Abstract
The prevalence of hypertension is about twofold higher in diabetic than in nondiabetic subjects. Hypertension aggravates the progression of diabetic complications, especially diabetic nephropathy. However, the mechanisms for the development of hypertension in diabetes have not been elucidated. We hypothesized that enhanced constrictive responsiveness of renal afferent arterioles (Af-Art) to angiotensin II (ANG II) mediated by ANG II type 1 (AT1) receptors contributes to the development of hypertension in diabetes. In response to an acute bolus intravenous injection of ANG II, alloxan-induced diabetic mice exhibited a higher mean arterial pressure (MAP) (119.1 ± 3.8 vs. 106.2 ± 3.5 mmHg) and a lower renal blood flow (0.25 ± 0.07 vs. 0.52 ± 0.14 ml/min) compared with nondiabetic mice. In response to chronic ANG II infusion, the MAP measured with telemetry increased by 55.8 ± 6.5 mmHg in diabetic mice, but only by 32.3 ± 3.8 mmHg in nondiabetic mice. The mRNA level of AT1 receptor increased by ~10-fold in isolated Af-Art of diabetic mice compared with nondiabetic mice, whereas ANG II type 2 (AT2) receptor expression did not change. The ANG II dose-response curve of the Af-Art was significantly enhanced in diabetic mice. Moreover, the AT1 receptor antagonist, losartan, blocked the ANG II-induced vasoconstriction in both diabetic mice and nondiabetic mice. In conclusion, we found enhanced expression of the AT1 receptor and exaggerated response to ANG II of the Af-Art in diabetes, which may contribute to the increased prevalence of hypertension in diabetes.
Keywords: AT1 receptor, Type I diabetes, angiotensin II, hypertension
diabetes mellitus and hypertension are two major health issues in the United States. The prevalence of diabetes was 9.3% as of 2012, and hypertension affects about one-third of the adult population (69). Diabetes and hypertension are interrelated and frequently coexist (91). The prevalence of hypertension in diabetic subjects is approximately twice that of the nondiabetic population (25, 51, 72, 93, 95). The presence of hypertension in diabetic patients further exacerbates their morbidity and mortality (25, 28, 52). However, the pathophysiological mechanisms for the development of hypertension in diabetes have not been completely elucidated.
The renin-angiotensin system (RAS) plays an essential role in regulating blood pressure and is one of the most important therapeutic targets for hypertension (21, 33, 34). RAS blockers, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, have been recognized as the first-line treatment with additional beneficial effects for hypertensive patients with diabetes, which suggests the involvement of RAS in the development of hypertension in diabetic subjects (4, 50, 82). Although plasma renin was suppressed (18, 27, 77) or normal (11, 61) in diabetic patients, the responsiveness of blood pressure to angiotensin II (ANG II) was demonstrated to be exaggerated in normotensive and mildly hypertensive patients with Type I diabetes (24, 100). However, the mechanism of the enhancement in blood pressure response to ANG II remains elusive.
A previous study with kidney cross-transplantation between wild-type and ANG II type 1 (AT1) receptor-deficient mice examined the action of AT1 receptors in the kidney. They demonstrated the predominant role of the renal AT1 receptors in the development and maintenance of ANG II-dependent hypertension (20). The afferent arterioles (Af-Arts) are the major resistance vessels in the kidney and play a critical role in regulating renal hemodynamics and blood pressure (23, 29, 53, 57). Therefore, we hypothesized in the present study that the increased constrictive responsiveness of the Af-Art to ANG II mediated by the AT1 receptor contributes to the enhanced response of renal hemodynamics to ANG II and the development of hypertension in diabetes. We examined the responses of blood pressure and renal hemodynamic changes to acute and chronic ANG II infusion in diabetic mice and measured the ANG II dose-response curve in the isolated perfused Af-Art.
METHODS
All studies were approved by the Institutional Animal Care and Use Committee at the University of South Florida, College of Medicine.
Induction of diabetes.
C57BL/6 mice (male, 13–15 wk old) were purchased from Jackson Laboratory. The mice were housed at 23 ± 1.0°C on a 12:12 h light-dark cycle with lights on at 6:00 AM and free access to standard chow and water. Diabetes was induced by an intravenous injection of alloxan (55 mg/kg in 100 µl saline) through the penile vein after overnight fasting. Blood glucose was measured twice a week starting 3 days after alloxan injection (Fig. 1). Mice with 300–500 mg/dl blood glucose levels were used in the present study. The mice with high blood glucose levels (>500 mg/dl) were treated with insulin (0.5 U/kg) to maintain the blood glucose at 300–500 mg/dl. The mice with an injection of 100 µl saline through the penile vein after overnight fasting served as nondiabetic animals. Measurement of blood glucose of the nondiabetic mice was the same as in the diabetic mice. All experiments were performed at 4 wk after alloxan or saline injection.
Fig. 1.
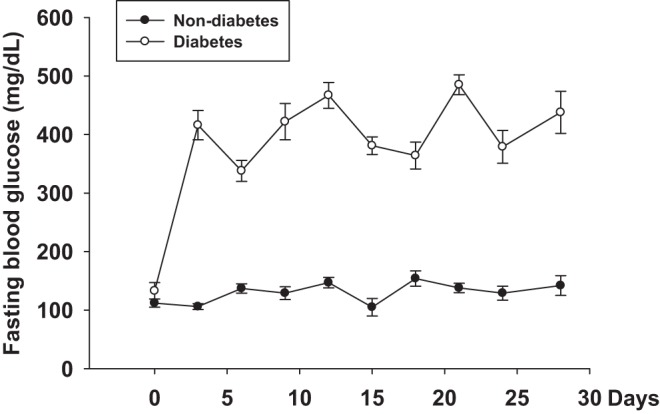
The change of blood glucose after alloxan injection.
Mean arterial pressure and renal hemodynamic responses to acute ANG II injection.
Mice were anesthetized with pentobarbital (50 mg/kg ip) and placed on a temperature-controlled operating table (Vestavia Scientific, Vestavia Hills, AL) to maintain body temperature at 37 ± 1.0°C. The carotid artery was catheterized for blood pressure measurement with a PowerLab (ADInstruments, Boulder, CO). Following an abdominal incision, the left renal artery was carefully separated from the vein, and a perivascular flow probe (Transonic Systems, Ithaca, NY) was placed around the left renal artery. The probe was then stabilized by a micromanipulator. Renal blood flow (RBF) measured with the flow probe was recorded via the PowerLab. After 30 min equilibration, mean arterial pressure (MAP) and RBF were measured for 5 min as a baseline. Then a bolus injection of 1 ng/kg ANG II (dissolved in 20 μl of 0.9% saline) was given through a penile vein (7). MAP and RBF were recorded for 15 min. Renal vascular resistance (RVR) was calculated as the renal artery pressure (same as MAP) divided by RBF.
MAP response to chronic ANG II infusion.
Telemetry transmitters (PA-C10, Data Sciences International) were implanted for measurement of MAP in conscious mice as we previously described (59, 102). Briefly, the mice were anesthetized with inhaled isoflurane (Butler chemicals) and placed on the temperature-controlled operating table. The left carotid artery was exposed by a small incision in the middle of the neck. The pressure catheter was implanted in the left carotid artery, and the telemetric device was placed subcutaneously in the right ventral flank of the mice. MAP was most stable from 1:00 to 5:00 PM during a 24 h period, so the MAP and heart rate were recorded for 10 s every 2 min for 4 h from 1:00 to 5:00 PM starting from 7 days after the transmitter implantation.
Five days after baseline MAP measurement, micro-osmotic pumps (model 1002, ALZET) were subcutaneously implanted as described previously (102). Briefly, micro-osmotic pumps were filled with ANG II (600 ng·kg−1·min−1) and incubated in sterile saline at 37°C overnight to reach steady state before implantation. Mice were anesthetized with isoflurane. A small incision was made in the skin between the scapulae, and a small pocket was formed by separating the subcutaneous connective tissues from the skin. The micro-osmotic pump was inserted into the pocket, and the wound was sutured. Fourteen days after implantation, the micro-osmotic pumps were removed from the animals and the new pumps filled with ANG II were subcutaneously implanted.
Isolation and microperfusion of Af-Art.
The isolation and microperfusion of the Af-Arts were the same as we described previously (58–60). Briefly, the mice without ANG II infusion were anesthetized with inhaled isoflurane, and the kidneys were removed and sliced. The kidney slices were placed in ice-cold Dulbecco's modified Eagle’s medium (DMEM). A single superficial intact glomerulus and its adherent Af-Art were microdissected under a stereomicroscope (SMZ1500; Nikon, Yuko, Japan). The microdissected sample was transferred to a temperature-regulated chamber mounted on an inverted microscope (Axiovert 100TV, Zeiss) together with DMEM. The glomerulus was held with micropipette, and Af-Art was cannulated and perfused with a set of micropipettes. The intraluminal pressure of the perfused Af-Art was maintained at 60 mmHg throughout the experiment. The chamber was perfused with DMEM with or without losartan (10−6 mol/l) at 1–1.5 ml/min at 37°C. After 30 min of an equilibration period, the dose-response curves of ANG II (10−12 to 10−6 mol/l in DMEM, in random order) with or without losartan (10−6 mol/l) were obtained. Each concentration of ANG II was perfused for 5 min, and the maximum constrictive response of the Af-Art was measured; the bath was then switched to DMEM for 15 min before next dose of ANG II stimulation.
Real-time PCR.
The same method was used to isolate the Af-Art as we reported previously (59, 60, 102). Af-Arts were isolated from kidney slices of the mice without ANG II infusion in ice-cold DMEM under a stereomicroscope. A single Af-Art was transferred into RLT buffer (RNeasy Mini Kit; Qiagen, Venlo, Netherlands) for RNA extraction. To avoid RNA degradation, the time for dissection was limited to 30 min after kidney removal.
Total RNAs (20–30 ng/μl) were extracted from an Af-Art using RNase Mini Kit according to the manufacturer's instructions. After digestion with RNase-free DNase (Promega) to eliminate the genomic contamination, the cDNAs were synthesized with a reverse transcription system using oligo d(T) primer and used as templates. Quantitative PCR analysis was performed using iQ SYBR Green Supermix (Bio-Rad) and CFX96 Real-Time Detection System (Bio-Rad) according to the manufacturer's protocol. Reaction conditions for AT1 and AT2 receptors were 95°C for 1 min, followed by 40 cycles of 95°C for 15 s, then 60°C for 30 s; and for Mas receptor were 95°C for 3 min, 1 cycle; 95°C for 10 s, 56°C for 30 s, 50 cycles; 72°C for 10 s, 1 cycle (75). The reaction of each sample was performed in triplicate. Dissociation analysis was performed at the end of each PCR reaction to confirm the amplification specificity. After the PCR program, data were analyzed and quantified with the comparative Ct method (2–ΔΔCt) based on Ct values to calculate the relative mRNA expression level. The primer sequences and accession numbers are listed in Table 1. The expected size of PCR products of AT1, AT2, and Mas was 240, 230, and 175 bp, respectively (75, 98).
Table 1.
Mouse primer sequences and accession numbers
| Gene Name | Sequences | Accession Number |
|---|---|---|
| AT1 | forward: 5′-ATCGCTACCTGGCCATTGTC-3′ | NM_177322.3 |
| reverse: 5′-GGAAGCCCAGGATGTTCTTG-3′ | ||
| AT2 | forward: 5′-TTACCAGCAGCCGTCCTTTT-3′ | NM_007429.5 |
| reverse: 5′-GTCAGCCAAGGCCAGATTGA-3′ | ||
| Mas | forward: 5′-AGGGTGACTGACTGAGTTTGG-3′ | NM_008552 |
| reverse: 5′-GAAGGTAAGAGGACAGGAGC-3′ | ||
| β-Actin | forward: 5′-GTCCCTCACCCTCCCAAAAG-3′ | NM_007393.3 |
| reverse: 5′-GCTGCCTCAACACCTCAACCC-3′ |
Statistics.
The number of mice in each experiment was determined by power analysis based on P value = 0.05 and a power of 80% (16, 45). Data are presented as means ± SE. A Student’s t-test was used to determine statistical differences. An ANOVA with post hoc test was used for within-group and between-group measurements. A two-way ANOVA was used to compare dose response curves in isolated arterioles. The difference was considered to be significant for a P value < 0.05.
RESULTS
MAP and renal hemodynamic responses to an acute ANG II injection.
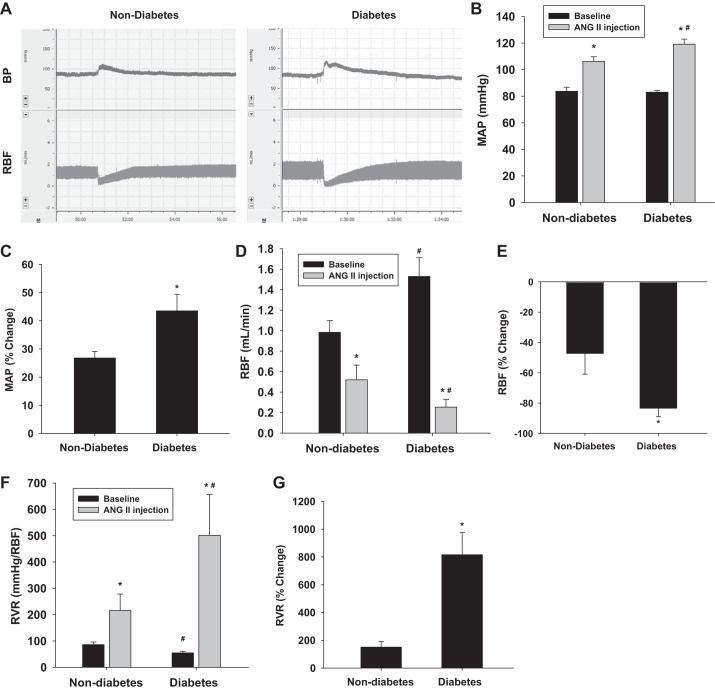
To determine whether the diabetic mice are more sensitive to acute ANG II stimulation, MAP and RBF were measured and compared between anesthetized nondiabetic and diabetic mice (Fig. 2A). Baseline MAP was 82.9 ± 1.1 and 83.7 ± 3.1 mmHg in diabetic and nondiabetic mice, respectively. MAP increased by 43.5 ± 2.6% to 119.1 ± 3.8 mmHg in diabetic mice following acute ANG II injection (P < 0.01 vs. baseline). However, MAP only increased by 26.8 ± 2.3% to 106.2 ± 3.5 mmHg in nondiabetic mice (Fig. 2, B and C) (P < 0.01 vs. baseline). Baseline RBF was significantly higher in diabetic mice (1.53 ± 0.18 ml/min) compared with nondiabetic mice (0.98 ± 0.12 ml/min) (P < 0.05 vs. nondiabetes). RBF decreased by 83.4 ± 5.4% to 0.25 ± 0.07 ml/min in diabetic mice (P < 0.01 vs. baseline) but only decreased by 47.1 ± 13.7% to 0.52 ± 0.14 ml/min in nondiabetic mice (P < 0.01 vs. baseline) following an acute ANG II injection (Fig. 2, D and E). Baseline RVR was significantly lower in diabetic mice compared with nondiabetic mice (Fig. 2F) (P < 0.05 vs. nondiabetes). RVR increased about eightfold in diabetic mice (P < 0.05 vs. baseline) and just increased ~1.5-fold in nondiabetic mice (Fig. 2G) (P < 0.05 vs. baseline). There was no functional response to an injection of 20 μl saline.
Fig. 2.
Responses of mean arterial pressure (MAP), renal blood flow (RBF), and renal vascular resistance (RVR) to acute ANG II injection. Representative image (A) demonstrates the change of MAP and RBF after acute ANG II injection. Acute ANG II injection induced a greater increase in MAP (B and C; *P < 0.01 vs baseline, #P < 0.05 vs nondiabetes) and a greater decrease in RBF (D and E; *P < 0.01 vs baseline, #P < 0.05 vs nondiabetes) in diabetic mice compared with nondiabetic mice. Calculated RVR was also greater in diabetic mice than in nondiabetic mice following ANG II infusion (F and G; *P < 0.05 vs. baseline, #P < 0.05 vs. nondiabetes) (diabetes, n = 10; nondiabetes, n = 7).
MAP response to chronic ANG II infusion.
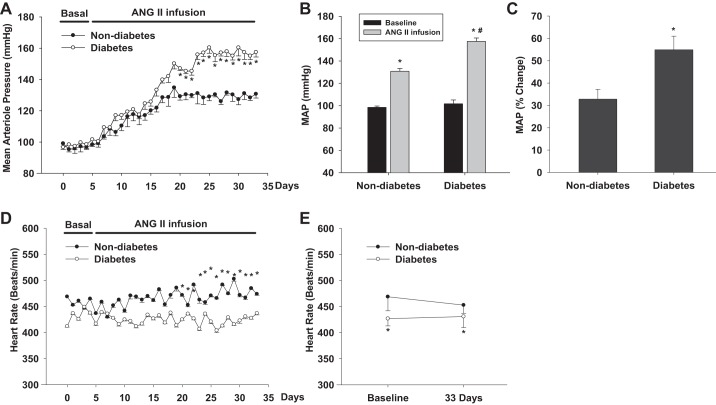
To determine whether the sensitivity of blood pressure to chronic ANG II infusion is enhanced in diabetes, we measured MAP with telemetry in diabetic mice infused with a slow pressor dose of ANG II and compared with nondiabetic mice (Fig. 3A). Baseline MAP was 101.6 ± 3.4 mmHg in diabetic mice and 98.4 ± 2.2 mmHg in nondiabetic mice. MAP increased by 54.9 ± 6.1% to 157.4 ± 4.6 mmHg in diabetic mice following 4-wk ANG II infusion (P < 0.01 vs. baseline). However, MAP only increased by 32.8 ± 4.4% to 130.7 ± 3.6 mmHg in nondiabetic mice (Fig. 3, B and C) (P < 0.01 vs. baseline). There were no significant differences in heart rate between diabetes and nondiabetes (Fig. 3D).
Fig. 3.
Changes in MAP in response to chronic ANG II infusion. Chronic ANG II infusion caused a greater increase in MAP in diabetic mice compared with nondiabetic mice. (n = 6) *P < 0.01 vs baseline; #P < 0.01 vs nondiabetes.
Diabetes enhances the constrictive effect of ANG II on Af-Art.
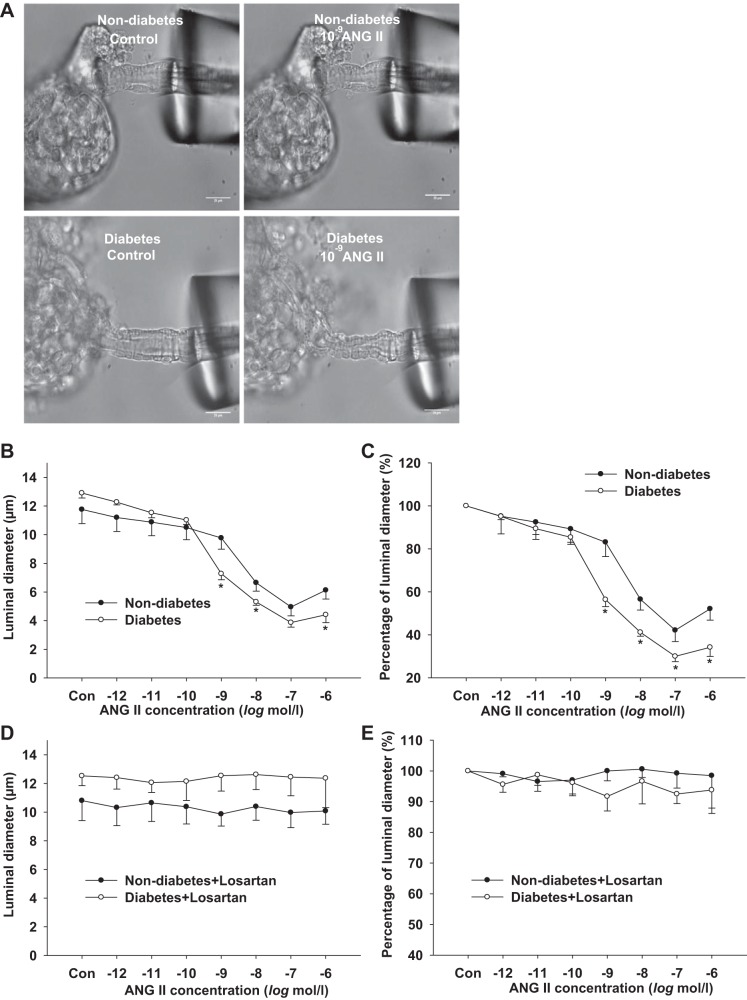
To determine whether ANG II-induced constriction of the Af-Art is enhanced in diabetes, we measured the dose-response curves for ANG II (10−12 to 10−6 mol/l) in isolated perfused Af-Art in diabetic mice and compared them with those of nondiabetic mice (Fig. 4A). In diabetic mice, the basal diameter of the Af-Art was 12.91 ± 0.3 μm. ANG II (10−12 to 10−6 mol/l) reduced the Af-Art diameter in a dose-dependent manner. The diameter was reduced to 56.3 ± 3.2% of baseline at 10−9 mol/l ANG II (P < 0.01 vs. baseline) and 34.1 ± 4.3% of baseline at 10−6 mol/l ANG II (P < 0.01 vs. baseline). In nondiabetic mice, the Af-Art diameter was 11.76 ± 0.9 μm at baseline and reduced to 83.1 ± 6.6% of baseline at 10−9 mol/l of ANG II (P < 0.05 vs. baseline) and to 52.1 ± 5.3% of baseline at 10−6 mol/l of ANG II (P < 0.01 vs. baseline). ANG II-induced contraction was significantly enhanced in diabetic mice (Fig. 4, B and C) (P < 0.05 vs. nondiabetes).
Fig. 4.
Dose-response curve of the afferent arteriole (Af-Art) to ANG II. Representative images (A) demonstrated Af-Art constriction induced by ANG II in nondiabetic and diabetic mice. ANG II-induced contraction was significantly enhanced in diabetic mice (B and C; *P < 0.05 vs. nondiabetes). (diabetes: n = 11, nondiabetes: n = 8). AT1 receptor antagonist losartan blocked ANG II-induced (10−12 to 10−6 mol/l) vasoconstriction of the Af-Art in both diabetic and nondiabetic mice. (D and E) (diabetes, n = 7; nondiabetes, n = 6).
Losartan blocked ANG II induced vasoconstriction.
To determine whether ANG II constricts Af-Art via activation of AT1 receptors, the dose-response curve for ANG II (10−12 to 10−6 mol/l) plus losartan (10−6 mol/l) was measured and compared between diabetic and nondiabetic mice. ANG II (10−12 to 10−6 mol/l) did not reduce the Af-Art diameter in the presence of losartan in diabetic and nondiabetic mice (Fig. 4, D and E).
mRNA levels of RAS receptors.
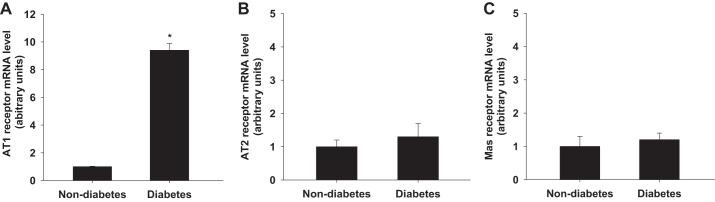
To determine whether diabetes alters the expression levels of angiotensin receptors, we dissected an Af-Art from nondiabetic and diabetic mice (8 wk after alloxan injection) respectively and measured the mRNA expressions of AT1, AT2 and Mas receptors by real-time PCR. The AT1 receptor mRNA level in the Af-Art was 9.4 ± 0.5 times higher in diabetic mice than that in nondiabetic mice (P < 0.01 vs. nondiabetes) (Fig. 5A). The AT2 and Mas receptor mRNA levels were similar in diabetic and nondiabetic mice (Fig. 5, B and C).
Fig. 5.
mRNA levels of AT1 and AT2 receptors in the Af-Art. AT1 receptor mRNA level was significantly higher in diabetic mice than that in nondiabetic mice in the isolated Af-Arts (A; *P < 0.01 vs. nondiabetes). AT2 and Mas receptors mRNA level in diabetic mice was similar as that in nondiabetic mice in the Af-Arts (B and C) (diabetes, n = 10; nondiabetes, n = 10).
DISCUSSION
In the present study, we demonstrated that the responses of MAP and RBF to both acute and chronic ANG II infusion were enhanced in alloxan-induced diabetic C57BL/6 mice. The constrictive response of the Af-Art to ANG II was exaggerated in diabetic mice associated with upregulated expression of AT1 receptor. AT1 receptor antagonist losartan blocked ANG II induced vasoconstriction in both nondiabetic and diabetic mice.
Diabetic patients have a higher prevalence of hypertension than nondiabetic subjects, but the reason has not been completely understood. RAS inhibition showed a distinctive benefit on cardiovascular outcomes for diabetic patients with hypertension (2, 78), suggesting a critical role of the RAS in the development of hypertension in diabetes (1, 71). Even though the plasma renin activity was suppressed (18, 27, 77) or normal (11, 61) in diabetes, the response of blood pressure to acute ANG II infusion was more sensitive in diabetic patients compared with nondiabetic subjects (10, 24, 100, 101). Similar findings have been reported in diabetic animal models. Plasma renin concentration (39, 94), renin activity (39, 47, 76), and ANG II activity (47) were decreased in diabetic models, but the response of blood pressure to chronic administration of AT1 receptor antagonist losartan was enhanced (85). In the present study, we compared MAP in diabetic and nondiabetic mice in response to either bolus intravenous injection or chronic infusion of ANG II. The higher MAP in the diabetic group indicates that the responsiveness of blood pressure to ANG II is exaggerated in diabetic mice.
Renal hemodynamics play an important role in control of salt-water balance and blood pressure, and hypertension is usually associated with decreased RBF and increased RVR (70, 74, 83). Type 1 diabetes is characterized by impairment in autoregulation, reduction in RVR, and elevation in RBF, particularly during the early stage of diabetes (8, 68, 92). In the present study, we demonstrated that the diabetic mice exhibited normal blood pressure but increased RBF compared with nondiabetics, suggesting the impaired autoregulation and decreased RVR in diabetes. Previous studies have demonstrated that both acute and chronic ANG II infusion intensify blood pressure along with a marked increase of RVR in nondiabetics (26, 35, 96). However, the renal hemodynamic responses to ANG II in diabetes are not known yet. In the present study, we compared RBF and calculated RVR between diabetic and nondiabetic mice in response to bolus intravenous injection of ANG II. We found that the renal hemodynamic response to acute ANG II infusion was greater in diabetic mice compared with nondiabetic mice.
As the major resistance vessels in the kidney, Af-Arts account for >50% of the RVR (6, 13, 15). To investigate the vascular response to ANG II in diabetes, we compared the ANG II dose-response curves of the Af-Arts from diabetic and nondiabetic mice. We found that the vasoconstriction of renal Af-Art in response to ANG II was significantly exaggerated in diabetic mice. Consistent with our finding, enhancement in ANG II-dependent vasoconstriction has been found in several different kinds of vessels, including mesenteric, carotid, and renal arteries from diabetic rats, indicating that increased responsiveness to ANG II may be a general characteristic of the vasculatures in diabetes (9).
Effects of ANG II on the Af-Arts are primarily mediated by two types of ANG II receptors, AT1 and AT2 receptors (31, 38, 43). Activation of the AT1 receptor in vascular smooth muscle cells stimulates Ca2+ influx and subsequently induces vasoconstriction (42, 56). On the contrary, AT2 receptor mediates the endothelium-dependent vasodilatation via stimulation of nitric oxide (NO) and bradykinin (5, 88, 89). The renal expression of angiotensin receptors in diabetes has been investigated by several laboratories. The renal AT1 receptor protein level in diabetic rats was reported to be higher than in nondiabetic rats (37). However, several other studies report that the kidney AT1 receptor was downregulated in diabetic animal models and diabetic patients (14, 65, 104). Also, a lower expression level of the AT1 receptor was found in hypertensive animals with diabetes (12, 14, 104). In addition, Wagner et al. (97) demonstrated that the mRNA level of the AT1 receptor was significantly lower in patients with Type II diabetes than nondiabetics in kidney biopsy samples. The reasons for the inconsistent observations in AT1 expression levels in diabetes are not clear but might be due to the different regions of the kidney that were examined. Regarding the AT2 receptor, a downregulation of expression in the kidney was demonstrated in both early streptozotocin-induced diabetic Sprague-Dawley rats (99) and spontaneously hypertensive rats with long-term diabetes (12). Moreover, the localization of ANG II receptors is widely distributed in the kidney. Both immunohistochemical and ANG II binding studies demonstrated that AT1 receptor was abundant in the cortical vasculature and S3 segment of the proximal tubules in the outer medulla, with lesser expression in the thick ascending limb and collecting ducts (63, 64, 67). The major distribution of AT2 receptor in the kidney was indicated in interlobular arteries, Af-Arts, glomeruli, proximal tubules, and collecting ducts (62, 87, 99). Thus, the expression levels of ANG II receptors in the kidney homogenate may not reflect their specific levels in Af-Art. To determine the expression of ANG II receptors exclusively in Af-Art, we isolated Af-Art and then measured the mRNA level of AT1 and AT2 receptors. We found that AT1 receptor expression was over 10-fold higher in mice at 8 wk of diabetes than in nondiabetic mice, whereas the level of AT2 receptor was similar. This finding is consistent with the study by Sodhi et al. (90) that found the medium with high glucose upregulated AT1 receptor expression in cultured vascular smooth muscle cells. However, our results are not inconsistent with the previous report that both AT1-A and AT1-B receptor protein levels in renal arterioles, measured by immunohistochemistry, were lower in STZ-induced diabetic Wistar rats (32, 81). The difference in blood pressure between diabetic and nondiabetic mice was not significant until 2 wk after ANG II infusion or 6 wk following alloxan injection. The reasons are not clear and may be due to the compensatory mechanisms or low expression level of AT1 in the Af-Art within the first few weeks of diabetes.
Besides the direct vascular reactions via AT1 and AT2 receptors, ANG II is converted by angiotensin converting enzyme-2 (ACE-2) into angiotensin 1–7 (ANG 1–7), which binds to G protein-coupled receptor Mas counteracting the AT1 receptor-mediated vasoconstriction. Previous studies demonstrated that the expression of renal ACE-2 and Mas receptor was decreased in 20 wk Akita mice (86). As the Mas receptor has an extensive distribution in the kidney, including the proximal tubule, thick ascending limb, and collecting duct, to determine the expression of Mas receptor exclusively in Af-Art, we isolated Af-Art and measured the mRNA level of Mas receptors. Inconsistent with the expression of Mas receptors in the kidney homogenate, our results showed that there is no significant change of Mas receptor expression in the Af-Arts between diabetic and nondiabetic mice, which suggests that the ACE-2/ANG 1–7/Mas pathway might not play a significant role in the enhancement of ANG II-induced Af-Art constriction in diabetic mice.
NO is an important modulator that negatively regulates the AT1 receptor-mediated vasoconstriction. NO in renal Af-Art is primarily generated in endothelial cells via endothelial nitric oxide synthase (NOS3). Previous evidence demonstrated that the expression of NOS3 in the endothelium of Af-Arts was markedly increased in alloxan-induced diabetic C57BL/6 mice (103), which suggests that NOS3-dependent NO generation should counteract the vasoconstriction of renal Af-Art in response to ANG II in diabetic mice. Both type 1 and type 2 cyclooxygenase (COX) are identified in renal Af-arts (22, 36). Prostaglandins (PGs) generated by the COX have been implicated in modulation of ANG II-induced Af-Art vasoconstriction (30, 80). Inhibition or knockout of COX2 augments the pressor effect of ANG II (48, 79). Vasodilatory PGs, such as prostacyclin and prostaglandin E2 (PGE2), are involved in the hyperfiltration in the early stage of diabetes (19, 40, 44). Even though the changes in COX expression of Af-Arts in diabetes are not clear, several studies have demonstrated that renal expression of COX2 (49, 54, 66) or PGE2 (17, 41) is upregulated in diabetes, which suggests that COX-derived PGs are unlikely to promote the ANG II-induced vasoconstriction of renal Af-Art in diabetic mice.
The synergistic interaction of contractile response between ANG II and adenosine on renal Af-Art has been recognized (15, 55, 73). In the presence of adenosine, the ANG II-induced vasoconstriction of Af-Art was significantly enhanced (55, 73). However, compared with wild-type mice, adenosine A1-receptor knockout mice exhibited similar renal hemodynamic changes in alloxan-induced diabetes (84), suggesting that adenosine is not the major cause of the enhanced renal hemodynamic responses or vasoconstriction of renal Af-Art to ANG II in diabetes.
Taken together, these findings indicate that exaggerated Af-Art vasoconstrictive response to ANG II, mediated by the increased expression of AT1 receptor, may contribute to the enhanced blood pressure and renal hemodynamic responses to ANG II in diabetes. Therefore, the current study reveals a mechanism that may contribute to the development of hypertension in diabetes.
Perspectives and Significance
Diabetic subjects have a higher prevalence of hypertension, but the mechanisms have not been fully elucidated. We demonstrated in the present study that diabetes exaggerates the constrictive responsiveness of the Af-Art to ANG II, which was mediated by the upregulation in the expression of AT1 receptor. This mechanism may promote the enhanced response of blood pressure and renal hemodynamic to ANG II in diabetes. Plasma renin-angiotensin II level is usually suppressed in early diabetes (3, 46), which may permit a low vascular tone of the Af-Art and a high glomerular hyperfiltration in early diabetes. On the other hand, mild or moderate elevation of circulating and/or local renin-angiotensin II that may not increase blood pressure in nondiabetic patients could induce hypertension in diabetes, which may be one of the mechanisms for the high prevalence of hypertension in diabetes. The findings of the present study reveal the significance of RAS as a potential mechanism for the hypertension in diabetes and justify the essential role of the RAS inhibition for the diabetic patients with hypertension.
GRANTS
This work was supported by AHA-15PRE2571306 (to J. Zhang) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-099276 and DK-098582 (to R. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z., J.W., and R.L. conceived and designed research; J.Z., J.S., J.W., and S.J. performed experiments; J.Z., H.Y.Q., J.S., J.W., S.J., and R.L. analyzed data; J.Z., H.Y.Q., and J.S. prepared figures; J.Z. and J.S. drafted manuscript; J.Z., J.S., J.W., S.J., Lei Wang, Liqing Wang, J.B., and R.L. approved final version of manuscript; H.Y.Q., J.S., J.W., S.J., Lei Wang, Liqing Wang, J.B., and R.L. edited and revised manuscript.
REFERENCES
- 1.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355: 253–259, 2000. doi: 10.1016/S0140-6736(99)12323-7. [DOI] [PubMed] [Google Scholar]
- 2.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288: 2981–2997, 2002. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 3.Amemiya S, Ishihara T, Higashida K, Kusano S, Ohyama K, Kato K. Altered synthesis of renin in patients with insulin-dependent diabetes: plasma prorenin as a marker predicting the evolution of nephropathy. Diabetes Res Clin Pract 10: 115–122, 1990. doi: 10.1016/0168-8227(90)90032-O. [DOI] [PubMed] [Google Scholar]
- 4.Arauz-Pacheco C, Parrott MA, Raskin P; American Diabetes Association . Hypertension management in adults with diabetes. Diabetes Care 27, Suppl 1: S65–S67, 2004. doi: 10.2337/diacare.27.2007.S65. [DOI] [PubMed] [Google Scholar]
- 5.Arima S, Endo Y, Yaoita H, Omata K, Ogawa S, Tsunoda K, Abe M, Takeuchi K, Abe K, Ito S. Possible role of P-450 metabolite of arachidonic acid in vasodilator mechanism of angiotensin II type 2 receptor in the isolated microperfused rabbit afferent arteriole. J Clin Invest 100: 2816–2823, 1997. doi: 10.1172/JCI119829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arima S, Ito S. The mechanisms underlying altered vascular resistance of glomerular afferent and efferent arterioles in diabetic nephropathy. Nephrol Dial Transplant 18: 1966–1969, 2003. doi: 10.1093/ndt/gfg263. [DOI] [PubMed] [Google Scholar]
- 7.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 47: 288–295, 2006. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 8.Bell TD, DiBona GF, Wang Y, Brands MW. Mechanisms for renal blood flow control early in diabetes as revealed by chronic flow measurement and transfer function analysis. J Am Soc Nephrol 17: 2184–2192, 2006. doi: 10.1681/ASN.2006030216. [DOI] [PubMed] [Google Scholar]
- 9.Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292: H666–H672, 2007. doi: 10.1152/ajpheart.00372.2006. [DOI] [PubMed] [Google Scholar]
- 10.Beretta-Piccoli C, Weidmann P. Exaggerated pressor responsiveness to norepinephrine in nonazotemic diabetes mellitus. Am J Med 71: 829–835, 1981. doi: 10.1016/0002-9343(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 11.Beretta-Piccoli C, Weidmann P, Keusch G. Responsiveness of plasma renin and aldosterone in diabetes mellitus. Kidney Int 20: 259–266, 1981. doi: 10.1038/ki.1981.129. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet F, Candido R, Carey RM, Casley D, Russo LM, Osicka TM, Cooper ME, Cao Z. Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens 20: 1615–1624, 2002. doi: 10.1097/00004872-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns KD. Angiotensin II and its receptors in the diabetic kidney. Am J Kidney Dis 36: 449–467, 2000. doi: 10.1053/ajkd.2000.16192. [DOI] [PubMed] [Google Scholar]
- 15.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 4: 303–306, 2013. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YJ, Santos M, Quilley J. Treatment of diabetic rats with a peroxynitrite decomposition catalyst prevents induction of renal COX-2. Am J Physiol Heart Circ Physiol 300: H1125–H1132, 2011. doi: 10.1152/ajpheart.00768.2010. [DOI] [PubMed] [Google Scholar]
- 18.Christlieb AR, Kaldany A, D’Elia JA. Plasma renin activity and hypertension in diabetes mellitus. Diabetes 25: 969–974, 1976. doi: 10.2337/diab.25.10.969. [DOI] [PubMed] [Google Scholar]
- 19.Craven PA, Caines MA, DeRubertis FR. Sequential alterations in glomerular prostaglandin and thromboxane synthesis in diabetic rats: relationship to the hyperfiltration of early diabetes. Metabolism 36: 95–103, 1987. doi: 10.1016/0026-0495(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 20.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005. doi: 10.1172/JCI23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMaria AN, Weir MR. Coxibs–beyond the GI tract: renal and cardiovascular issues. J Pain Symptom Manage 25, Suppl: S41–S49, 2003. doi: 10.1016/S0885-3924(02)00630-9. [DOI] [PubMed] [Google Scholar]
- 23.Dilley JR, Stier CT Jr, Arendshorst WJ. Abnormalities in glomerular function in rats developing spontaneous hypertension. Am J Physiol 246: F12–F20, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Drury PL, Smith GM, Ferriss JB. Increased vasopressor responsiveness to angiotensin II in type 1 (insulin-dependent) diabetic patients without complications. Diabetologia 27: 174–179, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension 19: 403–418, 1992. doi: 10.1161/01.HYP.19.5.403. [DOI] [PubMed] [Google Scholar]
- 26.Fagard RH, Cowley AW Jr, Navar LG, Langford HG, Guyton AC. Renal responses to slight elevations of renal arterial plasma angiotensin II concentration in dogs. Clin Exp Pharmacol Physiol 3: 531–538, 1976. doi: 10.1111/j.1440-1681.1976.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Cruz A Jr, Noth RH, Lassman MN, Hollis JB, Mulrow PJ. Low plasma renin activity in normotensive patients with diabetes mellitus: relationship to neuropathy. Hypertension 3: 87–92, 1981. doi: 10.1161/01.HYP.3.1.87. [DOI] [PubMed] [Google Scholar]
- 28.Gillow JT, Gibson JM, Dodson PM. Hypertension and diabetic retinopathy–what’s the story? Br J Ophthalmol 83: 1083–1087, 1999. doi: 10.1136/bjo.83.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göthberg G, Lundin S, Ricksten SE, Folkow B. Apparent and true vascular resistances to flow in SHR and NCR kidneys as related to the pre/postglomerular resistance ratio. Acta Physiol Scand 105: 282–294, 1979. doi: 10.1111/j.1748-1716.1979.tb06343.x. [DOI] [PubMed] [Google Scholar]
- 30.Green T, Gonzalez AA, Mitchell KD, Navar LG. The complex interplay between cyclooxygenase-2 and angiotensin II in regulating kidney function. Curr Opin Nephrol Hypertens 21: 7–14, 2012. doi: 10.1097/MNH.0b013e32834d9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res 11: 165–180, 2001. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 32.Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol 250: R960–R972, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Hall JE. Control of blood pressure by the renin-angiotensin-aldosterone system. Clin Cardiol 14, Suppl 4: IV6–IV21, 1991. doi: 10.1002/clc.4960141802. [DOI] [PubMed] [Google Scholar]
- 34.Hall JE. The renin-angiotensin system: renal actions and blood pressure regulation. Compr Ther 17: 8–17, 1991. [PubMed] [Google Scholar]
- 35.Hall JE, Guyton AC, Salgado HC, McCaa RE, Balfe JW. Renal hemodynamics in acute and chronic angiotensin II hypertension. Am J Physiol 235: F174–F179, 1978. [DOI] [PubMed] [Google Scholar]
- 36.Harris RC, Breyer MD. Update on cyclooxygenase-2 inhibitors. Clin J Am Soc Nephrol 1: 236–245, 2006. doi: 10.2215/CJN.00890805. [DOI] [PubMed] [Google Scholar]
- 37.Harrison-Bernard LM, Imig JD, Carmines PK. Renal AT1 receptor protein expression during the early stage of diabetes mellitus. Int J Exp Diabetes Res 3: 97–108, 2002. doi: 10.1080/15604280214483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112: 417–428, 2007. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Gallois Y, Bouby N, Bruneval P, Heudes D, Belair MF, Krege JH, Meneton P, Marre M, Smithies O, Alhenc-Gelas F. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci USA 98: 13330–13334, 2001. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen PK, Steven K, Blaehr H, Christiansen JS, Parving HH. Effects of indomethacin on glomerular hemodynamics in experimental diabetes. Kidney Int 29: 490–495, 1986. doi: 10.1038/ki.1986.26. [DOI] [PubMed] [Google Scholar]
- 41.Jia Z, Sun Y, Liu S, Liu Y, Yang T. COX-2 but not mPGES-1 contributes to renal PGE2 induction and diabetic proteinuria in mice with type-1 diabetes. PLoS One 9: e93182, 2014. doi: 10.1371/journal.pone.0093182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanaide H, Ichiki T, Nishimura J, Hirano K. Cellular mechanism of vasoconstriction induced by angiotensin II: it remains to be determined. Circ Res 93: 1015–1017, 2003. doi: 10.1161/01.RES.0000105920.33926.60. [DOI] [PubMed] [Google Scholar]
- 43.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 44.Kasiske BL, O’Donnell MP, Keane WF. Glucose-induced increases in renal hemodynamic function. Possible modulation by renal prostaglandins. Diabetes 34: 360–364, 1985. doi: 10.2337/diab.34.4.360. [DOI] [PubMed] [Google Scholar]
- 45.Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC, Kuzmuk V, Coward RJ, Goult J, Bull KR, Sharma R, Bharti K, Westenskow PD, Michael IP, Saleem MA, Friedlander M. VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest 127: 199–214, 2017. doi: 10.1172/JCI86418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennefick TM, Anderson S. Role of angiotensin II in diabetic nephropathy. Semin Nephrol 17: 441–447, 1997. [PubMed] [Google Scholar]
- 47.Kigoshi T, Imaizumi N, Azukizawa S, Yamamoto I, Uchida K, Konishi F, Morimoto S. Effects of angiotensin II, adrenocorticotropin, and potassium on aldosterone production in adrenal zona glomerulosa cells from streptozotocin-induced diabetic rats. Endocrinology 118: 183–188, 1986. doi: 10.1210/endo-118-1-183. [DOI] [PubMed] [Google Scholar]
- 48.Kim GH. Renal effects of prostaglandins and cyclooxygenase-2 inhibitors. Electrolyte Blood Press 6: 35–41, 2008. doi: 10.5049/EBP.2008.6.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YS, Jung DH, Sohn E, Lee YM, Kim CS, Kim JS. Extract of Cassiae semen attenuates diabetic nephropathy via inhibition of advanced glycation end products accumulation in streptozotocin-induced diabetic rats. Phytomedicine 21: 734–739, 2014. doi: 10.1016/j.phymed.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Kirpichnikov D, Sowers JR. Role of ACE inhibitors in treating hypertensive diabetic patients. Curr Diab Rep 2: 251–257, 2002. doi: 10.1007/s11892-002-0091-5. [DOI] [PubMed] [Google Scholar]
- 51.Klein BE, Klein R, Moss SE. Blood pressure in a population of diabetic persons diagnosed after 30 years of age. Am J Public Health 74: 336–339, 1984. doi: 10.2105/AJPH.74.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein R, Klein BE. Blood pressure control and diabetic retinopathy. Br J Ophthalmol 86: 365–367, 2002. doi: 10.1136/bjo.86.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knox FG, Ott C, Cuche JL, Gasser J, Haas J. Autoregulation of single nephron filtration rate in the presence and the absence of flow to the macula densa. Circ Res 34: 836–842, 1974. doi: 10.1161/01.RES.34.6.836. [DOI] [PubMed] [Google Scholar]
- 54.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107: 889–898, 2001. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006. doi: 10.1038/sj.ki.5001650. [DOI] [PubMed] [Google Scholar]
- 56.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca(2+) influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca(2+) entry. Circ Res 87: 551–557, 2000. doi: 10.1161/01.RES.87.7.551. [DOI] [PubMed] [Google Scholar]
- 57.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend Med 9: 103–111, 2012. doi: 10.1016/j.genm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1beta protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2016. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y, Zhang R, Ge Y, Carlstrom M, Wang S, Fu Y, Cheng L, Wei J, Roman RJ, Wang L, Gao X, Liu R. Identification and function of adenosine A3 receptor in afferent arterioles. Am J Physiol Renal Physiol 308: F1020–F1025, 2015. doi: 10.1152/ajprenal.00422.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luik PT, Kerstens MN, Hoogenberg K, Navis GJ, Dullaart RP. Low plasma aldosterone despite normal plasma renin activity in uncomplicated type 1 diabetes mellitus: effects of RAAS stimulation. Eur J Clin Invest 33: 787–793, 2003. doi: 10.1046/j.1365-2362.2003.01215.x. [DOI] [PubMed] [Google Scholar]
- 62.Matsubara H, Sugaya T, Murasawa S, Nozawa Y, Mori Y, Masaki H, Maruyama K, Tsutumi Y, Shibasaki Y, Moriguchi Y, Tanaka Y, Iwasaka T, Inada M. Tissue-specific expression of human angiotensin II AT1 and AT2 receptors and cellular localization of subtype mRNAs in adult human renal cortex using in situ hybridization. Nephron 80: 25–34, 1998. doi: 10.1159/000045121. [DOI] [PubMed] [Google Scholar]
- 63.Meister B, Lippoldt A, Bunnemann B, Inagami T, Ganten D, Fuxe K. Cellular expression of angiotensin type-1 receptor mRNA in the kidney. Kidney Int 44: 331–336, 1993. doi: 10.1038/ki.1993.248. [DOI] [PubMed] [Google Scholar]
- 64.Miyata N, Park F, Li XF, Cowley AW Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol 277: F437–F446, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Mogyorosi A, Sonkodi S. AT1 receptor antagonists: a challenge for ACE inhibitors in diabetic nephropathy. Diabetes Metab Res Rev 15: 55–58, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 66.Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G. Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol 181: 1991–2002, 2012. doi: 10.1016/j.ajpath.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mujais SK, Kauffman S, Katz AI. Angiotensin II binding sites in individual segments of the rat nephron. J Clin Invest 77: 315–318, 1986. doi: 10.1172/JCI112293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 17: 93–100, 1997. [PubMed] [Google Scholar]
- 69.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 51: 1142–1148, 2008. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 70.Parmer RJ, Stone RA, Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in response to changes in dietary sodium. Hypertension 24: 752–757, 1994. doi: 10.1161/01.HYP.24.6.752. [DOI] [PubMed] [Google Scholar]
- 71.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B; ADVANCE Collaborative Group . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370: 829–840, 2007. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 72.Pell S, D’Alonzo CA. Some aspects of hypertension in diabetes mellitus. JAMA 202: 104–110, 1967. doi: 10.1001/jama.202.1.104. [DOI] [PubMed] [Google Scholar]
- 73.Persson AE, Lai EY, Gao X, Carlström M, Patzak A. Interactions between adenosine, angiotensin II and nitric oxide on the afferent arteriole influence sensitivity of the tubuloglomerular feedback. Front Physiol 4: 187, 2013. doi: 10.3389/fphys.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Persson PB. Renal blood flow autoregulation in blood pressure control. Curr Opin Nephrol Hypertens 11: 67–72, 2002. doi: 10.1097/00041552-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Prasad T, Verma A, Li Q. Expression and cellular localization of the Mas receptor in the adult and developing mouse retina. Mol Vis 20: 1443–1455, 2014. [PMC free article] [PubMed] [Google Scholar]
- 76.Pratt JH, Parkinson CA, Weinberger MH, Duckworth WC. Decreases in renin and aldosterone secretion in alloxan diabetes: an effect of insulin deficiency. Endocrinology 116: 1712–1716, 1985. doi: 10.1210/endo-116-5-1712. [DOI] [PubMed] [Google Scholar]
- 77.Price DA, Porter LE, Gordon M, Fisher ND, De’Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10: 2382–2391, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre RN, Wagner EH, Furberg CD. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 277: 739–745, 1997. doi: 10.1001/jama.1997.03540330061036. [DOI] [PubMed] [Google Scholar]
- 79.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest 110: 61–69, 2002. doi: 10.1172/JCI0214752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quadri SS, Culver SA, Li C, Siragy HM. Interaction of the renin angiotensin and cox systems in the kidney. Front Biosci (Schol Ed) 8: 215–226, 2016. doi: 10.2741/s459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Razga Z, Kovacs G, Bódi N, Talapka P, Nyengaard JR. Heterogeneous downregulation of angiotensin II AT1-A and AT1-B receptors in arterioles in STZ-induced diabetic rat kidneys. BioMed Res Int 2014: 947506, 2014. doi: 10.1155/2014/947506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reboldi G, Gentile G, Angeli F, Verdecchia P. Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: update after recent clinical trials. Vasc Health Risk Manag 5: 411–427, 2009. doi: 10.2147/VHRM.S4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruilope LM, Lahera V, Rodicio JL, Carlos Romero J. Are renal hemodynamics a key factor in the development and maintenance of arterial hypertension in humans? Hypertension 23: 3–9, 1994. doi: 10.1161/01.HYP.23.1.3. [DOI] [PubMed] [Google Scholar]
- 84.Sällström J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 190: 253–259, 2007. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 85.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol 94: 648–658, 2009. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Angiotensin-(1–7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond) 128: 649–663, 2015. doi: 10.1042/CS20140329. [DOI] [PubMed] [Google Scholar]
- 87.Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Aldosterone Syst 11: 33–36, 2010. doi: 10.1177/1470320309347786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA 96: 6506–6510, 1999. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sodhi CP, Kanwar YS, Sahai A. Hypoxia and high glucose upregulate AT1 receptor expression and potentiate ANG II-induced proliferation in VSM cells. Am J Physiol Heart Circ Physiol 284: H846–H852, 2003. doi: 10.1152/ajpheart.00625.2002. [DOI] [PubMed] [Google Scholar]
- 91.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37: 1053–1059, 2001. doi: 10.1161/01.HYP.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 92.Takenaka T, Inoue T, Ohno Y, Miyazaki T, Nishiyama A, Ishii N, Suzuki H. Elucidating mechanisms underlying altered renal autoregulation in diabetes. Am J Physiol Regul Integr Comp Physiol 303: R495–R504, 2012. doi: 10.1152/ajpregu.00217.2012. [DOI] [PubMed] [Google Scholar]
- 93.Teuscher A, Egger M, Herman JB. Diabetes and hypertension. Blood pressure in clinical diabetic patients and a control population. Arch Intern Med 149: 1942–1945, 1989. doi: 10.1001/archinte.1989.00390090024005. [DOI] [PubMed] [Google Scholar]
- 94.Ubeda M, Hernandez I, Fenoy F, Quesada T. Vascular and adrenal reninlike activity in chronically diabetic rats. Hypertension 11: 339–343, 1988. doi: 10.1161/01.HYP.11.4.339. [DOI] [PubMed] [Google Scholar]
- 95.Vaishnava H, Bhasin RC. Hypertension in Indian diabetics. J Chronic Dis 21: 691–702, 1969. doi: 10.1016/0021-9681(69)90041-1. [DOI] [PubMed] [Google Scholar]
- 96.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–R1292, 2011. doi: 10.1152/ajpregu.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner J, Gehlen F, Ciechanowicz A, Ritz E. Angiotensin II receptor type 1 gene expression in human glomerulonephritis and diabetes mellitus. J Am Soc Nephrol 10: 545–551, 1999. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, Song J, Wang S, Buggs J, Chen R, Zhang J, Wang L, Rong S, Li W, Wei J, Liu R. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am J Physiol Renal Physiol 313: F326–F338, 2017. doi: 10.1152/ajprenal.00039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wehbi GJ, Zimpelmann J, Carey RM, Levine DZ, Burns KD. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol 280: F254–F265, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Weidmann P, Beretta-Piccoli C, Keusch G, Glück Z, Mujagic M, Grimm M, Meier A, Ziegler WH. Sodium-volume factor, cardiovascular reactivity and hypotensive mechanism of diuretic therapy in mild hypertension associated with diabetes mellitus. Am J Med 67: 779–784, 1979. doi: 10.1016/0002-9343(79)90734-4. [DOI] [PubMed] [Google Scholar]
- 101.Weidmann P, Beretta-Piccoli C, Trost BN. Pressor factors and responsiveness in hypertension accompanying diabetes mellitus. Hypertension 7: II33–II42, 1985. doi: 10.1161/01.HYP.7.6_Pt_2.II33. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Chandrashekar K, Lu Y, Duan Y, Qu P, Wei J, Juncos LA, Liu R. Enhanced expression and activity of Nox2 and Nox4 in the macula densa in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol 306: F344–F350, 2014. doi: 10.1152/ajprenal.00515.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang JH, Kawashima S, Yokoyama M, Huang P, Hill CE. Increased eNOS accounts for changes in connexin expression in renal arterioles during diabetes. Anat Rec A Discov Mol Cell Evol Biol 288: 1000–1008, 2006. doi: 10.1002/ar.a.20369. [DOI] [PubMed] [Google Scholar]
- 104.Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, Burns KD. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int 58: 2320–2330, 2000. doi: 10.1046/j.1523-1755.2000.00416.x. [DOI] [PubMed] [Google Scholar]