Abstract
Although many studies concerning the sensitivity mechanism of scorpion toxin-potassium channel interactions have been reported, few have explored the biochemical insensitivity mechanisms of potassium channel receptors toward natural scorpion toxin peptides, such as the KCNQ1 channel. Here, by sequence alignment analyses of the human KCNQ1 channel and scorpion potassium channel MmKv2, which is completely insensitive to scorpion toxins, we proposed that the insensitivity mechanism of KCNQ1 toward natural scorpion toxins might involve two functional regions, the turret and filter regions. Based on this observation, a series of KCNQ1 mutants were constructed to study molecular mechanisms of the KCNQ1 channel insensitivity toward natural scorpion toxins. Electrophysiological studies of chimera channels showed that the channel filter region controls KCNQ1 insensitivity toward the classical scorpion toxin ChTX. Interestingly, further residue mutant experiments showed that a single basic residue in the filter region determined the insensitivity of KCNQ1 channels toward scorpion toxins. Our present work showed that amino acid residue diversification at common sites controls the sensitivity and insensitivity of potassium channels toward scorpion toxins. The unique insensitivity mechanism of KCNQ1 toward natural scorpion toxins will accelerate the rational design of potent peptide inhibitors toward this channel.
Keywords: KCNQ1, Insensitivity, Sensitivity, Peptide design, Potassium channel
Highlights
-
•
Insensitivity mechanism of KCNQ1 towards scorpion toxins was still unclear.
-
•
A single basic residue in the KCNQ1 filter region controls its insensitivity.
-
•
Amino acid residue diversification controls KCNQ1 sensitivity and insensitivity.
-
•
Our work will accelerate rational design of KCNQ1 peptide inhibitors.
1. Introduction
The human genome encodes about 100 potassium channels, which serve various physiological functions ranging from the repolarization of neuronal and cardiac action potentials, to regulating Ca2+ signaling and cell volume, to driving cellular proliferation and migration; these potassium channels are also involved in various channelopathies, such as cancer, autoimmune diseases, and metabolic, neurological and cardiovascular disorders [1], [2], [3], [4], [5]. Some potassium channels were shown to be sensitive toward natural peptide toxins from the venoms of numerous animal species, such as scorpions, sea anemones, snakes, marine cone snails and spiders [6], [7], [8]; however, others were not sensitive toward natural peptide toxins [8], [9], [10]. Many studies concerning the sensitivity mechanism of animal toxin-potassium channel interactions have been reported, such as the KTX-Kv1.3 channel [11], ChTX-Kv1.3 channel [12], [13], BeKm-1-hERG channel [14], ChTX-BKCa channel [15], MTX-IKCa channel [16], and ScyTx-SKCa channel [17]. However, few studies have been conducted to explore the insensitivity mechanism of potassium channel receptors toward natural toxin peptides, such as the KCNQ1 channel [18], [19].
The scorpion is a notoriously venomous animal that has evolved a vast array of neurotoxins derived from its venom [20], [21], [22]. Interestingly, although scorpions possess the most potent weaponry of diverse neurotoxins, they are reported to be immune to the toxins of their own venom [23]. Recently, two scorpion potassium channels, MmKv1 and MmKv2, were characterized from Mesobuthus martensii in a whole-genome sequencing project [24]. Studies have shown that MmKv1 had a weaker sensitivity toward scorpion venom and the scorpion toxin ChTX than Kv1.3, a sensitive channel toward scorpion venom and many natural scorpion toxins. MmKv2 was found to be completely insensitive toward scorpion venom and the scorpion toxin charybdotoxin (ChTX). These findings provide us with new avenues to research the insensitivity mechanism of human potassium channel receptors toward scorpion toxins by comparing the human KCNQ1 channel with the scorpion MmKv2 channel.
In this work we have conducted sequence alignment analyses of the human KCNQ1 channel and the scorpion potassium channel MmKv2. We found that a conserved basic residue Lys318 in the channel filter region determines the insensitivity of the KCNQ1 channel toward natural scorpion toxins. Our present work showed a unique insensitivity mechanism of KCNQ1 toward natural scorpion toxins; this finding will accelerate the rational design of potent peptide inhibitors toward the KCNQ1 channel and other associated potassium channels.
2. Materials and methods
2.1. Structure Modeling and bioinformatics analyses
The structure of KCNQ1 was modeled using the KcsA (PDB code: 1BL8) as templates via the SWISSMODEL server as described previously [25], [26], [27]. The MmKv2 sequence was identified for open reading frames using ORFfinder (http://www.ncbi.nlm.nih.gov/projects/gorf/). After excluding signal peptides, the similarity was analyzed by searching against the GenBank NCBI database (http://www.ncbi.nlm.nih.gov/blast) using BLAST algorithms. Sequence alignments were performed using the Clustal_X 1.83 software followed by manual adjustment and viewing with the software Jalview.
2.2. Expression of the natural scorpion toxin peptide ChTX
The ChTX expression vector was constructed as described previously [28], [29]. The ChTX fragment was generated by the overlapping polymerase chain reaction (PCR). The PCR product of ChTX was digested with BamHI and XhoI, and then was inserted into a modified pGEX-4T-1 expression vector. After confirmation by sequencing, the plasmid was transformed into Escherichia coli Rosetta (DE3) cells for expression and characterization as previously described [28], [30]. For example, expression was induced by the addition of 1 mM isopropyl β-d-1-thiogalactopyranoside. After harvesting the cells by centrifugation, the fusion protein was purified. After cleavage, ChTX was further purified by high-pressure liquid chromatography (HPLC). Eluted fractions containing the isolated ChTX protein were lyophilized and stored at −20 °C.
2.3. Cloning and site-directed Mutagenesis for potassium channels
The cDNA of MmKv2 was amplified by PCR using total RNA isolated from M. martensii as template. The PCR product was cloned into the pTZ57R/T TA cloning vector (Fermentas, USA), transformed to E. coli, and then sequenced in both directions with an ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA). The cDNA encoding the KCNQ1 channel was cloned into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). The QuikChange Site-Directed Mutagenesis Kit (Stratagene, USA) was used to produce the mutant KCNQ1 channels based on the wild-type plasmid pcDNA3.1-KCNQ1 (Table S1). All mutant plasmids were verified by DNA sequencing before expression.
2.4. Electrophysiological studies
All vectors containing the entire coding channel sequences were transiently transfected into HEK293 cells (China Center for Type Culture Collection, Wuhan, China) using the SofastTM Transfection Reagent (Sunma Biotech, Xiamen, China), and channel currents were measured 1 to 3 days after transfection. Current measurements and data acquisition were obtained using an EPC 10 patch clamp amplifier (HEKA Elektronik, Lambrecht, Germany) that was controlled by PULSE software (HEKA Elektronik) as previously described [31], [32]. Data analyses were performed with IgorPro (WaveMetrics, Lake Oswego, OR).
3. Results and discussion
3.1. Two functional regions in scorpion toxin-sensitive potassium channels also exist in KCNQ1 and MmKv2 that are completely insensitive toward scorpion toxins
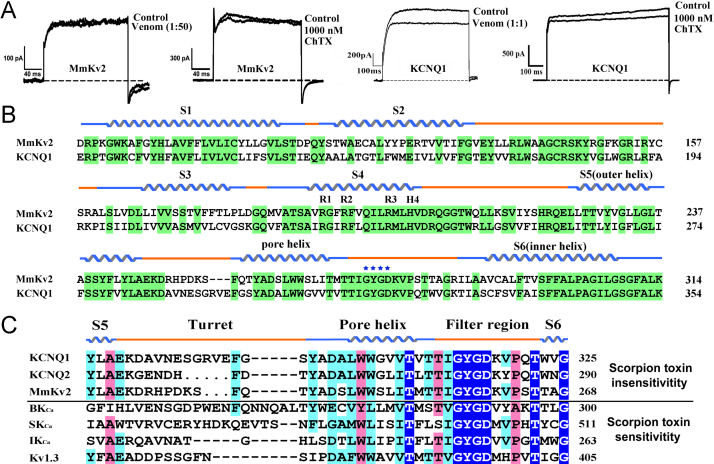
Our recent work showed that the scorpion potassium channel MmKv2 from M. martensii, which was characterized in a whole-genome sequencing project, was completely insensitive to scorpion whole venom and the classic scorpion toxin charybdotoxin (ChTX). These features are similar to the receptor KCNQ1 channel (Fig. 1A). Interestingly, primary structure blast analyses showed that MmKv2 belongs to the KCNQ potassium channel subfamily and is a KCNQ1-like potassium channel (Fig. 1B). Sequence analysis of the two classical functional regions, the turret and filter regions of scorpion toxin-sensitive channels and scorpion toxin-insensitive channels, showed that the insensitivity mechanism of KCNQ1 toward natural scorpion toxins might be similar to that of MmKv2 toward scorpion venoms, which also involved the two functional regions the turret and filter region (Fig. 1C). Two classical functional regions in scorpion toxin-sensitive potassium channels also exist in KCNQ1 and MmKv2 that were completely insensitive toward scorpion toxins, suggesting that the potassium channel turret and filter regions might also control KCNQ1 channel insensitivity toward scorpion toxins.
Fig. 1.
Two classical functional regions in scorpion toxin-sensitive potassium channels are also present in KCNQ1 and MmKv2, which are completely insensitive to scorpion toxins. A, KCNQ1 and MmKv2 are scorpion venom and scorpion toxin insensitivity channels. The venom of M. martensii was used to test the sensitivity of KCNQ1 and MmKv2 potassium channels, which was diluted using extracellular fluid as volume ratio 1:1 before using. B, KCNQ1 and MmKv2 belong to the same voltage-gated potassium channel subfamily. Conserved amino acid residues are indicated in bright green, and secondary structure elements are indicated above the sequences. The conserved positively charged residues in the voltage sensor of the S4 segment are marked, and the characteristic residues of the ion selectivity filter “GYGD” are also indicated. C, Sequence alignments of toxin-interacting regions (turret and filter regions) in KCNQ1 and KCNQ2 potassium channels, scorpion-derived potassium channel MmKv2, and scorpion-toxin-sensitive potassium channels, including the filter region and turret region. The complte conserved amino acid residues are indicated in blue, such as “GYGD”. Secondary structure elements are indicated above the sequences.
3.2. One conserved functional region determines potassium channel sensitivity and insensitivity toward the classical scorpion toxin
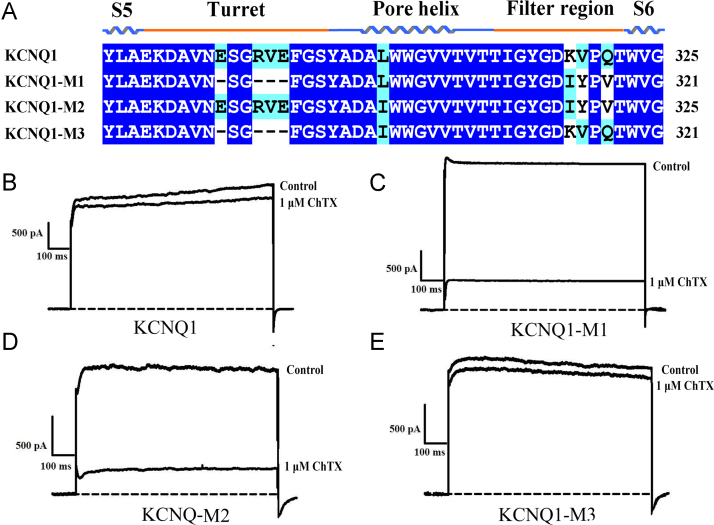
The vestibules of potassium channels are the determinants responsible for scorpion toxin binding. These channel vestibules are composed of turret and filter regions [31], [33], [34]. Thus far, many classical basic scorpion toxins and novel acidic scorpion toxins had little effect on the KCNQ1 channel. To investigate the molecular mechanism of KCNQ1 channel insensitivity toward scorpion toxins, we chose a classical scorpion toxin, ChTX, and constructed a human KCNQ1 channel chimera containing both turret and filter region mutants (KCNQ1-M1) (Fig. 2A). In contrast to the insensitivity of the KCNQ1 channel toward the scorpion toxin ChTX, pharmacological experiments showed that the currents of KCNQ1-M1 channel chimera was effectively inhibited by 1 μM of ChTX (Fig. 2B and C). These results suggest that turret region or filter region of the KCNQ1 channel might determine its insensitivity toward the scorpion toxin ChTX.
Fig. 2.
The filter region of the KCNQ1 potassium channel determines the insensitivity of KCNQ1 toward the scorpion toxin ChTX. A, Mutant designs for the KCNQ1 channel. KCNQ1-M1 is a double region mutant, KCNQ2-M1 is a turret region mutant, and KCNQ1-M3 is a filter region mutant. B-E, Sensitivities of the KCNQ1 channel and designed channel mutants toward the natural scorpion toxin ChTX.
To further explore the detailed region that determines KCNQ1 channel insensitivity. Two new KCNQ1 channel chimeras containing a turret region mutant (KCNQ1-M3) or a filter region mutant (KCNQ1-M2) were constructed (Fig. 2A). Similar to the insensitivity of the KCNQ1 channel toward the scorpion toxin ChTX, pharmacological experiments showed that the currents of the KCNQ1-M3 chimera channel were not effectively inhibited by 1 μM of the scorpion toxin ChTX (Fig. 2D). Interestingly, different from the insensitivity of the KCNQ1 channel toward the scorpion toxin ChTX, pharmacological experiments showed that the currents of the KCNQ1-M2 chimera channel were effectively inhibited by 1 μM of the scorpion toxin ChTX (Fig. 2E). These results suggest that the single filter region of KCNQ1 channel determined its insensitivity toward the scorpion toxin ChTX.
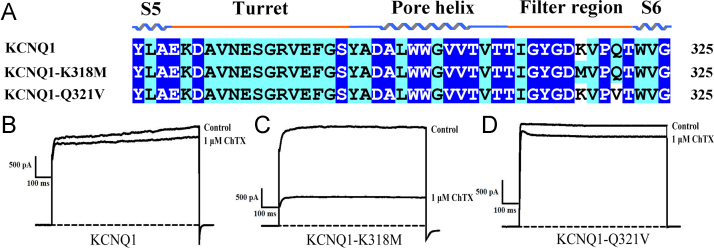
3.3. A single conserved basic residue in the potassium channel filter region controls KCNQ1 insensitivity toward the classical scorpion toxin
Electrostatic interactions play a dominant role in scorpion toxin-potassium channel sensitivity; therefore, different charged residues might also play an important role in scorpion toxin-potassium channel insensitivity. Sequence alignment of turret and filter region residues of scorpion toxin-sensitive and scorpion toxin-insensitive potassium channels showed that there is a special Lys318 residue in the KCNQ channel filter region that is similar to the scorpion MmKv2 channel. However, in the scorpion toxin-sensitive potassium channels, it is usually replaced by an uncharged residue, such as Met in the Kv1.3 channel (Fig. 1). Considering the dominant electrostatic interactions between the scorpion toxin and potassium channel, we proposed a bold conjecture that the unique basic residue Lys318 was expected to be unfavorable for the binding of the potent toxin with the KCNQ1 channel [35]. Subsequently, the differential residues between KCNQ1 and KCNQ1-M3 channels were further investigated by site-directed mutagenesis. As shown in Fig. 3, Lys318 in the KCNQ1 channel filter region, one of the different residues from those of the KCNQ1-M3 channel, was found to affect the binding of ChTX; however, another residue, Q321, did not affect the binding of ChTX. These data indicates that one single conserved basic residue, Lys318, located at the filter region, determines the insensitivity of the KCNQ1 potassium channel toward the natural scorpion toxins. Until now, no potent natural peptide toxin was found to interact with KCNQ1 channels [18]. Our present results showed that a single basic residue in the filter region of the KCNQ1 channel determined its insensitivity to scorpion toxins, thus highlighting the functional roles of basic residues located at the filter region of potassium channels in scorpion-toxin potassium channel interactions, and accelerating the rational design of potent KCNQ1 peptide inhibitors.
Fig. 3.
A single basic residue in filter region determines the insensitivity of KCNQ1 channels toward scorpion toxins. A, Sequence alignment of the scorpion toxin-sensitive channel Kv1.3, scorpion toxin-insensitive channel KCNQ1, and the KCNQ1 mutant channels targeting filter region. B-D, Sensitivities of the KCNQ1 channel and designed single residue mutants toward the natural scorpion toxin ChTX.
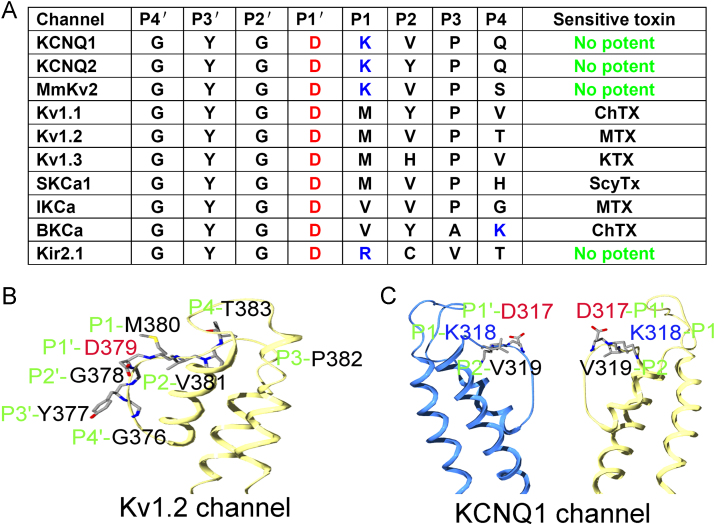
3.4. Amino acid residue diversification at common sites controls the sensitivity and insensitivity of potassium channels toward scorpion toxins
Regarding the interactions between scorpion toxins and insensitive potassium channels, there are two known functional regions in the vestibules of potassium channels: one is the turret region, and the other is the filter region [18], [34], [36], [37]. Previously, some pioneer works have shown that the potassium channel turret region can influence toxin-potassium channel interactions and plays an important role in potassium channel sensitivity toward natural scorpion toxins, such as the ChTX-BKCa channel, MTX-IKCa channel, and ScyTx-SKCa channel [15], [17], [38]. Studies of SKCa channels and BKCa+β4 channels showed that the turret regions of the potassium channel also played an important role in the potassium channel insensitivity toward scorpion toxins [28], [39] Based on the observation of filter region basic residues of the KCNQ1 channel and scorpion channel MmKv2, our present work investigated the influence of the potassium channel filter region on the sensitivity of scorpion toxin peptides. To explore its influence in detail, we named the filter region sequence GYGDKVPQ-322 (in KCNQ1 as an example) as P4′-P3′-P2′-P1′-P1-P2-P3-P4. In this system, we found the following: the P1′, P2′, P3′ and P4′ sites were always conserved; the P2′ and P4′ sites were always Gly, which was important to the conserved filter structure and ion selectivity of the potassium channel; the P1′ site was often Asp, and the P3′ site was often Tyr (Fig. 4A). Non-basic residues located at P2 can influence the activity and selectivity of potassium channels toward natural toxin peptides. Basic residues located at P2 can determine the insensitivity of some potassium channels, such as Kv1.4 channels (P2 is Lys) and Kv1.5 channels (P2 is Arg) [40]. Our present work showed that the complete insensitivity of potassium channels toward animal toxins was primarily determined by the basic residues Lys and Arg located at P1, such as in the Kir2.1 channels (P1 is Arg) [41], KCNQ1 channels (P1 site is Lys), KCNQ2 channels (P1 is Lys) [42], and MmKv1 channels (P1 is Arg) [24] (Fig. 4B and C).
Fig. 4.
One basic residue in the potassium channel filter region abolishes the potassium channel sensitivity toward scorpion toxins. To explore its influence in detail, we named the filter region sequence GYGDKVPQ-322 (in KCNQ1 as an example) as P4′-P3′-P2′-P1′-P1-P2-P3-P4. A, Representative residues of filter regions in the scorpion toxin-sensitive and -insensitive potassium channels. B, The P1 site, located at the entrance of K+ ion in potassium channels, controls channel sensitivity toward scorpion toxins.
Numous studies including ours have shown that the sensitivity and insensitivity mechanisms of potassium channels toward scorpion pore-blocking peptide toxins can be influenced by the turret region, but are primarily controlled by the filter region. Basic residues in both the potassium channel turret and filter regions play an important role in the sensitive and insensitive interactions. For the KCNQ1 channel, a single conserved basic residue in the filter region (P1 site) controls the complete KCNQ1 insensitivity to the natural scorpion toxins.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Sciences Foundation of China (Nos. 31470812, 31170789 and 31200557).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.07.003.
Contributor Information
Zongyun Chen, Email: chenzy2005@126.com.
Yingliang Wu, Email: ylwu@whu.edu.cn.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Heil B., Ludwig J., Lichtenberg-Frate H., Lengauer T. Computational recognition of potassium channel sequences. Bioinformatics. 2006;22:1562–1568. doi: 10.1093/bioinformatics/btl132. [DOI] [PubMed] [Google Scholar]
- 2.Miller C. An overview of the potassium channel family. Genome Biol. 2000;(1) doi: 10.1186/gb-2000-1-4-reviews0004. REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simeone T.A., Simeone K.A., Samson K.K., Kim do Y., Rho J.M. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol Dis. 2013;54:68–81. doi: 10.1016/j.nbd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahalan M.D., Chandy K.G. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wulff H., Castle N.A., Pardo L.A. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casewell N.R., Wuster W., Vonk F.J., Harrison R.A., Fry B.G. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol. 2013;28:219–229. doi: 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez de la Vega R.C., Schwartz E.F., Possani L.D. Mining on scorpion venom biodiversity. Toxicon. 2010;56:1155–1161. doi: 10.1016/j.toxicon.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Mouhat S., Andreotti N., Jouirou B., Sabatier J.M. Animal toxins acting on voltage-gated potassium channels. Curr Pharm Des. 2008;14:2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 9.Mulkey D.K., Hawkins V.E., Hawryluk J.M., Takakura A.C., Moreira T.S., Tzingounis A.V. Molecular underpinnings of ventral surface chemoreceptor function: focus on KCNQ channels. J Physiol. 2015 doi: 10.1113/jphysiol.2014.286500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung S.Y., Greenwood I.A. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange A., Giller K., Hornig S., Martin-Eauclaire M.F., Pongs O., Becker S., Baldus M. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature. 2006;440:959–962. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- 12.Chen R., Robinson A., Gordon D., Chung S.H. Modeling the binding of three toxins to the voltage-gated potassium channel (Kv1.3) Biophys J. 2011;101:2652–2660. doi: 10.1016/j.bpj.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werkman T.R., Kawamura T., Yokoyama S., Higashida H., Rogawski M.A. Charybdotoxin, dendrotoxin and mast cell degranulating peptide block the voltage-activated K+ current of fibroblast cells stably transfected with NGK1 (Kv1.2) K+ channel complementary DNA. Neuroscience. 1992;50:935–946. doi: 10.1016/0306-4522(92)90216-o. [DOI] [PubMed] [Google Scholar]
- 14.Yi H., Cao Z., Yin S., Dai C., Wu Y., Li W. Interaction simulation of hERG K+ channel with its specific BeKm-1 peptide: insights into the selectivity of molecular recognition. J Proteome Res. 2007;6:611–620. doi: 10.1021/pr060368g. [DOI] [PubMed] [Google Scholar]
- 15.Qiu S., Yi H., Liu H., Cao Z., Wu Y., Li W. Molecular Information of charybdotoxin blockade in the large conductance calcium-activated potassium channel. J Chem Inf Model. 2009;49:1831–1838. doi: 10.1021/ci900025n. [DOI] [PubMed] [Google Scholar]
- 16.Yi H., Qiu S., Wu Y., Li W., Wang B. Differential molecular information of maurotoxin peptide recognizing IK(Ca) and Kv1.2 channels explored by computational simulation. BMC Struct Biol. 2011;11:3. doi: 10.1186/1472-6807-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y., Cao Z., Yi H., Jiang D., Mao X., Liu H., Li W. Simulation of the interaction between ScyTx and small conductance calcium-activated potassium channel by docking and MM-PBSA. Biophys J. 2004;87:105–112. doi: 10.1529/biophysj.103.039156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z.Y., Zeng D.Y., Hu Y.T., He Y.W., Pan N., Ding J.P., Cao Z.J., Liu M.L., Li W.X., Yi H., Jiang L., Wu Y.L. Structural and functional diversity of acidic scorpion potassium channel toxins. PloS one. 2012;7:e35154. doi: 10.1371/journal.pone.0035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.H., Xu S.J., Bendahhou S., Wang X.L., Wang Y., Xu W.Y., Jin H.W., Sun H., Su X.Y., Zhuang Q.N., Yang Y.Q., Li Y.B., Liu Y., Xu H.J., Li X.F., Ma N., Mou C.P., Chen Z., Barhanin J., Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 20.Han S., Yi H., Yin S.J., Chen Z.Y., Liu H., Cao Z.J., Wu Y.L., Li W.X. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. The Journal of biological chemistry. 2008;283:19058–19065. doi: 10.1074/jbc.M802054200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S., Peigneur S., Gao B., Luo L., Jin D., Zhao Y., Tytgat J. Molecular diversity and functional evolution of scorpion potassium channel toxins. Mol Cell Proteomics. 2010;10 doi: 10.1074/mcp.M110.002832. M110 002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beeton C., Wulff H., Standifer N.E., Azam P., Mullen K.M., Pennington M.W., Kolski-Andreaco A., Wei E., Grino A., Counts D.R., Wang P.H., LeeHealey C.J., S.A. B, Sankaranarayanan A., Homerick D., Roeck W.W., Tehranzadeh J., Stanhope K.L., Zimin P., Havel P.J., Griffey S., Knaus H.G., Nepom G.T., Gutman G.A., Calabresi P.A., Chandy K.G. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legros C., Martin-Eauclaire M.F., Cattaert D. The myth of scorpion suicide: are scorpions insensitive to their own venom? J Exp Biol. 1998;201(Pt 18):2625–2636. doi: 10.1242/jeb.201.18.2625. [DOI] [PubMed] [Google Scholar]
- 24.Cao Z., Yu Y., Wu Y., Hao P., Di Z., He Y., Chen Z., Yang W., Shen Z., He X., Sheng J., Xu X., Pan B., Feng J., Yang X., Hong W., Zhao W., Li Z., Huang K., Li T., Kong Y., Liu H., Jiang D., Zhang B., Hu J., Hu Y., Wang B., Dai J., Yuan B., Feng Y., Huang W., Xing X., Zhao G., Li X., Li Y., Li W. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat Commun. 2013;4:2602. doi: 10.1038/ncomms3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renisio J.G., Romi-Lebrun R., Blanc E., Bornet O., Nakajima T., Darbon H. Solution structure of BmKTX, a K+ blocker toxin from the Chinese scorpion Buthus Martensi. Proteins. 2000;38:70–78. doi: 10.1002/(sici)1097-0134(20000101)38:1<70::aid-prot8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 27.Howard R.J., Clark K.A., Holton J.M., Minor D.L., Jr. Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron. 2007;53:663–675. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan G., Yi H., Chen M., Sun L., Li W., Wu Y., Ding J. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels, The. Journal of biological chemistry. 2008;283:24177–24184. doi: 10.1074/jbc.M800179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z., Hu Y., Hu J., Yang W., Sabatier J.M., De Waard M., Cao Z., Li W., Han S., Wu Y. Unusual binding mode of scorpion toxin BmKTX onto potassium channels relies on its distribution of acidic residues. Biochemical and biophysical research communications. 2014;447:70–76. doi: 10.1016/j.bbrc.2014.03.101. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z., Han S., Cao Z., Wu Y., Zhuo R., Li W. Fusion expression and purification of four disulfide-rich peptides reveals enterokinase secondary cleavage sites in animal toxins. Peptides. 2013;39:145–151. doi: 10.1016/j.peptides.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Yin S.J., Jiang L., Yi H., Han S., Yang D.W., Liu M.L., Liu H., Cao Z.J., Wu Y.L., Li W.X. Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. Journal of proteome research. 2008;7:4890–4897. doi: 10.1021/pr800494a. [DOI] [PubMed] [Google Scholar]
- 32.Fry B.G., Roelants K., Champagne D.E., Scheib H., Tyndall J.D., King G.F., Nevalainen T.J., Norman J.A., Lewis R.J., Norton R.S., Renjifo C., de la Vega R.C. Vol. 10. 2009. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms; pp. 483–511. (Annual review of genomics and human genetics). [DOI] [PubMed] [Google Scholar]
- 33.Broomand A., Osterberg F., Wardi T., Elinder F. Electrostatic domino effect in the Shaker K channel turret. Biophysical journal. 2007;93:2307–2314. doi: 10.1529/biophysj.107.104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu C.Q., Zhu S.Y., Chi C.W., Tytgat J. Turret and pore block of K+ channels: what is the difference? Trends in pharmacological sciences. 2003;24:446–448. doi: 10.1016/S0165-6147(03)00223-2. author reply 448–449. [DOI] [PubMed] [Google Scholar]
- 35.Han S., Yin S., Yi H., Mouhat S., Qiu S., Cao Z., Sabatier J.M., Wu Y., Li W. Protein-protein recognition control by modulating electrostatic interactions. Journal of proteome research. 2010;9:3118–3125. doi: 10.1021/pr100027k. [DOI] [PubMed] [Google Scholar]
- 36.Gross A., Abramson T., MacKinnon R. Transfer of the scorpion toxin receptor to an insensitive potassium channel. Neuron. 1994;13:961–966. doi: 10.1016/0896-6273(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee A., Lee A., Campbell E., Mackinnon R. Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. eLife. 2013;2:e00594. doi: 10.7554/eLife.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi H., Qiu S., Cao Z., Wu Y., Li W. Molecular basis of inhibitory peptide maurotoxin recognizing Kv1.2 channel explored by ZDOCK and molecular dynamic simulations. Proteins. 2008;70:844–854. doi: 10.1002/prot.21706. [DOI] [PubMed] [Google Scholar]
- 39.Feng J., Hu Y., Yi H., Yin S., Han S., Hu J., Chen Z., Yang W., Cao Z., De Waard M., Sabatier J.M., Li W., Wu Y. Two conserved arginine residues from the SK3 potassium channel outer vestibule control selectivity of recognition by scorpion toxins. J Biol Chem. 2013;288:12544–12553. doi: 10.1074/jbc.M112.433888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilquin B., Braud S., Eriksson M.A., Roux B., Bailey T.D., Priest B.T., Garcia M.L., Menez A., Gasparini S. A variable residue in the pore of Kv1 channels is critical for the high affinity of blockers from sea anemones and scorpions, The. Journal of biological chemistry. 2005;280:27093–27102. doi: 10.1074/jbc.M413626200. [DOI] [PubMed] [Google Scholar]
- 41.Millar I.D., Wang S., Brown P.D., Barrand M.A., Hladky S.B. Kv1 and Kir2 potassium channels are expressed in rat brain endothelial cells. Pflugers Archiv: European journal of physiology. 2008;456:379–391. doi: 10.1007/s00424-007-0377-1. [DOI] [PubMed] [Google Scholar]
- 42.Ru Q., Tian X., Wu Y.X., Wu R.H., Pi M.S., Li C.Y. Voltage-gated and ATP-sensitive K+ channels are associated with cell proliferation and tumorigenesis of human glioma. Oncol Rep. 2014;31:842–848. doi: 10.3892/or.2013.2875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material