Abstract
Kinesin family member 14 (KIF14), a microtubule-based motor protein, plays an important role in chromosomal segregation, congression, and alignment. Considerable evidence indicates that KIF14 is involved in cytokinesis, although little is known about its role in oral squamous cell carcinomas (OSCCs). In the current study, we functionally and clinically investigated KIF14 expression in patients with OSCC. Quantitative reverse transcriptase–polymerase chain reaction and immunoblotting analyses were used to assess the KIF14 regulatory mechanism in OSCC. Immunohistochemistry (IHC) was performed to analyze the correlation between KIF14 expression and clinical behavior in 104 patients with OSCC. A KIF14 knockdown model of OSCC cells (shKIF14 cells) was used for functional experiments. KIF14 expression was up-regulated significantly (P<0.05) in OSCCs compared with normal counterparts in vitro and in vivo. In addition, shKIF14 cells inhibited cellular proliferation compared with control cells by cell-cycle arrest at the G2/M phase through up-regulation of G2 arrest-related proteins (p-Cdc2 and cyclin B1). As expected, IHC data from primary OSCCs showed that KIF14-positive patients exhibited significantly (P<0.05) more larger tumors compared with KIF14-negative patients. The current results suggest for the first time that KIF14 is an indicator of tumoral size in OSCCs and that KIF14 might be a potential therapeutic target for development of new treatments for OSCCs.
Keywords: Kinesin family member 14 (KIF14), Oral squamous cell carcinoma, Tumoral size, Cell-cycle arrest, G2/M phase
1. Introduction
Kinesins are a family of the ATP-dependent motor proteins that travel unidirectionally along microtubule tracks to fulfill their many roles in intracellular transport or cell division [1], [2], [3]. Kinesins have so far been classified into 14 subfamilies (kinesin-1 family–kinesin-14 family) by phylogenetic analysis of the motor domain [1], [4] and are additionally composed of 45 kinesin superfamily proteins (KIFs) [1], [5]. KIFs reportedly transport organelles or participate in signal transduction, but mainly participate in cell mitosis, particularly in spindle formation, chromosomal and nuclear movement, and cytokinesis [5].
Previous studies also have indicated that KIFs play critical roles in several malignancies, including tumoral development and progression [1], [2], [4], [5], [6], [7], [8], [9], [10]. Among them, KIF14 protein is localized at the spindle midzone (the area formed between retreating chromosomes as they segregate toward the spindle poles in anaphase) and the midbody (the cytoplasmic bridge that connects two daughter cells at the end of cytokinesis in telophase) [11], [12], and is essential for cytokinesis and chromosome segregation. KIF14 has genomic gain at 1q31.3–1q32.1 with overexpressed gene levels in multiple cancers, i.e., breast, retinoblastoma, liver, renal, lung, laryngeal, and ovarian cancers and synovial sarcoma [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. However, the relationship between overexpression of KIF14 and clinical behavior of oral squamous cell carcinoma (OSCC) has not yet been clarified.
In the current study, we report that KIF14 expression in OSCCs is functionally and clinically linked to tumoral size in vitro and in vivo and show that KIF14 is closely related to the cell cycle. Therefore, KIF14 might be a potential therapeutic target for OSCCs.
2. Materials and methods
2.1. Ethics statement
The Ethical Committee of the Graduate School of Medicine, Chiba University approved the study protocol (approval number, 236); the study was performed in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent before participating in this research.
2.2. OSCC-derived cell lines and tissue specimens
Human OSCC-derived cell lines (HSC-2, HSC-3, HSC-4, KOSC-2, Ca9-22, Ho-1-N-1, Ho-1-u-1, and SAS) were obtained from the Human Science Research Resources Bank (Osaka, Japan) or the RIKEN BioResource Center (Ibaraki, Japan). Primary cultured human normal oral keratinocytes (HNOKs) were obtained from three healthy donors and served as normal controls [29], [30]. All cells were grown in Dulbecco's modified Eagle medium (Sigma-Aldrich Co, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma) and 50 units/ml penicillin and streptomycin (Sigma). Clinicopathologic staging was determined by the TNM classification of the International Union against Cancer [31].
2.3. Preparation of cDNA and protein
Total RNA and protein were isolated as described previously [28], [29].
2.4. mRNA expression analysis
Real-time quantitative reverse transcriptase–polymerase chain reaction (qRT–PCR) was conducted as described previously [28], [29]. Primers and universal probes were designed using the Universal Probe Library Assay Design Center (Roche Diagnostics GmbH), which specifies the most suitable set. The primer sequences used for qRT–PCR were: KIF14, forward, 5′-CCTGTCTTTTTGCTTATGGTCAG-3′; reverse, 5′-TCTTCACTAAATCCCATCATCG-3′; and universal probe #21, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward, 5′-AGCCACATCGCTCAGACAC-3′; reverse, 5′-GCCCAATACGACCAAATCC-3′; and universal probe #60.
2.5. Immunoblotting analysis
Immunoblotting analysis was performed as described previously [28], [29]. The antibodies were rabbit anti-KIF14 polyclonal antibody (Cat. no. A300-912A, Bethyl Laboratories, Montgomery, AL, USA), mouse anti-GAPDH monoclonal antibody (Cat. no. sc-32233, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-phospho-CDC2 polyclonal antibody (Cat. no. sc-101654, Santa Cruz Biotechnology), and rabbit anti-cyclin B1 polyclonal antibody (Cat. no. 12231, Cell Signaling Technology, Danvers, MA, USA).
2.6. IHC
IHC using the primary antibody was performed as previously described [32], [33]. To quantify the KIF14 protein expression in those components, we used the previously described IHC scoring system [32], [33]. To determine the cutoff points of KIF14 IHC scores, we analyzed the IHC scores of 104 patients using the receiver operating characteristic (ROC) curve. Cases with a score following over 95.0 (Youden Index and ROC curve for tumoral tissue) were considered KIF14-positive. Two independent pathologists from Chiba University Hospital, neither of whom had knowledge of the patients' clinical status, made these judgments.
2.7. Transfection with shRNA plasmid
A total of 1×105 cells from the Ho-1-N-1 and SAS cell lines were transfected with 10 ng/µl KIF14 shRNA (shKIF14) or 10 ng/µl control shRNA (shMock) vectors (Santa Cruz Biotechnology) using 1.25 µl Lipofectamine index LTX and 0.5 µl Plus Reagents (Invitrogen, Carlsbad, CA, USA). The stable shKIF14 and shMock cells were isolated using a culture medium containing 1 µg/ml puromycin (Santa Cruz Biotechnology).
2.8. Cellular growth
To evaluate the effect of KIF14 knockdown on cellular growth, we analyzed cellular growth in the shKIF14 and shMock cells. These cells were seeded in 6 cm plates at a density of 1×104 viable cells. A cellular growth assay was performed as described previously [28], [29].
2.9. Cell-cycle analysis
To synchronize cells at the G0/G1 or G2/M transition, the cells were cultured in serum free media for 48 h or treated with 200 ng/ml nocodazole (Sigma) for 12 h [34], [35]. Cell-cycle analysis was performed as described previously [28].
2.10. Statistical analysis
In comparisons of KIF14 expression levels, statistical significance was evaluated using the Mann–Whitney U-test. Relationships between the KIF14-IHC scores and clinicopathological profiles were evaluated using the Mann–Whitney U-test. P<0.05 was considered significant. The data are expressed as the mean±standard error of the mean (SEM).
3. Results
3.1. Evaluation of KIF14 expression in OSCC-derived cell lines
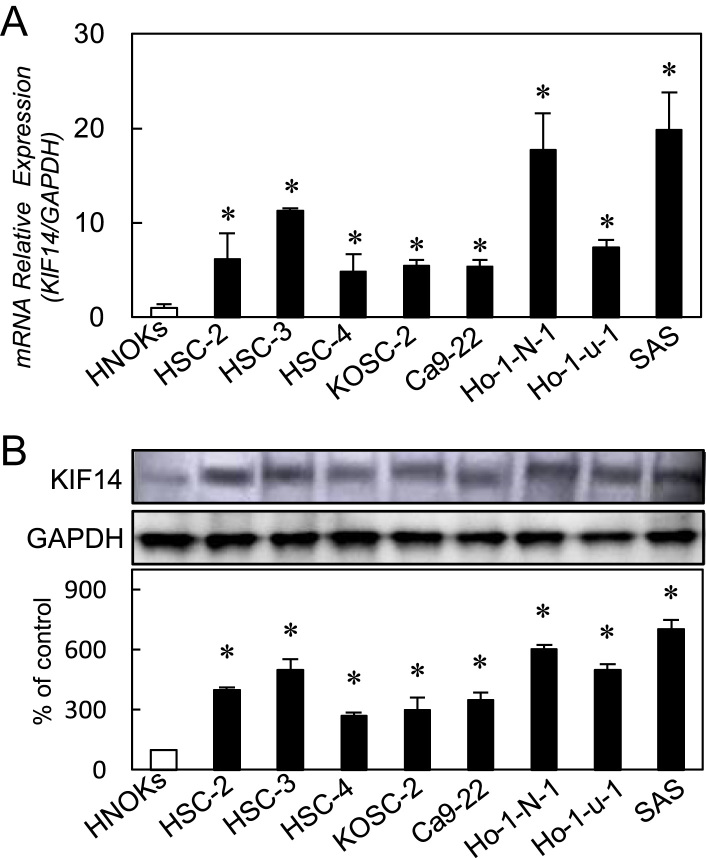
To investigate the expression status of KIF14, we performed qRT–PCR and immunoblotting analyses using eight OSCC-derived cell lines (HSC-2, HSC-3, HSC-4, KOSC-2, Ca9-22, Ho-1-N-1, Ho-1-u-1, and SAS) and HNOKs. KIF14 mRNA was up-regulated significantly (P<0.05) in all OSCC-derived cell lines compared with the HNOKs (Fig. 1A). Representative results of immunoblotting analysis are shown in Fig. 1B. The KIF14 protein expression was up-regulated significantly (P<0.05) in all OSCC-derived cell lines compared with the HNOKs.
Fig. 1.
Evaluation of KIF14 expression in OSCC-derived cell lines. (A) Quantification of KIF14 mRNA expression in OSCC-derived cellular lines by qRT-PCR analysis. Significant up-regulation of KIF14 mRNA is seen in eight OSCC-derived cellular lines compared with the HNOKs (P<0.05, Mann–Whitney U-test). Data are expressed as the means±SEM of triplicate results. (B) Immunoblotting analysis of KIF14 protein in the OSCC-derived cell lines and HNOKs. KIF14 protein expression is up-regulated in the OSCC-derived cell lines compared with that in the HNOKs. Densitometric KIF14 protein data are normalized to GAPDH protein levels.
3.2. Evaluation of KIF14 expression in primary OSCCs
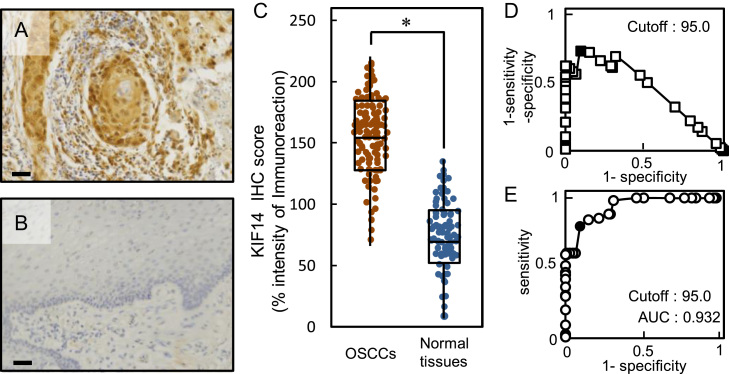
Representative IHC results for KIF14 protein in primary OSCCs and adjacent normal oral tissue are shown in Fig. 2A and B, respectively. Strong KIF14 immunoreactivity was detected in the nucleus of OSCC tissues, whereas the normal tissues showed almost negative immunostaining. We analyzed the KIF14 protein expression in primary OSCCs from 104 patients using the IHC scoring system [36], [37]. The KIF14 IHC scores in OSCCs and adjacent normal oral tissues ranged from 70 to 230 (median, 150) and 10–135 (median, 65), respectively. The IHC scores in primary OSCCs were significantly (P<0.05) higher than in normal oral tissues (Fig. 2C). To determine an optimal cutoff point of the identified IHC scores, we used the Youden Index and ROC curve analyses. In addition to the data from the Youden Index (sensitivity, 79.8%; specificity, 90.4%, P<0.05), ROC curve analysis showed that the cutoff value was 95.0 (Fig. 2D, E).
Fig. 2.
Evaluation of KIF14 expression in primary OSCCs. (A, B) Representative IHC results for KIF14 protein in primary OSCCs and normal oral tissue (scale bars, 10 μm). Strong KIF14 immunoreactivity is detected in the nuclei of primary OSCCs; normal oral tissues show almost negative immunostaining. (C) The state of KIF14 protein expression in primary OSCCs (n=104) and the normal counterparts. The KIF14 IHC scores for normal oral tissues and OSCCs range from 10 to 135 (median, 65) and 70 to 230 (median, 150), respectively. KIF14 protein expression levels in OSCCs are significantly (P<0.05, Mann–Whitney's U test) higher than in normal oral tissues. (D) Youden Index analysis shows that the optimal cutoff point is 95.0. (E) ROC curve analysis shows that the optimal cutoff point is 95.0.
3.3. Establishment of KIF14 knockdown cells
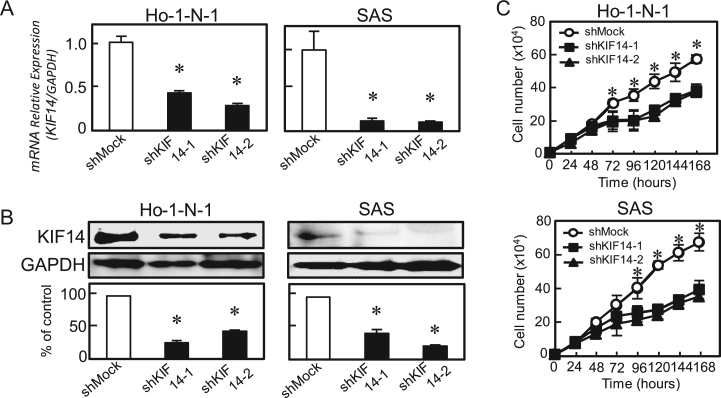
Since frequent up-regulation of KIF14 occurred in OSCC-derived cells (Fig. 1), the OSCC-derived cells (Ho-1-N-1 and SAS) were transfected with KIF14 shRNA and shMock as controls. To confirm the efficiency of shKIF14 transfection, we performed qRT–PCR and immunoblotting analyses (Fig. 3A, B). KIF14 mRNA expression in shKIF14 cells was significantly (P<0.05) lower than in the shMock cells (Fig. 3A). The KIF14 protein level in the shKIF14 cells also decreased compared with the shMock cells (Fig. 3B).
Fig. 3.
Establishment of KIF14 knockdown cells and cellular proliferation of KIF14 knockdown cells. (A) qRT-PCR shows that KIF14 mRNA expression in the shKIF14 cells (Ho-1-N-1-and SAS-derived transfectants; 2 clones each) is significantly (P<0.05, Mann–Whitney U-test) lower than in the shMock cells. (B) Immunoblotting analysis shows that the KIF14 protein levels in shKIF14 cells (Ho-N-1- and SAS-derived transfectants; 2 clones each) also have decreased markedly compared with that in the shMock cells. (C) To determine the effect of shKIF14 on cellular proliferation, shKIF14 and shMock cells were seeded in six-well plates at a density of 1×104 viable cells/well. Both transfectants were counted on seven consecutive days. The cellular growth of shKIF14 cells (Ho-1-N-1- and SAS-derived transfectants; 2 clones each) are significantly (P<0.05, Mann–Whitney U test) inhibited compared with the shMock cells after 72 (Ho-1-N-1) and 96 (SAS) hours. The results are expressed as the means±SEM of values from three assays.
3.4. Cellular proliferation of KIF14 knockdown cells
To evaluate the effect of KIF14 knockdown on cellular growth, we performed a cellular proliferation assay (Fig. 3C). We found a significant (P<0.05) decrease in cellular growth in shKIF14 cells compared with shMock cells. Therefore, the assays showed that KIF14 knockdown decreased cellular growth.
3.5. Cell-cycle analysis of KIF14 knockdown cells
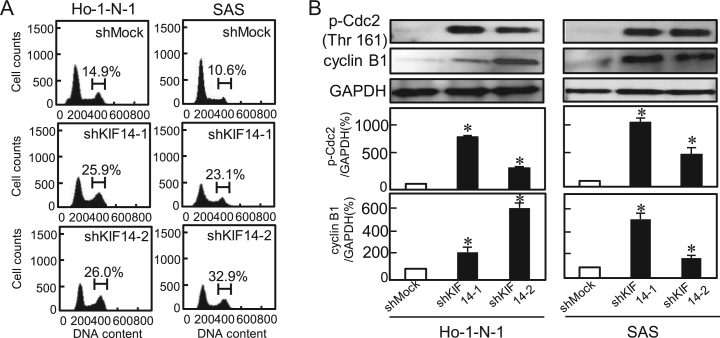
To investigate the mechanism by which KIF14 is related to cellular proliferation, we performed cell-cycle analysis of KIF14 knockdown cells using Ho-1-N-1 and SAS. The percentage of the shKIF14 cells in the G2/M phase was significantly (P<0.05) higher than in the Mock cells (Fig. 4A). We also assessed the expression levels of the G2 arrest-related proteins, p-Cdc2 and cyclin B1. As expected, these were up-regulated in shKIF14 cells (Fig. 4B). These results indicated that shKIF14 cells inhibited cellular proliferation by cell-cycle arrest at the G2/M phase.
Fig. 4.
Cell-cycle analysis of KIF14 knockdown cells. (A) Flow cytometric analysis was performed to investigate the cell cycle in shKIF14 and shMock cells. The percentage of the G2/M phase in shKIF14 cells (Ho-N-1- and SAS-derived transfectants; 2 clones each) has increased markedly compared with the shMock cells (P<0.05, Mann–Whitney U-test). (B) shKIF14 cells show up-regulation of p-Cdc2 and cyclin B1 (Ho-N-1- and SAS-derived transfectants; 2 clones each) compared with shMock cells (P<0.05, Mann–Whitney U test).
3.6. Correlation between KIF14 expression and clinical classifications in primary OSCCs
The correlations between the clinicopathological characteristics of the patients with OSCC and the status of KIF14 protein expression using the IHC scoring system are shown in Table 1. Among the clinical parameters, significant (P=0.019) differences in tumoral size in KIF14-positive patients with OSCC were seen compared with KIF14-negative patients.
Table 1.
Correlation between KIF14 expression and clinical classification in OSCCs.
| Clinical classification | Total |
Results of immunostaining no. of patients (%) |
Pvalue | |
|---|---|---|---|---|
| KIF14 negative | KIF14 positive | |||
| Age at surgery (years) | ||||
| <60 | 18 | 9 (50) | 9 (50) | 0.382 |
| ≧60, <70 | 24 | 8 (35) | 16 (65) | |
| ≧70 | 62 | 23 (37) | 39 (63) | |
| Gender | ||||
| Male | 65 | 24 (37) | 41 (63) | 0.438 |
| Female | 39 | 16 (41) | 23 (59) | |
| T-primary tumor | ||||
| T1 | 6 | 4 (67) | 2 (33) | 0.019⁎ |
| T2 | 54 | 25 (46) | 29 (54) | |
| T3 | 20 | 6 (30) | 14 (70) | |
| T4 | 24 | 5 (22) | 19 (78) | |
| N-regional lymph node | ||||
| Negative | 62 | 22 (35) | 40 (65) | 0.121 |
| Positive | 42 | 18 (44) | 24 (56) | |
| Stage | ||||
| I | 5 | 3 (60) | 2 (40) | 0.563 |
| II | 42 | 17 (37) | 25 (63) | |
| III | 19 | 6 (32) | 13 (68) | |
| IV | 38 | 14 (39) | 24 (61) | |
| Vascular invasion | ||||
| Negative | 85 | 36 (43) | 49 (57) | 0.439 |
| Positive | 19 | 4 (21) | 15 (79) | |
| Histopathologic type | ||||
| Well | 64 | 24 (38) | 40 (62) | 0.512 |
| Moderately | 33 | 13 (40) | 20 (60) | |
| Poorly | 7 | 3 (43) | 4 (57) | |
| Tumoral site | ||||
| Gingiva | 30 | 15 (50) | 15 (50) | 0.471 |
| Tongue | 58 | 20 (35) | 38 (65) | |
| Buccal mucosa | 10 | 4 (40) | 6 (60) | |
| Oral floor | 6 | 1 (17) | 5 (83) | |
P<0.05 was considered significant.
4. Discussion
The current study provided the first evidence that KIF14 overexpression occurs in OSCCs and is positively correlated with tumoral size. In other cancers, KIF14 expression was associated with lymph node metastasis, stage, and grade [38], [39]. Therefore, we assumed that KIF14 may have different functions in difference cancer types. In addition, our KIF14 knockdown experiments showed that KIF14 controlled cellular proliferation by arresting cell-cycle progression at the G2/M phase, suggesting that KIF14 plays a significant role in tumoral size in human OSCCs.
Genomic amplifications, 1q31.3–1q32.1, are observed in several types of cancer, leading to overexpression of KIF14 mRNA and protein [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [28]. In addition, overexpressed KIF14 mRNA and protein were reported as potential prognostic markers and therapeutic targets in retinoblastoma and breast, lung, and ovarian cancers [15], [16], [24], [28], [40]. In contrast, low expression of KIF14 was associated with poor overall survival in patients with lung adenocarcinoma [38]. Therefore, KIF14 plays pivotal roles in development and progression of several types of cancers.
Transient KIF14 knockdown cervical cancer cells showed significantly decreased proliferative and colony forming capabilities [39], [41]; however, the reasons for the cellular behaviors are unknown. Our previous study reported that KIF4A is closely related to the spindle assembly checkpoint (SAC) [36]; therefore, we speculated here that KIF14 is associated with cytokinesis and has a critical role in cell-cycle arrest [11], [12]. Consistent with our hypothesis, our KIF14 knockdown models showed cell-cycle arrest at the G2/M phase by activation of G2 arrest-related proteins. Interestingly, KIF14 did not participate in the SAC (data not shown), whereas KIF14 and KIF4A are similar kinesin superfamily proteins.
Radiation therapy is a major adjuvant treatment for patients with OSCC. The cells in the G2/M phase are highly radiosensitive, whereas the cells in the G0 and G1/S phases have low radiosensitivity. Since KIF14 knockdown led to cell-cycle arrest at the G2/M phase, combination radiation therapy with KIF14 inhibition seems critical for patients with OSCC.
In the current study, we found that KIF14 plays an important role in OSCC growth; therefore, KIF14 expression is likely to be a biomarker of proliferation and a potential therapeutic target for development of anticancer therapy for OSCC.
Conflict of interest statement
The authors have no competing interests to declare.
Acknowledgments
We thank Ms. Lynda C. Charters for editing this manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.07.008.
Contributor Information
Atsushi Kasamatsu, Email: kasamatsua@faculty.chiba-u.jp.
Katsuhiro Uzawa, Email: uzawak@faculty.chiba-u.jp.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Rath O., Kozielski F. Kinesins and cancer. Nat. Rev. Cancer. 2012;12:527–539. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 2.Miki H., Setou M., Hirokawa N. Kinesin superfamily proteins (KIFs) in the mouse transcriptome. Genome Res. 2003;13:1455–1465. doi: 10.1101/gr.984503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa T., Tanaka Y., Matsuoka E., Kondo S., Okada Y., Noda Y., Kanai Y., Hirokawa N. Identification and classification of 16 new kinesin superfamily (KIF) proteins in mouse genome. Proc. Nat. Acad. Sci. U.S.A. 1997;94:9654–9659. doi: 10.1073/pnas.94.18.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y., Feng Y.M. The role of kinesin family proteins in tumorigenesis and progression: potential biomarkers and molecular targets for cancer therapy. Cancer. 2010;116:5150–5160. doi: 10.1002/cncr.25461. [DOI] [PubMed] [Google Scholar]
- 5.Miki H., Okada Y., Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Kanehira M., Katagiri T., Shimo A., Takata R., Shuin T., Miki T., Fujioka T., Nakamura Y. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res. 2007;67:3276–3285. doi: 10.1158/0008-5472.CAN-06-3748. [DOI] [PubMed] [Google Scholar]
- 8.Shimo A., Nishidate T., Ohta T., Fukuda M., Nakamura Y., Katagiri T. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Sci. 2007;98:174–181. doi: 10.1111/j.1349-7006.2006.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniuchi K., Nakagawa H., Nakamura T., Eguchi H., Ohigashi H., Ishikawa O., Katagiri T., Nakamura Y. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005;65:105–112. [PubMed] [Google Scholar]
- 10.Zhang C., Zhu C., Chen H., Li L., Guo L., Jiang W., Lu S.H. Kif18A is involved in human breast carcinogenesis. Carcinogenesis. 2010;31:1676–1684. doi: 10.1093/carcin/bgq134. [DOI] [PubMed] [Google Scholar]
- 11.Carleton M., Mao M., Biery M., Warrener P., Kim S., Buser C., Marshall C.G., Fernandes C., Annis J., Linsley P.S. RNA interference-mediated silencing of mitotic kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Mol. Cell. Biol. 2006;26:3853–3863. doi: 10.1128/MCB.26.10.3853-3863.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruneberg U., Neef R., Li X., Chan E.H., Chalamalasetty R.B., Nigg E.A., Barr F.A. KIF14 and citron kinase act together to promote efficient cytokinesis. J. Cell Biol. 2006;172:363–372. doi: 10.1083/jcb.200511061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardini M., Lee C.H., Beheshti B., Prasad M., Albert M., Marrano P., Begley H., Shaw P., Covens A., Murphy J., Rosen B., Minkin S., Squire J.A., Macgregor P.F. Vol. 7. 2005. High-resolution mapping of genomic imbalance and identification of gene expression profiles associated with differential chemotherapy response in serous epithelial ovarian cancer; pp. 603–613. (Neoplasia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caserta D., Benkhalifa M., Baldi M., Fiorentino F., Qumsiyeh M., Moscarini M. Genome profiling of ovarian adenocarcinomas using pangenomic BACs microarray comparative genomic hybridization. Mol. Cytogenet. 2008;1:10. doi: 10.1186/1755-8166-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corson T.W., Gallie B.L. KIF14 mRNA expression is a predictor of grade and outcome in breast cancer. Int. J. Cancer. 2006;119:1088–1094. doi: 10.1002/ijc.21954. [DOI] [PubMed] [Google Scholar]
- 16.Corson T.W., Zhu C.Q., Lau S.K., Shepherd F.A., Tsao M.S., Gallie B.L. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin. Cancer Res.:Off. J. Am. Assoc. Cancer Res. 2007;13:3229–3234. doi: 10.1158/1078-0432.CCR-07-0393. [DOI] [PubMed] [Google Scholar]
- 17.Gorringe K.L., Jacobs S., Thompson E.R., Sridhar A., Qiu W., Choong D.Y., Campbell I.G. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2007;13:4731–4739. doi: 10.1158/1078-0432.CCR-07-0502. [DOI] [PubMed] [Google Scholar]
- 18.Gras E., Pons C., Machin P., Matias-Guiu X., Prat J. Loss of heterozygosity at the RB-1 locus and pRB immunostaining in epithelial ovarian tumors: a molecular, immunohistochemical, and clinicopathologic study. Int. J. Gynecol. Pathol.: Off. J. Int. Soc. Gynecol. Pathol. 2001;20:335–340. doi: 10.1097/00004347-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kiechle M., Jacobsen A., Schwarz-Boeger U., Hedderich J., Pfisterer J., Arnold N. Comparative genomic hybridization detects genetic imbalances in primary ovarian carcinomas as correlated with grade of differentiation. Cancer. 2001;91:534–540. [PubMed] [Google Scholar]
- 20.Kim T.M., Yim S.H., Shin S.H., Xu H.D., Jung Y.C., Park C.K., Choi J.Y., Park W.S., Kwon M.S., Fiegler H., Carter N.P., Rhyu M.G., Chung Y.J. Clinical implication of recurrent copy number alterations in hepatocellular carcinoma and putative oncogenes in recurrent gains on 1q. Int. J. Cancer. 2008;123:2808–2815. doi: 10.1002/ijc.23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J., Gao M., Lu Y., Feng X., Zhang J., Lin D., Xiao T., Hu Z., Yuan J., Su K., Shipley J., Xue J., Gao Y. Gain of 1q25-32, 12q23-24.3, and 17q12-22 facilitates tumorigenesis and progression of human squamous cell lung cancer. J. Pathol. 2006;210:205–213. doi: 10.1002/path.2050. [DOI] [PubMed] [Google Scholar]
- 22.Staebler A., Karberg B., Behm J., Kuhlmann P., Neubert U., Schmidt H., Korsching E., Burger H., Lelle R., Kiesel L., Bocker W., Shih M., Buchweitz Ie, O. Chromosomal losses of regions on 5q and lack of high-level amplifications at 8q24 are associated with favorable prognosis for ovarian serous carcinoma. Genes Chromosom. Cancer. 2006;45:905–917. doi: 10.1002/gcc.20356. [DOI] [PubMed] [Google Scholar]
- 23.Szponar A., Zubakov D., Pawlak J., Jauch A., Kovacs G. Three genetic developmental stages of papillary renal cell tumors: duplication of chromosome 1q marks fatal progression. Int. J. Cancer. 2009;124:2071–2076. doi: 10.1002/ijc.24180. [DOI] [PubMed] [Google Scholar]
- 24.Theriault B.L., Pajovic S., Bernardini M.Q., Shaw P.A., Gallie B.L. Kinesin family member 14: an independent prognostic marker and potential therapeutic target for ovarian cancer. Int. J. Cancer. 2012;130:1844–1854. doi: 10.1002/ijc.26189. [DOI] [PubMed] [Google Scholar]
- 25.Lagarde P., Przybyl J., Brulard C., Perot G., Pierron G., Delattre O., Sciot R., Wozniak A., Schoffski P., Terrier P., Neuville A., Coindre J.M., Italiano A., Orbach D., Debiec-Rychter M., Chibon F. Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2013;31:608–615. doi: 10.1200/JCO.2012.46.0147. [DOI] [PubMed] [Google Scholar]
- 26.Markowski J., Tyszkiewicz T., Jarzab M., Oczko-Wojciechowska M., Gierek T., Witkowska M., Paluch J., Kowalska M., Wygoda Z., Lange D., Jarzab B. Metal-proteinase ADAM12, kinesin 14 and checkpoint suppressor 1 as new molecular markers of laryngeal carcinoma. Eur. Arch. Oto-Rhino-laryngol.: Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. (EUFOS) 2009;266:1501–1507. doi: 10.1007/s00405-009-1019-3. [DOI] [PubMed] [Google Scholar]
- 27.Bowles E., Corson T.W., Bayani J., Squire J.A., Wong N., Lai P.B., Gallie B.L. Profiling genomic copy number changes in retinoblastoma beyond loss of RB1. Genes Chromosom. Cancer. 2007;46:118–129. doi: 10.1002/gcc.20383. [DOI] [PubMed] [Google Scholar]
- 28.Corson T.W., Huang A., Tsao M.S., Gallie B.L. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene. 2005;24:4741–4753. doi: 10.1038/sj.onc.1208641. [DOI] [PubMed] [Google Scholar]
- 29.Endo Y., Uzawa K., Mochida Y., Shiiba M., Bukawa H., Yokoe H., Tanzawa H. Sarcoendoplasmic reticulum Ca(2+) ATPase type 2 downregulated in human oral squamous cell carcinoma. Int. J. Cancer. 2004;110:225–231. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- 30.Kasamatsu A., Uzawa K., Nakashima D., Koike H., Shiiba M., Bukawa H., Yokoe H., Tanzawa H. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int. J. Mol. Med. 2005;16:269–273. [PubMed] [Google Scholar]
- 31.LH S., MK G., C W. Wiley-Liss; New York: 2009. UICC TNM Classification of Malignant Tumors. [Google Scholar]
- 32.Fukumoto C., Nakashima D., Kasamatsu A., Unozawa M., Shida-Sakazume T., Higo M., Ogawara K., Yokoe H., Shiiba M., Tanzawa H., Uzawa K. WWP2 is overexpressed in human oral cancer, determining tumor size and poor prognosis in patients: downregulation of WWP2 inhibits the AKT signaling and tumor growth in mice. Oncoscience. 2014;1:807–820. doi: 10.18632/oncoscience.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usukura K., Kasamatsu A., Okamoto A., Kouzu Y., Higo M., Koike H., Sakamoto Y., Ogawara K., Shiiba M., Tanzawa H., Uzawa K. Tripeptidyl peptidase II in human oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2013;139:123–130. doi: 10.1007/s00432-012-1307-y. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Rodriguez E., Alvarez-Fernandez S., Chen X., Paiva B., Lopez-Perez R., Garcia-Hernandez J.L., San Miguel J.F., Pandiella A. Deficient spindle assembly checkpoint in multiple myeloma. PloS One. 2011;6:e27583. doi: 10.1371/journal.pone.0027583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrero M., Ferragud J., Orlando L., Valero L., Sanchez del Pino M., Farras R., Font de Mora J. Phosphorylation of AIB1 at mitosis is regulated by CDK1/CYCLIN B. PloS One. 2011;6:e28602. doi: 10.1371/journal.pone.0028602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minakawa Y., Kasamatsu A., Koike H., Higo M., Nakashima D., Kouzu Y., Sakamoto Y., Ogawara K., Shiiba M., Tanzawa H., Uzawa K. Kinesin family member 4A: a potential predictor for progression of human oral cancer. PloS One. 2013;8:e85951. doi: 10.1371/journal.pone.0085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamatoji M., Kasamatsu A., Kouzu Y., Koike H., Sakamoto Y., Ogawara K., Shiiba M., Tanzawa H., Uzawa K. Dermatopontin: a potential predictor for metastasis of human oral cancer. Int. J. Cancer. 2012;130:2903–2911. doi: 10.1002/ijc.26328. [DOI] [PubMed] [Google Scholar]
- 38.Hung P.F., Hong T.M., Hsu Y.C., Chen H.Y., Chang Y.L., Wu C.T., Chang G.C., Jou Y.S., Pan S.H., Yang P.C. The motor protein KIF14 inhibits tumor growth and cancer metastasis in lung adenocarcinoma. PloS One. 2013;8:e61664. doi: 10.1371/journal.pone.0061664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang T., Zhang X.B., Zheng Z.M. Suppression of KIF14 expression inhibits hepatocellular carcinoma progression and predicts favorable outcome. Cancer Sci. 2013;104:552–557. doi: 10.1111/cas.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madhavan J., Coral K., Mallikarjuna K., Corson T.W., Amit N., Khetan V., George R., Biswas J., Gallie B.L., Kumaramanickavel G. High expression of KIF14 in retinoblastoma: association with older age at diagnosis. Investig. Ophthalmol. Vis. Sci. 2007;48:4901–4906. doi: 10.1167/iovs.07-0063. [DOI] [PubMed] [Google Scholar]
- 41.Zhu C., Zhao J., Bibikova M., Leverson J.D., Bossy-Wetzel E., Fan J.B., Abraham R.T., Jiang W. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol. Biol. Cell. 2005;16:3187–3199. doi: 10.1091/mbc.E05-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material