Abstract
Impaired diabetic wound healing is associated with a dermal extracellular matrix protein profile favoring proteolysis; within the healing diabetic wound, this is represented by an increase in activated matrix metalloproteinase (MMPs). Treatment of diabetic wounds with mesenchymal stem cells (MSCs) has been shown to improve wound healing; however, there has not yet been an assessment of their ability to correct dysregulation of MMPs in diabetic wounds. Furthermore, there has been no prior assessment of the role of microRNA29b (miR-29b), an inhibitory regulatory molecule that targets MMP-9 mRNA. Using in vitro models of fibroblast coculture with MSCs and in vivo murine wound healing models, we tested the hypothesis that MSCs correct dysregulation of MMPs in a microRNA-29b-dependent mechanism. In this study, we first demonstrated that collagen I and III protein content is significantly reduced in diabetic wounds, and treatment with MSCs significantly improves collagen I content in both nondiabetic and diabetic wounds. We then found that MMP-9 gene expression and protein content were significantly upregulated in diabetic wounds, indicating elevated proteolysis. Treatment with MSCs resulted in a decrease in MMP-9 gene expression and protein content level in diabetic wounds 3 and 7 days after wounding. Zymographic analysis indicated that MSC treatment also decreased the amount of activated MMP-9 present in diabetic wounds. Furthermore, miR-29b expression was inversely associated with MMP-9 gene expression; miR-29b expression was decreased in diabetic wounds and diabetic fibroblast. Following treatment of diabetic wounds with MSCs, as well as in diabetic fibroblasts cocultured with MSCs, miR-29b was significantly increased. These findings suggest a potential mechanism through which MSCs enhance diabetic wound healing by improving collagen I content in diabetic wounds through decreasing MMP-9 expression and increasing miR-29b expression.
Keywords: diabetes, wound healing, mesenchymal stem cells, extracellular matrix, proteolysis
the global burden of diabetes is increasing (18). In the United States alone, direct and indirect expenditures related to diabetes care surpassed $245 billion in 2012 (1). This significant burden of disease attributable to diabetes is associated with the development of multiple complications, including impaired wound healing. The impaired wound healing associated with diabetes is responsible for diabetes being the leading cause of nontraumatic lower extremity amputations in the United States, with up to 84% of amputations performed in diabetic patients preceded by a lower extremity ulcer (27, 30). The pathophysiology responsible for the abnormal wound healing displayed by patients with diabetes is far from being fully understood; decreased growth factors, an overactive inflammatory response, and poor angiogenesis have all been implicated (5, 6, 9). Diabetic wounds, specifically chronic, nonhealing ulcers, such as those that lead to amputation, have also been found to have very high levels of activated matrix metalloproteinases (MMPs) (21, 34). Elevated levels of activated MMPs could be central to the impaired healing of diabetic wounds (29). Indeed, dermal fibroblasts isolated from patients with diabetic ulcers showed reduced proliferation (13, 22), decreased collagen synthesis (28), and increased MMPs production (31) compared with nondiabetic dermal fibroblasts. The underlying mechanism of the impaired wound healing observed in diabetic wounds may be the result of increased proteolysis leading to increased tissue turnover, resulting in decreased collagen content.
Mesenchymal stem cells (MSCs) are being increasingly explored as a novel and cost-effective therapy directed at improving wound healing, among other applications (2, 7, 33). The unique capacities of MSCs, such as pluripotency, auto-renewal, and the ease of isolation from adult tissue (14, 17, 25), make MSCs an attractive target for wound healing research. Studies indicate that MSCs can produce growth factors integral to the wound healing process, differentiate into cells necessary for wound contraction and healing, and can interact with the extracellular matrix (ECM) (14, 17, 25, 33). In our previous reports, we demonstrated that diabetic skin has a progressive impairment of biomechanical properties at baseline due to imbalances in collagen synthesis and degradation in diabetic compared with nondiabetic skin (4, 38). We have also demonstrated that treatment with MSCs can not only increase collagen content and correct the impaired biomechanical properties of diabetic skin (4, 38), but also correct the delay in wound healing seen in the Db/Db mouse model of Type II diabetes (2, 10); however, the exact molecular mechanisms of this correction have not been well characterized.
MicroRNAs (miRNAs) are small, noncoding RNAs that negatively regulate gene expression at a posttranscriptional/translational level (3). Alteration of miRNA regulation is now believed to be responsible for multiple disease states; specifically, miRNAs have been shown to be involved in many aspects of diabetic wound healing (36, 37). Among them, miR-29a has been associated with collagen synthesis in our previous work, by directly targeting collagen I gene expression (38). In this study, we focus on miR-29b (26, 32, 35), and it has been shown to directly target MMP-9 (8, 23, 24).
Therefore, we hypothesize that the improved healing in diabetic wounds following treatment with MSCs may be due to the creation of conditions that favor net collagen production within these wounds. To test this hypothesis in vivo, diabetic (Db/Db) and nondiabetic (Db/+) mice were wounded with an 8 mm punch biopsy and treated with either MSCs or a control [phosphate-buffered saline (PBS)]. The wounds were then harvested on days 3 and 7 after wounding, and collagen gene expression, collagen protein content, expression and activity of MMP-9, and levels of miR-29b were assessed. To test this hypothesis in vitro, dermal fibroblasts were cultured from the skin of diabetic and nondiabetic mice and then cocultured with MSCs before undergoing assessment of miR-29b expression.
MATERIALS AND METHODS
Animal model.
We obtained 12 wk old female mice homozygous for the Leprdb mutation (Db/Db) and age-matched female nondiabetic heterozygous littermates (Db/+) from the Jackson Laboratory (Bar Harbor, ME) and used them in these experiments, as described in previous work (37). Animals were given standard rodent chow and water ad libitum. All experiments were approved and performed under the guidelines of the University of Colorado Denver Anschutz Medical Campus Animal Care and Use Committee.
Mouse wound healing model.
Inhaled isoflurane was used for anesthesia for all procedures. After induction and maintenance of anesthesia, the dorsal skin of each mouse was shaved, depilated, and prepared with isopropyl alcohol before being wounded. Each mouse (n = 4 in each control and treatment groups, at each time point) underwent a single dorsal full-thickness wound (including panniculus carnosus) with an 8 mm punch biopsy (Miltex, York, PA). All wounds were dressed with Tegaderm (3M, St. Paul, MN), which was subsequently removed on postoperative day 2. At day 3 and 7 after wounding, a 1 mm area of skin surrounding the wound edges was harvested for RNA isolation and ELISA analysis. A 2 mm are of skin surrounding the wound edges was taken for histological analysis.
MSC isolation and treatment.
MSCs were isolated from the bone marrow of transgenic adult mice expressing GFP, as previously described (16, 36, 37). Eight millimeter full-thickness wounds were created as described above, and diabetic (Db/Db) and nondiabetic (Db/+) wounds were treated with either 1 × 106 MSCs or control (PBS) alone. Wound treatment (MSC or vehicle) was performed immediately after wounding by administration of four intradermal injections spaced in a radial pattern from the wound edge for a total volume of 40 μl (10 μl per injection) with a Hamilton syringe.
Dermal fibroblast isolation and culture.
For dermal fibroblast isolation, skin from adult nondiabetic and diabetic mice was placed dermal side down, to allow fibroblasts to adhere and outgrow. Dermal fibroblasts were cultured in DMEM supplemented with 10% fetal bovine serum, antibiotics, and antimycotics. Cultures were maintained in a humidified incubator, with an atmosphere of 5% CO2 and 95% air. For coculture, MSCs were seeded at 200 cells/cm2 in inserts that were then added to the fibroblast wells. Controls consisted of fibroblasts cocultured with an empty insert.
Enzyme-linked immunosorbent assay.
Wounds were harvested at day 3 and 7 after wounding and subsequently homogenized in a solution of 750 ml of tissue lysis buffer (Qiagen, Valencia, CA). This solution was then centrifuged for 10 min at 10,000 rpm. The supernatant was collected and frozen at −80°C for further analysis. Protein concentration was quantified with a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL). Enzyme-linked immunosorbent assay (ELISA) was then used to quantify the concentration of MMP-9 protein levels (R&D Systems, Minneapolis, MN). The ELISA kits that were used quantified only the total amounts of MMP-9 and did not differentiate between pro- and active forms of MMP-9 or whether either protein was bound by other proteins. Values were normalized to the total protein concentration of the samples.
Gelatin zymography for MMP-9 activity.
Protein was extracted from wounds at day 3 and 7 with Shirmer strips (Alcon; Novartis, Basel, Switzerland). Shirmer strips were placed directly over the wounds and covered for 6 h with a Tegaderm. After 6 h, the strip was removed and protein was eluted from them with a buffer solution made of 50 mM Tris, 50 mM NaCl, 0.05% Brij-35 detergent (Fischer Scientific Waltham, MA) at 4°C for 3 h. We then normalized the protein concentration between the samples by diluting them with sample buffer. The samples were then run at room temperature using a Novex 10% zymogram gel (gelatin) (Invitrogen, Life Technologies), per the manufacturer’s instructions. Murine MMP-9 standard (R&D Systems) was loaded as a control for later quantifying proteolytic activity. Likewise, whole protein was added to later evaluate the ratios of pro-MMP to active MMP. Gels were allowed to renature for 1 h and incubated overnight at 37°C. Band intensity was quantified by using NIH Image J software version 1.49 (https://imagej.nih.gov/ij/).
Collagen extraction and Western blot for collagens I and III.
Collagen was extracted by previously published methods with some modification (4). In brief, wound samples were cut into pieces and were subsequently homogenized in a 0.5 M acetic acid solution containing 1 × protease inhibitor cocktail and 5 mmol/l EDTA. The protein concentration of the supernatant was quantified with a bicinchoninic acid protein assay. Western blot was performed with a standard technique. A total of 20 μg protein per sample was loaded on the gel, together with a lane of 3 μg collagen I protein or 15 μg collagen III protein (BD 354236 and 7535, respectively; BD Bioscience, San Jose, CA) as positive controls for collagen 1 and collagen 3, respectively. Tris-acetate gels (3% to 8%) were run at 150 V for 1 h and were then transferred at 30 V overnight at 4°C. Antibodies to collagen I (ab34710), collagen III (ab7778), and GAPDH (ab8245) (Abcam, Cambridge, MA) were diluted 1:1,000, and blots were incubated with diluted antibody for 1 h at room temperature and then washed with Tris-buffered saline with 0.1% Tween. They were subsequently incubated with secondary antibody (anti-rabbit IgG horseradish peroxidase; GE Healthcare, Piscataway, NJ) at 1:10,000 for 1 h at room temperature. Band intensity was performed with NIH Image J software version 1.49 (https://imagej.nih.gov/ij/). Collagen absolute levels were calculated relative to the band intensity of the positive control.
Real-time quantitative PCR.
Wound samples from day 3 and day 7 postinjury were homogenized in TRIzol (Life Technologies, Invitrogen), and total cellular RNA isolated and purified following the manufacturer’s instructions. mRNA analysis and miRNA analysis were described in our previous publication (37). For mRNA analysis, total RNA was extracted and mRNA was converted into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). Real-time quantitative (q)PCR was performed with the CFX96 real-time PCR thermal cycler (Bio-Rad, Hercules, CA) to amplify samples in triplicate. TaqMan gene expression assay for MMP-9 and tissue inhibitor of matrix metalloproteinase (TIMP)1 was applied. Relative gene product amounts were reported for each gene compared with 18S ribosomal RNA. We paid specific attention to miR-29b, given the previous research demonstrating the association between miR-29b expression and remodeling of the ECM (8, 20, 23, 26). miR-29b expression was examined with the TaqMan MicroRNA Assay Kit. MultiScribe Reverse Transcriptase was used for RT, and TaqMan primers for has-miR-29b (assay ID 000413) were used to monitor miRNA expression. U6 (assay ID 001973) was used as an internal miRNA control. Results are reported as means ± SD.
Statistical analysis.
In vitro data, such as ELISA and zymography, are reported as means ± SD of three to five independent experiments. Real-time qPCR results were reported using 2-ΔΔCT values of each sample. Values were compared by the Student’s t-test or with one-way ANOVA when three or more groups were present using GraphPad software Prism 7.0 (GraphPad Software). In vivo data are expressed as means ± SD, and statistical analyses were carried out with GraphPad Prism 7.0 (GraphPad Software). A two-tailed Student’s t-test was applied for two-group comparison. A P value < 0.05 was considered as statistically significant.
RESULTS
Decreased collagen I and III content in diabetic wounds.
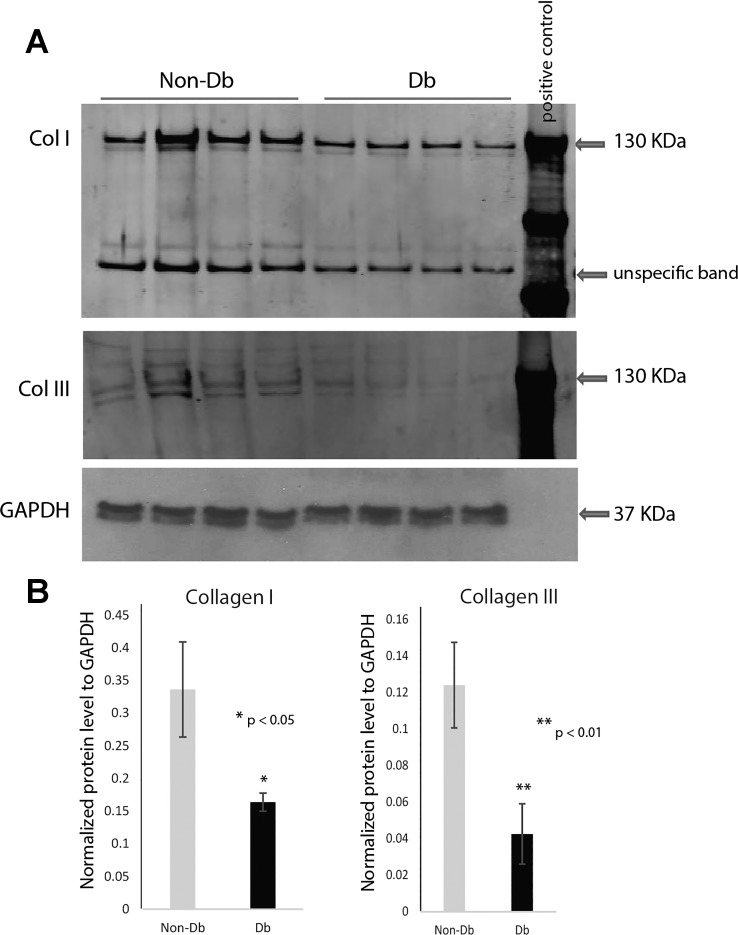
Western blot analysis of collagen types I and III demonstrated significant differences between diabetic and nondiabetic wounds 7 days after injury (Fig. 1). Murine diabetic wounds had significantly decreased type I collagen content compared with nondiabetic murine wounds (n = 4), (P < 0.05). Diabetic murine wounds also demonstrated significantly decreased type III collagen content compared with nondiabetic murine wounds (n = 4, P < 0.05).
Fig. 1.
Western blot analysis demonstrating decreased collagen content of diabetic wounds. A: Western blot analysis of collagen I and III protein content and loading control GAPDH in nondiabetic (Non-Db, Db/+) (n = 4) and diabetic (Db, Db/Db) (n = 4) wounds 7 days after injury (top). Equal amounts of protein samples were loaded to each lanes: 4 for nondiabetic wounds, 4 for diabetic wounds, together with 1 lane for positive control. The positive control was loaded with standardized either collagen I or collagen III protein. B: normalized collagen I and III content was calculated after normalizing with internal loading control (bottom) using Image J analysis. Results are presented as means ± SD; *P value <0.05 considered statistically significant by Student’s t-test. Comparison was performed between Db/Db wounds and Db/+ wounds. Diabetic wounds showed significantly lower level on collagen I or collagen III expression.
MMP-9 was significantly higher in diabetic wounds.
Real-time PCR analysis of MMP-9 gene expression demonstrated that diabetic wounds had significantly increased relative gene expression of MMP-9 when compared with nondiabetic wounds at 3 and 7 days after wounding (Fig. 2, A and B). We also measured MMP-9 protein level by ELISA and demonstrated that levels of MMP-9 were significantly higher (P < 0.01) in murine diabetic wounds compared with those of nondiabetic mice at both 3 days and 7 days after wounding (Fig. 2, D and E). As TIMPs are natural inhibitors for MMP-9, we also measured TIMP1 gene and protein expression. At day 3 postwounding, diabetic wounds demonstrated slightly lower but not significant differences in gene expression (Fig. 2C) and significantly lower levels in protein content (Fig. 2F) compared with nondiabetic wounds.
Fig. 2.
Matrix metalloproteinase (MMP)-9 was significantly higher in diabetic wounds. Real-time quantitative PCR analysis for MMP-9 gene expression at day 3 (A), day 7 (B) wounds, and tissue inhibitor of matrix metalloproteinase (TIMP)1 at day 3 wounds (C). MMP-9 gene expression was significantly upregulated in diabetic wounds at both day 3 and day 7, while TIMP1 gene expression showed no significant difference. ELISA analysis of protein level for MMP-9 in Db/+ (n = 4) and Db/Db (n = 4) wounds at 3 (D) or 7 days (E) after injury, and TIMP1 protein at day 3 wounds (F). Similar to gene expression level, MMP-9 protein level was significantly higher in diabetic wounds, while TIMP1 protein level was significantly lower in diabetic wounds. Results are presented as means ± SD; *P value <0.05 by Student’s t-test. Comparison was performed between Db/Db wounds and Db/+ wounds.
MSC treatment corrected collagen content in diabetic wounds.
MSC treatment of diabetic wounds resulted in a significant improvement in wound closure, similar to previously described observations (2, 16). We measured the wounds size using imageJ, which indicated that MSC-treated diabetic wounds (n = 8) have significantly reduced wound size compared with vehicle-treated diabetic wounds (n = 8) at day 7 after wounding (42.1 ± 1.4 mm2 vs. 51.5 ± 3.2 mm2, mean ± SD, P < 0.03). The effect of MSC-mediated correction of collagen content in diabetic wounds was assessed by Western blot. Seven days after wounding, MSC-treated diabetic wounds exhibited significantly increased collagen I protein (n = 4 per group, P < 0.05) and collagen III protein (n = 4 per group, P < 0.05) content compared with vehicle-treated controls in diabetic wounds (Fig. 3).
Fig. 3.
Mesenchymal stem cell (MSC) treatment increased collagen I content in diabetic wounds. A: Western blot analysis of collagen I and III protein content and loading control GAPDH in nondiabetic (Non-Db, Db/+) (n = 4) and diabetic (Db, Db/Db) (n = 4) wounds 7 days after injury, with or without MSC treatment (top). Equal amounts of protein samples were loaded to each lanes: 2 for nondiabetic wounds treated with PBS, 2 for diabetic wounds treated with PBS, 2 for nondiabetic treated with MSC, 2 for diabetic wounds treated with MSC, together with 1 lane for positive control. The positive control was loaded with standardized either collagen I or collagen III protein. B: collagen content was calculated after normalizing with standardized collagen control (bottom) using Image J analysis. Results are presented as means ± SD; *P value <0.05 considered statistically significant by 1-way ANOVA statistical test. Comparison was performed between Db/Db wounds and Db/+ wounds. Diabetic wounds showed significantly higher collagen I and III protein level with treatment of MSC.
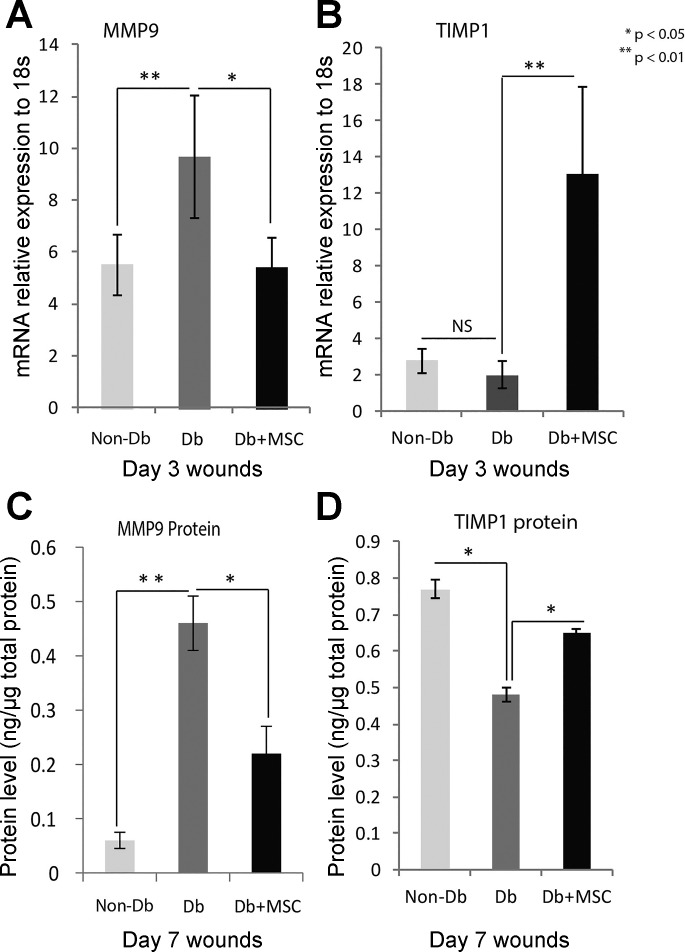
MSC treatment reduced MMP-9 expression.
Following MSC application, we measured MMP-9 gene expression and protein level. Real-time PCR analysis of MMP-9 gene expression demonstrated that diabetic wounds had significantly decreased relative gene expression of MMP-9 with MSC treatment (Fig. 4A). As for protein levels as measured by ELISA, diabetic wounds had a significant reduction in levels of MMP-9 (P < 0.05) at 7 days after wounding when compared with diabetic wounds not treated with MSCs (Fig. 4C). We also measured TIMP1 gene and protein expression. TIMP1 gene expression was significantly higher in MSC treated-diabetic wounds at day 3 postwounding (Fig. 4B), and TIMP1 protein expression was also significantly higher in MSC-treated day 7 diabetic wounds (Fig. 4D).
Fig. 4.
MMP-9 gene and protein expression levels were altered with MSC treatment in day 3 or day 7 wounds. Real-time quantitative PCR analysis for gene MMP-9 (A) and TIMP1 (B) mRNA in Db/+ (n = 4) and Db/Db (n = 4) wounds at 3 days after injury, with or without treatment of MSCs. MMP-9 gene expression was significantly higher in diabetic wounds and significantly reduced when diabetic wounds were treated with MSC. There was no difference in TIMP1 gene expression between nondiabetic and diabetic wounds. However, MSC treatment significantly induced TIMP1 gene expression in diabetic wounds. We used ELISA to analyze protein levels for MMP-9 (C) and TIMP1 (D) in Db/+ (n = 4) and Db/Db (n = 4) wounds at 7 days after injury, with or without MSC treatment. MMP-9 protein level was significantly higher in diabetic wounds and significantly reduced when diabetic wounds were treated with MSC. We found significantly lower levels of TIMP1 protein when we compared nondiabetic and diabetic wounds. MSC treatment significantly induced TIMP1 protein levels in diabetic wounds. Results are presented as means ± SD; *P value <0.05, **P value <0.01. Comparison was performed between Db/Db wounds and Db/+ wounds, as well as Db/Db wounds with and without MSC treatment by 1-way ANOVA statistical test with multiple comparisons. NS, not significant.
ECM proteolytic activity was increased in diabetic wounds and attenuated by MSC treatment.
Quantitative zymography demonstrated a significant difference in MMP-9 activity in diabetic and nondiabetic wounds. Figure 5A displays the zymogram comparing diabetic and nondiabetic specimens; ratios of MMP-9 to pro-MMP-9 in the diabetic group were higher than in the nondiabetic group. Furthermore, active MMP-9 concentrations were higher in the diabetic group at both 3 days and 7 days after wounding, and treatment with MSCs significantly decreased the level of MMP-9 proteolytic activity (Fig. 5, B and C).
Fig. 5.
MSC treatment decreased MMP-9 function as shown by gelatin zymography analysis. Gelatin zymography analysis of MMP-9 function in Db/+ wounds, Db/Db wounds, or Db/Db wounds treated with MSCs at day 3 or day 7 following injury (n = 4 per group). The band densitometry was measured by Image J software. We used 1-way ANOVA statistical test for comparison, **P value <0.01. The data of MMP-2 are not related to this study.
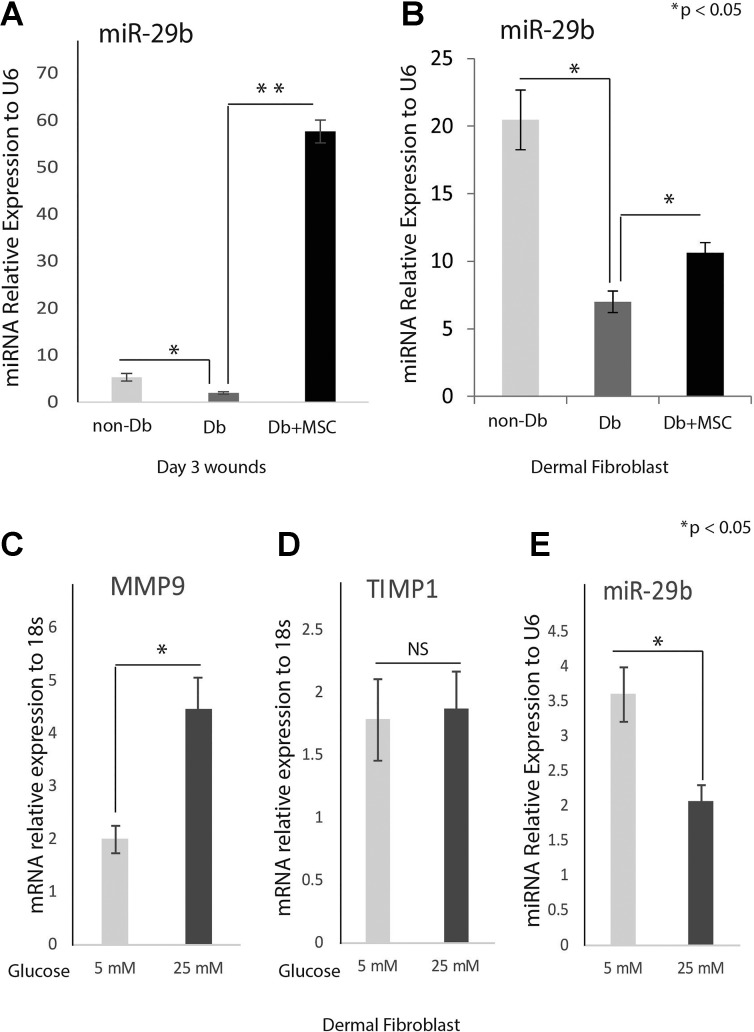
MSC treatment increased miR-29b expression in diabetic wounds and fibroblasts.
We performed real-time PCR analysis to examine the expression levels of miR-29b in wounds 7 days after injury. Figure 6A shows that diabetic wounds have significantly lower miR-29b levels compared with nondiabetic wounds 3 days after injury. These expression patterns are inversely correlated with the MMP-9 expression pattern in wounds (Fig. 2A). We did linear regression analysis between MMP-9 expression and miR-29b expression in diabetic wounds, and the R square was 0.917, indicating a high degree of correlation.
Fig. 6.
MSCs upregulate miR-29b levels in diabetic wounds and fibroblasts. Real-time quantitative PCR analysis of miR-29b in Db/+ (n = 4) and Db/Db (n = 4) wounds 7 days after injury with or without MSC treatment (A) and in dermal fibroblasts with MSC or vehicle coculture (B). The expression of miR-29b was significantly lower in diabetic wounds or diabetic fibroblasts. Treatment with MSC significantly induced miR-29b expression in diabetic wounds or diabetic fibroblasts. Real-time quantitative PCR analysis for MMP-9 (C), TIMP1(D), and miR-29b (E) in normal dermal fibroblasts under 5 mM (low glucose) or 25 mM (high glucose) conditions. MMP-9 was induced while miR-29b was reduced by high glucose. Results are presented as means ± SD. *P value <0.05, **P value <0.01. Comparison was performed between Db/Db and Db/+, as well as Db/Db with and without MSC treatment by 1-way ANOVA statistic test with multiple comparisons.
Next, we analyzed miR-29b expression after treatment with MSCs. We found that following treatment with MSCs, miR-29b expression dramatically increased in both nondiabetic wounds and diabetic wounds 3 days after injury (Fig. 6A).
We further analyzed miR-29b expression in diabetic fibroblasts. miR-29b was significantly downregulated in diabetic dermal fibroblasts (Fig. 6B) compared with nondiabetic fibroblasts. The expression level of miR-29a in fibroblasts was reversely correlated with MMP-9 gene expression; the determination of coefficient was supported by R-squared analysis (R2 = 0.84), while diabetic fibroblasts cocultured with MSCs demonstrated significant upregulation of miR-29b (Fig. 6B), similar to the data we found in diabetic wounds. In another set experiment, we treated nondiabetic fibroblasts with 5 mM (normal glucose) or 25 mM (high glucose). Real-time PCR analysis indicated that MMP-9 gene was significantly induced by high glucose (Fig. 6C), while TIMP1 gene expression showed no significant difference (Fig. 6D). We also checked miR-29b expression and found that miR-29b was significantly reduced by high glucose (Fig. 6E).
DISCUSSION
We have demonstrated that MSCs enhance diabetic wound healing impairment partly through decreasing proteolysis. To begin, we showed that protein levels of collagen I and III were significantly decreased in diabetic wounds. Next, we demonstrated that MSC treatment can increase protein levels of collagen I in diabetic wounds. We further analyzed levels of MMP-9, one of the primary enzymes responsible for collagen degradation, in diabetic wounds both with and without MSC treatment. We found that MMP-9 gene expression and protein content were significantly elevated in diabetic wounds, and treatment of diabetic wounds with MSCs attenuated MMP-9 expression, indicating decreased proteolysis in diabetic wounds treated with MSCs. Moreover, we showed that MMP9 activity is upregulated in diabetic wounds and treatment with MSCs decreased this activity. Furthermore, the downregulation of MMP-9 expression following treatment with MSCs may be modulated through upregulation of the MMP-9 negative regulator, miR-29b, in a paracrine fashion.
Impaired wound healing following injury in patients with diabetes represents a major clinical problem, resulting in prolonged hospitalizations and significant healthcare expenditures. Of nontraumatic amputations, 84% are preceded by a diabetic wound, and every 30 s a lower limb is lost to a diabetic wound (27). The impaired healing of diabetic wounds is multifactorial. Normal wound healing is a dynamic process involving many different cell types during three overlapping phases: the inflammatory phase, the proliferative phase, and the remodeling phase. Proper and timely wound healing requires a balanced interaction of various cell types, cytokines, growth factors, proteases and ECM components. However, this balance is interrupted in diabetic wounds. The impaired healing observed in diabetic wounds is tied to multiple factors, including decreased collagen content (4, 21). Collagen is one of the most important components of ECM and plays an important role in the wound healing process. However, in diabetic wounds, the imbalance of MMP and TIMPs contributes to impaired ECM, failure to heal, and the formation of chronic wounds (19). Studies have demonstrated that chronic diabetic wounds possess higher levels of the proteolytic MMPs and lower levels of their inhibitors, TIMPs (19). Our studies again confirm that elevated levels of proteinases, especially MMPs, can alter the balance of proteins within the ECM, impairing normal healing.
It has previously been shown that MSCs can migrate to the site of an injury in response to chemotactic signals, and further studies have shown that MSCs play an important role in mediating each phase of wound healing (14). Two mechanisms have been suggested for the improved tissue repair mediated by MSCs: 1) through differentiation into resident cells and 2) through paracrine signaling. In a fate mapping experiment, Fathke et al. (2004) (11) reported that normal skin is a target organ for MSCs, with 15–20% of spindle-shaped dermal fibroblasts being derived from bone marrow MSCs. These data indicated that MSCs can selectively migrate to the skin, contributing to the maintenance of skin architecture by direct differentiation into dermal fibroblasts. Moreover, the bone marrow-derived cells contributed toward increased contraction of the collagen matrix and were able to transcribe both collagen I and collagen III, whereas the skin-resident cells transcribed only type I collagen. In our study, we found that MSC treatment enhanced the collagen I content of diabetic skin; together with our previous studies showing that MSC treatment increases myofibroblasts in diabetic wounds (2), we cannot eliminate the possibility that MSCs enhanced the collagen content of diabetic wounds through cellular differentiation into resident cells. However, ample evidence suggests that MSCs may elicit the majority of their wound healing properties through a paracrine mechanism. Previous studies have indicated that MSCs secrete many growth factors, cytokines, and chemokines that are essential for wound healing (15). Multiple cell types, such as fibroblasts, endothelial cells, and inflammatory cells, have been shown to respond to the paracrine signaling of MSCs (12, 15).
To further understand the mechanism by which MSCs correct MMP-9 expression, we looked into novel regulation of the relationship between MMP-9 and MSCs by miR-29b. MiRNAs are small, noncoding RNAs that negatively regulate gene expression at a posttranscriptional/translational level (3). Alteration of miRNA regulation is now believed to be responsible for multiple disease states. Specifically, miRNAs have been shown to be involved in many aspects of diabetic wound healing (36, 37). Among them, miR-29b is gaining attention (26, 32, 35). MMP-9 has also been well documented to be a direct target of miR-29b (8, 23, 24). In this study, we provide evidence that MMP-9 expression is inversely associated with its novel regulator, miR-29b. In vivo, in a murine model of diabetic wounds, we found elevated levels of MMP-9 and decreased miR-29b expression. In vitro, we also found that miR-29b expression was lower in murine diabetic fibroblasts compared with nondiabetic fibroblasts. Moreover, we found that, when miR-29b is cocultured with MSCs, its expression was induced in diabetic murine fibroblasts, indicating MSCs alter miR-29b expression in a paracrine fashion.
In summary, we have demonstrated that MSCs have the potential to enhance diabetic wound healing by decreasing proteolysis and correcting collagen I levels in the ECM partly through modulation of MMP-9 expression in an miR-29b-dependent manner.
GRANTS
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 7DP2 DK-083085-01 and a Department of Surgery AEF grant to J. Xu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.X. conceived and designed research; J.X., C.Z., R.C.C., and J.H. performed experiments; J.X., C.Z., R.C.C., J.H., and K.W.L. analyzed data; J.X., C.Z., M.H., R.C.C., J.H., and K.W.L. interpreted results of experiments; J.X., C.Z., and M.H. prepared figures; J.X., C.Z., M.H., R.C.C., and K.W.L. drafted manuscript; J.X., C.Z., M.H., and K.W.L. edited and revised manuscript; J.X. and K.W.L. approved final version of manuscript.
ACKNOWLEDGMENTS
K. W. Liechty is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
REFERENCES
- 1.Centers of Diseases Control and Prevention , https://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html.
- 2.Badillo AT, Redden RA, Zhang L, Doolin EJ, Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res 329: 301–311, 2007. doi: 10.1007/s00441-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez DM, Herdrich BJ, Xu J, Lind R, Beason DP, Mitchell ME, Soslowsky LJ, Liechty KW. Impaired biomechanical properties of diabetic skin implications in pathogenesis of diabetic wound complications. Am J Pathol 178: 2215–2223, 2011. doi: 10.1016/j.ajpath.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 23: 594–608, 2006. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219–1222, 2007. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooke G, Cook M, Blair C, Han R, Heazlewood C, Jones B, Kambouris M, Kollar K, McTaggart S, Pelekanos R, Rice A, Rossetti T, Atkinson K. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol 18: 846–858, 2007. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J 25: 1718–1728, 2011. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- 9.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 10.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13: 1299–1312, 2007. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 11.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 22: 812–822, 2004. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hehenberger K, Kratz G, Hansson A, Brismar K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J Dermatol Sci 16: 144–151, 1998. doi: 10.1016/S0923-1811(97)00042-X. [DOI] [PubMed] [Google Scholar]
- 14.Herdrich BJ, Lind RC, Liechty KW. Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy 10: 543–550, 2008. doi: 10.1080/14653240802345820. [DOI] [PubMed] [Google Scholar]
- 15.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 316: 2213–2219, 2010. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javazon EH, Keswani SG, Badillo AT, Crombleholme TM, Zoltick PW, Radu AP, Kozin ED, Beggs K, Malik AA, Flake AW. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen 15: 350–359, 2007. doi: 10.1111/j.1524-475X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 17.Keating A. Mesenchymal stromal cells. Curr Opin Hematol 13: 419–425, 2006. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21: 1414–1431, 1998. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 19.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 10: 26–37, 2002. doi: 10.1046/j.1524-475X.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Wang B, Lin J, Zhao L. microRNA-29b: an emerging player in human cancer. Asian Pac J Cancer Prev 15: 9059–9064, 2014. doi: 10.7314/APJCP.2014.15.21.9059. [DOI] [PubMed] [Google Scholar]
- 21.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 45: 1011–1016, 2002. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 22.Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res 291: 93–99, 1999. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- 23.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 122: 497–506, 2012. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melo SA, Kalluri R. miR-29b moulds the tumour microenvironment to repress metastasis. Nat Cell Biol 15: 139–140, 2013. doi: 10.1038/ncb2684. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Poudyal D, Cui X, Le PM, Hofseth AB, Windust A, Nagarkatti M, Nagarkatti PS, Schetter AJ, Harris CC, Hofseth LJ. A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS One 8: e75034, 2013. doi: 10.1371/journal.pone.0075034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, Boulton AJ. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 22: 157–162, 1999. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 28.Seibold JR, Uitto J, Dorwart BB, Prockop DJ. Collagen synthesis and collagenase activity in dermal fibroblasts from patients with diabetes and digital sclerosis. J Lab Clin Med 105: 664–667, 1985. [PubMed] [Google Scholar]
- 29.Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, Hocking AM. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 316: 48–54, 2010. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Department of Health and Human Services National Diabetes Fact Sheet. Atlanta, GA: Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 31.Wall SJ, Sampson MJ, Levell N, Murphy G. Elevated matrix metalloproteinase-2 and -3 production from human diabetic dermal fibroblasts. Br J Dermatol 149: 13–16, 2003. doi: 10.1046/j.1365-2133.2003.05262.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Li W, Liu H, Yang L, Liao Q, Cui S, Wang H, Zhao L. miR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial-mesenchymal transition. Cell Death Dis 5: e1335, 2014. doi: 10.1038/cddis.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25: 2648–2659, 2007. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 101: 64–68, 1993. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Zhang Q, Cheng W, Zhang Z, Wang J, Ge J. Effect of miR-29b-1* and miR-29c knockdown on cell growth of the bladder cancer cell line T24. J Int Med Res 41: 1803–1810, 2013. doi: 10.1177/0300060513505266. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, Liechty KW. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes 61: 2906–2912, 2012. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Zgheib C, Hu J, Wu W, Zhang L, Liechty KW. The role of microRNA-15b in the impaired angiogenesis in diabetic wounds. Wound Repair Regen 22: 671–677, 2014. doi: 10.1111/wrr.12217. [DOI] [PubMed] [Google Scholar]
- 38.Zgheib C, Hodges M, Hu J, Beason DP, Soslowsky LJ, Liechty KW, Xu J. Mechanisms of mesenchymal stem cell correction of the impaired biomechanical properties of diabetic skin: The role of miR-29a. Wound Repair Regen 24: 237–246, 2016. doi: 10.1111/wrr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]