccharin was found to be independent of temperature. These stimulus-dependent effects have implications for mechanism, as discussed below.
Thermal effects on sweet taste reported in Green and Nachtigal’s work were frequently enhanced at more extreme cold temperatures. From this, it was concluded that sweetness perception operates largely independently of temperature and could be impacted by cooling mainly when consuming refrigerated foods and beverages (56). One consideration for this interpretation, though, is that Green and Nachtigal (56) tested sugar concentrations that were considerably strong and possibly less sensitive to temperature effects than lower stimulus densities. For instance, their work used a sucrose concentration of 0.42 M, which is very near or at the plateau for sweet taste intensity and liking ratings in other human studies (69) and approaches the half-molar concentration of approximate thermal invariance for sucrose noted in other data (8, 18, 54, 145). Thus 0.42 M sucrose may be expected to induce gustatory responses that tend to resist modification by temperature change. It is curious if broader perceptual sensitivity to cooling and warming would emerge in humans for reduced concentrations of sucrose, and other sweet stimuli, delivered to controlled, restricted oral receptive fields. As above, weak and low concentrations of sucrose tend to induce activity with the highest susceptibility to change with thermal influence (8, 145).
Stimulus concentration notwithstanding, Green and Nachtigal’s data revealed that perceptual gustatory adaptation processes for sugars could be affected by thermal preadaptation of the tongue (56), which hints to a role for oral mucosa temperature in the processing of sugar taste. This postulate is also supported by earlier human work from Green’s group that showed tongue cooling caused greater inhibition of sugar sweetness than cooling taste solutions (53, 54). For example, cooling reduced the perceived intensity of sucrose only when the tongue and solution were similarly cooled to 20°C, as stimulus cooling caused only a slight decrease in sucrose taste intensity when the tongue was preadapted to a warm, 36°C temperature (54). Similar effects of oral thermal preadaptation on sucrose taste processing have been reported in rodent electrophysiological work. Recordings from mouse NTS neurons showed that unit firing to oral presence of 25°C sucrose was suppressed when the oral mucosa was precooled to ~25°C, albeit extended adaptation of the oral cavity to physiological warm (35°C) could, at some stimulus concentrations, rescue and augment activity to 25°C sucrose (77).
Sweet thermogustation: possible receptor mechanisms.
The ability of temperature to impact perceptual and neural responses to sweet stimuli is at least partly tied to thermal sensitivity by gustatory receptor mechanisms for these inputs. Talavera and colleagues (133) used patch-clamp electrophysiology applied to an expression system to discover that the TRPM5 ion channel, which mediates membrane currents during the transduction of sweet stimuli (30, 109, 155), is strongly sensitive to increasing temperature, with warming from around 15°C to 40°C causing a marked increase in TRPM5 open probability and current flow. This work also used in vivo electrophysiological recordings to show that mice genetically deficient for TRPM5 show suppressed CT nerve responses to oral presence of sugars, such as sucrose and glucose, and artificial sweeteners, including saccharin and SC45647, where remaining residual nerve activity to these stimuli gave no increase or change in magnitude with warming from 15°C to 35°C; such warming increased nerve activity to these inputs in wild-type mice (133). These data identified the warmth-sensitive TRPM5 ion channel as a molecular contributor to thermal influence on sweet taste and suggest there is a peripheral contribution to warmth-induced effects on sweetness perception. This postulate is also supported by the human phenomenon of “thermal taste,” where in the absence of taste stimulation, delivery of warmth to the tongue tip, poised to activate TRPM5 in sweet taste cells that may communicate with the CT nerve, can cause perceptions of sweetness in some subjects (29).
Nevertheless, it is debatable whether temperature sensitivity by TRPM5 is sufficient to account for sweet thermogustation. Initial studies on the involvement of TRPM5 with taste suggested this channel was critical for the transduction of diverse types of sweet tastants, including carbohydrates and artificial sweeteners (30, 133, 155), predicting TRPM5-mediated temperature effects should generalize across sweet stimuli. Yet as above, psychophysical investigations have found nonuniform effects of temperature on gustatory perceptual responses and adaptation among sugars and synthetic sweet tastants (56). What is more, some reports have revealed that mice genetically deficient for TRPM5 show residual CT nerve responses to lingual presence of glucose that significantly increase with warming from 15°C to 35°C (104), suggesting TRPM5 is not wholly necessary to induce warmth facilitation of responses to select sweet inputs. It is curious how temperature might influence TRPM5-independent components of the sweet taste signaling process (56). One possibility is that temperature may affect the receptor proteins that initiate sweet taste transduction (55, 144) and differentially impact ligand-receptor binding across sweet stimuli, although this remains to be experimentally studied.
Effects of Temperature on Other Taste Qualities
There are comparably fewer data on the effects of temperature on the taste qualities of bitter, sour, and umami. In fact, the first paper to study thermal influence on umami taste perception in humans was published in 2016. In this work, the perceived umami intensity of monopotassium glutamate increased with warming between 10°C and 37°C, albeit temperature change affected only the perceived saltiness, not umami, of the better known amino acid taste stimulus monosodium glutamate (MSG) delivered to the whole mouth (51). Although these effects appear differential, the latter agrees with neural and behavioral data showing that the gustatory signal for MSG, tested alone, is dominated by its sodium component (e.g., 75, 92, 147). MSG has been discussed to induce an umami sensation when mixed with flavor-enhancing nucleotides (e.g., 32, 92, 146). Considering this finding, electrophysiological data from mouse NTS neurons have revealed gustatory activity to MSG mixed with disodium inosinate (IMP) decorrelates with sodium and is substantially inhibited and facilitated by, respectively, cooling and warming of taste solutions (144). Moreover, warming from 22°C to 30°C causes superadditive enhancement of NTS unit activation to MSG-IMP mixtures, similar to the superadditive effect of warming on central gustatory activity to sucrose (144; Fig. 5). Thus neural messages concerning umami taste can, under certain conditions, show a marked susceptibility to increase in magnitude with warming.
Concerning mechanism, it seems probable that warmth facilitation of umami taste is at least partly contributed by the actions of temperature on current flow through the warmth-sensitive TRPM5 ion channel, a molecular component of the gustatory transduction cascade for amino acids (30, 155). In accord with a peripheral locus of effect, electrophysiological studies have revealed that, over a broad range of cool and warm temperatures, innocuous warming to ~30° to 35°C causes maximal responding by the CT nerve to oral presence of MSG-nucleotide mixtures and also MSG with its sodium component pharmacologically suppressed (78, 93). Comparable electrophysiological data from genetically TRPM5-deficient mice have reported that the CT nerve in these mutants displays gustatory responses to MSG that are insensitive to temperature (133). However, this work also reported that temperature did not influence nerve firing to MSG in wild-type mice. One consideration for this lack of thermal effect is that responses were based on only MSG alone; a mostly salty stimulus from the perspective of gustatory coding (75). It is curious if neural thermotaste responses to MSG-nucleotide mixtures would differ between TRPM5-deficient and control animals. Additional direct data on thermogustatory receptor mechanisms for umami stimuli await collection.
The available perceptual and neural data on sour thermogustation show variable results. Considering human work, Moskowitz (90) reported that 5°C steps in temperature between 25°C and 50°C could significantly impact the slope of the gustatory psychophysical function for citric acid, albeit these changes in slope were not systematic and statistical significance was lost if data from the 50°C condition were omitted. Other human psychophysical work revealed oral cooling had no effect on the psychophysical function relating stimulus concentration to the perceived intensity of citric acid (54). Moreover, electrophysiological studies on the rodent CT nerve have reported that responses to lingual presence of HCl, and other acids in some cases, are modulated (149), only weakly modulated (78), or unaffected (1) by change in temperature between cool (10°C to 23°C) and warm (~40°C to 45°C) values. This discrepancy could be related to the whole nerve preparation where the summed activity of many random fibers is sampled at once, which may facilitate or mask effects depending on the recorded fiber types. Along this line, single-neuron recordings from the rat geniculate ganglion (16) and also mouse NTS (144) have identified subpopulations of taste-active cells where innocuous warming could significantly increase neuronal firing to acidic taste stimuli. More work is needed to draw a consensus on how temperature impacts the processing of sour stimuli across species.
Temperature appears to induce stimulus-dependent effects on bitter taste. For instance, select oral cooling and warming conditions in humans can, respectively, suppress and enhance the perceived bitterness of the alkaloids caffeine (54) and quinine (52). However, perceptual gustatory adaptation to quinine increases with warming from 21°C to 37°C, whereas caffeine adaptation is unaffected by temperature change (52); this same study also reported that adaptation to the bitters denatonium benzoate and naringin was insensitive to temperature, further showing quinine can demonstrate unique sensitivity to temperature among select bitter tastants. Moreover, temperature effects on quinine gustation are, as with sodium and sweet taste, evidenced to involve interplay with stimulus concentration. Concentration-detection thresholds for oral quinine are reportedly lowest at moderately cool temperatures but rise with warming or extreme cooling (86).
Mechanisms supporting quinine thermogustation remain unclear. Electrophysiological studies have found that innocuous warming can facilitate CT nerve and unit responses to oral presence of quinine in rats (16, 149) and gradually increase CT nerve activity to quinine in mice (78). Taste-active NTS neurons in mice do show facilitated spiking to oral quinine with warming from 22°C to 35°C. However, the magnitude of this warmth-enhanced firing is substantially less than observed for sweet (cf., Fig. 6A) or umami stimuli and, unlike sweet and umami, does not follow a superadditive pattern on average across cells (144). The warmth-sensitive ion channel TRPM5 is implicated to participate in the gustatory transduction of bitters in addition to its role in sweet and umami taste (30, 109, 155) and, thus, seems probable to contribute to thermal effects on bitter taste processing. However, the differential effect of temperature on the growth of neuronal activity to TRPM5-dependent bitter and appetitive taste stimuli (144) and the observation that temperature causes nonuniform perceptual effects across bitter ligands (52) further question the sufficiency of TRPM5 to fully account for GPCR thermogustation.
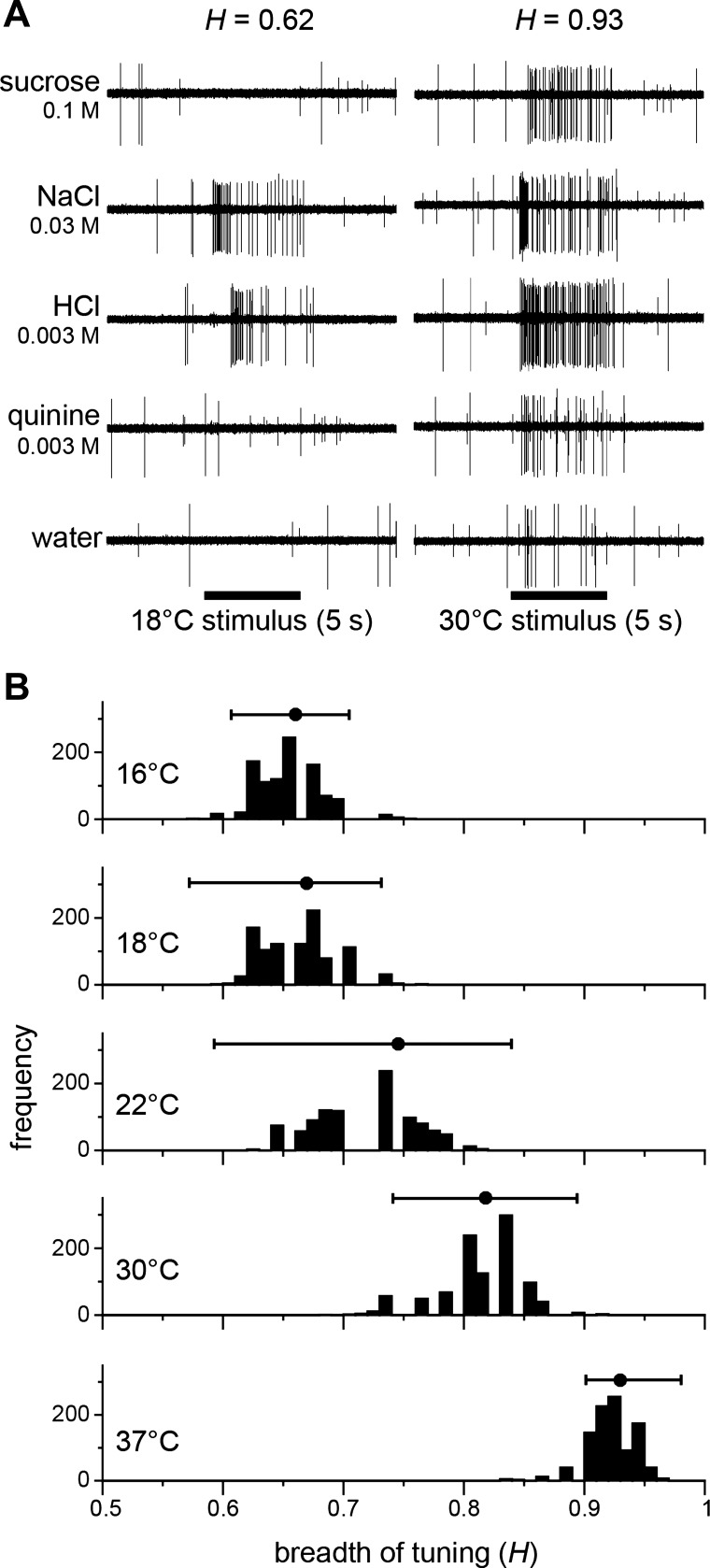
Fig. 6.
Temperature can change the breadth of neural responsiveness across taste qualities. A: example raw electrophysiological sweeps depicting spiking activity by an individual taste-active neuron in the mouse nucleus tractus solitarius (NTS) to oral delivery of sucrose (“sweet”), NaCl (“salty”), HCl (“sour”), quinine (“bitter), and water at 18°C (left) and 30°C (right). The H metric, which quantitatively gauges the breadth of tuning of individual neurons to gustatory stimuli (123), is given at the top of the column for each temperature condition. For four taste stimuli as shown here, an H that that approaches 0 indicates narrow responsiveness by a neuron to only one taste stimulus, a value around 0.5 can reflect robust firing to two stimuli, and a value approaching 1 can reflect responding by a cell across all four tastants (e.g., 128, 144). This unit fired spikes to only NaCl and HCl when stimuli were tested at 18°C. The cell showed increased breath of tuning and responded to all tastants when stimuli were warmed to 30°C. Note that for this cell, spike firing to warmed sucrose and quinine is greater than elicited by warming alone, which is represented by the sweep for 30°C water. Neuron from Wilson and Lemon (144). B: analysis of temperature effects on the breath of gustatory neural tuning. Depicted are histograms of bootstrapped median H values computed from electrophysiological responses by 39 mouse NTS neurons to oral delivery of 0.1 M sucrose, 0.03 M NaCl, 0.003 M HCl, and 0.003 M quinine delivered at each of five stimulus temperatures. The sample median H value and its bootstrapped ± 95% confidence interval is given as a point and whiskers above each histogram. Neural H values increased with warming, as revealed by no overlap among confidence intervals for the coolest (16°C and 18°C) and warmest (30°C and 37°C) temperatures. Thus cooling can narrow and warming can broaden the tuning of gustatory neurons to taste qualities. Data are from neurons described in Wilson and Lemon (144).
Nevertheless, TRP ion channels appear to underlie thermogustatory activity to select bitter stimuli in invertebrates. A gustatory electrophysiological study in Manduca hornworms revealed neural responses to salts, sugars, and caffeine were insensitive to temperature change, although neural impulse discharge to aristolochic acid, which tastes bitter to humans (45), was suppressed by cooling and enhanced by warming relative to 22°C (3). This modulatory effect of temperature was completely blocked by application of a selective antagonist of the ion channel TRP ankyrin 1 (TRPA1), which appears to function in part as a thermotaste receptor for aristolochic acid in Manduca (3). Implicated for roles in somatosensory and pain processing (7, 66), TRPA1 appears to not contribute to mammalian gustatory transduction; oral presence of a partial agonist of TRPA1 does not stimulate taste-active nerves in mice (105). Given the unique feeding niches of mice and Manduca, this species difference may reflect a role for ecological variables in the emergence of mechanisms supporting thermal sensitivity to taste, albeit this is not fully understood (3).
Other Questions and Issues
Although progress has been made, it is obvious from discussions above there remain many gaps in our understanding of thermogustatory effects. In addition to the relatively reduced amount of thermal data available for some taste qualities, basic questions about thermogustatory processing remain open, particularly concerning mechanism. Moreover, the ability of temperature to modify gustatory processing in nerves and neurons may have important implications for understanding the organization of taste circuits. Brief discussions of these and other issues follow.
Elucidating receptors and potential trigeminal involvement with thermogustation.
From the perspective of establishing its cause, clear delineation of the mechanisms underlying thermogustation is still lacking. Establishing mechanism will provide important clues to thermogustatory function: does this process originate from temperature modulation of taste pathways or also involve interactions between taste and oral somatosensory processes? Considering the former, probably the most discussed thermoreceptor mechanism associated with gustation is the warmth-sensitive TRPM5 ion channel involved with umami, sweet, and bitter taste transduction. However, the unbalanced effect of temperature on neural and perceptual responses across and among sweet and bitter inputs (52, 56, 144) suggests there may be multiple receptor components or effectors that mediate thermogustatory activity for these qualia. As discussed above and elsewhere (56, 77, 144), explanations based solely on TRPM5 partly strain to account for all thermogustatory effects observed for GPCR-transduced tastants. Moreover, although biophysical work indicates ENaCs, involved with salt taste reception, are imbued with temperature sensitivity (5, 25, 26), most prior investigations of salt taste transduction and processing involving this channel have not studied thermotaste effects.
Beyond receptors, there appears to be strong potential for thermogustatory interactions to involve interplay between gustatory-active and trigeminal-somatosensory neurons, with the latter carrying the major signals about oral temperature (72). Interaction between the trigeminal and gustatory systems is evidenced to possibly arise at multiple levels of the neuraxis, beginning at the periphery. Stimulation of the trigeminal nerve can modulate taste transmission in CT fibers possibly through axon reflex release of neuromodulators (142). Lingual application of capsaicin, an agonist of the nocisensor TRPV1 expressed by trigeminal fibers, can modulate taste processing in rat NTS neurons via a peripheral mechanism (120). Furthermore, capsaicin-sensitive trigeminal fibers are implicated to innervate taste papillae and influence the ontogeny of taste buds (106), reflective of a form of crossmodal developmental interaction between somatosensory and gustatory pathways. Finally, several lines of work have shown that trigeminal afferents and brain structures are anatomically and physiologically linked to central nuclei for taste, such as the NTS (12, 13, 15, 27, 28, 38, 58, 85, 143, 154). Nevertheless, there are no published studies on how direct manipulation of trigeminal circuits could modify oral thermal activity or combined taste-temperature processing in gustatory-active neurons. If present, trigeminal contributions to oral responding in thermogustatory cells could suggest involvement of these neurons with true multisensory function, integrating gustatory and flavor-related somatosensory signals conveyed by separate cranial nerves.
Temperature: a latent variable in studies of gustatory coding.
When compared with gustatory intensity or sensitivity, there is only a dearth of data on how temperature may influence the processing of taste quality. Thermal stimulation of the human tongue in the absence of taste can, depending on temperature and locale, induce select gustatory perceptions through the thermal taste effect (29), as above. But does temperature modulate and guide how the nervous system represents information involved with the recognition and discrimination of different taste qualities? Newer data speak to a potential role for temperature in understanding the neural code for gustatory quality–a longstanding topic in sensory neuroscience that still awaits resolution.
Multiple theories have been proposed to account for taste coding, with the most popular involving spatial neural transmission. Although there are different, argued to be opposing, variants (e.g., 70, 74), models of spatial coding in taste all posit that, through some format, a change in gustatory quality is signaled by a substitutive change in the neurons that markedly fire to taste input. Considering this, a recent neurophysiological study carried out in the mouse NTS demonstrated that the breadth of taste qualities that gustatory-sensitive cells will fire to can depend on temperature (144). Under stimulus cooling, neurons recorded in this work predominantly responded to, on average, electrolyte taste stimuli, such as moderate concentrations of salts and acids. Yet when taste stimuli were warmed, some of these same cells could increase their firing to electrolytes and also begin to show notable coresponsiveness to stimuli of other taste qualities, such as sweet and bitter (e.g., Fig. 6A). When indexed using a standard quantitative metric, the breadth of tuning of NTS gustatory neurons was found to significantly and substantially increase with warming steps (144; Fig. 6B). Furthermore, additional work has shown that temperature can determine, in some cases, how many neurons will fire to gustatory stimulation, with stimulus warming increasing the number mouse NTS neurons that significantly respond to perithreshold and low concentrations of sucrose (145). Altogether, these data reveal that stimulus cooling and warming may have critical influence on spatial neural representations for particular tastes through recruiting and releasing neuronal signals to and from gustatory response patterns. Control of temperature, alongside concentration, would seem critical for interpretation of neural activity with respect to models of spatial gustatory processing.
Nevertheless, the available electrophysiological and imaging data specifically focused on gustatory quality coding and discrimination are largely based on temperature-uncontrolled stimuli. The norm in such works, as so in prior papers I coauthored, was to test only “room temperature” taste solutions, with no real standardization of this temperature across studies. If one assumes that room temperature in a laboratory is ~22°C (72°F), functional studies conducted with stimuli isothermal to laboratory air temperature would index responses to taste stimuli at a relatively cool value that can cause, for instance, suppression of gustatory nerve and NTS unit firing to the sweet prototype sucrose (16, 104, 133, 144, 145; see Fig. 5). Testing temperature-uncontrolled taste stimuli in functional work could lead to only partial characterization of neuronal taste profiles or the subpopulations of cells excited by select taste stimuli (144, 145). This may distort measurement of spatial neural activity presumably associated with the representation of gustatory quality.
However, it remains unclear how temperature-induced shifts in tuning breadth or activated neurons would impact neural messages for taste quality. Although these shifts could indeed broaden or narrow spatial response patterns to tastes across a cellular population, such change could simply relate to modulation of gustatory signal strength as opposed to quality. Distinguishing these possibilities would rely in part on determining the functions served by different classes of neurons and the networks into which they are embedded. Yet related to taste quality, there has been some discussion in perceptual works on how temperature may influence the time course over which taste profiles evolve (53). There also exist neurophysiological data that show particular temperature conditions can markedly affect the speed of onset of gustatory neural activity to select stimuli, such as sucrose (77, 145). Temperature-imbued change in taste response latency may influence the effect of time on the qualitative recognition of taste cues (49) and could also affect the timing of interactions between signals for multiple taste and flavor qualities bound in a stimulus mixture (53, 145).
Exploring thermogustatory behavior in rodents.
On relating neuronal effects to perception, there is a notable gap in our knowledge of how temperature influences gustatory detection and ingestive behaviors in rodents, which have been important models for functional studies on thermogustation. There exists a sparse body of papers on the effects of solution temperature on saline/water intake (46–48, 68) and orosensory conditioning (125), but studies with a focus on temperature effects on gustatory-guided intake behavior in rodents are almost nonexistent. One notable investigation used a custom Peltier-based system for thermal control of taste solutions to reveal effects of solution temperature on rodent preference behavior toward carbohydrates that were conditioned on thirst. In this work, Torregrossa et al. (134) showed water-restricted rats preferred cold (10°C) water over multiple concentrations of sucrose tested at 30°C or 40°C, albeit preference behavior displayed by these animals shifted toward sucrose upon reinstatement of normal water availability. Furthermore, brief-access orosensory preference tests revealed chilling sucrose to 10°C suppressed intake compared with 20°C sucrose (134), which suggests cooling reduces the palatability of sweet stimuli to rodents. This finding agrees with neurophysiological data indicating cooling can suppress sweet taste transmission in gustatory-sensitive neurons (e.g., 16, 145, 149). However, Torregrossa and colleagues identified that warming to 40°C could, in some cases, also suppress rat orosensory preference toward sucrose (134), whereas some neurophysiological data show the rat CT nerve gives enhanced firing to sucrose at 40°C compared with cold temperatures (16). To further explore how temperature operates on sensory processes associated with ingestion in rodents it may prove fruitful to monitor and perturb the actions of thermogustatory neurons in awake animals actively sampling thermally varied taste substances.
Synopsis
Knowledge gaps notwithstanding, there are certain trends that can be gleaned from the available data on the influence of temperature on taste. For one, several studies discussed above suggest thermogustatory effects, when observed, show stimulus dependence. A given thermal shift can differently influence neural activity to taste stimuli of different qualities, such as the ability of cooling to facilitate and suppress nerve activity to low concentrations of, respectively, sodium and acidic stimuli (e.g., 149; Fig. 2). Furthermore, warmth can cause superadditive increases in neural firing to appetitive umami and sweet stimuli, albeit this effect is generally not observed with avoided bitters (144). Finally, multiple studies have identified that temperature effects can vary among stimuli considered to compose a singular taste quality class (1, 52, 56, 104), as exemplified by the differential effects of temperature on perceived sweetness intensity ratings and adaptation for different “sweet” ligands (56). These data were discussed as potential evidence that multiple mechanisms support thermal modulation of sweet taste, as above, or sweet taste transduction (56). Although both are possible, the latter postulate agrees with other results suggesting multiple effectors could mediate sweet taste (e.g., 104, 116, 151) and highlights a utility of temperature for exploring this issue (see 55, 104).
Several neural and perceptual studies have demonstrated that temperature effects on taste importantly depend on stimulus concentration, with some data showing temperature exerts systematically greater influence on responses to low or weakly concentrated taste solutions as opposed to high (e.g., 145, 148). Thus, temperature appears to operate as a partial parameter of taste response magnitude. This postulate is also supported by the observation that focal thermal stimulation of the tongue can, by itself, induce perceptions of select taste qualities in some human subjects (29), suggesting there exist temperature-sensitive gustatory neurons that largely generate signals about taste (29, 72) with temperature capable of modulating their gustatory information output.
Some functional and psychophysical data suggest thermal influence on taste may also involve effects contributed by mouth temperature (53, 54, 77). This phenomenon may have relevance to applied scenarios, as oral mucosa temperature may vary during eating partly as a function of heat exchange between intraoral surfaces and warmed or chilled foods and drinks. In this context, sipping cooled or heated water can rapidly change the temperature inside the human mouth, measured on the inner molar surface, by several degrees Celsius (107). It is also possible that oral behaviors associated with feeding may modulate oral temperature. Green (50) showed the temperature of the human tongue surface is reduced when the mouth is closed for 30 s compared with 60 s, which suggests mouth openings as arising during eating or accompanying conversation facilitate evaporative heat loss by oral epithelia. Variation in oral temperature during eating may conceivably contribute to a temporally dynamic relationship between the temperature of oral skin surface and taste substance that could, in some cases, impact the generation of gustatory signals.
Conclusion
Neural and perceptual responses to select taste stimuli can display substantial and impressive changes with shifts in temperature. Nonetheless, major gaps in our knowledge remain concerning the mechanisms, neural basis, and consequences of thermogustation. Because temperature is an inherent component of gustatory experience, resolving open questions concerning the thermoreceptors, circuits, and operational principles underlying thermogustatory activity may prove critical for ultimately unravelling the organization of gustatory pathways and their roles in multisensory processing relevant to flavor perception. From a functional perspective, it remains unclear as to why some elements of taste perception appeared to evolve susceptibility to change with the oral thermal environment. Was this evolution purposeful with, to use a speculative postulate, the heighted sensitivity of umami sensations to warming possibly associated with detection by carnivores of proteinaceous tastes of freshly-killed meat in warm-blooded prey. Or, is gustatory thermal sensitivity partly accidental and due to conservation and reuse by the nervous system of effectors with apparent multipurpose function, as exemplified by gustatory transduction cascades that partly make use of temperature-sensitive ion channels. Whatever the catalyst, temperature appears to play important roles in the processing and perception of particular taste sensations. Exploration of thermogustation has old roots but is also relatively nascent.
GRANTS
This paper was supported in part by National Institutes of Health Grant DC011579 to C. H. Lemon.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
C.H.L. analyzed data; C.H.L. interpreted results of experiments; C.H.L. prepared figures; C.H.L. drafted manuscript; C.H.L. edited and revised manuscript; C.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. Jinrong Li for critical reading of this paper and artwork contribution.
REFERENCES
- 1.Abbott P. The Effect of Temperature on Taste in the White Rat (PhD Thesis) Brown University, 1953. [Google Scholar]
- 2.Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 136: 91–98, 2005. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Afroz A, Howlett N, Shukla A, Ahmad F, Batista E, Bedard K, Payne S, Morton B, Mansfield JH, Glendinning JI. Gustatory receptor neurons in Manduca sexta contain a TrpA1-dependent signaling pathway that integrates taste and temperature. Chem Senses 38: 605–617, 2013. doi: 10.1093/chemse/bjt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai T, Ohkuri T, Yasumatsu K, Kaga T, Ninomiya Y. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience 165: 1476–1489, 2010. doi: 10.1016/j.neuroscience.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 5.Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001. doi: 10.1073/pnas.111155398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awayda MS, Shao W, Guo F, Zeidel M, Hill WG. ENaC–membrane interactions: regulation of channel activity by membrane order. J Gen Physiol 123: 709–727, 2004. doi: 10.1085/jgp.200308983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 8.Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav 28: 905–910, 1982. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- 9.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 10.Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol 190: 259–282, 1980. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- 11.Beidler LM. A theory of taste stimulation. J Gen Physiol 38: 133–139, 1954. doi: 10.1085/jgp.38.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomquist AJ, Antem A. Localization of the terminal of the tongue afferents in the nucleus of the solitary tract. J Comp Neurol 124: 127–130, 1965. doi: 10.1002/cne.901240110. [DOI] [PubMed] [Google Scholar]
- 13.Boucher Y, Simons CT, Faurion A, Azérad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res 973: 265–274, 2003. doi: 10.1016/S0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- 14.Bradley RM, Mistretta CM, Nagai T. Demonstration of sensory innervation of rat tongue with anterogradely transported horseradish peroxidase. Brain Res 367: 364–367, 1986. doi: 10.1016/0006-8993(86)91620-3. [DOI] [PubMed] [Google Scholar]
- 15.Braud A, Vandenbeuch A, Zerari-Mailly F, Boucher Y. Dental afferents project onto gustatory neurons in the nucleus of the solitary tract. J Dent Res 91: 215–220, 2012. doi: 10.1177/0022034511429569. [DOI] [PubMed] [Google Scholar]
- 16.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 17.Calviño AM. Effects of concentration and temperature on gustatory persistence. Percept Mot Skills 58: 647–650, 1984. doi: 10.2466/pms.1984.58.2.647. [DOI] [PubMed] [Google Scholar]
- 18.Calviño AM. Perception of sweetness: the effects of concentration and temperature. Physiol Behav 36: 1021–1028, 1986. doi: 10.1016/0031-9384(86)90474-9. [DOI] [PubMed] [Google Scholar]
- 19.Cardello A, Maller O. Acceptability of water, selected beverages and foods as a function of serving temperature. J Food Sci 47: 1549–1552, 1982. doi: 10.1111/j.1365-2621.1982.tb04980.x. [DOI] [Google Scholar]
- 20.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol 80: 465–492, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R64–R76, 2007. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 22.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 23.Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240: 153–160, 1985. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chraïbi A, Horisberger JD. Dual effect of temperature on the human epithelial Na+ channel. Pflugers Arch 447: 316–320, 2003. doi: 10.1007/s00424-003-1178-9. [DOI] [PubMed] [Google Scholar]
- 26.Chraïbi A, Horisberger JD. Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J Gen Physiol 120: 133–145, 2002. doi: 10.1085/jgp.20028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst 6: 303–322, 1982. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- 28.Corson J, Aldridge A, Wilmoth K, Erisir A. A survey of oral cavity afferents to the rat nucleus tractus solitarii. J Comp Neurol 520: 495–527, 2012. doi: 10.1002/cne.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz A, Green BG. Thermal stimulation of taste. Nature 403: 889–892, 2000. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 30.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 31.Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39–45, 1973. doi: 10.1016/0031-9384(73)90120-0. [DOI] [PubMed] [Google Scholar]
- 32.Delay ER, Beaver AJ, Wagner KA, Stapleton JR, Harbaugh JO, Catron KD, Roper SD. Taste preference synergy between glutamate receptor agonists and inosine monophosphate in rats. Chem Senses 25: 507–515, 2000. doi: 10.1093/chemse/25.5.507. [DOI] [PubMed] [Google Scholar]
- 33.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351–357, 2006. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- 34.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28: 566–575, 2008. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamant H, Funakoshi M, Ström L, Zotterman Y. Electrophysiological studies on human taste nerves. In: Olfaction and Taste, edited by Zotterman Y. Oxford: Pergamon, 1963, p. 193–203. doi: 10.1016/B978-1-4831-9834-7.50020-2. [DOI] [Google Scholar]
- 36.Dodt E, Zotterman Y. Mode of action of warm receptors. Acta Physiol Scand 26: 345–357, 1952. doi: 10.1111/j.1748-1716.1952.tb00916.x. [DOI] [PubMed] [Google Scholar]
- 37.Escanilla OD, Victor JD, Di Lorenzo PM. Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. J Neurosci 35: 6284–6297, 2015. doi: 10.1523/JNEUROSCI.3526-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felizardo R, Boucher Y, Braud A, Carstens E, Dauvergne C, Zerari-Mailly F. Trigeminal projections on gustatory neurons of the nucleus of the solitary tract: a double-label strategy using electrical stimulation of the chorda tympani and tracer injection in the lingual nerve. Brain Res 1288: 60–68, 2009. doi: 10.1016/j.brainres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Fernstrom JD, Munger SD, Sclafani A, de Araujo IE, Roberts A, Molinary S. Mechanisms for sweetness. J Nutr 142: 1134S–1141S, 2012. doi: 10.3945/jn.111.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 41.Fishman IY. Single fiber gustatory impulses in rat and hamster. J Cell Comp Physiol 49: 319–334, 1957. doi: 10.1002/jcp.1030490213. [DOI] [PubMed] [Google Scholar]
- 42.Fontanini A, Katz DB. State-dependent modulation of time-varying gustatory responses. J Neurophysiol 96: 3183–3193, 2006. doi: 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- 43.Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, Katz DB. State dependence of olfactory perception as a function of taste cortical inactivation. Nat Neurosci 13: 158–159, 2010. doi: 10.1038/nn.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol 65: 1452–1463, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Glendinning JI, Brown H, Capoor M, Davis A, Gbedemah A, Long E. A peripheral mechanism for behavioral adaptation to specific “bitter” taste stimuli in an insect. J Neurosci 21: 3688–3696, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold RM, Kapatos G, Prowse J, Quackenbush PM, Oxford TW. Role of water temperature in the regulation of water intake. J Comp Physiol Psychol 85: 52–63, 1973. doi: 10.1037/h0034881. [DOI] [PubMed] [Google Scholar]
- 47.Gold RM, Laforge RC, Sawchenko PE, Fraser JC, Pytko D. Cool water’s suppression of water intake: persistence across deprivation conditions, ages, sexes, and osmolarities. Physiol Behav 18: 1047–1053, 1977. doi: 10.1016/0031-9384(77)90010-5. [DOI] [PubMed] [Google Scholar]
- 48.Gold RM, Prowse J. Water temperature preference shifts during hydration. Physiol Behav 13: 291–296, 1974. doi: 10.1016/0031-9384(74)90047-X. [DOI] [PubMed] [Google Scholar]
- 49.Graham DM, Sun C, Hill DL. Temporal signatures of taste quality driven by active sensing. J Neurosci 34: 7398–7411, 2014. doi: 10.1523/JNEUROSCI.0213-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green BG. Oral perception of the temperature of liquids. Percept Psychophys 39: 19–24, 1986. doi: 10.3758/BF03207579. [DOI] [PubMed] [Google Scholar]
- 51.Green BG, Alvarado C, Andrew K, Nachtigal D. The Effect of Temperature on Umami Taste. Chem Senses 41: 537–545, 2016. doi: 10.1093/chemse/bjw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green BG, Andrew K. Stimulus-dependent effects of temperature on bitter taste in humans. Chem Senses 42: 153–160, 2017. doi: 10.1093/chemse/bjw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav 43: 515–519, 1988. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 54.Green BG, Frankmann SP. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses 12: 609–619, 1987. doi: 10.1093/chemse/12.4.609. [DOI] [Google Scholar]
- 55.Green BG, Nachtigal D. Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav 107: 488–495, 2012. doi: 10.1016/j.physbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green BG, Nachtigal D. Temperature affects human sweet taste via at least two mechanisms. Chem Senses 40: 391–399, 2015. doi: 10.1093/chemse/bjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577, 1984. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- 59.Harada S, Smith DV. Gustatory sensitivities of the hamster’s soft palate. Chem Senses 17: 37–51, 1992. doi: 10.1093/chemse/17.1.37. [DOI] [Google Scholar]
- 60.Hayama T, Ito S, Ogawa H. Receptive field properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res 68: 458–465, 1987. doi: 10.1007/BF00249790. [DOI] [PubMed] [Google Scholar]
- 61.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 62.Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees II: single chorda tympani fibers. Physiol Behav 61: 829–841, 1997. doi: 10.1016/S0031-9384(96)00562-8. [DOI] [PubMed] [Google Scholar]
- 63.Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res 513: 24–34, 1990. doi: 10.1016/0006-8993(90)91085-U. [DOI] [PubMed] [Google Scholar]
- 64.Heyer BR, Taylor-Burds CC, Mitzelfelt JD, Delay ER. Monosodium glutamate and sweet taste: discrimination between the tastes of sweet stimuli and glutamate in rats. Chem Senses 29: 721–729, 2004. doi: 10.1093/chemse/bjh081. [DOI] [PubMed] [Google Scholar]
- 65.Jones LM, Fontanini A, Katz DB. Gustatory processing: a dynamic systems approach. Curr Opin Neurobiol 16: 420–428, 2006. doi: 10.1016/j.conb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 67.Kadohisa M, Rolls ET, Verhagen JV. Orbitofrontal cortex: neuronal representation of oral temperature and capsaicin in addition to taste and texture. Neuroscience 127: 207–221, 2004. doi: 10.1016/j.neuroscience.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 68.Kapatos G, Gold RM. Tongue Cooling during Drinking: A Regulator of Water Intake in Rats. Science 176: 685–686, 1972. doi: 10.1126/science.176.4035.685. [DOI] [PubMed] [Google Scholar]
- 69.Kareken DA, Dzemidzic M, Oberlin BG, Eiler WJ II. A preliminary study of the human brain response to oral sucrose and its association with recent drinking. Alcohol Clin Exp Res 37: 2058–2065, 2013. doi: 10.1111/acer.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katz DB, Nicolelis MA, Simon SA. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol 12: 448–454, 2002. doi: 10.1016/S0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- 71.Kim JW, Samant SS, Seo Y, Seo HS. Variation in saltiness perception of soup with respect to soup serving temperature and consumer dietary habits. Appetite 84: 73–78, 2015. doi: 10.1016/j.appet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Lemon CH, Kang Y, Li J. Separate functions for responses to oral temperature in thermo-gustatory and trigeminal neurons. Chem Senses 41: 457–471, 2016. doi: 10.1093/chemse/bjw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lemon CH. Perceptual and neural responses to sweet taste in humans and rodents. Chemosens Percept 8: 46–52, 2015. doi: 10.1007/s12078-015-9177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemon CH, Katz DB. The neural processing of taste. BMC Neurosci 8, Suppl 3: S5, 2007. doi: 10.1186/1471-2202-8-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009. doi: 10.1152/jn.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Lemon C. Trigeminal convergence onto oral sensory neurons in the mouse nucleus of the solitary tract associates with gustatory and thermal tuning (Abstract). Chem Senses 40: 575, 2015. [Google Scholar]
- 77.Li J, Lemon CH. Influence of stimulus and oral adaptation temperature on gustatory responses in central taste-sensitive neurons. J Neurophysiol 113: 2700–2712, 2015. doi: 10.1152/jn.00736.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu B, Breza JM, Contreras RJ. Temperature influences chorda tympani nerve responses to sweet, salty, sour, umami, and bitter stimuli in mice. Chem Senses 41: 727–736. 2016. 10.1093/chemse/bjw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav 107: 533–539, 2012. doi: 10.1016/j.physbeh.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Lundy RF Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Lundy RF Jr, Contreras RJ. Neural responses of thermal-sensitive lingual fibers to brief menthol stimulation. Brain Res 641: 208–216, 1994. doi: 10.1016/0006-8993(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 82.Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makous W, Nord S, Oakley B, Pfaffmann C. The gustatory relay in the medulla. In: Olfaction and Taste, edited by Zotterman Y. Oxford: Pergamon, 1963, p. 381–393. doi: 10.1016/B978-1-4831-9834-7.50034-2 [DOI] [Google Scholar]
- 84.Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the tranganglionic transport of horseradish peroxidase. J Comp Neurol 203: 785–798, 1981. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- 85.Marfurt CF, Rajchert DM. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J Comp Neurol 303: 489–511, 1991. doi: 10.1002/cne.903030313. [DOI] [PubMed] [Google Scholar]
- 86.McBurney DH, Collings VB, Glanz LM. Temperature dependence of human taste responses. Physiol Behav 11: 89–94, 1973. doi: 10.1016/0031-9384(73)90127-3. [DOI] [PubMed] [Google Scholar]
- 87.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 88.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J 30: 582–593, 2011. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montavon P, Hellekant G, Farbman A. Immunohistochemical, electrophysiological, and electron microscopical study of rat fungiform taste buds after regeneration of chorda tympani through the non-gustatory lingual nerve. J Comp Neurol 367: 491–502, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 90.Moskowitz HR. Effect of solution temperature on taste intensity in humans. Physiol Behav 10: 289–292, 1973. doi: 10.1016/0031-9384(73)90312-0. [DOI] [PubMed] [Google Scholar]
- 91.Nagaki J, Yamashita S, Sato M. Neural response of cat to taste stimuli of varying temperatures. Jpn J Physiol 14: 67–89, 1964. doi: 10.2170/jjphysiol.14.67. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura M, Kurihara K. Canine taste nerve responses to monosodium glutamate and disodium guanylate: differentiation between umami and salt components with amiloride. Brain Res 541: 21–28, 1991. doi: 10.1016/0006-8993(91)91069-D. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura M, Kurihara K. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol 261: R1402–R1408, 1991. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura M, Kurihara K. Temperature dependence of amiloride-sensitive and -insensitive components of rat taste nerve response to NaCl. Brain Res 444: 159–164, 1988. doi: 10.1016/0006-8993(88)90923-7. [DOI] [PubMed] [Google Scholar]
- 95.Nejad MS. The neural activities of the greater superficial petrosal nerve of the rat in response to chemical stimulation of the palate. Chem Senses 11: 283–293, 1986. doi: 10.1093/chemse/11.3.283. [DOI] [Google Scholar]
- 96.Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res 451: 319–325, 1988. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- 97.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol 150: 217–237, 1973. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 98.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 91: 99–117, 1975. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- 99.Oakley B. Taste responses of human chorda tympani nerve. Chem Senses 10: 469–481, 1985. doi: 10.1093/chemse/10.4.469. [DOI] [Google Scholar]
- 100.Ogawa H, Hayama T, Ito S. Convergence of input from tongue and palate to the parabrachial nucleus neurons of rats. Neurosci Lett 28: 9–14, 1982. doi: 10.1016/0304-3940(82)90200-2. [DOI] [PubMed] [Google Scholar]
- 101.Ogawa H, Hayama T, Ito S. Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res 68: 449–457, 1987. doi: 10.1007/BF00249789. [DOI] [PubMed] [Google Scholar]
- 102.Ogawa H, Hayama T, Yamashita Y. Thermal sensitivity of neurons in a rostral part of the rat solitary tract nucleus. Brain Res 454: 321–331, 1988. doi: 10.1016/0006-8993(88)90833-5. [DOI] [PubMed] [Google Scholar]
- 103.Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chordat typani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol 199: 223–240, 1968. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol 296: R960–R971, 2009. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature 494: 472–475, 2013. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omelian JM, Samson KK, Sollars SI. Chronic oral capsaicin exposure during development leads to adult rats with reduced taste bud volumes. Chemosens Percept 9: 95–104, 2016. doi: 10.1007/s12078-016-9214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pangborn RM, Chrisp RB, Bertolero LL. Gustatory, salivary, and oral thermal responses to solutions of sodium chloride at four temperatures. Percept Psychophys 8: 69–75, 1970. doi: 10.3758/BF03210177. [DOI] [Google Scholar]
- 108.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 109.Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176, 2002. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 110.Pfaffmann C, Erickson RP, Frommer GP, Halpern BP. Gustatory discharge in the rat medulla and thalamus. In: Sensory Communication, edited by Rosenblith WA. New York: Wiley, 1961, p. 455–473. [Google Scholar]
- 111.Pittman DW, Contreras RJ. Responses of single lingual nerve fibers to thermal and chemical stimulation. Brain Res 790: 224–235, 1998. doi: 10.1016/S0006-8993(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 112.Ren Z, Rhyu MR, Phan TH, Mummalaneni S, Murthy KS, Grider JR, DeSimone JA, Lyall V. TRPM5-dependent amiloride- and benzamil-insensitive NaCl chorda tympani taste nerve response. Am J Physiol Gastrointest Liver Physiol 305: G106–G117, 2013. doi: 10.1152/ajpgi.00053.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roper SD. TRPs in taste and chemesthesis. Handb Exp Pharmacol 223: 827–871, 2014. doi: 10.1007/978-3-319-05161-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samuelsen CL, Fontanini A. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci 1926–16, 2016. doi: 10.1523/JNEUROSCI.1926-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato M. Gustatory response as a temperature-dependent process. Contrib Sens Physiol 2: 223–251, 1967. doi: 10.1016/B978-1-4831-6749-7.50011-8. [DOI] [PubMed] [Google Scholar]
- 116.Schier LA, Spector AC. Behavioral evidence for more than one taste signaling pathway for sugars in rats. J Neurosci 36: 113–124, 2016. doi: 10.1523/JNEUROSCI.3356-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schiffman SS, Sattely-Miller EA, Graham BG, Bennett JL, Booth BJ, Desai N, Bishay I. Effect of temperature, pH, and ions on sweet taste. Physiol Behav 68: 469–481, 2000. doi: 10.1016/S0031-9384(99)00205-X. [DOI] [PubMed] [Google Scholar]
- 118.Shigemura N, Ohkuri T, Sadamitsu C, Yasumatsu K, Yoshida R, Beauchamp GK, Bachmanov AA, Ninomiya Y. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC α-subunit in mice. Am J Physiol Regul Integr Comp Physiol 294: R66–R75, 2008. doi: 10.1152/ajpregu.00420.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7: 890–901, 2006. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 120.Simons CT, Boucher Y, Carstens E. Suppression of central taste transmission by oral capsaicin. J Neurosci 23: 978–985, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol 92: 1892–1903, 2004. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- 122.Smith DV, St John SJ. Neural coding of gustatory information. Curr Opin Neurobiol 9: 427–435, 1999. doi: 10.1016/S0959-4388(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 123.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses 4: 215–229, 1979. doi: 10.1093/chemse/4.3.215. [DOI] [Google Scholar]
- 124.Smith KR, Treesukosol Y, Paedae AB, Contreras RJ, Spector AC. Contribution of the TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. Am J Physiol Regul Integr Comp Physiol 303: R1195–R1205, 2012. doi: 10.1152/ajpregu.00154.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith PL, Smith JC, Houpt TA. Interactions of temperature and taste in conditioned aversions. Physiol Behav 99: 324–333, 2010. doi: 10.1016/j.physbeh.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spector AC, Guagliardo NA, St. John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci 16: 8115–8122, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci 22: 1937–1941, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 129.Stapleton JR, Lavine ML, Nicolelis MA, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Front Neurosci 1: 161–174, 2007. doi: 10.3389/neuro.01.1.1.012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steen I, Waehrens SS, Petersen MA, Münchow M, Bredie WL. Influence of serving temperature on flavour perception and release of Bourbon Caturra coffee. Food Chem 219: 61–68, 2017. doi: 10.1016/j.foodchem.2016.09.113. [DOI] [PubMed] [Google Scholar]
- 131.Stone H, Oliver S, Kloehn J. Temperature and pH effects on the relative sweetness of suprathreshold mixtures of dextrose and fructose. Percept Psychophys 5: 257–260, 1969. doi: 10.3758/BF03209557. [DOI] [Google Scholar]
- 132.Sweazey RD, Bradley RM. Responses of neurons in the lamb nucleus tractus solitarius to stimulation of the caudal oral cavity and epiglottis with different stimulus modalities. Brain Res 480: 133–150, 1989. doi: 10.1016/0006-8993(89)91576-X. [DOI] [PubMed] [Google Scholar]
- 133.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 134.Torregrossa AM, Bales MB, Breza JM, Houpt TA, Smith JC, Contreras RJ. Water restriction and fluid temperature alter preference for water and sucrose solutions. Chem Senses 37: 279–292, 2012. doi: 10.1093/chemse/bjr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- 136.Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol 73: 2144–2162, 1995. [DOI] [PubMed] [Google Scholar]
- 137.Travers SP, Smith DV. Responsiveness of neurons in the hamster parabrachial nuclei to taste mixtures. J Gen Physiol 84: 221–250, 1984. doi: 10.1085/jgp.84.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R1799–R1809, 2007. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- 139.Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol 89: 265–275, 2003. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- 140.Verhagen JV, Kadohisa M, Rolls ET. Primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol 92: 1685–1699, 2004. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- 141.von Békésy G. Duplexity theory of taste. Science 145: 834–835, 1964. doi: 10.1126/science.145.3634.834. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y, Erickson RP, Simon SA. Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J Neurophysiol 73: 1468–1483, 1995. [DOI] [PubMed] [Google Scholar]
- 143.Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol 220: 378–395, 1983. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]
- 144.Wilson DM, Lemon CH. Modulation of central gustatory coding by temperature. J Neurophysiol 110: 1117–1129, 2013. doi: 10.1152/jn.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilson DM, Lemon CH. Temperature systematically modifies neural activity for sweet taste. J Neurophysiol 112: 1667–1677, 2014. doi: 10.1152/jn.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamaguchi K. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J Food Sci 32: 473–478, 1967. doi: 10.1111/j.1365-2621.1967.tb09715.x. [DOI] [Google Scholar]
- 147.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. III. Neural and behavioral measures compared. J Neurophysiol 53: 1370–1386, 1985. [DOI] [PubMed] [Google Scholar]
- 148.Yamashita S, Ogawa H, Kiyoara T, Sato M. Modification by temperature change of gustatory impulse discharges in chorda tympani fibres of rats. Jpn J Physiol 20: 348–363, 1970. doi: 10.2170/jjphysiol.20.348. [DOI] [PubMed] [Google Scholar]
- 149.Yamashita S, Sato M. The effects of temperature on gustatory response of rats. J Cell Physiol 66: 1–17, 1965. doi: 10.1002/jcp.1030660102. [DOI] [PubMed] [Google Scholar]
- 150.Yamashita S, Yamada K, Sato M. The effect of temperature on neural taste response of cats. Jpn J Physiol 14: 505–514, 1964. doi: 10.2170/jjphysiol.14.505. [DOI] [PubMed] [Google Scholar]
- 151.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108: 5431–5436, 2011. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yokota Y, Bradley RM. Receptive field size, chemical and thermal responses, and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons. J Neurophysiol 115: 3062–3072, 2016. doi: 10.1152/jn.00045.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol 97: 966–978, 2007. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]
- 154.Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, Nosjean A. Trigemino-solitarii-facial pathway in rats. J Comp Neurol 487: 176–189, 2005. doi: 10.1002/cne.20554. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003. doi: 10.1016/S0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 156.Zotterman Y. Action potentials in the glossopharyngeal nerve and in the chorda tympani. Skand Arch Physiol 72: 73–77, 1935. doi: 10.1111/j.1748-1716.1935.tb00412.x. [DOI] [Google Scholar]