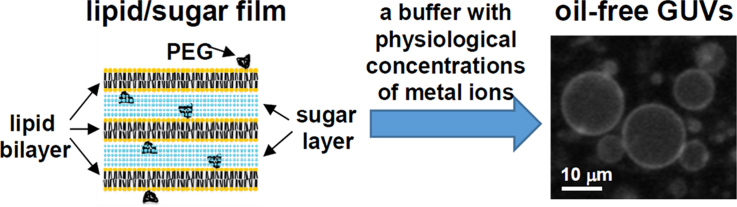
Abstract
We report a new and improved method to prepare, by gentle hydration of lipid films, oil-free giant unilamellar vesicles (GUVs), in which enzymatic reactions can be encapsulated. The traditional method of gentle hydration requires very low concentrations of metal ions, whereas enzymatic reactions generally require mono- and divalent metal ions at physiological concentrations. In order to improve the production of oil-free GUVs that can confine enzymatic reactions, we developed a novel method also based on gentle hydration, but in which the precursor lipid film was doped with both 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEGylated lipid) and sugar. Close examination of the size, shape, and lamellarity of vesicles prepared in this manner demonstrated that the process improves the production of oil-free GUVs even at low temperatures and physiological salt concentrations. PEGylated lipid and sugar were found to synergistically improve GUV formation. Finally, we demonstrate the successful enzymatic synthesis of RNA within oil-free GUVs that were prepared on ice.
Abbreviations: PSGH, PEGylated-lipid-and-sugar-doped gentle hydration; GUV, giant unilamellar vesicle; GMV, giant multilamellar vesicle; PEGylated lipid, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; Nile red, 9-(diethylamino)-5 H-benzo(α)phenoxazin-5-one
Keywords: GUV, Liposome, Gentle hydration, Flow cytometry, Synthetic biology, Membrane protein
Graphical abstract
Highlights
-
•
Preparation of oil-free GUVs at physiological metal ion concentrations on ice.
-
•
Synergistic effects of PEGylated lipid and sugar on improvement in GUV formation.
-
•
Enzymatic RNA synthesis with RNA polymerase in oil-free GUVs prepared on ice.
1. Introduction
A giant unilamellar vesicle (GUV) is a single, closed lipid bilayer membrane of diameter >1 μm. GUVs have been used to study biomembranes, membrane proteins, and models of living cells, and utilized in synthetic biology [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. In these studies, GUVs are often used as systems to mimic cells.
The water in oil (W/O) emulsion centrifuge method [12] and the oil-supported microfluidic method [13], [14], [15] are the standard methods to prepare GUVs that can confine enzymatic reactions. These techniques can generate GUVs in the presence of metal ions at concentrations required for enzymatic reactions. The encapsulation yields of these W/O droplet-based methods are generally high. The lipid-coated ice droplet hydration method recently developed, for instance, obtains very high entrapment yields for water-soluble enzymes into giant vesicles (GVs) [16]. However, as large amounts of oil are used, it could potentially penetrate GUV membranes. In fact, the microfluidic jetting technique, an oil-supported microfluidic method, produces GUVs contaminated with oil [13], [17]. Oil contamination may affect membrane properties such as thickness, stability, and permeability, as well as the activity of integral membrane proteins. Therefore, an oil-free method to prepare GUVs is urgently needed to investigate confined enzymatic reactions and active proteins.
Gentle hydration of lipid films is a traditional technique to prepare oil-free GUVs [18]. This technique gives a lower encapsulation yield than the W/O droplet-based schemes, but the GUVs prepared do not contain any oil at all. However, the technique is not compatible with enzymatic reactions, as it requires very low concentrations of metal ions (<0.1 mM) [19], whereas enzymatic reactions generally require physiological concentrations. Electroformation improves this technique by enabling GUV preparation at physiological concentrations of metal ions [20], [21]. However, there is no report of GUVs prepared with electroformation at a low temperature, such as on ice, and at physiological concentrations of mono- and divalent metal ions. GUV preparation on ice is often required to encapsulate enzymatic reactions and active proteins. The agarose matrix-assisted procedure [22], which is another form of gentle hydration, also enables GUV preparation at physiological concentrations of mono- and divalents. However, GUV preparation through this technique has also not been tested on ice; furthermore, GUVs are typically attached to a surface, and are often arrayed in several layers above the surface [22]. These features would not be suitable for some applications. Thus, current methods of gentle hydration should be further improved.
Here, we describe a novel process of gentle hydration, both at physiological levels of mono- and divalent ions and low temperatures. The method is termed PEGylated lipid-and-sugar-doped Gentle Hydration (PSGH) of lipid films; the defining characteristic of the technique is the doping of lipid films with PEGylated lipid and sugar. GVs prepared using this method were closely examined by microscopy and flow cytometry to characterize size, shape, and lamellarity. Consequently, we found that PSGH improved the production of oil-free GUVs even at physiological concentrations of metal ions and low temperatures. A synergistic effect of PEGylated lipid and sugar on GUV productivity was clearly demonstrated. We also demonstrated the successful enzymatic synthesis of RNA within GUVs prepared on ice.
2. Materials and methods
2.1. Reagents
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DOPE-PEG2000: PEGylated lipid) were purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). d-Fructose and 9-(diethylamino)-5H-benzo(α)phenoxazin-5-one (Nile red), a red fluorescent dye, were obtained from Wako Pure Chemical Industries (Osaka, Japan). Solutions of 1 M Tris–HCl(aq.) (pH 8.0), 500 mM MgCl2(aq.), and 500 mM ethylenediamine-N,N,N,N-tetraacetic acid (EDTA(aq.) pH 8.0) were from Nacalai Tesque (Kyoto, Japan). Mag-Fluo-4 was procured from Invitrogen (Carlsbad, CA, U.S.A.). An NTP mix was purchased from New England Biolabs (Ipswich, MA, USA), while bovine serum albumin (BSA) and tris(2-carboxyethyl)phosphine (TCEP) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Thermo T7 RNA polymerase (50 unit/μL) and TTh RNaseH (10 unit/μL) were obtained from Toyobo (Osaka, Japan). Oligonucleotides were synthesized by Sigma-Aldrich Japan (Tokyo, Japan). The sequence of the oligonucleotides are as follows: non-template strand, 5′-TTTTTTAATACGACTCACTATAGGGATCTTCAGACACACGCTTGCATAGTTTTGCTTTG-3′; template strand, 5′-CAAAGCAAAACTATGCAAGCGTGTGTCTGAAGATCCCTATAGTGAGTCGTATTAAAAAA-3′; molecular beacon probe, 5′-[FAM]-CTATGCAAGCGTGTGTCTGAAGATGCATAG-[BHQ1]-3′. The T7 promoter is underlined. BHQ1 refers to black hole quencher version 1.
2.2. Vesicle preparation by PSGH
In PSGH, vesicle preparation is initiated by formation of a lipid/sugar film, followed by hydration with a buffer solution. To form the film, 20 μL of 10 mM DOPC in CHCl3, 2 μL of 1 mM DOPE-PEG2000 in CHCl3, 40 μL of 50 mM d-fructose in MeOH, 178 μL of CHCl3, and 60 μL of MeOH were mixed. The amount of PEGylated lipid was 1 mol% of DOPC, which is the same ratio used by Yamashita et al. to prepare GUVs in a buffer containing monovalent ions only [23]. The amount of sugar was 10×DOPC, as used by Tsumoto et al. [24]. The solution was put into a 10 mL round-bottom glass flask. The organic solvent was removed with a rotary evaporator (N-1000, EYELA, Japan) equipped with a vacuum pump (DIVAC, ULVAC, USA). The evaporator was set at a speed of 180 rpm, exhaust rate of 1.2 L/min, and temperature of 40 °C. After evaporation for five minutes, a lipid/sugar film of diameter ~2 cm is formed at the bottom of flask. To remove residual solvent, the flask was placed for 17 h in a vacuum desiccator set at 10 mmHg and room temperature. To hydrate, 2 mL of a solution containing 10 mM Tris–HCl (pH 8.0), 100 mM NaCl, and 10 mM MgCl2 was heated to 37 °C and gently poured into the flask. The flask was then sealed and incubated at 37 °C for 2 h to form vesicles. A sample of 1 mL was taken, and filtered through 40 μm mesh nylon (Cell strainer, BD Falcon, USA).
2.3. Preparation of GV reference
To determine lamellarity of thin-walled GVs, a reference mixture containing uni-, bi-, and trilamellar GVs was prepared according to Yamashita’s method [23]. The composition of lipids in the reference was identical to that of vesicles prepared by PSGH to enable a direct quantitative comparison of fluorescence intensity. Thus, the reference mixture was prepared from a lipid film consisting of DOPC and DOPE-PEG2000 (1 mol% of DOPC), which was gently hydrated with deionized water for 2 h at 37 °C, and then filtered through 40 μm mesh nylon.
2.4. Analysis of synergism between PEGylated lipid and sugar
To test whether PEGylated lipid and sugar synergistically improve GUV productivity, two vesicle samples were prepared identically, except that either PEGylated lipid or sugar was excluded during preparation. Thus, a lipid film of DOPC (0.67 mM), doped either with PEGylated lipid (1 mol% of DOPC) or sugar (10×DOPC), was hydrated at 37 °C for 2 h with buffer containing 10 mM Tris–HCl (pH 8.0), 100 mM NaCl, and 10 mM MgCl2.
2.5. Fluorescent staining
All the vesicle membranes were stained with Nile red, which is a lipophilic red fluorescent dye. Nile red dissolved in CHCl3 was added to phospholipid mixtures at 0.2 mol% of DOPC. To stain the encapsulated aqueous pool, Mag-Fluo-4, a hydrophilic green fluorescent dye was added at a final concentration of 1 μM to hydration buffers. The concentration of Nile red was the same as that used by Akashi et al. [25] to determine the lamellarity of thin-walled GVs. The concentration of Mag-Fluo-4 was a manufacturer-recommended one, at which the fluorescence intensity is proportional to the dye concentration.
2.6. Microscopy
Vesicle samples were placed between two cover glass slides, sealed with FrameSeal (Invitrogen, Carlsbad, CA, USA) and characterized with a phase contrast and fluorescence microscope (IX71, Olympus, Japan) equipped with a 20× objective lens and a CCD camera (Model C4742-95-12ER, Hamamatsu Photonics, Japan). Red and green fluorescence images were obtained using corresponding filter and dichroic mirror units (WIG, excitation 520–550 nm/emission>580 nm for red fluorescence; and NIBA, excitation 470–490 nm/emission>510–550 nm for green fluorescence, Olympus, Japan). AquaCosmos (ver. 2.5, Hamamatsu Photonics, Japan) was used to analyze images.
2.7. Determination of lamellarity
The lamellarity of thin-walled GVs was determined according to the method previously described by Akashi et al. [25]. This technique is based on microscopic analysis of GVs stained with Nile red; the fluorescence intensity of the vesicle boundary (i.e., peripheral fluorescence intensity) is proportional to the number of lamellae. Uni-, di-, tri-, and tetralamellar vesicles are clearly distinguishable, based on well-separated averages of the fluorescence intensity at four peripheral sites per GV.
2.8. Flow cytometry
Vesicle samples were diluted 5-fold with the buffer used for vesicle preparation. As Mag-Fluo-4 was intended to stain the encapsulated aqueous pool, EDTA was added to a final concentration of 20 mM to quench fluorescence of dyes that had not been encapsulated. Also, to prevent blockage of flow lines, samples were filtered through a 40 μm mesh (Cell strainer, BD Falcon). Vesicle samples were thus analyzed using the EPICS ALTRA HyPerSort (Beckman-Coulter, Fullerton, CA, USA) flow cytometer. The stream was irradiated by an Ar ion laser beam (λ=488 nm), and fluorescence signals were separated by appropriate dichroic mirrors and band pass filters. Compensation was performed according to the manufacturer’s protocol to correct red and green fluorescence intensities. The number of vesicles observed in a single analysis was 10,000.
2.9. In vitro transcription in oil-free GUV
GUVs used in in vitro transcription experiments were prepared on ice to minimize enzymatic activity during preparation. A 500 μL sample of the transcription solution (40 mM Tris–HCl pH 8.0, 50 mM NaCl, 10 mM MgCl2, 1 mM NTP mix, 0.1 μM template DNA, 1 μM molecular beacon probe, 0.6 unit/μL thermo T7 RNA polymerase, 0.02% BSA, and 1 mM TCEP) was cooled on ice, and added to a flask containing a lipid/sugar film, which had also been cooled on ice. The resulting mixture was left on ice for 30 min. A sample of 400 μL was then filtered through a 40 μm mesh (Cell strainer, BD Falcon). Finally, RNaseH at 0.01 unit/μL was added to quench fluorescence from transcription products that were not encapsulated. Vesicles were incubated at 37 °C for 2 h. Finally, the sample was analyzed using microscopy and flow cytometry.
3. Results and discussion
3.1. PSGH
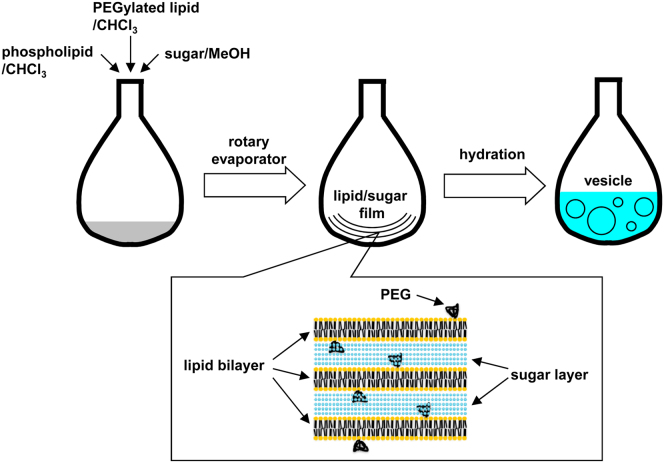
PSGH is based on gentle hydration of a lipid/sugar film (Fig. 1), which is formed by evaporation, under reduced pressure, of organic solvent from a mixture of phospholipid, PEGylated lipid and sugar. Due to the hydrophilic nature of both the PEG group and the sugar molecule, the PEG moiety probably projects out from the lipid bilayer, while sugar molecules settle between lipid bilayers. This might result in alternating layers of sugar and lipid in the film (Fig. 1, lower panel). Gentle hydration with an aqueous solution results in spontaneous formation of vesicles. As oil is never used, the resulting vesicles are oil-free.
Fig. 1.
Outline of PEGylated lipid-and-sugar-doped gentle hydration (PSGH).
3.2. Microscopic analysis
To prepare GUVs at physiological concentrations of mono- and divalent metal ions, we used a buffer containing 100 mM NaCl and 10 mM MgCl2, which are similar to cellular concentrations, and are suitable for protein-based activity [26]. Lipid/sugar films were hydrated at 37 °C, a temperature ideal for cells.
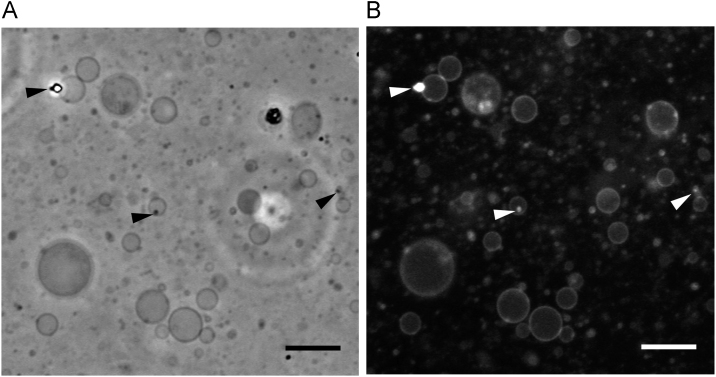
Phase contrast and fluorescence microscopy were used to examine vesicle samples prepared using PSGH (Fig. 2). A number of thin-walled GVs and myelin-like multilamellar vesicles (arrowhead) were observed. Myelin-like multilamellar vesicles have cross sections similar to the myelin sheath covering nerve fibers. On the other hand, thin-walled GVs were mostly spherical and 1–22 μm in diameter. The size range is sufficient to provide models of the cell, as well as compartments for synthetic biology.
Fig. 2.
Phase contrast (A) and fluorescence (B) microscopy of GVs prepared by PSGH. Arrowheads indicate myelin-like multilamellar vesicles. Scale bars are 20 μm.
3.3. Lamellarity
To validate the PSGH process, we analyzed the lamellarity of thin-walled GVs produced. However, the resolution of a phase contrast microscope is in the order of sub-micrometers, whereas the distance between lipid bilayers in a giant multilamellar vesicle (GMV) usually approaches the order of nanometers. Therefore, GMVs cannot be distinguished from GUVs under phase contrast. Akashi et al. reported a fluorescence-based technique to determine the lamellarity of GVs [25]. In this method, lamellarity is determined from peripheral fluorescence of GVs stained with the lipophilic fluorescent dye Nile red. Thin-walled GVs are thus categorized according to peripheral fluorescence intensity, with GUVs exhibiting the least intense fluorescence.
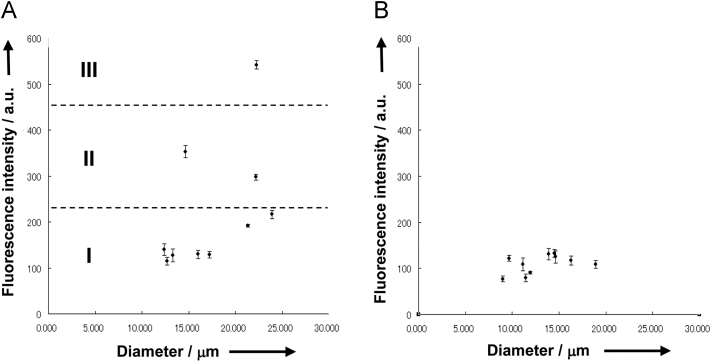
Thus, we determined the lamellarity of thin-walled GVs in this manner. Fig. 3 plots peripheral fluorescence intensity versus the diameter of vesicles from a reference mixture and from a preparation obtained by PSGH. Vesicles in the reference mixture were categorized into groups I, II, and III, which correspond to uni-, bi-, and trilamellar GVs, respectively (Fig. 3a). On the other hand, vesicles prepared by PSGH were concentrated in the region corresponding to group I (Fig. 3b), indicating that these thin-walled GVs are unilamellar.
Fig. 3.
Plots of peripheral red fluorescence intensity versus diameter of thin-walled GVs. (A) A reference preparation containing uni-, bi-, and trilamellar vesicles. (B) A sample of vesicles prepared by PSGH. Error bars are standard error. Myelin-like multilamellar vesicles were excluded from analysis.
3.4. Flow cytometry
Microscopy can be used to examine only a small number of GVs. To analyze large populations of GVs, we used flow cytometry. Since almost all GVs were dispersed (Fig. 2), vesicles could be detected individually. To perform flow cytometry, vesicle membranes and encapsulated aqueous pools were stained with the Nile red and Mag-Fluo-4, respectively (Fig. 4a). To quench fluorescence from unencapsulated reactions, we added excess EDTA to chelate Mg2+, which is necessary for Mag-Fluo-4 to fluoresce.
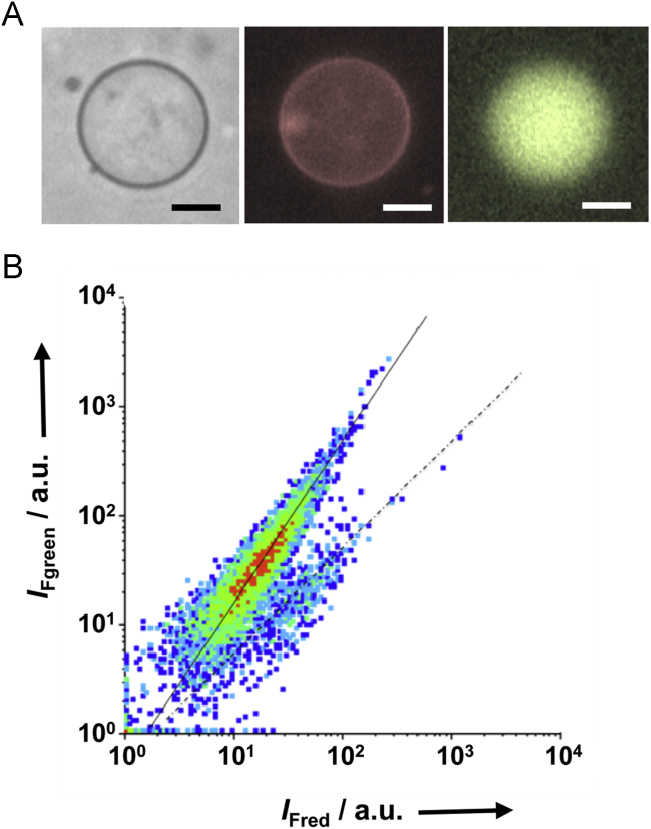
Fig. 4.
Flow cytometry of GVs prepared by the PSGH method. (A) Phase contrast (left), and red (center) and green (right) fluorescence images of samples used in flow cytometry. Vesicle membranes and encapsulated aqueous pools were stained with a red (Nile red) and a green (Mag-Fluo-4) fluorescent dye, respectively. Scale bars are 10 μm. (B) Log–log density plot of fluorescence intensity, with red fluorescence intensity (IFred) on the horizontal axis and green fluorescence intensity (IFgreen) on the vertical axis. The slopes of the solid and dashed lines are 1.5 and 1.0, respectively.
The logarithm of green fluorescence intensity from doubly stained GUVs is proportional to that of red fluorescence intensity. The intensity of red fluorescence, IFred, is proportional to the surface area, S, while that of green fluorescence, IFgreen, is proportional to the volume, V. Therefore, the following equations hold, given constants k and k′.
| (1) |
| (2) |
Assuming that the GUV is a complete sphere, its surface area and volume are related according to the equation
| (3) |
When Eqs. (1), (2) are substituted into Eq. (3) and transformed logarithmically, Eq. (4) is obtained, with .
| (4) |
Therefore, for spherical GUVs of various sizes, a plot of versus should be a line with slope 1.5.
Fig. 4b shows such a plot of 10,000 vesicles prepared using PSGH. The vesicles were distributed into two groups that lie along lines with slopes 1.5 (solid line) and 1.0 (dashed line). More than 8000 vesicles fell along the first line, indicating that more than 80% of the PSGH preparation was spherical GUVs. The remaining vesicles were distributed around the second line, indicating they were myelin-like and multilamellar. For these vesicles, the total surface area of the lamellae is proportional to the volume, because lamellae also occupy the encapsulated space. Therefore, both red and green fluorescence intensities are proportional to the volume, resulting in a line with slope 1.0. In summary, the distribution of vesicles in Fig. 4b demonstrates that PSGH efficiently generated oil-free GUVs in a buffer containing both 100 mM NaCl and 10 mM MgCl2.
3.5. Synergistic effects of PEGylated lipid and sugar
To examine whether PEGylated lipid and sugar synergistically improved GUV production at physiological salt concentrations, we conducted two experiments, in which either PEGylated lipid or sugar was excluded during preparation of lipid films. As before, hydration products were characterized by fluorescence microscopy and flow cytometry.
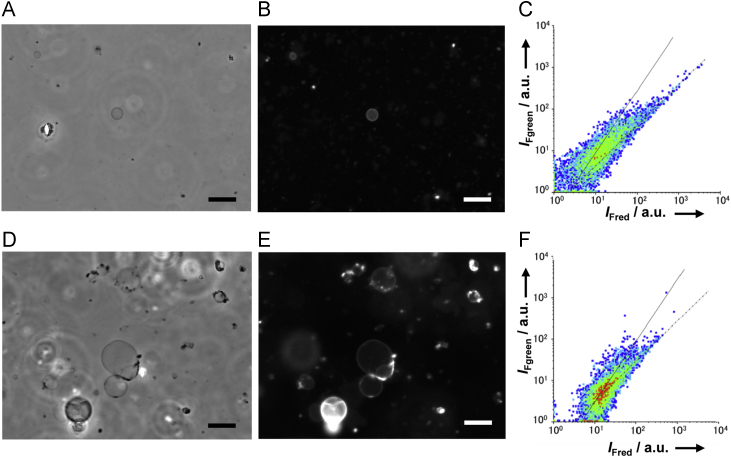
Samples prepared by doping with PEGylated lipid only contained few thin-walled GVs and some myelin-like multilamellar vesicles that strongly fluoresced (Fig. 5a, b). The number of lipid aggregates was quite low, indicating that many lipid bilayers in the film did not separate. In Fig. 5c, most of these vesicles were distributed along a line with slope 1.0, proving that the major lipid aggregates were myelin-like and multilamellar. It has been reported that gentle hydration of lipid film doped with only PEGylated lipid can be used to prepare GUVs in buffers containing up to 2 M NaCl [23]. However, as Fig. 5c demonstrates, GUVs could not be prepared in the presence of both 100 mM NaCl and 10 mM MgCl2.
Fig. 5.
Phase contrast and fluorescence microscopy and flow cytometry of GV samples prepared by doping with either PEGylated lipid only (A, B, C) or sugar only (D, E, F). Scale bars are 20 μm. Horizontal and vertical axes are the red and the green fluorescence intensities, respectively, in arbitrary units. The slopes of the solid and dashed lines are 1.5 and 1.0, respectively.
Additionally, microscopic images of vesicles prepared with sugar only showed a few thin-walled GVs, together with many myelin-like multilamellar vesicles (Fig. 5d, e). The majority of lipid aggregates adhered to one another. These observations are consistent with results from flow cytometry (Fig. 5f), data from which fell along a line with slope 1.0, implying either the presence of multilamellar vesicles, or adhesion of lipid aggregates. It has been reported that gentle hydration using sugar only is suitable to prepare dispersed GUVs in 100 mM NaCl [24]. However, when we attempt this technique in 100 mM NaCl and 10 mM MgCl2, adhesion among lipid aggregates, which is undesirable, occurred to a significant extent.
These experiments strongly suggest that PEGylated lipid and sugar are individually insufficient to separate lipid bilayers. However, PEGylated lipid and sugar synergistically promote separation of lipid bilayers during PSGH. This synergism is essential to increase production of oil-free GUVs in a buffer containing both 100 mM NaCl and 10 mM MgCl2.
3.6. In vitro transcription in oil-free GUVs
We demonstrated the encapsulation of active enzymes, in particular RNA polymerase, which transcribes RNA from DNA. GUVs for this experiment were prepared by gentle hydration on ice, instead of 37 °C, to minimize RNA synthesis prior to encapsulation. Actually, RNA that was synthesized with RNA polymerase during vesicle formation on ice did not reach a detectable level. Therefore, the GUV preparation on ice allowed us to observe RNA that was synthesized only after the vesicle formation.
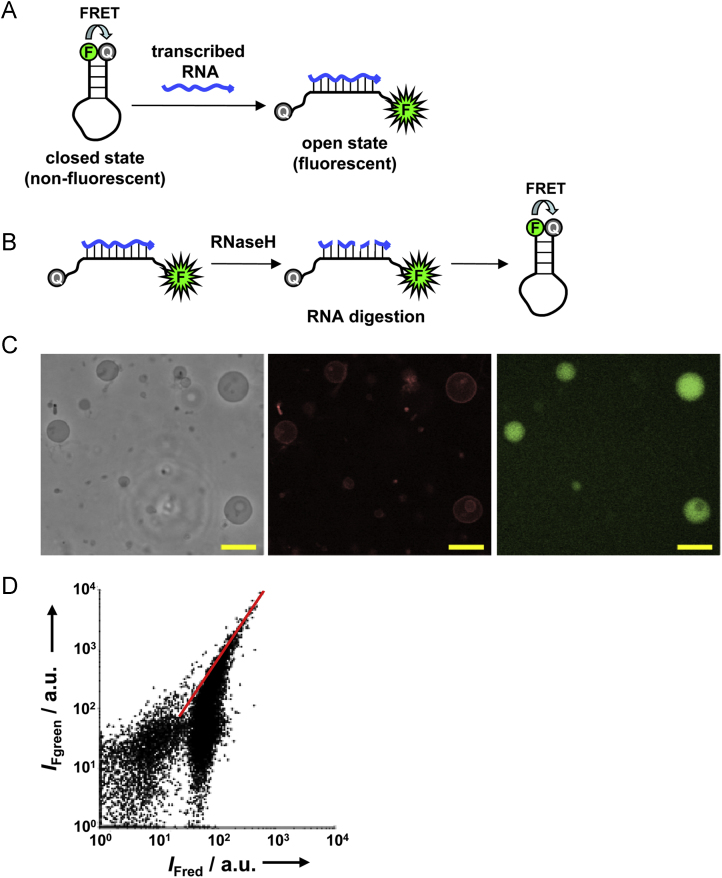
Transcribed RNA was specifically detected using a molecular beacon (MB) probe (Fig. 6a), which is a hairpin DNA labeled with a fluorescent and a quenching dye at the 5′ and the 3′ end, respectively [27]. In the absence of transcribed RNA, the MB probe is folded, and does not fluoresce due to 3′ quenching via fluorescence resonance energy transfer. However, in the presence of transcribed RNA, the probe unfolds and anneals to transcribed RNA so that quenching does not occur. In this case, the probe fluoresces in proportion to the amount of transcribed RNA.
Fig. 6.
Encapsulated RNA synthesis in oil-free GUVs. (A) RNA synthesis was detected using the molecular beacon (MB) probe. F, green fluorescent dye; Q, quencher. (B) Reduction by RNAseH of fluorescence from unencapsulated reactions. RNA strands annealed to MB are specifically digested by RNaseH, and the probe spontaneously refolds back to the hairpin structure, in which fluorescence is quenched by FRET. (C) Phase contrast (left), red fluorescence (center) and green fluorescence (right) images of GUVs encapsulating transcription reactions. Scale bars are 20 μm. (D) Flow cytometry of vesicles with encapsulated enzymes. The slope of the red solid line is 1.5.
Fluorescence due to RNA synthesis outside vesicles would interfere with microscopy and flow cytometry. Therefore, RNaseH was added to degrade any RNA transcribed outside vesicles (Fig. 6b). Since RNaseH does not penetrate the lipid bilayer membrane, RNA strands produced in encapsulated reactions are protected from RNaseH digestion.
Fig. 6c shows microscopic images of GVs that have encapsulated RNA polymerase. These vesicles were prepared on ice, and even though yield decreased to ~30% of production at 37 °C, many thin-walled GVs were still obtained. Peripheral red fluorescence from these vesicles has similar intensity, as is observed for GUVs in Fig. 2, suggesting they are also unilamellar. These GVs also emitted green fluorescence from the encapsulated aqueous pool, indicating that RNA synthesis had occurred in confinement. These results demonstrate that RNA polymerase was active in oil-free GUVs obtained by PSGH.
We performed flow cytometry to analyze a large number of GVs with encapsulated enzyme, and results are shown in Fig. 6d. The distribution of vesicles changed distinctly with the surface area, which in this plot is indirectly measured by red fluorescence on the horizontal axis. Vesicles with red fluorescence exceeding 1.5×102 arbitrary units were distributed along the red solid line with slope 1.5. In these larger vesicles, all MB probes would have unfolded and annealed to transcribed RNA, so that green fluorescence intensity should be proportional to the volume of vesicle. Therefore, these vesicles were GUVs like those shown in Fig. 4b. Among smaller vesicles, which have red fluorescence intensity between 2×101 and 1.5×102 arbitrary units, only about 20% were distributed along the red line; the rest were spread downward from this line. This suggests that enzymatic activity might be inhibited due to frequent collisions between RNA polymerase and the inner surface of these vesicles. These collisions are more frequent because of increased area to volume ratio. Finally, vesicles with fluorescence below 2×101 arbitrary units of red fluorescence are indistinguishable from the background noise, and analysis of these vesicles was not pursued. Taken together, results demonstrate that vesicle-encapsulated RNA polymerase successfully synthesized RNA.
4. Conclusions
We achieved preparation of oil-free GUVs in a buffer containing 100 mM NaCl and 10 mM MgCl2, which are typically needed for enzyme and other protein-based activity. We found that PEGylated lipid and sugar synergistically improved GUV production by PSGH. We demonstrated enzymatic RNA synthesis in reactions confined within oil-free GUVs prepared on ice. The encapsulation yield of PSGH is likely to be similar to that of the lipid film gentle hydration-based methods and therefore is lower than that of the W/O droplet-based methods. The oil-free GUV prepared by PSGH is a suitable compartment for use as a platform in synthetic biology, and is especially useful to capture membrane proteins, which may be sensitive to oil contamination. Since the PSGH method relies on a synergistic effect of PEGylated lipid and sugar, the method is not suitable for experiments that do not tolerate the presence of PEGylated lipid or sugar. The use of oil-free GUVs to study membrane proteins will pioneer new avenues in synthetic biology.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas (24104001-5 to K.S., 23119007 to A.S.) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.07.005.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Walde P., Cosentino K., Engel H., Stano P. Giant vesicles: preparations and applications. ChemBioChem. 2010;11:848–865. doi: 10.1002/cbic.201000010. [DOI] [PubMed] [Google Scholar]
- 2.Kahya N. Protein–protein and protein–lipid interactions in domain-assembly: lessons from giant unilamellar vesicles. Biochim. Biophys. Acta. 2010;1798:1392–1398. doi: 10.1016/j.bbamem.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Szostak J.W., Bartel D.P., Luisi P.L. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 4.Noireaux V., Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. 2004;101:17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda Y.T., Nakadai T., Shin J., Uryu K., Noireaux V., Libchaber A. Assembly of MreB filaments on liposome membrane: a synthetic biology approach. ACS Synth. Biol. 2012;1:53–59. doi: 10.1021/sb200003v. [DOI] [PubMed] [Google Scholar]
- 6.Chiarabelli C., Stano P., Luisi P.L. Chemical approaches to synthetic biology. Curr. Opin. Biotechnol. 2009;20:492–497. doi: 10.1016/j.copbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Stano P., Luisi P.L. Semi-synthetic minimal cells: origin and recent developments. Curr. Opin. Biotechnol. 2013;24:633–638. doi: 10.1016/j.copbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura K., Matsuura T., Nishimura K., Sunami T., Suzuki H., Yomo T. Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir. 2012;28:8426–8432. doi: 10.1021/la3001703. [DOI] [PubMed] [Google Scholar]
- 9.Soga H., Fujii S., Yomo T., Kato Y., Watanabe H., Matsuura T. In vitro membrane protein synthesis inside cell-sized vesicles reveals the dependence of membrane protein integration on vesicle volume. ACS Synth. Biol. 2014;3:372–379. doi: 10.1021/sb400094c. [DOI] [PubMed] [Google Scholar]
- 10.Saito H., Kato Y., Le Berre M., Yamada A., Inoue T., Yoshikawa K., Baigl D. Time-resolved tracking of a minimum gene expression system reconstituted in giant liposomes. ChemBioChem. 2009;10:1640–1643. doi: 10.1002/cbic.200900205. [DOI] [PubMed] [Google Scholar]
- 11.Nourian Z., Roelofsen W., Danelon C. Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane. Angew. Chem. Int. Ed. 2012;51:3114–3118. doi: 10.1002/anie.201107123. [DOI] [PubMed] [Google Scholar]
- 12.Pautot S., Frisken B.J., Weitz D.A. Production of unilamellar vesicles using an inverted emulsion. Langmuir. 2003;19:2870–2879. [Google Scholar]
- 13.Funakoshi K., Suzuki H., Takeuchi S. Formation of giant lipid vesicle like compartments from a planar lipid membrane by a pulsed jet flow. J. Am. Chem. Soc. 2007;129:12608–12609. doi: 10.1021/ja074029f. [DOI] [PubMed] [Google Scholar]
- 14.Ota S., Yoshizawa S., Takeuchi S. Microfluidic formation of monodisperse, cell-sized, and unilamellar vesicles. Angew. Chem. Int. Ed. 2009;48:6533–6537. doi: 10.1002/anie.200902182. [DOI] [PubMed] [Google Scholar]
- 15.Matosevic S., Paegel B.M. Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J. Am. Chem. Soc. 2011;133:2798–2800. doi: 10.1021/ja109137s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroiwa T., Fujita R., Kobayashi I., Uemura K., Nakajima M., Sato S., Walde P., Ichikawa S. Efficient preparation of giant vesicles as biomimetic compartment systems with high entrapment yields for biomacromolecules. Chem. Biodivers. 2012;9:2453–2472. doi: 10.1002/cbdv.201200274. [DOI] [PubMed] [Google Scholar]
- 17.Kirchner S.R., Ohlinger A., Pfeiffer T., Urban A.S., Stefani F.D., Deak A., Lutich A.A., Feldmann J. Membrane composition of jetted lipid vesicles: a Raman spectroscopy study. J. Biophotonics. 2012;5:40–46. doi: 10.1002/jbio.201100058. [DOI] [PubMed] [Google Scholar]
- 18.Reeves J.P., Dowben R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969;73:49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- 19.Mueller P., Chien T.F., Rudy B. Formation and properties of cell-size lipid bilayer vesicles. Biophys. J. 1983;44:375–381. doi: 10.1016/S0006-3495(83)84311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelova M.I., Dimitrov D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986;81:303–311. [Google Scholar]
- 21.D’Onofrio T.G., Hatzor A., Counterman A.E., Heetderks J.J., Sandel M.J., Weiss P.S. Controlling and measuring the interdependence of local properties in biomembranes. Langmuir. 2003;19:1618–1623. [Google Scholar]
- 22.Horger K.S., Estes D.J., Capone R., Mayer M. Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. J. Am. Chem. Soc. 2009;131:1810–1819. doi: 10.1021/ja805625u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita Y., Oka M., Tanaka T., Yamazaki M. A new method for the preparation of giant liposomes in high salt concentrations and growth of protein microcrystals in them. Biochim. Biophys. Acta. 2002;1561:129–134. doi: 10.1016/s0005-2736(02)00338-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsumoto K., Matsuo H., Tomita M., Yoshimura T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf. B: Biointerfaces. 2009;68:98–105. doi: 10.1016/j.colsurfb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Akashi K., Miyata H., Itoh H., Kinosita K., Jr. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 1996;71:3242–3250. doi: 10.1016/S0006-3495(96)79517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. fifth ed. Garland Science, Taylor & Francis; New York: 2008. Membrane transport of small molecules and the electrical properties of membranes; pp. 651–694. (Molecular Biology of the Cell). [Google Scholar]
- 27.Tyagi S., Bratu D.P., Kramer F.R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material